The formation of tooth enamel takes place before the tooth erupts in a confined extracellular environment between dentin and ameloblast cells (enamel-making cells). A series of physiological and chemical events including gene expression, protein secretion, protein folding and assembly, mineral growth, and protein degradation are involved in making enamel. Because mature enamel post-tooth eruption does not contain cells and does not remodel, synthetic enamel is necessary for enamel regeneration.
Scientists now understand how the cells form enamel and how their gene products function, enabling the exploration of different approaches to producing synthetic or regenerated enamel. Cell-based strategies and biomimetic approaches (imitating biological processes) have both been incorporated into preparing synthetic enamel. It is only a matter of time before these efforts provide a successful result.
Educational Objectives
After reading this course, the participant should be able to:
Identify the organic matrix components of forming enamel and recognize how they differ from that of bone.
Understand the unique mechanical properties of dental enamel.
List the intrinsic and extrinsic factors that affect dental enamel leading to its loss.
Discuss the potential of enamel to be regenerated in the laboratory.
The Hierarchical Structure of Enamel
The structure of enamel at high magnification resembles a perfect pattern for knitting or crochet (Figure 1). Mature enamel’s packing order ranges from the nanoscale of long fluoridated calcium hydroxyapatite crystals to the microscale level where the crystals are aligned together in bundles to form 3 μm to 5 μm diameter prisms or rods. Each enamel prism has approximately the same diameter as an ameloblast and is most likely the product of a single cell.
Figure 1.
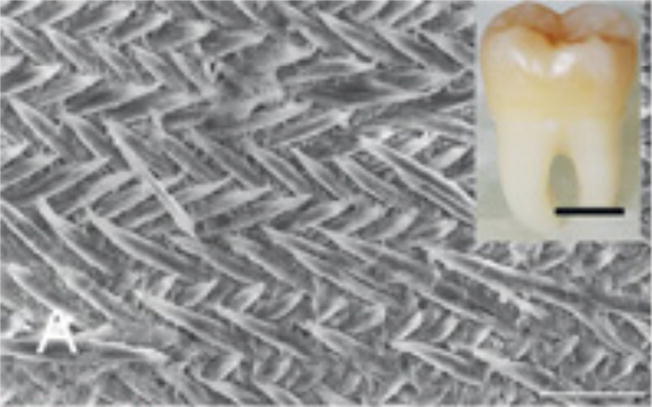
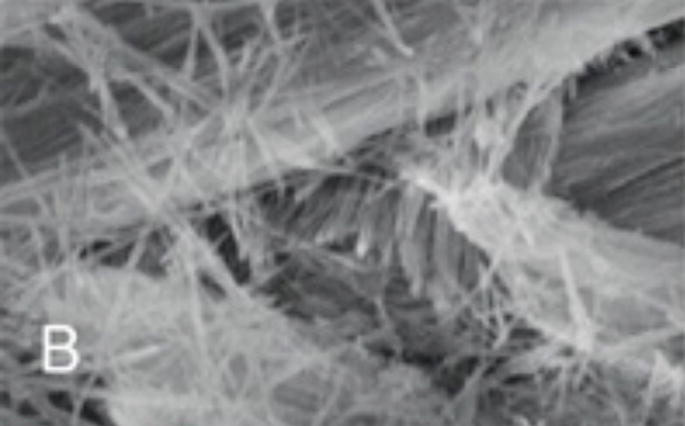

Figure 1A: Scanning electron microscope (SEM) images of etched surface of tooth enamel (mouse incisor) showing the interwoven arrangement of the prisms (scale 10 μm). Inset: A human third molar 6 (scale 1 cm).
Figure 1B: Higher magnification SEM image showing the calcium fluoridated hydroxyapatite crystals arranged in parallel bundles within the prisms (scale 1 μm).
Figure 1C: Transmission electron micrograph of a section of forming enamel (mouse molars) showing the arrangement black lines) within the protein-rich organic matrix (white circles) (scale 50 nanometers).
The next level of order is the interwoven arrangement of the prisms with the interprismatic enamel. The packing of the prisms is probably the result of an orchestrated receding of the ameloblast cell layer. The final product is an approximately 2.5 mm thick mineralized tissue that is translucent and varies in color from yellowish-white to grayish-white.1 The key to achieving such precisely organized architecture lies not only in the cell movement but also in the highly controlled expression of proteins and enzymes, and the manner in which these organic molecular components interact with each other, with cell surfaces, and, most important, with the forming mineral.
Among the three mineralized tissues composing the complex tooth organ, enamel is the hardest. This protective cover of the tooth is the most highly mineralized tissue in the human body and has remarkable mechanical characteristics. A natural bioceramic, enamel is hard yet resistant to fracture and wear. To create a material with a combination of such contradictory mechanical properties (flexibility and hardness), nature has used a clever composition of mineral, water, and organic material organized into various structural hierarchies (Figure 1). The underlying dentino-enamel junction provides additional mechanical support that prevents enamel deformation, which might otherwise result from excessive external forces.
The Extracellular Microenvironment
Enamel formation follows the general organic matrix-mediated biomineralization process, meaning that the protein components in the extracellular space control the initiation, orientation, and packing of the crystals. The timing of expression and secretion of the required proteins and proteinases are well controlled by various genes and signaling pathways. This process occurs in an enclosed microenvironment, isolated from circulating blood and located in the extracellular space between the columnar epithelial cells (the ameloblasts) and the underlying dentin formed by the odontoblast cells. Enamel development (amelogenesis) is the result of a series of complex and programmed cellular activities.2 The cellular, chemical, and physiological events involved in tooth enamel formation are dynamic and occur in various stages. These range from the secretory stage when cells secrete the majority of the proteins and proteinases needed for mineralization to the maturation stage when massive protein degradation allows the simultaneous growth of crystals to fill the space the proteins leave behind. These two stages are separated by the transition stage, when protein secretion declines and crystal growth increases. Critical extracellular events include protein self-assembly, stepwise processing of proteins by specific enzymes, ion transport, and control of local pH.3 These dynamic events transform the matrix that is 70% water and organic material (mostly protein) with only 30% mineral by weight to a highly organized structure that is more than 99% inorganic (mostly calcium hydroxyapatite crystals). The smallest inorganic units—apatite crystals—grow in length at the secretory stage and mainly grow in width and thickness at the transition and maturation stages.
The major structural protein of the organic matrix is amelogenin, which constitutes more than 90% of the protein content. The second most abundant protein is ameloblastin, which has cell adhesion properties and most likely controls ameloblast cell differentiation. Another protein found in much smaller quantities is enamelin, which is also thought to control apatite nucleation and growth in conjunction with amelogenin. Proteinases, such as matrix metalloproteinase MMP-20 and KLK4, function to process and degrade amelogenin and other enamel proteins at different stages of amelogenesis.4
Besides calcium, fluoride, and phosphate, the extracellular environment contains other ions such as sodium, magnesium, potassium, chloride, and bicarbonate. These ions enter from blood vessels on the surface of enamel organ cells. Through controlled or perhaps facilitated movement, these ions have to cross a distance of 50 μm to 100 μm (two or three different cell layers) to travel from the blood stream to the developing enamel surface.
Enamel apatite crystals incorporate sodium, magnesium, potassium, fluoride, carbonate, and hydrogen phosphate (HPO4)-3 in their structures. One of the most important ions incorporated into the enamel apatite structure is fluoride. Fluoride substitutes for hydroxyl ions in apatite and stabilizes the lattice as the result of hydrogen bonds with neighboring OH- ions. The resulting fluorohydroxyapatite is less soluble than hydroaxyapatite, has better crystallinity, and is less susceptible to acid dissolution and caries progression. Fluoride uptake mostly occurs during the transition/maturation stage and continues after the ameloblasts cease secretion. The enamel surface also absorbs fluoride from the surrounding tissue fluid prior to eruption of the tooth. However, excessive fluoride consumption during enamel development results in the formation of fluorosed or mottled enamel.5 Since significant acidity is generated in the enamel extracellular matrix microenvironment following the precipitation of enamel hydroxyapatite, the pH buffering function in the system is critical when progressive and rapid growth of enamel crystals is occurring during enamel maturation. Bicarbonate is another essential component of enamel fluid involved in buffering the extracellular environment.
During the entire process of amelogenesis, which begins in humans during the third trimester of pregnancy, ameloblasts pass through a series of differentiation stages characterized by changes in cell morphology and function. Once the enamel is fully mineralized and the organic matrix is degraded and removed—6 months after birth in humans— the ameloblasts stop functioning and undergo regression. They shrink dramatically and in the oral cavity can lead to dental caries and/or dental erosion.7 Both caries and erosion are the result of enamel mineral loss due to an acidic environment, while carries formation specifically involves the presence of bacteria.
In addition to the possibility of such damage, the formation of enamel can also be defective from early developmental stages due to mutations in ameloblast gene products. The result is a malfunction of one of the proteins or proteinases that are responsible for controlling the processes of mineral formation and for the organization and processing of the organic matrix. Mutation in any of the genes encoding amelogenin, enamelin, MMP-20, or KLK4 leads to one of a series of inherited diseases of enamel malformation called amelogenesis imperfecta.4 Depending on the protein affected and the developmental stage involved, the defective enamel could be thin (hypoplastic) or have normal thickness but a soft (hypomineralized) structure.
Rebuilding Enamel
Because mature enamel does not contain cells and cannot remodel itself, scientists must create synthetic enamel for use as a future alternative dental restorative material.8,9 The hydroxyapatite crystals isolated from mature enamel are unusually long and different in shape from those synthesized under ambient conditions in the laboratory. However, the synthesis of enamel-like hydroxyapatite nanorods is possible by applying certain techniques under extreme laboratory conditions. The majority of these synthesis methods were developed under conditions of high temperature, high pressure, and extremely acidic pH, or in the presence of chemicals such as surfactants.10,11 Clearly the development of improved biomaterials that do not require such harsh conditions is essential if these materials are to find their way into the clinic.
Hope comes from scientists in the field of materials chemistry who have adopted ideas from nature for synthesis of biomaterial with desirable structures.8 In biomimetic synthesis biomaterials are prepared in the laboratory under conditions close to those of the physiological microenvironment. Biomimetic strategies integrate key elements of the composition, structure, and function of biological systems into the material formulation.
Understanding the timing and pattern of the ameloblast gene products—amelogenin, enamelin, and ameloblastin—as well as the nature of the mineral phase allows scientists to develop techniques for preparing enamel-like material in the laboratory in a cell-free system.13 The key is to carefully control the appropriate conditions (ie, pH) and the timing of adding the proteins to the system. In our laboratory we use a strategy based on the application of proteins that are known to control the processes of crystal initiation, crystal shape, and packing organization. For example, a device with a special membrane that allows only calcium ions to enter the system in a unidirectional manner could mimic the ameloblast cell membrane when synthetic amelogenin protein trapped between two layers of membranes is used as the organic matrix to initiate mineralization.12 Such a device has been used successfully to grow apatite crystals with similar organization to that of enamel within the prisms (Figure 2). The addition of fluoride also promoted the growth of organized rod-like apatite crystals. In another case, amelogenin protein was carefully added to a crystallization solution, which promoted the initiation of unusually elongated ribbons of apatite crystals.14 Macromolecular selfassembly controls the oriented and elongated growth of the carbonate-containing fluoridated hydroxyapatite crystals within enamel prisms. Proteinase can also be applied during in vitro mineralization to mimic the dynamic process of protein degradation in enamel.15
Figure 2.
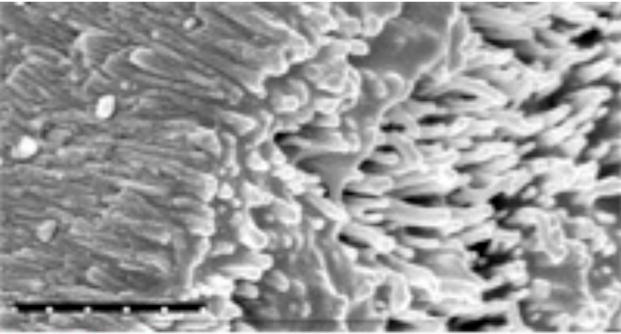
SEM of enamel-like hydroxy apatite crystals grown in the laboratory using the cation selective membrane system 12 (scale 1 μm).
Numerous in vitro experimental approaches have been implemented to demonstrate amelogenin’s ability to control particular aspects of calcium phosphate mineralization: crystal initiation, crystal shape, and direction. Because of its significant potential for controlling key steps in apatite mineralization, amelogenin has also been applied in different in vitro systems for the synthesis of uniquely ordered composite material similar to enamel. For example, when amelogenin was applied in a biomimetic deposition system to remineralize the surface of etched enamel, organized needle-like fluoridated hydroxyapatite crystals grew on the surface.6 The presence of amelogenin promoted the formation of organized bundles of crystals. Although not exactly a reconstruction of enamel, the formation of this material is one of the first steps toward the development of synthetic enamel in a cell-free system.
Taking Advantage of What Cells can Do
Stem cell biology is important to dental research because of its potential to regenerate not just enamel but the entire tooth.16,17 One of the first steps in regenerating the entire tooth structure was an explant culture strategy where early stage tooth buds were used.18 In another approach, nondental stem cells from mice, including embryonic stem cells, neural stem cells, and adult bone marrow-derived cells, were stimulated after recombination with embryonic oral epithelium to express odontogenic genes and develop into tooth structures and associated bone. Single-cell suspensions derived from dissociated pig or rat tooth buds have also been seeded onto a synthetic polymer scaffold and the whole construct grown in vivo to generate tooth structures. These examples illustrate that the regeneration of a tooth is probable. However, controlling spatial growth of the tooth structures in three dimensions to fit a size scale suitable for humans is still a challenge.18
Another potential strategy to regenerate dental enamel involves the application of genes that are known to control the development of enamel-forming cells.19 Ameloblasts have an epithelial origin and their differentiation is tightly linked to odontoblast differentiation. Odontoblasts are dentin-making cells that develop from the mesenchymal cells underlying the epithelium. The progressive differentiation of ameloblasts and odontoblasts is intimately related to tooth morphogenesis, which is regulated by a chain of epithelial-mesenchemal interactions and controlled by various signaling molecules. The controlled differentiation of ameloblasts eventually leads to amelogenesis through programmed secretion of amelogenin, enamelin, ameloblastin, and amelotin. Artificial bioactive materials (nano fibers) have been designed with a signaling function on their surfaces and have been used to facilitate the attachment, proliferation, and differentiation of ameloblast-like cells.20 In studies done on mice this process promoted enamel regeneration in embryonic incisors.20
Challenges for the Future
Our understanding of the mechanisms of enamel formation has advanced greatly in the past 2 decades. The knowledge we now have of the genetics and biochemistry of enamel provides a valuable foundation for the development of cell-free strategies for enamel reconstruction. A variety of feasible sources for stem cells are available to use in whole tooth regeneration and to establish a stable cell line that will perform the functions of ameloblasts. Efforts to regenerate enamel are ongoing but remain at the level of laboratory investigation. The main challenge in cell-free fabrication of synthetic enamel is the creation of enamel’s complex hierarchical prism and interprismatic structures. However, it is only a matter of time before an artificial material with enamel-like optical, mechanical, and esthetic properties will be available to replace conventional restorative materials. In the dental operatory, this advancement may lead to patients receiving a dental device (a night guard for example) that contains the appropriate organic and inorganic materials for release into the oral cavity that will help enamel to regrow overnight. Also, the oral health professional may one day be able to inject stem cells into the tooth cavity to repair damaged enamel. Eventually, nothing will be better than enamel—except enamel itself!
Acknowledgments
The author would like to thank Dr. Yuwei Fan, Dr. Chang Du, and Dr. Iijima Mayumi for the use of Figures 1 and 2, as well as the National Institute of Dental and Craniofacial Research, National Institutes of Health, for financial support made through grants DE- 13414, DE-15332, and DE-15644 to JMO.
Biography

Janet Moradian-Oldak, MSc, PhD, is associate professor at the University of Southern California School of Dentistry, Center for Craniofacial Molecular Biology, Los Angeles. She is currently working on two research projects: “The Kinetics of Mineralization of Teeth” funded by the State University of New York at Buffalo and the National Institute of Dental and Craniofacial Research (NIDCR), and “Matrix-based Mineral Enamel Biomimetics” funded by NIDCR. Moradian-Oldak is also the author of more than 70 peer reviewed papers and six textbook chapters.
References
- 1.Nanci A. Ten Cate’s Oral Histology Development, Structure, and Function. St. Louis: Mosby Elsevier; 2008. Enamel: composition, formation, and structure; pp. 141–190. [Google Scholar]
- 2.Thesleff I. Tooth morphogenesis. Adv Dent Res. 1995;9(3 Suppl):12. doi: 10.1177/0895937495009003S0301. [DOI] [PubMed] [Google Scholar]
- 3.Moradian-Oldak J, Paine ML. Mammalian. Enamel formation. In: Astrid S, Sigel H, Sigel RKO, editors. Metal Ions In Life Sciences. Chichester, United Kingdom: John Wiley & Sons Ltd; 2008. pp. 507–546. [Google Scholar]
- 4.Simmer J, Hu J. Dental enamel formation and its impact on clinical dentistry. J Dent Educ. 2001;65:896–905. [PubMed] [Google Scholar]
- 5.Aoba T, Fejerskov O. Dental fluorosis: chemistry and biology. Crit Rev Oral Biol Med. 2002;13:155–170. doi: 10.1177/154411130201300206. [DOI] [PubMed] [Google Scholar]
- 6.Fan Y, Sun Z, Moradian-Oldak J. Controlled reminer alization of enamel in the presence of amelogenin and fluoride. Biomaterials. 2009;30:478–483. doi: 10.1016/j.biomaterials.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honorio HM, Rios D, Santos CF, Magalhaes AC, Buzalaf MA, Machado MA. Effects of erosive, cariogenic or combined erosive/cariogenic challenges on human enamel: an in situ/ex vivo study. Caries Res. 2008;42:454–459. doi: 10.1159/000163021. [DOI] [PubMed] [Google Scholar]
- 8.Mann S. The Biomimetics of Enamel: a Para digm for Organized Biomaterials Synthesis. Chi chester, United Kingdom: John Wiley & Sons Ltd; 1997. [Google Scholar]
- 9.Slavkin HC. Enamel revisited. Dimensions of Dental Hygiene. 2007;5(6):16–18. [Google Scholar]
- 10.Yamagishi K, Onuma K, Suzuki T, et al. Materials chemistry: a synthetic enamel for rapid tooth repair. Nature. 2005;433:819. doi: 10.1038/433819a. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Clarkson B, Sun K, Mansfield J. Selfassembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J Colloid Interface Sci. 2005;288:97–103. doi: 10.1016/j.jcis.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y, Sun Z, Wang R, Abbott C, Moradian-Oldak J. Enamel inspired nano composite fabrication through amelogenin supra mole cular assembly. Biomaterials. 2007;28:3034–3042. doi: 10.1016/j.biomaterials.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iijima M, Moradian-Oldak J. Control of apatite crystal growth in a fluoride containing amelogeninrich matrix. Biomaterials. 2005;26:1595–1603. doi: 10.1016/j.biomaterials.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Guan X, Yin H, Moradian-Oldak J, Nancollas G. Mimicking the self-organized microstructure of tooth enamel. J Phys Chem C Nanomater Interfaces. 2008;112:5892–5899. doi: 10.1021/jp077105+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uskokovic V, Kim MK, Li W, Habelitz S. Enzymatic processing of amelogenin during continuous crystallization of apatite. J Mater Res. 2008;23:3184–3195. doi: 10.1557/JMR.2008.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snead M. Whole-tooth regeneration: it takes a village of scientists, clinicians, and patients. J Dent Educ. 2008;72:903–911. [PMC free article] [PubMed] [Google Scholar]
- 17.Chai Y, Slavkin H. Prospects for tooth regeneration in the 21st century: a perspective. Microsc Res Tech. 2003;60:469–479. doi: 10.1002/jemt.10287. [DOI] [PubMed] [Google Scholar]
- 18.Du C, Moradian-Oldak J. Tooth regeneration: challenges and opportunities for biomedical material research. Biomed Mater. 2006;1:R10–17. doi: 10.1088/1748-6041/1/1/R02. [DOI] [PubMed] [Google Scholar]
- 19.Catón J, Luder H, Zoupa M, et al. Enamel-free teeth: Tbx1 deletion affects amelogenesis in rodent incisors. Dev Biol. 2009;328:493–505. doi: 10.1016/j.ydbio.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, Sargeant T, Hulvat J, et al. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J Bone Miner Res. 2008;23:1995–2006. doi: 10.1359/JBMR.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]


