Abstract
Background and Aims
Reproductive isolation is a mechanism that separates species, and is classified into two types: prezygotic and postzygotic. Inviability of hybrids, or hybrid lethality, is a type of postzygotic isolation and is observed in some plant species, including Nicotiana species. Previous work has shown that the Q chromosome, which belongs to the S subgenome of N. tabacum, encodes one or more genes leading to hybrid lethality in some crosses.
Methods
Interspecific crosses of eight wild species were conducted in section Suaveolentes (which consists of species restricted to Australasia and Africa) with the cultivated species Nicotiana tabacum. Hybrid seedlings were cultivated at 28, 34 or 36 °C, and PCR and chromosome analysis were performed.
Results and Conclusions
Seven of eight wild species produced inviable hybrids after crossing. Hybrid lethality, which was observed in all crosses at 28 °C, was Type II lethality, with the characteristic symptoms of browning of hypocotyl and roots; lethality was suppressed at elevated temperatures (34 or 36 °C). Furthermore, one or more genes on the Q chromosome of N. tabacum were absolutely responsible for hybrid lethality, suggesting that many species of section Suaveolentes share the same factor that triggers hybrid lethality by interaction with the genes on the Q chromosome. Exceptionally, only one wild species, N. fragrans, produced 100 % viable hybrids after crossing with N. tabacum, suggesting that N. fragrans has no factor triggering hybrid lethality.
Keywords: Hybrid lethality, interspecific cross, Nicotiana section Suaveolentes, Q chromosome, reproductive isolation, tobacco
INTRODUCTION
The genus Nicotiana includes 76 species classified into 13 sections (Knapp et al., 2004). Many researchers have attempted to reveal the origin and evolution of this complex genus. In particular, the origin of cultivated tobacco, Nicotiana tabacum, has been extensively studied and it has been well characterized by studies based on interspecific crosses (Lim et al., 2006), chloroplast and mitochondrial DNA (Gray et al., 1974; Bland et al., 1985), and chromosome painting (Lim et al., 2000). Furthermore, phylogenetic studies based on analysis of internal transcribed spacer (ITS) regions of rDNA (Chase et al., 2003), plastid genes (Aoki and Ito, 2000; Clarkson et al., 2004) and nuclear-encoded chloroplast-expressed glutamine synthetase (ncpGS; Clarkson et al., 2010) have been conducted using almost all species of the genus Nicotiana. Phylogenetic relationships have also been inferred by random amplified polymorphic DNA (RAPD) and amplified fragment length polymorphism (AFLP) analyses (Ren and Timko, 2001; Khan and Narayan, 2007), genome size (Leitch et al., 2008) and analysis of five low-copy nuclear genes other than ncpGS (Kelly et al., 2010). Because information on phylogenetic relationships has been accumulated from various angles, the genus Nicotiana can act as a model to understand the evolution of species.
Species of the genus Nicotiana are distributed mainly in the Americas and Australia. Section Suaveolentes includes 25 species restricted to Australasia and one African species, N. africana, which is the only known Nicotiana species in Africa (Knapp et al., 2004). These 26 species in section Suaveolentes are geographically isolated from the majority of species in other sections, most of which are distributed in the Americas. Recent studies based on ITS region, plastid genes and ncpGS have indicated that section Suaveolentes is a monophyletic group (Chase et al., 2003; Clarkson et al., 2004, 2010). However, the relationship among species of this section and its progenitors is less well understood and requires further explanation.
Reproductive isolation is a mechanism that separates species. It is considered to play a crucial role in the evolution of animals and plants. Reproductive isolation is divided into two types of barriers, namely prezygotic and postzygotic. In plants, a typical prezygotic barrier observed after pollination is pollen–pistil incongruity. Postzygotic barriers include seed abortion and, in F1 or later generations, weakness, inviability and sterility. Inviability of hybrids, often referred to as hybrid lethality, is observed in some plant species, including Nicotiana species (Yamada et al., 1999), rice (Ichitani et al., 2007), wheat (Chu et al., 2006) and Arabidopsis thaliana (Bomblies et al., 2007). In the genus Nicotiana, hybrid lethality is classified into four types based on surface symptoms as follows: Type I, browning of shoot apex and root tip; Type II, browning of hypocotyl and roots; Type III, yellowing of true leaves; and Type IV, formation of multiple shoots (Yamada et al., 1999).
Nicotiana tabacum, which belongs to section Nicotiana, has two subgenomes, S and T, which are similar to the genomes of its progenitors, N. sylvestris and N. tomentosiformis, respectively (Lim et al., 2000, 2006). According to the classical numbering system, each chromosome of N. tabacum is lettered alphabetically (A–Z, excluding X and Y); chromosomes A–L belong to the T subgenome and M–Z to the S subgenome. A complete set of 24 monosomic lines of N. tabacum (Haplo-A to Z) has been established in the genetic background of the cultivar ‘Red Russian’. They are classified mainly based on morphological characteristics and their missing chromosome has been assigned to the S or T subgenome based on observation of chromosome pairing in hybrids (2n – 1) from crosses of monosomic lines with N. sylvestris (Clausen and Cameron, 1944; Cameron, 1959). Monosomic lines are useful for locating genes on specific chromosomes and these lines, especially Haplo-Q, have been used in research on hybrid lethality (Tezuka and Marubashi, 2006a; Tezuka et al., 2007). Involvement of the Q chromosome in the S subgenome was also confirmed using progenitors of N. tabacum and Q-chromosome-specific DNA markers (Tezuka and Marubashi, 2006a).
Nicotiana suaveolens and N. debneyi, which both belong to section Suaveolentes, produce inviable hybrids after crosses with N. tabacum. Previously, using monosomic lines of N. tabacum, it was demonstrated that the Q chromosome, which belongs to the S subgenome of N. tabacum, encodes one or more genes leading to hybrid lethality in these crosses (Tezuka and Marubashi, 2006a; Tezuka et al., 2007). In addition to these two species, other species in section Suaveolentes, N. gossei, N. megalosiphon and N. africana, are reported to yield inviable hybrids after crosses with N. tabacum; however, hybrid lethality was not described in any detail (Tanaka, 1961; Gerstel et al., 1979). Only for N. africana is the H chromosome presumed to be responsible for hybrid lethality through crosses with monosomic lines of N. tabacum (Gerstel et al., 1979), as described in the Discussion. In the present study, study of hybrid lethality in crosses with N. tabacum was extended to another eight species of section Suaveolentes.
MATERIALS AND METHODS
Plant materials
Eight wild species of section Suaveolentes, namely Nicotiana africana (2n = 46), N. excelsior (2n = 38), N. fragrans (2n = 48), N. goodspeedii (2n = 40), N. gossei (2n = 36), N. maritima (2n = 32), N. megalosiphon (2n = 40) and N. velutina (2n = 32), were used in this study. These species were crossed with N. tabacum (2n = 48, SSTT) ‘Red Russian’ and ‘Samsun NN’ in both directions. Two other species of section Suaveolentes, N. suaveolens (2n = 32) and N. debneyi (2n = 48), were also crossed with N. tabacum ‘Red Russian’ as the male parent to collect hybrid seeds used as controls. N. tabacum monosomic lines Haplo-Q (2n = 47) and F1 progeny (2n = 47) derived from the cross Haplo-Q × N. tabacum ‘Samsun NN’, the latter identified by Q-chromosome-specific DNA markers (Tezuka et al., 2004), were used as the female parent in crosses with the other seven species. Two viable hybrids from the cross N. tabacum ‘Red Russian’ × N. debneyi obtained in a previous study (Tezuka and Marubashi, 2006b) were used to identify the presence or absence of Q-chromosome-specific DNA markers. All plants were cultivated in a greenhouse, except for N. africana, which was cultivated in a phytotron (natural lighting conditions, 26 °C).
Interspecific crosses
Flowers of plants used as female parents were emasculated 1 d before anthesis and pollinated with pollen of plants used as male parents. F1 seeds were soaked in 0·5 % gibberellic acid (GA3) solution for 30 min and sterilized with 5 % sodium hypochlorite for 15 min. The sterilized seeds were sown in Petri dishes (60 mm diameter, 17 mm deep) containing 8 mL of half-strength MS medium (Murashige and Skoog, 1962) supplemented with 1 % sucrose and 0·2 % Gelrite (pH 5·8), and were cultured at 28 °C under continuous illumination (approx. 150 µmol m−2 s−1).
Ovule culture was carried out as follows. Flowers of female parents were collected at 7–10 d after pollination (DAP) and their corolla, sepals and styles were removed. The ovaries were surface-sterilized with 70 % ethanol for 30 s and with 5 % sodium hypochlorite for 5 min. The ovary walls were peeled to expose the placentas with intact ovules. Fertilized and enlarged ovules were excised, placed in Petri dishes containing 8 mL of half-strength MS medium supplemented with 3 % sucrose and 0·8 % agar (pH 5·8), and cultured at 28 °C under continuous illumination.
Test-tube pollination in combination with ovule culture was carried out as previously described (Tezuka and Marubashi, 2004). Fertilized and enlarged ovules at 10–14 DAP were excised from placentas and cultured as described above.
Cultivation of hybrid seedlings
Hybrid seedlings were cultured at 28 °C under continuous illumination. Some seedlings were transferred to flat-bottomed test tubes (25 mm diameter, 100 mm length) that contained 10 mL of half-strength MS medium supplemented with 1 % sucrose and 0·2 % Gelrite (pH 5·8) immediately after germination and were cultured at 28, 34 or 36 °C under continuous illumination. Hybrid seedlings cultured at 34 or 36 °C for 30 d after germination (DAG) were transferred to 28 °C under continuous illumination. Hybrid seedlings surviving for more than 30 d after transfer were potted and cultivated in a greenhouse under natural lighting conditions.
PCR analysis
Total DNA was extracted from leaves of each plant using a cetyltrimethylammonium bromide (CTAB) method (Murray and Thompson, 1980). Q-chromosome-specific DNA markers, OPB-07870, OPB-131400, QCS1, QCS2, QCS3 and QCS4, were detected as previously described (Tezuka et al., 2004; Tezuka and Marubashi, 2006a). RAPD analysis was carried out using 20 random 10-mer oligonucleotide primers (Kit A; Operon Technologies, Inc., Alameda, CA, USA) as previously described (Tezuka et al., 2007).
Chromosome analysis
Root tips were pretreated with distilled water for 24 h at 4 °C and with 2 mm 8-hydroxyquinoline for 4 h at 18 °C, then fixed in ethanol/acetic acid (3 : 1) overnight to determine chromosome numbers. The root tips were hydrolysed in 1 m HCl for 8 min at 60 °C, stained with Schiff's reagent and squashed in 45 % acetic acid. The number of chromosomes in at least five root tip cells for each plant was counted under a light microscope (Eclipse E600; Nikon, Tokyo, Japan).
RESULTS
Production of hybrid seedlings between N. tabacum and eight species of section Suaveolentes
Reciprocal crosses were carried out between N. tabacum and eight species of section Suaveolentes using conventional cross-pollination. Two cultivars of N. tabacum, ‘Red Russian’ and ‘Samsun NN’, were used for the crosses. The results of the crosses are shown in Table 1. In general, conventional crossing was more successful in crosses using N. tabacum as the male parent than crosses in the opposite direction; i.e. good seeds that could germinate were obtained when six species, N. excelsior, N. goodspeedii, N. gossei, N. maritima, N. megalosiphon and N. velutina, were pollinated by N. tabacum. Conversely, flowers of N. tabacum dropped at about 7 DAP and seeds were never obtained when pollinated by N. goodspeedii, N. maritima or N. velutina. This suggests that fertilization did not occur in these crosses. When N. excelsior, N. gossei or N. megalosiphon were used as the male parent in crosses with N. tabacum, many N. tabacum flowers dropped at about 7 DAP, but some flowers produced a few capsules containing seeds. These seeds germinated poorly, suggesting that there was some kind of postzygotic barrier during seed development as well as prezygotic barriers preventing fertilization. Conventional crosses using two other species, N. africana and N. fragrans, showed different tendencies from the crosses mentioned above for the other six species of section Suaveolentes. Seeds could be obtained with comparative ease and the percentage of seed germination was relatively high in reciprocal crosses between N. africana and N. tabacum. In crosses using N. fragrans, seeds could be obtained in all cross combinations except the cross using ‘Red Russian’ as the female parent. Only hybrid seeds from the cross ‘Samsun NN’ × N. fragrans germinated.
Table 1.
Conventional crossing between N. tabacum and eight species of section Suaveolentes
| Cross combination | No. of flowers pollinated | No. of capsules obtained | No. of seeds sown | No. of hybrids obtained |
|---|---|---|---|---|
| N. africana × ‘Red Russian’ | 8 | 4 | 161 | 134 |
| ‘Red Russian’ × N. africana | 20 | 10 | 200 | 141 |
| N. africana × ‘Samsun NN’ | 3 | 3 | 120 | 69 |
| ‘Samsun NN’ × N. africana | 9 | 7 | 118 | 103 |
| N. excelsior × ‘Red Russian’ | 10 | 6 | 140 | 123 |
| ‘Red Russian’ × N. excelsior | 14 | 3 | 120 | 2 |
| N. excelsior × ‘Samsun NN’ | 5 | 4 | 120 | 108 |
| ‘Samsun NN’ × N. excelsior | 4 | 4 | 119 | 0 |
| N. fragrans × ‘Red Russian’ | 2 | 2 | 119 | 0 |
| ‘Red Russian’ × N. fragrans | 9 | 0 | – | – |
| N. fragrans × ‘Samsun NN’ | 1 | 1 | 120 | 0 |
| ‘Samsun NN’ × N. fragrans | 7 | 5 | 120 | 100 |
| N. goodspeedii × ‘Red Russian’ | 20 | 19 | 198 | 168 |
| ‘Red Russian’ × N. goodspeedii | 20 | 0 | – | – |
| N. goodspeedii × ‘Samsun NN’ | 20 | 19 | 116 | 113 |
| ‘Samsun NN’ × N. goodspeedii | 20 | 0 | – | – |
| N. gossei × ‘Red Russian’ | 20 | 20 | 199 | 196 |
| ‘Red Russian’ × N. gossei | 27 | 2 | 198 | 6 |
| N. gossei × ‘Samsun NN’ | 21 | 15 | 115 | 93 |
| ‘Samsun NN’ × N. gossei | 20 | 1 | 120 | 26 |
| N. maritima × ‘Red Russian’ | 8 | 6 | 150 | 142 |
| ‘Red Russian’ × N. maritima | 20 | 0 | – | – |
| N. maritima × ‘Samsun NN’ | 6 | 6 | 120 | 117 |
| ‘Samsun NN’ × N. maritima | 20 | 0 | – | – |
| N. megalosiphon × ‘Red Russian’ | 20 | 20 | 195 | 176 |
| ‘Red Russian’ × N. megalosiphon | 20 | 3 | 313 | 0 |
| N. megalosiphon × ‘Samsun NN’ | 20 | 17 | 119 | 84 |
| ‘Samsun NN’ × N. megalosiphon | 9 | 6 | 117 | 2 |
| N. velutina × ‘Red Russian’ | 20 | 17 | 159 | 123 |
| ‘Red Russian’ × N. velutina | 20 | 0 | – | – |
| N. velutina × ‘Samsun NN’ | 11 | 10 | 120 | 109 |
| ‘Samsun NN’ × N. velutina | 20 | 0 | – | – |
Ovule culture is effective in bypassing ovule abortion in some interspecific crosses (Reed and Collins, 1978; Chung et al., 1988). Ovule culture was thus carried out in two crosses that yielded few hybrid seedlings from seeds obtained through conventional crossing, i.e. N. tabacum × N. excelsior and N. tabacum × N. megalosiphon. When conventional crossing and crosses using ovule culture were compared, the percentage of total hybrid seedlings obtained from the cultured seeds or ovules increased from the 0·8 % of conventional crossing to 3·5 % in the cross N. tabacum × N. excelsior (Tables 1 and 2). Ovule culture also improved the results from the cross ‘Red Russian’ × N. megalosiphon, for which no hybrid seedlings were obtained through conventional crossing.
Table 2.
Production of hybrid seedlings between N. tabacum and two species of section Suaveolentes through ovule culture
| Cross combination | Days after pollination | No. of ovaries used | No. of ovules cultured | No. of hybrids obtained |
|---|---|---|---|---|
| ‘Red Russian’ × N. excelsior | 7 | 1 | 489 | 6 |
| 8 | 1 | 362 | 12 | |
| 9 | 1 | 512 | 34 | |
| 10 | 1 | 422 | 17 | |
| ‘Samsun NN’ × N. excelsior | 7 | 1 | 724 | 2 |
| 8 | 1 | 758 | 16 | |
| 9 | 1 | 387 | 42 | |
| 10 | 1 | 888 | 28 | |
| ‘Red Russian’ × N. megalosiphon | 7 | 1 | 504 | 1 |
| 8 | 1 | 438 | 1 | |
| 9 | 1 | 322 | 1 | |
| 10 | 1 | 418 | 6 | |
| ‘Samsun NN’ × N. megalosiphon | 7 | 1 | 358 | 0 |
| 8 | 1 | 944 | 2 | |
| 9 | 1 | 803 | 3 | |
| 10 | 2 | 1413 | 7 |
Hybrid lethality is observed in most crosses between N. tabacum and species of section Suaveolentes
Hybrid seedlings obtained from crosses between N. tabacum and species of section Suaveolentes died at 28 °C, except from crosses with N. fragrans (Table 3). The day of appearance of first symptoms and of death varied depending on the parental species and cultivars used for the cross. Characteristic symptoms of hybrid lethality – obvious browning of hypocotyl and roots – were observed in all crosses using seven species of section Suaveolentes (Table 3, Fig. 1). These symptoms were identical to those in crosses between N. suaveolens or N. debneyi and N. tabacum, indicating that hybrid lethality in crosses between N. tabacum and these seven species of section Suaveolentes is of Type II.
Table 3.
Viability of hybrid seedlings between N. tabacum and eight species of section Suaveolentes at 28 °C
| No. of hybrids |
||||
|---|---|---|---|---|
| Cross combination | No. of hybrids cultured | Viable | Inviable | Lethality type* |
| N. africana × ‘Red Russian’ | 114 | 0 | 114 | II |
| ‘Red Russian’ × N. africana | 115 | 0 | 115 | II |
| N. africana × ‘Samsun NN’ | 69 | 1 | 68 | II |
| ‘Samsun NN’ × N. africana | 103 | 0 | 103 | II |
| N. excelsior × ‘Red Russian’ | 113 | 0 | 113 | II |
| ‘Red Russian’ × N. excelsior | 61 | 0 | 61 | II |
| N. excelsior × ‘Samsun NN’ | 108 | 0 | 108 | II |
| ‘Samsun NN’ × N. excelsior | 78 | 0 | 78 | II |
| ‘Samsun NN’ × N. fragrans | 100 | 100 | 0 | – |
| N. goodspeedii × ‘Red Russian’ | 158 | 0 | 158 | II |
| N. goodspeedii × ‘Samsun NN’ | 113 | 0 | 113 | II |
| N. gossei × ‘Red Russian’ | 186 | 0 | 186 | II |
| ‘Red Russian’ × N. gossei | 3 | 0 | 3 | II |
| N. gossei × ‘Samsun NN’ | 93 | 0 | 93 | II |
| ‘Samsun NN’ × N. gossei | 20 | 0 | 20 | II |
| N. maritima × ‘Red Russian’ | 132 | 0 | 132 | II |
| N. maritima × ‘Samsun NN’ | 117 | 0 | 117 | II |
| N. megalosiphon × ‘Red Russian’ | 166 | 1 | 165 | II |
| ‘Red Russian’ × N. megalosiphon | 4 | 0 | 4 | II |
| N. megalosiphon × ‘Samsun NN’ | 84 | 0 | 84 | II |
| ‘Samsun NN’ × N. megalosiphon | 6 | 0 | 6 | II |
| N. velutina × ‘Red Russian’ | 113 | 0 | 113 | II |
| N. velutina × ‘Samsun NN’ | 109 | 0 | 109 | II |
*Type I, browning of shoot apex and root tip; Type II, browning of hypocotyl and roots; Type III, yellowing of true leaves; Type IV, formation of multiple shoots (Yamada et al., 1999).
Fig. 1.
Observation of the characteristic early symptoms of hybrid lethality, browning of hypocotyl and roots, in hybrid seedlings at 28 °C. Hybrid seedlings between each species of section Suaveolentes and N. tabacum ‘Red Russian’ were photographed. (A) Hybrids of N. africana at 5 DAG; (B) hybrids of N. excelsior at 10 DAG; (C) hybrids of N. maritima at 10 DAG.
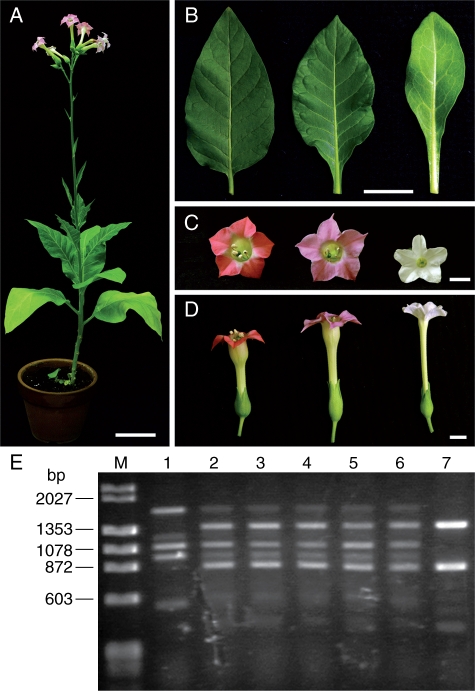
Among the eight tested species of section Suaveolentes, only N. fragrans yielded 100 % viable hybrid seedlings in the cross with N. tabacum. All hybrid seedlings from the cross N. tabacum × N. fragrans were still viable at 30 DAG at 28 °C (Table 3). Twenty hybrid seedlings were selected at random and cultivated in a greenhouse. These seedlings grew to maturity and flowered (Fig. 2A). The morphological characteristics of hybrid plants were uniform. The leaf shape and flower shape of hybrid plants were intermediate in appearance between those of parents (Fig. 2B–D). Five hybrid plants were analysed and all had 48 chromosomes, which is the sum of the number of haploid chromosomes of the parents. RAPD analysis was carried out with 20 random primers on five hybrid plants to confirm that these plants were true hybrids. All 20 random primers gave RAPD patterns showing clear polymorphisms between the parents; 32 bands were detected only in ‘Samsun NN’ and 41 bands were detected only in N. fragrans. Hybrid plants had all 73 bands characteristic of both parents, indicating that they were true hybrids. RAPD patterns obtained with the primer OPA-19 are shown in Fig. 2E. Hybrid plants from the cross N. tabacum × N. fragrans were self-sterile and cross-sterile with their parents.
Fig. 2.
Hybrids from the cross N. tabacum ‘Samsun NN’ × N. fragrans. (A) Shape of a hybrid plant that has grown to maturity and flowered. (B) Leaves of ‘Samsun NN’, a hybrid plant and N. fragrans (left to right). (C, D) Flowers of ‘Samsun NN’, a hybrid plant and N. fragrans (left to right). Scale bars = 5 cm (A, B) and 1 cm (C, D). (E) Confirmation of hybrid formation by RAPD analysis. M, DNA markers (λ/Hind III and φX174/Hae III). Lane 1, ‘Samsun NN’; lanes 2–6, hybrid plants; lane 7, N. fragrans.
Hybrid lethality is suppressed at elevated temperatures
Hybrid lethality in crosses of three species, N. debneyi, N. suaveolens and N. gossei, with N. tabacum was reported to be suppressed at 34, 36 and 37 °C, respectively (Mino et al., 2002; Yamada and Marubashi, 2003; Tezuka et al., 2007). Whether hybrid lethality in crosses between N. tabacum and the seven species of section Suaveolentes, N. africana, N. excelsior, N. goodspeedii, N. gossei, N. maritima, N. megalosiphon and N. velutina, is also suppressed at elevated temperatures was assessed. Hybrid lethality in all crosses was completely suppressed at 34 °C for 30 DAG except in crosses using N. africana as a parent (Table 4). Hybrid seedlings from reciprocal crosses between N. africana and N. tabacum did not die but the base of the stem frequently turned brown at 34 °C at 30 DAG. Hybrid lethality in these crosses was completely suppressed for 30 DAG by culture at 36 °C (Table 4).
Table 4.
Effects of elevated temperatures on hybrid lethality observed in hybrid seedlings between N. tabacum and seven species of section Suaveolentes
| No. of hybrids showing lethal symptoms |
||||
|---|---|---|---|---|
| Cross combination | Temperature (°C) | No. of hybrids cultured | By 30 DAG | After transfer to 28 °C* |
| N. africana × ‘Red Russian’ | 34 | 10 | 6 | 4 |
| 36 | 10 | 0 | 10 | |
| ‘Red Russian’ × N. africana | 34 | 16 | 16 | – |
| 36 | 10 | 0 | 10 | |
| N. excelsior × ‘Red Russian’ | 34 | 10 | 0 | 10 |
| ‘Red Russian’ × N. excelsior | 34 | 10 | 0 | 10 |
| ‘Samsun NN’ × N. excelsior | 34 | 10 | 0 | 10 |
| N. goodspeedii × ‘Red Russian’ | 34 | 10 | 0 | 10 |
| N. gossei × ‘Red Russian’ | 34 | 10 | 0 | 10 |
| ‘Red Russian’ × N. gossei | 34 | 3 | 0 | 3 |
| ‘Samsun NN’ × N. gossei | 34 | 6 | 0 | 6 |
| N. maritima × ‘Red Russian’ | 34 | 10 | 0 | 10 |
| N. megalosiphon × ‘Red Russian’ | 34 | 10 | 0 | 10 |
| ‘Red Russian’ × N. megalosiphon | 34 | 5 | 0 | 5 |
| ‘Samsun NN’ × N. megalosiphon | 34 | 8 | 0 | 8 |
| N. velutina × ‘Red Russian’ | 34 | 10 | 0 | 10 |
*Hybrid seedlings that did not show lethal symptoms at 30 DAG were transferred from the elevated temperature to 28 °C.
Hybrid seedlings cultured at 34 or 36 °C were transferred to 28 °C to confirm that hybrid lethality was induced at 28 °C. Hybrid seedlings from all crosses died after transfer (Table 4).
Production of hybrid seedlings between N. tabacum monosomic lines lacking the Q chromosome and seven species of section Suaveolentes
Next, two monosomic lines of N. tabacum lacking the Q chromosome, namely Haplo-Q and F1 progeny derived from the cross Haplo-Q × N. tabacum ‘Samsun NN’ (Tezuka et al., 2004), were used for crosses with seven species of section Suaveolentes, N. africana, N. excelsior, N. goodspeedii, N. gossei, N. maritima, N. megalosiphon and N. velutina. Monosomic lines were used as female parents for the crosses, as pollen of Haplo-Q aborts at a high frequency (Cameron, 1959) and the transmission of the monosomic condition through pollen is very low (Olmo, 1935). Methods to produce hybrid seedlings were determined based on results of crosses using disomic N. tabacum (Tables 1 and 2). Conventional crossing was conducted for crosses using N. africana or N. gossei. Ovule culture was carried out after conventional crossing using N. excelsior or N. megalosiphon. For N. goodspeedii, N. maritima and N. velutina, as mentioned above, fertilization did not occur on pollination by N. tabacum. In such cases, hybrid seedlings could be obtained by test-tube pollination in combination with ovule culture (Tezuka and Marubashi, 2004; Tezuka et al., 2007). Thus, test-tube pollination and ovule culture were carried out in crosses of N. tabacum to these three species. As a result, hybrid seedlings between monosomic lines of N. tabacum and all seven species of section Suaveolentes were obtained (Tables 5–7). Hybrid seedlings were cultured at 34 or 36 °C, temperatures at which hybrid lethality was suppressed (Table 4).
Table 5.
Production of hybrid seedlings in conventional crosses between monosomic lines of N. tabacum lacking the Q chromosome and two species of section Suaveolentes
| Cross combination | No. of flowers pollinated | No. of capsules obtained | No. of seeds sown | No. of hybrids obtained |
|---|---|---|---|---|
| Haplo-Q × N. africana | 7 | 3 | 80 | 72 |
| (Haplo-Q × ‘Samsun NN’) × N. africana | 1 | 1 | 80 | 72 |
| Haplo-Q × N. gossei | 18 | 4 | 259 | 12 |
| (Haplo-Q × ‘Samsun NN’) × N. gossei | 27 | 21 | 196 | 30 |
Table 6.
Production of hybrid seedlings between monosomic lines of N. tabacum lacking the Q chromosome and two species of section Suaveolentes by ovule culture
| Cross combination | Days after pollination | No. of ovaries used | No. of ovules cultured | No. of hybrids obtained |
|---|---|---|---|---|
| (Haplo-Q × ‘Samsun NN’) × N. excelsior | 8 | 3 | 253 | 27 |
| 9 | 1 | 59 | 16 | |
| (Haplo-Q × ‘Samsun NN’) × N. megalosiphon | 7 | 1 | 605 | 23 |
| 8 | 2 | 922 | 19 | |
| 9 | 1 | 898 | 54 | |
| 10 | 2 | 795 | 47 |
Table 7.
Production of hybrid seedlings between monosomic lines of N. tabacum lacking the Q chromosome and three species of section Suaveolentes by test-tube pollination and ovule culture
| Cross combination | No. of placentas pollinated | No. of ovules cultured | No. of hybrids obtained |
|---|---|---|---|
| (Haplo-Q × ‘Samsun NN’) × N. goodspeedii | 14 | 565 | 37 |
| (Haplo-Q × ‘Samsun NN’) × N. maritima | 7 | 423 | 32 |
| (Haplo-Q × ‘Samsun NN’) × N. velutina | 48 | 576 | 8 |
The Q chromosome encodes gene(s) leading to hybrid lethality in all crosses between N. tabacum and seven species of section Suaveolentes
Two Q-chromosome-specific DNA markers, QCS2 and QCS3 (Tezuka et al., 2004; Tezuka and Marubashi, 2006a), were used to determine whether the Q chromosome was present in hybrid seedlings between monosomic lines of N. tabacum and seven species of section Suaveolentes. Hybrid seedlings from respective crosses were divided into two types, hybrids possessing the Q chromosome and those lacking the Q chromosome, as summarized in Table 8. Some ovules yielded primary roots without shoots. These hybrids were excluded from analysis as genomic DNA could not be isolated.
Table 8.
Relationship between the Q chromosome and hybrid lethality observed in crosses between monosomic lines of N. tabacum lacking the Q chromosome and seven species of section Suaveolentes
| No. of hybrids |
||||
|---|---|---|---|---|
| Cross combination | DNA markers* | Total | Viable | Inviable |
| Haplo-Q × N. africana | + | 16 | 0 | 16 |
| − | 56 | 56 | 0 | |
| (Haplo-Q × ‘Samsun NN’) × N. africana | + | 13 | 0 | 13 |
| − | 59 | 59 | 0 | |
| (Haplo-Q × ‘Samsun NN’) × N. excelsior | + | 5 | 0 | 5 |
| − | 19 | 19 | 0 | |
| (Haplo-Q × ‘Samsun NN’) × N. goodspeedii | + | 12 | 0 | 12 |
| − | 18 | 18 | 0 | |
| Haplo-Q × N. gossei | + | 4 | 0 | 4 |
| − | 8 | 8 | 0 | |
| (Haplo-Q × ‘Samsun NN’) × N. gossei | + | 2 | 0 | 2 |
| − | 28 | 28 | 0 | |
| (Haplo-Q × ‘Samsun NN’) × N. maritima | + | 10 | 0 | 10 |
| − | 21 | 21 | 0 | |
| (Haplo-Q × ‘Samsun NN’) × N. megalosiphon | + | 7 | 0 | 7 |
| − | 125 | 125 | 0 | |
| (Haplo-Q × ‘Samsun NN’) × N. velutina | + | 3 | 0 | 3 |
| − | 5 | 5 | 0 | |
*DNA markers used were QCS2 and QCS3 (Tezuka and Marubashi, 2004, 2006a). A ‘ + ’ indicates that Q-chromosome-specific DNA markers were detected and a ‘–’ indicates that they were not.
If the Q chromosome is responsible for hybrid lethality, hybrid seedlings possessing the Q chromosome will die and those lacking the Q chromosome will live at 28 °C. To verify this assumption, hybrid seedlings cultured at 34 or 36 °C were transferred to 28 °C. In all crosses, hybrid seedlings possessing the Q chromosome died and those lacking the Q chromosome survived (Table 8). The hybrid seedlings lacking the Q chromosome grew to maturity and flowered without symptoms of hybrid lethality when they were potted and cultivated in a greenhouse. Hybrid plants were morphologically uniform within each cross combination and were intermediate between those of the parents.
Analysis of rare viable hybrids obtained from crosses with disomic N. tabacum
One of 68 hybrid seedlings from the cross N. africana × N. tabacum ‘Samsun NN’ and one of 166 hybrid seedlings from the cross N. megalosiphon × N. tabacum ‘Red Russian’ were viable at 28 °C (Table 3). When these two viable hybrids were potted and cultivated in a greenhouse, they grew to maturity and flowered. Hybrid plants were morphologically intermediate between those of the parents. The hybrid plant from the cross N. africana × N. tabacum had 46 chromosomes, which is less than the 47 chromosomes expected for a true hybrid. The hybrid plant from the cross N. megalosiphon × N. tabacum had 44 chromosomes, which is the sum of the number of haploid chromosomes of the parents.
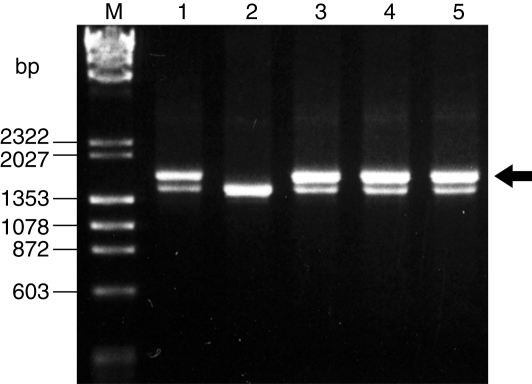
Whether the Q chromosome was present in the hybrid plant from the cross N. africana × N. tabacum and from the cross N. megalosiphon × N. tabacum was investigated. In addition, two viable hybrids from the cross N. tabacum ‘Red Russian’ × N. debneyi (Tezuka and Marubashi, 2006b), for which hybrid seedlings are usually inviable, were investigated. When six Q-chromosome-specific DNA markers, OPB-07870, OPB-131400, QCS1, QCS2, QCS3 and QCS4, were tested, all markers were detected in hybrid plants from crosses N. megalosiphon × N. tabacum and N. tabacum × N. debneyi. Conversely, none of the markers was detected in the hybrid plant from the cross N. africana × N. tabacum (Fig. 3).
Fig. 3.
Detection of the marker QCS2 in viable hybrid seedlings from crosses between wild species of section Suaveolentes and N. tabacum. The marker QCS2, indicated by an arrow, was not detected in hybrid seedlings from the cross N. africana × N. tabacum. M, DNA markers (λ/Hind III and φX174/Hae III). Lane 1, N. tabacum ‘Red Russian’; lane 2, hybrid seedling from the cross N. africana × N. tabacum ‘Samsun NN’; lane 3, hybrid seedling from the cross N. megalosiphon × N. tabacum ‘Red Russian’; lanes 4, 5, hybrid seedlings from the cross N. tabacum ‘Red Russian’ × N. debneyi (Tezuka and Marubashi, 2006b).
DISCUSSION
Among the eight species of section Suaveolentes used in this study, seven yielded inviable hybrids after crosses with N. tabacum. In reciprocal crosses of N. tabacum with four species, N. africana, N. excelsior, N. gossei and N. megalosiphon, inviable hybrids could be obtained in both directions through conventional crossing or ovule culture. Conversely, in crosses of N. tabacum with N. goodspeedii, N. maritima or N. velutina, inviable hybrids were obtained when these wild species were used as the female parent, whereas no hybrids were obtained in crosses using them as the male parent. However, we confirmed that hybrid lethality was observed in hybrids between N. tabacum and these three wild species through crosses using monosomic lines of N. tabacum lacking the Q chromosome. Hybrid lethality was observed in reciprocal crosses between these seven wild species and N. tabacum, suggesting that hybrid lethality is due to the interaction of coexisting heterologous genomes, and not to a cytoplasmic effect.
Hybrid lethality observed in crosses between N. tabacum and seven species of section Suaveolentes was of Type II, for which characteristic symptoms are browning of hypocotyl and roots. The present observation that hybrid lethality in these crosses was suppressed at 34 or 36 °C supported the suggestion that Type II lethality in all crosses might be suppressed at elevated temperatures by Yamada et al. (1999). In the present study, hybrid lethality in reciprocal crosses between N. tabacum and N. africana was not completely suppressed at 34 °C, whereas hybrid lethality in other crosses was completely suppressed at this temperature. Although the mechanism of suppression of hybrid lethality at elevated temperatures is not clear, it may be common for each cross. It is interesting that more elevated temperatures than 34 °C were required to suppress hybrid lethality only in crosses using N. africana as the parent. Hamada and Inoue (1999) also reported that hybrid lethality in the cross between N. tabacum and N. africana was suppressed at 36 °C but not at 32 °C.
In crosses between monosomic lines of N. tabacum lacking the Q chromosome and seven species of section Suaveolentes, hybrid seedlings possessing the Q chromosome were inviable and those lacking the Q chromosome were viable at 28 °C. Therefore, we concluded that the Q chromosome, which belongs to the S subgenome in N. tabacum, encodes gene(s) leading to hybrid lethality in crosses with seven species of section Suaveolentes. This is the same conclusion as that arrived at in crosses between N. tabacum and two species of section Suaveolentes, N. suaveolens and N. debneyi (Tezuka and Marubashi, 2006a; Tezuka et al., 2007). For N. africana, however, other interesting results were reported; when N. africana was crossed with all 24 monosomic lines of N. tabacum (Haplo-A to Z), only Haplo-H produced a great number of viable hybrids (Gerstel et al., 1979). Based on these results, the H chromosome, which belongs to the T subgenome in N. tabacum, is considered to be related to hybrid lethality. As it was not clear whether the viable hybrids from the cross Haplo-H × N. africana lacked the H chromosome in their study, further studies will be needed to verify H chromosome involvement in hybrid lethality.
Programmed cell death (PCD) accompanied by apoptotic features is observed in Type II lethality in crosses of N. suaveolens or N. debneyi with N. tabacum (Yamada et al., 2000; Marubashi and Kobayashi, 2002; Tezuka and Marubashi, 2004, 2006b). In these crosses, gene(s) on the Q chromosome cause hybrid lethality (Tezuka and Marubashi, 2006a; Tezuka et al., 2007). Hybrid lethality observed in crosses between seven species of section Suaveolentes and N. tabacum was also Type II and was caused by the interaction of one or more genes on the Q chromosome and some factor in wild species. Considering the common points, hybrid lethality observed in crosses using the seven section Suaveolentes species would also be expected to involve PCD accompanied by apoptotic features. However, a different type of PCD is observed in hybrid lethality in the cross N. gossei × N. tabacum (Mino et al., 2005). PCD in this cross is accompanied by vacuolar collapse and apoptotic features are never observed. Therefore, these observations suggest that, in hybrid lethality caused by the interaction of a gene on the Q chromosome and an interacting factor in wild species, (1) both PCD pathways are present within a cross combination or (2) a particular type of PCD is involved depending on the cross combination.
Section Suaveolentes is a monophyletic group in analyses of the ITS region, plastid genes and ncpGS (Chase et al., 2003; Clarkson et al., 2004, 2010). This section is considered to have originated from a single polyploid event some 10 Mya, followed by speciation to produce the species known today (Leitch et al., 2008). Furthermore, two progenitors of the section are proposed: N. sylvestris would be the maternal progenitor and section Trigonophyllae would be the paternal progenitor (Clarkson et al., 2010). However, the data obtained from these analyses were insufficient to reveal the relationship among species of section Suaveolentes and its progenitors, as incongruously genomic DNA of N. sylvestris failed to show hybridization with species from section Suaveolentes in genomic in situ hybridization experiments (Chase et al., 2003). Hybrid lethality, a type of reproductive isolation, might be useful for the classification of section Suaveolentes species. Based on the results of the present study and previous studies (Tezuka and Marubashi, 2006a; Tezuka et al., 2007), ten species of section Suaveolentes can be divided into two groups: species yielding inviable hybrids and species yielding viable hybrids on crosses with N. tabacum. The former group consists of nine species: N. africana, N. debneyi, N. excelsior, N. goodspeedii, N. gossei, N. maritima, N. megalosiphon, N. suaveolens and N. velutina. This group may be further divided into two subgroups based on type of PCD observed, as mentioned above. The latter group consists of only one species, N. fragrans, which is distributed in islands of the South Pacific. N. fragrans is geographically isolated from Australian species and is extremely far from N. suaveolens, which approximates the morphological mean of section Suaveolentes species (Wheeler, 1945).
It is interesting how and why only N. fragrans yields 100 % viable hybrids in crosses with N. tabacum. F1 hybrids or allopolyploids with genomes from more than one species present the nucleus with a totally different situation. This can result in an irreversible burst of reorganization and modification of the genomes involved, leading to stability of F1 hybrids or allopolyploids (Jones and Hegarty, 2009). Because both N. fragrans and N. tabacum have 48 chromosomes and are polyploids, such reorganization and modification might be related to the production of 100 % viable hybrids. However, N. debneyi, which also has 48 chromosomes, yielded inviable hybrids after crosses with N. tabacum (Marubashi and Kobayashi, 2002; Tezuka and Marubashi, 2006b; Tezuka et al., 2007). Alternatively, N. fragrans may not possess any factor triggering hybrid lethality by interaction with gene(s) on the Q chromosome. Considering that the common factor triggering hybrid lethality is widely distributed in species of section Suaveolentes and the presumed single origin of the section as mentioned above, an ancestral species involved in the formation of the section might already have had the factor triggering hybrid lethality. The factor might have been lost through speciation, probably including reorganization and modification of the genomes, leading to the formation of N. fragrans. We are currently carrying out studies on hybrid lethality using species in sections other than Suaveolentes. These studies will provide additional results to clarify how section Suaveolentes evolved.
Many hybrid seedlings lacking the Q chromosome were obtained compared with those possessing the Q chromosome in certain crosses between monosomic lines of N. tabacum and species of section Suaveolentes. For example, seven hybrids possessed the Q chromosome and 125 hybrids lacked the Q chromosome in crosses using N. megalosiphon. A small number of hybrids were obtained from crosses between N. tabacum disomic and N. megalosiphon through conventional crossing or ovule culture. These results suggest that hybrid lethality observed in seedlings is also likely to be involved in embryonic development after fertilization. However, this idea is not applicable to crosses using N. africana. In the cross N. tabacum × N. africana, hybrid seeds germinated well and many hybrid seedlings were obtained. Because these hybrid seedlings were considered to have the Q chromosome, hybrid lethality is quite unlikely to be involved in embryonic development in this case. Therefore, it seems that n – 1 gametes lacking the Q chromosome are more likely to be fertilized than n gametes in this interspecific cross using monosomic lines of N. tabacum lacking the Q chromosome.
A few viable hybrids are occasionally obtained from several hundred hybrid seedlings that are usually inviable in some interspecific crosses in the genus Nicotiana (Tezuka and Marubashi, 2006b). A viable hybrid from the cross N. africana × N. tabacum and another from the cross N. megalosiphon × N. tabacum are apparent examples of such infrequent viable hybrids. Previously, it was shown that PCD related to hybrid lethality does not occur in viable hybrids of N. tabacum × N. debneyi (Tezuka and Marubashi, 2006b). However, the reason why PCD does not occur in viable hybrids is still unclear. The present study has shown that a viable hybrid from the cross N. africana × N. tabacum lacked the Q chromosome. Therefore, deletion of the chromosome encoding genes leading to hybrid lethality contributes to some of the cases in which a few viable hybrids are occasionally obtained.
Climate change, including climate warming, can affect ecosystems (Thomas et al., 2004). As revealed in our present and previous studies (Yamada et al., 1999; Marubashi and Kobayashi, 2002), hybrid lethality in interspecific crosses in the genus Nicotiana is temperature sensitive. In other plant taxa such as Gossypium (Phillips, 1977) and Arabidopsis thaliana (Bomblies et al., 2007), hybrid lethality could be suppressed at higher temperatures than typical temperatures for their growth. Therefore, climate warming could produce hybrid-lethality-suppressed hybrids from species now separated by hybrid lethality. If such hybrids produce progeny, they may ultimately give rise to new species. Special attention might be needed for changes in ecosystems triggering such a mechanism breaking down hybrid lethality.
ACKNOWLEDGEMENTS
We thank Japan Tobacco Inc., Iwata, Japan, for providing seeds of cultivated and wild species of the genus Nicotiana and Dr T. Kubo, a former director of the Iwata tobacco experiment station of Japan Tobacco Inc., for the gift of Haplo-Q. We also thank two anonymous referees for their constructive comments. This work was partly supported by Grants-in-Aid for Scientific Research (A) No. 13306003 and (C) No. 15580003 from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a Grant-in-Aid for Young Scientists (Start-up) No. 20880024 from the Japan Society for the Promotion of Science, Japan.
LITERATURE CITED
- Aoki S, Ito M. Molecular phylogeny of Nicotiana (Solanaceae) based on the nucleotide sequence of the matK gene. Plant Biology. 2000;2:316–324. [Google Scholar]
- Bland MM, Matzinger DF, Levings CS. Comparison of the mitochondrial genome of Nicotiana tabacum with its progenitor species. Theoretical and Applied Genetics. 1985;69:535–541. doi: 10.1007/BF00251100. [DOI] [PubMed] [Google Scholar]
- Bomblies K, Lempe J, Epple P, et al. Autoimmune response as a mechanism for a Dobzhansky–Muller-type incompatibility syndrome in plants. PLoS Biology. 2007;5:e236. doi: 10.1371/journal.pbio.0050236. doi:10.1371/journal.pbio.0050236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DR. The monosomics of Nicotiana tabacum. Tobacco Science. 1959;3:164–166. [Google Scholar]
- Chase MW, Knapp S, Cox AV, et al. Molecular systematics, GISH and the origin of hybrid taxa in Nicotiana (Solanaceae) Annals of Botany. 2003;92:107–127. doi: 10.1093/aob/mcg087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C-G, Faris JD, Friesen TL, Xu SS. Molecular mapping of hybrid necrosis genes Ne1 and Ne2 in hexaploid wheat using microsatellite markers. Theoretical and Applied Genetics. 2006;112:1374–1381. doi: 10.1007/s00122-006-0239-9. [DOI] [PubMed] [Google Scholar]
- Chung CS, Nakajima T, Takeda G. Interspecific hybridization between Nicotiana trigonophylla Dun. and N. tabacum L. through ovule culture. Japanese Journal of Breeding. 1988;38:319–326. [Google Scholar]
- Clarkson JJ, Knapp S, Garcia VF, Olmstead RG, Leitch AR, Chase MW. Phylogenetic relationships in Nicotiana (Solanaceae) inferred from multiple plastid DNA regions. Molecular Phylogenetics and Evolution. 2004;33:75–90. doi: 10.1016/j.ympev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Clarkson JJ, Kelly LJ, Leitch AR, Knapp S, Chase MW. Nuclear glutamine synthetase evolution in Nicotiana: phylogenetics and the origins of allotetraploid and homoploid (diploid) hybrids. Molecular Phylogenetics and Evolution. 2010;55:99–112. doi: 10.1016/j.ympev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Clausen RE, Cameron DR. Inheritance in Nicotiana tabacum. XVIII. Monosomic analysis. Genetics. 1944;29:447–477. doi: 10.1093/genetics/29.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstel DU, Burns JA, Burk LG. Interspecific hybridizations with an African tobacco, Nicotiana africana Merxm. Journal of Heredity. 1979;70:342–344. [Google Scholar]
- Gray JC, Kung SD, Wildman SG, Sheen SJ. Origin of Nicotiana tabacum L. detected by polypeptide composition of Fraction I protein. Nature. 1974;252:226–227. doi: 10.1038/252226a0. [DOI] [PubMed] [Google Scholar]
- Hamada K, Inoue M. Effect of high temperature on overcoming inviability in interspecific hybrids between Nicotiana tabacum L. and N. africana Merxm. Kinki Journal of Crop Science and Breeding. 1999;44:13–16. (in Japanese with English summary) [Google Scholar]
- Ichitani K, Namigoshi K, Sato M, et al. Fine mapping and allelic dosage effect of Hwc1, a complementary hybrid weakness gene in rice. Theoretical and Applied Genetics. 2007;114:1407–1415. doi: 10.1007/s00122-007-0526-0. [DOI] [PubMed] [Google Scholar]
- Jones RN, Hegarty M. Order out of chaos in the hybrid plant nucleus. Cytogenetic and Genome Research. 2009;126:376–389. doi: 10.1159/000266171. [DOI] [PubMed] [Google Scholar]
- Kelly LJ, Leitch AR, Clarkson JJ, Hunter RB, Knapp S, Chase MW. Intragenic recombination events and evidence for hybrid speciation in Nicotiana (Solanaceae) Molecular Biology and Evolution. 2010;27:781–799. doi: 10.1093/molbev/msp267. [DOI] [PubMed] [Google Scholar]
- Khan MQ, Narayan RKJ. Phylogenetic diversity and relationships among species of genus Nicotiana using RAPDs analysis. African Journal of Biotechnology. 2007;6:148–162. [Google Scholar]
- Knapp S, Chase MW, Clarkson JJ. Nomenclatural changes and a new sectional classification in Nicotiana (Solanaceae) Taxon. 2004;53:73–82. [Google Scholar]
- Leitch IJ, Hanson L, Lim KY, et al. The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae) Annals of Botany. 2008;101:805–814. doi: 10.1093/aob/mcm326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KY, Matyášek R, Lichtenstein CP, Leitch AR. Molecular cytogenetic analyses and phylogenetic studies in the Nicotiana section Tomentosae. Chromosoma. 2000;109:245–258. doi: 10.1007/s004120000074. [DOI] [PubMed] [Google Scholar]
- Lim KY, Souckova-Skalicka K, Sarasan V, et al. A genetic appraisal of a new synthetic Nicotiana tabacum (Solanaceae) and the Kostoff synthetic tobacco. American Journal of Botany. 2006;93:875–883. doi: 10.3732/ajb.93.6.875. [DOI] [PubMed] [Google Scholar]
- Marubashi W, Kobayashi M. Temperature-dependent apoptosis detected in hybrids between Nicotiana debneyi and N. tabacum expressing lethality. Plant Biotechnology. 2002;19:267–270. [Google Scholar]
- Mino M, Maekawa K, Ogawa K, Yamagishi H, Inoue M. Cell death process during expression of hybrid lethality in interspecific F1 hybrid between Nicotiana gossei Domin and Nicotiana tabacum. Plant Physiology. 2002;130:1776–1787. doi: 10.1104/pp.006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino M, Misaka Y, Ueda J, Ogawa K, Inoue M. Hybrid lethality of cultured cells of an interspecific F1 hybrid of Nicotiana gossei Domin and N. tabacum L. Plant Cell Reports. 2005;24:179–188. doi: 10.1007/s00299-005-0923-2. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmo HP. Genetical studies of monosomic types of Nicotiana tabacum. Genetics. 1935;20:286–300. doi: 10.1093/genetics/20.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LL. Interspecific incompatibility in Gossypium. IV. Temperature-conditional lethality in hybrids of G. klotzschianum. American Journal of Botany. 1977;64:914–915. [Google Scholar]
- Reed SM, Collins GB. Interspecific hybrids in Nicotiana through in vitro culture of fertilized ovules. Journal of Heredity. 1978;69:311–315. [Google Scholar]
- Ren N, Timko MP. AFLP analysis of genetic polymorphism and evolutionary relationships among cultivated and wild Nicotiana species. Genome. 2001;44:559–571. [PubMed] [Google Scholar]
- Tanaka M. The effect of irradiated pollen grains on species crosses of Nicotiana. Bulletin of the Hatano Tobacco Experiment Station. 1961;51:1–38. (in Japanese with English summary) [Google Scholar]
- Tezuka T, Marubashi W. Apoptotic cell death observed during the expression of hybrid lethality in interspecific hybrids between Nicotiana tabacum and N. suaveolens. Breeding Science. 2004;54:59–66. [Google Scholar]
- Tezuka T, Marubashi W. Hybrid lethality in interspecific hybrids between Nicotiana tabacum and N. suaveolens: evidence that the Q chromosome causes hybrid lethality based on Q-chromosome-specific DNA markers. Theoretical and Applied Genetics. 2006a;112:1172–1178. doi: 10.1007/s00122-006-0219-0. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Marubashi W. Genomic factors lead to programmed cell death during hybrid lethality in interspecific hybrids between Nicotiana tabacum and N. debneyi. SABRAO Journal of Breeding and Genetics. 2006b;38:69–81. [Google Scholar]
- Tezuka T, Onosato K, Hijishita S, Marubashi W. Development of Q-chromosome-specific DNA markers in tobacco and their use for identification of a tobacco monosomic line. Plant and Cell Physiology. 2004;45:1863–1869. doi: 10.1093/pcp/pch204. [DOI] [PubMed] [Google Scholar]
- Tezuka T, Kuboyama T, Matsuda T, Marubashi W. Possible involvement of genes on the Q chromosome of Nicotiana tabacum in expression of hybrid lethality and programmed cell death during interspecific hybridization to Nicotiana debneyi. Planta. 2007;226:753–764. doi: 10.1007/s00425-007-0522-2. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Cameron A, Green RE, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Wheeler H-M. A contribution to the cytology of the Australian–South Pacific species of Nicotiana. Proceedings of the National Academy of Sciences of the USA. 1945;31:177–185. doi: 10.1073/pnas.31.7.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Marubashi W. Overproduced ethylene causes programmed cell death leading to temperature-sensitive lethality in hybrid seedlings from the cross Nicotiana suaveolens × N. tabacum. Planta. 2003;217:690–698. doi: 10.1007/s00425-003-1035-2. [DOI] [PubMed] [Google Scholar]
- Yamada T, Marubashi W, Niwa M. Detection of four lethality types in interspecific crosses among Nicotiana species through the use of three rescue methods for lethality. Breeding Science. 1999;49:203–210. [Google Scholar]
- Yamada T, Marubashi W, Niwa M. Apoptotic cell death induces temperature-sensitive lethality in hybrid seedlings and calli derived from the cross of Nicotiana suaveolens × N. tabacum. Planta. 2000;211:614–622. doi: 10.1007/s004250000329. [DOI] [PubMed] [Google Scholar]