Abstract
The P2X7 receptor (P2X7R) is a purinoceptor expressed predominantly by cells of immune origin, including microglial cells. P2X7R has a role in the release of biologically active proinflammatory cytokines such as IL-1β, IL-6 and TNFα. Here we demonstrate that when incubated with lipopolysaccharide (LPS), glial cells cultured from brain of P2X7R−/− mice produce less IL-1β compared to glial cells from brains of wild-type mice. This is not the case for TNFα and IL-6. Our results indicate a selective effect of the P2X7R gene deletion on release of IL-1β release but not of IL-6 and TNFα. In addition, we confirm that only microglial cells produce IL-1β, and this release is dependent on P2X7R and ABC1 transporter. Because IL-1β is a key regulator of the brain cytokine network and P2X7R is an absolute requirement for IL-1β release, we further investigated whether response of brain cytokines to LPS in vivo was altered in P2X7R−/− mice compared to wild-type mice. IL-1β and TNFα mRNAs were less elevated in the brain of P2X7R−/− than in the brain of wild-type mice in response to systemic LPS. These results show that P2X7R plays a key role in the brain cytokine response to immune stimuli, which certainly applies also to cytokine-dependent alterations in brain functions including sickness behavior.
Keywords: P2X7R knockout mice, Microglia, Astrocyte, Brain, ABC1 transporter, IL-1β, IL-6, TNFα, RT-PCR, LPS
1. Introduction
Proinflammatory cytokines including interleukin-1 (IL-1), tumor necrosis factor α (TNFα) and IL-6, are expressed in the healthy and injured brain (Besedovsky et al., 1991; Kent et al., 1992; Kluger, 1991). In the healthy brain, pro-inflammatory cytokines are produced by both glial and neuronal cells, and they regulate memory and learning (Avital et al., 2003; Pearson et al., 1999; Wang et al., 1994). The production of proinflammatory cytokines by macrophage-like cells and microglia is greatly enhanced in the brain in response to activation of the peripheral innate immune system, and this leads to the development of sickness behavior, an adaptive response to infectious pathogens (Dantzer and Kelley, 2007). However, when overexpressed, brain proinflammatory cytokines induce neuronal death, astrogliosis and demyelination (Perry, 2004). Although much knowledge has been accumulated on the expression of proinflammatory cytokines and their receptors in the brain during the last decade, much less is known about the mechanisms of release of proinflammatory cytokines.
Extracellular ATP has been reported to trigger the release of proinflammatory cytokine through its binding to type 2 purinergic receptors (P2 receptors) (Ferrari et al., 2006). These receptors are subdivided into P2Y metabotropic (P2YR), that are G protein-coupled receptors, and P2X ionotropic receptors (P2XR), that are ligand-gated ion channels (Di Virgilio et al., 2001). Interestingly, microglial cells, the main source of proinflammatory cytokine in the brain, express P2X7R (James and Butt, 2002; Norenberg et al., 1994). Using a microglial cell line, Sanz and Di Virgilio have demonstrated that extracellular ATP triggers maturation and release of IL-1β (Sanz and Di Virgilio, 2000). IL-1β is synthesized as an inactive precursor of 35 kDa which is processed into a 17-kDa active form by a specific cysteine protease, the IL-1β-converting enzyme (ICE or caspase 1) (Thornberry et al., 1992). Caspase 1 activation requires increased K+ efflux via the P2X7R activation (Kahlenberg and Dubyak, 2004; Sanz and Di Virgilio, 2000). Although the P2X7R is clearly involved in the processing of LPS-induced IL-1β in macrophages, mechanisms of release of mature IL-1β are still ill-defined. Because of the lack of secretory signal sequence (Lomedico et al., 1984), IL-1β does not follow the canonical endoplasmic reticulum-to-Golgi exocytic pathway (Singer et al., 1988). ATP-binding cassette (ABC) transporters form a large family of transmembrane proteins implicated in the movement of a wide variety of molecules across biological membranes including leaderless secretory proteins like IL-1β (Hamon et al., 1997; Marty et al., 2005). This prompted us to investigate if ABC1 was involved in the P2X7R-mediated release of mature IL-1β in microglial cells.
By blocking the brain IL-1 type I receptor with the specific antagonist of the IL-1 receptor IL-1ra in vivo, we have previously demonstrated that IL-1 is a key component of the proinflammatory cytokine network. Indeed, intracerebroventricular administration of IL-1ra totally blocked the enhanced expression of hypothalamic inflammatory cytokines that is normally induced by peripheral administration of LPS (Laye et al., 2000). These results indicate that endogenous release of IL-1 in the brain is a key event of the regulation of the cytokine network. However, since IL-1ra blocks access of both IL-1β and IL-1α to the type I IL-1 receptor, these results did not allow us to determine whether IL-1β is more important than IL-1α in the organization of the brain cytokine network response to LPS. We therefore used P2X7R−/− mice to further assess the role of IL-1β in the organization of the brain cytokine response to LPS.
Although P2X7R is likely to play a key role in IL-1β processing, its possible involvement in the processing of TNFα and IL-6 remains to be determined. ATP-induced IL-6 release appears to be dependent on P2Y receptors (Shigemoto-Mogami et al., 2001). P2X7R−/− mice release less IL-6 and IL-1 in the peritoneal cavity in response to an intraperitoneal injection of lipopolysaccharide (LPS), an endotoxin which induces the cytokine production. Moreover, macrophages from P2X7R−/− mice do not release IL-1β in response to ATP (Solle et al., 2001). In accordance with these results, P2X7R−/− mice are resistant to the development of collagen-induced arthritis (Labasi et al., 2002), have excess osteoclast activity (Ke et al., 2003) and are less sensitive to chronic inflammatory pain (Chessell et al., 2005).
To assess the role of the P2X7R in the release of brain cytokines in response to LPS, we evaluated the release of IL-1β, TNFα and IL-6, by mixed glial cell cultures of wild-type mice in which the P2X7R was blocked by a specific antagonist and by primary glial cell cultures of P2X7R−/− mice. We show here that microglial cells from P2X7R−/− mice are unable to release mature IL-1β. We confirmed the in vivo relevance of these data by assessing the expression of cytokines in the brain of P2X7R−/− and wild-type mice.
2. Methods
2.1. Animals and treatments
Homozygous P2X7 receptor knockout mice (P2X7R−/−) were raised on a background of C57Bl/6 and were kindly provided by Dr. Gabel (Pfizer, Groton, USA) (Solle et al., 2001). Wild-type (WT) C57BL/6 and CD1 mice were supplied by Charles River (France). Lack of the P2X7 receptor was confirmed in KO mice using PCR as previously described (Le Feuvre et al., 2002). The investigators adhered to the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals. Studies were carried out according to the Quality Reference System of INRA (http://www.international.inra.fr/content/download/947/11111/file/requirements) and approved by the local ethical committee for care and use of animals (AP 2/3/2004).
For in vivo studies of cytokine expression in plasma (ELISA) and in the brain (real-time PCR), WT and P2X7R−/− male mice were treated as follow. Mice were weighed and handled at least 1 week before the experiments. All mice were given an intraperitoneal injection of sterile saline (0.9% NaCl) or LPS from E. coli (Sigma, 0127:B8; 5 μg/mouse in saline; 0.2 ml/mouse) and sacrificed 2 or 4 h later as in previous studies (Laye et al., 1994). The hypothalamus was collected, frozen on dry ice and stored at −80 °C until further investigation.
2.2. Cell culture and treatments
Mixed glial cell cultures were performed from 2-day-old CD1, C57Bl/6 and P2X7R−/− mice. After decapitation, brains were carefully isolated and meninges were removed in ice-cold Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen). Forebrains were homogenized by mechanical dissociation and centrifugated (285 g, 10 min) at 4 °C to collect cells. Then, 4 × 105 cells were plated on 100 mm Petri Dishes in 5 ml of DMEM, 10 % fetal calf serum (FCS, Eurobio) and 0.5 % gentamicin (Eurobio). Under these conditions, neurons do not survive the mechanical dissociation (as determined by a NEUN labeling, data not shown) and only glial cells (mainly astrocytes and microglial cells) grow (Chauvet et al., 2001). Unattached cells were harvested 24 h later. Mixed glial cells were maintained in culture (95% O2, 5% CO2 at 37 °C) for 15 days until use.
When cells reached 80% confluence, they were serum deprived for 24 h to avoid synergistic or additive effects with proteins contained in serum. Then, glial cells were treated with LPS for 6 h (1 μg/ml, Sigma). Time and dose of LPS were chosen on the basis of both preliminary and previously published results (Chauvet et al., 2001). To enhance IL-1β release, ATP was added to cells 30 min before the end of LPS treatment (1 mM, Sigma) as previously described (Bianco et al., 2005; Colomar et al., 2003; Marty et al., 2005). In some experiments, oxidized ATP (oATP, 300 μM, Sigma) a specific P2X7R antagonist, was added during the last 90 min of LPS stimulation (Colomar et al., 2003; Marty et al., 2005). In another set of experiments, glybenclamide (0.1 mM, Sigma), a pharmacological inhibitor of the ABC1 transporter was used 30 min before the end of LPS treatment as previously described by our group (Marty et al., 2005). Neither oATP nor glybenclamide had any effect on cytokine release as determined in pilot experiments (data not shown). Media were collected and adherent cells scrapped off and kept at −20 °C until further investigation by ELISA or western blot.
After treatments, cell viability was evaluated by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) test as previously decribed (Chauvet et al., 2001). Cell death was assessed by the cytotoxicity detection kit (Roche) which measures release of lactate dehydrogenase (LDH) from dying cells, according to the manufacturer’s instructions. Total LDH release was achieved by adding Triton X-100 (1% in DMEM, Sigma, France) to untreated control cells. MTT and LDH assays demonstrated no effect of the treatments on cell viability or cell death (data not shown).
2.3. Western blot
Cell culture media were concentrated from 4 ml to 100 μl by several centrifugations at 792g for 10 min with 5 kDa Amicon ultra centrifugal device (Millipore). Proteins were extracted from cell pellets with 100 μl cold lysis buffer (TRIS 20 mM pH 7.5, anti protease cocktail (Sigma P2714), 5 mM MgCl2, 1 mM DTT, 0.5 M EDTA, 1 mM NaOV, 1 mM NaF) and centrifuged (30 min at 13,000 rpm at 4 °C). Protein concentration of samples was measured with a BCA assay (Uptima, France).
Equal amount of proteins (10 μg) were loaded on SDS–PAGE gels (12%) and transferred onto PVDF membrane (Millipore). Briefly, blots were blocked 1 h with TBST (50 mM Tris, 250 mM NaCl, 0.1 % Tween 20, pH 7.5) containing 5% skim milk powder, washed three times with TBST, and incubated with anti-IL-1β antibody (1:1000, R&D) diluted in TBST 3% BSA overnight at 4 °C. After washing three times with TBST, blots were incubated with peroxidase-conjugated anti-goat IgG antibody (1:10,000, Jackson) diluted in 5% skim-milk TBST for 1 h, washed and incubated with chemiluminescent substrate (ECL-Plus Western blotting detection system (Perkin-Elmer) for 5 min. Images were captured on hyperfilm ECL (Amersham). Optical density analysis of signals was performed using a calibrated densitometer (GS-800, Bio-Rad, France). After IL-1β detection, membranes were incubated 10 min at 70 °C in stripping buffer (0.065 M Tris, pH 6.7, 1% SDS, 0.7% β-mercapto-ethanol) in order to erase IL-1β signal. Actin was revealed by using the same procedure as described above (polyclonal rabbit anti-actin antibody, 1:2500 in 3% BSA–TBST, Sigma; followed by peroxidase-conjugated anti-rabbit antibody, 1:5000 in 5% milk-TBST, Jackson).
2.4. Cytokine measurement
The levels of IL-1β (both pro and mature) released into the cell culture medium and in plasma of WT and P2X7R−/− mice, was quantified by using a specific mouse sandwich ELISA. IL-1β ELISA reagents were kindly supplied by Dr. S. Poole (NIBSC, UK) and ELISA was performed as previously described (Laye et al., 2000). Assay detection limits were <1.9 pg/ml. TNFα (assay detection limits <5 pg/ml) and IL-6 (assay detection limits <15.6 pg/ml, were measured in WT and P2X7R−/− plasma by using Immunoassays from R&D Systems according to the manufacturer’s instructions. All assay plates were read on a microplate reader (Perkin-Elmer, France) at the appropriate wavelength. Data are expressed as picograms per milliliter of medium culture or plasma.
2.5. Immunocytochemistry
Cells were seeded on 13-mm glass coverslips in 100 mm Petri dishes. After treatments, cells were fixed with 4% paraformaldehyde for 10 min and blocked in TBS, 1% BSA, 2% FCS (Pousset et al., 2000). Astrocytes were detected with rabbit anti-glial fibrillary acidic protein antibody (1:100, 1 h at RT, Dako) and Alexa 594-conjugated anti rabbit (1:250, 1 h at RT, Molecular Probes). Microglia were detected with FITC-conjugated rat anti-CD68 (1:100, 1 h at RT, Serotec). The use of anti-CD11b or isolectinB4 gave similar results (data not shown). IL-1β was detected with goat anti-IL-1β antibody (1:100, 1 h at RT, R&D system), biotinylated-conjugated anti-goat IgG (1:500, 1 h at RT, Amersham Biosciences) and Alexa 594-conjugated streptavidin (1:500, 1 h at RT, Molecular Probes). Antibodies were diluted in TBS 1% BSA 2% FCS. Fluorescent mounting medium (Dako, France) was used to mount all preparations and cells were examined using a fluorescent microscope (Nikon Eclipse E400) with ACT-1 imaging software (Nikon, France). Total cells were visualized by 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) staining (1:500, 5 min, Merck). Negative controls were performed by omitting primary antibodies (data not shown). The number of cells expressing both CD68 and IL-1β was counted and normalized to DAPI-positive cells by cover slip.
2.6. Quantitative real-time PCR
One microgram of RNA obtained from hypothalamus was reverse transcribed using RETROscript Kit (Ambion). Quantitative analysis of cytokine mRNA expression was performed using the Applied Biosystems (Foster, CA) assay-on demand gene expression protocol as described previously (Godbout et al., 2005). The primers (target of IL-6, IL-1β, TNFα genes and of a reference gene (glyceraldehyde-3 phosphate dehydrogenase, GAPDH)) were used to amplify the corresponding cDNAs by using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). Fluorescence was determined on an ABI PRISM 7900-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative 2−ΔΔCt method (Livak and Schmittgen, 2001) and the results were expressed as relative fold change. Each quantitative PCR experiment included a minimum of five animals per treatment group and time point.
2.7. Statistics
For real-time PCR studies, a 3-way ANOVA (strain × treatment × time) was used to determine the significance of the effects of the treatments and strain under investigation. Between-group differences were determined by post hoc comparison with least significant difference test. All results were summarized and presented as means ± SEM.
3. Results
3.1. Microglial cells produce IL-1β in mixed glial cell culture in reponse to LPS
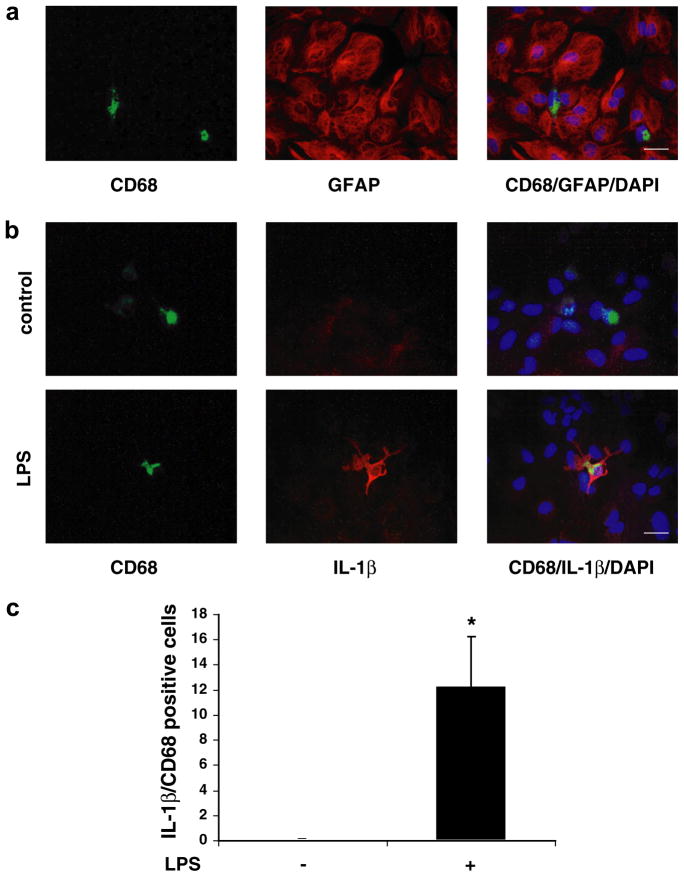
To confirm that only microglia and astrocytes were present in mixed glial cell culture performed from newborn mice, we used a double immunohistochemistry approach to identify microglial cells (CD68 staining) and astrocytes (GFAP staining). Total cell number was determined by the visualisation of cell nuclei with a DAPI staining. Fig. 1a shows that only two types of cells were present in the primary culture: microglial cells and astrocytes. Moreover, all nuclei stained with DAPI were colocalized with either GFAP or CD68. Next, the cellular origin of IL-1β was investigated by using CD68/IL-1β/DAPI immunocytochemistry on glial cell cultures 6 h after a LPS treatment (1 μg/ml) or its solvent (control). As depicted in Fig. 1b, no IL-1β staining was detectable in control cells whereas only microglial cells were immunoreactive for IL-1β staining after LPS treatment. The counting of CD68 positive cells expressing IL-1β normalized to DAPI-positive cells by coverslip revealed a statistically significant effect of LPS (Fig. 1c; p < 0.05).
Fig. 1.
IL-1β is synthesized by LPS-treated microglial cells in mixed glial cell cultures. In mixed glial cell cultures, microglial cells were characterized by FITC-conjugated rat anti-CD68 (green), whereas astrocytes were identified by rabbit monoclonal antibodies directed against GFAP and revealed by anti-rabbit Alexa 595 labelling (red) (a). Nuclei were revealed by DAPI staining (blue).(Scale bar 10 μm). Glial cells were incubated with LPS or its solvent for 6 h (b). IL-1β was immunodetected by goat anti-IL-1β antibody followed by biotinylated-conjugated anti-goat IgG and revealed by Alexa 594-conjugated streptavidin (red) (scale bar 10 μm). IL-1βCD-68 co-labeled cells were quantified (c). Each bar represents the mean number of IL-1βCD-68 positive cells normalized to DAPI-positive cells by coverslip ± SEM of four independent experiments (*p < 0.05). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
3.2. P2X7R and ABC1 are necessary for the release of IL-1β by mixed glial cells in culture
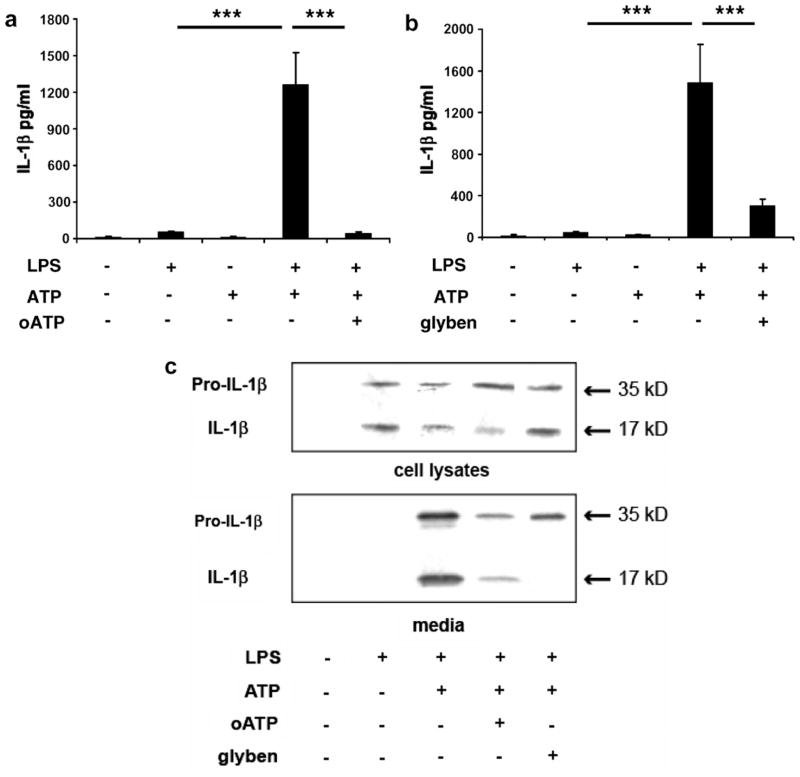
In order to assess the mechanisms underlying IL-1β synthesis and release by microglial cells in glial cell cultures, CD1 cells were treated with LPS before being treated with ATP which induces IL-1β release (Kahlenberg and Dubyak, 2004), oATP a potent P2X7R antagonist (Colomar et al., 2003; Marty et al., 2005; Murgia et al., 1993) and glybenclamide, an inhibitor of the transporter ABC1 (Hamon et al., 1997; Marty et al., 2005). IL-1β was measured in cell media by ELISAs (Fig. 2a and b). As revealed by statistical analysis by one-way ANOVA on the treatment factor, IL-1β levels were modified by the treatments under study (Fig. 2a: F(4,20) = 21.96, p < 0.0001; Fig. 2b: F(4,20) = 13.91, p < 0.0001). IL-1β levels were low in control media and media from cultures treated with ATP or LPS (Fig. 2a and b). The addition of ATP to LPS significantly increased IL-1β levels (Fig. 2a and b: p < 0.001). The addition of oATP (Fig. 2a, p < 0.001) or glybenclamide (Fig. 2b, p < 0.001) significantly decreased LPS + ATP induced IL-1β release. Taken together, these results show that the P2X7R and ABC1 transporter mediate IL-1β release by microglial cells. Analysis of IL-1β expression by western-blot confirmed these last results (Fig. 2c). Western blot analysis of cell lysates revealed that control treatment did not induce pro-IL-1β nor mature IL-1β synthesis whereas LPS treatment induced the expression of both forms of IL-1β (Fig. 2c). Addition of ATP in the medium did not affect intracellular expression of precursor and mature IL-1β as compared to LPS treatment (Fig. 2c). As shown in Fig. 2c, LPS + ATP + oATP treatment decreased mature IL-1β expression in cell lysate as compared to LPS + ATP treatment, whereas glybenclamide treatment had no effect on mature IL-1β expression. Measurement of IL-1β expression by Western blot in cell media showed that control and LPS treatment did not trigger the release of any form of IL-1β contrary to LPS + ATP treatment which induced the release of both precursor and mature IL-1β. Addition of oATP or glybenclamide in the medium altered the release of both forms with a stronger effect of glybenclamide on mature IL-1β release (Fig. 2c).
Fig. 2.
IL-1β release by microglial cells in culture is mediated by P2X7 receptor (P2X7R) activation and ABC1 transporter. Mixed glial cells cultures from CD1 mice were treated with LPS, ATP and oATP (a and c) or glybenclamide (b and c). Quantitative measurements of released IL-1β in cell media were performed by ELISA (a and b). Pro-IL-1β and IL-1β were measured in cell lysates and cell media by Western blot analysis (c). Western blots presented are representative results of 2–3 independent experiments. Data are given as the mean level of IL-1β ± SEM of five independent experiments (***p < 0.001).
3.3. P2X7R deficiency abrogates IL-1β but not TNFα and IL-6 release by mixed glial cells culture
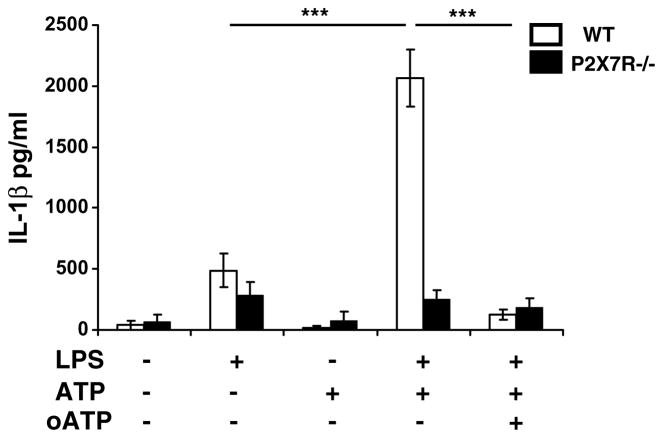
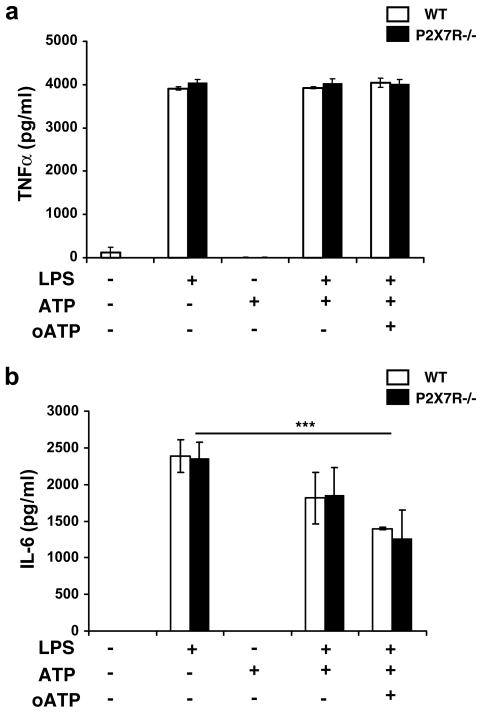
By using glial cell culture performed from WT and P2X7R−/− mice, we determined by ELISA whether P2X7R deficiency abrogated ATP-induced IL-1β release. WT glial cells behaved as CD1 cells in response to LPS, LPS + ATP and LPS + ATP + oATP treatments as represented in Fig. 3. A 2-way ANOVA (treatment × strain) revealed a significant effect of treatment (F(1,20) = 39.96, p < 0.0001), strain (F(1,20) = 32.047, p < 0.0001) and treatment × strain interaction (F(1,20) = 30.13, p < 0.0001). Post hoc analysis revealed an additive effect of ATP on LPS-induced IL-1β release (p < 0.0001) that was abrogated by the addition of oATP (p < 0.0001). By contrast, there was no increase in IL-1β release in LPS-treated P2X7R−/− glial cells in response to ATP. Fig. 4a shows that intracellular pro-IL-1β expression was not modified in P2X7R−/− as compared to WT glial cells in response to LPS + ATP and LPS + ATP + oATP treatments in contrast to intracellular mature IL-1β expression. Thus, mature IL-1β expression was significantly decreased in P2X7R−/− cells in response to LPS + ATP treatment (WT vs P2X7R−/−, p < 0.05). Moreover, LPS + ATP + oATP stimulation in WT cells did not significantly alter pro-IL-1β expression (Fig. 4b) but reduced expression of the mature form of IL-1β (LPS + ATP vs LPS + ATP + oATP, p = 0.05) (Fig. 4c). All together, these results show that IL-1β maturation and release by microglial cells is mediated by binding of ATP to the P2X7R. To assess whether this mode of release is common to other inflammatory cytokines, we analyzed TNFα and IL-6 release from WT and P2X7R−/− glial cells treated with LPS and treated with ATP and oATP (Fig. 5). As revealed by a 2-way ANOVA (strain × treatment), LPS significantly induced both TNFα (Fig. 5a, F(1,20) = 1638, p < 0.0001) and IL-6 release (Fig. 5b, F(1,18) = 35.92, p < 0.0001) independently of the strain. Post hoc analysis revealed a significant effect of LPS on both IL-6 (Fig. 5b, p < 0.0001) and TNFα release (Fig. 5a, p < 0.0001) but no additive effect of ATP. However, while nor ATP neither oATP modified TNFα release (Fig. 5a), IL-6 levels were altered by a oATP treatment (Fig. 5b, p = 0.0004). This last result indicates that IL-6 release although not linked to P2X7R activation could be regulated by the activation of another ATP receptor.
Fig. 3.
LPS treated microglial cells from P2X7R−/− do not release IL-1β. Mixed glial cell cultures from WT and P2X7R−/− were treated with LPS, ATP and/or oATP. IL-1β was measured by ELISA in cell media. Data are given as the mean level of IL-1β ± SEM of three independent experiments (**p < 0.01, ***p < 0.001).
Fig. 4.
Mature IL-1β expression is strongly decreased in LPS treated microglial cells from P2X7R−/−. Mixed glial cell cultures from WT and P2X7R−/− were incubated with LPS and ATP and/or oATP. Pro-IL-1β and IL-1β were detected in cell lysates by Western blot (a). Pro-IL-1β (top) and IL-1β (bottom) optical density was normalized to actin (b). Data are given as means ± SEM of three independent experiments (*p < 0.05).
Fig. 5.
LPS-treated microglial cells from P2X7R−/− release TNFα and IL-6. Mixed glial cell cultures from WT and P2X7R−/− were treated with LPS, ATP and/or oATP. TNFα (a) and IL-6 (b) were measured by ELISA in cell media. Data are given as the mean level of cytokines ± SEM of three independent experiments (*p < 0.05).
3.4. P2X7R deficiency attenuates response of brain cytokines to LPS in vivo
In the hypothalamus, a network of inflammatory cytokines is induced in response to an intraperitoneal injection of LPS. IL-1β is the main inflammatory cytokine in this network as intracerebroventricular administration of its antagonist totally blocks LPS-induced expression of brain inflammatory cytokines (Laye et al., 2000). We used quantitative real-time PCR on the hypothalamus of WT and P2X7R−/− mice submitted to an intraperitoneal injection of saline or LPS (5 μg/mouse) to determine whether the LPS-induced brain cytokine network expression was altered by the absence of P2X7R. LPS significantly increased IL-1β (F(1, 49) = 59.29, p < 0.001), IL-6 (F(1, 49) = 49.17, p < 0.001) and TNFα (F(1, 49) = 103, p < 0.001) mRNA expression in the hypothalamus of both strains 2 and 4 h after treatment with no time effect (Fig. 6). The strain × treatment interaction was significant for IL-1β (F(1, 49) = 4.05, p < 0.05) and TNFα (F(1, 49) = 6.01, p < 0.05), but not for IL-6 (F(1, 49) = 2.43, p = 0.12) (Fig. 6). These findings indicate that the the lack of P2X7R attenuates LPS-induced IL-1β and TNFα mRNA expression in the hypothalamus.
Fig. 6.
LPS-induced TNFα mRNA expression is decreased in hypothalamus of P2X7R−/− mice. Wild-type (WT) and P2X7R−/− mice were injected with either saline or LPS. IL-1β (a), TNFα (b) and IL-6 (c) mRNA were measured by quantitative real-time PCR in hypothalamus collected 2 and 4 h after. Data are given as the mean level of relative fold changes ± SEM (n = 6–8/experimental group).
4. Discussion
The overall aim of this study was to characterize the role of P2X7R in the proinflammatory cytokine release by microglial cells in response to LPS. We demonstrated that IL-1β release by microglial cells in culture was strongly related to the functionality of the P2X7R and ABC1 transporter whereas TNFα and IL-6 release was not dependent on P2X7R activity. We also observed that LPS-induced expression of hypothalamic cytokines in vivo was partially altered by P2X7R deficiency.
Microglial cells have been identified as the main source of IL-1β in the brain (Chauvet et al., 2001; Konsman et al., 1999) although there is evidence that after brain damage, astrocytes can contribute to the production of this cytokine (Pearson et al., 1999; Wang et al., 1994). By using CD68/IL-1β/DAPI immunocytochemistry we confirmed that microglia, but not astrocytes, expressed IL-1β in LPS-stimulated mixed glial cell cultures. Our immunocytological results indicate that IL-1β immunocytochemical staining was mainly located in the cytosol. The cytosolic accumulation of IL-1β is in accordance with the lack of a conventional secretory peptide (Andrei et al., 1999). It is important to note that we used an antibody that recognizes both forms of IL-1β (precursor and mature) as revealed by Western blot performed with the same antibody (Figs. 2 and 4). Therefore, we cannot dissociate the cellular localization of precursor and mature IL-1β by immunohistochemistry. Interestingly, we showed that LPS-stimulated glial cocultures expressed both precursor and mature forms of IL-1β in the intracellular compartment (Figs. 2 and 4). IL-1β maturation is likely due to the activation of ICE which is expressed in microglial cells (Yao and Johnson, 1997). The activation of ICE could be linked to the endogenously release of ATP as demonstrated in glial cells after a LPS treatment (Bianco et al., 2005; Ferrari et al., 1997; Verderio and Matteoli, 2001). Detection of the mature form of IL-1β in cells after a LPS treatment is intriguing. Previous studies performed on rat primary glial cells did not reveal the presence of the mature form in the cellular compartment following a LPS treatment (Chauvet et al., 2001). These contradictory results could be explained by an increase in LPS-induced ATP release by microglial cells or astrocytes (Bianco et al., 2005; Ferrari et al., 1997; Verderio and Matteoli, 2001). This LPS-induced ATP release could be sufficient to induce pro-IL-1β maturation but not to induce IL-1β release in cell media. Addition of extracellular ATP resulted in a strong extracellular release of IL-1β as measured by ELISA. By analyzing extracellular IL-1β by Western blot, we demonstrated that both forms of IL-1β were released. In accordance with previous studies on peripheral immune cells, we observed that treatment with oxidized ATP, a potent inhibitor of the P2X7R, decreased IL-1β release induced by a co-treatment with LPS and ATP (Colomar et al., 2003; Mehta et al., 2001). This result indicates an implication of P2X7R in microglial IL-1β release. In parallel, intracellular mature IL-1β expression was decreased, indicating an involvement of ICE in microglial IL-1β maturation.
The mechanism involved in ATP-induced IL-1β release remains ill-defined and several hypotheses have been proposed to account for it, including cell lysis (Hogquist et al., 1991a), fusion of endolysosome-related vesicles (Andrei et al., 1999), bleb formation, shedding of membrane vesicles (MacKenzie et al., 2001) through a membrane multimeric complex induced by ICE (Singer et al., 1995) and specific transporters such as ABC1 (Hamon et al., 1997; Marty et al., 2005). More recently, Pannexin-1 (Panx1), a mammalian protein that functions as a hemi-channel when ectopically expressed, has been shown to be required for the processing and release of mature IL-1β by macrophages (Pelegrin and Surprenant, 2006). However, in a follow up study (Pelegrin and Surprenant, 2007) reported that Panx1 was probably not the pathway involved in the P2X7R-mediated release of mature IL-1β, but was instead responsible for the immediate activation of caspase-1 processing by the inflammasome.
In the present study, we demonstrate that glybenclamide, an ABC1 blocker, strongly decreased (LPS + ATP)-induced IL-1β release, identified by Western blot as the mature form. These results indicate that both the P2X7R and ABC1 transporter are necessary for IL-1β release from microglia. In the light of Pelegrin and Surprenant (Pelegrin and Surprenant, 2006, 2007) reports, our results with glybenclamide can be interpreted to suggest that ABC1 transporter is acting downstream Panx1. Our study does not allow us to determine the localisation of the ABC1 transporter, that could be either on the plasma membrane or in shed vesicles as described by Bianco et al. who demonstrated that ATP induced the formation and shedding of membrane vesicles containing IL-1β from microglial cells (Bianco et al., 2005). IL-1β efflux from shed vesicles was enhanced by ATP stimulation and inhibited by a pre-treatment with oATP, thus indicating a crucial involvement of the P2X7R in the release of the cytokine from vesicles. Consistent with these results, shed vesicles contain all the machinery necessary for IL-1β processing, including P2X7R (Bianco et al., 2005). Pro-IL-1β could be processed into mature form following P2X7R activation in shed vesicles and released through ABC1 transporter. IL-1β efflux requires the activation of a chloride conductance by P2X7 receptor to enhance export activity through ABC1 transporter in Schwann cells (Marty et al., 2005) but such a mechanism has not been described yet in microglia. Pro-IL-1β release was not affected by oATP, indicating that P2X7R is not involved in such a process. Pro-IL-1β is devoid of any biological activity (Mosley et al., 1987). The release of pro-IL-1β has been proposed to be a consequence of cell death induced by a toxic dose of LPS (Hauser et al., 1986; Hogquist et al., 1991a,b; Young et al., 1988). However, such a mechanism is unlikely to occur in our cell system since there was no cell death in response to the treatments under investigation (data not shown).
Measurement of the release of TNFα and IL-6 in response to LPS in mixed glial cell cultures of P2X7R−/− mice shows that, unlike IL-1β, the release of these cytokines was not dependent on P2X7R. Our results are in contradiction with those recently obtained by Choi in human microglia (Choi et al., 2007). In that study, oATP was effective in attenuating LPS-induced mRNA expression of IL-1β, IL-6 and TNFα. Moreover, Hide et al. reported that ATP triggered TNFα release from rat microglial cells (Hide et al., 2000), while we could not measure TNFα release after a single ATP stimulation at the same concentration as the one used by Hide (1 mM for 6 h, data not shown). These discrepancies could be due to differences in culture systems since Choi and Hide used purified microglial culture whereas we used mouse glial coculture. In our study IL-6 release, but not TNFα, was altered by oATP treatment. Although oATP is often presented as a specific P2X7R antagonist (Murgia et al., 1993), it can act as a partial antagonist of other P2 purinergic receptors. In agreement with this, Shigemoto-Mogami et al. have shown in a mouse microglial cell line that IL-6 release is induced by ATP fixation on P2Y receptors (Shigemoto-Mogami et al., 2001). These data are relevant as microglial cells highly express P2Y receptors and in particular P2Y6 (Light et al., 2006).
The impact of the P2X7R deficiency on LPS-induced brain cytokine expression was measured by using real-time PCR in the hypothalamus of LPS-treated WT and P2X7R−/− mice. LPS-induced IL-1β and TNFα, but not IL-6, mRNA expression was significantly altered in P2X7R−/− hypothalamus as compared to WT mice. We have previously shown that an icv injection of IL-1ra strongly reduces LPS-induced IL-1β and TNFα mRNA expression, indicating that IL-1 induces its own synthesis and the expression of TNFα in the hypothalamus (Laye et al., 2000). Our results demonstrating that LPS-induced IL-1β and TNFα mRNA expression was significantly decreased in the hypothalamus of P2X7R−/− mice are consistent with this hypothesis. The main cellular sources of IL-1β and TNFα in the brain are microglia that are well known to express P2X7R (Buttini and Boddeke, 1995; Ferrari et al., 1997; Nadeau and Rivest, 1999; van Dam et al., 1992). Indeed the reduced production of TNFα in the hypothalamus of LPS-treated P2X7R−/− mice can be attributed to the lack of release of IL-1β by microglial cells of P2X7R−/− as we demonstrated in vitro. However, contribution of the P2X7R in both IL-1β and TNFα expression is only partial as their expression is not abrogated by P2X7R deficiency. Other mechanisms, such as binding of LPS on its receptors beared by microglia (Chakravarty and Herkenham, 2005), could be responsible for the residual IL-1 and TNF mRNA expression in the hypothalamus of P2X7R−/− mice. Further studies are needed to clarify this issue.
The fact that LPS-induced IL-6 mRNA expression was not significantly reduced in P2X7R−/− as compared to WT mice indicates that IL-6 expression is independent on P2X7R-induced IL-1β release. As shown by our in vitro results, LPS-induced IL-6 production is probably mediated by an effect of ATP on other purinergic receptors than P2X7R. Both microglia and astrocytes are important sources of IL-6 (Lee et al., 1993; van Wagoner and Benveniste, 1999). As both microglia and astrocytes express TLR4 (Bowman et al., 2003; Chakravarty and Herkenham, 2005) and P2Y receptors (Dixon et al., 2004; Franke et al., 2004; Fumagalli et al., 2003; Yamakuni et al., 2002) it is therefore possible that LPS as well as other inflammatory cytokines directly trigger IL-6 release by these cells independently of IL-1β.
In conclusion, the present results show that maturation and release of microglial IL-1β involves both P2X7R and ABC1 transporter. Unlike IL-1β release, the release of TNFα and IL-6 is not dependent on P2X7R activation. In vivo, the release of mature IL-1β appears to be an important component in the organization of the brain cytokine response to LPS. These results are important because a better understanding of the molecular mechanism by which the P2X7 receptor controls the maturation and release of brain IL-1β could lead to novel avenues for the treatment of brain inflammatory diseases.
Acknowledgments
This work has been supported by INRA, CNRS, Région Aquitaine (20030305001A2Bbis and 20051203008AB to S.L.). This research was supported in part by grants from the National Institutes of Health (NIH) to K.W.K. (R01 AG 029573) and R.D. (MH071349).
References
- Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1beta involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A, Klusman I, Furukawa H, Monge Arditi G, Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1991;40:613–618. doi: 10.1016/0960-0760(91)90284-c. [DOI] [PubMed] [Google Scholar]
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Buttini M, Boddeke H. Peripheral lipopolysaccharide stimulation induces interleukin-1 beta messenger RNA in rat brain microglial cells. Neuroscience. 1995;65:523–530. doi: 10.1016/0306-4522(94)00525-a. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhe-matopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet N, Palin K, Verrier D, Poole S, Dantzer R, Lestage J. Rat microglial cells secrete predominantly the precursor of interleukin-1beta in response to lipopolysaccharide. Eur J Neurosci. 2001;14:609–617. doi: 10.1046/j.0953-816x.2001.01686.x. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomar A, Marty V, Medina C, Combe C, Parnet P, Amedee T. Maturation and release of interleukin-1beta by lipopolysaccharide-primed mouse Schwann cells require the stimulation of P2X7 receptors. J Biol Chem. 2003;278:30732–30740. doi: 10.1074/jbc.M304534200. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Yu R, Panupinthu N, Wilson JX. Activation of P2 nucleotide receptors stimulates acid efflux from astrocytes. Glia. 2004;47:367–376. doi: 10.1002/glia.20048. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Grosche J, Heine C, Hartig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127:431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D’Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43:218–230. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Hamon Y, Luciani MF, Becq F, Verrier B, Rubartelli A, Chimini G. Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- Hauser C, Saurat JH, Schmitt A, Jaunin F, Dayer JM. Interleukin 1 is present in normal human epidermis. J Immunol. 1986;136:3317–3323. [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991a;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogquist KA, Unanue ER, Chaplin DD. Release of IL-1 from mononuclear phagocytes. J Immunol. 1991b;147:2181–2186. [PubMed] [Google Scholar]
- James G, Butt AM. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]
- Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Kelley K, Dantzer R. Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience. 1999;89:535–548. doi: 10.1016/s0306-4522(98)00368-6. [DOI] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–R98. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- Laye S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J Biol Chem. 2002;277:3210–3218. doi: 10.1074/jbc.M104388200. [DOI] [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- Light AR, Wu Y, Hughen RW, Guthrie PB. Purinergic receptors activating rapid intracellular Ca increases in microglia. Neuron Glia Biol. 2006;2:125–138. doi: 10.1017/S1740925X05000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lomedico PT, Gubler U, Hellmann CP, Dukovich M, Giri JG, Pan YC, Collier K, Semionow R, Chua AO, Mizel SB. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 1984;312:458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by micro-vesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- Marty V, Medina C, Combe C, Parnet P, Amedee T. ATP binding cassette transporter ABC1 is required for the release of interleukin-1beta by P2X7-stimulated and lipopolysaccharide-primed mouse Schwann cells. Glia. 2005;49:511–519. doi: 10.1002/glia.20138. [DOI] [PubMed] [Google Scholar]
- Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987;262:2941–2944. [PubMed] [Google Scholar]
- Murgia M, Hanau S, Pizzo P, Rippa M, Di Virgilio F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J Biol Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol. 1999;58:61–77. doi: 10.1097/00005072-199901000-00008. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Langosch JM, Gebicke-Haerter PJ, Illes P. Characterization and possible function of adenosine 5′-triphosphate receptors in activated rat microglia. Br J Pharmacol. 1994;111:942–950. doi: 10.1111/j.1476-5381.1994.tb14830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson VL, Rothwell NJ, Toulmond S. Excitotoxic brain damage in the rat induces interleukin-1beta protein in microglia and astrocytes: correlation with the progression of cell death. Glia. 1999;25:311–323. [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pousset F, Palin K, Verrier D, Bristow A, Dantzer R, Parnet P, Lestage J. Production of interleukin-1 receptor antagonist isoforms by microglia in mixed rat glial cells stimulated by lipopolysaccharide. Eur Cytokine Netw. 2000;11:682–689. [PubMed] [Google Scholar]
- Sanz JM, Di Virgilio F. Kinetics and mechanism of ATP-dependent IL-1 beta release from microglial cells. J Immunol. 2000;164:4893–4898. doi: 10.4049/jimmunol.164.9.4893. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78:1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- Singer, Scott S, Chin J, Bayne EK, Limjuco G, Weidner J, Miller DK, Chapman K, Kostura MJ. The interleukin-1 beta-converting enzyme (ICE) is localized on the external cell surface membranes and in the cytoplasmic ground substance of human monocytes by immuno-electron microscopy. J Exp Med. 1995;182:1447–1459. doi: 10.1084/jem.182.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, Scott S, Hall GL, Limjuco G, Chin J, Schmidt JA. Interleukin 1 beta is localized in the cytoplasmic ground substance but is largely absent from the Golgi apparatus and plasma membranes of stimulated human monocytes. J Exp Med. 1988;167:389–407. doi: 10.1084/jem.167.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- van Dam AM, Brouns M, Louisse S, Berkenbosch F. Appearance of interleukin-1 in macrophages and in ramified microglia in the brain of endotoxin-treated rats: a pathway for the induction of non-specific symptoms of sickness? Brain Res. 1992;588:291–296. doi: 10.1016/0006-8993(92)91588-6. [DOI] [PubMed] [Google Scholar]
- van Wagoner NJ, Benveniste EN. Interleukin-6 expression and regulation in astrocytes. J Neuroimmunol. 1999;100:124–139. doi: 10.1016/s0165-5728(99)00187-3. [DOI] [PubMed] [Google Scholar]
- Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol. 2001;166:6383–6391. doi: 10.4049/jimmunol.166.10.6383. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, Barone FC, White RF, Gagnon RC, Feuerstein GZ. Concomitant cortical expression of TNF-alpha and IL-1 beta mRNAs follows early response gene expression in transient focal ischemia. Mol Chem Neuropathol. 1994;23:103–114. doi: 10.1007/BF02815404. [DOI] [PubMed] [Google Scholar]
- Yamakuni H, Kawaguchi N, Ohtani Y, Nakamura J, Katayama T, Nakagawa T, Minami M, Satoh M. ATP induces leukemia inhibitory factor mRNA in cultured rat astrocytes. J Neuroimmunol. 2002;129:43–50. doi: 10.1016/s0165-5728(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Yao J, Johnson RW. Induction of interleukin-1 beta-converting enzyme (ICE) in murine microglia by lipopolysaccharide. Brain Res Mol Brain Res. 1997;51:170–178. doi: 10.1016/s0169-328x(97)00235-0. [DOI] [PubMed] [Google Scholar]
- Young PR, Hazuda DJ, Simon PL. Human interleukin 1 beta is not secreted from hamster fibroblasts when expressed constitutively from a transfected cDNA. J Cell Biol. 1988;107:447–456. doi: 10.1083/jcb.107.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]