Abstract
Purpose
Preoperative axillary lymph node ultrasound (US) and fine-needle aspiration (FNA) biopsy can identify a proportion of node-positive patients and avoid sentinel lymph node (SLN) surgery and direct surgical treatment. We compared the costs with preoperative US/FNA to without US/FNA (standard of care) for invasive breast cancer.
Methods
Using decision-analytic software we constructed a model to assess the costs associated with the two preoperative strategies. Diagnostic test sensitivities and specificities were obtained from literature review. Costs were derived from Medicare payment rates and actual resource utilization. Base-case results were fully probabilistic to capture parameter uncertainty in economic results.
Results
Base-case results estimate total mean costs per patient of $10,947 (“$” indicates US dollars throughout) with the US/FNA strategy and $10,983 with standard of care, an incremental cost savings of $36, on average, per patient [95% confidence interval (CI) of cost difference: −$248 to $179]. Most (63%) of the simulations resulted in cost saving with axillary US/FNA. One-way sensitivity analyses suggest that results are sensitive to assumed diagnostic and surgical costs and selected diagnostic test parameters. US/FNA approach was similar in costs or cost saving relative to the standard of care for all tumor stages.
Conclusions
The additional cost of performing axillary US with possible FNA in every patient is balanced, on average, by the savings from avoiding SLN in cases where metastasis can be documented preoperatively. Routine use of preoperative axillary US with FNA to guide surgical planning can decrease the overall cost of patient care for invasive breast cancer.
Preoperative axillary staging of breast cancer plays an important role in surgical treatment planning and assists in consideration of neoadjuvant therapy. It has become widely apparent that clinical examination of the axillary lymph nodes is not a good indicator of the presence or absence of axillary lymph node metastasis in breast cancer.1,2
Recently, multiple studies have shown that the use of routine preoperative ultrasound (US) examination of the axilla with fine-needle aspiration (FNA) biopsy of suspicious axillary lymph nodes to evaluate for metastatic disease is effective for determining patients who are lymph node positive prior to surgery.1–5 The advantage of documenting lymph node involvement preoperatively is that it allows the patient to proceed directly to axillary lymph node dissection (ALND) and avoids the need for sentinel lymph node (SLN) surgery. Additional advantages of preoperative identification of node positivity include decreased anesthesia and operative time and associated costs by eliminating the need to identify and resect the SLNs and intraoperative pathological analysis, as well as avoiding radioactive isotope and blue dye injection and associated possible complications.
Preoperative identification of node positivity may also prepare the patient better for surgery, as the patient’s education can be tailored preoperatively regarding postoperative recovery after ALND, drain management, and lymphedema prevention. The patient can also be considered for neoadjuvant chemotherapy. Postmastectomy radiation may be considered for node-positive patients, aiding preoperative counseling regarding immediate reconstruction.
The disadvantages of preoperative axillary US with FNA biopsy are that it is not 100% sensitive and requires additional procedural resources. Since a significant portion of patients are lymph node negative, and also a portion of positive lymph nodes are not detected on US with FNA biopsy, the procedure of US with possible FNA biopsy only stands to benefit patients found to be node positive by directing the surgical treatment more appropriately, while increasing resource utilization for all patients.2–7 Given that performing preoperative axillary US on all patients followed by FNA biopsy of suspicious nodes could increase overall resource use, there is a clear tradeoff of these added costs against the benefits (and cost savings) of decreased SLN procedures. The aim of this study, therefore, was to estimate and compare the costs of performing routine axillary ultrasound with FNA biopsy of any morphologically abnormal-appearing lymph nodes in all patients with invasive breast cancer with those incurred by alternatively performing SLN surgery without the use of preoperative axillary ultrasound (standard of care).
METHODS
Decision-Analytic Model
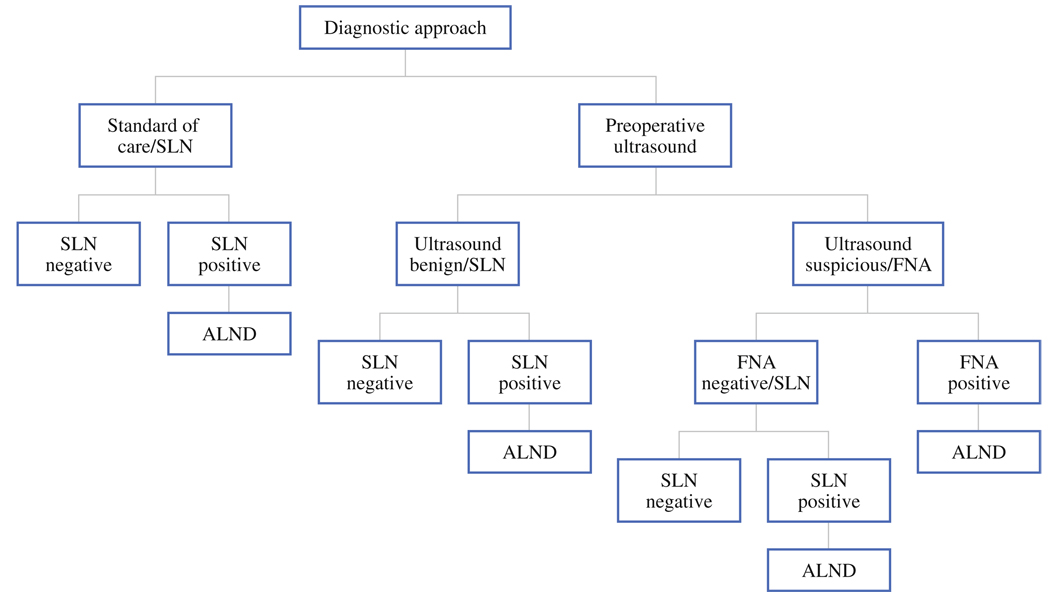
Institutional Review Board approval for this study was obtained. A decision tree was constructed using TreeAge Pro 2007 to estimate and compare the direct medical costs associated with the two alternative diagnostic strategies for invasive breast cancer patients: axillary US with FNA biopsy of any morphologically abnormal-appearing lymph nodes compared with SLN surgery without the use of preoperative axillary US. The target population for the study was operable cases with clinical stage I–III at presentation. The schematic shown in Fig. 1 outlines the sequence of events considered in our analyses, which occur with uncertainty and associated costs. Standard of care consists of all patients undergoing SLN surgery, with those having a positive result after histologic examination continuing on to ALND. Alternatively, patients undergo preoperative axillary US; those without axillary nodes seen on US, or those with morphologically normal-appearing nodes, do not undergo FNA. Patients with morphologically suspicious lymph nodes on US would continue on to FNA biopsy. Positive results of the FNA biopsy result in the patient going directly to ALND. Patients having benign results for US or FNA would continue on with the standard of care with SLN surgery, and ALND only in cases where the SLN was positive.
FIG. 1.
Decision tree for alternative diagnostic approaches to identify metastasis for invasive breast cancer patients
The modeling timeframe focused on short-term direct medical costs related to preoperative diagnostics and surgical treatment. Costs occurring beyond the day of surgery were not considered, as we did not feel that diagnostic approach impacted clinical or economic outcomes beyond surgical intervention.
Model Inputs
The probabilities of nodal metastasis and test parameters were obtained from comprehensive review of the published literature in the English language. Parameter estimates used in the base-case analysis and their ranges considered in one-way sensitivity analyses are given in Table 1.2,3,5–12 We weighted published diagnostic test sensitivities and specificities by study sample size to obtain our base-case parameter estimates used in analyses. The prevalence of nodal metastasis was assumed to be 30% at baseline. US sensitivity and specificity were 63% and 91%, respectively, while FNA sensitivity and specificity were similar: 65% and 99%, respectively. The specificity of SLN surgery was reported as (or assumed to be) 99.9% in references to date.
TABLE 1.
Base-case probability and cost estimates and their ranges for sensitivity analysis
| Base-case estimate (min/max) | References | |
|---|---|---|
| Probabilities | ||
| Metastasis prevalence | 0.30 (0.15/0.45) | 2,3 |
| Ultrasound sensitivity | 0.63 (0.26/0.92) | 5,7 |
| Ultrasound specificity | 0.91 (0.44/0.98) | 5,7 |
| FNA sensitivity | 0.65 (0.06/0.95) | 5,7 |
| FNA specificity | 0.99 (0.90/1.00) | 5,7 |
| SLN surgery sensitivity | 0.93 (0.77/0.98) | 6,8–12 |
| SLN surgery specificity | 0.99 (0.90/1.00) | 6,8–12 |
| Costs | ||
| SLN surgery | $10,078 ($6,522/$17,473) | Patient data |
| ALND | $11,045 ($7,250/$19,315) | Patient data |
| SLN surgery and ALND | $13,321 ($8,402/$23,537) | Patient data |
| FNA with imaging guidance | $128 ($64/$512) | Medicare reimbursement |
| Ultrasound of breast | $98 ($49/$392) | Medicare reimbursement |
| Echo guidance for biopsy | $206 ($103/$824) | Medicare reimbursement |
| Cytopathology evaluation for FNA | $142 ($71/$568) | Medicare reimbursement |
ALND axillary lymph node dissection, FNA fine-needle aspiration, SLN sentinel lymph node
Costs associated with surgical treatment for patients with invasive breast cancer were estimated from administrative data sources among patients treated at the Mayo Clinic Rochester between January 1, 2004 and December 31, 2006. Because of well-known discrepancies between billed charges and true resource use, we valued utilization using standard methods by grouping services into the Medicare part A and B classification: part A billed charges were adjusted using hospital cost-to-charge ratios at the departmental level and wage indexes. Costs associated with part B physician services were proxied based on Medicare reimbursement rates. Costs presented reflect 2007 constant dollars and reflect utilization that occurred on the day of surgery within the identified hospital episode. Costs pertaining to US, FNA biopsy, and subsequent histologic examination were derived from 2007 Medicare reimbursement rates by appropriate CPT4 billing code.
Base-Case Analysis
An analysis of costs per patient for each strategy was conducted using base-case parameter estimates. Incremental costs between strategies were calculated. Increasingly, guidelines for decision-analytic modeling strongly suggest that all estimates of input parameters in a model be specified as full probability distributions to appropriately capture the uncertainty surrounding parameter estimates.13,14 Thus, in our base-case analysis, we captured sampling uncertainty using Monte Carlo simulation techniques and prespecified parameter distributions. Choice of the distribution associated with each parameter was based on suggestions as noted by Briggs et al.14 A distribution of mean incremental costs from the simulations was produced along with 95% confidence intervals using the percentile method. The frequency of each strategy being least costly was also calculated from the simulations. These modeling and simulation techniques have been performed previously and reported in the published literature.15–18
Sensitivity Analysis
One-way deterministic sensitivity analyses were performed for all parameters where the parameter of interest was varied; all other parameters remained constant at base-case point estimates (Table 1). Probability parameter ranges considered were based on the minimum and maximum values reported in the literature. Only one study reported specificities of SLN or FNA below 100% (99% and 97%, respectively). Minimum values for each of these variables were set more conservatively at 90%. The range in cost estimates considered for surgical intervention was based on the estimated 95% confidence interval from patient-level analyses. Diagnostic cost estimate ranges were set at 50% and 400% of base-case values.
For variables identified in one-way sensitivity analyses as influencing the choice of economically preferred diagnostic option, we also assessed, via threshold analysis, the parameter value at which the preferred option changed. Finally, in an attempt to determine whether results hinged on patient tumor stage, we reran analyses holding the metastasis prevalence rates constant at 15%, 41%, and 75%, corresponding to tumor stages T1, T2, and T3 or greater, respectively.
RESULTS
Base-Case Analysis
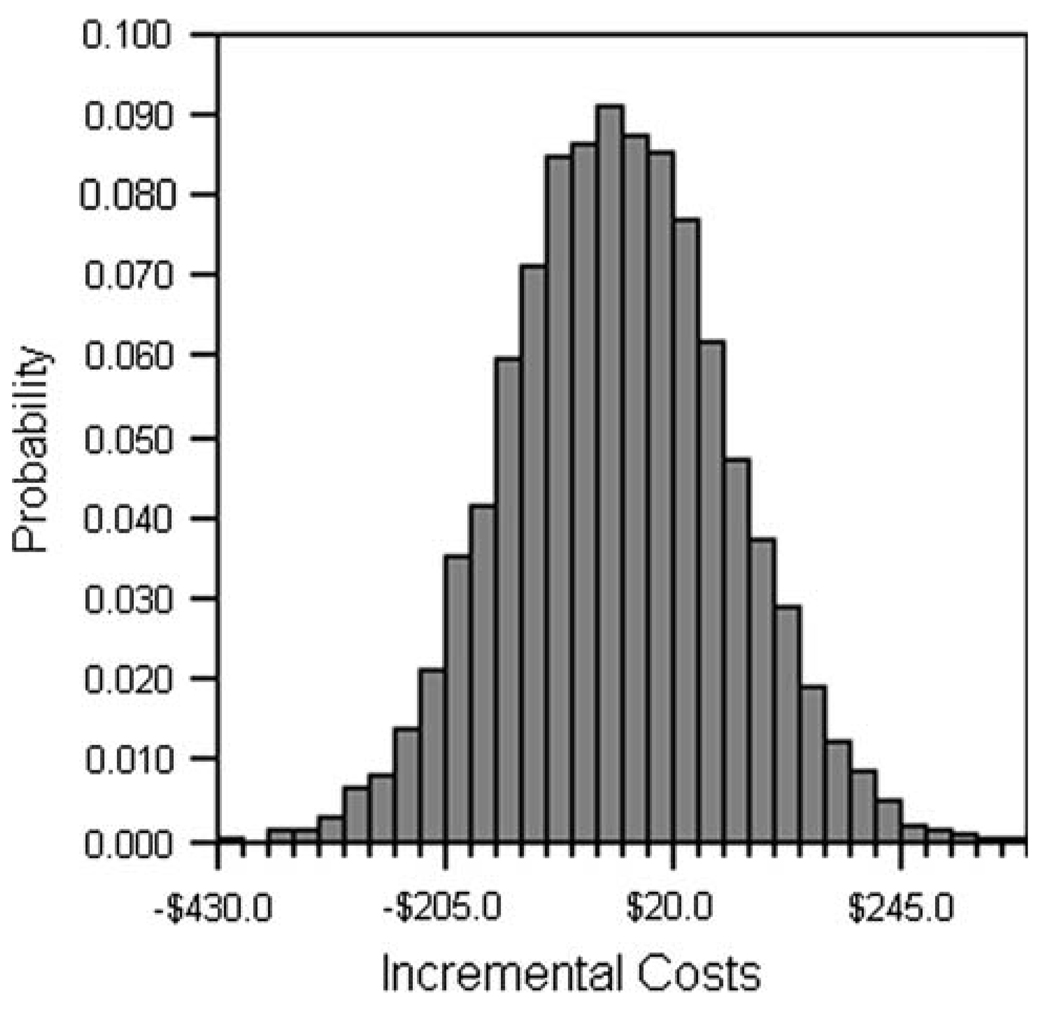
Figure 2 shows the base-case results using 10,000 Monte Carlo simulations capturing the global effect of uncertainty in all model parameters. Costs per patient with the US/FNA diagnostic strategy were estimated at $10,947 and with the standard of care at $10,983, an incremental cost savings of $36, on average, per patient (95% CI of cost difference: −$248 to $179). Approximately 63% of the simulations suggested incremental cost savings in favor of the US/FNA approach.
FIG. 2.
Incremental costs comparing the preoperative US/FNA approach with the standard of care (without US/FNA) for invasive breast cancer
Sensitivity Analyses
One-way sensitivity analysis of cost results comparing preoperative US/FNA approach and the standard of care (without US/FNA) are shown in Table 2. Model parameters having an effect on the economically favorable diagnostic strategy included: prevalence of metastasis, US sensitivity and specificity, FNA sensitivity, SLN sensitivity, surgical costs for ALND, and surgical costs for combined SLN and ALND procedures. Diagnostic costs (US costs, FNA costs, costs for ultrasonic guidance for FNA, and cytopathology evaluation of FNA) also impacted whether or not US/FNA was found to be a cost-saving approach. Threshold analyses found the US/FNA strategy to be cost-saving as long as US costs were $132 or lower (135% of the base-case estimate). FNA costs could be as high as $264 (206% of the base-case estimate) and ultrasonic guidance costs could be as high as $342 (166% of the base-case estimate), and the US/FNA strategy would remain cost-saving.
TABLE 2.
One-way sensitivity analysis cost results comparing preoperative US/FNA approach and the standard of care (without US/FNA) for invasive breast cancer
| Minimum | Maximum | |||||
|---|---|---|---|---|---|---|
| US/FNA | Standard of care | Incremental costs | US/FNA | Standard of care | Incremental costs | |
| Probabilities | ||||||
| Metastasis prevalence | $10,584 | $10,531 | $53 | $11,311 | $11,431 | −$120 |
| Ultrasound sensitivitya | $11,042 | $10,981 | $61 | $10,874 | $10,981 | −$107 |
| Ultrasound specificitya | $11,106 | $10,981 | $125 | $10,925 | $10,981 | −$56 |
| FNA sensitivitya | $11,175 | $10,981 | $194 | $10,832 | $10,981 | −$149 |
| FNA specificity | $10,953 | $10,981 | −$28 | $10,947 | $10,981 | −$34 |
| SLN surgery sensitivity | $10,858 | $10,829 | $29 | $10,978 | $11,034 | −$56 |
| SLN surgery specificity | $11,172 | $11,206 | −$34 | $10,945 | $10,979 | −$34 |
| Costs | ||||||
| SLN surgery | $8,414 | $8,416 | −$2 | $16,215 | $16,317 | −$102 |
| ALNDa | $10,480 | $10,981 | −$501 | $11,965 | $10,981 | $984 |
| SLN surgery and ALNDa | $10,138 | $9,611 | $527 | $12,628 | $13,826 | −$1,198 |
| FNA with imaging guidancea | $10,931 | $10,981 | −$50 | $11,043 | $10,981 | $62 |
| Ultrasound of breasta | $10,898 | $10,981 | −$83 | $11,241 | $10,981 | $260 |
| Echo guidance for biopsya | $10,922 | $10,981 | −$59 | $11,101 | $10,981 | $120 |
| Cytopathology evaluation for FNAa | $10,930 | $10,981 | −$51 | $11,053 | $10,981 | $72 |
ALND axillary lymph node dissection, FNA fine-needle aspiration, SLN sentinel lymph node, US ultrasound
Results shown are estimated using the range of values for each parameter as indicated in Table 1
Parameters which influence economically optimal decision in one-way analysis
Table 3 provides base-case results by tumor stage and respective metastasis rates. With an assumed metastasis rate of 15% for T1 tumors, costs for the US/FNA strategy decreased to $10,584 per patient and standard of care costs decreased to $10,531, resulting in an incremental cost of $53. For patients assumed to have T3 or T4 disease, and a corresponding 75% metastasis rate, costs associated with the US/FNA strategy were estimated at $12,038 and with the standard of care strategy at $12,331, an incremental cost savings of $293. Estimated results for patients with T2 disease showed an estimated cost savings of $97 in favor of the US/FNA diagnostic approach.
TABLE 3.
Base-case results by tumor stage and respective metastasis rate
| Tumor stage |
Metastasis rate |
US/ FNA |
Standard of care |
Incremental cost |
|---|---|---|---|---|
| T1 | 15% | $10,584 | $10,531 | $53 |
| T2 | 41% | $11,214 | $11,311 | −$97 |
| T3 + T4 | 75% | $12,038 | $12,331 | −$293 |
| All stages | 30% | $10,947 | $10,983 | −$36 |
DISCUSSION
Our objective was to compare the costs of performing routine axillary ultrasound with FNA biopsy of any morphologically abnormal-appearing lymph nodes in all patients with invasive breast cancer with those incurred by alternatively performing SLN surgery without the use of preoperative axillary ultrasound (standard of care). Our results suggest that, on average, the routine use of US, with FNA when appropriate, provides a small incremental cost savings compared with the standard of care and, most importantly, did not increase the overall cost of care of patients with invasive breast cancer. The US/FNA diagnostic approach remained economically favorable even with extreme assumed parameter values and, in fact, 63% of simulations in base-case analyses found US/FNA to be a cost-saving alternative. Cost savings associated with US/FNA increased with tumor stage and associated metastasis prevalence.
Multiple studies in the literature have reported on the use of preoperative axillary ultrasound with FNA biopsy of suspicious lymph nodes. Overall, this technique can decrease SLN procedures by between 8% and 26% and possibly decrease the false-negative rate of SLN surgery while increasing the SLN identification rate.1–3,6 It is rapidly becoming used in breast centers across the nation; however, data on the cost implications are just emerging.
Recently Genta et al. evaluated the use of axillary US with FNA, sentinel node surgery, and frozen-section analysis of the sentinel nodes to assess the effectiveness and cost of this approach.5 Overall they found a cost savings to the Italian National Heath Care System, mainly related to the benefit of one-step axillary surgery in patients found to be node positive. Our study focuses on axillary US and FNA and estimates the economic impact of alternative diagnostic approaches from the perspective of a provider in the USA.
Alternative techniques of imaging the axilla such as mammogram, breast magnetic resonance imaging, and positron emission tomography (PET) scan can provide information regarding the axillary lymph nodes, although none of these techniques are 100% sensitive. Ultrasmall superparamagnetic iron oxide (USPIO)-enhanced magnetic resonance (MR) imaging has been reported to have sensitivity of 98% and 100% in very small studies.19,20 These modalities do not provide tissue for histological analysis and, therefore, without tissue diagnosis, SLN surgery is still recommended for axillary evaluation.
For patients with ductal carcinoma in situ, axillary US and FNA adds additional cost; therefore, axillary US and FNA is usually limited to diagnosed invasive breast cancer. For T1 tumors, axillary US and FNA does incur a minimal increase in cost, calculated at $53 in this study, and therefore this strategy is not cost-saving in this group. From the clinical standpoint, identifying patients with T1 tumors who are node positive is important, as these are the patients who are anticipated to be node negative and in whom detection of nodal metastases prior to surgery will have a greater impact to assist with patient expectations and adjuvant treatment planning. Studies have shown that axillary US and FNA detects a similar proportion of node-positive patients in invasive lobular carcinoma (ILC) as in invasive ductal carcinoma (IDC), and therefore this cost modeling applies to both ILC and IDC.21
Limitations
We acknowledge limitations to our analysis, including the fact that some of our data comes from a high-volume referral center and that practice patterns and associated costs may differ in other settings. Some centers, for instance, perform core-needle biopsy of the axillary lymph node, which allows more tissue to be obtained and the lymph node to be evaluated histologically. This may be more expensive than FNA, and our study focused on axillary US with FNA, as predominantly performed at our institution. Core-needle biopsy uses a larger biopsy needle and there is a theoretical concern that core-needle biopsy may have a higher complication rate and associated costs. Moreover, US-guided core-needle biopsy has higher sensitivity (77%) and specificity (94%) than FNA biopsy.22 This increased sensitivity and specificity could lead to greater cost savings if incorporated into our model. We modeled based on FNA, as this is far more commonly used than core biopsy of axillary lymph nodes, which is done at only a few centers.
Our analysis also focused on short-term direct medical costs associated with the alternative diagnostic approaches. We did not consider indirect costs associated with this clinical practice change, such as the increased time attributable to diagnostic workup. However, many breast surgeons perform their own breast ultrasound and therefore the addition of axillary US when evaluating a patient with breast cancer adds minimal time to patient workup. Similarly, in academic settings where ultrasound is often performed by breast radiologists, implementing axillary US at the time of ultrasound of a suspicious breast lesion can be easily accommodated into clinical practice. We also did not consider the additional advantages that preoperative axillary US with possible FNA provides in terms of surgical treatment planning, time allocation, and patient education and expectations, which may further support the routine use of preoperative axillary US with FNA.
CONCLUSIONS
The additional cost of performing axillary US with possible FNA in every patient is balanced, on average, by the savings from avoiding SLN. Routine use of preoperative axillary US with FNA to guide surgical planning can decrease the overall cost of patient care for invasive breast cancer.
Footnotes
Presented in part at the Society of Surgical Oncology, March 2008 and the 2008 Breast Cancer Symposium, September 2008.
REFERENCES
- 1.Mathijssen IMJ, Strijdhorst H, Kiestra SK, et al. Added value of ultrasound in screening the clinically negative axilla in breast cancer. J Surg Oncol. 2006;94:364–367. doi: 10.1002/jso.20590. [DOI] [PubMed] [Google Scholar]
- 2.van Rijk MC, Deurloo EE, Nieweg OE, et al. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol. 2006;13:31–35. doi: 10.1245/ASO.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Deurloo EE, Tanis PJ, Gilhuijs KGA, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer. 2003;39:1068–1073. doi: 10.1016/s0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 4.Krishnamurthy S, Sneige N, Bedi DG, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 5.Genta F, Zanon E, Camanni M, et al. Cost/accuracy ratio analysis in breast cancer patients undergoing ultrasound-guided fine-needle aspiration cytology, sentinel node biopsy, and frozen section of node. World J Surg. 2007;31:1155–1163. doi: 10.1007/s00268-007-9009-3. [DOI] [PubMed] [Google Scholar]
- 6.Sato K, Tamaki K, Tsuda H, et al. Utility of axillary ultrasound examination to select breast cancer patients suited for optimal sentinel node biopsy. Am J Surg. 2004;187:679–683. doi: 10.1016/j.amjsurg.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez S, Añorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. Am J Roentgenol. 2006;186:1342–1348. doi: 10.2214/AJR.05.0936. [DOI] [PubMed] [Google Scholar]
- 8.Fraile M, Rull M, Julian FJ, et al. Sentinel node biopsy as a practical alternative to axillary lymph node dissection in breast cancer patients: an approach to its validity. Ann Oncol. 2000;11:701–705. doi: 10.1023/a:1008377910967. [erratum appears in Ann Oncol. 2000 Dec;11(12):1619]. [DOI] [PubMed] [Google Scholar]
- 9.D’Eredita’ G, Giardina C, Ingravallo G, et al. Sentinel lymph node biopsy in multiple breast cancer using subareolar injection of the tracer. Breast. 2007;16:316–322. doi: 10.1016/j.breast.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez A, Cortes M, Benito E, et al. Gamma probe sentinel node localization and biopsy in breast cancer patients treated with a neoadjuvant chemotherapy scheme. Nucl Med Commun. 2001;22:361–366. doi: 10.1097/00006231-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Kang SH, Kim SK, Kwon Y, et al. Decreased identification rate of sentinel lymph node after neoadjuvant chemotherapy. World J Surg. 2004;28:1019–1024. doi: 10.1007/s00268-004-7367-7. [DOI] [PubMed] [Google Scholar]
- 12.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymphnode dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 13.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 14.Briggs A, Claxton K, Sculpher M. Decision modeling for health economic evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 15.Aujesky D, Smith KJ, Cornuz J, et al. Cost-effectiveness of low-molecular-weight heparin for treatment of pulmonary embolism. Chest. 2005;128:1601–1610. doi: 10.1378/chest.128.3.1601. [DOI] [PubMed] [Google Scholar]
- 16.Coco A. The cost-effectiveness of expanded testing for primary HIV infection. Ann Fam Med. 2005;3:391–399. doi: 10.1370/afm.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konig HH, Barry JC. Cost effectiveness of treatment for amblyopia: an analysis based on a probabilistic Markov model. Br J Ophthalmol. 2004;88:606–612. doi: 10.1136/bjo.2003.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao G, Freemantle N, Calvert MJ, et al. The long-term cost-effectiveness of cardiac resynchronization therapy with or without an implantable cardioverter-defibrillator. Eur Heart J. 2007;28:42–51. doi: 10.1093/eurheartj/ehl382. [DOI] [PubMed] [Google Scholar]
- 19.Memarsadeghi M, Riedl CC, Kaneider A, et al. Axillary lymph node metastases in patients with breast carcinomas: assessment with nonenhanced versus uspio-enhanced MR imaging. Radiology. 2006;241:367–377. doi: 10.1148/radiol.2412050693. [DOI] [PubMed] [Google Scholar]
- 20.Stadnik TW, Everaert H, Makkat S, et al. Breast imaging. Preoperative breast cancer staging: comparison of USPIO-enhanced MR imaging and 18F-fluorodeoxyglucose (FDC) positron emission tomography (PET) imaging for axillary lymph node staging–initial findings. Eur Radiol. 2006;16:2153–2160. doi: 10.1007/s00330-006-0276-4. [DOI] [PubMed] [Google Scholar]
- 21.Boughey JC, Middleton LP, Harker L, et al. Utility of ultrasound and fine-needle aspiration biopsy of the axilla in the assessment of invasive lobular carcinoma of the breast. Am J Surg. 2007;94:450–455. doi: 10.1016/j.amjsurg.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Nathanson SD, Burke M, Slater R, et al. Preoperative identification of the sentinel lymph node in breast cancer. Ann Surg Oncol. 2007;14:3102–3110. doi: 10.1245/s10434-007-9494-5. [DOI] [PubMed] [Google Scholar]