Abstract
Background and Aims
Aerenchyma provides a low-resistance O2 transport pathway that enhances plant survival during soil flooding. When in flooded soil, soybean produces aerenchyma and hypertrophic stem lenticels. The aims of this study were to investigate O2 dynamics in stem aerenchyma and evaluate O2 supply via stem lenticels to the roots of soybean during soil flooding.
Methods
Oxygen dynamics in aerenchymatous stems were investigated using Clark-type O2 microelectrodes, and O2 transport to roots was evaluated using stable-isotope 18O2 as a tracer, for plants with shoots in air and roots in flooded sand or soil. Short-term experiments also assessed venting of CO2 via the stem lenticels.
Key Results
The radial distribution of the O2 partial pressure (pO2) was stable at 17 kPa in the stem aerenchyma 15 mm below the water level, but rapidly declined to 8 kPa at 200–300 µm inside the stele. Complete submergence of the hypertrophic lenticels at the stem base, with the remainder of the shoot still in air, resulted in gradual declines in pO2 in stem aerenchyma from 17·5 to 7·6 kPa at 13 mm below the water level, and from 14·7 to 6·1 kPa at 51 mm below the water level. Subsequently, re-exposure of the lenticels to air caused pO2 to increase again to 14–17 kPa at both positions within 10 min. After introducing 18O2 gas via the stem lenticels, significant 18O2 enrichment in water extracted from roots after 3 h was confirmed, suggesting that transported O2 sustained root respiration. In contrast, slight 18O2 enrichment was detected 3 h after treatment of stems that lacked aerenchyma and lenticels. Moreover, aerenchyma accelerated venting of CO2 from submerged tissues to the atmosphere.
Conclusions
Hypertrophic lenticels on the stem of soybean, just above the water surface, are entry points for O2, and these connect to aerenchyma and enable O2 transport into roots in flooded soil. Stems that develop aerenchyma thus serve as a ‘snorkel’ that enables O2 movement from air to the submerged roots.
Keywords: Aerenchyma, oxygen transport, soybean (Glycine max), flooding, root aeration, hypertrophic lenticels, soil waterlogging
INTRODUCTION
In flooded soils, the root environment supplies insufficient O2 because the diffusion of gases in water is approx. 10 000 times slower than in air (Armstrong, 1979) and diffusion of atmospheric O2 to the roots is largely prevented. Many crop plants exhibit poor root growth, injury, and death when exposed to saturated soil. On the other hand, wetland plants are well-adapted and can survive prolonged flooding. Enlarged intercellular gas-filled spaces, called ‘aerenchyma’, are present throughout most of the body of most wetland plants. Therefore, aerenchyma provides a low-resistance pathway for transport of O2 from the shoot to the roots, and enables plant roots to maintain respiration. For example, some Rumex species that inhabit frequently flooded environments produce high volumes of aerenchyma in their root cortex and have high flood tolerance, whereas species that seldom inhabit flooded environments develop less aerenchyma and have low flood tolerance (Laan et al., 1989). These anatomical differences have been observed in many plants (Smirnoff and Crawford, 1983; Justin and Armstrong, 1987), suggesting that aerenchyma is an important anatomical characteristic that helps plants to survive soil flooding.
Many field crops, including soybean (Glycine max), are very sensitive to flooding stress. Soybean suffers from such stress during its vegetative stages, leading to decreased yield (Sojka, 1985; Griffin and Saxton, 1988; Scott et al., 1989; Linkemer et al., 1998). According to Shimamura et al. (2003), however, stems, roots and root nodules of soybean develop aerenchyma within a few weeks under flooded conditions. This aerenchyma arises from successive cell division by the phellogen, and is composed of white and porous (spongy) tissues that are referred to as ‘secondary aerenchyma’ (Arber, 1920; Fraser, 1931; Williams and Barber, 1961; Jackson and Armstrong, 1999). This type of aerenchyma is the tissue of secondary origin, and morphologically and anatomically different from cortical (primary) aerenchyma (i.e. lysigenous and schizogenous aerenchyma), which can be distinguished by their process of formation. Lysigenous aerenchyma is created through cell disintegration (death), and schizogenous aerenchyma is created by cell separation (Evans, 2003; Visser and Voesenek, 2004). Both aerenchymas occur in the primary cortex of roots and shoots, whereas secondary aerenchyma occurs not in the primary cortex but in the phellogen region and is homologous with cork tissue. In addition, hypertrophy of secondary aerenchyma enhances formation of large cracks (i.e. hypertrophic lenticels) on the surface of stems and roots, and the aerenchyma is exposed to the atmosphere through the lenticels (e.g. for Sesbania javanica see fig. 1 in Jackson et al., 2009), which may facilitate O2 entry into the aerenchyma. Flood-tolerant leguminous plants, such as Sesbania aculeata (Scott and Wager 1888), Sesbania rostrata (Saraswati et al., 1992; Shiba and Daimon, 2003), Neptunia oleracea (Metcalfe, 1931), and Viminaria juncea (Walker et al., 1983), produce secondary aerenchyma in their stems, roots and root nodules. Thomas et al. (2005) reported that when soybean plants are flooded, N2 fixation decreases quickly in their root nodules but recovers when aerenchyma formation begins. Similarly, when both petroleum jelly and film are applied to the soybean hypocotyl surface to prevent the entry of atmospheric O2 into the secondary aerenchyma through hypertrophic lenticels under flooding, root growth is sharply inhibited compared with shoot growth (Shimamura et al., 2003). At the same time, the activity of root nodules is significantly restricted (Shimamura et al., 2002). These reports indicate that formation of hypertrophic lenticels at the stem base and development of aerenchyma are important acclimations enabling O2 supply into roots and root nodules of soybean in flooded soil.
Fig. 1.
Development of secondary aerenchyma and hypertrophic lenticels in soybean plants after approx. 5 weeks of flooding. The broken line on the stem indicates the water level at 3·5 cm above the soil surface. Secondary aerenchyma (white tissue) was developed in the stem and the adventitious roots, and hypertrophic lenticels were observed just above the water surface. Therefore, when water level was raised from 3·5 cm to 6 cm, the hypertrophic lenticels were completely submerged. Scale bar = 20 mm.
Internal O2 movement from shoot to roots via the aerenchyma has been demonstrated in various ways. Drew et al. (1985) found a higher adenylate energy charge [defined as (ATP + ½ADP)/(ATP + ADP + AMP)] in adventitious roots of Zea mays under hypoxic conditions that developed aerenchyma, compared with roots in which formation of aerenchyma was blocked by the application of silver ions to inhibit ethylene action. These results indicate that aerenchyma supplies O2 to hypoxic roots in Z. mays. One drawback of the approach using adenylate energy charge assessments is that it requires destructive samplings, and thus cannot measure changes over time within a single plant. As an alternative, the O2 concentration in aerenchyma can be directly measured in intact plants using a Clark-type O2 microelectrode (Armstrong et al., 2000). In completely submerged plants, photosynthetic O2 is transported from shoots to roots in Halosarcia pergranulata (Pederson et al., 2006) and rice (Oryza sativa); Colmer and Pedersen, 2008), because O2 in aerenchymatous roots decreases sharply when photosynthetic tissues are placed in darkness. Use of O2 isotopes is another technique that can be used to study plant aeration. For example, Dacey and Klug (1982) showed exposure of leaves of the yellow waterlily (Nuphar luteum) to the stable-isotope 18O2 (used as a tracer) confirmed movement of 18O2 via aerenchyma to the rhizomes. In the present paper, the O2 dynamics of aerenchymatous soybean stems were investigated using Clark-type O2 microelectrodes after preventing the entry of atmospheric O2 into secondary aerenchyma through hypertrophic stem lenticels. Also stable-isotope 18O2 was used as a tracer to confirm O2 transport from stem to the roots via aerenchyma and demonstrate that this O2 was used to sustain root aerobic respiration.
MATERIALS AND METHODS
Experiment 1: O2 dynamics in aerenchymatous stems
Plant material and experimental set-up
Seeds of soybean (Glycine max ‘Asoaogari’) were sown in 200 mL of silica sand (passed through an 18–26 mesh) without chemical fertilizer in 400-mL plastic pots (one seed per pot). The plants were grown in a growth cabinet under artificial light (25 °C, 14 h light and 10 h dark, 590 µmol m−2 s−1 PAR). Ten days after sowing, the pots were placed inside tanks 11 cm in diameter × 14 cm in height (one pot per tank) and the plants were grown under continuously flooded conditions, with the water level maintained at 6–7 cm above the sand surface. Approximately 14 d after flooding, plants that developed secondary aerenchyma from the bottom of the stems to just above the water level were used for the experiment (Table 1).
Table 1.
Plant growth and development of secondary aerenchyma and lenticels after approx. 14 d flooding: the area of stele (vascular cylinder), secondary aerenchyma and stem in cross-sections of stems at the water surface
| Shoot dry weight (g) | 0·29 ± 0·03 |
| Root dry weight (g) | 0·15 ± 0·01 |
| Stele area (mm2) | 2·91 ± 0·15 |
| Secondary aerenchyma area (mm2) | 8·37 ± 1·45 |
| Stem area (mm2) | 12·7 ± 1·48 |
| Percentage of aerenchyma area in stems (%) | 63·8 ± 4·74 |
| Lenticel height at stems above water surface (mm) | 6·60 ± 0·51 |
Aerenchyma area indicates the area not of gas spaces but of aerenchymatous tissues in cross-sections. Percentage of aerenchyma area indicates not porosity but the percentage of stem area occupied by aerenchymatous tissue area in cross-sections.
Digital images of stem cross-sections were photographed using a microscope and analysed with ImageJ 1·38x software (National Institutes of Health, USA) to measure each tissue area.
Values are the mean (±s.e.) of five plants.
The plant was placed in a tank in the same growth cabinet, and the water level was subsequently maintained at 7 mm below the top of the aerenchymatous stem so that hypertrophic lenticels were exposed to the atmosphere. A Clark-type O2 microelectrode with a guard cathode and a tip diameter of 25 µm (OX-25; Unisense A/S, Aarhus, Denmark) was used. The microelectrode was connected to a pA meter (PA2000; Unisense A/S) and the output was logged at 1 s intervals on a computer using an analogue-to-digital converter (ADC-16; Pico Technology, St Neots, Cambridgeshire, UK). The electrode was calibrated immediately before measurement in air and in O2-free N2. The O2 concentration of the water was 228 µmol L−1 on average at 3 cm below the water surface.
Radial O2 profile across an aerenchymatous stem of intact soybean plants
The microelectrode tip was positioned at the surface of the aerenchymatous stem 15 mm below the water level using a micromanipulator (MM5; Marzhauser, Wetzlar, Germany). The microelectrode was set to move toward the stele in 100-μm steps and set at a frequency of a step every 10 s. After obtaining measurements, a transverse section of the fresh stem was cut on a plant microtome (MTH-1; Nippon Medical & Chemical Instruments, Osaka, Japan), and photographed using a microscope.
O2 movement in the stem aerenchyma
The microelectrodes were inserted into aerenchymatous stem tissue at 13 and 51 mm below the water level using the micromanipulator, and the tips were positioned within the aerenchyma approx. 1 mm from the stem surface. After confirming the presence of stable signals from both sensors, the hypertrophic stem lenticels were completely submerged by raising the water level to 3 mm above the uppermost lenticels. After 2 h, the water level was reduced to the level at the start of the experiment to re-expose the hypertrophic lenticels to air.
Experiment 2: O2 transport from stem lenticels to roots via the aerenchyma
Plant material and growth conditions
Three seeds of ‘Asoaogari’ were sown in low-humic andosols containing 4 g of inorganic fertilizer (3 % N, 10 % P2O5, 10 % K2O), 1·4 g of dolomite and 3 g of fused magnesium phosphate in a plastic pot (one of two sizes: either 11·5 or 14 cm in height and 11 cm in diameter). The plants were grown in a growth cabinet under artificial light (25 °C, 14 h light and 10 h dark, approx. 800 µmol m−2 s−1 PAR). After germination, seedlings were thinned to one plant per pot. When the primary leaves had fully expanded, some pots were placed inside 3·7-L tanks (one pot per tank) and the plants were grown under continuously flooded soil conditions, with the water level maintained 3·5 cm above the soil surface. The remaining plants were used as non-flooded (i.e. drained) controls. Approximately 5 weeks after flooding, plants that had developed secondary aerenchyma in the stems, roots and root nodules were used for the experiment, and hypertrophic lenticels and aerenchyma in the stems were formed just above the water level (Fig. 1 and Table 2). The O2 concentration of the flood water was 175 µmol L−1 on average at 3 cm below the water surface just before 18O2 treatment.
Table 2.
Plant growth and development of secondary aerenchyma after approx. 5 weeks flooding: the area of stele, secondary aerenchyma and stem in cross-sections of stems at 3·5 cm above the soil surface (control plants) or at the water surface (flooded plants)
| Control | Flooded | |
|---|---|---|
| Shoot dry weight (g) | 9·10 ± 0·38 | 5·58 ± 0·72 |
| Root dry weight (g) | 3·93 ± 0·20 | 3·73 ± 0·37 |
| Stele area (mm2) | No data | 12·6 ± 1·71 |
| Secondary aerenchyma area (mm2) | 0* | 43·2 ± 8·73 |
| Stem area (mm2) | No data | 62·0 ± 11·2 |
| Percentage of aerenchyma area in stems (%) | 0* | 68·8 ± 2·38 |
Aerenchyma area indicates the area not of gas spaces but of aerenchymatous tissues in cross-sections. Percentage of aerenchyma area indicates not porosity but the percentage of stem area occupied by aerenchymatous tissue area in cross-sections. The development of secondary aerenchyma was not observed in control plants, so the percentage of aerenchyma area in the stems was zero (*).
Digital images of stem cross-sections were photographed using a microscope and analysed with ImageJ 1·38x software (National Institutes of Health, USA) to measure each tissue area.
Values are the mean (±s.e.) of six plants.
Tracing 18O2 transport via the aerenchyma
In aerobic respiration with l8O in plants, carbohydrates are metabolized as follows:
Thus, to measure the uptake of 18O2 by root metabolism, the 18O content of the water produced by root respiration was measured (Yoshida and Eguchi, 1994).
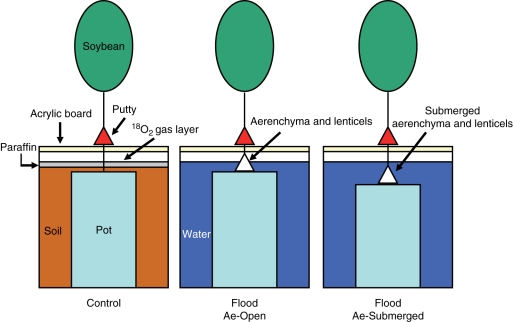
The control pots (14 cm in height) were placed inside 3·7-L tanks (one pot per tank), and were embedded in the soil. The surface was covered with paraffin to prevent O2 diffusion into soils. After covering the tank with an acrylic board (with the space where the plant stem passed through the board sealed with putty), the gas space (a gas layer about 7 mm thick with a volume of about 150 mL) was filled between the paraffin layer and the acrylic board with approx. 100 % O2 gas (95 atom % excess 18O) to supply 18O2 to the basal region of the stem that lacked both aerenchyma and lenticels (Fig. 2). Similarly, flooded plants were treated with 18O2 gas. In the flooded taller pots (14 cm in height), the water level was maintained at 3·5 cm above the soil surface, so aerenchyma and lenticels just above the water surface could be exposed to 18O2 gas layer (Flood Ae-Open conditions). In contrast, in the flooded pots of small size (diameter = 11 cm; height = 11·5 cm), water level was raised from 3·5 cm to 6 cm above the soil surface to cover both aerenchyma and lenticels on the stem, which was 2·5 cm higher than the level of pre-flooded conditions. Therefore, aerenchyma and lenticels of stem base were completely submerged. The basal region of the stem, but above the region that contained aerenchyma and lenticels, was exposed to 18O2 gas layer (Flood Ae-Submerged conditions).
Fig. 2.
The experimental system used to supply 18O2 gas to the base of the stems of soybean plants. Control: 18O2 was supplied to the basal region of the stem of irrigated control plants that lacked both aerenchyma and hypertrophic lenticels. Flood Ae-Open (Ae, aerenchyma): 18O2 was supplied to the basal region of the stem aerenchyma and hypertrophic lenticels of flooded plants. Water level was maintained at 3·5 cm that was the same level as in pre-flooded conditions, so aerenchyma and lenticels just above the water surface could be exposed to 18O2 gas layer. Flood Ae-Submerged: 18O2 was supplied to the basal region of the stem, but above the region that contained aerenchyma and lenticels in flooded plants. Aerenchyma and hypertrophic lenticels were well-developed in the basal stem, but the water level was raised to cover them. Water level was maintained at 6 cm that was 2·5 cm higher than the level of pre-flooded conditions, so aerenchyma and lenticels of the stem base were completely submerged.
Plants were harvested before exposure to the 18O2 gas, and at 0·5, 1·0 and 3·0 h after exposure to the 18O2. They were then separated into shoots and roots (including nodules). The fresh weights (FW) of the samples were measured, and they were then placed in a cryogenic vacuum distillation apparatus for several hours to extract water from the tissues. The dry weights (DW) of the shoots and roots were then measured after oven-drying at 80 °C for 48 h. The water samples extracted from the shoots and roots were analysed to determine their O2 isotope ratios using an isotope-ratio mass spectrometer (Delta plus LX; Thermo Fisher Scientific, Waltham, MA, USA) with an automated CO2–O2 equilibration unit. All O2 isotope ratios were expressed as the δ18O values (‰) relative to the Vienna-standard mean ocean water (V-SMOW) values:
where R = the 18O/16O ratio for the sample and the standard (respectively).
The weight of plant water (=FW−DW) and O2 isotope ratios of the extracted water were used to estimate the volume of 18O2 gas transported to each tissue over the 3-h period:
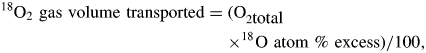 |
where O2total is the total amount of O2 (mol) in the plant water after 3 h of exposure, and 18O atom % excess is the increase in the 18O atom % after 3 h in the extracted water.
Experiment 3: Gas exchange through the aerenchyma
The plant materials and experimental conditions were the same as in expt 2 (Fig. 2), and non-planted conditions were used as blank controls. However, the gas layer in each pot was supplied with air or approx. 100 % O2 gas. The concentrations of O2 and CO2 in the gas layer were then measured in each treatment. Gas samples (0·2 mL) were extracted from the initial gas layer and the gas layer 3 h after exposure and were analysed using a gas chromatograph (GC-8APT; Shimadzu, Kyoto, Japan) with 3 mm (i.d.) × 2 m column of 60/80 mesh Molecular Sieve 5A for O2 and 3 mm (i.d.) × 2 m column of 50/80 mesh Porapak N for CO2, a column temperature of 80 °C, and helium as the carrier gas.
RESULTS
Experiment 1: O2 dynamics in aerenchymatous stems
Radial distribution of O2 in aerenchymatous stems
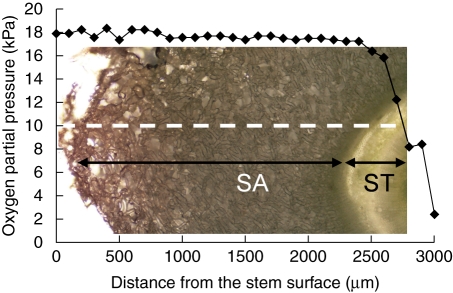
The radial distribution of O2 in an aerenchymatous stem at 15 mm below the water level is shown in Fig. 3. At the stem surface, pO2 was 18 kPa. As the microelectrode began moving into the tissues at 100 µm per step, the internal pO2 in the aerenchymatous layers (approx. 2500 µm in thickness) between the stem surface and the stele remained relatively stable at about 17 kPa, but pO2 gradually decreased to between 15 and 16 kPa at the boundary of the stele. As the electrode tip entered the stele, pO2 decreased rapidly, reaching 8 kPa at a distance of 200–300 µm inside the stele.
Fig. 3.
Radial O2 profile across an aerenchymatous stem of an intact soybean plant at a position 15 mm below the water level. The white broken line represents the path of the O2 microelectrode inserted from the stem surface through the secondary aerenchyma and into the stele. The experiment was repeated twice (not shown). SA, Secondary aerenchyma; ST, stele (vascular cylinder).
O2 movement in the stem aerenchyma
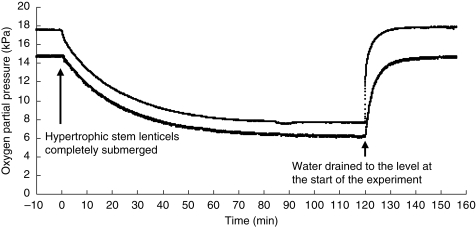
Two microelectrode tips were positioned within the aerenchyma approx. 1 mm from the stem surface, and internal pO2 values of 17·5 kPa measured at a depth of 13 mm below the water level and 14·7 kPa at 51 mm. The pO2 values had remained stable for >10 min before the start of the experiment (Fig. 4). After raising the water level enough to submerge the hypertrophic stem lenticels completely, pO2 gradually declined for the first 60 min, then slowly decreased for the next 60 min, and finally reached 7·6 kPa (13 mm) and 6·1 kPa (51 mm), representing decreases to about 40 % of the values at the start of experiment. When the water level was reduced to the same level as at the start of experiment, thereby re-exposing the hypertrophic stem lenticels to the air, internal pO2 values increased dramatically and reached approximately the same levels as at start of the experiment after only 10 min. The pO2 profiles remained stable thereafter.
Fig. 4.
The effect of complete submergence of hypertrophic stem lenticels on pO2 values in aerenchyma in the lower stem of soybean. The upper and lower lines represent the pO2 values in the stem aerenchyma at 13 and 51 mm below the water level, respectively. The experiment was repeated twice (not shown)
Experiment 2: O2 supply from the stem to the roots via the aerenchyma
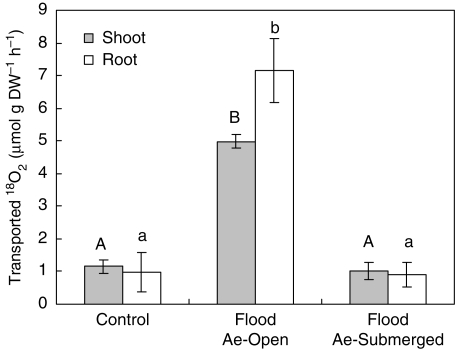
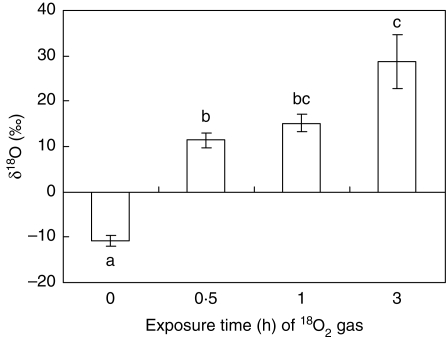
After the non-aerenchymatous stems of the control plants were supplied with 18O2 for 3 h, the volume of 18O2 transported was about 1 µmol g DW−1 h−1 in both the shoots and the roots of the control plants (Fig. 5). Similarly, in plants continuously flooded for about 5 weeks, after exposure of the basal stem without aerenchyma or lenticels to 18O2 for 3 h (Flood Ae-Submerged conditions), the volume of 18O2 transported in the roots and shoots of plants in this treatment was not significantly different from the value of about 1 µmol g DW−1 h−1 in the control plants (Fig. 5). In contrast, when the basal region of the flooded stem with the lenticels was exposed to 18O2 for 3 h (Flood Ae-Open conditions), the volume of 18O2 transported (5·0 µmol g DW−1 h−1 in shoots and 7·2 µmol g DW−1 h−1 in roots) was significantly higher than in the control and the Flood Ae-Submerged plants (Fig. 5). Moreover, the δ18O values in the roots from 0 to 3 h increased steadily over time, with significant (P < 0·05) 18O2 enrichment detected after only 0·5 h of exposure to the 18O2 (Fig. 6).
Fig. 5.
Volume of 18O2 transported from the stem to the roots with or without exposure of the aerenchyma to the 18O2 gas in each treatment. See Fig. 2 for an illustration of the treatment conditions. Flood Ae-Open, aerenchyma and lenticels above the water; Flood Ae-Submerged, submerged aerenchyma and lenticels. Values are the mean (±s.e.) of four or five plants. Means in the shoot followed by the same upper-case letter and means in the root followed by the same lower-case letter do not differ significantly (P < 0·01, Tukey–Kramer's test).
Fig. 6.
δ18O enrichment (‰) of water extracted from flooded soybean root tissues during 3 h of exposure of aerenchyma in the basal stem to 18O2 gas. Values are the mean (±s.e.) of three plants. Bars labelled with the same letter do not differ significantly (P < 0·05, Tukey's test).
Experiment 3: Gas exchange through the aerenchyma
Table 3 shows the rates of decrease in O2 and increase in CO2 in the gas layer in each treatment. The rate of decrease in O2 (56·6 µmol g DWRoot−1 h−1 in air and 191 µmol g DWRoot−1 h−1 in O2 treatments) in the Flood Ae-Open plants was significantly higher than in the control and the Flood Ae-Submerged plants. Similarly, the rate of increase in CO2 (22·2 µmol g DWRoot−1 h−1 in air and 33·4 µmol g DWRoot−1 h−1 in O2 treatments) in the Flood Ae-Open plants was significantly higher than in the other conditions. Aerenchyma and lenticels at the base of the flooded stem enhanced exchange of these gases between flooded tissues and gas layer.
Table 3.
Rates of decrease in O2 and increase in CO2 in the gas space above the water in each treatment
| Gas treatment in gas space | Plant conditions | Decreased O2 (μmol g−1 DWRoot h−1) | Increased CO2 (μmol g−1 DWRoot h−1) |
|---|---|---|---|
| Air | Control | 1·72 ± 0·56a | 0·98 ± 0·10a |
| Flood Ae-Open | 56·6 ± 3·49b | 22·2 ± 0·27c | |
| Flood Ae-Submerged | 12·2 ± 0·98a | 7·49 ± 0·82b | |
| Oxygen | Control | 15·8 ± 6·84a | 0·82 ± 0·22a |
| Flood Ae-Open | 191 ± 18·3b | 33·4 ± 1·68c | |
| Flood Ae-Submerged | 35·2 ± 13·4a | 8·12 ± 0·34b |
See Fig. 2 for an illustration of the treatment conditions.
Flood Ae-Open, Aerenchyma and lenticels above the water; Flood Ae-Submerged, submerged aerenchyma and lenticels.
Values are the mean (±s.e.) of three plants.
Means followed by the same letter within each column for the treatments do not differ significantly (P < 0·01, Tukey's test).
DISCUSSION
Radial O2 profile in stem aerenchyma
In the distribution of O2 within aerenchymatous tissues, it has been reported that radial O2 profile was studied for cortical aerenchyma in several plants (Armstrong et al., 2000; Darwent et al., 2003; De Simone et al., 2003), but there is no information about the profile of secondary aerenchyma. The O2 profile of soybean stems in the present study remained stable at pO2 values between 15 and 17 kPa in the aerenchymatous layers (secondary aerenchyma), but decreased rapidly to about 2 kPa in the stele (Fig. 3). This profile was similar to that of root with cortical aerenchyma; e.g. Armstrong et al. (2000) demonstrated that pO2 remained stable at approx. 15 kPa in the aerenchymatous cortex of Phragmites australis at 100 mm from the root apex, but decreased to 12 kPa in the stele at that position. Similarly, the O2 levels were higher in cortical lysigenous aerenchyma than in the stele at different positions along a Z. mays root (Darwent et al., 2003). Therefore, the O2 levels were stable and high from the outer to the inner aerenchyma (both cortical and secondary); in contrast, O2 levels decreased at all positions as the microelectrode penetrated into the stele.
O2 transport through lenticels and aerenchyma
The swelling of the stem and the hypertrophic lenticels in submerged portions of the lower stem appear to be the first entry points for O2. These structures facilitate O2 entry into the aerenchyma of nearby adventitious roots in various herbaceous dicots and woody species (Jackson and Ricard, 2003). Stevens et al. (2002) reported that artificial disruption of the continuity of the aerenchymatous tissues that develop in submerged portions of the lower shoot in Lythrum salicaria caused a significant reduction in root O2 levels, indicating inhibition of O2 transport from the atmosphere into the roots. Salix viminalis (Jackson and Attwood, 1996) and some woody plants (Armstrong, 1968) show inhibition of O2 transport to the roots and reduced plant growth when hypertrophic lenticels at the base of the stem are blocked.
In soybean, hypertrophic stem lenticels also appear to be the first entry points of O2 into the aerenchyma because experimental blocking of these lenticels inhibits plant growth and root nodule activity (Shimamura et al., 2002, 2003). This was confirmed by the present experiments, in which pO2 gradually decreased for 120 min within aerenchyma in the lower stem when the hypertrophic stem lenticels were completely submerged (Fig. 4). The decrease in pO2 in the aerenchyma would result from both O2 diffusion into the surrounding water such as radial O2 loss and from O2 consumption by living cells of the stem, roots and aerenchyma. A similar reaction was reported in Alnus glutinosa: the O2 concentration in the rhizosphere decreased from 100 µmol L−1 to almost 0 µmol L−1 within a few minutes when N2 gas was applied to the base of stems with well-developed lenticels under flooded conditions (Dittert et al., 2006). In the present study, internal pO2 in the aerenchyma of the submerged stems increased steeply when lenticels in the upper stem were exposed to the air for a few minutes (Fig. 4), and there was little difference in the response time for the increased influx of atmospheric O2 between the two sensor positions, even though the distance between the sensors was 38 mm. These results indicate high gas diffusibility in the aerenchyma and that aerenchyma development in the stem provides a ‘snorkel’ that supplies flooded tissues with O2.
The O2 isotope experiments also indicated that the aerenchyma provides O2 transport into flooded roots. When the basal region of a stem that contained lenticels and aerenchyma was exposed to 18O2, significant enrichment of δ18O in water extracted from the root system was confirmed within 0·5 h (Fig. 6), and 7·2 µmol g DW−1 h−1 of 18O2 was transported into the roots (Fig. 5). In addition, the volume of 18O2 transported to the shoots was 5·0 µmol g DW−1 h−1 under the same conditions. On the other hand, slight enrichment of 18O2 in the roots and the shoot was observed after exposure of non-aerenchymatous stem tissue to 18O2 under control and flooded conditions (Fig. 5), indicating that the non-aerenchymatous stem has a low ability to transport O2 to the roots, and that little water was produced by respiration using 18O2 in the stem. So, it seems that the volume of 18O2 in the shoot under flooded Ae-Open conditions, which was 5 times the value observed under the other two experimental conditions (Fig. 5), was due to greater production of water by root respiration and its movement into the shoots via the xylem. Dittert et al. (2006) reported that the O2 transport rate into the root system of 2-year-old A. glutinosa averaged 0·12 mmol h−1 plant−1 in a flooded stem with well-developed lenticels, versus 0·01 mmol h−1 plant−1 in a non-flooded stem with poorly developed lenticels. By the way, the rate of decrease in O2 in the gas layer was somewhat higher in the Flood Ae-Submerged plants than in the control plants (Table 3), which might mean the difference in O2 diffusivity between paraffin and water, i.e. O2 was easily dissolved in water. However, these results did not agreed with the 18O2 experiment (Fig. 5) which did not show the difference in the volume of 18O2 in roots between the Flood Ae-Submerged plants and the control plants. So an effect of O2 diffusion from the gas layer to water in order to supply flooded tissues was not confirmed in the present studies.
In the present experiments, 18O2 gas transport was calculated equal to 7·2 µmol g DW−1 h−1 based on the enrichment of δ18O in the water of root tissues after 3 h of supplying 18O2 gas to the lenticels in the basal region of the stem (Fig. 5). However, this volume might have been underestimated because the water produced by root respiration is available for cellular metabolism and for leaf transpiration. In addition, the rate of decrease in O2 in the gas layer (Fig. 2) was 191 µmol g DWRoot−1 h−1 under the same conditions (Table 3).
The diffusion of CO2, produced in the roots by respiration and in the soil by micro-organisms under flooding, to the atmosphere is very slow, and a high CO2 level in the root zone inhibits growth of soybean under flooding and anoxia (Boru et al., 2003; Araki, 2006). However, aerenchyma and lenticels at the base of the flooded stem accelerated venting of CO2 (Table 3), so secondary aerenchyma may reduce the likelihood of CO2 accumulating in the flooded soils and root tissues.
Conclusions
The results showed that hypertrophic lenticels in the lower stem of soybean, just above the water surface, facilitate O2 entry into the aerenchyma, after which the aerenchyma enhance O2 transport into roots in the flooded soil. Although soybean plants can produce aerenchyma in their root systems, they are nonetheless very sensitive to flooding stress (Table 2). The development of an aerenchymatous network in stems, adventitious roots and root nodules requires a few weeks after flooding begins, so the diffusion of atmospheric O2 into flooded tissues is limited to O2 that penetrates the non-aerenchymatous stem and O2 absorbed at the water surface. Thus, soybean plants suffer from flooding stress until they develop a sufficient aerenchymatous network. In contrast, many wetland plants possess aerenchyma in their roots even under aerobic conditions (Justin and Armstrong, 1987), and are thus able to adapt rapidly to sudden flooding. Therefore, to improve the flood tolerance of soybean, it will be necessary to investigate the potential for improvement of aerenchyma characteristics, such as diffusibility, porosity, amount, and rate of formation. Further research will also be needed to elucidate the regulation of aerenchyma formation to accelerate aerenchyma formation under aerobic conditions.
ACKNOWLEDGEMENTS
We thank Drs T. Mochizuki and S. Yoshida of Kyushu University, Japan, for technical advice on the experiment, and Drs N. Nakayama and S. Hiraga of the National Institute of Crop Science, Japan, for helpful comments and discussion during this study. This work was funded by the Development of Innovative Crops through the Molecular Analysis of Useful Genes Program (2205, 2209) of the National Agriculture and Food Research Organization of Japan.
LITERATURE CITED
- Araki H. Water uptake of soybean (Glycine max L. Merr.) during exposure to O2 deficiency and field level CO2 concentration in the root zone. Field Crops Research. 2006;96:98–105. [Google Scholar]
- Arber A. Water plants: a study of aquatic angiosperms. Cambridge: Cambridge University Press; 1920. [Google Scholar]
- Armstrong W. Oxygen diffusion from the roots of woody species. Physiologia Plantarum. 1968;21:539–543. [Google Scholar]
- Armstrong W. Aeration in higher plants. Advances in Botanical Research. 1979;7:225–332. [Google Scholar]
- Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Annals of Botany. 2000;86:687–703. [Google Scholar]
- Boru G, VanToai T, Alves J, Hua D, Knee M. Responses of soybean to oxygen deficiency and elevated root-zone carbon dioxide concentration. Annals of Botany. 2003;91:447–453. doi: 10.1093/aob/mcg040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmer TD, Pedersen O. Oxygen dynamics in submerged rice (Oryza sativa) New Phytologist. 2008;178:326–334. doi: 10.1111/j.1469-8137.2007.02364.x. [DOI] [PubMed] [Google Scholar]
- Dacey JWH, Klug MJ. Tracer studies of gas circulation in Nuphar: 18O2 and 14CO2 transport. Physiologia Plantarum. 1982;56:361–366. [Google Scholar]
- Darwent MJ, Armstrong J, Armstrong W, Beckett PM. Exploring the radial and longitudinal aeration of primary maize roots by means of Clark-type oxygen microelectrodes. Russian Journal of Plant Physiology. 2003;50:722–732. [Google Scholar]
- De Simone O, Junk WJ, Schmidt W. Central Amazon floodplain forests: root adaptations to prolonged flooding. Russian Journal of Plant Physiology. 2003;50:848–855. [Google Scholar]
- Dittert K, Wötzel J, Sattelmacher B. Responses of Alnus glutinosa to anaerobic conditions: mechanisms and rate of oxygen flux into the roots. Plant Biology. 2006;8:212–223. doi: 10.1055/s-2005-873041. [DOI] [PubMed] [Google Scholar]
- Drew MC, Saglio PH, Pradet A. Larger adenylate energy charge and ATP/ADP ratios in aerenchymatous roots of Zea mays in anaerobic media as a consequence of improved internal oxygen transport. Planta. 1985;165:51–58. doi: 10.1007/BF00392211. [DOI] [PubMed] [Google Scholar]
- Evans DE. Aerenchyma formation. New Phytologist. 2003;161:35–49. [Google Scholar]
- Fraser L. The reaction of Viminaria denudata to increased water content of the soil. Proceedings of the Linnean Society of New South Wales. 1931;56:391–406. [Google Scholar]
- Griffin JL, Saxton AM. Response of solid-seeded soybean to flood irrigation. II. Flood duration. Agronomy Journal. 1988;80:885–888. [Google Scholar]
- Jackson MB, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology. 1999;1:274–287. [Google Scholar]
- Jackson MB, Attwood PA. Roots of willow (Salix viminalis L.) show marked tolerance to oxygen shortage in flooded soils and in solution culture. Plant and Soil. 1996;187:37–45. [Google Scholar]
- Jackson MB, Ricard B. Physiology, biochemistry and molecular biology of plant root systems subjected to flooding of the soil. In: De Koon H, Visser EJW, editors. Root ecology. Berlin: Springer; 2003. pp. 193–213. [Google Scholar]
- Jackson MB, Ishizawa K, Osamu I. Evolution and mechanisms of plant tolerance to flooding stress. Annals of Botany. 2009;103:137–142. doi: 10.1093/aob/mcn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justin SHFW, Armstrong W. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist. 1987;105:465–495. [Google Scholar]
- Laan P, Berrevoets MJ, Lythe S, Armstrong W, Blom CWM. Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. Journal of Ecology. 1989;77:693–703. [Google Scholar]
- Linkemer G, Board JE, Musgrave ME. Waterlogging effect on growth and yield components of late-planted soybean. Crop Science. 1998;38:1576–1584. doi: 10.2135/cropsci1998.0011183x003800060028x. [DOI] [PubMed] [Google Scholar]
- Metcalfe CR. Bulletin of miscellaneous information. London: His Majesty's Stationery Office; 1931. The ‘aerenchyma’ of Sesbania and Neptunia; pp. 151–154. [Google Scholar]
- Pedersen O, Vos H, Colmer TD. Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant, Cell & Environment. 2006;29:1388–1399. doi: 10.1111/j.1365-3040.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Saraswati R, Matoh T, Sekiya J. Nitrogen fixation of Sesbania rostrata: contribution of stem nodules to nitrogen acquisition. Soil Science and Plant Nutrition. 1992;38:775–780. [Google Scholar]
- Scott DH, Wager H. On the floating-root of Sesbania aculeata, Pers. Annals of Botany. 1888;1:308–314. [Google Scholar]
- Scott HD, De Angulo J, Daniels MB, Wood LS. Flood duration effects on soybean growth and yield. Agronomy Journal. 1989;81:631–636. [Google Scholar]
- Shiba H, Daimon H. Histological observation of secondary aerenchyma formed immediately after flooding in Sesbania cannabina and S. rostrata. Plant and Soil. 2003;255:209–215. [Google Scholar]
- Shimamura S, Mochizuki T, Nada Y, Fukuyama M. Secondary aerenchyma formation and its relation to nitrogen fixation in root nodules of soybean plants (Glycine max) grown under flooded conditions. Plant Production Science. 2002;5:294–300. [Google Scholar]
- Shimamura S, Mochizuki T, Nada Y, Fukuyama M. Formation and function of secondary aerenchyma in hypocotyl, roots and nodules of soybean (Glycine max) under flooded conditions. Plant and Soil. 2003;251:351–359. [Google Scholar]
- Smirnoff N, Crawford RMM. Variation in the structure and response to flooding of root aerenchyma in some wetland plants. Annals of Botany. 1983;51:237–249. [Google Scholar]
- Sojka RE. Soil oxygen effects on two determinate soybean isolines. Soil Science. 1985;140:333–343. [Google Scholar]
- Stevens KJ, Peterson RL, Reader RJ. The aerenchymatous phellem of Lythrum salicaria (L.): a pathway for gas transport and its role in flood tolerance. Annals of Botany. 2002;89:621–625. doi: 10.1093/aob/mcf088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AL, Guerreiro SMC, Sodek L. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Annals of Botany. 2005;96:1191–1198. doi: 10.1093/aob/mci272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Voesenek LACJ. Acclimation to soil flooding – sensing and signal – transduction. Plant and Soil. 2004;254:197–214. [Google Scholar]
- Walker BA, Pate JS, Kuo J. Nitrogen fixation by nodulated roots of Viminaria juncea (Schrad. & Wendl.) Hoffmans. (Fabaceae) when submerged in water. Australian Journal of Plant Physiology. 1983;10:409–421. [Google Scholar]
- Williams WT, Barber DA. The functional significance of aerenchyma in plants. Symposia of the Society for Experimental Biology. 1961;15:132–144. [Google Scholar]
- Yoshida S, Eguchi H. Environmental analysis of aerial O2 transport through leaves for root respiration in relation to water uptake in cucumber plants (Cucumis sativus L.) in O2-deficient nutrient solution. Journal of Experimental Botany. 1994;45:187–192. [Google Scholar]