Abstract
Background and Aims
Monoecious plants have the capacity to allocate resources separately to male and female functions more easily than hermaphrodites. This can be advantageous against environmental stresses such as leaf herbivory. However, studies showing effects of herbivory on male and female functions and on the interaction with the plant's pollinators are limited, particularly in tropical plants. Here, the effects of experimental defoliation were examined in the monoecious shrub Croton suberosus (Euphorbiaceae), a wasp-pollinated species from a Mexican tropical dry forest.
Methods
Three defoliation treatments were applied: 0 % (control), 25 % (low) or 75 % (high) of plant leaf area removed. Vegetative (production of new leaves) and reproductive (pistillate and staminate flower production, pollen viability, nectar production, fruit set, and seed set) performance variables, and the abundance and activity of floral visitors were examined.
Key Results
Defoliated plants overcompensated for tissue loss by producing more new leaves than control plants. Production of staminate flowers gradually decreased with increasing defoliation and the floral sex ratio (staminate : pistillate flowers) was drastically reduced in high-defoliation plants. In contrast, female reproductive performance (pistillate flower production, fruit set and seed set) and pollinator visitation and abundance were not impacted by defoliation.
Conclusions
The asymmetrical effects of defoliation on male and female traits of C. suberosus may be due to the temporal and spatial flexibility in the allocation of resources deployed by monoecious plants. We posit that this helps to maintain the plant's pollination success in the face of leaf herbivory stress.
Keywords: Euphorbiaceae, floral sex ratio, foliar herbivory, leaf production, nectar production, Neotropical dry forest, plant–insect interactions, pollen production, pollination success, pollinator activity
INTRODUCTION
Herbivory may decrease plant photosynthates, and this can drastically influence vegetative and/or reproductive performance because, typically, plant resources are limited (Whitham et al., 1991; Marquis, 1992; Obeso, 2002; Hanley and Fegan, 2007). The impact of herbivory on plant reproduction varies among and within species and, on individual plants, it can affect male reproductive traits (e.g. pollen production or pollen performance), female reproductive traits (e.g. ovule production) or both, as well as secondary sexual characteristics (e.g. corolla size or nectar production; Quesada et al., 1995; Lehtilä and Strauss, 1999; Thomson et al., 2004; Avila-Sakar and Stephenson, 2006). In most cases, herbivory may have a direct effect on male or female reproductive output, affecting male or female gamete production (Quesada et al., 1995; Mutikainen and Delph, 1996; Mothershead and Marquis, 2000; Steets et al., 2006). In other cases, the effects on the reproductive output may by indirect, as the changes produced on floral traits may in turn influence the activity of pollinators (Strauss et al., 1996; Mothershead and Marquis, 2000; Ashman and Penet, 2007). Ultimately, the direct and indirect effects of herbivory on reproductive traits can have variable consequences for male and/or female fitness (Gronemeyer et al., 1997; Strauss et al., 2001; Penet et al., 2009).
Plants with monomorphic (e.g. monoecy or hermaphroditism) or dimorphic (e.g. dioecy, androdioecy or gynodioecy) breeding systems do not respond consistently to herbivory because of the markedly different male/female resource allocation pattern of each system (Charlesworth, 1999; Zhang, 2006). In gender-dimorphic plants, herbivores seem to preferentially attack male individuals (for reviews, see Ashman, 2002; Cornelissen and Stiling, 2005), and this strongly influences resource allocation, thus playing an important role in sexual-system evolution (Ashman, 2002; Verdú et al., 2004). The effects of herbivory on gender-monomorphic plants are more difficult to interpret because both male and female functions are on the same individual. Studies on the effects of herbivory on gender-monomorphic species show that it can affect specific components of male and/or female output (e.g. Gronemeyer et al., 1997; Lehtilä and Strauss, 1999; Paige et al., 2001; Strauss et al., 2001). However, although a well-defined pattern of the consequences of herbivory on male/female fitness has not emerged, most of our understanding is based on work with hermaphroditic species.
In contrast to hermaphroditic species, monoecious species can separate allocation to male and female functions more easily (Charlesworth and Morgan, 1991; de Jong et al., 2008), and thus the depletion of resources caused by folivory (leaf herbivory) may differentially influence male and female functions. In an influential review of the effect of herbivores in the evolution of sexual systems, Ashman (2002) found that foliar herbivory often causes a plastic shift towards maleness. In this context, sex allocation theory predicts that folivory should decrease preferentially female function because it is more expensive in terms of plant resources (Lloyd, 1979; Charlesworth and Morgan, 1991). Thus, it is known for example, that herbivory affects monoecious gymnosperms by increasing maleness (Whitham and Mopper, 1985; Snyder, 1993). In monoecious angiosperms, the expected decline of female function is not always observed, and the impact of herbivory on male and female output has been found to vary, depending on the species. For instance, in Cucurbita texana, leaf damage decreases the number of pistillate and staminate flowers, pollen grains per flower and pollen performance (Quesada et al., 1995). In another Cucurbit species, Cucumis sativus, continuous herbivory decreased the number of staminate flowers and fruit production (Thomson et al., 2003, 2004). In Cnidosculus aconitifolius, folivory decreases male flowers production, but the effect on the proportion of female to total flowers varies among sites (Parra-Tabla et al., 2004; Arceo-Gómez et al., 2009). Thus, a variety of factors must be at play in determining how folivory alters the relative allocation of resources to male and female functions. For instance, if pistillate and staminate flowers are produced at different positions within the inflorescence, one of the two types of flowers may receive a greater amount of resources than the other solely due to ‘architectural effects’ (Diggle, 2002). Furthermore, if pistillate flowers are produced earlier than staminate flowers, the latter may suffer a pre-emption of resources due to plastic responses in the reallocation of resources to develop fruits by pistillate flowers (Diggle, 2002; Diggle and Miller, 2004). Recently, the adaptive significance of differential resource allocation among sequentially blooming flowers in response to temporal variation in herbivory was highlighted in a study with a hermaphroditic species (Kliber and Eckert, 2004).
Despite the variety of studies conducted on the effects of foliar herbivory in monoecious plants, two crucial aspects have not yet been considered. In addition to the bias of studies with hermaphroditic and dioecious plant, studies have not generally been conducted under natural populations (but see Parra-Tabla et al., 2004; Arceo-Gómez et al., 2009) and this is critical for our understanding of the ecological and evolutionary significance of herbivory (Parmesan, 2000). Because the effect of herbivores on plant reproductive traits does not occur in isolation from other biotic agents (Hanley et al., 2009), and indeed may influence mutualistic relationships with pollinators (Mothershead and Marquis, 2000; Ashman and Penet, 2007; Irwin, 2009), studies need to improve realism by considering both herbivores and pollinators in their interaction with the plant. In this paper, the effects of experimental defoliation on vegetative and reproductive (male and female) performance of plants are examined in a natural population of Croton suberosus (Euphorbiaceae), a monoecious shrub occurring in tropical deciduous forests of Mexico. Although this plant is defoliated by caterpillars and grasshoppers (Dominguez et al., 1989; R. Dirzo and E. Narbona, unpubl. res.), it has a rare but effective defensive system against herbivores. The vespid wasp Polistes instabilis is rewarded by the floral nectar of C. suberosus and, in so doing, the wasps act as defenders because they kill herbivores that happen to be feeding on the plant's leaves (Domínguez et al., 1989). Recently, it has been demonstrated that, although C. suberosus plants are visited by a variety of insects, it is mainly pollinated by medium/large-size wasps, including, notably, P. instabilis (Narbona and Dirzo, 2010). The obligate interaction with insects for effective pollination, because C. suberosus has a monoecious sexual system, makes it especially interesting to investigate how herbivory can affect vegetative and reproductive traits and to examine its effects on the interaction with flower visitors.
Assuming that monoecy is a relatively flexible sexual system and that female function is more costly than male function (Lloyd, 1979; Freeman et al., 1981; Charlesworth and Morgan, 1991), it was hypothesized that foliar herbivory in C. suberosus would predominantly produce a decrease in female reproductive success (pistillate flower production, fruit set or seed set). Furthermore, if herbivory affects floral traits that influence pollinator attraction or effectiveness, it was predicted that this could be reflected in a decline in the abundance and activity of flower visitors and, consequently, on pollination success. Here, these issues are addressed by applying experimental defoliation to plants in the wild. Specifically, the following questions were posed. (a) Does defoliation differentially affect staminate and pistillate flower production, and therefore floral sex ratio? (b) Does defoliation alter reproductive traits (pollen production and viability, and nectar production), and pollination success (fruit set and seed set)? (c) Is there variation in insect visitation to staminate or pistillate flowers depending on defoliation treatments?
MATERIALS AND METHODS
Study species and study area
Croton suberosus Kunth is a deciduous shrub endemic to the south Pacific coast of Mexico (locally distributed in the states of Guerrero, Jalisco, Michoacan and Oaxaca states; Rzedowski, 1978; Domínguez et al., 1989). The plant produces its leaves during the rainy season, just before flowering time (July–August). Individuals usually have a basal branch which produces successive secondary branches. Dense racemose inflorescences are developed on the apex of branches. Croton suberosus develops acropetally with pistillate flowers at the base of the inflorescence and staminate flowers at the apex. Inflorescence anthesis duration is about 5 d for the pistillate phase and 17 d for the staminate phase (Domínguez and Bullock, 1989). Pistillate flowers are apetalous and have three large bifid styles. Staminate flowers have five white petals and 15 stamens (E. Narbona, pers. obs.). This species lacks extra-floral nectaries but the floral nectaries present in both types of flowers produce nectar during daylight hours (Domínguez et al., 1989; Narbona and Dirzo, 2010). Croton suberosus is a self-compatible species (Domínguez and Bullock, 1989). Fruits have an explosive dehiscence mechanism and contain three seeds that mature approx. 20 d after fertilization.
The study population is located in the Chamela Biological Station in Jalisco, Mexico (19°30′N, 105°03′W). The climate in the study site is characterized by a mean annual temperature of 24·6 °C and 852 mm of annual precipitation; however, rainfall is extremely seasonal, with most of the rains concentrated between June and October (García-Oliva et al., 2002). The predominant vegetation is seasonally dry tropical forest, in which the plants produce their new leaves just before the rainy season, and loose them by the end of season (Bullock and Solís-Magallanes, 1990). Insect herbivory in the forest is restricted to the rainy season, with a peak in July (Dirzo and Domínguez, 2002).
Defoliation experiment
Sixty reproductive individuals of C. suberosus that were similar in size (mean ± s.e. = 51·3 ± 5·7 cm height) were selected and tagged. Each plant was randomly assigned to one of three treatments: (1) 25 % defoliation (hereafter low defoliation), in which a quarter of the area from all leaves of the plant was removed; (2) 75 % defoliation (hereafter high defoliation), in which three-quarters of the leaf area of all leaves of the plant was removed; and (3) undamaged control. These percentages of leaf removed were chosen to reflect the natural levels of folivory of the non-flowering individuals in the study area. However, mean leaf area loss to insect herbivory on C. suberosus is typically low (<5 %; Dirzo and Domínguez, 2002) because P. instabilis seems to be highly effective in defending the flowering plants (Domínguez et al., 1989). Yet, individuals lacking inflorescences due to delayed flowering show high levels of defoliation (about 40 % of plants have >25 % leaf area defoliated and in some cases defoliation is complete; R. Dirzo and E. Narbona, unpubl. res.).
The defoliation treatments were performed by cutting off the designated leaf area with scissors, leaving the central midrib intact (as is typically observed in the field). Defoliation was conducted on 8 July 2006, just before the peak of abundance of foliar herbivores on the population of C. suberosus. Control plants that had appreciable levels of herbivory before the treatments were applied were discarded. At the same time, a larva of Hipercombe sp. (Lepidoptera), a common herbivore of C. suberosus (Domínguez et al., 1989), was placed on a leaf enclosed within a nylon bag to insure that chemical defences were produced upon herbivory if this is the case for this plant. To maintain the desired experimental levels of damage, plants of the three treatments were sprayed with a pyrethroid insecticide (Deltametrin, Agromundo, Mexico City, Mexico) to avoid any possible additional herbivory. Insecticide was applied 2 d after defoliation and again 2 weeks later. It was confirmed that the application of the insecticide had no effect on vegetative and reproductive development of the experimental plants (results not shown). Additionally, the new leaves produced by plants of the low- and high-defoliation treatments were defoliated twice at regular time intervals until the experiment was finished.
Variables measured
Vegetative growth performance was estimated as the production of new leaves. Overall number of leaves of each plant was counted upon application of the treatments and 28 d later, when the experiment was finished. A ‘leaf production index’ was calculated by subtracting the number of leaves present at the end of experiment from the number of leaves at the beginning and dividing this by the initial number of leaves. If treated plants show a leaf production index higher than those of the control plants, overcompensation for defoliation exists, whereas the reverse pattern would indicate undercompensation.
The first inflorescence bud developed by the plant after the application of the defoliation treatment was marked and monitored every 3–4 d during the flowering period. The mean number of inflorescences per plant in the flowering season was 3·2 ± 0·26 (median = 2), and this was not statistically different among treatments (F2 = 2·4, P = 0·101). One or two plants in each treatment produced no flowers before the experiment finished and were not considered for the analysis. The number of pistillate and staminate flowers which developed on each inflorescence was counted. The floral sex ratio of individuals was estimated as the ratio of the number of staminate to pistillate flowers of the inflorescence. This index has been usefully used in other monoecious species (Bickel and Freeman, 1993).
Pollen production was assessed in two staminate flowers from 11 or 12 randomly chosen plants of each treatment. Staminate flowers were always collected from the mid-position of the inflorescence. Well-developed flower buds were sampled and analysed in the laboratory. Each staminate flower develops 15 stamens arranged in three parallel rings. To avoid positional effects, the number of pollen grains per flower was estimated by counting the pollen of one stamen of each ring and extrapolating for the five stamens of the ring. Each stamen was dissected on a slide and all its pollen grains were counted under an optical microscope (×40). Additionally, to assess if the number of stamens per flower is constant regardless of treatment, the number of stamens of these flowers was counted.
Pollen viability was analysed in ten plants of each defoliation treatment. Two flowers from the mid-part of the inflorescence were collected from each plant, and one stamen of the middle part of the ring was analysed in each one. Anthers were bagged the day before recollection to prevent insect visitation and removal. Pollen viability was estimated by assessing peroxidase activity in pollen grains using the Fast DAB technique (3,3′-diaminobenzidine tablets; Sigma D 4168; Dafni et al., 2005). Pollen viability was scored under a microscope for all the grains in the anthers.
Nectar production was measured in 61 staminate flowers (25 of nine control plants, six of two low-defoliation plants and 30 of nine high-defoliation plants) and in 38 pistillate flowers (12 of five control plants, 10 of four low-defoliation plants and 16 of eight high-defoliation plants). Inflorescences with some well-developed buds were bagged in situ for 24 h using plastic bags to exclude insects. In the morning, the nectar volume of the five nectaries of the flowers was extracted with 5-μL capillary tubes and the sugar concentration was measured with a portable refractometer (range 0–32 ° Brix). The volume and concentration of nectar were used to estimate the weight of sugar produced per flower, following Cruden and Hermann (1983).
Pollination success was estimated by measuring fruit set as the proportion of pistillate flowers setting fruit, and seed set was estimated as the number of ovules setting seed in all the developed capsules of the inflorescence. Because of intense rains at the end of the flowering period, and accidental falling of tree branches, tags of two to four plants in each treatment were lost. Ovules of C. suberosus develop in the early stages of fertilization and may abort during later stages (E. Narbona, pers. obs.). To avoid bias in the measurements due to this problem, capsules were left on the plant until completely mature; the inflorescences were covered with a nylon bag just before maturation to prevent the loss of seeds.
Insect abundance and activity on flowers was observed in six plants of each treatment during 2 d of the pistillate flowering peak. Ten days later, at the staminate flowering peak, insect activity was observed in a set of six additional plants of each treatment during 2 d. On each census day, plants with a similar numbers of flowers in anthesis per inflorescence were selected. Random censuses of 15 min duration for the different herbivory treatments were carried out during the maximum activity of flower visitors (0900–1700 h; Domínguez et al., 1989; E. Narbona, pers. obs.). The total number of censuses was 101 for pistillate flowers (35 in control plants, 32 in low-defoliation plants and 34 in high-defoliation plants) and 83 for staminate flowers (30 in control plants, 27 in low-defoliation plants and 26 in high-defoliation plants). The sampling effort for each treatment accounted for a considerable proportion of the total species diversity (>78 %, based on the Incidence-based Coverage Estimator) in this population (Narbona and Dirzo, 2010). During each census, the following were recorded: (a) the insect species, (b) the number of visits of each taxon to the inflorescence, and (c) the duration of each visit. ‘Visitation rate’ was recorded as the number of visits per 15-min observation period (Fishbein and Venable, 1996), and ‘bout length’ as the time spent foraging on an inflorescence by an insect (Potts, 2005). Functional groups of flower visitors were considered as insects that interact with the flowers in a similar manner and having similar size and foraging behaviour (Wilson et al., 2004). In the calculation of visitation rates or duration, six functional insects groups were considered: wasps, bees, beetles, butterflies, flies and ants (Narbona and Dirzo, 2010). Reduviidae (Hemiptera) and Membracidae (Homoptera) were not included because they were casual visitors.
Statistical analyses
Generalized linear models (GLMs) were used to test the effects of defoliation treatments, and different link functions and error distributions were specified depending on the type of response variable modelled (Crawley, 2007); model selection was carried out using the Akaike's Information Criterion (Lindsey, 1997; Crawley, 2007). An identity link function and a gamma error distribution were specified for the evaluation of leaf production rate. A log link function and a Poisson error distribution were used to analyse production of pistillate, staminate and total flowers. Floral sex ratio was modelled using an inverse Gaussian distribution with a log link function. Fruit set, seed set and pollen viability were evaluated using a probit link function and binomial error structure. A log link function and a Poisson error distribution were used to analyse pollen production, in which the factor ‘plant’ was nested within ‘defoliation treatment’. A log link function and a gamma error distribution were used to compare sugar production between pistillate and staminate flowers. Differences in sugar production among treatments were modelled using a log link function and gamma error structure, in which the factor ‘plant’ was nested in ‘treatment’. Visitation rate of pistillate and staminate flowers were modelled using a Gaussian distribution with a log link function considering the factors ‘treatment’, ‘insect group’ and their interaction; data were square-root transformed. The same procedure was used to analyse duration of visits, but considering a gamma error distribution with a log link function of pistillate flowers and gamma distribution with a power link function. Comparisons of visitation rate and bout length between specific insect groups and defoliation treatments were performed by separate GLMs. To compare the insect visitor spectra among treatments, a generalized estimating equations analysis was used, considering the presence and absence of insect taxa as repeated measurements in the three treatments; binomial distribution of errors and logit link function were used.
Quasi-Poisson and quasi-binomial were used instead of Poisson and binomial error distributions to correct for data over-dispersion (Crawley, 2007), applying F-test for analysis of deviance performed with R version 2.5.0 (R Development Core Team, 2007). All other GLMs and generalized estimating equations were carried out using SPSS 15·0 (SPSS, Inc., Chicago, IL, USA). When the GLMs showed significant differences, the means of treatments were compared using t-tests based on the standard errors calculated from the specific model. To control for experiment-wise type I error produced by multiple comparisons (García, 2004), the sequential Bonferroni test was applied in the analysis of pistillate, staminate, total flowers and floral sex ratio, and in the visitation rate and bout length.
RESULTS
Effects of defoliation on leaf production
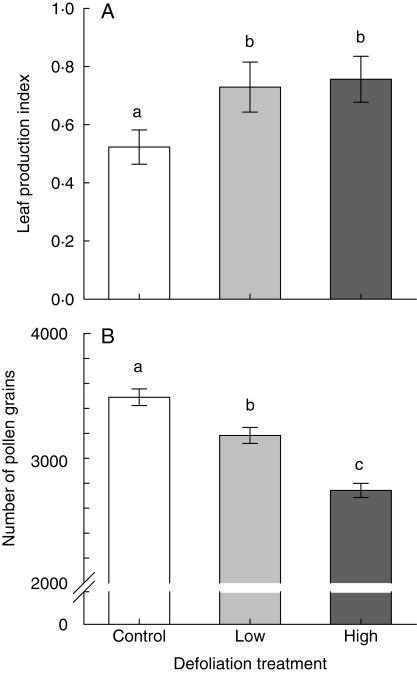
Leaf production index of experimental plants ranged between 0·20 and 0·92 for control plants, 0·18 and 1·35 for low-defoliation plants, and 0·11 and 1·57 for high-defoliation plants. On average, low- and high-defoliation plants had, respectively, 28·3 % and 30·8 % higher mean leaf production index than the control (Fig. 1A), and this difference was statistically significant (Wald χ22,51 = 7·00, P = 0·03).
Fig. 1.
The effect of defoliation treatments on leaf production rate (A) and on pollen production per flower (B) in plants of Croton suberosus. Values are back-transformed means ± s.e. Bars with different letters are significantly different at P < 0·05.
Effects of defoliation on pistillate and staminate flower production and sex ratio
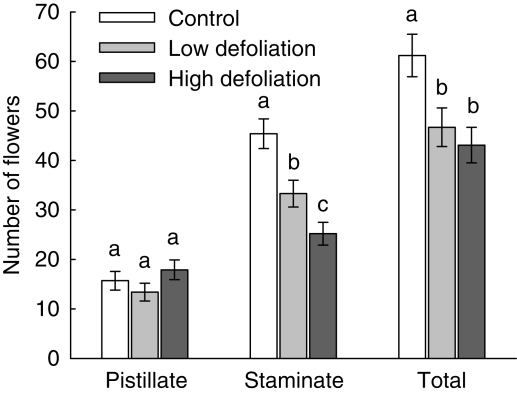
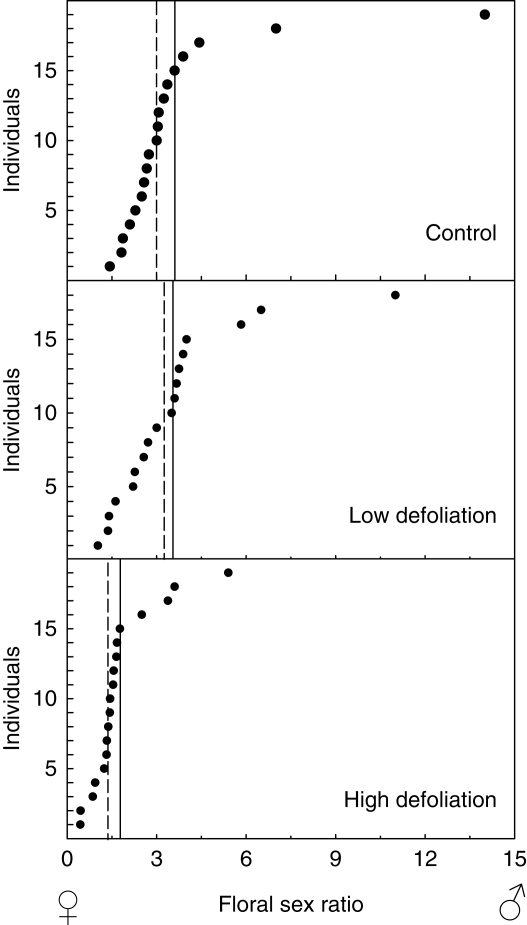
Pistillate flower production per inflorescence did not differ significantly across treatments (F2,54 = 2·81, P = 0·42; Fig. 2). In contrast, defoliation had a significant effect on the production of staminate flowers (F2,54 = 113·44, P < 0·0001; Fig. 2). Low- and high-defoliation plants bore, respectively, 26·7 % and 44·5 % fewer staminate flowers than the control plants (Fig. 2). Finally, total flowers per inflorescence was significantly different across treatments (F2,54 = 62·01, P < 0·01) and, in this case both low- and high-defoliation plants produced fewer flowers than control plants (Fig. 2). As a consequence, defoliation significantly affected the mean flower sex ratio (Wald χ22,54 = 17·31, P < 0·0001). Plants in the low-defoliation treatment had a reduced number of staminate and total flowers but this did not translate into differences in the floral sex ratio when compared with control plants (P = 0·94; Fig. 3). In contrast, as the decline in the number of staminate flowers was more pronounced in the high-defoliation treatment, the floral sex ratio was significantly reduced compared with the control (P = 0·004) and low-defoliation plants (P = 0·006; Fig. 3). Plants of the high-defoliation treatment showed a narrow range of values in comparison with the other two treatments, and four plants exhibited values smaller than 1 (Fig. 3), i.e. plants developed inflorescences with a smaller proportion of staminate flowers, thus maleness was reduced with high defoliation.
Fig. 2.
Production of pistillate and staminate flowers per inflorescence in plants of Croton suberosus under the three defoliation treatments. Values are back-transformed means ± s.e. Different letters indicate significant differences between treatments at P < 0·05. All comparisons remain significant at the sequential Bonferroni-corrected alpha level (0·05/3 = 0·017).
Fig. 3.
Floral sex ratio (staminate/pistillate flowers) of plants of Croton suberosus from the three defoliation treatments. Plants were ordered by the relative value. Horizontal continuous and dashed lines represent the mean and median, respectively.
Effects of defoliation on male reproductive traits
Leaf damage caused a significant reduction in the number of pollen grains produced per staminate flower (Wald χ22,66 = 1972·5, P < 0·001). The mean number of pollen grains per flower of low- and high-defoliation plants was 2742 and 3183, respectively, whereas that of the control plants was 3489 (Fig. 1B). Pollen production was not significantly different among plants within treatments (Wald χ22,34 = 94·1, P = 0·40). In contrast, all anthers analysed had high pollen viability: mean ± s.e. = 97·9 ± 2·1 for control plants, 97·8 ± 2·1 for low-defoliation and 98·7 ± 1·3 for high-defoliation plants. These differences were not statistically significant (Wald χ22,28 = 5·44, P = 0·53).
Effects of defoliation on pollination success
Fruit set was not affected by herbivory treatments. Although high-defoliation plants had a slightly smaller fruit set (mean ± s.e. = 0·58 ± 0·06) than the control and low-defoliation plants (0·68 ± 0·07 and 0·69 ± 0·08, respectively), this difference was not significant (F2,45 = 1·35, P = 0·27). Similarly, seed set did not differ significantly among treatments (mean ± s.e. = 0·71 ± 0·05 for control, 0·66 ± 0·06 for low defoliation and 0·75 ± 0·05 for high defoliation; F2,39 = 1·24, P = 0·54).
Effects of defoliation on nectar production
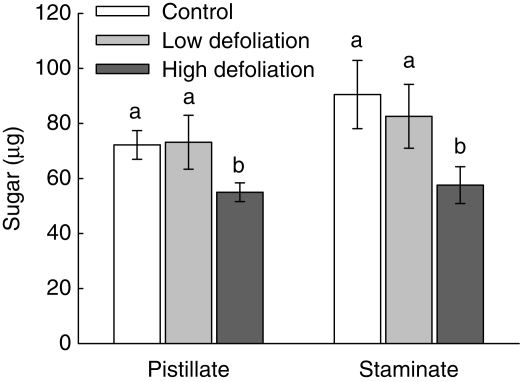
The pistillate flowers of control plants produced a slightly lower amount of sugar (mean ± s.e. = 72·2 ± 5·2) compared with those of the staminate flowers (90·5 ± 12·4), but the difference between both types of flowers was not statistically significant (Wald χ236 = 2·70, P = 0·10). The same was true for the low- and high-defoliation treatment (Wald χ215 = 0·29, P = 0·59 and Wald χ235 = 0·82, P = 0·37, respectively).
Although the amount of sugar produced in pistillate flowers was highly variable in all the three treatments (21–291 µg for control, 38–101 µg for low defoliation and 23–369 µg for high defoliation), the mean amount of sugar was significantly different among treatments (Wald χ22,40 = 9·67, P < 0·01) and among plants (Wald χ218,40 = 159·65, P < 0·0001). Plants of the high-defoliation treatment produced, on average, a 31 % and 33 % smaller amount of sugar than those of the control and low defoliation, respectively (Fig. 4). Similarly, sugar production of staminate flowers was highly variable (24–161 µg for control, 12–220 µg for low defoliation and 14–260 µg for high defoliation), and the mean amount of sugar was significantly different among treatments (Wald χ22,25 = 7·41, P < 0·05) and among plants (Wald χ213,25 = 46·00, P < 0·0001). Again, plants in the high-defoliation treatment produced a 57 % and 45·7 % lower amount of sugar than those in the control and low-defoliation treatments (Fig. 4).
Fig. 4.
The effect of defoliation treatments on the quantity of sugar produced by pistillate and staminate flowers plants of Croton suberosus. Values are back-transformed means ± s.e. Bars with different letters are significantly different at P < 0·05.
Effects defoliation on flower-visitor abundance and activity
Pistillate and staminate flowers of C. suberosus were visited by at least 22 and 25 insect taxa, respectively, belonging to six orders (Appendix). Pistillate flowers were visited by 19 taxa in the control plants, 18 taxa in the low-defoliation, and 15 in the high-defoliation treatment (Appendix), and the relative abundance of these taxa across the plants of different treatments was not significantly different (Wald χ22,20 = 2·28, P = 0·32). Staminate flowers were visited by 24 taxa in the control plants, 19 taxa in the low-defoliation, and 19 in the high-defoliation plants (Appendix). Again, differences of the relative abundance of taxa among treatments were not significant (Wald χ22,23 = 3·56, P = 0·17).
The mean visitation rate of insect groups did not differ significantly among treatments in both staminate and pistillate flowers (Tables 1 and 2). Insect groups showed statistically different visitation rates in staminate flowers but not in pistillate ones; the interaction treatment × insect was not significant in both flowers types (Table 1). The visitation rate of individual insect groups and the mean of each treatment were highly variable (Table 2). All the insect groups showed similar visitation rates among treatments in both staminate and pistillate flowers (P > 0·32 and P > 0·35, respectively), with the exception of wasps and ants in pistillate flowers, which showed marginally significant differences (P = 0·067 and P = 0·061, respectively), although both were considered non-significant after application of the Bonferroni correction for multiple tests (α = 0·05/6 = 0·008).
Table 1.
Factorial analysis of deviance comparing visitation rate and bout length among treatments and insect groups
| Visitation rate |
Bout length |
|||||
|---|---|---|---|---|---|---|
| Flower type | Source of variation | d.f. | Wald χ2 | P | Wald χ2 | P |
| Pistillate | Treatment | 2 | 0·33 | 0·848 | 1·62 | 0·445 |
| Insect group | 5 | 7·76 | 0·170 | 167·82 | < 0·0001 | |
| Treatment × insect group | 10 | 6·63 | 0·760 | 19·74 | 0·032 | |
| Staminate | Treatment | 2 | 0·05 | 0·975 | 2·995 | 0·224 |
| Insect group | 5 | 55·75 | < 0·0001 | 866·53 | < 0·0001 | |
| Treatment × insect group | 10 | 11·62 | 0·311 | 34·41 | < 0·0001 | |
Significant P values after Bonferroni adjustment are marked in bold.
Table 2.
Visitation rate and bout length of the insect groups that visit inflorescences with pistillate or staminate flowers of C. suberosus in each defoliation treatment
| Visitation rate (no. of visits/15 min) |
Bout length (s) |
||||||
|---|---|---|---|---|---|---|---|
| Flower type | Insect group | Control | Low defoliation | High defoliation | Control | Low defoliation | High defoliation |
| Pistillate | Wasps | 0·413 (0·097) | 1·327 (0·496) | 0·373 (0·115) | 6·8 (1·9) | 10·9 (2·3) | 15·6 (6·0) |
| Bees | 0·129 (0·063) | 0·289 (0·205) | 0·042 (0·038) | 52·0 (38·3) | 21·5 (9·7) | 17·0 (12·5) | |
| Ants | 1·271 (0·692) | 0·679 (0·328) | 3·622 (3·058) | 303·4 (57·1) | 189·9 (66·2) | 222·9 (56·3) | |
| Beetles | 0·242 (0·103) | 0·242 (0·122) | 0·483 (0·117) | 432·6 (147·6) | 264·6 (101·9) | 248·1 (95·6) | |
| Butterflies | 0·735 (0460) | 0·129 (0·086) | 0·533 (0·451) | 74·3 (16·0) | 189·7 (69·9) | 203·6 (47·5) | |
| Flies | 0·068 (0·044) | 0·629 (0·180) | 0·125 (0·078) | 187·7 (138·4) | 36·2 (11·9) | 41·0 (30·3) | |
| Mean | 0·476 (0·133) | 0·549 (0·112) | 0·863 (0·516) | 93·0 (18·2) | 66·3 (9·6) | 68·8 (14·1) | |
| Staminate | Wasps | 2·506 (0·446) | 1·792 (0·235) | 1·767 (0·451) | 38·9 (4·9) | 27·4 (4·5) | 26·7 (4·3) |
| Bees | 0·511 (0·145) | 0·914 (0·230) | 1·492 (0·351) | 34·7 (11·3) | 66·2 (17·1) | 85·6 (19·5) | |
| Ants | 0·750 (0·144) | 0·567 (0·243) | 0·100 (0·091) | 303·1 (83·6) | 235·7 (86·6) | 535·7 (340·9) | |
| Beetles | 2·350 (0·409) | 4·653 (1·818) | 3·714 (0·660) | 517·2 (63·7) | 723·2 (65·3) | 593·0 (63·2) | |
| Butterflies | 0·561 (0·078) | 0·289 (0·200) | 0·414 (0·149) | 176·0 (41·4) | 141·8 (39·1) | 60·7 (15·4) | |
| Flies | 0·111 (0·101) | 0·250 (0·199) | 0·414 (0·150) | 84·5 (46·6) | 13·8 (4·8) | 23·7 (7·5) | |
| Mean | 1·131 (0·109) | 1·411 (0·314) | 1·355 (0·151) | 258·2 (27·4) | 317·3 (27·2) | 329·4 (94·4) | |
Data are means and s.e. in brackets. None of the comparisons between treatments (columns) was significant with Bonferroni-corrected alpha level.
As for visitation rate, the time invested by insects in a visit was highly variable (Table 2). Mean bout lengths of insect groups did not differ significantly across treatments in either staminate or pistillate flowers but insect groups showed statistically different bout length in both flower types (Tables 1 and 2). Although significant treatment × insect interaction was found in staminate flowers, insect groups did not differ in the time they remained on inflorescences of the three treatments in both staminate and pistillate flowers (P > 0·30 and P > 0·19, respectively).
DISCUSSION
The results show that experimental defoliation in the wild causes differential effects on the vegetative and reproductive traits of Croton suberosus. Effects were also differential, depending on sexual function. Defoliated plants overcompensated for tissue loss by producing more new leaves than the control plants. On the other hand, an asymmetrical effect was found on reproductive components, whereby defoliation reduces only the reproductive output via the male function, thus affecting floral sex ratio and gender expression. Furthermore, it has been found that defoliation did not have an indirect effect on visitation rates and number of visitors of C. suberosus.
The ability of C. suberosus to overcompensate for defoliation has been reported in other species (Whitman et al., 1991; Agrawal, 2000; Parra-Tabla et al., 2004). Generally, the capacity to generate new vegetative tissues is constrained by the allocation of resources to reproduction, mainly affecting fruit and seed production (Domínguez and Dirzo, 1994; Parra-Tabla et al., 2004; Boege, 2005). Following defoliation, C. suberosus not only overcompensated by producing new leaves but also in terms of fruit and seed set (see below), highlighting its high capacity to respond to foliar herbivory. Plant capacity to compensate or overcompensate depends of the ability to increase carbohydrate production via increasing photosynthetic capacity or efficiency (see Thomson et al., 2003), or by increased mobilization of carbohydrates from stored reserves (e.g. stem bases or roots) (Whitham et al., 1991). Plants of C. suberosus could have responded in either of these ways; however, the fact that plants have a great capacity of resprouting after cutting total above-ground biomass (E. Narbona and R. Dirzo, per. obs.) indicates that they accumulate reserves in the perennial root. However, the ability of C. suberosus to overcompensate should be treated with caution as it is known that some species, including shrubs from seasonally dry forests, may show negative effects only after subsequent post-defoliation years (Domínguez and Dirzo, 1994).
Does defoliation differentially affect staminate and pistillate flower production?
The production of staminate flowers gradually decreased with defoliation whereas production of pistillate flowers was not affected. As a result, the floral sex ratio (number of staminate relative to pistillate flowers) was drastically reduced in high-defoliation plants. Similar results were found in other monoecious species subject to herbivory (Parra-Tabla et al., 2004; Thomson et al., 2004; Arceo-Gómez et al., 2009) or to other stressful environmental conditions (Shaanker and Ganeshaiah, 1984; Ackerly and Jasiefiski, 1990; Traveset, 1992). However, these results do not support theoretical predictions based on sex allocation theory (Lloyd, 1979; Freeman et al., 1981). Thus, a hypothesis of phenotypic plasticity during flower development within the inflorescence (Diggle, 2002) was invoked as follows.
The pistillate and staminate flowers in the inflorescence are developed at different times and positions: first, pistillate flowers at the base, and later staminate flowers at the top. This means that during inflorescence development, pistillate flowers are affected only by exhaustion of resources due to defoliation, whereas staminate flowers are not only affected by defoliation but also by competition for resources for fruit and seed development (Diggle and Miller, 2004: Kliber and Eckert, 2004). Thus, defoliated plants fail to develop the most apical, staminate flowers. In C. suberosus, the position of the unisexual flowers within the inflorescence, together with the plasticity of resource allocation would explain that a reduction in resources due to defoliation selectively affects male reproductive traits (Miller and Diggle, 2003). This is consistent with the fact that, under the stressful conditions encountered by plants of C. suberosus growing in shady conditions, there is a reduction in the male floral sex ratio (Domínguez and Bullock, 1989). The ability of C. suberosus to change the number of staminate flowers on each inflorescence allows flexibility in allocation of resources. This can be advantageous when a shift in environmental stresses, such as defoliation, occurs during development (Dorken and Barrett, 2004; Kliber and Eckert, 2004; de Jong et al., 2008).
Is flower-visitor assemblage indirectly affected by defoliation?
Foliar herbivory in C. suberosus had no effect on flower visitor abundance, visitation rate or bout length at both staminate and pistillate flowers. In studies analysing the effect of herbivory on floral display and the relationship with pollinators, most of them in hermaphroditic species, herbivory changes floral morphology, and this in turn translates into reductions in pollinator abundance and/or visitation rate (Strauss et al., 1996; Mothershead and Marquis, 2000; Steets and Ashman, 2004; but see Arceo-Gómez et al., 2009). The fact that other secondary sexual characteristics such as petal size of staminate flowers, or number of open flowers of inflorescences per day, were not affected by the defoliation treatments (E. Narbona, per. obs.), could have influenced the finding that defoliation did not have an indirect effect on visitation rates and number of visitors of C. suberosus.
On the other hand, intense defoliation reduced the amount of sugar produced in both pistillate and staminate flowers, and the numbers of pollen grains per flower. The amount of nectar or pollen may influence the time spent by insects on the flowers (Heinrich and Raven, 1972). As insect visitors of C. suberosus feed on both nectar and pollen (Narbona and Dirzo, 2010), at least a reduction in visitation duration time would be expected. However, bout length did not vary across treatments in either type of flower. This was applicable to both total number of insects and individual insect groups, including wasps and bees, which are the only true pollinators (Narbona and Dirzo, 2010). The present finding is unexpected since in a similar study with Raphanus raphanistrum it was found that pollen and nectar production is reduced, and this in turn reduces insect visitation time (Strauss et al., 1996). We posit that this might be due to two reasons: (1) the assemblage of pollinators of R. raphanistrum (largely bees and syrphid flies) differs considerably from that of C. suberosus; and (2) insect visitors of C. suberosus spend a considerable time extracting nectar from the nectaries because they are concealed at the base of the sepals, which may mask the differences in time taken to gather nectar (see Narbona and Dirzo, 2010).
Does defoliation alter pollination success?
Pollination success in C. suberosus was not affected by the defoliation treatments. These results contrast with those reported with other hermaphroditic species (e.g. Dominguez and Dirzo, 1994; Mothershead and Marquis, 2000; Steets and Asham, 2004; but see Lehtilä and Strauss, 1999), including some from this same forest ecosystem (Dominguez and Dirzo, 1994), and with most of the monoecious or andromonoecious species studied so far (Parra-Tabla et al., 2004; Avila-Sakar and Stephenson, 2006; Wise and Cummins, 2006). Pollen and resource limitation are the main factors that affect both fruit and seed set (Knight et al., 2005). The present findings imply that (a) defoliation did not directly affect resource allocation to female function, and (b) pollen receipt on stigmas of defoliated plants was not affected. The first possibility can be explained by the fact that maintenance of the female function after leaf herbivory is possibly due to the reallocation of resources away from the male function, as proposed for other species (Lehtilä and Strauss, 1999; Arceo-Gómez et al., 2009; but see Vallejo-Marín and Rausher, 2007). Alternatively, maintaining the female function in C. suberosus may represent only a slight cost to the plant because the production of pistillate flowers is likely to require little effort as they do not produce petals and only have three ovules, in contrast to other species that usually produce many ovules (Burd et al., 2009). Thus, the depletion of resources caused by defoliation may preferentially affect the more costly staminate flowers. The fact that pollen receipt on stigmas of defoliated plants was not affected is it not surprising, given that experimental defoliation was applied only on a small proportion of plants of the whole population. Thus the observed reduction in pollen in the defoliated plants may not represent a significant proportion of the population's pollen pool and flow. Furthermore, some characteristics of the pollination system of C. suberosus render reduced pollination success after defoliation stress improbable. First, a larger number of male gametes is produced relative to female ones: three ovules per pistillate flower and about 2000 pollen grains per staminate flower. Also, the number of staminate flowers in the inflorescences is about three times that of pistillate flowers. Secondly, a single visit of a pollinator could ensure female reproductive success, as proposed for other Euphorbiaceae species (Narbona et al., 2005, 2008), because the flower has only three ovules. Thirdly, the diversity, abundance and activity of pollinators are very high. Finally, and as a consequence of the previous points, the population studied of C. suberosus is not pollen limited (Narbona and Dirzo, 2010). Under these circumstances, the potential for herbivores to indirectly affect the pollination success of C. suberosus would seem limited, as Mothershead and Marquis (2000) and Ashman and Penet (2007) pointed out for the case of herbivores that consume vegetative or reproductive structures.
Conclusions
Croton suberosus exhibits a plastic response to defoliation given that it overcompensates in terms of leaf production but compensates or undercompensates in terms of the reproductive characteristics. The present data show an asymmetrical effect on reproductive traits, reducing only the reproductive output via the male function, thus affecting floral sex ratio and gender expression. Monoecy in C. suberosus provides temporal and spatial flexibility in allocation of resources to male and female function allowing the maintenance of pollination success, which may play an important role in the evolution and maintenance of this sexual system (Charlesworth and Morgan, 1991; Huang et al., 2002; de Jong et al., 2008).
A multiplicative effect of defoliation was found in male traits via the reduction of staminate flowers and the number of pollen grains per flower. Although pollen viability was not affected, this does not indicate that all pollen had the same performance; in fact herbivory can affect pollen vigour and its capacity to sire seeds (Quesada et al., 1995; Mutikainen and Delph, 1996). The effect of defoliation on male reproductive traits may or may not necessarily translate into variation in paternal components of fitness (Wilson et al., 1994). Of the three studies already published regarding the relationship between herbivory and fitness, in two of them, an increase in male reproductive traits translated into an increase of male fitness (Gronemeyer et al., 1997; Paige et al., 2001); in the other, an increase in male fitness was found without any evident effect of herbivory on reproductive traits (Strauss et al., 2001). In contrast, as male reproductive traits decreased in C. suberosus, a paternity analysis is needed to confirm if, as is expected, defoliation will effectively reduce male fitness.
ACKNOWLEDGEMENTS
We thank to Pedro Jordano, Mick Hanley and two anonymous reviewers for critically reading the manuscript and offering constructive comments. We thank Tim Lambert, Josh Kuempel, Blair Beverly and Stan Tram (Stanford University), and Ana López Llandes (EEZA-CSIC, Almería) for their assistance in the field. Katherine Renton and Karina Boege provided logistical support and advice during field work. Insect determinations were provided by Ricardo Ayala, Harry Braylovsky, Deborah Gordon, Felipe A. Noguera, Enrique Ruiz, Sean O'Donnell and Santiago Zaragoza. This work was supported by a grant to E. Narbona from the Junta de Andalucía and Plan Propio de Investigación (Univ. Pablo de Olavide) and by Stanford's VPUE programme of summer research support. We are grateful to the Chamela Research Station for the support provided for field work.
APPENDIX
Insect visitors on pistillate and staminate flowers of C. suberosus. Letters indicate the presence of a given taxon in censuses on plants of the control (C), low-defoliation (L) and high-defoliation (H) treatments.
| Flower type |
|||||
|---|---|---|---|---|---|
| Order | Functional group | Family | Insect species | Pistillate | Staminate |
| Hymenoptera | Wasps | Sphecidae | Ammophila sp. | C-L-H | C-L-H |
| Vespidae | Eumenes sp. | C-L-H | C-L-H | ||
| Vespidae | Montezumia mexicana | C-H | |||
| Vespidae | Polistes instabilis | C-L-H | C-L-H | ||
| Vespidae | Polybia sp. | L | C-H | ||
| Vespidae | Zethus sp. | C-L | |||
| Vespidae | Eumeninae sp. 1 | C-L-H | C-L-H | ||
| Bees | Megachilidae | Ashmeadiella sp. | C-L | C-L-H | |
| Apidae | Trigona fulviventris | C-L-H | C-L | ||
| Ants | Formicidae | Camponotus sp. | C-L-H | C-L | |
| Formicidae | Cephalotes sp. | C | |||
| Formicidae | Crematogaster sp. | C-L-H | C-L-H | ||
| Formicidae | Pseudomyrmex sp. | C-H | C | ||
| Coleoptera | Beetles | Cerambycidae | Rhopalophora | C | C-L-H |
| Cerambycidae | Stenobatyle eburata | C-L-H | C-L-H | ||
| Scarabaeidae | Trigonopeltastes burmeister | C-L | C | ||
| Carabidae | Carabidae | C-L-H | |||
| Chrysomelidae | Chrysomelidae 1 | L-H | C-L-H | ||
| Chrysomelidae | Chrysomelidae 2 | C-L-H | |||
| Lepidoptera | Butterflies | Nymphalidae | Euptoieta hegesia | C-L-H | C-L-H |
| Nymphalidae | Microtia elva | C-L-H | C-L-H | ||
| Lycaenidae | Nicolaea ophia | L | C-L | ||
| Lycaenidae | Theclinae | C-L-H | C-H | ||
| Diptera | Flies | Muscidae | Muscidae | C-L-H | C-L-H |
| Tachiniidae | Tachiniidae | L-H | |||
| Hemiptera | Reduviidae | Apiomerus sp. | C-H | ||
| Homoptera | Membracidae | Membracidae | C | C-L-H | |
LITERATURE CITED
- Ackerly DD, Jasiefiski M. Size-dependent variation of gender in high density stands of the monoecious annual, Ambrosia artemisiifolia (Asteraceae) Oecologia. 1990;82:474–477. doi: 10.1007/BF00319788. [DOI] [PubMed] [Google Scholar]
- Agrawal AA. Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends in Plant Science. 2000;5:309–313. doi: 10.1016/s1360-1385(00)01679-4. [DOI] [PubMed] [Google Scholar]
- Arceo-Gómez G, Parra-Tabla V, Navarro J. Changes in sexual expression as result of defoliation and environment in a monoecious shrub in Mexico: implications for pollination. Biotropica. 2009;41:435–441. [Google Scholar]
- Ashman T-L. The role of herbivores in the evolution of separate sexes from hermaphroditism. Ecology. 2002;83:1175–1184. [Google Scholar]
- Ashman T-L, Penet L. Direct and indirect effects of a sex-biased antagonist on male and female fertility: consequences for reproductive trait evolution in a gender dimorphic plant. American Naturalist. 2007;169:595–608. doi: 10.1086/513150. [DOI] [PubMed] [Google Scholar]
- Avila-Sakar G, Stephenson AG. Effects of the spatial pattern of leaf damage on growth and reproduction: whole plants. International Journal of Plant Sciences. 2006;167:1021–1028. [Google Scholar]
- Bickel AM, Freeman DC. Effects of pollen vector and plant geometry on floral sex ratio in monoecious plants. American Midland Naturalist. 1993;130:239–247. [Google Scholar]
- Boege K. Influence of plant ontogeny on compensation to leaf damage. American Journal of Botany. 2005;92:1632–1640. doi: 10.3732/ajb.92.10.1632. [DOI] [PubMed] [Google Scholar]
- Bullock SH, Solís-Magallanes JA. Phenology of canopy trees of a tropical deciduous forest in Mexico. Biotropica. 1990;22:22–35. [Google Scholar]
- Burd M, Ashman TL, Campbell DR, et al. Ovule number per flower in a world of unpredictable pollination. American Journal of Botany. 2009;96:1159–1167. doi: 10.3732/ajb.0800183. [DOI] [PubMed] [Google Scholar]
- Charlesworth D. Theories of the evolution of dioecy. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. Berlin: Springer-Verlag; 1999. pp. 33–60. [Google Scholar]
- Charlesworth D, Morgan MT. Allocation of resources to sex functions in flowering plants. Philosophical Transactions of the Royal Society of London B. 1991;332:91–102. [Google Scholar]
- Cornelissen T, Stiling P. Sex-biased herbivory: a meta-analysis of the effects of gender on plant-herbivore interactions. Oikos. 2005;111:488–500. [Google Scholar]
- Crawley MJ. The R book. Chichester: John Wiley and Sons; 2007. [Google Scholar]
- Cruden RW, Hermann SM. Studying nectar? Some observation on the art. In: Bentley B, Elias T, editors. The biology of nectaries. New York, NY: Columbia; 1983. pp. 223–241. [Google Scholar]
- Dafni A, Pacini E, Nepi M. Pollen and stigma biology. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge: Enviroquest; 2005. pp. 83–146. [Google Scholar]
- Diggle PK. A developmental morphologist's perspective of plasticity. Evolutionary Ecology. 2002;16:267–283. [Google Scholar]
- Diggle PK, Miller JS. Architectural effects mimic floral sexual dimorphism in Solanum (Solanaceae) American Journal of Botany. 2004;91:2030–2040. doi: 10.3732/ajb.91.12.2030. [DOI] [PubMed] [Google Scholar]
- Dirzo R, Domínguez CA. Interacciones planta-herbívoro en la selva baja caducifolia de Chamela. In: Nogera FA, Vega JH, García AN, Quesada M, editors. Historia natural de Chamela. Mexico City: Instituto de Biología-UNAM; 2002. pp. 453–459. [Google Scholar]
- Domínguez CA, Bullock SH. La reproducción de Croton suberosus (Euphorbiaceae) en luz y sombra. Revista de Biología Tropical. 1989;37:1–10. [Google Scholar]
- Domínguez CA, Dirzo R. Effects of defoliation on Erythroxylum havanense, a tropical proleptic species. Ecology. 1994;75:1896–1902. [Google Scholar]
- Domínguez CA, Dirzo R, Bullock SH. On the function of floral nectar in Croton suberosus (Euphorbiaceae) Oikos. 1989;56:109–114. [Google Scholar]
- Dorken ME, Barrett SCH. Phenotypic plasticity of vegetative and reproductive traits in monoecious and dioecious populations of Sagittaria latifolia (Alismataceae): a clonal aquatic plant. Journal of Ecology. 2004;92:32–44. [Google Scholar]
- Fishbein M, Venable DL. Diversity and temporal change in the effective pollinators of Asclepias tuberosa. Ecology. 1996;77:1061–1073. [Google Scholar]
- Freeman DC, McArthur ED, Harper KT, Blauer AC. Influence of environment on the floral sex ratio of monoecious plants. Evolution. 1981;35:194–197. doi: 10.1111/j.1558-5646.1981.tb04875.x. [DOI] [PubMed] [Google Scholar]
- García LV. Escaping the Bonferroni iron claw in ecological studies. Oikos. 2004;105:657–663. [Google Scholar]
- García-Oliva F, Camou F, Maass JM. El clima de la región central de la costa del Pacifico mexicano. In: Nogera FA, Vega JH, García AN, Quesada M, editors. Historia natural de Chamela. Mexico City: Instituto de Biología-UNAM; 2002. pp. 3–10. [Google Scholar]
- Gronemeyer P, Dilger BJ, Bouzat JL, Paige KN. The effects of herbivory on paternal fitness in scarlet gilia: better moms also make better pops. American Naturalist. 1997;150:592–602. doi: 10.1086/286083. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Fegan EL. Timing of cotyledon damage affects growth and flowering in mature plants. Plant, Cell & Environment. 2007;30:812–819. doi: 10.1111/j.1365-3040.2007.01671.x. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Armbruster WS. Pollination and plant defence traits co-vary in Western Australian Hakeas. New Phytologist. 2009;182:251–260. doi: 10.1111/j.1469-8137.2008.02709.x. [DOI] [PubMed] [Google Scholar]
- Heinrich B, Raven PH. Energetics of pollination. Science. 1972;176:597–602. doi: 10.1126/science.176.4035.597. [DOI] [PubMed] [Google Scholar]
- Huang S, Sun S, Takahashi Y, Guo Y. Gender variation of sequential inflorescences in a monoecious plant Sagittaria trifolia (Alismataceae) Annals of Botany. 2002;90:613–622. doi: 10.1093/aob/mcf236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RE. Realized tolerance to nectar robbing: compensation to floral enemies in Ipomopsis aggregata. Annals of Botany. 2009;103:1425–1433. doi: 10.1093/aob/mcp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong TJ, Shmida A, Thuijsman F. Sex allocation in plants and the evolution of monoecy. Evolutionary Ecology Research. 2008;10:1087–1109. [Google Scholar]
- Kliber A, Eckert CG. Sequential decline in allocation among flowers within inflorescences: proximate mechanisms and adaptive significance. Ecology. 2004;85:1675–1687. [Google Scholar]
- Knight TM, Steets JA, Vamosi JC, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Annual Review of Ecology, Evolution, and Systematics. 2005;36:467–497. [Google Scholar]
- Lehtilä K, Strauss SY. Effects of foliar herbivory on male and female reproductive traits of wild radish, Raphanus raphanistrum. Ecology. 1999;80:116–124. [Google Scholar]
- Lindsey JK. Applying generalized linear models. New York, NY: Springer-Verlag; 1997. [Google Scholar]
- Lloyd DG. Parental strategies of angiosperms. New Zealand Journal of Botany. 1979;17:595–606. [Google Scholar]
- Marquis RJ. Selective impacts of herbivores. In: Fritz RS, Simms EL, editors. Plant resistance to herbivores and pathogens: ecology, evolution, and genetics. Chicago, IL: The University of Chicago Press; 1992. pp. 301–325. [Google Scholar]
- Miller JS, Diggle PK. Diversification of andromonoecy in Solanum section Lasiocarpa (Solanaceae): the roles of phenotypic plasticity and architecture. American Journal of Botany. 2003;90:707–715. doi: 10.3732/ajb.90.5.707. [DOI] [PubMed] [Google Scholar]
- Mothershead K, Marquis RJ. Fitness impacts of herbivory through indirect effects on plant–pollinator interactions in Oenothera macrocarpa. Ecology. 2000;81:30–40. [Google Scholar]
- Mutikainen P, Delph LF. Effects of herbivory on male reproductive success in plants. Oikos. 1996;75:353–358. [Google Scholar]
- Narbona E, Dirzo R. A reassessment of the function of floral nectar in Croton suberosus (Euphorbiaceae): a reward for plant defenders and pollinators. American Journal of Botany. 2010;97:672–679. doi: 10.3732/ajb.0900259. [DOI] [PubMed] [Google Scholar]
- Narbona E, Ortiz PL, Arista M. Dichogamy and sexual dimorphism in floral traits in the andromonoecious Euphorbia boetica. Annals of Botany. 2005;95:779–787. doi: 10.1093/aob/mci077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbona E, Ortiz PL, Arista M. Sexual dimorphism in the andromonoecious Euphorbia nicaeensis: effects of gender and inflorescence development. Annals of Botany. 2008;101:717–726. doi: 10.1093/aob/mcn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Paige KN, Williams B, Hickox T. Overcompensation through the paternal component of fitness in Ipomopsis arizonica. Oecologia. 2001;128:72–76. doi: 10.1007/s004420100647. [DOI] [PubMed] [Google Scholar]
- Parmesan C. Unexpected density-dependent effects of herbivory in a wild population of the annual Collinsia torreyi. Journal of Ecology. 2000;88:392–400. [Google Scholar]
- Parra-Tabla V, Rico-Gray V, Carbajal M. Effect of defoliation on leaf growth, sexual expression and reproductive success of Cnidoscolus aconitifolius (Euphorbiaceae) Plant Ecology. 2004;173:153–160. [Google Scholar]
- Penet L, Collin CL, Ashman T-L. Florivory increases selfing: an experimental study in the wild strawberry, Fragaria virginiana. Plant Biology. 2009;11:38–45. doi: 10.1111/j.1438-8677.2008.00141.x. [DOI] [PubMed] [Google Scholar]
- Potts SG. Recording pollinator behaviour on flowers. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge: Enviroquest; 2005. pp. 332–339. [Google Scholar]
- Quesada M, Bollman K, Stephenson AG. Leaf damage decreases pollen production and hinders pollen performance in Cucurbita texana. Ecology. 1995;76:437–443. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. [Google Scholar]
- Rzedowski J. Vegetación de México. Mexico City: Limusa; 1978. [Google Scholar]
- Shaanker RU, Ganeshaiah KN. Age-specific sex ratio in a monoecious species Croton bonplandianum. New Phytologist. 1984;97:523–532. [Google Scholar]
- Snyder MA. Interactions between Albert's squirrel and Ponderosa pine: the relationship between selective herbivory and host plant fitness. American Naturalist. 1993;141:866–879. [Google Scholar]
- Steets JA, Ashman T-L. Herbivory alters the expression of a mixed mating system. American Journal of Botany. 2004;91:1046–1051. doi: 10.3732/ajb.91.7.1046. [DOI] [PubMed] [Google Scholar]
- Steets JA, Hamrick JL, Ashman T-L. Consequences of vegetative herbivory for maintenance of intermediate outcrossing in an annual plant. Ecology. 2006;87:2717–2727. doi: 10.1890/0012-9658(2006)87[2717:covhfm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Strauss SY, Conner JK, Rush SL. Foliar herbivory affects floral characters and plant attractiveness to pollinators: implications for male and female plant fitness. American Naturalist. 1996;147:1098–1107. [Google Scholar]
- Strauss SY, Conner JK, Lehtila KP. Effects of foliar herbivory by insects on the fitness of Raphanus raphanistrum: damage can increase male fitness. American Naturalist. 2001;158:496–504. doi: 10.1086/323116. [DOI] [PubMed] [Google Scholar]
- Thomson VP, Cunningham SA, Ball MC, Nicotra AB. Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia. 2003;134:167–175. doi: 10.1007/s00442-002-1102-6. [DOI] [PubMed] [Google Scholar]
- Thomson VP, Nicotra AB, Cunningham SA. Herbivory differentially affects male and female reproductive traits of Cucumis sativus. Plant Biology. 2004;6:621–628. doi: 10.1055/s-2004-821236. [DOI] [PubMed] [Google Scholar]
- Traveset A. Sex expression in a natural population of the monoecious annual, Ambrosia artemisiifolia (Asteraceae) American Midland Naturalist. 1992;127:309–315. [Google Scholar]
- Vallejo-Marín M, Rausher MD. The role of male flowers in andromonoecious species: energetic costs and siring success in Solanum carolinense L. Evolution. 2007;61:404–412. doi: 10.1111/j.1558-5646.2007.00031.x. [DOI] [PubMed] [Google Scholar]
- Verdú M, García-Fayos P, Gleiser G. Mites attack males of the sexually polymorphic tree Acer opalus more harmfully and more often. Functional Ecology. 2004;18:592–597. [Google Scholar]
- Whitham TG, Mopper S. Chronic herbivory: impacts on architecture and sex expression of pinyon pine. Science. 1985;228:1089–1091. doi: 10.1126/science.228.4703.1089. [DOI] [PubMed] [Google Scholar]
- Whitham TG, Maschinski J, Larson KC, Paige KN. Plant responses to herbivory: the continuum from negative to positive and underlying physiological mechanisms. In: Price PW, Lewinsohn TW, Benson W, Fernandez GW, editors. Plant-animal interactions: evolutionary ecology in tropical and temperate regions. New York, NY: Wiley-Interscience; 1991. pp. 227–256. [Google Scholar]
- Wilson P, Thomson JD, Stanton ML, Rigney LP. Beyond floral batemania: gender biases in selection for pollination success. American Naturalist. 1994;143:283–296. [Google Scholar]
- Wilson P, Castellanos MC, Hogue JN, Thomson JD, Armbruster WS. A multivariate search for pollination syndromes among Penstemons. Oikos. 2004;104:345–361. [Google Scholar]
- Wise MJ, Cummins JJ. Strategies of Solanum carolinense for regulating maternal investment in response to foliar and floral herbivory. Journal of Ecology. 2006;94:629–636. [Google Scholar]
- Zang D-Y. Evolutionary stable reproductive investment and sex allocation in plants. In: Harder LD, Barret SCH, editors. Ecology and evolution of flowers. New York, NY: Oxford University Press; 2006. pp. 41–60. [Google Scholar]