Abstract
Purpose
To quantify intraretinal cystoid spaces (ICS) and the outer nuclear layer (ONL) using optical coherence tomography (OCT) in patients with neovascular age-related macular degeneration (AMD), and to investigate the correlation of these parameters with visual acuity.
Methods
StratusOCT images from 53 patients receiving their initial treatment with intravitreal ranibizumab were collected. Images were analyzed using custom software (entitled “OCTOR”) that allows accurate manual segmentation of OCT B-scans, and provides thickness/volume measurements of ICS, ONL, neurosensory retina, pigment epithelial detachments (PEDs), subretinal fluid (SRF) and subretinal tissue (SRT). Univariate and multivariate analyses were used to correlate OCT parameters with best-corrected Snellen visual acuity. Reproducibility was assessed with weighted Kappa statistics and intraclass correlation coefficients.
Results
A multivariate linear regression model with adjusted R2 values showed that ONL volume and SRT thickness significantly correlated with Snellen visual acuity (R2=0.15, p=0.002 and R2=0.19, p=0.001, respectively) with an overall model R2 of 0.34. Adjustment of ONL volume for ICS did not improve correlation with visual acuity, and ICS volume did not independently correlate with visual acuity. Weighted Kappa statistics showed excellent intergrader agreement for both ICS and ONL measurements.
Conclusions
This study suggests that an increased total volume of the ONL is associated with decreased visual acuity in neovascular AMD, and that the total volume of ICS is not correlated with visual acuity. Although the correlations detected in this study are modest, quantitative subanalysis of OCT images may be of greater clinical relevance in the context of more advanced OCT technology.
Keywords: Age-Related Macular Degeneration, Quantitative Image Analysis, Optical Coherence Tomography
Introduction
Age-related macular degeneration (AMD) is the leading cause of severe visual loss and blindness in the developed world among people over the age of 50 years.1 Current treatment options for the neovascular form of AMD include thermal laser photocoagulation, photodynamic therapy with verteporfin, and anti-angiogenic therapies such as ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA).2–5 The efficacy of these treatments is determined primarily by assessment of visual acuity (VA), and secondarily by assessment of fluorescein angiography (FA) and optical coherence tomography (OCT) parameters.2, 4–6
StratusOCT (Carl Zeiss Meditec, Dublin, CA) includes image analysis software that provides a measure of central retinal thickness, and this parameter has been widely adopted for clinical trials.6–8 Despite its usage across the spectrum of macular disease, the complex morphology of choroidal neovascularization (CNV) exposes the limitations of the StratusOCT automated analysis – errors commonly occur in retinal boundary detection, and the software is unable to provide quantitative information regarding many important features of CNV such as subretinal fluid (SRF), subretinal tissue (SRT), and pigment epithelial detachment (PED).9 To address these issues we have developed a software tool (entitled “OCTOR”) that allows accurate manual segmentation of OCT images, as well as facilitating the quantification of any morphologic area of interest in the neurosensory retina.10–15
Using the publicly available OCTOR software, we have previously demonstrated that the presence of increased SRT thickness/volume on OCT, and to a lesser extent increased neurosensory retinal thickness/volume, is associated with decreased visual acuity in patients with neovascular AMD.13 The correlations, however, were modest and only a small proportion of the variability in vision was explained by the retinal volume. To explore potential explanations for the lack of a stronger correlation, we sought to evaluate other morphologic characteristics of the retina in CNV lesions. We hypothesized that the integrity of the outer nuclear layer (ONL) and the presence of cystoid spaces are two features that may influence visual function. Photoreceptor loss, as reflected by a loss of ONL volume could be associated with visual loss.16 Accumulation of cystoid spaces,17 however, could result in an increased ONL volume despite a loss of retinal cellular material.
In this report, we use the OCTOR software to quantify the ONL and cystoid spaces of eyes with active neovascular AMD prior to therapy, and correlate these measurements with VA.
Methods
Data Collection
A consecutive series of patients receiving their initial treatment with ranibizumab for neovascular AMD were identified from the procedure logs at the Doheny Eye Institute. Data were collected and analyzed in accordance with the policies and procedures of the institutional review board of the University of Southern California and the tenets set forth in the Declaration of Helsinki.
For inclusion in the study, patients were required to have StratusOCT imaging performed prior to receiving their initial treatment with intravitreal ranibizumab. Although dense spectral domain OCT (SDOCT) data is now becoming available for patients with neovascular AMD, StratusOCT data was utilized for this study due to the availability of manual grading tools and the impracticality of manually segmenting boundaries on the large number of B-scans in dense SDOCT volume scans. All images were obtained using the Radial Lines protocol of 6 high-resolution B-scans on a single StratusOCT machine. Data for each case were exported to an external hard drive using the export feature available in the StratusOCT version 4.0 analysis software.
Each patient’s best-corrected visual acuity was recorded at the time of initial diagnosis using Snellen visual acuity charts. The number and type of any previous treatments, for CNV secondary to AMD in the study eye, were recorded. Other data collected included patient age and gender, as well as the color photos and fluorescein angiographic images for each patient.
Computer-Assisted Grading Software
The software used for OCT analysis, OCTOR, was written by Doheny Image Reading Center software engineers to facilitate manual grading of OCTs. OCTOR is publicly available at http://www.diesel.la and has been described and validated in previous reports.11, 15 OCTOR imports data from the StratusOCT machine as a raw data file and allows the user to delineate boundaries of interest in each radial line B-scan (Figure 1). After annotation of the desired layers in each B-scan, OCTOR calculates the distance in pixels between the manually drawn boundary lines for each of the various defined spaces and converts these to micrometers to yield a thickness measurement at each location. The thickness of all unsampled locations is interpolated based on a polar approximation to yield a thickness map analogous to the StratusOCT analysis. Thickness values are converted into volumes (mm3) by multiplying the average thickness measurements by the sampled area. The interpolation algorithm, intergrader reliability, and intragrader reproducibility have been previously reported.11, 15
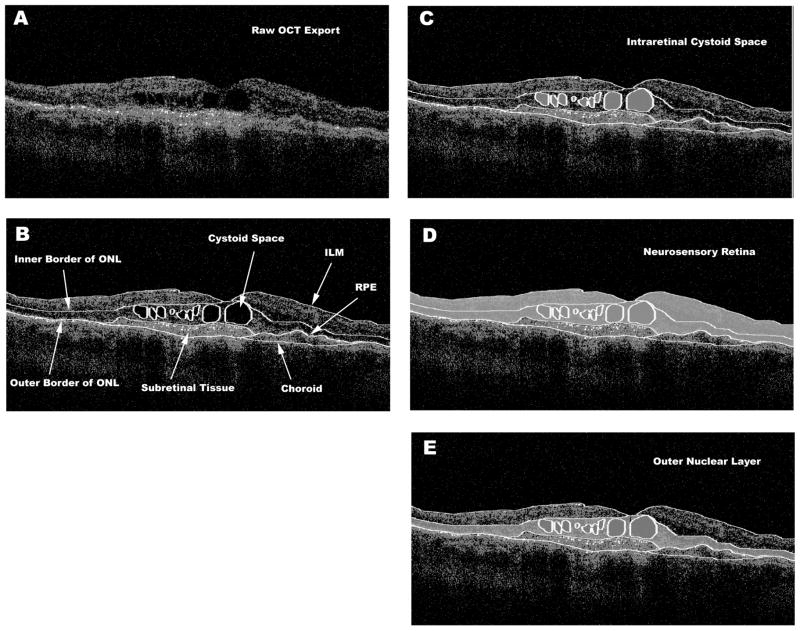
Figure 1.
Optical coherence tomography B-scan [A] demonstrating intraretinal cystoid spaces (ICS) and subretinal tissue. The clinically relevant boundaries [B] are graded using OCTOR (a computer-assisted manual grading tool) software, which then computes the volumes of the spaces defined by these boundaries. Demonstration of [C] graded ICS shaded grey, [D] total volume of the neurosensory retina shaded grey, and [E] total volume of the ONL shaded grey. ONL = Outer Nuclear Layer, ILM = Internal Limiting Membrane, RPE = Retinal Pigment Epithelium
Analogous to the StratusOCT software, OCTOR provides a report showing the calculated thickness and volume values for the 9 Early Treatment Diabetic Retinopathy Study (ETDRS) macular subfields. The means and standard deviations of the foveal center point (FCP) thickness are also calculated. In contrast to the StratusOCT output, OCTOR provides separate maps for the various macular spaces (e.g. subretinal tissue, subretinal fluid, retina, pigment epithelial detachment, intraretinal cystoid space, outer nuclear layer etc).
Grading Protocol
Boundaries drawn in each of the 6 OCT B-scans for this study included the internal limiting membrane, outer border of the photoreceptors, borders of subretinal fluid and subretinal tissue (if present), inner surface of the RPE and estimated normal position of the RPE layer (in cases of RPE elevation), inner border of the ONL and boundaries of intraretinal cystoid spaces. All boundaries were drawn in accordance with the standard OCT grading protocol of the Doheny Image Reading Center as previously described,11 except for the additional boundaries of intraretinal cystoid spaces and the ONL, which are described below. After completion of the grading, OCTOR was used to calculate output parameters for the various spaces including the entire neurosensory retina as well as the ONL alone.
The outer nuclear layer (ONL) was identified as the hyporeflective layer located just internal to the hyperreflective band believed to correspond to the inner segment – outer segment (IS-OS) junction (Figure 1E). In some cases, as a result of the destructive effects of the choroidal neovascular process, the IS-OS junction could not be reliably identified. In those cases, other more anterior layers of the retina (e.g. inner nuclear layer, outer plexiform layer) were used as a reference in order to localize the ONL. Similar to the grading protocol for other boundaries described in previous reports, the borders of the ONL were initially drawn in the periphery of the B-scans where disease pathology was usually least severe and retinal layers were most easily distinguishable.11 Additional segments of the ONL boundaries were then drawn in areas where these boundaries could be recognized with the greatest confidence. Discontinuities between segments of ONL boundaries (i.e. areas of uncertainty, usually due to pathology) were interpolated using adjacent areas in which the boundaries were clearly identifiable.
An intraretinal cystoid space (ICS) was defined as any intraretinal hyporeflective space that was greater than 5 × 5 pixels (Figure 1B, C). Smaller areas were not easily drawn or reliably recognized on successive grading of the same OCT scans, and were therefore not included. Tissue septae were often observed within larger cystoid spaces. To provide the most precise assessment of the cystoid space volume, these septae were excluded when drawing the boundaries of the cystoid space. To study the influence of the cystoid spaces, adjusted ONL and neurosensory retinal volumes were calculated by subtracting the ICS volume from the ONL and total retinal volumes, respectively. All boundary determinations were assessed by two graders and differences were adjudicated as described in previous reports.
Statistical Methods
The mean and standard deviation of the FCP thickness, as well as the total volume (subfields 1–9), were calculated for each space in each case. Volume was measured in cubic millimeters while thickness was measured in micrometers. Snellen visual acuity was converted to LogMAR visual acuity to facilitate statistical analysis. Univariate and multivariate regression was used to test for associations between LogMAR visual acuity, age, gender, and OCT parameters. SAS programming language (SAS Institute, Cary, NC) was used for all the analyses.
The reliability of ONL and ICS grading was determined using weighted Kappa (κ) statistics and intraclass correlation coefficients (ICC) in a subset of cases (the first 28 consecutive cases included in the study – i.e. more than half of the study cohort) that were graded independently by a second grader. As described in previous reports, the κ statistic is a measure of intergrader concordance on categorical scales that adjusts for chance agreement.11 κ statistics were interpreted using the ranges described earlier: 0–0.20 slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement and >0.80 almost perfect agreement.11 ICC is a separate measure of correlation between graders that takes into account the differences in individual ratings. Previous studies have suggested that these two statistics present two different types of information regarding agreement and both were used here to increase the confidence in our assessments. Bland-Altman plots were generated to demonstrate the level of agreement between graders.11
Results
Baseline Characteristics
53 consecutive patients presenting for initial ranibizumab therapy for neovascular AMD, met the inclusion criteria for the study. Demographic characteristics of the population included 19 (36%) males and 34 (64%) females. The average age of all patients was 79 ± 7 years (mean± SD) with a range of 55–92 years. Mean visual acuity at time of initial treatment was 20/100. The neovascular lesions were categorized by fluorescein angiography as either retinal angiomatous proliferation (3 eyes, 6%), predominantly classic (8 eyes, 15%), minimally classic (7 eyes, 13%), or as occult with no classic (32 eyes, 60%). Baseline characteristics of the study population are summarized in Table 1.
Table 1.
Baseline Characteristics of Study Population
| Mean Visual Acuity | 0.77 ± 0.44 (LogMAR) |
| 20/100 (Snellen) | |
| Angiographic Classification | |
| Occult with no classic CNV | 32 (60%) |
| Predominantly classic CNV | 8 (15%) |
| Minimally classic CNV | 7 (13%) |
| RAP | 3 (6%) |
| Not Available | 3 (6%) |
| Total | 53 eyes |
| Pretreatment | |
| None | 26 (49%) |
| Avastin | 9 (17%) |
| Macugen | 4 (8%) |
| Photodynamic Therapy | 5 (9%) |
| Multiple Treatments | 8 (15%) |
| Other | 1 (2%) |
| Total | 53 |
CNV = choroidal neovascularization
RAP = Retinal Angiomatous Proliferation
Multiple Treatments = Patients who received more than one treatment modality
Reproducibility Analysis
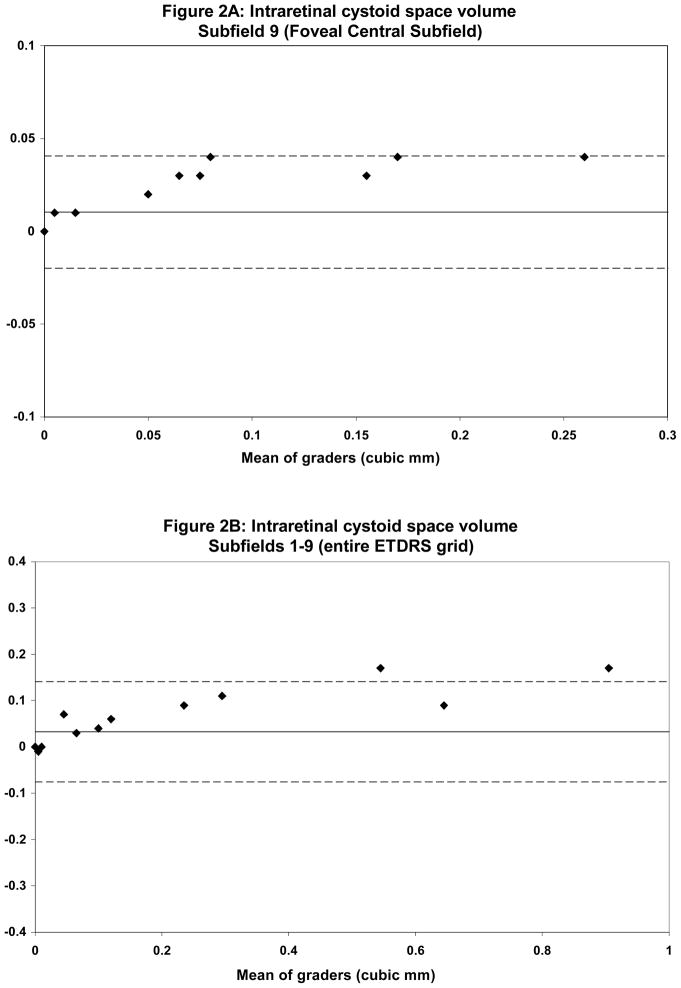
28 consecutive sets of radial line scans (168 scans total), from 28 patients, were graded and analyzed for reproducibility of ONL and ICS grading. Mean total ICS volumes ranged from 0.14 mm3 for grader 1 to 0.10 mm3 for grader 2. Qualitative comparison of discordant cases revealed that the largest differences between graders was evident in the grading of smaller cysts that were eccentrically located, irregularly shaped and had poorly demarcated borders. Despite this qualitative difference, weighted κ for the total ICS volume (entire EDTRS grid) was 0.83. Agreement on grading of cystic spaces located in the central subfield was excellent (weighted κ = 1.00).
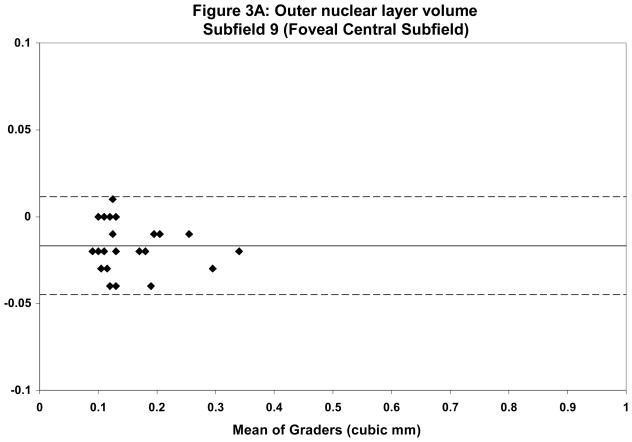
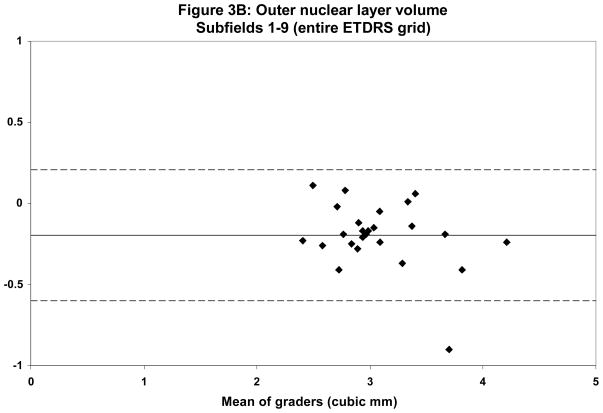
Mean total ONL volume ranged from 2.98 mm3 for grader 1 to 3.17 mm3 for grader 2. Qualitative assessment of discordant cases revealed that the largest discrepancies occurred in areas of severe disease pathology, particularly those with extensive SRT and SRF. These areas often had profound loss of both the IS-OS junction as well as the hyporeflective-hyperreflective boundary between the ONL and OPL. Overall κ statistics for ONL volume in all ETDRS areas was 0.58 with much better agreement in the central subfield (κ = 0.80). Table 2 summarizes the reproducibility analysis between the two graders. Bland-Altman plots were generated to show the level of agreement between graders (Figures 2 and 3).
Table 2.
Intergrader Comparison
| OCT Parameter | Grader 1 Mean | Grader 2 Mean | Mean absolute difference (median, maximum) | ICC | Weighted Kappa |
|---|---|---|---|---|---|
| ICS | |||||
| Volume (9) | 0.04 | 0.03 | 0.01 (0, 0.04) | 0.96 | 1.00 |
| Volume (1–9) | 0.14 | 0.10 | 0.03 (0, 0.17) | 0.97 | 0.83 |
| FCP | 72.76 | 59.56 | 13.76 (0, 79) | 0.98 | 0.96 |
| ONL | |||||
| Volume (9) | 0.14 | 0.16 | 0.02 (0.02, 0.04) | 0.94 | 0.80 |
| Volume (1–9) | 2.98 | 3.17 | 0.22 (0.19, 0.90) | 0.81 | 0.58 |
| FCP | 211.40 | 228.08 | 25.16 (26, 76) | 0.95 | 0.77 |
ICC = Intraclass correlation coefficient
FCP = Foveal center point
ONL = Outer nuclear layer
ICS = Intraretinal cystoid space
Figure 2.
Bland-Altman plots demonstrating intergrader reproducibility for intraretinal cystoid spaces (ICS) grading in the foveal central subfield (A) and entire ETDRS grid (B). Dashed lines indicate the 95% limits of agreement.
Figure 3.
Bland-Altman plots demonstrating intergrader reproducibility for outer nuclear layer (ONL) grading in foveal central subfield (A) and entire ETDRS grid (B). Dashed lines indicate the 95% limits of agreement.
Univariate Analysis
Univariate linear regression model with adjusted R2 values showed no significant correlation between ONL volume and Snellen visual acuity (R2=0.06, p=0.09). Adjustment of ONL volume by subtraction of cystoid space volume did not improve the correlation with visual acuity. Overall retinal volume was significantly correlated with visual acuity (R2=0.13, p=0.008). Adjustment of overall retinal volume by subtraction of ICS volume also did not improve the correlation with visual acuity. Total ICS volume did not independently correlate with visual acuity (R2=0.02, p=0.26). As we have shown previously, SRT volume did correlate significantly with Snellen visual acuity (R2=0.11, p=0.01) whereas PED and SRF volume did not (R2=0.004, p=0.64 and R2=0.002, p=0.73 respectively).13 Table 3 summarizes the data for the univariate analysis.
Table 3.
Univariate correlation between OCT parameters and Snellen visual acuity
| OCTOR Measured Parameter | Mean ± SD | Correlation Coefficient, r | Model R2 | P Value |
|---|---|---|---|---|
| Intraretinal Cystoid Space | ||||
| FCP (μm) | 53.85 ± 103.84 | 0.14 | 0.02 | 0.29 |
| Total Vol. (mm3) | 0.25 ± 0.84 | 0.14 | 0.02 | 0.26 |
| Outer Nuclear Layer | ||||
| FCP (μm) | 198.66 ± 98.28 | 0.08 | 0.007 | 0.54 |
| Total Vol. (mm3) | 3.12 ± 0.64 | 0.24 | 0.06 | 0.09 |
| Outer Nuclear Layer (Adjusted for ICS) | ||||
| FCP (μm) | 144.81 ± 65.66 | 0.1 | 0.01 | 0.45 |
| Total Vol. (mm3) | 2.87 ± 1.01 | 0.00 | 0.00 | 0.89 |
| Neurosensory Retina | ||||
| FCP (μm) | 276.79 ± 132.60 | 0.37 | 0.14 | 0.007 |
| Total Vol. (mm3) | 7.67 ± 1.06 | 0.37 | 0.13 | 0.008 |
| Neurosensory Retina (Adjusted for ICS) | ||||
| FCP (μm) | 222.94 ± 114.21 | 0.3 | 0.09 | 0.03 |
| Total Vol. (mm3) | 7.42 ± 1.32 | 0.2 | 0.04 | 0.17 |
| Pigment Epithelium Detachment | ||||
| FCP (μm) | 102.40 ± 130.96 | 0.2 | 0.04 | 0.16 |
| Total Vol. (mm3) | 3.19 ± 17.10 | 0.06 | 0.004 | 0.64 |
| Subretinal Tissue | ||||
| FCP (μm) | 48.98 ± 54.33 | 0.36 | 0.13 | 0.009 |
| Total Vol. (mm3) | 0.25 ± 0.48 | 0.33 | 0.11 | 0.01 |
| Subretinal Fluid | ||||
| FCP (μm) | 21.09 ± 39.66 | 0.00 | 0.00 | 0.95 |
| Total Vol. (mm3) | 0.24 ± 0.38 | 0.04 | 0.002 | 0.73 |
FCP = foveal center point
ICS = intraretinal cystoid spaces
OCT = optical coherence tomography
Multivariate Analysis
Two multivariate linear regression models were generated controlling for age and sex. In Model 1, independent variables for stepwise selection were only those variables where the univariate model had p < 0.10, excluding total retinal volume. In this model SRT thickness in the central macular subfield and total ONL volume were both significantly correlated with visual acuity (R2=0.19, p=0.001 and R2=0.15, p=0.002 respectively). The model R2 for this analysis was 0.34. In Model 2, independent variables for stepwise selection were similar to Model 1 but also included total retinal volume. In this model SRT thickness and total retinal volume were significantly correlated with visual acuity (R2=0.19, p=0.001 and R2=0.15, p=0.001 respectively). The results of the analyses are summarized in Table 4.
Table 4.
Multivariate correlation between OCT parameters and Snellen visual acuity
| OCTOR Measured Parameter | Model 1 | Model 2 |
|---|---|---|
| Intraretinal Cystoid Space | NC | NC |
| Outer Nuclear Layer | Correlated | NC |
| Volume ETDRS 1–9 | R2=0.15, p=0.002 | NC |
| Outer Nuclear Layer (Adjusted for ICS) | NC | NC |
| Neurosensory Retina | ||
| Total Vol. (mm3) | Excluded | R2=0.15, p=0.001 |
| Neurosensory Retina (Adjusted for ICS) | Excluded | NC |
| Pigment Epithelial Detachment (PED) | NC | NC |
| Subretinal Tissue | Correlated | Correlated |
| ETDRS 9 (μm) | R2=0.19, p=0.001 | R2=0.19, p=0.001 |
| Subretinal Fluid | NC | NC |
| Overall R2 | 0.34 | 0.44 |
FCP = foveal center point
ICS = intraretinal cystoid spaces
OCT = optical coherence tomography
NC = no correlation
Discussion
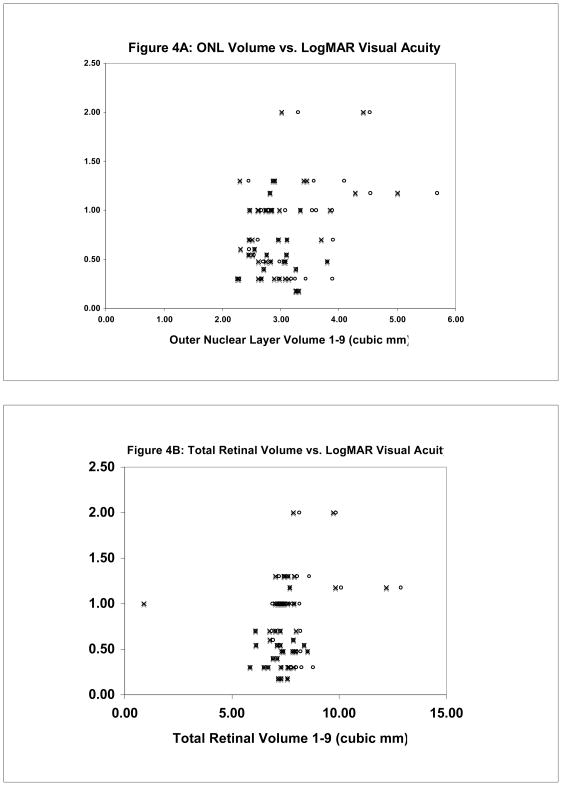
In this report, we describe the association between outer nuclear layer and cystoid space volume on best-corrected Snellen visual acuity in patients with active neovascular AMD. An increase in total neurosensory thickness/volume was found to modestly correlate with a decrease in Snellen visual acuity. Other investigators have also reported similar, modest correlations.18 We also confirm our previous findings that SRT thickness correlates modestly with a decrease in visual acuity.13 We have hypothesized that consideration of additional morphologic characteristics of CNV lesions on OCT could improve the observed correlations. The ONL, which contains the photoreceptor nuclei, constitutes only a fraction of the overall retinal thickness. Whereas photoreceptor loss is a well-established mechanism of irreversible vision loss in patients with neovascular AMD, the inner retina in these patients is generally well preserved.19 Consequently, small changes in ONL volume from photoreceptor loss may be masked if the whole neurosensory retina is considered for correlation with visual function. On the other hand, accumulation of fluid in cystoid spaces in the retina may thicken the retina without any increase in the cellular content of the retina. Similarly, cystoid spaces may mask the presence of retinal neuronal cell loss if the whole retinal or ONL volume is considered without adjusting for the cystoid spaces. In the present study, however, quantification of ONL alone and adjustment of the ONL and retinal volume by subtraction of the ICS volume did not result in an improved correlation with visual acuity (Tables 3 and 4; Figure 4).
Figure 4.
Scatter plot of Outer Nuclear Layer (ONL) versus LogMAR Snellen visual acuity (A). Circles represent uncorrected ONL measurements. Crosses represent ONL adjusted for ICS (ONL – ICS). Total retinal volume versus LogMAR Snellen visual acuity (B). Circles represent uncorrected total retinal volume (TRV) measurements. Crosses represent total retinal volume adjusted for ICS (TRV – ICS).
There are many potential reasons why a better correlation was not observed. The grading of the ONL can often be difficult in the setting of severe disease pathology that destroys landmarks including the IS-OS junction and the high contrast boundaries between retinal layers. In our grading of the ONL, we attempted to use the IS-OS junction and the outer border of the OPL to define the ONL anatomically. In most cases, one or both of these landmarks were readily identifiable in some areas, if not most, of the B-scan. This allows interpolation of ONL boundaries in sections where anatomic landmarks are not as evident. Qualitative analysis of the grading show that there was very good agreement between two independent graders in defining the ONL boundaries. The most significant disagreements between graders occurred in more eccentric scan locations and are reflected in the lower weighted κ for subfields 1–9 versus the central subfield alone (0.58 versus 0.80 respectively). ICC statistics showed a similar trend. Grading of ICS was similarly very reliable overall, but more reliable in the central subfield compared with more eccentric scan locations (weighted κ 0.83 versus 1.00 respectively). Based on subsequent qualitative comparison of discordant cases, this appears to be due to the much smaller size and poorly demarcated boundaries of more eccentric cystoid spaces. The results of these reproducibility analyses would suggest, however, that grading reproducibility is not the main factor explaining the lack of correlation between the ONL or ICS and visual acuity.11
There are a number of other potential explanations for the lack of correlation between these OCT parameters and visual acuity. First, the consideration of the ONL and cystoid spaces are only attempts to more precisely quantify the “dry” retinal volume and provide anatomic evidence of retinal neuronal preservation. In active or recent-onset neovascular AMD, however, vision loss may be due to disruption of photoreceptor function rather than photoreceptor loss. It is possible, that quantification of the ONL and cystoid spaces may show better correlation with vision in patients with eyes with chronic or long-standing CNV. In addition, it is possible that these parameters may be more predictive of visual outcome or prognosis rather than visual acuity at presentation. Second, subtraction of cystoid spaces alone still does not provide a quantification of the true dry retinal volume. For example, fluid exudation into the neurosensory retina could result in diffuse thickening without accumulation in cystoid spaces. In the present study, spaces smaller than 5 × 5 pixels in size were not considered as we determined that smaller spaces could not be graded reproducibly. Third, there are likely a number of other OCT parameters that may have shown better correlation, which were not considered. For example, eccentricity of the edema from the foveal center was not accounted for. Fourth, although distance visual acuity did not correlate, it is possible that other parameters of visual functions such as reading speed or contrast sensitivity may have shown better results.
One additional OCT parameter that may correlate with visual acuity, which was not considered in this study, is the integrity of the IS-OS junction. This parameter may be an early indicator of the health and function of the photoreceptors prior to the development of frank cellular loss.20, 21 While we were able to use the approximate location of the IS-OS junction to delimit the ONL, we found it difficult to reliably quantify the volume of the IS-OS junction itself since it was only a few pixels in width. The higher resolution and speed afforded by new SD-OCT technology may eventually facilitate identification and reliable quantification of the IS-OS boundary (as well as the photoreceptor inner and outer segments themselves) in the setting of pathology, but better automated segmentation algorithms may be required.
Finally, there are a number of potential reasons for lack of correlation, related to the limitations of the study design. For example, only best-corrected (frequently pinhole) Snellen visual acuity was used and protocol refractions were not performed. It is of course, well known that Snellen visual acuity measurements are more variable than ETDRS measured visual acuity, particularly in the visual acuity range greater than 20/100.22 Among our 28 patients, 25 had visual acuity better than 20/100 and 28 patients had vision that was equal to or worse than 20/100. In addition, the retrospective nature of the study introduces the possibility of unknown biases that may have confounded the analysis. Furthermore, the sample size is relatively small and the study may have been underpowered to detect a relationship. Unfortunately, manual tracing of all of the cysts in each B-scan is an extremely time-consuming process and limits the feasibility of conducting a much larger study. Future development of automated algorithms to segment retinal cysts may allow this limitation to be addressed. The OCT technology itself is another limitation of this study. Since the six radial line scans from the StratusOCT were used for this analysis, calculation of cyst volumes required interpolation between scan lines. Fortunately, most of the cysts were in the central macula, thus reducing the extent of interpolation. Nonetheless, interpolation can introduce significant artifacts when considering small structures such as cysts compared with larger structures such as the whole retina. Dense volume scanning with SDOCT technology may help address this problem, but are impractical for manual segmentation due to the large number of B-scans that must be assessed.
Despite these limitations, our study does appear to confirm previous findings of a weak to modest correlation between SRT and neurosensory retina volume and visual acuity. Of note, the correlation between total retinal volume or ONL volume and visual acuity in our statistical models was very similar. This may suggest that ONL volume is the main component of retinal volume that is driving the observed correlation with visual acuity. Although the correlations are weak, they are also in line with previous studies of OCT and visual acuity in other diseases. Several studies of eyes with diabetic macular edema have shown that the correlation between foveal center point and foveal central subfield retinal thickness and visual acuity ranges from 0.08 to 0.54 in diabetic patients.23–32 Our correlation between visual acuity and retinal thickness in neovascular AMD fall within the range of correlations reported for diabetic macular edema.33
In summary, consideration of outer nuclear layer and intraretinal cystoid spaces did not improve the correlation of StratusOCT morphologic parameters with Snellen distance visual acuity in this small series of patients with active neovascular AMD. Further study of these and other parameters, however, may be warranted when automated sub-analysis of spectral domain OCT volume scans becomes feasible.
Acknowledgments
Supported in part by NIH Grant EY03040 and NEI Grant R01 EY014375
Abbreviations
- FCP
foveal center point
- AMD
age-related macular degeneration
- CNV
choroidal neovascularization
- FA
fluorescein angiography
- OCT
optical coherence tomography
- RPE
retinal pigment epithelium
- SRF
subretinal fluid
- SRT
subretinal tissue
- PED
pigment epithelium detachment
- ONL
outer nuclear layer
- ICS
intraretinal cystoid space
- SDOCT
spectral domain optical coherence tomography
- ICC
intraclass correlation coefficient
- VA
Visual Acuity
- κ
Kappa
Footnotes
Drs. Walsh and Sadda are co-inventors of Doheny intellectual property related to optical coherence tomography that has been licensed by Topcon Medic al Systems. However, it is not related to the article’s subject matter.
References
- 1.Bressler NM, Bressler SB, Congdon NG, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–1624. doi: 10.1001/archopht.121.11.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 3.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–1231. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 4.Barbazetto I, Burdan A, Bressler NM, et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin: fluorescein angiographic guidelines for evaluation and treatment--TAP and VIP report No. 2. Arch Ophthalmol. 2003;121:1253–1268. doi: 10.1001/archopht.121.9.1253. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser PK, Blodi BA, Shapiro H, Acharya NR, Group MS. Angiographic and Optical Coherence Tomographic Results of the MARINA Study of Ranibizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2007;114:1868–75. doi: 10.1016/j.ophtha.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Regillo CD, Brown DM, Abraham P, et al. Randomized, Double-Masked, Sham-Controlled Trial of Ranibizumab for Neovascular Age-related Macular Degeneration: PIER Study Year 1. Am J Ophthalmol. 2008;145:239–248.e235. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Sadda SR, Wu Z, Walsh AC, et al. Errors in retinal thickness measurements obtained by optical coherence tomography. Ophthalmology. 2006;113:285–293. doi: 10.1016/j.ophtha.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Joeres S, Kaplowitz K, Brubaker JW, et al. Quantitative Comparison of Optical Coherence Tomography after Pegaptanib or Bevacizumab in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2008;115:347–354. doi: 10.1016/j.ophtha.2007.03.082. [DOI] [PubMed] [Google Scholar]
- 11.Joeres S, Tsong JW, Updike PG, et al. Reproducibility of quantitative optical coherence tomography subanalysis in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:4300–4307. doi: 10.1167/iovs.07-0179. [DOI] [PubMed] [Google Scholar]
- 12.Keane PA, Liakopoulos S, Chang KT, et al. Comparison of the optical coherence tomographic features of choroid neovascular membranes in pathological myopia versus age-related macular degeneration, using quantitative subanalysis. Br J Ophthalmol. 2008;92:1081–5. doi: 10.1136/bjo.2008.138891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keane PA, Liakopoulos S, Chang KT, et al. Relationship between Optical Coherence Tomography Retinal Parameters and Visual Acuity in Neovascular Age-Related Macular Degeneration. Ophthalmology. 2008;115:2206–14. doi: 10.1016/j.ophtha.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keane PA, Liakopoulos S, Ongchin SC, et al. Quantitative Subanalysis of Optical Coherence Tomography after treatment with Ranibizumab for Neovascular Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2008;49:3115–20. doi: 10.1167/iovs.08-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadda SR, Joeres S, Wu Z, et al. Error correction and quantitative subanalysis of optical coherence tomography data using computer-assisted grading. Invest Ophthalmol Vis Sci. 2007;48:839–848. doi: 10.1167/iovs.06-0554. [DOI] [PubMed] [Google Scholar]
- 16.Kim SY, Sadda SR, Pearlman J, et al. Morphometric analysis of the macula in eyes with disciform age-related macular degeneration. Retina. 2002;22:471–477. doi: 10.1097/00006982-200208000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Ting TD, Oh M, Cox TA, Meyer CH, Toth CA. Decreased visual acuity associated with cystoid macular edema in neovascular age-related macular degeneration. Arch Ophthalmol. 2002;120:731–737. doi: 10.1001/archopht.120.6.731. [DOI] [PubMed] [Google Scholar]
- 18.Moutray T, Alarbi M, Mahon G, Stevenson M, Chakravarthy U. Relationships between clinical measures of visual function, fluorescein angiographic and optical coherence tomography features in patients with subfoveal choroidal neovascularisation. Br J Ophthalmol. 2008;92:361–364. doi: 10.1136/bjo.2007.123976. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Sadda SR, Humayun MS, de Juan E, Melia BM, Green WR. Morphometric analysis of the macula in eyes with geographic atrophy due to age-related macular degeneration. Retina. 2002;22:464–470. doi: 10.1097/00006982-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Ota M, Tsujikawa A, Murakami T, Kita M, et al. Association between integrity of foveal photoreceptor layer and visual acuity in branch retinal vein occlusion. Br J Ophthalmol. 2007;91:1644–9. doi: 10.1136/bjo.2007.118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ota M, Tsujikawa A, Murakami T, et al. Foveal photoreceptor layer in eyes with persistent cystoid macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2008;145:273–80. doi: 10.1016/j.ajo.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Falkenstein IA, Cochran DE, Azen SP, et al. Comparison of Visual Acuity in Macular Degeneration Patients Measured with Snellen and Early Treatment Diabetic Retinopathy Study Charts. Ophthalmology. 2008;115:319–23. doi: 10.1016/j.ophtha.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandello F, Polito A, Del Borrello M, Zemella N, Isola M. “Light” versus “classic” laser treatment for clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:864–870. doi: 10.1136/bjo.2004.051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catier A, Tadayoni R, Paques M, et al. Characterization of macular edema from various etiologies by optical coherence tomography. Am J Ophthalmol. 2005;140:200–206. doi: 10.1016/j.ajo.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 25.Goebel W, Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy: a study using optical coherence tomography (OCT) Retina. 2002;22:759–767. doi: 10.1097/00006982-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995;113:1019–1029. doi: 10.1001/archopht.1995.01100080071031. [DOI] [PubMed] [Google Scholar]
- 27.Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–1179. doi: 10.1136/bjo.2003.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 29.Massin P, Audren F, Haouchine B, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–224. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 30.Massin P, Duguid G, Erginay A, Haouchine B, Gaudric A. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169–177. doi: 10.1016/s0002-9394(02)01837-8. [DOI] [PubMed] [Google Scholar]
- 31.Otani T, Kishi S. Tomographic findings of foveal hard exudates in diabetic macular edema. Am J Ophthalmol. 2001;131:50–54. doi: 10.1016/s0002-9394(00)00661-9. [DOI] [PubMed] [Google Scholar]
- 32.Ozdemir H, Karacorlu M, Karacorlu SA. Regression of serous macular detachment after intravitreal triamcinolone acetonide in patients with diabetic macular edema. Am J Ophthalmol. 2005;140:251–255. doi: 10.1016/j.ajo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Browning DJ, Glassman AR, Aiello LP, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]