Abstract
Although dyspnea and fatigue are hallmark symptoms of heart failure (HF), the burden of pain may be underrecognized. This study assessed pain in HF and identified contributing factors. As part of a multicenter study, 96 veterans with HF (96% male, 67 ± 11 years) completed measures of symptoms, pain (Brief Pain Inventory [BPI]), functional status (Functional Morbidity Index), and psychological state (Patient Health Questionnaire-2 and Generalized Anxiety Disorder-2). Single items from the BPI interference and the quality of life-end of life measured social and spiritual well-being. Demographic and clinical variables were obtained by chart audit. Correlation and linear regression models evaluated physical, emotional, social, and spiritual factors associated with pain. Fifty-three (55.2%) HF patients reported pain, with a majority (36 [37.5%]) rating their pain as moderate to severe (pain ≥ 4/10). The presence of pain was reported more frequently than dyspnea (67 [71.3%] vs. 58 [61.7%]). Age (P = 0.02), psychological (depression: P = 0.002; anxiety: P = 0.001), social (P < 0.001), spiritual (P = 0.010), and physical (health status: P = 0.001; symptom frequency: P = 0.000; functional status: P = 0.002) well-being were correlated with pain severity. In the resulting model, 38% of the variance in pain severity was explained (P < 0.001); interference with relations (P < 0.001) and symptom number (P = 0.007) contributed to pain severity. The association of physical, psychological, social, and spiritual domains with pain suggests that multidisciplinary interventions are needed to address the complex nature of pain in HF.
Keywords: Heart failure, symptoms, spiritual well-being, social work, total pain, palliative care, social well-being, conceptual framework, PHQ-2, BPI
Introduction
Awareness of the burden of pain and symptoms in chronic heart failure (HF) is growing.1–4 Because HF is a progressive disease, treatment is focused on slowing disease progression and palliating symptoms.4–6 Approximately 5.3 million Americans are currently diagnosed with HF,7 with more than 550,000 new cases each year.6 The prevalence of HF, which is primarily a disease of the elderly,6,8 increases with age; approximately 80% of cases occur in patients older than 65 years.6,9 In the United States, HF accounts for 12–15 million office visits a year and 6.5 million hospital days.6 HF hospital discharges increased 171% from 1979 to 2005,7 and hospital admissions for HF consume more Medicare dollars than any other diagnosis.10
The disease trajectory of HF is characterized by periods of acute symptomatic exacerbation followed by returns to nearly baseline.11 Despite the symptom burden of advanced HF12 and because dyspnea and fatigue are considered the hallmarks of HF,6,8,13 other symptoms amenable to palliation, including pain, may go unnoticed.12 Awareness of pain in HF dates to the SUPPORT study14 in which family caregivers reported that four of 10 patients suffered severe pain “most of the time” during their last three days of life. Although few studies have investigated pain in HF, limited studies suggest that the incidence of pain ranges from 5% to 78%.2,13,15–18 In two studies of HF patients in late life, the symptom of pain was second only to dyspnea in prevalence.13,18 The present study was conducted to elucidate the nature of pain in the population with advanced HF.
Conceptual Framework
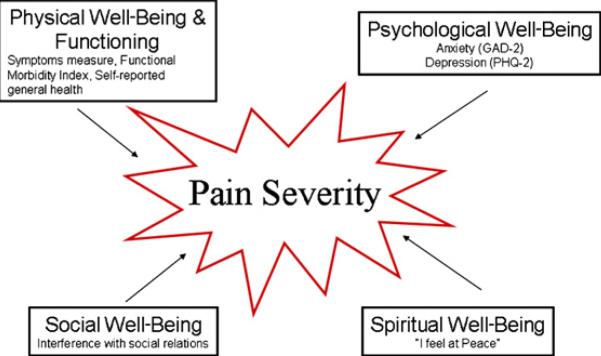
In an effort to more accurately understand the phenomena of pain in HF, a conceptual framework was chosen to guide this investigation. Saunders's19 total pain theory posited that physical, psychological, social, and spiritual domains influence pain and suggested that clinicians should explore for mental, social, and spiritual dimensions of pain.19 Ferrell et al.20 operationalized Saunders's work into a conceptual framework, “pain impacts quality of life” (Fig. 1). Functional status and symptoms are considered as key factors in physical well-being.20 Current research across populations suggests that, as pain or physical discomfort increase, functional status deteriorates.21–24 Although HF patients are known to experience numerous symptoms,2,8,25–28 little is known about their influence on pain severity. Anxiety and depression, aspects of psychological distress that may be more common in HF,8,15,16,29 appear to be associated with increased physical symptomatology.16,30 Studies examining the influence of the spiritual domain on pain are limited, but in a cohort of sickle cell patients, as pain increased, spiritual well-being declined.31 Finally, research suggests a lack of social support or resources may contribute to pain experiences.23,32,33
Fig. 1.
Pain impacts quality of life. Adapted with permission from Ferrell et al.20
Although awareness of pain prevalence in chronic HF is growing, less is known about ambulatory HF populations and factors that contribute to pain severity.17 Guided by this framework, the aim of this research is to identify correlates of pain severity in a population of ambulatory HF patients.
Methods
Study Design
This secondary data analysis was a cohort study nested within a larger Veterans Administration (VA)-funded investigation to evaluate routine pain management practices.
Sample and Setting
The aim of the Help Veterans Experience Less Pain Study (HELP-Vets) was to evaluate routine pain screening and management among outpatient veterans from March 2006 to June 2007 at two hospitals and six affiliated community sites in three large counties (Los Angeles, Ventura, and Orange). Of the 19 participating clinics, five offer oncology and cardiology services and 14 offer primary care. This study was approved by the institutional review boards at each respective medical center.
Trained research assistants approached veterans after a visit with their provider and screened for inclusion criteria. Veterans who had their vital signs measured during their visit, were able to pass a brief cognitive screening test,34 had intact hearing, spoke and understood English, had not participated in the study before, and agreed to have their medical records reviewed, were offered the opportunity to participate. In an effort to ensure an adequate sample of both healthy and frail subjects, research assistants interviewed every other eligible veteran who reported their health as excellent, very good, or good and every veteran who reported their health as fair or poor.
After confirmation of eligibility and consent, research assistants interviewed veterans immediately and provided a $20 check in return for participation in the study. For this analysis, we selected only veterans with a medical record documentation of systolic, diastolic, or mixed HF; who did not have a heart transplant; and who had complete data on the severity subscale of the Brief Pain Inventory (BPI).
After the interview, medical records were reviewed for all participants. The principal and co-principal investigators (PI and co-PI) identified key diagnostic categories for the chart review by administrative data diagnosis codes. One abstractor, with more than five years of full-time experience as a chart reviewer for the VA's national surgical quality monitoring program, was used for the abstraction process. Initial chart reviews were overread by the PI and co-PI. At scheduled intervals, the PI or co-PI reviewed additional abstractions for accuracy and conducted regular meetings to clarify issues that arose during the review process. Other details related to the study methods are provided elsewhere.35,36
Measures
The patient survey was developed for the HELP-Vets study by a multidisciplinary team of palliative care and health service researchers and included existing pain instruments as well as team-derived items of clinical interest. The survey was refined after piloting and cognitive testing to the current form used in this study. We used the pain severity scale of the BPI as the dependent variable for this analysis.37 The BPI was originally developed for use in cancer patients, but subsequent research demonstrated the psychometric properties of this tool in other populations,38 including post-coronary bypass patients.39 The pain severity scale is composed of four items that ask the patients to rate their current pain and their pain at its worst, least, and average in the last week. Each item is rated on a 0–10 scale, and items are averaged to provide a composite score. Higher numbers indicate more severe pain. In a study investigating chronic pain in a population of patients recovering from coronary artery bypass graft surgery,39 criterion validity was established by comparing it with the bodily pain scale of the Medical Outcomes Study Short Form Health Survey (MOS-SF).40 Cronbach's alpha coefficients for the MOS-SF were 0.84–0.89.39 The Cronbach's alpha coefficient in our investigation was 0.91.
As independent variables, we assessed each of the four domains of well-being associated with pain in the total pain theory (physical, psychological, social, and spiritual). With regard to the physical domain, functional status was assessed by an index using age, gender, and specific activities (dependence in bathing, dependence in shopping, difficulty walking several blocks, and difficulty pushing/pulling heavy objects) to stratify elders into groups at varying risk of all-cause mortality (range 0–10, continuous). Higher scores are associated with poorer function and higher mortality. The index demonstrated good discrimination, with a c-statistic of 0.76 in the development cohort and 0.74 in the validation cohort,41 and is comparable with other prognostic indices.42,43
We assessed a broad range of physical symptoms and modified existing measures of somatic symptoms44,45 to create a measure of overall symptom prevalence. Dyspnea, anorexia, cough, paresthesias, vertigo, palpations, weight gain, weight loss, diarrhea, constipation, syncope, vomiting, nausea/gas/indigestion, and swelling in the extremities were symptoms included in the measure (range 0–14, continuous). We assessed various pain symptoms (e.g., chest pain, back pain) but did not include them in the measure to avoid endogeneity. We also included an item related to the patient's perception of general health (very poor, poor, fair, good, and excellent; range 1–5, continuous).46
The psychological domain was evaluated using brief screening measures of depression (Patient Health Questionaire-2 [PHQ-2])47 and anxiety (Generalized Anxiety Disorder-2 [GAD-2]).48 The PHQ-2 is derived from the 9- item Patient Health Questionnaire (PHQ)-9 and measures depressed mood and anhedonia over the past two weeks. Patients were asked how often they had “little interest or pleasure in doing things” and felt “down, depressed, or hopeless.” Anxiety was measured by two items (GAD-2) derived from the 7- item Generalized Anxiety Disorders (GAD-7), asking how often they felt “nervous, anxious, or on edge” and not able “to stop or control worrying.” Items were scored as 0 (not at all) to 3 (nearly every day). The PHQ-2 and GAD-2 demonstrate excellent validity as screening tools as evidenced by area under the curve scores of 0.80–0.91.47,48 Both measures were analyzed as continuous variables (range 0–6), with higher scores indicating greater dysphoria.
The social domain was measured using a single item from the BPI interference scale, asking veterans to rank the amount of interference with social relations that resulted from pain (range 0–10, no interference to complete interference, continuous).37
The spiritual domain was assessed with a single item modified from the Quality of Life-End of Life scale,49 asking veterans to rank their agreement with the statement: “I feel at peace.” Responses ranged from “not at all true” to “completely true” (range 1–5, continuous). This item was chosen because it assesses spirituality across a range of religious traditions and for individuals who may consider themselves spiritual but not religious.50
Items related to sociodemographic characteristics including race (African American, Caucasian, and other), age in years, gender (male and female), and educational levels (less than high school graduate, high school graduate, college graduate, and postgraduate work) were included in the questionnaire. Specific comorbidities and ejection fractions were abstracted from chart review and are listed in Table 1. Physicians selected comorbidities commonly associated with pain in other research studies. “Problem lists” were also evaluated as a source of additional important comorbidities.
Table 1.
Baseline Demographic and Clinical Characteristics of HF Patients (n = 96)
| Demographics | n (%)a |
|---|---|
| Age, years | |
| Mean (range, SD) | 67.18 (45–87, 10.98) |
| Male | 92 (95.83) |
| Educational level | |
| Junior high | 9 (9.8) |
| High school graduate, some college, no degree | 49 (51) |
| Associate arts, vocational, or college degree | 23 (24) |
| Postgraduate work or degree | 11 (11.5) |
| Race | |
| African American | 26 (27.1) |
| Caucasian | 56 (58.3) |
| Other | 14 (14.6) |
| Comorbidities | |
| Coronary artery disease | 64 (66.7) |
| Prior myocardial infarction | 36 (37.5) |
| Ischemic chest pain | 26 (27.1) |
| Diabetes mellitus | 47 (49.0) |
| COPD, emphysema, bronchitis, or asthma | 45 (46.9) |
| Depression | 40 (41.7) |
| Low back pain | 26 (27.1) |
| Osteoarthritis | 26 (27.1) |
| PTSD or anxiety disorder | 25 (26.0) |
| Cancer (other than nonmelanoma skin) | 20 (20.8) |
| Peripheral vascular disease | 16 (16.8) |
| Stroke | 16 (16.8) |
| Clinical variables | |
| Ejection fraction mean (n = 87) (range, SD) | 39.1% (15–70, 16.7) |
| Pain ≥ 1 "right now" | 53 (55.2) |
| Pain moderate or severe (≥4)b | 36 (37.5) |
COPD = chronic obstructive pulmonary disease; PTSD = post-traumatic stress disorder.
Unless otherwise noted.
Pain “right now.”
Data Analysis
All data were transferred into SPSS Version 15 for analysis. Measures of central tendency, including frequencies, means, ranges, and standard deviations (SDs), described sample characteristics. Residuals were examined for normality, heteroscedasticity, and linearity. In bivariate analyses, Chi squares compared categorical variables and Pearson coefficient correlations compared continuous variables. To address non-normality, the GAD-2 and the PHQ-2 were transformed with a natural log transformation and these values were used in bivariate and multivariate analyses. Sensitivity analyses using the nontransformed GAD-2 and PHQ-2 found similar results. A linear regression model controlled for age and included the variables measuring the four domains of well-being (Functional Morbidity Index, symptom measure, self-reported health, GAD-2, PHQ-2, interference with social relations, and feelings of peace). Because missing data across variables were low (≤3%), we used mean substitution techniques for independent variables; sensitivity tests deleting the few participants with missing data found similar results. For multivariate analysis, with alpha set at 0.05, an effect size of 0.20, a sample size of 96 yields a power of 0.84 to evaluate eight covariates, critical F (8,87) = 2.0476.51 Although brief screening measures for anxiety and depression were moderately correlated, as a sensitivity analysis, we examined regression models using these measures individually and together and results did not change significantly. Variance inflation factor and tolerance values revealed no problems with multicollinearity.
Results
Of the 6138 people approached for study inclusion, 939 were eligible and 650 veterans completed the interview (69.2% response rate). Age, race, and pain levels were similar for eligible veterans who did and did not choose to participate (P > 0.05). Age, race, and pain levels were similar for eligible veterans who did and did not choose to participate (P > 0.05).
Ninety-six veterans (14.8% of the total sample) met inclusion criteria for this analysis. Table 1 summarizes sample characteristics. The mean age of the sample was 67.18 years (SD = 10.98), 92 (95.83%) were male, and approximately one-third reported some college education. Slightly less than half of the sample described their ethnicity as non-Caucasian. In this sample of veterans with both systolic and diastolic HF, the mean ejection fraction was 39.1% (n = 87).
Veterans suffered significant comorbidities, especially diabetes (n = 47, 49%) and chronic obstructive pulmonary disease (n = 45, 47%). Although the correlations of individual comorbidities with pain severity were not statistically significant, degenerative joint disease and angina were the comorbidities most strongly associated with pain severity (results not shown). The lack of statistical significance may be related to the small sample size or the historical nature of the data available for analysis.
The incidence of pain was significant, with 53 of the 96 veterans (55.2%) reporting pain ≥1 “right now.” More than one-third (38%) of all HF veterans reported moderate or severe (≥4) pain “right now.”52 Medical record documentation of ischemic chest pain was present for one-quarter (27%) of veterans, but one-third (35%) stated that they experienced chest pain during the last four weeks. Whereas one-quarter of veterans had medical record documentation of osteoarthritis or low back pain, more than two-thirds reported pain in the arms, legs, or joints during the last four weeks. These data suggest that some veterans experience unrecognized pain (and may “suffer silently”).53
Table 2 describes the frequencies, means, and SDs of variables measuring physical, psychological, social, and spiritual well-being domains. Table 3 describes symptom frequencies; of all symptoms evaluated, the most common symptoms were fatigue (n = 63, 75.9%); pain in arms, legs, and joints (n = 67, 71.3%); and shortness of breath (n = 58, 61.7%). With regard to the overall symptom burden measure, veterans reported an average number of 5 ± 2.8 symptoms (range 0–11). The mean Functional Morbidity Index was 4.57, indicating that this sample is at moderate risk (11%–12%) for a two-year mortality.41
Table 2.
Domains of Physical, Psychological, Social, and Spiritual Well-Being Related to Pain Severity (n = 96)
| Domains | n (%)a |
|---|---|
| Physical domain | |
| General health | |
| Very poor | 8 (8.5) |
| Poor | 17 (18.1) |
| Fair | 44 (46.8) |
| Good | 20 (21.3) |
| Excellent | 5 (5.3) |
| Overall symptoms, mean (range, SD) | 5 (0–11, 2.8) |
| Functional Morbidity Index, mean (range, SD) | 4.57 (0–9, 1.83) |
| Assistance with bathing or showering | 10 (10.64) |
| Assistance with shopping | 15 (16.1) |
| Difficulty walking several blocks | 57 (62.64) |
| Difficulty pushing/pulling large objects | 50 (54.9) |
| Psychological domain | |
| GAD-2, mean (range, SD) | 1.62 (0–6, 1.97) |
| PHQ-2, mean (range, SD) | 1.56 (0–6, 1.94) |
| Social domain | |
| Interference with relations, mean (range, SD) | 2.67 (0–10, 3.62) |
| Spiritual domain | |
| "I feel at peace" | |
| Not at all true | 7 (7.4) |
| A little bit true | 18 (19.1) |
| A moderate amount true | 15 (16) |
| Quite a bit true | 27 (28.7) |
| Completely true | 27 (28.7) |
Unless otherwise noted.
Table 3.
Symptom Frequency (n = 94)
| Symptom | n (%) |
|---|---|
| Tired, low energya | 63 (75.9) |
| Pain in arms, legs, or joints | 67 (71.3) |
| Shortness of breathb | 58 (61.7) |
| Trouble sleepinga | 47 (56.6) |
| Coughb | 52 (55.3) |
| Swelling in legs/ankleb | 51 (54.3) |
| Back pain | 52 (55.3) |
| Numbness in hands and feetb | 48 (51.1) |
| Dizzinessb | 42 (44.7) |
| Nausea, gas, indigestionb | 42 (44.7) |
| Weight lossb | 33 (35.1) |
| Chest pain | 33 (35.1) |
| Feeling heart pound or raceb | 33 (35.1) |
| Weight gainb | 31 (33.7) |
| Stomach pain | 30 (31.9) |
| Loose bowel (diarrhea)b | 26 (27.7) |
| Headaches | 25 (26.6) |
| Lack of appetiteb | 23 (24.5) |
| Constipationb | 21 (22.3) |
| Fainting spellsb | 8 (8.5) |
| Vomitingb | 8 (8.5) |
n = 83, not included in the symptom measure.
Items included in the symptom measure.
In correlation analyses with sociodemographic variables, only age was significantly correlated with pain severity (r = –0.237, P = 0.02). Each of the domains of well-being was independently correlated with pain severity. Results of the correlation analysis are displayed in Table 4.
Table 4.
Bivariate Correlates of Pain Severity (n = 96)
| Variable | r | P-value |
|---|---|---|
| Sociodemographics | ||
| Male gender | 0.02 | 0.86 |
| Age (years) | –0.24 | 0.02 |
| Educational level | 0.11 | 0.30 |
| Race | ||
| African American | 0.16 | 0.13 |
| Caucasian | –0.03 | 0.77 |
| Other | 0.14 | 0.19 |
| Physical domain | ||
| General health | –0.36 | <0.001 |
| Functional statusa | 0.31 | 0.002 |
| Overall symptoms | 0.54 | <0.001 |
| Psychological domain | ||
| Anxiety (GAD-2)b | 0.33 | 0.001 |
| Depression (PHQ-2)c | 0.32 | 0.002 |
| Spiritual domain | ||
| "I feel at peace" | –0.26 | 0.01 |
| Social domain | ||
| Interference with relations | 0.57 | <0.001 |
Functional Morbidity Index.
Natural log transformation of Generalized Anxiety Disorder-2.
Natural log transformation of PHQ-2.
Table 5 shows the results of the multivariate analysis. The overall model explained 44% of the variance in pain severity (R2 = 0.436; F (8, 87) = 8.38, P < 0.000); lower social well-being (greater interference with social relations) and increased number of symptoms were independently associated with pain severity. Self-reported health status was also positively related to pain severity, although it was only marginally significant (P = 0.057).
Table 5.
Factors Independently Associated with Pain Severity
| Variable | β | t | P-value |
|---|---|---|---|
| Age | –0.03 | –0.31 | 0.76 |
| Overall symptoms | 0.29 | 2.74 | 0.008 |
| General health | –0.19 | –1.89 | 0.06 |
| Functional statusa | –0.04 | –0.33 | 0.74 |
| Anxiety (GAD-2)b | –0.08 | –0.62 | 0.53 |
| Depression (PHQ-2)c | <0.00 | –0.01 | 0.99 |
| "I feel at peace" | 0.07 | 0.65 | 0.52 |
| Interference with relations | 0.45 | 4.05 | <0.001 |
Full model information when controlling for age: R2 = 0.436; F (8, 87) = 8.38; P < 0.0001.
Functional Morbidity Index.
Natural log transformation of Generalized Anxiety Disorder-2.
Natural log transformation of PHQ-2.
Discussion
The prevalence of pain in this study was high, and the frequency of patients suffering from moderate or severe pain was clinically important. More than half of all veterans reported pain “right now,” and one-third of veterans reported their pain as moderate or severe (≥4 on a 0–10 scale). This suggests a need for more aggressive pain management in primary care settings. Although physical, psychological, social, and spiritual domains of well-being were individually related to pain severity, only interference with social relations and symptom number were independently associated with pain.
The severity of pain witnessed in this study may result from a variety of issues faced by these patients. For example, patients may experience physical pain from multiple comorbidities.8 Musculoskeletal pain was reported by the majority of patients, which may exacerbate physical deconditioning common in HF and interfere with self-management. Spiritual/existential pain may occur with a loss of function and independence that occurs with disease progression. Psychological pain may occur secondary to uncertain disease trajectories,11,54 the “What next?” of HF progression. In previous studies, patients experiencing higher levels of psychological distress, including anxiety and/or depression, testified to greater levels of pain or symptomatology.32,55,56 In a review of the literature, the median prevalence of major depression in HF was 17% compared with 5% in the general population.57 Fatigue and shortness of breath, so common in HF, may limit patient ability to interact with family and peers, contributing to isolation and social pain as support systems contract.54
The positive relationship between symptom number and pain severity seen in this investigation is consistent with research outside of HF that associates a variety of symptoms with pain perception.58–60 HF patients rarely experience a symptom in isolation; more commonly, they experience multiple concurrent symptoms such as pain, dyspnea, and fatigue. The patients in this sample suffered multiple comorbidities, contributing to the overall diversity of symptoms and adding to the complexity of symptom analysis. Furthermore, the literature suggests symptom clusters, defined as two or more symptoms experienced concurrently61 and not simply individual symptoms, that may be an important patient-related outcome to be investigated in future studies. The prevalence of symptoms in this study (mean 5; range 0–11; SD 2.8) suggests that symptoms may not receive adequate attention and present an opportunity for improved management.
Pain severity was associated with interference with social relations in this sample of HF veterans. Chronic HF patients frequently experience a protracted disease trajectory that contributes to social isolation for patients and their families.54 Life-limiting diseases such as HF lead to changes in roles and relationships, affection/sexual function, and physical appearance. Because of the progressive nature of HF, maintaining supportive relationships may be extremely challenging. Limited research suggests an independent association between social isolation and poor cardiovascular outcomes.62–66
Few studies have investigated the influence of social well-being on pain perception in HF. In 58 HF patients, Carels et al.32 found that greater social conflict was associated with physical symptoms (chest pain or heaviness, shortness of breath) longitudinally.32 A comparison of 20 advanced HF patients with 20 lung cancer patients suggested that social isolation and hopelessness were greater in HF. The primary concern for cancer patients and caregivers was facing death; unlike cancer patients, frustration, progressive losses, social isolation, and the stress of balancing and monitoring a complex medication regimen dominated the lives of patients with HF. Moreover, more health and social services (including hospice services and financial benefits) were available to lung cancer patients.54
Our findings reinforce the need to identify individuals with HF who are at risk for social isolation. Although social workers have not consistently been associated with the day-to-day operations of HF clinics, these colleagues have expertise in supporting patient and family emotional well-being and in meeting needs for practical and social support. Strategies that enhance access to such support may be key to minimizing pain and improving quality of life for patients facing the progressive nature of chronic HF.
Limitations
Our measures of the specific domains of the total pain model were limited. Nonetheless, our findings suggest actionable targets for future interventions. Because of the cross-sectional study design, it is difficult to ascertain whether pain severity contributed to social isolation and increased symptomatology or whether social isolation and increased symptomatology contributed to pain severity. The item measuring interference with social relations had a mean of 2.67 (range 0–10), suggesting that HF veterans experience significant social isolation. Still, because the item measuring social relations contains the word “pain,” there is concern for endogeneity with this variable. Although this study is unique in including veterans with both systolic and diastolic dysfunction, the results may not be generalizable to patients with isolated systolic or diastolic dysfunction. Yet by including both types of dysfunction, this sample may more closely reflect a population encountered in a community practice. Finally, we oversampled for veterans with fair or poor health, and our findings may more closely reflect veterans who have a greater overall burden of illness. However, choosing this vulnerable population allowed us to focus on a population in whom the opportunities and importance of palliating symptomatic concerns is greatest.
Conclusions
HF patients report substantial levels of pain in outpatient settings. This study suggests that veterans who report more interference with social relations and a larger number of symptoms suffer more severe pain. Treating pain severity more aggressively may improve social relations, symptom prevalence, and other domains of well-being. The association of physical, psychological, social, and spiritual domains with pain severity suggests that multidisciplinary interventions are needed to address the complex nature of pain in HF.
References
- 1.Ekman I, Cleland JGF, Swedberg K, et al. Symptoms in patients with heart failure are prognostic predictors: insights from COMET. J Card Fail. 2005;11:288–292. doi: 10.1016/j.cardfail.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Levenson J, McCarthy E, Lynn J, Davis R, Phillips R. The last six months of life for patients with congestive heart failure. J Am Geriatr Soc. 2000;48(Suppl 5):S101–S109. doi: 10.1111/j.1532-5415.2000.tb03119.x. [DOI] [PubMed] [Google Scholar]
- 3.Zambroski C, Moser D, Bhat G, Zeigler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4:198–206. doi: 10.1016/j.ejcnurse.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MJ. Management of end stage cardiac failure. Postgrad Med J. 2007;83(980):395–401. doi: 10.1136/pgmj.2006.055723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodlin S, Hauptman P, Arnold R, et al. Consensus statement: palliative and supportive care in advanced heart failure. J Card Fail. 2004;10:200–209. doi: 10.1016/j.cardfail.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline update for the diagnosis and management of chronic heart failure in the adult—a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:1825–1852. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 7.American Heart Association [March 1, 2009];Heart disease and stroke statistics—2008 update. Available from http://www.americanheart.org/downloadable/heart/1200078608862HS_Stats%202008.final.pdf.
- 8.Addington-Hall J, Rogers A, McCoy A, Gibbs J. Heart disease. In: Morrison R, Meier D, Capello C, editors. Geriatric palliative care. Oxford University Press; New York: 2003. pp. 110–122. [Google Scholar]
- 9.Masoudi F, Havranek E, Krumholz H. The burden of chronic congestive heart failure in older persons: magnitude and implications for policy and research. Heart Fail Rev. 2002;7:9–16. doi: 10.1023/a:1013793621248. [DOI] [PubMed] [Google Scholar]
- 10.Massie B, Shah N. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133:703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein N, Lynn J. Trajectory of end-stage heart failure: the influence of technology and implications for policy change. Perspect Biol Med. 2006;49:8–10. doi: 10.1353/pbm.2006.0008. [DOI] [PubMed] [Google Scholar]
- 12.Anderson H, Ward C, Eardley A, et al. The concerns of patients under palliative care and a heart failure clinic are not being met. Palliat Med. 2001;15:279–286. doi: 10.1191/026921601678320269. [DOI] [PubMed] [Google Scholar]
- 13.Norgren L, Sorensen S. Symptoms experienced in the last six months of life in patients with end-stage heart failure. Eur J Cardiovasc Nurs. 2003;2:213–217. doi: 10.1016/S1474-5151(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 14.Lynn J, Teno J, Phillips R, et al. Perception by family members of the dying experience of older and seriously ill patients. Ann Intern Med. 1997;216:97–105. doi: 10.7326/0003-4819-126-2-199701150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan M, Levy W, Russo J, Spertus J. Depression and health status in patients with advanced heart failure: a prospective study in tertiary care. J Card Fail. 2004;10:390–396. doi: 10.1016/j.cardfail.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13:643–648. doi: 10.1016/j.cardfail.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey CM, Harrison MB, Friedberg E, Medves JM, Tranmer JE. The symptom of pain in individuals recently hospitalized for heart failure. J Cardiovasc Nurs. 2007;22:368–374. doi: 10.1097/01.JCN.0000287035.77444.d9. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy M, Lay M, Addington-Hall J. Dying from heart disease. J R Coll Physicians Lond. 1996;30:325–328. [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders C, Baines M. Living with dying. 2nd ed. Oxford University Press; New York: 1984. [Google Scholar]
- 20.Ferrell B, Grant M, Padilla G, Vermuri S, Rhiner M. The experience of pain and perceptions of quality of life: validation of a conceptual model. Hosp J. 1991;7:9–23. doi: 10.1080/0742-969x.1991.11882702. [DOI] [PubMed] [Google Scholar]
- 21.Desbiens N, Wu A, Broste S, et al. Pain and satisfaction with pain control in seriously ill hospitalized adults: findings from the SUPPORT research investigations. For the SUPPORT investigators. Study to understand prognoses and preferences for outcomes and risks of treatment. Crit Care Med. 1996;24:1953–1961. doi: 10.1097/00003246-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Fagring AJ, Gaston-Johansson F, Kjellgren KI, Welin C. Unexplained chest pain in relation to psychosocial factors and health-related quality of life in men and women. Eur J Cardiovasc Nurs. 2007;6:329–336. doi: 10.1016/j.ejcnurse.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Evers AWM, Kraaimaat FW, Geenen R, Jacobs JWG, Bijlsma JWJ. Pain coping and social support as predictors of long-term functional disability and pain in early rheumatoid arthritis. Behav Res Ther. 2003;41:1295–1310. doi: 10.1016/s0005-7967(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 24.Westerbotn M, Hillerås P, Fastbom J, Agüero-Torres H. Pain reporting by very old Swedish community dwellers: the role of cognition and function. Aging Clin Exp Res. 2008;20:40–46. doi: 10.1007/BF03324746. [DOI] [PubMed] [Google Scholar]
- 25.Walke L, Gallo W, Tinetti M, Fried T. The burden of symptoms among community-dwelling older persons with advanced chronic disease. Arch Intern Med. 2004;164:2321–2324. doi: 10.1001/archinte.164.21.2321. [DOI] [PubMed] [Google Scholar]
- 26.Boyd K, Murray S, Kendall M, et al. Living with advanced heart failure: a prospective, community based study of patients and their carers. Eur J Heart Fail. 2004;6:585–591. doi: 10.1016/j.ejheart.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Pantilat SZ, Steimle AE. Palliative care for patients with heart failure. JAMA. 2004;291(20):2476–2482. doi: 10.1001/jama.291.20.2476. [DOI] [PubMed] [Google Scholar]
- 28.Zambroski CH, Moser DK, Roser LP, Heo S, Chung ML. Patients with heart failure who die in hospice. Am Heart J. 2005;149:558–564. doi: 10.1016/j.ahj.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Koenig HG. Depression in hospitalized older patients with congestive heart failure. Gen Hosp Psychiatry. 1998;20:29–43. doi: 10.1016/s0163-8343(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 30.Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2009;38:115–123. doi: 10.1016/j.jpainsymman.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison M, Edwards C, Koenig H, et al. Religiosity/spirituality and pain in patients with sickle cell disease. J Nerv Ment Dis. 2005;193:250–257. doi: 10.1097/01.nmd.0000158375.73779.50. [DOI] [PubMed] [Google Scholar]
- 32.Carels RA, Musher-Eizenman D, Cacciapaglia H, et al. Psychosocial functioning and physical symptoms in heart failure patients: a within-individual approach. J Psychosom Res. 2004;56:95–101. doi: 10.1016/S0022-3999(03)00041-2. [DOI] [PubMed] [Google Scholar]
- 33.López-Martínez AE, Esteve-Zarazaga R, Ramírez-Maestre C. Perceived social support and coping responses are independent variables explaining pain adjustment among chronic pain patients. J Pain. 2008;9:373–379. doi: 10.1016/j.jpain.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Goebel JR, Doering LV, Evangelista LS, et al. A comparative study of pain in heart failure and non-heart failure veterans. J Card Fail. 2009;15:24–30. doi: 10.1016/j.cardfail.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherbourne C, Asch S, Shugarman L, et al. Early identification of co-occurring pain, depression and anxiety. J Gen Intern Med. 2009;24:620–625. doi: 10.1007/s11606-009-0956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleeland CS, Ryan K. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 38.Keller S, Bann C, Dodd S, et al. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Gjeilo KH, Stenseth R, Wahba A, Lydersen S, Klepstad P. Validation of the Brief Pain Inventory in patients six months after cardiac surgery. J Pain Symptom Manage. 2007;34:648–656. doi: 10.1016/j.jpainsymman.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Ware J. [March 9, 2004];SF-36.org: a community for measuring health outcomes using SF tools. Available from http://www.sf-36.org/tools/sf36.shtml.
- 41.Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a Functional Morbidity Index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19:1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 43.Desai MM, Bogardus ST, Williams CS, Vitagliano G, Inouye SK. Development and validation of a risk-adjustment index for older patients: the high-risk diagnoses for the elderly scale. J Am Geriatr Soc. 2002;50:474–481. doi: 10.1046/j.1532-5415.2002.50113.x. [DOI] [PubMed] [Google Scholar]
- 44.Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Portenoy RK, Thaler HT, Kornblith AB, et al. The memorial symptom assessment scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 46.DeSalvo KB, Fan VS, McDonell MB, Fihn SD. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40:1234–1246. doi: 10.1111/j.1475-6773.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroenke K, Spitzer R, Williams J. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 48.Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 49.Steinhauser KE, Bosworth HB, Clipp EC, et al. Initial assessment of a new instrument to measure quality of life at the end of life. J Palliat Med. 2002;5:829–841. doi: 10.1089/10966210260499014. [DOI] [PubMed] [Google Scholar]
- 50.Steinhauser KE, Voils CI, Clipp EC, et al. “Are you at peace?”: one item to probe spiritual concerns at the end of life. Arch Intern Med. 2006;166:101–105. doi: 10.1001/archinte.166.1.101. [DOI] [PubMed] [Google Scholar]
- 51.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. [Google Scholar]
- 52.Jensen MP, Smith DG, Ehde DM, Robinsin LR. Pain site and the effects of amputation pain: further clarification of the meaning of mild, moderate, and severe pain. Pain. 2001;91:317–322. doi: 10.1016/S0304-3959(00)00459-0. [DOI] [PubMed] [Google Scholar]
- 53.Ferrell B, Coyle N. The nature of suffering and the goals of nursing. Oxford University Press; New York: 2008. [DOI] [PubMed] [Google Scholar]
- 54.Murray S, Boyd K, Kendall M, et al. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their careers in the community. Br Med J. 2002;325:929–934. doi: 10.1136/bmj.325.7370.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butler LD, Koopman C, Cordova MJ, et al. Psychological distress and pain significantly increase before death in metastatic breast cancer patients. Psychosom Med. 2003;65:416–426. doi: 10.1097/01.psy.0000041472.77692.c6. [DOI] [PubMed] [Google Scholar]
- 56.Carr E, Nicky Thomas V, Wilson-Barnet J. Patient experiences of anxiety, depression and acute pain after surgery: a longitudinal perspective. Int J Nurs Stud. 2005;42:521–530. doi: 10.1016/j.ijnurstu.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Johansson P, Dahlstrom U, Brostrom A. The measurement and prevalence of depression in patients with chronic heart failure. Prog Cardiovasc Nurs. 2006;21:28–36. doi: 10.1111/j.0197-3118.2006.04644.x. [DOI] [PubMed] [Google Scholar]
- 58.Jump R, Robinson M, Armstrong A, et al. Fatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social support. J Rheumatol. 2005;32:1699–1705. [PubMed] [Google Scholar]
- 59.Theadom A, Cropley M, Humphrey K-L. Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res. 2007;62:145–151. doi: 10.1016/j.jpsychores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Schillinger M, Domanovits H, Paulis M, et al. Clinical signs of pulmonary congestion predict outcome in patients with acute chest pain. Wien Klin Wochenschr. 2002;114(21–22):917–922. [PubMed] [Google Scholar]
- 61.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 62.Moser DK. Psychosocial factors and their association with clinical outcomes in patients with heart failure: why clinicians do not seem to care. Eur J Cardiovasc Nurs. 2002;1:183–188. doi: 10.1016/S1474-5151(02)00033-6. [DOI] [PubMed] [Google Scholar]
- 63.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 64.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. Systematic review of prospective cohort studies. [Clinical Research Ed]. BMJ. 1999;318(7196):1460–1467. doi: 10.1136/bmj.318.7196.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- 66.Lomas J. Social capital and health: implications for public health and epidemiology. Soc Sci Med. 1998;47:1181–1188. doi: 10.1016/s0277-9536(98)00190-7. [DOI] [PubMed] [Google Scholar]