Abstract
Background and Aims
Phoenix dactylifera (date palm) is a dioecious species displaying strong dimorphism between pistillate and staminate flowers. The mechanisms involved in the development of unisexual flowers are as yet unknown.
Methods
This paper describes the results of inflorescence and flower development studies using different histological and molecular cytological approaches. Nuclear integrity and cell division patterns in reproductive organs were investigated through DAPI staining and in situ hybridization using a histone H4 gene probe.
Key Results
The earliest sex-related difference in flower buds is observed at an otherwise ‘bisexual’ stage, at which the number of cells in the gynoecium of pistillate flowers is higher than in their staminate counterparts. In the pistillate flower, staminodes (sterile stamens) display precocious arrest of development followed by cell differentiation. In the staminate flower, pistillodes (sterile gynoecium) undergo some degree of differentiation and their development ceases shortly after the ovule has been initiated. Staminode and pistillode cells exhibit nuclear integrity although they did not show any accumulation of histone H4 gene transcripts.
Conclusions
These results strongly suggest that the developmental arrest of sterile sex organs and the subsequent unisexuality of date palm flowers result from a cessation of cell division and precocious cell differentiation rather than from cell death.
Keywords: Date palm, reproductive development, cell division patterns, sex determination
INTRODUCTION
Sex determination is a fundamental question that needs to be addressed in order to understand how plants achieve allogamy (Tanurdzic and Banks, 2004). It is the process that leads to the development of unisexual flowers by the selective abortion of male or female reproductive organs, a condition known as dicliny in angiosperms. Pistillate and staminate flowers may be produced on separate plants (in dioecious species such papaya and date palm) or on the same plant (in monoecious species such maize and oil palm). During the last few decades, the genetics of sex differentiation and determination have been investigated in a number of diclinous species. In papaya, sex determination was found to be localized in a small, specific and non-recombining region (MSY) of the homomorphic Y chromosome (Ming et al., 2007). In Silene latifolia, two independent regions (female suppressor and male activator) located in the heteromorphic Y chromosome control the sex of the plant (Farbos et al., 1999). A recent study showed that sex determination in melon is governed by the interaction between CmACS-7, a gene encoding an ethylene biosynthesis enzyme that leads to the development of female flowers by stamen repression, and CmWIP1, a gene encoding a zinc finger transcription factor that is required for carpel abortion in the male flower (Boualem et al., 2008; Martin et al., 2009). Marguerit et al. (2009) showed that the sex of grapevine plants is under the control of a single major genomic region. Interestingly, these authors demonstrated that the sex locus co-segregated with various inflorescence development characters and also with the gene corresponding to CmACS-7 previously identified in melon.
Regarding the developmental process of sex differentiation in diclinous species, unisexual flowers result in general from either the absence of androecium/gynoecium or the non-functionality of male or female organs due to developmental arrest of the stamen or carpel primordia at a particular stage. Some species, such as spinach and Nypa fruticans palm, produce unisexual flowers devoid of any vestiges of either male or female organs. Flower development bypasses the hermaphrodite flower bud stage and either the carpel or the stamen primordium is never initiated (Uhl, 1972; Sherry et al., 1993). In most diclinous species, male and female flower buds are initially bisexual in appearance, developing both carpel and stamen primordia. Flowers can become unisexual at an early stage by abortion of sterile sex organs as seen in the premature developmental arrest of pistillodes (sterile gynoecium) in staminate flowers of maize and S. latifolia (Cheng et al., 1983; Farbos et al., 1997). Sexual dimorphism can also be expressed very late during flower development. For example, in Vitis vinifera, the carpel of the staminate flowers becomes sterile as a result of abortion of the embryo sac in a fully developed ovule. Similarly, the pistillate flowers of Raphis subtilis become functionally unisexual after pollen fails to form in the anthers (Caporali et al., 2003; Dransfield et al., 2008).
The molecular mechanisms leading to the developmental arrest of sterile sex organs are as yet unknown for the majority of diclinous species. A cell death process induces pistil abortion in staminate flowers of maize and male sterility in pistillate flowers of Actinidia deliciosa (Calderon-Urrea and Dellaporta, 1999; Coimbra et al., 2004). In cucumber, the developmental arrest of sterile stamens in pistillate flowers is closely associated with DNA damage (Hao et al., 2003). Unisexuality in S. latifolia occurs through specific cell division patterns in whorls 3 and 4 (Matsunaga et al., 2004). These examples show that for each diclinous species, different processes are controlling the transition from a bisexual to a unisexual state.
Palms (Arecaceae) are a particularly interesting family for the study of dicliny, as they display great diversity in their reproductive morphology and more than 85 % of palm genera comprise species producing single sex flowers (Dransfield et al., 2008). On the basis of its phylogenetic distribution, dioecy appears to have evolved separately several times within the palm family (Weiblen et al., 2000). The present study focuses on date palm, within the genus Phoenix which contains only dioecious species. Few data are available on sex determination in date palm. Currently, there is no way to distinguish between male and female plants prior to the first flowering, which occurs 5–8 years after planting. Date palm progenies consist of male and female individuals in equal proportion, which has led to the hypothesis that sex is determined genetically. Based on cytological studies with chromomycin staining, Siljak-Yakovlev et al. (1996) proposed the existence of sex chromosomes in the date palm. However, no gene associated with sex determination has been reported to date, nor has the process of developmental arrest in sterile sex organs been studied in detail.
In order to understand the molecular and physiological basis of sex differentiation, a useful prerequisite is to study the developmental process at the histo-cytological level. De Mason et al. (1982) provided the first description of floral development in date palm. The author identified a ‘bisexual stage’ in early floral development when all organ primordia had been initiated. More recently, developmental stages were described during the development of female flowers (Masmoudi et al., 2008).
The present study provides a detailed analysis of inflorescence and flower development in date palm. In particular, it compares the development of the fertile gynoecium and androecium with that of the sterile gynoecium (pistillodes) and androecium (staminodes), respectively. The study focuses on when and how the first morphological differences appear between male and female reproductive organs. Nuclear integrity and cell division patterns in floral buds were investigated in order to better understand into the mechanisms underlying the arrest of sterile sex organ development. The results revealed an absence of nuclear degradation and cell cycle activity in the residual sex organs. These data support the hypothesis that the developmental arrest observed in sterile sex organs and the subsequent unisexuality of date palm flowers result from cell cycle arrest rather than from cell death.
MATERIALS AND METHODS
Plant material
Flower buds used for this study were sampled from date palms (Phoenix dactylifera L.) growing in Ambouli (Djibouti) and Bordighera (Italy) during the 2008 and 2009 flowering seasons. All the sampled seed-grown palms were from different genotypes. They were selected for their size (higher than 4 m), age (at least 20 years) and well-established reproductive phase. Palms were cut down and dissected as described by Adam et al. (2005), the axillary buds being removed gradually from the base to apex. At early developmental stages, prophylls were removed and the inflorescences were fixed for further histological studies.
Histological analysis
All samples (undetermined buds, inflorescence buds and young inflorescences) were fixed in 4 % PFA (paraformaldehyde) – 2× PBS (phosphate-buffered saline) solutions under vacuum for 1 h, and then incubated for 16 h at 4 °C. Samples were then embedded in serial solutions of 1× PBS and then dehydrated through a graded ethanol series (50, 70, 80, 90 and 100 %, v/v) for 2 h and stored at 4 °C. They were lastly transferred into absolute butanol for 1 month and then embedded in resin (Technovit resin, Heraeus Kulzer, Wehrheim, Germany). Histological sections (3·5 µm) were made using a rotary microtome (Microm HM 355 S). Sections were double stained with periodic acid-Schiff's reagent (Sigma-Aldrich, Lyon, France) and naphthol blue-black (NBB, Sigma-Aldrich). Slides were then observed with a Leitz DMRB microscope equipped with a Leica DFC300FX camera.
Measurement of the size of whorl 4
Measurement of the width and height of whorl 4 was made using floral median longitudinal sections at two developmental stages (IV, n = 9 and V, n = 10). Width was considered as the maximum width of the gynoecium as shown in Fig. 4(A, B), and height was determined as the distance between the base and upper limit of the primordium. The total number of cells and cell sizes in the primordium were estimated from histological sections of both male (n = 5) and female (n = 5) flowers. Measurements were carried out using ImageJ software (http://rsbweb.nih.gov/ij/). Data were analysed statistically by ANOVA using the SAS software package (SAS Institute, Inc., Cary, NC, USA).
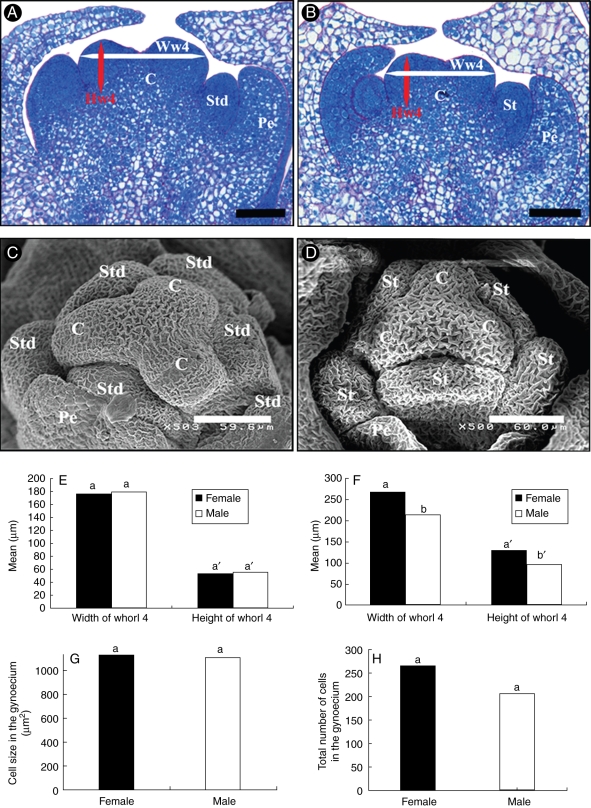
Fig. 4.
Biometric analysis of flower bud immediately after the initiation of organ primordia (stage V). (A) Longitudinal section of ‘bisexual’ pistillate flower bud at stage V. (B) Longitudinal section of ‘bisexual’ staminate flower bud at stage V. (C) SEM observation of pistillate flower bud at ‘bisexual’ stage showing the emergence of three carpel primordia in a triangular shape in the inner whorl and six stamens at their periphery. (D) SEM observation of staminate flower bud at ‘bisexual’ stage V. (E) Histogram of mean widths and heights of gynoecium in staminate and pistillate flowers at stage IV; no significant difference is observed in width (P = 0·45, n = 9) or height (P = 0·54, n = 9). (F) At ‘bisexual’ stage V, carpel widths (P = 0·0016, n = 10) and heights (P = 0·0005, n = 10) are significantly different, the gynoecium of pistillate flowers being on average larger than that of the staminate flower. (G,H) Histogram of mean cell sizes (G) and total number of cells (H) as measured from median sections in both staminate and pistillate primordia. The results revealed no significant difference in cell size (P = 0·59, n = 5) while the two gynoecium primordium types displayed a significant difference in total cell number (P = 0·0001, n = 5) Abbreviations: C, carpel; Pe, petal; St, stamen; Hw4, height of whorl 4; Ww4, width of whorl 4. Means with the same letter are not significantly different. Scale bars: (A, B) = 100 µm.
Scanning electron microscopy
Flower buds at stages IV, V and VI were fixed and dehydrated as for histological analysis. Samples were dehydrated by hexamethyldisilazine for 2 min. Subsequently, they were sputter coated with an approx. 10-nm-thick gold film and then examined under a scanning electron microscope (Hitachi S4000, CRIC, Montpellier, France) using a lens detector with an acceleration voltage of 10 kV at calibrated magnifications.
In situ hybridization and DAPI staining
Fixation of inflorescences and in situ hybridization were performed as described by Adam et al. (2007). The specific accumulation of histone H4 transcripts was used as a marker for cells undergoing G1-S phase of the cell cycle (Krizek, 1999; Gaudin et al., 2000).
PCR amplifications were performed with gene-specific antisense primers tailed with a T7 RNA polymerase binding site. PCRs were undertaken using primers EgH4S1 (AAAAGAAGGGAGAGAGCTCG) and EgH4AS1-T7 (GCGAAATTAATACGACTCACTATAGGGCGAAAGACCGAGCCCGAAAACGC), which were designed from oil palm gene sequences, in order to produce a specific RNA probe corresponding to H4 transcripts (HISTONE 4, accession no. DQ400915). The resulting DNA fragments were used directly as templates for synthesizing antisense riboprobes incorporating UTP-digoxigenin (Roche, Paris, France) in conjunction with a T7 Maxi Script kit (Ambion, Montrouge, France).
After staining to visualize H4 transcript accumulation, sections were incubated in a 0·3 mm solution of 4′,6-diamidino-2phenylindole dihydrochloride (DAPI; Boehringer Mannheim, Germany) for 5 min at room temperature. They were then washed in PBS solution and mounted in mowiol. Photographs were taken via a Zeiss AX10 microscope equipped with a Leica DFC300FX camera and digital images were processed using Adobe Photoshop software.
RESULTS
Initiation and development of inflorescences
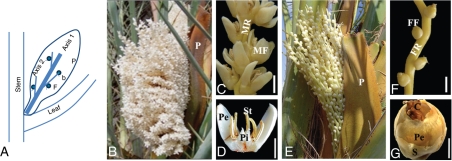
In Djibouti, date palm flowering occurs from December to April, peaking between the end of January and early February. During this period, date palms produce inflorescences in the axils of their subtending leaves. Male and female inflorescences show the same overall structure and axis organization. The date palm inflorescence consists of a rachis (axis 1) bearing numerous rachillae (axis 2) upon which the flowers occur (Fig. 1A). A single prophyll encloses the whole inflorescence. At anthesis, inflorescences show differences in shape and size between the two sexes. Male inflorescences (Fig. 1B) have shorter peduncles. The male rachis carries many dense and short rachillae, each bearing about 50 solitary flowers (Fig. 1C, D). Female inflorescences, by contrast, have large and elongated peduncles (Fig. 1E). The female rachis has fewer branches and each female rachilla bears about 40 solitary flowers which are less densely assembled than in the male rachilla (Fig. 1F, G).
Fig. 1.
Reproductive development in date palm. (A) Structure of inflorescence. Axis 1 and axis 2 correspond to the rachis and rachilla respectively. (B) Male inflorescence at anthesis. (C) Mature flowers densely distributed on axis of staminate rachilla. (D) Longitudinal section of mature staminate flower. (E) Female inflorescence. (F) Pistillate rachilla. (G) Mature pistillate flower. Abbreviations: b, bract; C, carpel; F, flower; FF, female flower; FM, male flower; FR, female rachilla; MR, male rachilla; P, prophyll; Pe, petal; Pi, pistillode; S, sepals; St, stamens. Scale bars: (C, F) = 500 µm, (D, G) = 250 µm.
Date palm inflorescences are initiated from axillary buds. Three types of axillary buds were observed in adult date palms. Undetermined buds (Fig. 2A) were found at the base of immature leaves, which were not yet photosynthetic and which were located within the palm heart. The development of undetermined buds into inflorescence buds was observed at later stages, at which the leaves were photosynthetically active and spiny. Inflorescence buds show an elongated, compact structure and a trapezoidal shape (Fig. 2B). The apices of pre-inflorescence buds consist of a meristematic zone and a basal region containing parenchyma cells with a large number of starch grains and numerous vascular bundles (Fig. 2C). No obvious morphological differences could be observed between male and female inflorescence buds.
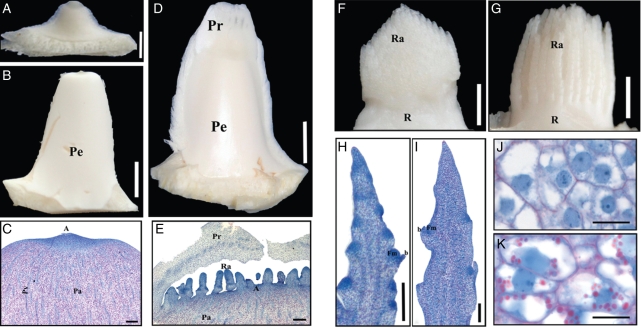
Fig. 2.
Initiation and development of date palm inflorescences. (A) Undetermined axillary bud subtended by young leaves in the crown. (B) Inflorescence bud subtended by adult leaves before flowering. (C) Longitudinal section of inflorescence bud. (D) Young inflorescence bud shortly after rachilla and prophyll initiation. (E) Longitudinal section of young inflorescence bud. (F–K) Structure of young inflorescence after removal of prophyll rachilla. (F) Male inflorescence (around 10 mm in length). (G) Female inflorescence (around 10 mm in length). (H) Longitudinal section of the male rachilla. (I) Longitudinal section of the female rachilla. (J,K) Detail of cellular structures showing no starch accumulation in male rachilla (J) but high starch accumulation in female rachilla (K, starch bodies in pink). Abbreviations: A, apex; b, bract; Fm, floral meristem; Pa, parenchyma; Pe, peduncle of inflorescence; Pr, prophyll; Pv, provascular strand; R, rachis; Ra, rachillae. Scale bars: (A) = 250 µm, (C, E, H, I) = 300 µm, (B, D, F, G) = 5 mm, (J, K) = 20 µm.
Inflorescence buds develop into young inflorescences (Fig. 2D) under favourable flowering conditions. The basal part of the inflorescence shows the same basic organization as the inflorescence bud (Fig. 2C). The rachillae and prophyll developed simultaneously from the dome of the young inflorescence (Fig. 2E). No bracts were observed at the base of the developing rachillae. The prophyll was composed of vacuolated parenchyma and provascular strands (Fig. 2E). The intense NBB staining of the apex indicated that the young inflorescence was in an active growth phase at this point.
At the time of flower primordium initiation, male and female inflorescences show a similar structural organization (Fig. 2F, G). However, several morphological and cytological differences were observed between the two sexes at this early stage. First, during early development (before floral organogenesis), young male and female inflorescences are similar in size (around 10 mm) although they show distinguishable shapes. Indeed, the male inflorescence (Fig. 2F) is conical whereas the female inflorescence (Fig. 2G) has a more rounded shape. Secondly, longitudinal sections of young rachillae reveal differences in the density of floral meristems which develop laterally on them in male and female inflorescences. At the time of their initiation, floral meristems are generally more densely distributed on the axis of the male rachilla (Fig. 2H) than on the female rachilla (Fig. 2I). A third difference observed between male and female inflorescences prior to floral organogenesis was their pattern of starch accumulation. Starch grains were more abundant in the parenchyma cells of the female rachilla (Fig. 2K) compared with those of the male rachilla (Fig. 2J). During the development and growth of rachillae, starch accumulated progressively. No starch was detected in floral tissues during the early stages of floral organogenesis; however, starch became visible in sepals and petals during sexual differentiation.
In conclusion, the young male and female inflorescences of date palm were found to display morphological differences during the earliest stages of development, thus indicating that sexual dimorphism exists before the differentiation of flowers in this species.
Floral morphogenesis
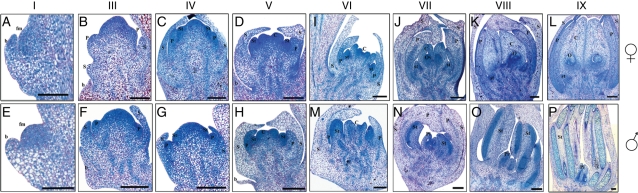
In order to identify key developmental stages and pinpoint structural differences between pistillate and staminate flowers, histological sections of flower buds throughout morphogenesis were analysed. This allowed us to define nine specific stages, starting from the floral meristem and culminating in the appearance of mature unisexual flowers (Fig. 3, Table 1).
Fig. 3.
Stages of flower development in date palm. Histological sections of female (A–D, I–L) and male (E–H, N–P) flowers. Stage I (A, E), Stage III (B, F), Stage IV (C, G), Stage V (D, H), Stage VI (I, M), Stage VII (J, N), Stage VIII (K, O), Stage IX (L, P). For description of each stage and the corresponding inflorescence size, see Table 1. Abbreviations: b, bract; C, carpel; fm, floral meristem; O, ovule; Ov, ovular meristem; P, petal; Pi, pistillode; St, stamen; st, staminode; S, sepal. Scale bars: (A, E) = 100 µm, (B–D, F–P) = 200 µm.
Table 1.
Morphological events in date palm flower development
| Stage | Sex | Morphological events | Overall length of rachillae (cm) | Figures |
|---|---|---|---|---|
| 0 | F-M | Initiation of inflorescence meristem; initiation of rachis and rachilla | 0·2–0·5 | 2C, E |
| 1 | F-M | Floral meristem initiation | 0·5–1 | 3A, E |
| 2 | F-M | Emergence of sepal primordia | 1·5–3 | |
| 3 | F-M | Emergence of petal primordia | 2·5–3·5 | 3B, F |
| 4 | F-M | Initiation of stamen primordia | 3–4·5 | 3C, G |
| 5 | F-M | Initiation of carpel primordia | 3·5–4·5 | 3D, H |
| Early | Comparable size of carpel primordia in both sexes (bisexual stage) | 4C, D | ||
| Late | Enlargement in carpel primordia of pistillate flower (first morphological differences) | |||
| 6 | M | Stamen and pistillode primordia elongated | 5·5–7 | 3M, 5A |
| F | Carpel primordium enlargement and slight differentiation to carpel. Staminode primordia with active cells | 3I, 6A | ||
| 7 | M | Stamen differentiates to produce anther and filament | 7–13 | 3N |
| F | Carpel elongated. Initiation of ovular meristem. No size increase in staminodes: arrest of development | 3J | ||
| 8 | M | Locules differentiated in stamen. Meiosis in anthers. Pistillodes: carpel is slightly elongated. Ovular meristem is initiated | 13–20 | 3O |
| F | Initiation of nucellus in young carpel. At end, carpel further expands and differentiates into style in upper part, internal integument and sporangial tissues are initiated in ovule. Staminodes show cellular differentiation | 3K | ||
| 9 | M | Stamens increase in size; binuclear pollen appears in pollen sac. Pistillodes: no change in size but ovular meristem gives rise to nucellus. No further changes in pistillodes: arrest of development | 23–30 | 3P |
| F | Initiation of stigma. External integument is formed, pre-meiotic macrospore is visible. Staminodes: cells continue to vacuolate and some of them differentiate into vascular bundles. | 3L |
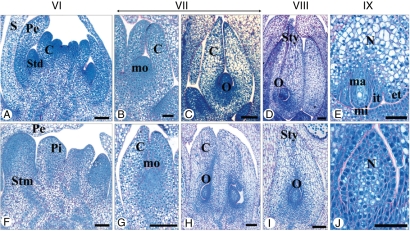
Both male and female floral meristems were initiated from a lateral meristematic zone of the rachilla next to a subtending bract. The emergence of the floral meristem was designated as stage I (Fig. 3A, E, Table 1). The floral meristem consisted of meristematic cells with intense NBB coloration. The floral meristem was seen to develop via periclinal and anticlinal divisions, the initiation of floral organ primordia occurring in an acropetal order to produce successive and opposite trimerous whorls. The appearance of the primordia of sepals, petals, stamens and carpels was used to define stages II to V, respectively (Fig. 3B–D, F–H, Table 1). The developing staminate and pistillate flowers displayed very similar morphologies until stage V. At this stage, the flower bud is ‘bisexual’ in appearance, immediately after the emergence of all floral organ primordia (Figs 3D, H and 4A–D, Table 1). The differentiation of sex organs occurred during developmental stages VI and VII, leading to the development of pistillate and staminate flowers displaying highly divergent morphologies (Fig. 3I, J, M, N, Table 1). Thus, the fertile sex organs (carpels and stamens of pistillate and staminate flowers, respectively) increased in size and became more differentiated than the sterile sex organs (staminodes and pistillodes of pistillate and staminate flowers, respectively). Therefore, the sex of the flower can be clearly identified from stage VI onwards. At stages VIII and IX, flower differentiation was fully achieved and fertile sex organs were observed whereas the sterile sex organs displayed incomplete development (Fig. 3K, L, O, P, Table 1).
Morphological changes associated with sexual dimorphism in date palm flowers
In order to identify the initial developmental differences between the two sexes, morphological studies were performed by measuring the size of whorl 4 in flower buds at stages IV and V for both sexes (Fig. 4A–D). At stage IV, the widths and heights of carpel primordia were comparable in pistillate and staminate flower buds (Fig. 4E). In contrast, at ‘bisexual stage’ V, the carpel heights and widths were significantly different (Fig. 4F), the developing gynoecium of the pistillate flower being larger than that of the staminate flower. Mean cell sizes in the developing gynoecium of staminate and pistillate flowers did not differ significantly (Fig. 4G). However, in sections of the two different types of developing gynoecium, a difference in total number of cells was observed (Fig. 4H). Thus, the larger size of carpel primordia in pistillate flowers was associated with a higher cell number, this being the earliest sex-specific morphological difference observed in date palm flowers.
Development of the androecium in staminate and pistillate flowers
A precise comparison of androecium development in the staminate flower (fertile stamens) and in the pistillate flower (sterile staminodes) was carried out (Fig. 5). At stage VI, the stamen primordia of male flowers were elongate through numerous anticlinal and periclinal divisions (Fig. 5A). Stamen cells were characterized by a large nucleus and intense NBB coloration of the cytoplasm (Fig. 5A). These active cells were associated with the early development of stamens. At stage VII, fertile stamens enlarged and became constricted at the base, the first sign of their differentiation into anther and filament (Fig. 5B). Differentiation within the anthers was first observed as an onset of zonal polarization. At this point, the abaxial side of anthers consisted of cells densely stained with NBB, destined to form sporogenic cells and ultimately pollen sacs. In contrast, the adaxial side contained vacuolated parenchyma cells. Scanning electron microscopy (SEM) observations showed that stamen primordia became bilobed at this point (data not shown). At stage VIII, the anthers continued their differentiation. Meiosis occurred within the microspore mother cells (Fig. 5C) and tetrad cells were observed at the end of stage VIII. At stage IX, stamens showed an increase in size (Fig. 5D) and tetrads developed into microspores, which will generate mature pollen grains. Pollen sacs were surrounded by a differentiated tapetum and contained binuclear pollen.
Fig. 5.
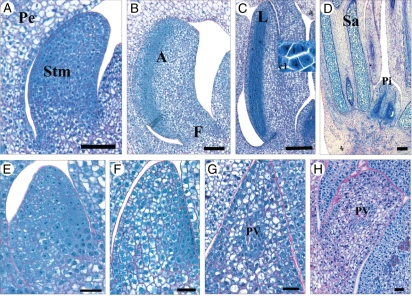
Development of the androecium in staminate and pistillate flowers. (A) Early stamen primordium at stage VI. (B) Early differentiation of the stamen into anther and filament at stage VII. (C) Bilobed stamens at stage VIII. First meiotic divisions appear in locules of anthers. (D) Stamens at stage IX with more developed pollen sac. (E) Slight growth of staminode primordium at stage VI. In contrast to stamens at the same stage (A), no significant elongation of staminodes was observed. (F) At stage VII, staminode cells became vacuolated and first signs of cell differentiation were observed. (G,H) Stages VIII and IX, respectively; cellular vacuolation increased strongly and some cells differentiated into provascular strands. Abbreviations: A, anther; F, filament; L, locule of anther; Pe, petal; Pi, pistillode; PV, provascular strands; Sa, pollen sac. Scale bars: (E–H) = 30 µm, (A,B) = 90 µm, (C,D) = 200 µm.
In contrast to fertile stamens, staminodes in the pistillate flower displayed an arrested development. The developmental changes underlying this process which occurred between stages VI and IX were investigated in detail. At stage VI, staminode primordia were slightly elongated (Fig. 5E). Staminodes were less elongated than developing stamens at the same stage (Fig. 5A). Staminode cells were densely stained with NBB and displayed a large nucleus and dense cytoplasm and they were undifferentiated. At stage VII, no further elongation or enlargement of staminode primordia was observed, indicating that development was blocked (Fig. 5F). From stage VII and through the following stages VIII and IX, the staminode primordia showed cellular differentiation involving an increase in cell vacuolation and a decrease in cytoplasm density (Fig. 5F–H). At stages VIII and IX, the staminodes contained cells with large vacuoles and a small laterally positioned nucleus while vascular bundles were centrally differentiated (Fig. 5G, H). In conclusion, the precocious developmental arrest of staminodes followed by cell differentiation appears to be a key feature for the formation of unisexual female flowers in date palm.
Development of the gynoecium in pistillate and staminate flowers
The development of the gynoecium in both pistillate and staminate flowers was compared (Fig. 6) with the aim of pinpointing histo-cytological changes occurring in pistillodes during the establishment of sexual dimorphism.
Fig. 6.
Development of the gynoecium in pistillate and staminate flowers. (A–E) Sexual differentiation and maturation of the fertile gynoecium in pistillate flower. (A) Carpel primordium initiation at stage VI. (B) Elongation of carpel primordium at stage VII. (C) Ovule initiation revealing the presence of proximal/distal symmetry at stage VII. (D) At stage VIII, the carpel primordium is elongated, the external integuments are formed and the ovule occupies an anatropous position. (E) Detail of ovule at stage IX. The internal integuments are formed and the macrospore remains in a premeiotic state. (F–J) Development of sterile gynoecium (pistillode) in staminate flower. (F) At stage VI, the pistillode primordium in the staminate flower shows weak growth. (G) At stage VII, the pistillode primordium has elongated and ovular meristem is initiated. (H) At stage VIII, the nucellus is initiated in the ovule of the pistillode. The carpel shows a proximal/distal symmetry. (I,J) At stage IX, pistillodes display a developed nucellus (I). (J) Detail of ovule in pistillodes. Abbreviations: C, carpel; et, external integument; it, internal integument; mi, micropyle; mo, ovular meristem; ma, macrospore; N, nucellus; O, ovule; pe, petal; Pi, pistillode; S, sepal; Std, staminodes; Stm, stamens; sty, style. Scale bars: (B, G, E, J) = 50 µm, (A, C, D, F, H, I) = 100 µm.
At stage VI, the carpel primordia of the pistillate flower (Fig. 6A) were slightly more enlarged and elongated than at stage V (Fig. 3D). The differentiation of the carpel was initially characterized by the appearance of a small depression in the centre of the carpel primordium (Fig. 6A). At stage VII, the young carpel was elongated and the ovular meristem had initiated (Fig. 6B). The ovular meristem contained cells that were densely stained by NBB and appeared to be metabolically active (Fig. 6B). At stage VII, the nucellus of the functional pistil had initiated and was composed of very dense epidermal cells and meristematic cells located in the centre (Fig. 6C). The immature ovule showed a proximal/distal axis of symmetry. At stage VIII, the carpel had undergone a substantial increase in size (Fig. 6D), the style and ovule being clearly distinguishable. The inner integument was formed and sporangial tissue was clearly identifiable within the nucellus. The ovule was anatropous and its differentiation was characterized by the acquisition of abaxial/adaxial symmetry. During stage IX, the carpel enlarged and elongated greatly (Fig. 6E). A differentiated stigma was observed, the placenta was highly vascularized and the outer integuments were in place. Located on the micropyle side, the sporangial cells were seen to have produced a macrospore mother cell which, after meiosis, gave rise to the embryo sac (data not shown). Prior to flower maturity, the gynoecium of the pistillate flower was composed of three uniovulate carpels.
Compared with the fertile gynoecium, the pistillodes of the staminate flower displayed a lower degree of development. At stage VI, the pistillode primordia were elongated and slightly wider (Fig. 6F). Pistillode primordia cells were less dense than those of functional carpel primordium (Fig. 6A). At stage VII, the pistillodes were further elongated and the ovular meristem contained active cells that were densely stained by NBB (Fig. 6G). The pistillodes were slightly more elongated at stage VIII and had undergone differentiation in the distal region to form a very short style and stigma (Fig. 6H). The ovular meristem gave rise to the nucellus in which epidermal cells were densely stained. At stage IX, the centre of the nucellus was occupied by vacuolate cells containing large nuclei, a characteristic of quiescent cells (Fig. 6I, J). The ovule did not differentiate to produce integuments, nor was any chalazal vascularization seen. The nucellus did not initiate any sporangial tissue. A proximal/distal axis of symmetry was easily identifiable in the incompletely developed ovule.
Overall, the comparison between gynoecia from flowers of both sexes showed that pistillodes showed a structure similar to that of fertile carpels during stages VI and VII. However, pistillode growth was slower and ceased prematurely when compared with the fertile carpel. In the staminate flower, pistillodes were found to remain small and the development of the ovule was blocked. Furthermore, precocious cell differentiation was observed in the pistillode.
Cytological changes accompanying the development of unisexual flowers
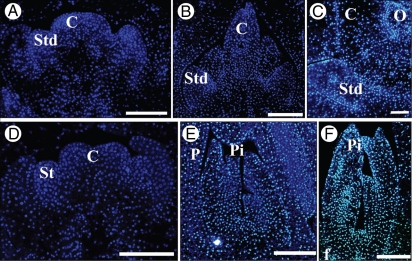
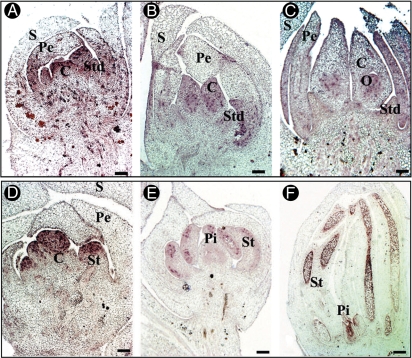
In order to investigate the cellular processes involved in the arrest of development of the residual organ, nuclear integrity in pistillodes and staminodes was examined. In this regard, paraffin-embedded tissue sections stained with DAPI at three different stages of development (stages V, VI and VII) were observed. DAPI staining revealed the presence of intact nuclei in the cells from staminodes (Fig. 7A–C) and pistillodes (Fig. 7D–F) at stage V (Fig. 7A, C), VI (Fig. 7B, E) and VII (Fig. 7C, F) In parallel, in situ hybridization was used to study the accumulation of histone H4 transcripts (a marker of cells in the DNA replication phase of mitosis) at three different stages (V, VI and VII). At the sexually undifferentiated stage of the pistillate flower, the accumulation of H4 transcripts was detected in the primordia of both staminodes and carpels (Fig. 8A). However, during stages VI and VII, H4 transcripts were only detected in the cells of the fertile developing carpels and later in the cells of ovule primordia (Fig. 8B, C). No significant signal corresponding to H4 transcripts was detected in the staminode primordia (Fig. 8B, C). In staminate flowers, H4 transcripts were detectable in all organ primordia at stage V (Fig. 8D), in the microspores of stamens (Fig. 8E, F) and especially in the ovular meristem of pistillodes following the initiation of the nucellus at stage VII (Fig. 8F). The presence of H4 signal in the ovules of pistillodes is presumably associated with nucellus initiation, as seen previously (Fig. 6H). However, no H4 signal was detected in the cells of pistillodes at stage VI at the time when stamens were undergoing differentiation to generate anther and filament (Fig. 8E).
Fig. 7.
DAPI staining of longitudinal sections of date palm flower at three stages of development. With DAPI (blue fluorescent signal), nuclei appear in blue. Staminodes (A–C) and pistillodes (D–F) show intact nuclei at ‘bisexual’ stages V (A,D), VI (B,E) and VII (C,F). Abbreviations: C, carpel; O, ovule; Pi, pistillode; Std, staminodes; St, stamens. Scale bars = 100 µm.
Fig. 8.
In situ hybridization analysis of histone H4 gene expression in longitudinal sections of date palm flower at three stages of development. Cells that accumulate histone H4 gene transcripts are stained in purple. In the pistillate flower, histone H4 gene transcripts were accumulated in all primordium at ‘bisexual’ stage V (A) and in the ovule (B,C) while no accumulation of H4 transcripts was observed in staminodes at stages VI (B) and VII (C). In the staminate flower, histone H4 gene expression was observed throughout the primordia at the ‘bisexual’ stage (D), in stamens (E,F), in ovule of pistillodes (F) but no expression was seen in the pistillode at stage VI (E). Abbreviations: C, carpel; O, ovule; Pe, petal; Pi, pistillode; Std, staminodes; St, stamens; S, sepal. Scale bars = 200 µm.
In both arrested staminode and pistillode cells of pistillate and staminate flowers, respectively, there wer no signs of nuclear degeneration, although a cessation of cell cycle activity was observed. These data are compatible with the hypothesis that the developmental arrest observed in sterile sex organs results from an absence of cell division rather than from a cell death mechanism.
DISCUSSION
Inflorescence development and sexual dimorphism
Date palm inflorescences are large, single-order, monopodially branched structures. Male and female inflorescences emerge simultaneously and show the same overall structural organization. However, at the early stages of inflorescence development, three different characters distinguished the female and male inflorescences, namely form and size, floral meristem density, and polysaccharide accumulation. Indeed, these differences were observable shortly after the emergence of the rachillae. The high starch accumulation in the female inflorescence may reflect the high demand on the resources of the plant between pollination and fruit maturation. Dimorphism between staminate and pistillate inflorescences is common in diclinous plants. For example, in oil palm, the female inflorescence displays a smaller number of rachillae with differences in bract number and form (Adam et al., 2005). In Phytelephas equatorialis, male and female inflorescences differ in the number of branches and in the number of flowers per branch (four in the male and one in the female inflorescence) (Uhl and Dransfield, 1984). Similarly, inflorescence structure in papaya depends on the sex of the tree, the male trees being characterized by a long and many-flowered inflorescence while female trees bear short and fewer-flowered inflorescences (Ming et al., 2007).
These differences in inflorescence structure between the two sexes are not directly related to gynoecium and androecium development and can be considered as secondary sexual characters which emerged subsequently to the appearance of unisexual flowers (Lloyd and Webb, 1977; Dellaporta and Calderon-Urrea, 1993). Fossils of Phoenix flower displaying strong floral dimorphism have been recovered from sediments dating from the Middle Miocene, around 50 Mya (Dransfield et al., 2008), suggesting that dioecy is an ancient character in the Phoenix genus. Only a few genes involved in the control of secondary sex characters in diclinous species have been reported. In maize, TASSELSEED 4 and 6 genes were found to regulate both sex determination and meristem fate, the latter process affecting inflorescence branching (Juarez and Banks, 1998). More recently, several quantitative trait loci controlling inflorescence morphological traits (such as size and number of inflorescences) were identified in grapevine and found to be close to the sex locus (Marguerit et al., 2009). Further investigation will be necessary to understand the developmental link between secondary sex characters and sex determination in date palm.
Sexual differentiation of flowers
The first morphological gender difference appears at stage V when the carpel primordium is slightly larger in size in the pistillate flower. Despite their similar cell size, these initial differences in cell number between the two types of carpel primordium may be related to a difference in meristem activity during floral development between two sexes. Similar morphological differences were observed in S. latifolia. In this case, the gynoecium of the staminate flower is significantly smaller than the corresponding structure in the pistillate flower as soon as all floral organ primordia are initiated (Grant et al., 1994). SLM2 (PISTILLATA homologue) and SLM3 (APTALA3 homologue) MADS-box genes were found to be more expressed in the central region of the staminate flower meristem compared with that of pistillate flowers (Lebel-Hardenack and Grant, 1997). The authors suggested that these identity genes might play a key role in limiting the growth of the gynoecium in the staminate flower. It is interesting to note that in S. latifolia, orthologues of the Arabidopsis SHOOTMERISTEMLESS and CUC SHAPED COTYLEDON 1 and 2 genes show sex-dependent differences in their spatial expression patterns before any morphological differences can be observed between staminate and pistillate flower buds (Zluvova et al., 2006). A number of mutations that affect plant floral meristem size have been identified. Indeed, mutations in the CLAVATA3 gene in Arabidopsis, as well as the FLORAL ORGAN NUMBER 1 gene in rice, result in an over-proliferation of the floral meristem, and an enlargement of the meristem with an increase in organ number (Suzaki et al., 2004; Kazama et al., 2009). In Arabidopsis, the AGAMOUS and SUPERMAN genes act to regulate cell proliferation in the third and fourth whorls of the flower (Meyerowitz, 1997; Jenik and Irish, 2000). The INHIBITOR OF MERISTEM ACTIVITY (IMA) gene, which acts as a repressor of the genes regulating stem cell fate (WUS, STM, KN1) and which consequently participates in the process of floral meristem determinacy, was recently described in tomato (Sicard et al., 2008). It will be interesting to determine whether orthologues of these genes might play a role in the processes of cell proliferation in whorls 3 and 4 and sex determination in date palm.
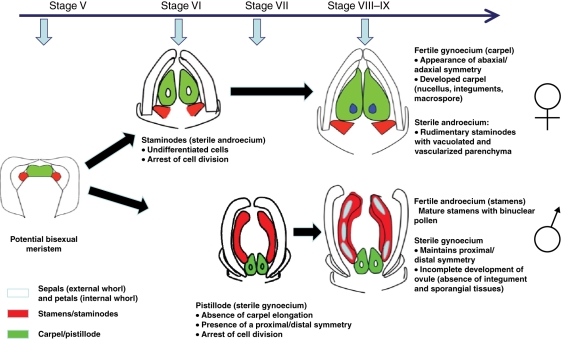
The data here clearly indicate a difference in the timing of residual sex organ arrest between staminate and pistillate flowers of date palm (Fig. 9). Similarly, the exact timing of floral organ developmental arrest varies widely in other diclinous species. In oil palm, staminodes form a rudimentary structure whereas in S. latifolia and Cucumis sativus, the developmental arrest of staminodes occurs later after the differentiation of the anthers and filament (Bai et al., 2004; Adam et al., 2005). Pistillodes remain rudimentary and are arrested early in oil palm and in Silene whereas in other species such as Vitis vinifera they are more developed and degenerated after embryo sac formation (Grant et al., 1994; Farbos et al., 1997; Caporali et al., 2003; Bai et al., 2004; Adam et al., 2005). These variations in the timing of the developmental arrest of sterile male and female organs suggest different underlying mechanisms which may be independent of each other.
Fig. 9.
Major events leading to the generation of unisexual flowers in date palm.
Arrest in the cell cycle of sterile reproductive organs
This is the first report on a detailed cytological study of reproductive development in date palm with particular attention to the expression of cell cycle-specific genes, aimed at understanding the process governing the transition from a ‘bisexual’ floral bud to a unisexual flower. The data suggest that the abortion of sterile sex organs in date palm results from cell cycle arrest rather than from cell death. Cell cycle arrest at the S phase, as demonstrated by the disappearance of H4 gene transcripts, is consistent with the lack of cell activity and subsequent limited growth of the sterile sex organ. Similarly, in Silene, the cessation of growth of residual sex organs results from a dramatic reduction in cell division activity, as shown by the expression patterns of both the histone H4 (SlH4) and cyclin A1 (SlCycA1) genes (Matsunaga et al., 2004). Developmental studies in cucumber revealed that staminode developmental arrest in pistillate flowers is associated with DNA damage and chromatin condensation without any link with programmed cell death process (Hao et al., 2003). In maize, stamen arrest in the ear flower is caused by cell cycle arrest, as demonstrated by the lack of cyclin B gene expression, whereas in the tassel and secondary ear, pistil abortion is associated with a cell death process controlled by the action of TASSELSEED 1 and 2 genes (Calderon-Urrea and Dellaporta, 1999; Kim et al., 2007). Whether similar mechanisms govern the developmental arrest of sterile sex organs in date palm remain to be determined.
The absence of nuclear degeneration in cells from sterile sex organs can explain the floral plasticity observed in date palm under specific in vitro or in planta environments. The results are consistent with the observed plasticity of residual sex organs in response to hormone induction. The in vitro reactivation of pistillodes in staminate flowers is obtained in the presence of the synthetic auxin 2,4-D (De Mason and Tisserat, 1980). Recently, in pistillate flowers of date palm, Masmoudi-Allouche et al. (2008) reported on the in vitro reactivation of staminodes and their subsequent development into fertile stamens in specific in vitro conditions. This was achieved by modifying the balance between auxin (AIB, aminoisobutyric acid) and cytokinin (BA, benzyladenine).
The unisexuality of date palm flowers results from the developmental arrest of sterile sex organs through the cessation of cell division and precocious cell differentiation rather than cell degeneration. Further ultrastructural observations of the arrested sex organ will be necessary to investigate whether DNA degeneration or chromatin condensation is associated with the observed cell cycle arrest. These data form a useful starting point to study the processes controlling sex differentiation and determination in date palm.
ACKNOWLEDGEMENTS
We thank Dr Alain Rival (CIRAD) for critical reading of the manuscript and Dr Jean-Luc Verdeil for his useful comments on the cytological analysis of flower development. Chantal Cazevieille and Cécile Sanchez (Centre de Ressources en Imagerie Cellulaire, IURC, Montpellier, France) provided technical assistance. We are grateful to Claudio Littardi and Robert Castellana from Centro Studi e Ricerche per le Palme, Italy, for material harvesting assistance. This work was supported by the French Ministry of Foreign Affairs via a CORUS 2 project and by IRD through institutional funding and a JEAI project (Djibpalm).
LITERATURE CITED
- Adam H, Jouannic S, Escoute J, Duval Y, Verdeil JL, Tregear J. Reproductive developmental complexity in the african oil palm (Elaeis guineensis, Arecaceae) American Journal of Botany. 2005;92:1836–1852. doi: 10.3732/ajb.92.11.1836. [DOI] [PubMed] [Google Scholar]
- Adam H, Jouannic S, Orieux Y, et al. Functional characterization of MADS box genes involved in the determination of oil palm flower structure. Journal of experimental Botany. 2007;58:1245–1259. doi: 10.1093/jxb/erl263. [DOI] [PubMed] [Google Scholar]
- Bai S-L, Peng YB, Cui JX, et al. Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.) Planta. 2004;220:230–240. doi: 10.1007/s00425-004-1342-2. [DOI] [PubMed] [Google Scholar]
- Boualem A, Fergany M, Fernandez R, et al. A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science. 2008;321:836–838. doi: 10.1126/science.1159023. [DOI] [PubMed] [Google Scholar]
- Calderon-Urrea A, Dellaporta SL. Cell death and cell protection genes determine the fate of pistils in maize. Development. 1999;126:435–441. doi: 10.1242/dev.126.3.435. [DOI] [PubMed] [Google Scholar]
- Caporali E, Spada A, Marziani G, Failla O, Scienza A. The arrest of development of abortive reproductive organs in the unisexual flower of Vitis vinifera ssp. silvestris. Sexual Plant Reproduction. 2003;15:291–300. [Google Scholar]
- Cheng PC, Gryson RI, Walden DB. Organ initiation and the development of unisexual flowers in the tassel and ear of Zea mays. American Journal of Botany. 1983;70:450–462. [Google Scholar]
- Coimbra S, Torrao L, Abreu I. Programmed cell death induces male sterility in Actinidia deliciosa female flowers. Plant Physiology and Biochemistry. 2004;42:537–541. doi: 10.1016/j.plaphy.2004.05.004. [DOI] [PubMed] [Google Scholar]
- De Mason DA, Tisserat B. The occurrence and structure of apparently bisexual flowers in the date palm, Phoenix dactylifera L. (Arecaceae) Botanical Journal of the Linnean Society. 1980;81:283–292. [Google Scholar]
- De Mason DA, Stolte KW, Tisserat B. Floral development in Phoenix dactylifera. Canadian Journal of Botany. 1982;60:1439–1446. [Google Scholar]
- Dellaporta SL, Calderon-Urrea A. Sex determination in flowering plants. Plant Cell. 1993;5:1241–1251. doi: 10.1105/tpc.5.10.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield J, Uhl NW, Amussen CB, Baker WJ, Harley M, Lewis CL. Genera Palmarum. The evolution and classification of palm. Kew: Royal Botanic Gardens; 2008. [Google Scholar]
- Farbos I, Veuskens J, Vyskot B, Negrutiu I, Mouras A. Sex organ determination and differentiation in the dioecious plant Melandrium album (Silene latifolia): a cytological and histological analysis. Sexual Plant Reproduction. 1994;10:155–167. [Google Scholar]
- Farbos I, Oliveira M, Negrutiu I, Mouras A. Sex organ determination and differentiation in the dioecious plant Melandrium album (Silene latifolia): a cytological and histological analysis. Sexual Plant Reproduction. 1997;10:155–167. [Google Scholar]
- Farbos I, Veuskens J, Vyskot B, et al. Sexual dimorphism in white campion: deletion on the Y chromosome results in floral asexual phenotype. Genetics. 1999;151:1187–1196. doi: 10.1093/genetics/151.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin V, Lunness PA, Fobert PR, et al. The expression of D-cyclin genes defines distinct developmental zones in snapdragion apicals meristems an dis locally regulated by the CYCLOIDEA gene. Plant Physiology. 2000;122:1137–1148. doi: 10.1104/pp.122.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Hunkirchen B, Saedler H. Developmental differences between male and female flowers in the dioecious plant Silene latifolia. The Plant Journal. 1994;6:471–480. [Google Scholar]
- Hao YJ, Wang DH, Peng YB, et al. DNA damage in the early primordial anther is closely correlated with stamen arrest in the female flower of cucumber (Cucumis sativus L.) Planta. 2003;217:888–895. doi: 10.1007/s00425-003-1064-x. [DOI] [PubMed] [Google Scholar]
- Jenik PD, Irish VF. Regulation of cell proliferation patterns by homeotic genes during Arabidopsis floral development. Development. 2000;127:1267–1276. doi: 10.1242/dev.127.6.1267. [DOI] [PubMed] [Google Scholar]
- Juarez C, Banks JA. Sex determination in plants. Current Opinion in Plant Biology. 1998;1:68–72. doi: 10.1016/s1369-5266(98)80130-1. [DOI] [PubMed] [Google Scholar]
- Kazama Y, Fujiwara MT, Koizumi A, et al. A SUPERMAN-like gene is exclusively expressed in female flowers of the dioecious plant Silene latifolia. Plant and Cell Physiology. 2009;50:1127–1141. doi: 10.1093/pcp/pcp064. [DOI] [PubMed] [Google Scholar]
- Kim JC, Laparra H, Calderon-Urrea A, Mottinger JP, Moreno MA, Dellaporta SL. Cell cycle arrest of stamen initials in maize sex determination. Genetics. 2007;177:2547–2551. doi: 10.1534/genetics.107.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA. Ectopic expression of AINTEGUMENTA in Arabidopsis plants results in increased growth of floral organs. Developmental Genetics. 1999;25:224–236. doi: 10.1002/(SICI)1520-6408(1999)25:3<224::AID-DVG5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Lebel-Hardenack S, Grant S. Genetics of sex determination in flowering plants. Trends in Plant Sciences. 1997;2:130–136. [Google Scholar]
- Lloyd DG, Webb CJ. Secondary sex characters in seed plants. Botanical Reviews. 1977;43:177–216. [Google Scholar]
- Marguerit E, Boury C, Manicki A, et al. Genetic dissection of sex determinism, inflorescence morphology and downy mildew resistance in grapevine. Theoretical and Applied Genetics. 2009;118:1261–1278. doi: 10.1007/s00122-009-0979-4. [DOI] [PubMed] [Google Scholar]
- Martin A, Troadec C, Boualem A, et al. A transposon-induced epigenetic change leads to sex determination in melon. Nature. 2009;461:1135–1138. doi: 10.1038/nature08498. [DOI] [PubMed] [Google Scholar]
- Masmoudi-Allouche F, Anissa Châari-Rkhis A, et al. In vitro hermaphrodism induction in date palm female flower. Plant Cell Reports. 2008;28:1–10. doi: 10.1007/s00299-008-0611-0. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, Uchida W, Kawano S. Sex-specific cell division during development of unisexual flowers in the dioecious plant Silene latifolia. Plant and Cell Physiology. 2004;45:795–802. doi: 10.1093/pcp/pch081. [DOI] [PubMed] [Google Scholar]
- Meyerowitz EM. Genetic control of cell division patterns in developing plants. Cell. 1997;88:299–308. doi: 10.1016/s0092-8674(00)81868-1. [DOI] [PubMed] [Google Scholar]
- Ming R, Yu Q, Moore PH. Sex determination in papaya. Seminars in Cell and Developmental Biology. 2007;18:401–408. doi: 10.1016/j.semcdb.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Sherry RA, Eckard KJ, Lord EM. Flower development in dioecious Spinacia oleracea (Chenopodiaceae) American Journal of Botany. 1993;80:283–291. [Google Scholar]
- Sicard A, Petit J, Mouras A, Chevalier C, Hernould M. Meristem activity during flower and ovule development in tomato is controlled by the mini zinc finger gene INHIBITOR OF MERISTEM ACTIVITY. The Plant Journal. 2008;55:415–427. doi: 10.1111/j.1365-313X.2008.03520.x. [DOI] [PubMed] [Google Scholar]
- Siljak-Yakovlev S, Cerbah M, Sarr A, et al. Chromosomal sex determination and heterochromatin structure in date palm. Sexual Plant Reproduction. 1996;9:127–132. [Google Scholar]
- Suzaki T, Sato M, Ashikari M, Miyoshi M, Nagato Y, Hirano HY. The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development. 2004;131:5649–5657. doi: 10.1242/dev.01441. [DOI] [PubMed] [Google Scholar]
- Tanurdzic M, Banks JA. Sex determining mechanisms in land plants. The Plant Cell. 2004;16:61–71. doi: 10.1105/tpc.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl NW. Inflorescence and flower structure in NYPA FRUTICANS (Palmae) American Journal of Botany. 1972;59:729–743. [Google Scholar]
- Uhl NW, Dransfield J. Development of the inflorescence, androecium, and gynoecium with reference to palms. In: White RA, Dickison WC, editors. Contemporary problems in plant anatomy. New York: Academic Press; 1984. pp. 397–449. [Google Scholar]
- Weiblen GD, Oyama RK, Donoghue MJ. Phylogenetic analysis of dioecy in monocotyledons. American Naturalist. 2000;155:46–58. doi: 10.1086/303303. [DOI] [PubMed] [Google Scholar]
- Zluvova J, Nicolas M, Berger A, Negrutiu I, Moneger F. Premature arrest of the male flower meristem precedes sexual dimorphism in the dioecious plant Silene latifolia. Proceedings of the National Academy of Sciences. 2006;103:18854–18859. doi: 10.1073/pnas.0606622103. [DOI] [PMC free article] [PubMed] [Google Scholar]