Abstract
Background and Aims
Heterostylous plants have been characterized by the presence of two or three discrete morphs that differ in their sex organ position within populations. This polymorphism is widely distributed among the angiosperms, but detailed studies are limited to few taxonomic groups. Although a small representation, evolutionary meaningful variations of the heterostylous syndrome have been reported when precise measurements of the sexual whorls were taken. A thorough exploration of groups where heterostyly has been reported should offer new opportunities to further testing the evolutionary hypotheses explaining heterostyly. Here, the traits defining heterostyly were explored in half of the species in Nivenia, the only genus of Iridiaceae where heterostyly has been reported.
Methods
Detailed morphometric analysis of the flower sexual whorls and some traits considered as ancillary are supplied to determine for each population (a) the kind of stylar polymorphism, (b) the morph ratio and (c) the degree of reciprocity between sexual whorls. Also the rates of assortative (within morph) versus disassortative (between morphs) pollen transfer were estimated by analysing pollen loads on stigmas. The association between floral phenotypic integration and the reciprocity between sexual whorls was estimated; both characteristics have been quoted as dependent on the accuracy of the fit between pollinators and flowers and therefore related to the efficiency of pollen transfer.
Key Results
Different types of polymorphism, differing in their degree of reciprocity, were found in Nivenia. Effective disassortative mating appears to be common, since (a) all dimorphic populations show equal morph-ratios (isoplethy), and (b) the pollen placed on the stigmas of each morph is likely to be coming from the other (complementary) morph. The most reciprocal populations of the heterostylous species have also the highest values of phenotypical integration.
Conclusions
Stigma height dimorphism, as opposed to distyly, is proven for the first time in Nivenia. The presence of different types of polymorphism within the genus is consistent with hypotheses of the evolution of heterostyly. The role of the pollinators as the leading force of the transition seems to be apparent, since floral integration is related to reciprocity.
Keywords: Heterostyly, Nivenia, phenotypic integration, reciprocal herkogamy, reciprocity degree, disassortative mating
INTRODUCTION
Discrete sexual polymorphisms in plants, i.e. the existence of two or more morphs affecting sexual organs within single populations, is a pervasive trait in angiosperms which has attracted evolutionary biologists ever since Darwin's seminal work (Darwin, 1877). The species having these genetically based polymorphisms are excellent model systems to study the functioning and evolution of the mechanisms for disassortative mating. Among these polymorphisms, heterostyly is by far the most common. Its functional significance is thought to be simultaneously the avoidance of self-interference between stigmas and anthers by spatially separating them (herkogamy), and the promotion of accurate pollen transfer between plants by making this separation reciprocal within populations (reciprocal herkogamy; Webb and Lloyd, 1986; Lloyd and Webb, 1992a; Barrett 2002). Different hypotheses have been proposed to explain the evolution of this polymorphism. One of the most recent and the most comprehensive is that of Lloyd and Webb (1992a, b). This hypothesis recalls the arguments which Darwin (1877) proposed, neglected for most of 20th century. In short, Lloyd and Webb (1992a, b) propose that the ancestral condition is an approach herkogamous flower (stigmas above anthers, the commonest arrangement in angiosperms; Webb and Lloyd, 1986), then an intermediate stage of stigma-height dimorphism (two morphs with different style lengths but the anthers at the same level). Stigma-height dimorphism is considered to be unstable because of lack of precision in pollen transfer between morphs [although Lloyd and Webb (1992b) provide specific conditions for its maintenance; for a test case, see Cesaro and Thompson, 2004], thus a reciprocal positioning of sexual whorls between morphs would be selected for. The selective factor is therefore the promotion of the efficiency in pollen transfer driven by the particular behaviour of the pollinators collecting and delivering pollen grains in precise parts of their bodies. Usually heterostylous plants also have other typical features, such a diallelic incompatibility system which only permits crosses between morphs (for a review, see Barrett and Cruzan, 1994), and some between-morph differences in floral traits (ancillary traits: e.g. pollen and stigma size and shape, corolla size, etc.; Ganders, 1979). In Lloyd and Webb's (1992a) model, these features are interpreted as devices to increase the efficiency of the system by avoiding gamete wastage. Thus incompatibility would appear after reciprocal herkogamy, both traits not necessarily being linked.
In recent times the depicted evolutionary scenario has gained increasing support from diverse areas such as pollination ecology, flower ontogeny, breeding systems and phylogenetics. Nevertheless, despite the wide taxonomic representation of heterostyly among angiosperms [28 families (Barrett, 1992) and 164 genera (Ganders, 1979)], most studies have somewhat concentrated on only a few taxa [e.g. Pontederiaceae (Barrett et al., 1989), Turneraceae (Shore et al., 2006), Primulaceae (Nishihiro et al., 2000), Rubiaceae (Faivre and McDade, 2001), Oxalidaceae (Weller et al., 2007), Amarillydaceae (Barrett and Harder, 2005; Pérez-Barrales et al., 2006), Boraginaceae (Schoen et al., 1997; Brys et al., 2008a, b; Ferrero et al., 2009)]. For example, stigma-height dimorphism (as opposed to distyly, sensu Barrett et al., 2000) has only recently been shown to be the most likely intermediate step in Narcissus (Graham and Barrett 2004; Pérez-Barrales et al., 2006) and Lithodora sensu lato (Ferrero et al., 2009). Although stigma-height dimorphism had been reported in other groups where heterostyly does exist [Anchusa (Philipp and Schou, 1981), Quichamalium (Riveros et al., 1987)], the evolutionary relationship between distyly and dimorphism remains to be proven. Since stigma-height dimorphism has been sometimes mistakenly taken as distyly (e.g. Fernandes, 1964; Phillip and Schou, 1981; Riveros et al., 1987; Richards and Koptur, 1993) stigma-height dimorphism is likely to be more frequent than was previously thought, and therefore distyly overestimated. Detailed floral morphometric studies from different groups where heterostyly and monomorphism are present are thus necessary. Such morphometric studies would throw light on at least two complementary aspects: (1) measurements of sexual organs could show the presence of the supposed intermediate stages, and are needed to estimate the contribution of each variant to fitness; (2) perianth traits could reveal the importance of precise pollinators, which may be inferred from restrictive corollas (Lloyd and Webb, 1992a) and strong correlations among floral traits (Berg, 1960). In fact, higher correlations among floral traits (phenotypic integration sensu Wagner, 1984) were found in style polymorphic populations than in monomorphic populations (Pérez-Barrales et al., 2007). Moreover, a positive correlation between reciprocity and floral phenotypic integration has been found (Ferrero et al., 2011).
Nivenia is the only genus within the Iridaceae containing heterostylous species, and has been reported to include distylous and monomorphic species (Mulcahy, 1965; Ornduff, 1974; Goldblatt, 1993). The taxonomic monograph by Goldblatt (1993) reported four monomorphic species and five heterostylous species. Afterwards Goldblatt (1997) described N. parviflora, and recently Manning and Goldblatt (2007) have described a new species, N. inaequalis, both considered to be distylous. The most comprehensive study on the floral biology of Nivenia is that of Goldblatt and Bernhardt (1990) which reports data on flower morphology, breeding systems, and pollinators for all the distylous species known up to that date. Unfortunately, absolute measurements of sexual organs were not included there, and the relative lengths provided do not allow to determine if the considered distylous species are truly so, nor their degree of reciprocity, although some apparent variability deserves further investigation. Nor has the incompatibility system been conclusively studied for the genus, contrasting the results of Goldblatt and Bernhardt (1990) that suggest at least partial morph-compatibility based on the growth of pollen tubes in the pistils, with those by Ornduff (1983) who reported morph-incompatibility for N. corymbosa based on seed production.
Here, variation in floral traits related to style polymorphism and pollination biology is reported in five species of Nivenia, to explore if this variation meets the requirements of any model for the evolution of heterostyly. A definite test would need to reconstruct evolutionary transitions of these variations on the phylogeny, which is not yet available.
The specific aims of this work are (a) to analyse the variation of floral traits in order to quantitatively characterize the type of floral polymorphism and its reciprocity level; (b) to determine the morph ratio in the populations; (c) to determine the pollen load on stigmas and to estimate the pollen transfer rate between morphs, and hence the efficiency of style polymorphism, using the between-morph variability in pollen size; and (d) to estimate the levels of floral phenotypic integration and its relationship with the polymorphism and the pollinator types, as well as the possible role of any particular floral trait in pollination success.
MATERIALS AND METHODS
Study species and population sampling
The genus Nivenia Vent. (Iridaceae, subfam. Nivenioideae) is endemic to the Western Cape Province (South Africa), and all species are restricted to sand-stones of the montane layer of the Cape System, on soils of the Table Mountain Series (Goldblatt and Bernhardt, 1990; Goldblatt, 1993). It comprises small to tall evergreen shrubs with woody underground stems. Inflorescences are terminal, forming a pseudopanicle with short to long axillary branches, thus becoming corymbose or raceme-like. Flowers, from one to many per inflorescence, are blue with well-developed perianth tubes and outspread tepals. The filaments of the stamens (three per flower) are adnate to the tube resulting from the connation of the six tepals. All three anthers of the flower are placed at the same height level except for N. inaequalis, which takes its name from this trait. The ovary is inferior, globose and usually two-ovulate (for a thorough description, see Goldblatt, 1993; Manning and Goldblatt, 2007).
Six populations of five species were surveyed between 1998 and 2005 (Table 1). Given the narrow endemicity of most species, sampling was non-destructive at the plant level; in each population, only one apparently healthy recently opened flower per plant was collected in 100 randomly selected plants when available (just one flower from dense clumps to avoid oversampling of genets), assuming a similar age for the collected flowers. Flowers were preserved in 70 % ethanol until their measurement in the laboratory. In addition, one flower-bud per plant was also sampled in 50 plants to estimate the size and production of pollen grains. According to our experience, that sampling number is representative of the populations, with a number in the population of a few hundreds at most; note that in populations with reduced population size, like N. argentea at Aasvoëlkrans, all flowering individuals were sampled.
Table 1.
Summarized data of the localities of the five species of Nivenia studied
| Species | Location | Altitude (m) | No. of plants sampled (L/S morphs) |
|---|---|---|---|
| N. inaequalis | Rooiberg Massif, near Teeboskop peak, slope facing south (33°41′S, 21°34′E) | 1021 | 100 (53/47) |
| N. argentea | Phesantefontein, near Aasvoëlkraans peak, slope facing south (33°56′S, 21°08′E) | 955 | 10 (6/4) |
| N. argentea | Peak south Langkloof, near Garcia pass, crest and slope south (33°57′S, 21°15′E) | 1033 | 100 (59/41) |
| N. binata | Swartberg Pass (33°21′S, 22°03′E) | 1228 | 18 (13/5) |
| N. corymbosa | Wellington, Bain's Kloof Pass (33°38′S, 19°05′E) | 451 | 84 (35/52) |
| N. fruticosa | Perdeberg (33°56′S, 21°04′E) | 1144 | 18 |
Morphometric measurements and morph ratios
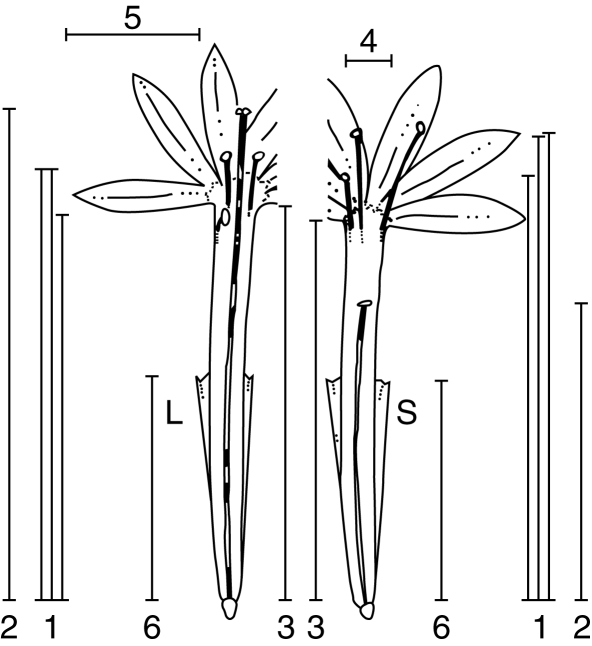
To characterize and evaluate the degree of reciprocity in each population, flowers were photographed, longitudinally dissected, and then photographed again. Measurements were taken from the digital photos with the image analyser software analySIS 5·0. Floral traits measured were: (a) stamens height (up to the insertion of the filament on the anther for each one of them); (b) stigma height; (c) corolla length; (d) tube width; (e) tepal limb length; (f) bract length; and (g) anther length (Fig. 1). Sample sizes ranged between 10 and 100 (Table 1). Individual flowers were classed as L (long) morphs when the stigma level was above the level of the anthers, or S (short) morphs when the stigma level was below the level of the anthers.
Fig. 1.
Main floral parts measured in the flowers sampled of both morphs (L and S): (1) height of all stamens, (2) stigma height, (3) corolla tube length, (4) tube width, (5) length of the tepal limb, (6) bract length. Anther lengths were also measured but are not indicated in the figure due to small size (schematic drawing modified from Goldblatt, 1993).
To characterize each population as style-dimorphic (all stamens of both morphs at the same height level) or truly heterostylous (different stamen level for each morph), t-tests were performed to compare the heights of anthers between morphs. When anther heights do not show significant between-morph differences the population was considered as style-dimorphic. Also the fit between anther and stigma heights for each morph was calculated with regression. The regression coefficients were compared to find out if both sexual whorls co-varied at the same rate in both morphs.
Also the degree of reciprocity between sexual whorls for each population was calculated with the index of Sánchez et al. (2008). Unlike previous indices, it provides meaningful values that can be compared across populations and species because it compares stigma–stamen height gaps for all potential legitimate crosses (i.e. each and every stamen of a morph versus each and every stigma of the complementary morph measured in a population) while considering also the dispersion of the data, and it is not skewed by the more frequent sex (stamens). Unlike previous indices based only on a measure for stamen and stigma heights, this index needs a lot of computation to deal with all the available data of a population (for a complete description, see Sánchez et al., 2008; computational software available at http://webs.uvigo.es/plantecology/software.es.html).
Among the studied ancillary traits were (a) the total number of pollen grains per anther and (b) pollen grain size. To this end, each anther was placed in a drop of 50 % glycerine over a microscope slide, opened and squashed beneath a cover slip; the pollen grains were counted under a light microscope (magnification ×100) differentiating among healthy and apparently aborted (collapsed) grains. The pollen preparations were photographed under the optical microscope and measurements of polar axis in 50 grains were made with the image analyser software analySIS 5·0.
Differences between morphs in each population were compared with a t-test for all traits, except when the available number of flowers was small (N. binata and N. argentea at Aasvoëlkrans) which were analysed with the non-parametric Mann–Whitney test. The numbers of aborted pollen grains were compared between morphs with ANCOVA, considering the total number of grains per anther as a co-variable, except N. binata and N. argentea at Aasvoëlkrans, where Mann–Whitney test was used.
The same flowers collected for floral measurements were used to estimate the morph ratio in each polymorphic population. Deviations from isplethy (equal morph-ratio) were tested by means of the chi-square test.
Pollen transfer within populations
Between-morph differences in pollen reception were tested by comparing pollen loads on stigmas. According to Goldblatt and Bernhardt (1990) and the present observations, pollen size dimorphism in the polymorphic species of Nivenia could allow to discrimination between the pollen produced by each morph. Based on this possibility, the polar axis of pollen grains on stigmas was measured with the image analyser software analySIS 5·0, to assess the level of assortative and disassortative pollen flow within the populations.
Hence, styles were cleared and softened with 8 n sodium hydroxide for 24 h, rinsed in distilled water and stained overnight with 0·05 % aniline blue prepared in 0·1 m potassium phosphate (Dafni et al., 2005). Pistils were then placed on a microscope slide with a drop of 50 % glycerine and squashed beneath a cover slip. Samples were observed and photographed through a Nikon Eclipse 80i epifluorescence microscope (Nikon Instruments, Kanagawa, Japan) with a UV-2A filter cube and the following variables were measured on these photographs: (a) number of pollen grains on the stigma; (b) polar axis diameter of five, when possible, pollen grains randomly chosen in each stigma; and (c) number of pollen tubes inside the style. Pollen loads on stigmas were estimated as the addition of the number of pollen grains on the stigma plus the number of pollen tubes in the style when not linked to a pollen grain.
To compare pollen reception between morphs 2 × 2 contingency tables for each population were analysed, considering the morph (long/short styled) and presence/absence of pollen on the stigma. Since the size of the pollen ranges overlapped between morphs (and therefore it cannot be conclusively decided by which morph a particular pollen grain was produced), the prevalence of assortative or disassortative mating was assessed by comparing (t-test) the mean sizes of the pollen grains on the L and S stigmas.
Phenotypic integration
To depict if all flower traits respond together to selective pressures related to precise pollen delivery and deposition by pollinators (Berg, 1960), flower phenotypic integration indices were estimated (Herrera et al., 2002; Pérez-Barrales et al., 2007) in morphs and populations of those species with a large enough sample size, including both sex and perianth traits. A precise pollination would imply a high reciprocity between the sex organs of the complementary morphs, and also a high correlation (i.e. integration) among all other floral traits affecting the behaviour of the pollinator in the flower (Ferrero et al., 2011). Phenotypic integration (after Wagner, 1984) was estimated through the eigenvalues of a correlation matrix of floral traits, namely corolla length, corolla width, style length and anther height. The magnitude of phenotypic integration is represented by the integration index (the variance of those eigenvalues). Because sample size varied among populations, the integration index was corrected by subtracting the expected value of integration under the assumption of random co-variation of traits [random integration = (no. of characters –1)/no. of plants; after Wagner, 1984; Herrera et al., 2002]. The integration index was also expressed as a percentage of the maximum possible value, which is the number of traits considered (Herrera et al., 2002; Pérez-Barrales et al., 2007; Ordano et al., 2008). For each population two values were calculated, one for each morph.
Relationship between floral traits and pollination success
To assess if any floral trait was determining the probability of pollen transfer, logistic regression analyses were performed for the three populations with the highest sample size (see Table 1), with all the floral traits studied as independent variables, and presence/absence of pollen on the stigmas as the response variable. Independent analyses were carried out for each morph because their different architecture may be responsible for different pollinator efficiency.
RESULTS
Morphometric analysis and morph ratio
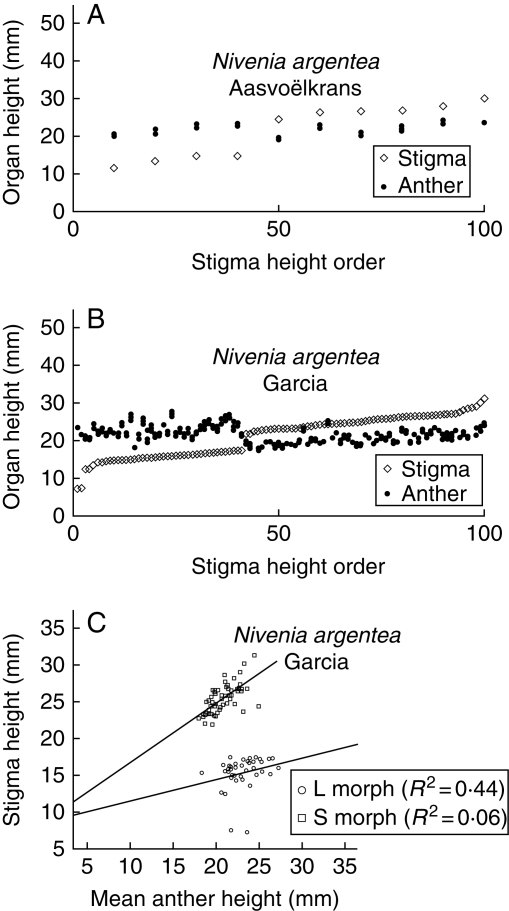
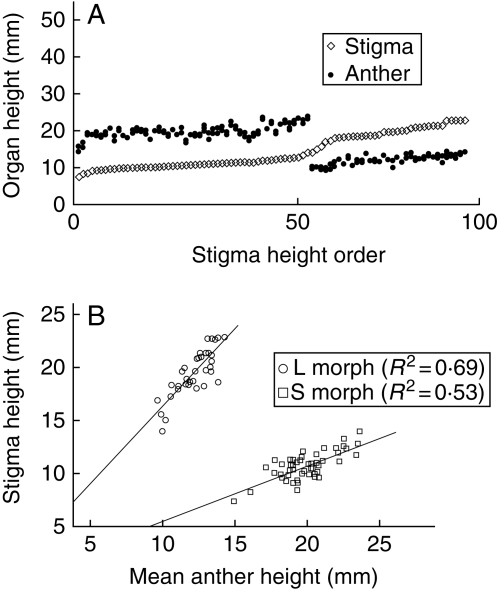
Average values of the measurements of the floral traits for each population and index of reciprocity between sexual whorls are shown in Tables 2 and 3, respectively. Variation of sexual whorls is represented in comparative plots in Figs 2–5. Among the species studied, only N. fruticosa is monomorphic, presenting clearly approach herkogamous flowers where the style is located well above the anthers (Table 2). For the species with two floral morphs, stigma height was significantly different in all of them (Table 2), and the length of the stamens was statistically different between morphs in all cases, except for the population of N. argentea at Aasvoëlkrans (Table 2). Thus, those were considered as distylous, and N. argentea at Aasvoëlkrans as stigma-height dimorphic since two style-length morphs are present but anther heights remain indistinguishable between morphs (see Fig. 3A). It has been thus confirmed that this is not a biased sample from a larger distylous population, but a truly stigma-height dimorphic, with a bootstrapping simulation on the data of the population of N. argentea at Garcia (distylous, n = 100). Re-sampled subpopulations with the same sample size of the population at Aasvoëlkrans (n = 10, 6 large- + 4 short-styled flowers) were randomly extracted, and the mean stamen height compared between morphs at each iteration (t-test, P < 0·01) in MsExcel®. Since the probability of obtaining stigma-height dimorphic subsamples from the large heterostylous population at Garcia is negligible (1000 iterations, P < 0·0001), it can be concluded that the population at Aasvoëlkrans is truly stigma-height dimorphic.
Table 2.
Morphometric characteristics of the studied species and populations of the genus Nivenia
|
N. inaequalis |
N. argentea Aasvoëlkraans |
N. argentea Garcia |
N. binata |
N. corymbosa |
N. fruticosa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morph | n | Mean ± s.d. | n | Mean ± s.d. | n | Mean ± s.d. | n | Mean ± s.d. | n | Mean ± s.d. | n | Mean ± s.d. | |
| Floral characteristics (mm) | |||||||||||||
| Stamen height | L | 158 | 35·25 ± 4·74** | 18 | 22·09 ± 1·64 | 166 | 20·91 ± 1·65** | 39 | 13·73 ± 1·62* | 102 | 12·24 ± 1·28** | 54 | 22·51 ± 2·39 |
| S | 138 | 37·28 ± 3·84** | 12 | 21·93 ± 1·18 | 120 | 23·14 ± 1·90** | 15 | 16·05 ± 1·12* | 104 | 19·89 ± 1·84** | |||
| Stigma height | L | 53 | 41·38 ± 2·95** | 6 | 27·19 ± 1·84** | 59 | 25·48 ± 2·02** | 13 | 18·05 ± 1·76** | 35 | 19·46 ± 2·20** | 18 | 27·60 ± 2·87 |
| S | 47 | 30·90 ± 2·80** | 4 | 13·62 ± 1·55** | 41 | 15·41 ± 2·18** | 5 | 12·03 ± 1·60** | 52 | 10·68 ± 1·27** | |||
| Tube diameter | L | 53 | 02·80 ± 0·32 | 6 | 03·34 ± 0·44 | 59 | 03·00 ± 0·45 | 13 | 2·28 ± 0·20 | 35 | 02·51 ± 0·35 | 18 | 02·96 ± 0·58 |
| S | 47 | 02·81 ± 0·33 | 4 | 03·29 ± 0·34 | 41 | 03·03 ± 0·40 | 5 | 2·19 ± 0·23 | 52 | 02·49 ± 0·34 | |||
| Tube length | L | 53 | 33·63 ± 3·31** | 6 | 20·98 ± 1·62* | 59 | 19·94 ± 1·65 | 13 | 10·95 ± 1·41 | 35 | 11·54 ± 1·37** | 18 | 20·75 ± 2·20 |
| S | 47 | 32·28 ± 2·93** | 4 | 17·90 ± 0·98* | 41 | 19·42 ± 1·54 | 5 | 10·31 ± 0·82 | 52 | 12·43 ± 1·51** | |||
| Tepal limb length | L | 53 | 13·40 ± 1·66 | 6 | 11·11 ± 1·03 | 59 | 12·44 ± 1·49* | 13 | 7·59 ± 0·92 | 35 | 09·41 ± 1·25 | 18 | 10·51 ± 1·05 |
| S | 47 | 13·47 ± 1·50 | 4 | 11·28 ± 0·89 | 41 | 11·70 ± 1·64* | 5 | 7·64 ± 0·64 | 52 | 09·17 ± 1·55 | |||
| Bract length | L | 53 | 23·56 ± 2·38 | 6 | 20·76 ± 2·95 | 59 | 20·88 ± 2·17 | 13 | 9·17 ± 0·86 | 35 | 5·41 ± 1·11 | 18 | 13·94 ± 1·04 |
| S | 47 | 22·83 ± 2·85 | 4 | 17·59 ± 3·40 | 41 | 20·02 ± 2·66 | 5 | 9·49 ± 0·86 | 50 | 5·73 ± 0·66 | |||
| Anther length | L | 133 | 01·73 ± 0·24 | 18 | 01·88 ± 0·18** | 164 | 02·22 ± 0·24** | 13 | 1·89 ± 0·18 | 97 | 01·76 ± 0·17** | 54 | 02·19 ± 0·18 |
| S | 65 | 01·80 ± 0·21 | 12 | 02·25 ± 0·20** | 108 | 02·33 ± 0·34** | 5 | 1·95 ± 0·17 | 94 | 01·94 ± 0·20** | |||
| Pollen characteristics in buds [n = number of anthers (number of flowers)] | |||||||||||||
| Total no. of pollen grains per anther | L | 21 (7) | 874·1 ± 184·9 | 15 (5) | 2169·2 ± 322·4 | 21 (7) | 2083·5 ± 209·1 | – | – | 21 (7) | 2236·7 ± 405·5 | 30 (10) | 787·3 ± 246·2 |
| S | 21 (7) | 917·0 ± 228·1 | 12 (4) | 2383·8 ± 275·4 | 21 (7) | 2199·1 ± 360·6 | – | – | 21 (7) | 2295·8 ± 387·3 | |||
| Aborted pollen grains per anther | L | 21 (7) | 12·1 ± 10·1 | 15 (5) | 46·2 ± 44·5 | 21 (7) | 111·8 ± 121·7 | – | – | 21 (7) | 125·5 ± 219·1 | 30 (10) | 142·5 ± 310·5 |
| S | 21 (7) | 20·7 ± 21·5 | 12 (4) | 32·8 ± 15·1 | 21 (7) | 90·1 ± 74·3 | – | – | 21 (7) | 29·2 ± 20·7 | |||
| [n: number of grains (number of flowers)] | |||||||||||||
| Pollen grain size (μm) | L | 525 (7) | 53·35 ± 4·63** | 375 (5) | 45·81 ± 4·77** | 525 (7) | 42·81 ± 4·19** | 225 (7) | 42·77 ± 4·69** | 525 (7) | 39·81 ± 4·00** | 750 (10) | 55·34 ± 7·05 |
| S | 525 (7) | 54·67 ± 4·92** | 300 (4) | 44·25 ± 4·45** | 525 (7) | 46·82 ± 4·39** | 75 (7) | 46·16 ± 3·66** | 525 (7) | 40·75 ± 4·48** | |||
Approach herkogamous flowers were categorized as L, and reverse herkogamous as S. Morphs within populations were compared with a t-test, except that (a) comparisons within the populations of N. binata and N. argentea were performed with non-parametric test (Mann–Whitney) due the small sample size; (b) the number of aborted pollen grains was compared with ANCOVA considering the total number of grains per anther as a co-variable, except for N. binata and N. argentea, where the proportion of aborted pollen grains was compared with non-parametric test (Mann–Whitney).
Values in bold differ significantly between morphs (* P < 0·05, ** P < 0·001).
Table 3.
Reciprocity values (R) after Sánchez et al. (2008) (note that the higher the value, the lower the reciprocity); floral phenotypic integration for each morph as a percentage of the maximum possible value (number of traits considered); proportion of pollinated flowers; and pollen load range and median in pollinated flowers, for the style polymorphic populations
| Population | R | Phenotypic integration (%) |
No. of flowers pollinated/unpollinated (%) | Pollen load ranges in pollinated flowers [min–max (median)] | |
|---|---|---|---|---|---|
| L | S | ||||
| N. inaequalis | 0·019 | 44·1 | 48·7 | 44/56n.s. (44·0) | 1–23 (4·5) |
| N. argentea Aasvoëlkraans | 0·043 | – | – | 6/4n.s. (60·0) | 2–33 (11·5) |
| N. argentea Garcia | 0·031 | 47·0 | 32 .9 | 65/35** (65·0) | 1–113 (15) |
| N. binata | 0·021 | – | – | 7/19n.s. (38·9) | 2–14 (9) |
| N. corymbosa | 0·019 | 52·2 | 51·7 | 50/38n.s. (56·8) | 1–90 (7·5) |
Phenotypic integration was not calculated for populations with a sample size which was too small.
Differences between the number of pollinated and unpollinated flowers were tested with chi-square (n.s., not significant; ** P < 0·001).
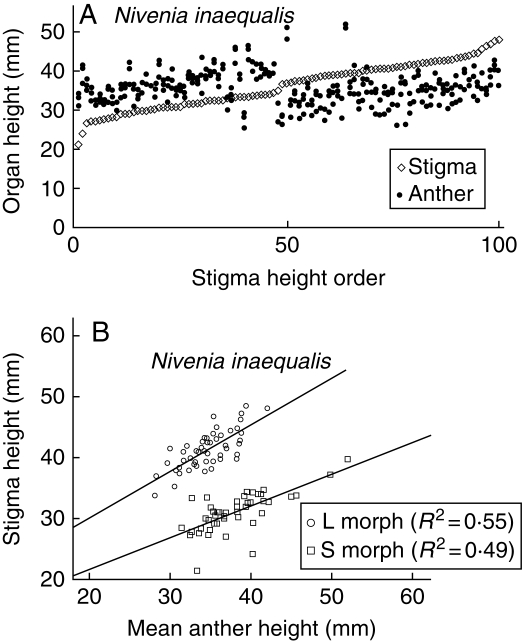
Fig. 2.
(A) Variation in the position of the stigmas and every anther in the flowers of N. inaequalis. Values are ordered by increasing stigma height. (B) Relationship between stigma height and mean stamen height for each flower of the two morphs.
Fig. 3.
Variation in the position of the stigmas and every anther in the flowers of N. argentea at the populations of (A) Aasvoëlkrans and (B) Garcia. Floral values are ordered by increasing stigma height. (C) Relationship between stigma height and mean stamen height for all flowers of both morphs in the Garcia population.
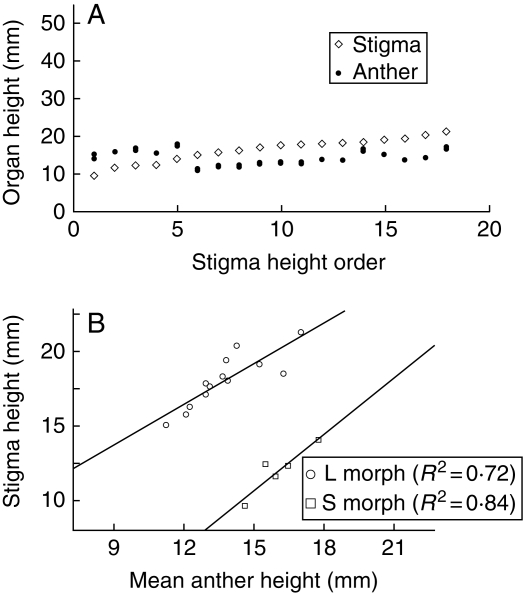
Fig. 4.
(A) Variation in the position of the stigmas and every anther in the flowers of N. binata. Floral values are ordered by increasing stigma height. (B) Relationship between stigma height and mean stamen height for each flower of the two morphs.
Fig. 5.
(A) Variation in the position of the stigmas and every anther in the flowers of N. corymbosa. Floral values are ordered by increasing stigma height. (B) Relationship between stigma height and mean stamen height for each flower of the two morphs.
The results show that N. inaequalis has higher and more dispersed stamens and stigmas than N. argentea (Figs 2 and 3). A larger dispersion is in part due to the fact that in N. inaequalis one of the three stamens is clearly placed under the level of the other two. When comparing stigma and mean stamen height for N. inaequalis (Fig. 2B) two facts can be readily noticed: (1) the high dispersion of the values; (2) their fitted lines are close to parallel (i.e. their regression coefficients are not statistically different, t = 1·92, P = 0·06), meaning that the height of stamens and stigmas varies isometrically in both morphs. This species presents high between-morph reciprocity, that is, a low R index (Table 3).
The two populations of N. argentea (Aasvoëlkraans and Garcia) are represented in Fig. 3. As previously stated, the population at Aasvoëlkraans is stigma-height dimorphic; while the population at Garcia is distylous, but the stigmas of the short-styled morph seem to be lacking reciprocal stamens, since the anthers of the long-styled morph are placed constantly above the level of the short-styled stigmas (Fig. 3B). The relationship between stigmas and stamens at the intra-flower level for the population with a large sample size (Garcia, Fig. 3C) shows a diverging pattern between the fitted lines of the morphs (i.e. statistically significant difference between the regression coefficients, t = 2·45, P = 0·02), reflecting an allometric behaviour of the sexual organ heights between morphs. This pattern reflects certain independence between the ontogenetic growth of stigmas and stamens, especially for the short morph where the fitted line is closer to horizontal. The reciprocity degree for this species is the lowest (the highest R-values) of the four species studied (Table 3).
Nivenia binata is distylous with a small separation between the heights of stamens of the short and long morphs (Fig. 4A), and a high reciprocity degree (low R-values; Table 3). The relationship between stigma and mean-stamen heights for both morphs results in two fitted lines close to parallel (i.e. their regression coefficients are not statistically different, t = −0·81, P = 0·43), indicating an isometric pattern of both morphs (Fig. 4B).
Nivenia corymbosa is the species with the two most clearly separated sexual whorls (Fig. 5A) and the highest degree of reciprocity between morphs (the lowest R-value; Table 3). In addition, when stigma height is compared with the mean height of the stamens for each flower (Fig. 5B), two clearly diverging fitted lines emerge, suggesting an allometric pattern (i.e. statistically significant difference between the regression coefficients, t = 5·70, P < 0·001). As for N. argentea at Garcia, this suggests different ontogenetic rates for stigmas and stamens, so an increase in the height of the stigmas in the long-morph is not followed by an increase of stamen height at the same rate but lower, and the opposite is true for the other floral morph.
With regard to perianth characteristics, there are between-morph differences in tube length for three species, N. inaequalis, N. argentea and N. corymbosa, of different sign. The two former have larger tubes in the short-morph, while the latter has significantly shorter tubes in the short-morph. Tepal limb length varies between morphs but only in the population of N. argentea at Garcia (Table 2).
Anthers and pollen grains are slightly bigger in the short-styled flowers for all species (Table 2). In relation to pollen grain production, the anthers of both morphs produce a similar amount of pollen grains in each population, either healthy or aborted (Table 2).
The morph ratio of all polymorphic populations was isoplethic, i.e. 50 % of each morph in the population (χ2 test, P ranging between 0·06 (N. binata) and 0·55 (N. inaequalis)).
Pollen transfer within populations
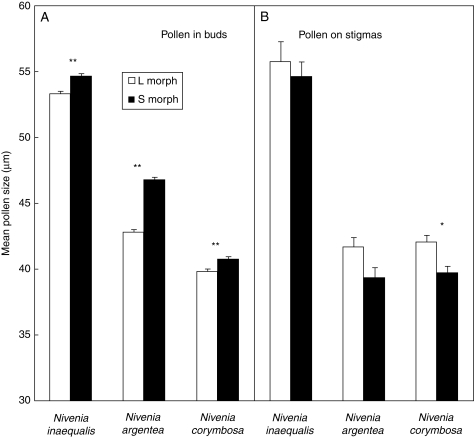
Pollen transfer in the distylous populations of Nivenia analysed is mainly disassortative: pollen grains on stigmas of the long-styled flowers are on average larger and on the short-styled flowers are smaller (Fig. 6), although the difference is statistically significant only for N. corymbosa (t = 2·90, d.f. = 156, P = 0·004).
Fig. 6.
Comparison between the mean (± s.e.) sizes of the pollen grains within the floral buds (A) and the pollen grains on the stigmas (B). Note that the between-morph relationships for the pollen on the stigmas are the inverse to those in the anthers (prior to pollen release) in all cases, suggesting a preferential dissortative (between morph) mating. Size measure is the diameter of the polar axis; between-morph differences were tested with t-tests (*P < 0·01, **P < 0·001).
At the population level, no correlation has been found between reciprocity and the two components of the female fitness studied, i.e. number of flowers pollinated or mean pollen load found on stigmas of pollinated flowers (rs = 0·1, rs = 0·2, respectively, P > 0·05; Table 3).
The results showed a relationship between morph and presence/absence of pollen only for N. inaequalis, where long-styled flowers seemed to be more efficient in capturing pollen. Significant differences were not found in any of the other species studied (Table 4).
Table 4.
Number of flowers with and without pollen on the stigma for the five dimorphic populations
|
N. inaequalis |
N. argentea Aasvoëlkrans |
N. argentea Garcia |
N. binata |
N. corymbosa |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Morph | L | S | L | S | L | S | L | S | L | S |
| Present/absent | 33/18 | 11/37 | 5/1 | 1/3 | 37/21 | 28/10 | 6/6 | 1/4 | 23/12 | 27/26 |
| χ2 | 17·49** | 3·40n.s. | 1·03n.s. | 1·31n.s. | 1·88n.s. | |||||
The association between morph and pollen presence was tested with chi-square tests (n.s. not significant; ** P < 0·001).
Phenotypic integration
The average value of flower phenotypic integration for the populations was 46·7 %. Variation among populations, species and morphs was high (range: 32·9 % in the short-styled morph of N. argentea Garcia to 52·2 % in the long-styled morph of N. corymbosa). Morphs have similar values in N. inaequalis and N. corymbosa, but short-styled morph of N. argentea (at Garcia) had a much lower integration than the long-styled morph (see Table 3). The monomorphic N. fruticosa presented high integration values (50·1 %), close to the values of N. corymbosa.
Relationship between individual floral traits and pollination success
The results of the logistic regression analyses show that there is only a slight influence on the probability of having pollen on stigmas for the following cases: ‘stigma height’ of the long-styled flowers of N. inaequalis (R2 = 0·30, B of stigma height = −0·42, P = 0·05), ‘corolla tube diameter’ for the long morph of N. argentea (R2 = 0·19, B of tube diameter = 2·07, P = 0·02), and ‘corolla tube length’ for the short morph of N. corymbosa (R2 = 0·19, B of tube length = 1·11, P = 0·02). No significant effect was found for the remaining floral traits analysed.
DISCUSSION
Morphological variation
The comprehensive model explaining the evolution of heterostyly of Lloyd and Webb (1992a) assumes a transition from ancestral approach herkogamy through an intermediate stage of stigma-height dimorphism and then to reciprocal herkogamy (distyly). The absence of previous reports on the intermediate stage may stem mainly from the lack of detailed morphometric measurements in heterostylous groups, which in turn are necessary to get insight on the functional significance of these variations (e.g. Eckert and Barrett, 1994). Here a small genus, Nivenia, was selected as it contains both distylous and monomorphic species, and is thus appropriate for exploring morphological variations associated to heterostyly. Half of the species in the genus including four out of the seven species previously described as distylous were checked (Brown, 1933; Goldblatt and Bernhardt, 1990; Goldblatt, 1993, 1997; Manning and Goldblatt, 2007). Indeed, three (N. concinna, N. levynsiae and N. stokoei) out of the six unsampled species are approach herkogamous monomorphic according to taxonomic descriptions (Goldblatt, 1993), like the one here confirmed as monomorphic based on detailed measurements (N. fruticosa). Interestingly, among the four polymorphic species sampled, variation was found in the patterns of polymorphism, both at population and species levels, as a result of our detailed morphometric analysis. Stylar polymorphism is by definition a population trait, and taxonomic information based on limited sampling within populations may produce spurious results or ignore small but meaningful variations (see, for example, Barrett et al., 1996, 1997; Armbruster et al., 2006; Sánchez et al., 2008).
Broadly, the present data fit the morphometric description of these species provided by Goldblatt (1993) for all floral traits except in the case of N. argentea. This is not surprising since Goldblatt (1993) characterized N. argentea with data of specimens from the population at Rooiberg (also studied here, Table 1), but this population has been recently described as the new species N. inaequalis by Manning and Goldblatt (2007), splitting it off N. argentea mostly because the inequality of the stamens of the former. Therefore, the measurements of the flowers of both N. argentea and N. inaequalis, taken in the present study, fit the description of Manning and Goldblatt (2007). Nivenia argentea shows a greater variation since the two populations studied have different types of polymorphism, namely distyly at Garcia and stigma-height dimorphism at Aasvoëlkrans. As far as is known this is the first report of such variation within a single species, although it has been reported within some genera, e.g. Narcissus (Barrett et al., 1996) and Lithodora (Ferrero et al., 2009). It could be argued that the stigma-height dimorphism of the Aasvoëlkrans population is the result of their small population size, and that the array of phenotypes is a biased ‘sample’ of the general pattern of the species; in such a scenario stigma-height dimorphism would be a spurious pattern resulting from the random colonization by long-styled flowers of relatively higher long stamens and short styled flowers of shorter stamens (see Fig. 3B for the distylous distribution at Garcia). However, the probability of this randomly driven fixation has been discarded with the re-sampling simulation used: it can be concluded that the stigma-height dimorphism of the Aasvoëlkrans population is real and not an artefact. Moreover, this population, though small, shows an isoplethic morph ratio; both factors (dimorphism and isoplethy) seem to indicate that this population has achieved high levels of disassortative mating. It would be worth, however, exploring more populations to ascertain the extent of the variation within N. argentea.
The lack of differences in the ancillary floral traits except pollen size (Table 2) supports the idea that they are not so critically tightened to the sexual polymorphism, as has been found in many studies (Ganders, 1979; Dulberger, 1992).
Functional aspects
Reciprocity values turned out to be the highest, and equal, in distylous N. corymbosa and N. inaequalis. Close values of reciprocity have been obtained for the distylous N. binata. The two populations of N. argentea were clearly less reciprocal than N. inaequalis (Table 3). Surprisingly, the higher degree of reciprocity of N. inaequalis is largely due to the higher dispersion of its anther heights (see Fig. 2A), given the displacement of the shortest stamen with respect to the level of the other two (Manning and Goldblatt, 2007; and this study). For this species, it was checked (and discarded) that the value of reciprocity was different when each anther height was considered separately: if that were the case, some anthers could have different probability of between-morph outcrossing, and therefore a different performance as male. In contrast, all three anthers of N. argentea flowers are placed at the same level. The greater dispersion of the anthers in N. inaequalis makes it possible that all stigmas have at least some reciprocal anthers (i.e. anthers of the other morph) at its level. This distinctly different arrangement of the androecium of N. inaequalis within the genus could be the result of directional selection on the length of one of the stamens in order to increase the probability of delivering pollen to legitimate stigmas. A similar process has been described for Lithodora by Ferrero et al. (2009). Both morphs are equally good in receiving pollen, as in other truly distylous species (N. binata and N. corymbosa; Table 3).
The greater ontogenetic independence of styles and stamens in N. corymbosa and N. argentea at Garcia is noteworthy. It is hypothesized that such independence allows the sexual organs to respond differently to the selective pressures imposed by the pollinators, which is the main force driving the evolution of heterostyly according to the Lloyd and Webb (1992a) model. Indeed such a scenario could have driven N. corymbosa to higher values of reciprocity than the other distylous species (Table 3).
Pollen transfer within populations
Although the presence of a diallelic incompatibility system may determine by itself an isoplethic morph ratio (Charlesworth and Charlesworth, 1979; Heuch, 1979), the available data for Nivenia (Goldblatt and Bernhardt, 1990) are not conclusive. There is increasing evidence that heterostyly and heteromorphic incompatibility are not necessarily linked (Barrett et al., 1997; Pérez-Barrales et al., 2006; Ferrero et al., 2009). Based on controlled hand pollinations, Goldblatt and Bernhardt (1990) have reported that pollen tubes could grow along the styles of either morph, independently of the morph from where the pollen was coming, even self-pollen. Nonetheless, it has been shown that some heterostylous and stigma-height dimorphic species present a late-acting type of incompatibility system (Dulberger, 1964; Phillip and Schou, 1981; Sage et al., 1999). Nivenia could have a similar system as indicated by the results of Ornduff (1983) on N. corymbosa, but this still needs to be ascertained in order to know if it stands for the observed isoplethy in the genus. Since the size of pollen grains is different between morphs (Goldblatt, 1993; present data), at least it was possible to explore if the observed polymorphism is functional in promoting disassortative pollen transfer, irrespective of the mating system in the populations. Unfortunately those differences are small enough to prevent the source (morph) of each individual pollen grain being unequivocally ascertained, as it was the case in a number of other studies (for some examples, see Ganders, 1979). Therefore, it was only possible to have a rough estimate of the levels of disassortative pollen transfer by comparing the average pollen size on the stigmas of each morph. For the three distylous species analysed a higher disassortative than assortative pollen transfer was the rule (Fig. 6). This prevalence of disassortative crosses is to be expected according to the Lloyd and Webb (1992b) model for the selection and maintenance of distyly.
Goldblatt and Bernhardt (1990) and Goldblatt (1993) suggested that the maintenance of distyly in Nivenia is related to a peculiar and specialized pollination system by flies with very long mouth parts of the families Nemestrinidae (Moegistorhynchus) and Tabanidae (Philoliche), or by long-tongued anthophorine bees. Those features allow them to collect nectar, which is the main reward they are looking for in the Nivenia flowers. The interaction between long-tongued pollinators and long and narrow floral tubes has been suggested as the promoter of the evolution of heterostyly, since those traits are usually related to precise location of pollen grains on insect bodies (Lloyd and Webb, 1992a).
Floral phenotypic integration has also been suggested to reflect specialized pollination systems (in terms of fitting, rather than species identity; Berg, 1959, 1960; Armbruster et al., 1999; Pérez-Barrales et al., 2007). In accordance with this, all Nivenia species analysed presented high values of integration (32·9–52·2 %), compared with the data base examined by Ordano et al. (2008) (average of 21·5 % in their sample of 36 species). In addition, there seems to be a trend in the populations of Nivenia studied, since those having higher floral integration presented also higher values of reciprocity (see Table 3), which seems to indicate that specialized pollination can be related to the maintenance of the stylar polymorphism.
Evolutionary implications
In this work, it has been possible to demonstrate a wide variation in style polymorphism in Nivenia, even including a case of within-species differences and a type (stigma-height dimorphism) previously unreported for the genus. Goldblatt (1993) has suggested that heterostyly would be an ancestral condition to monomorphism, although based mainly on morphological data. However, loss of heterostyly usually results in a homostylous condition, i.e. stamens and stigmas at the same height (i.e. non-herkogamous), a process associated with reproductive assurance and selfing (e.g. Schoen et al., 1997; Mast et al., 2006; Barrett et al., 1989). Thus it is improbable that conspicuous approach herkogamous monomorphism in Nivenia has arisen as a derived condition from heterostyly (although, for an alternative view, see Mast et al., 2004). Moreover, our report of stigma-height dimorphism in N. argentea could fill a gap in the building-up of heterostyly within the genus. A robust molecular phylogeny, not yet available for Nivenia, is also needed to ascertain if the morphological variations found fit the transition stages suggested by the models of the evolution of heterostyly.
Whereas it was not possible to proceed further on the evolutionary history of stigma-height polymorphism, the data do provide valuable information supporting the functional role of reciprocal herkogamy on promoting between-morph pollen transfer (and therefore likely disassortative mating) leading to isoplethic morph ratios. Moreover, there is some indication that the interplay between a restrictive floral morphology (deep and narrow tubes) and pollinator type and behaviour may promote this disassortative mating, as the data on flower phenotypic integration seem to point out. It is worth noting that N. parviflora (not included in this study) has a generalist pollinator array (Goldblatt and Manning, 2006) and much shorter flower tubes. On the other hand, N. fruticosa, even though monomorphic, has long corolla tubes and high floral integration values, which probably indicates that such a specialized pollination is a necessary but not a sufficient condition for heterostyly to evolve. Although there are still many open questions, it has become clear that Nivenia constitutes an excellent model system for further prospective studies on the evolution and function of different flower morphologies, including variable reciprocal herkogamy and floral shapes, as well as their interaction with pollinators.
ACKNOWLEDGMENTS
The authors thank Dr Koos Roux, Curator of the Compton Herbarium for their help in locating populations of Nivenia, Dr John Manning, the Compton Herbarium at the Kirstenbosch National Botanical Garden, for his initial encouragement to study heterostyly in Nivenia, and Dr Jose M Matías for his statistical advice. This work was supported by the Spanish Dirección General de Investigación, Ciencia y Tecnología (DGICYT) (grant numbers BOS2003-07924-CO2-02 and CGL2006-13847-CO2-01-02) and the Xunta de Galicia (grant numbers PGIDT04PXIC31003PN and PGIDT05RF031001PR). The work of V. Ferrero was funded by the Spanish Ministerio de Educación y Ciencia through a PhD scholarship (grant number AP-2004-6394).
LITERATURE CITED
- Armbruster WS, Di Stilio VS, Tuxill JD, Flores TC, Velasquez Runk JL. Covariance and decoupling of floral and vegetative traits in nine neotropical plants: a re-evauation of Berg's correlation-pleiades concept. American Journal of Botany. 1999;86:39–55. [PubMed] [Google Scholar]
- Armbruster WS, Pérez-Barrales R, Arroyo J, Edwards ME, Vargas P. Three-dimensional reciprocity of floral morphs in wild flax (Linum suffruticosum): a new twist on heterostyly. New Phytologist. 2006;171:581–590. doi: 10.1111/j.1469-8137.2006.01749.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. Heterostylous genetic polymorphisms: model systems for evolutionary analysis. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992. pp. 1–29. [Google Scholar]
- Barrett SCH. The evolution of plant sexual diversity. Nature Reviews Genetics. 2002;3:274–284. doi: 10.1038/nrg776. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Cruzan MB. Incompatibility in heterostylous plants. In: Williams EG, Knox RB, Clarke AE, editors. Genetic control of self-incompatibility and reproductive development. Dordrecht: Kluwer Academic; 1994. pp. 189–219. [Google Scholar]
- Barrett SCH, Harder LD. The evolution of polymorphic sexual systems in daffodils (Narcissus) New Phytologist. 2005;165:45–53. doi: 10.1111/j.1469-8137.2004.01183.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Morgan MT, Husband BC. The dissolution of a complex polymorphism: the evolution of self-fertilization in tristylous Eichhornia paniculata (Pontederiaceae) Evolution. 1989;43:1398–1416. doi: 10.1111/j.1558-5646.1989.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Barrett SCH, Lloyd DG, Arroyo J. Stylar polymorphisms and the evolution of heterostyly. In: Lloyd DG, Barrett SCH, editors. Floral biology: studies on floral evolution in animal-pollinated plants. New York, NY: Chapman & Hall; 1996. pp. 339–376. [Google Scholar]
- Barrett SCH, Cole WW, Arroyo J, Cruzan MB, Lloyd DG. Sexual polymorphisms in Narcissus triandrus (Amaryllidaceae): is this species tristylous? Heredity. 1997;78:135–145. [Google Scholar]
- Barrett SCH, Jesson LK, Baker AM. The evolution and function of stylar polymorphisms in flowering plants. Annals of Botany. 2000;85:253–265. [Google Scholar]
- Berg RL. A general evolutionary principle underlying the origin of developmental homeostasis. American Naturalist. 1959;93:103–105. [Google Scholar]
- Berg RL. The ecological significance of correlation pleiades. Evolution. 1960;14:171–180. [Google Scholar]
- Brown NE. Nivenia, Vent. and Nivenia, R. Br. Transactions of the Royal Society of South Africa. 1933;13:175–195. [Google Scholar]
- Brys R, Jacquemyn H, Beeckman T. Morph-ratio variation, population size and female reproductive success in distylous Pulmonaria officinalis (Boraginaceae) Journal of Evolutionary Biology. 2008a;21:1281–1289. doi: 10.1111/j.1420-9101.2008.01569.x. [DOI] [PubMed] [Google Scholar]
- Brys R, Jacquemyn H, Hermy M, Beeckman T. Pollen deposition rates and the functioning of distyly in the perennial Pulmonaria officinalis (Boraginaceae) Plant Systematics and Evolution. 2008b;273:1–12. [Google Scholar]
- Cesaro AC, Thompson JD. Darwin's cross-promotion hypothesis and the evolution of stylar polymorphism. Ecology Letters. 2004;7:1209–1215. [Google Scholar]
- Charlesworth D, Charlesworth B. A model for the evolution of distyly. American Naturalist. 1979;114:467–498. [Google Scholar]
- Dafni A, Kevan PG, Husband BC. Practical pollination biology. Haifa, Israel: Institute of Evolution, University of Haifa; 2005. [Google Scholar]
- Darwin CR. The different forms of flowers on plants of the same species. London: John Murray; 1877. [Google Scholar]
- Dulberger R. Flower dimorphism and self-compatibility in Narcissus tazetta L. Evolution. 1964;18:361–363. [Google Scholar]
- Dulberger R. Floral polymorphisms and their functional significance in the heterostylous syndrome. In: Barrett SCH. ed. Evolution and function of heterostyly. 1992:41–84. Berlin: Springer-Verlag. [Google Scholar]
- Eckert CG, Barrett SCH. Tristyly, self-compatibility and floral variation in Decodon verticillatus (Lythraceae) Biological Journal of the Linnean Society. 1994;53:1–30. [Google Scholar]
- Faivre AE, McDade LA. Population-level variation in the expression of heterostyly in three species of Rubiaceae: does reciprocal placement of anthers and stigmas characterize heterostyly? American Journal of Botany. 2001;88:841–853. [PubMed] [Google Scholar]
- Fernandes A. Contribution á la connaissance de la génétique de l'hétérostilie chez le genre Narcissus L. I. Résultats de quelques croisements. Boletim da Sociedade Broteriana, Sér. 2. 1964;38:81–96. [Google Scholar]
- Ferrero V, Arroyo J, Vargas P, Navarro L. Evolutionary transitions of style polymorphism in Lithodora (Boraginaceae) Perspectives in Ecology, Evolution and Systematics. 2009;11:111–125. [Google Scholar]
- Ferrero V, Chapela I, Arroyo J, Navarro L. Reciprocal style polymorphisms are not so easily categorized: the case of heterostyly in Lithodora and Glandora (Boraginaceae) Plant Biology. 2011 doi: 10.1111/j.1438-8677.2009.00307.x. (in press). doi:10·1111/j.1438-8677·2009·00307.x. [DOI] [PubMed] [Google Scholar]
- Ganders FR. The biology of heterostyly. New Zealand Journal of Botany. 1979;17:607–635. [Google Scholar]
- Goldblatt P. Portland, OR: Timber Press; 1993. The woody Iridaceae: systematics, biology and evolution of Nivenia, Klattia and Witsenia. [Google Scholar]
- Goldblatt P. A new species of Nivenia (Iridaceae) Bothalia. 1997;27:101–103. [Google Scholar]
- Goldblatt P, Bernhardt P. Pollination biology of Nivenia (Iridaceae) and the presence of heterostylous self-compatibility. Israel Journal of Botany. 1990;39:93–111. [Google Scholar]
- Goldblatt P, Manning JC. Radiation of pollination systems in the Iridaceae of sub-Saharan Africa. Annals of Botany. 2006;97:317–344. doi: 10.1093/aob/mcj040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SW, Barrett SCH. Phylogenetic reconstruction of the evolution of stylar polymorphisms in Narcissus (Amaryllidaceae) American Journal of Botany. 2004;91:1007–1021. doi: 10.3732/ajb.91.7.1007. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Cerdá X, García MB, et al. Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. Journal of Evolutionary Biology. 2002;15:108–121. [Google Scholar]
- Heuch I. Equilibrium populations of heterostylous plants. Theoretical Population Biology. 1979;15:43–57. [Google Scholar]
- Lloyd DG, Webb CJ. The evolution of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992a. pp. 151–178. [Google Scholar]
- Lloyd DG, Webb CJ. The selection of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer-Verlag; 1992b. pp. 179–207. [Google Scholar]
- Manning JC, Goldblatt P. Nivenia argentea misunderstood and the new species Nivenia inaequalis (Nivenioideae) Bothalia. 2007;37:192–196. [Google Scholar]
- Mast AR, Feller DM, Kelso S, Conti E. Buzz-pollinated Dodecatheon originated from within the heterostylous Primula subgenus Auriculastrum (Primulaceae): a seven-region cpDNA phylogeny and its implications for floral evolution. American Journal of Botany. 2004;91:926–942. doi: 10.3732/ajb.91.6.926. [DOI] [PubMed] [Google Scholar]
- Mast AR, Kelso S, Conti E. Are any primroses (Primula) primitively monomorphic? New Phytologist. 2006;171:605–616. doi: 10.1111/j.1469-8137.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- Mulcahy DL. Heterostyly within Nivenia (Iridaceae) Britonia. 1965;17:349–51. [Google Scholar]
- Nishihiro J, Washitani I, Thomson JD, Thomson BA. Patterns and consequences of stigma height variation in natural population of a distylous plant, Primula sieboldii. Functional Ecology. 2000;14:502–512. [Google Scholar]
- Ordano M, Fornoni J, Boege K, Domínguez CA. The adaptive value of phenotypic floral integration. New Phytologist. 2008;179:1183–1192. doi: 10.1111/j.1469-8137.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- Ornduff R. Heterostyly in South African flowering plants: a conspectus. Journal of South African Botany. 1974;40:169–187. [Google Scholar]
- Ornduff R. Studies on the reproductive system of Nivenia corymbosa (Iridaceae), an apparently androdioecious species. Annals of the Missouri Botanical Garden. 1983;70:146–148. [Google Scholar]
- Pérez-Barrales R, Vargas P, Arroyo J. New evidence for the Darwinian hypothesis of heterostyly: breeding systems and pollinators in Narcissus sect. Apodanthi. New Phytologist. 2006;171:553–567. doi: 10.1111/j.1469-8137.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Barrales R, Arroyo J, Armbruster WS. Differences in pollinator faunas may generate geographic differences in floral morphology and integration in Narcissus papyraceus (Amaryllidaceae) Oikos. 2007;116:1904–1918. [Google Scholar]
- Philipp M, Schou O. An unusual heteromorphic incompatibility system: distyly, self-incompatibility, pollen load and fecundity in Anchusa officinalis (Boraginaceae) New Phytologist. 1981;89:693–703. [Google Scholar]
- Richards JH, Koptur S. Floral variation and distyly in Guetarda scabra (Rubiaceae) American Journal of Botany. 1993;80:31–40. [Google Scholar]
- Riveros M, Arroyo MTK, Humaña AM. An unusual kind of distyly in Quinchamalium chilense (Santalaceae) on Volcan Casablanca, Southern Chile. American Journal of Botany. 1987;74:313–320. [Google Scholar]
- Sage TL, Strumas F, Cole WW, Barrett SCH. Differential ovule development following self-and cross pollination: the basis of self-sterility in Narcissus triandrus (Amaryllidaceae) American Journal of Botany. 1999;86:855–870. [PubMed] [Google Scholar]
- Sánchez JM, Ferrero V, Navarro L. A new approach to the quantification of degree of reciprocity in distylous (sensu lato) plant populations. Annals of Botany. 2008;102:463–472. doi: 10.1093/aob/mcn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen DJ, Johnston MO, L'Heureux A-M, Marsolais JV. Evolutionary history of the mating system in Amsinckia (Boraginaceae) Evolution. 1997;51:1090–1099. doi: 10.1111/j.1558-5646.1997.tb03956.x. [DOI] [PubMed] [Google Scholar]
- Shore JS, Arbo MM, Fernández A. Breeding system variation, genetics and evolution in the Turneraceae. New Phytologist. 2006;171:539–551. doi: 10.1111/j.1469-8137.2006.01807.x. [DOI] [PubMed] [Google Scholar]
- Wagner GP. On the eigenvalue distribution of genetic and phenotypic dispersion matrices: evidences for a non-random organization of quantitative character variation. Journal of Mathematical Biology. 1984;21:77–95. [Google Scholar]
- Webb CJ, Lloyd DG. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany. 1986;24:163–178. [Google Scholar]
- Weller SG, Domínguez CA, Molina-Freaner FE, Fornoni J, LeBuhn G. The evolution of distyly from tristyly in populations of Oxalis alpina (Oxalidaceae) in the Sky Islands of the Sonoran Desert. American Journal of Botany. 2007;94:972–985. doi: 10.3732/ajb.94.6.972. [DOI] [PubMed] [Google Scholar]