Abstract
Glioblastoma multiforme (GBM) is the most common and most aggressive primary brain tumor in humans. Systemic immunity against gene therapy vectors has been shown to hamper therapeutic efficacy; however, helper-dependent high-capacity adenovirus (HC-Ad) vectors elicit sustained transgene expression, even in the presence of systemic anti-adenoviral immunity. We engineered HC-Ads encoding the conditional cytotoxic herpes simplex type 1 thymidine kinase (TK) and the immunostimulatory cytokine fms-like tyrosine kinase ligand 3 (Flt3L). Flt3L expression is under the control of the regulatable Tet-ON system. In anticipation of a phase I clinical trial for GBM, we assessed the therapeutic efficacy, biodistribution, and clinical and neurotoxicity with escalating doses of HC-Ad-TetOn-Flt3L + HC-Ad-TK in rats. Intratumoral administration of these therapeutic HC-Ads in rats bearing large intracranial GBMs led to long-term survival in ~70% of the animals and development of antiglioma immunological memory without signs of neuropathology or systemic toxicity. Systemic anti-adenoviral immunity did not affect therapeutic efficacy. These data support the idea that it would be useful to develop HC-Ad vectors further as a therapeutic gene-delivery platform to implement GBM phase I clinical trials.
Glioblastoma multiforme (GBM), the most common primary brain tumor in adults, carries the dismal prognosis of 15–21 months median survival.1–3 Its current clinical management comprises surgical resection followed by chemotherapy and radiotherapy.4–6 However, given GBM's highly infiltrative nature, total resection is not possible and the tumor inevitably recurs. Recurrent GBM is resistant to chemotherapy and radiotherapy and eventually leads to the patient's death.2 Immunotherapeutic approaches, currently under preclinical and clinical testing, constitute a promising adjuvant treatment for GBM.7–13
We previously engineered first-generation adenoviral vectors (Ads) that deliver a combination of therapeutic transgenes for the treatment of GBM. The conditionally cytotoxic herpes simplex type 1 thymidine kinase (TK)8,12 kills proliferating tumor cells in the presence of the prodrug ganciclovir (GCV), and human soluble fms-like tyrosine kinase ligand 3 (Flt3L) recruits bone marrow–derived dendritic cells into the brain tumor milieu, triggering an anti-GBM-specific immune response.8–9,13 It has been shown that the administration of Ad-TK + Ad-Flt3L into the tumor mass leads to long-term survival in rats bearing intracranial CNS-1, 9L, F98, and RG2 tumors12,13 and in mice bearing intracranial GL26, GL261, and B16-F10 tumors.8 In addition, Ad-Flt3L + Ad-TK induces GBM-specific immunological memory that improves survival in intracranial multifocal and recurrent models of GBM.8,9,14,15
The outcome of delivering TK alone has been studied in clinical trials using first-generation Ads.16 Injection of Ad-TK into the margins of the tumor cavity after surgical resection of the GBM was well tolerated in more than 70 patients in six early clinical trials.17,18 Final results from a large, multicenter phase III trial are eagerly awaited; however, the interim data, although they are promising, reveal that there is room for improvement with respect to the efficacy of the treatment.19 Systemic anti-adenoviral immunity, which is present in most human patients20 and which curtails transgene expression,21–23 could limit the therapeutic efficacy of Ad-TK.
In an effort to evade the immune system, “gutless” high-capacity adenovirus (HC-Ad) vectors have been developed that are devoid of all viral genes.24,25 In the presence of anti-adenoviral immunity, transgene expression from first-generation Ads is eliminated within 4 weeks, whereas expression from HC-Ad vectors remains stable in the brain for at least 1 year.26–28 We recently showed the lack of efficacy of first-generation Ad-TK vectors in tumor-bearing rats preimmunized against Ads.23 Conversely, HC-Ad-mediated expression of TK led to long-term survival in >50% of the preimmunized rats.23 In the light of our earlier finding that single-cytotoxic therapies do not elicit antiglioma immunity,8–10,12 we combined the HC-Ad-TK vector with a HC-Ad vector encoding Flt3L under the control of a tightly regulatable mCMV-TetOn expression system (HC-Ad-TetOn-Flt3L29–31) for future implementation in GBM clinical trials.
This study involved dose escalation of intratumoral injections of HC-Ad-TetOn-Flt3L + HC-Ad-TK in rats bearing intracranial GBM. We assessed the biodistribution and transgene expression as well as putative systemic and neurological toxicity. Therapeutic efficacy was evaluated in naive and preimmunized rats. This is the first study involving the combination of two HC-Ad vectors encoding a conditional cytotoxic gene and a tightly regulatable immunostimulatory gene in a preclinical animal model of brain cancer. Taken together, these data demonstrate the high safety profile and therapeutic efficacy of the HC-Ad vector platform for the delivery of therapeutic genes to the brain and suggest that there are potential advantages in pursuing further downstream process development for their eventual use in phase I clinical trials in human patients.
RESULTS
In vitro characterization of HC-Ad-TetOn-Flt3L + HC-Ad-TK
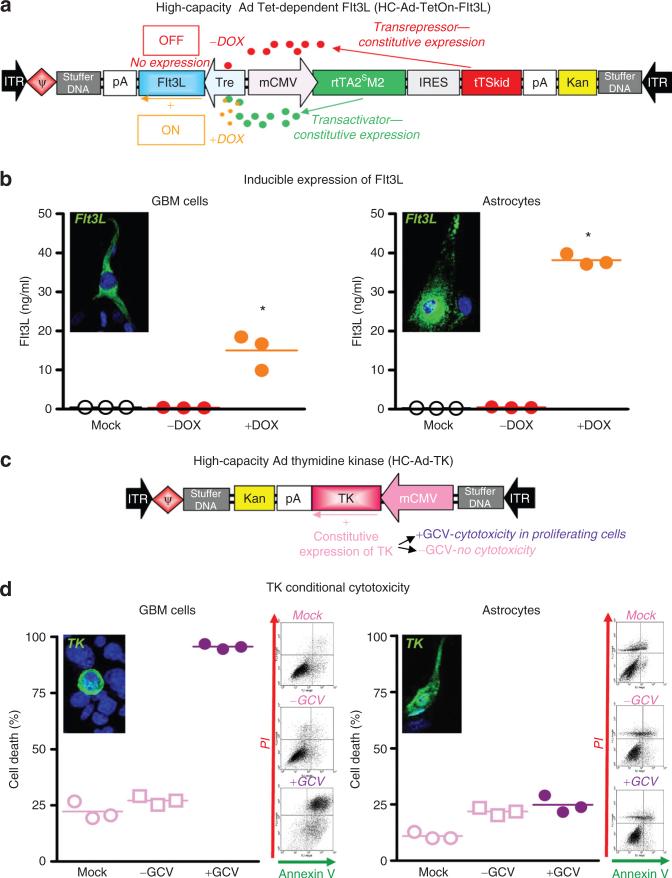
Characterization of HC-Ads was performed in vitro in rat CNS-1 GBM cells and in primary astrocyte cultures. Infection with HC-Ad-TetON-Flt3L (Figure 1a) led to doxycycline-dependent Flt3L release in both GBM cells and astrocytes, with negligible release in the OFF state (Figure 1b). Flt3L expression was also confirmed by immunofluorescence. Expression of TK in cells infected with HC-Ad-TK (Figure 1c) was readily observed by immunofluorescence in GBM cells and astrocytes. However, the presence of the prodrug GCV exerted a cytotoxic effect only in CNS-1, leaving primary astrocytes unaffected (Figure 1d).
Figure 1.
Structure and in vitro characterization of therapeutic HC-Ads. (a) Structure and transcriptional regulation of HC-Ad-TetOn-Flt3L. (b) CNS-1 GBM cells and astrocytes in primary culture were infected with HC-Ad-TetOn-Flt3L with or without the inducer, DOX. Flt3L expression was assessed by immunofluorescence and ELISA. *P < 0.05 vs. mock infection. One-way ANOVA followed by Tukey's test. (c) Illustration depicting structure and function of HC-Ad-TK. (d) CNS-1 GBM cells and astrocytes in primary culture were infected with HC-Ad-TK and incubated with or without the prodrug GCV. TK expression was assessed by immunofluorescence, and cell death was determined by flow cytometric analysis of Annexin V/PI-stained cells. *P < 0.05 vs. mock infection. One-way ANOVA followed by Tukey's test. ANOVA, analysis of variance; DOX, doxycycline; ELISA, enzyme-linked immunosorbent assay; Flt3L, fms-like tyrosine kinase ligand 3; GBM, glioblastoma multiforme; GCV, ganciclovir; HC-Ad, high-capacity adenovirus; PI, propidium iodide; TK, thymidine kinase.
Intratumoral delivery of HC-Ad-TetOn-Flt3L + HC-Ad-TK mediates long-term survival up to 1 year after treatment
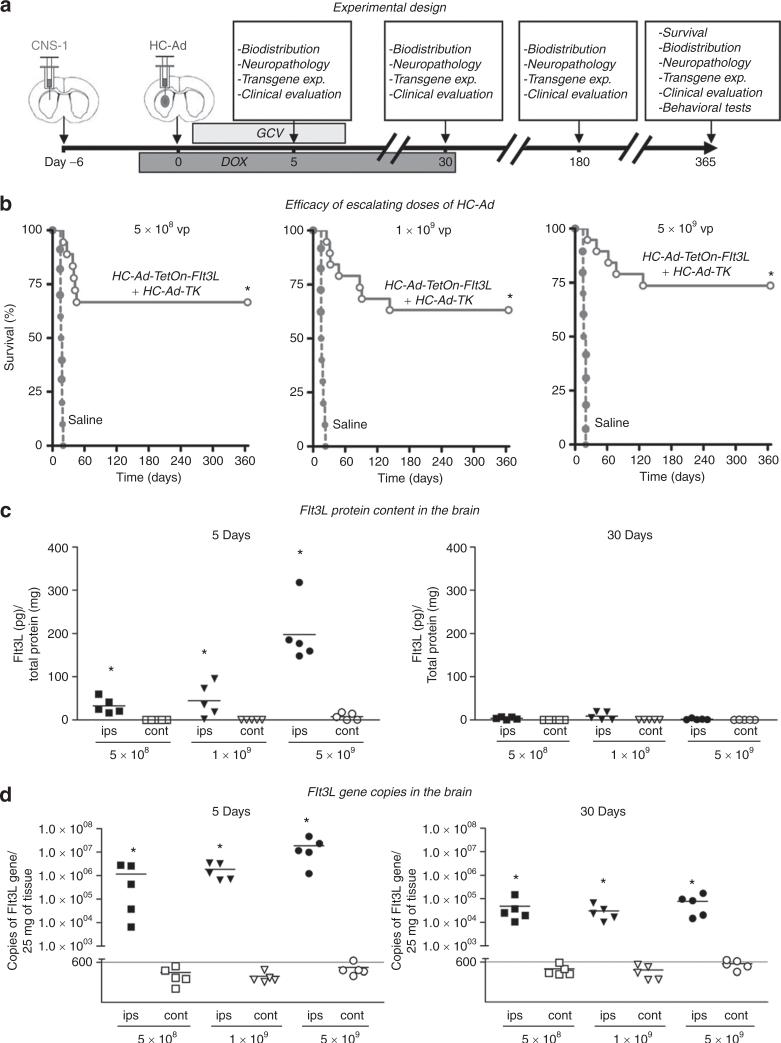
As a prelude to a phase I clinical trial in GBM patients, a dose-escalation study was performed in rats to assess the efficacy and neuropathology of intratumoral delivery of HC-Ad-TetOn-Flt3L and HC-Ad-TK (Figure 2a). We also assessed the biodistribution of vector genomes and transgene expression at 5 days, 30 days, 6 months, and 1 year after treatment. Each rat bearing an intracranial CNS-1 tumor received an intratumoral injection of either 5 × 108 vp, 1 × 109 vp, or 5 × 109 vp of each HC-Ad vector or saline. GCV was administered twice daily for 10 days. The rats received doxycycline-containing chow for 4 weeks because this schedule was found to be optimal with respect to efficacy of the therapy (see Supplementary Figure S1 online). All three doses led to tumor regression and survival for at least 1 year in ~70% of rats (Figure 2b).
Figure 2.
Efficacy and Flt3L expression after intratumoral injection of escalating doses of HC-Ads. (a) Three escalating doses of HC-Ad-TK and HC-Ad-TetOn-Flt3L (5 × 108 vp, 1 × 109 vp, and 5 × 109 vp, n = 18–19/group) or saline (n = 10/group) were delivered intratumorally into 6-day intracranial CNS-1 tumors in rats. (b) Kaplan–Meier survival curves show efficacy of HC-Ad treatment. *P < 0.05 vs. saline (log-rank test). (c) Levels of Flt3L protein in brain hemispheres ipsilateral and contralateral to HC-Ad injection site were assessed by ELISA at 5 and 30 days after treatment (n = 5/group). *P < 0.05 vs. contralateral (Student's t-test). (d) DNA was isolated from both brain hemispheres (n = 5/group), and Flt3L transgene copies were quantified using quantitative PCR at 5 and 30 days after HC-Ad delivery. *P < 0.05 vs. contralateral (Student's t-test). cont, contralateral; ELISA, enzyme-linked immunosorbent assay; Flt3L, fms-like tyrosine kinase ligand 3; GCV, ganciclovir; HC-Ad, high-capacity adenovirus; ips, ipsilateral; TK, thymidine kinase.
Flt3L protein expression and copies of Flt3L transgene decrease within 30 days of HC-Ad delivery
The levels of Flt3L protein and transgene copies were determined in the brain 5 and 30 days after intratumoral administration of HC-Ads. Using an enzyme-linked immunosorbent assay, Flt3L protein was detected only in the injected hemisphere of the brain at day 5 after the treatment and became undetectable at day 30 (Figure 2c). Flt3L protein was not found in the contralateral hemisphere of the brain. We also quantified the copies of the Flt3L transgene, using real-time quantitative PCR. Flt3L transgene was detected only in the injected hemisphere of the brain at both day 5 and day 30 after the HC-Ad injection (Figure 2d).
Biodistribution of HC-Ad vector genomes is restricted to the brain hemisphere ipsilateral to the injection site at all vector doses and time points tested
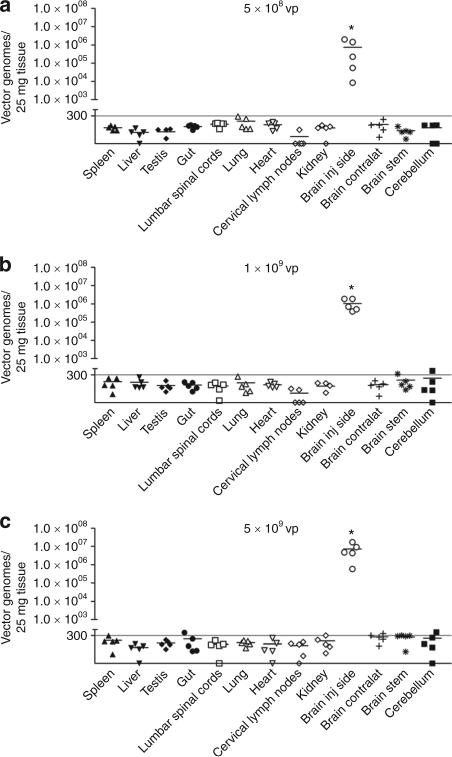
We assessed the biodistribution of HC-Ad vector genomes in the CNS and in peripheral organs at 5 days, 30 days, 6 months, and 1 year after treatment, using quantitative PCR analysis. At all doses and time points, HC-Ad genomes were restricted to the injected hemisphere of the brain (Figure 3 and see Supplementary Figure S3 online), where a substantial decrease in HC-Ad vector genomes was detected between days 5 and 30 after the administration of HC-Ad. These findings are in agreement with the decline in Flt3L transgene copy number observed during the same time period (Figure 2d). Importantly, HC-Ad vector genomes were below detectable limits in all peripheral organs, including other regions of the CNS and the liver, even at the highest dose tested at all time points (Figure 3 and see Supplementary Figure S3 online), thereby indicating the high safety profile of this combined approach.
Figure 3.
Biodistribution of vector genomes in tumor-bearing animals. HC-Ad vector genomes were quantified in the tissues indicated at 5 days after intratumoral injection of escalating doses of HC-Ad-TetOn-Flt3L and HC-Ad-TK (a, 5 × 108 vp; b, 1 × 109 vp; and c, 5 × 109 vp). The dotted line indicates the detection limit. Flt3L, fms-like tyrosine kinase ligand 3; HC-Ad, high-capacity adenovirus; TK, thymidine kinase.
Analysis of neuropathology and clinical laboratory parameters
To examine the effects of HC-Ad-TetOn-Flt3L and HC-Ad-TK delivery and subsequent brain tumor regression on brain architecture and inflammation, we performed an extensive neuropathological analysis of brain sections at 5 days, 1 month, 6 months, and 1 year after intratumoral HC-Ad delivery. The three doses led to similar findings with respect to neuropathology. Nissl staining revealed a dramatic reduction in tumor burden within 5 days of HC-Ad delivery as compared to saline-treated animals. Myelin basic protein and tyrosine hydroxylase immunoreactivity indicated rapid restoration of brain architecture, even at this early time point. Profuse infiltration of CD8+ T cells, macrophages, and major histocompatibility complex II+ cells was localized only within the tumor-bearing hemisphere of the brain (Figure 4).
Figure 4.
Neuropathological analysis of samples from tumor-bearing Lewis rats treated with HC-Ads. Neuropathological analysis of the brains of rats at 5 days after intratumoral administration of escalating doses of HC-Ad-TetOn-Flt3L and HC-Ad-TK. Flt3L, fms-like tyrosine kinase ligand 3; MBP, myelin basic protein; MHC II, major histocompatibility complex II; TH, tyrosine hydroxilase; TK, thymidine kinase.
One month after the treatment, although the infiltration of CD8+ T cells in the brain declined, sustained infiltration of macrophages and major histocompatibility complex II+ immune cells was observed (see Supplementary Figure S4 online). At this time point, all the saline-treated rats had succumbed to their tumor burden, whereas no remnants of the tumors were visible in ~70% of the rats that had received HC-Ad treatment, irrespective of the dose (see Supplementary Figure S4 online). At 6 months and at 1 year after HC-Ad delivery, we found complete restoration of the normal brain architecture—with the exception of minor ventriculomegaly, which was more accentuated in the rats that received the highest dose—in the brain hemisphere that harbored the tumor (Figure 5 and see Supplementary Figure S5 online). Residual macrophages were localized in the scar left after tumor regression.
Figure 5.
Long-term survivors do not show evidence of neuropathology at 1 year after treatment. Neuropathological analysis was performed of the brains of the rats that were long-term survivors, at 1 year after intratumoral administration of escalating doses of HC-Ad-TetOn-Flt3L and HC-Ad-TK. Flt3L, fms-like tyrosine kinase ligand 3; HC-Ad, high-capacity adenovirus; MBP, myelin basic protein; MHC II, major histocompatibility complex II, TH, tyrosine hydroxilase; TK, thymidine kinase.
Immunocytochemical characterization of transgene expression revealed abundant cells expressing TK and Flt3L within the tumor mass at day 5 after the treatment (Figure 4). Along with tumor regression, the presence of transgene-expressing cells declined with time; at 6 months and at 1 year after the treatment, only a few positive cells, probably non-neoplastic ones, remained in the scar area (Figure 5 and see Supplementary Figure S5 online). These data further highlight the fact that the conditional cytotoxicity of TK in combination with GCV is such that it does not kill normal brain cells and is a safe cytotoxic approach for brain cancer.
Serum chemistry tests were carried out and blood cell counts were assessed in the blood of tumor-bearing rats at 5 days, 30 days, 6 months, and 1 year after treatment. Analysis of biochemical laboratory parameters indicated normal liver and renal function, with concentrations of aspartate aminotransferase, alanine aminotransferase, bilirubin, urea, and creatinine within the normal range as seen in age-matched naive animals, at all time points and vector doses tested (see Supplementary Tables S1–S4 online). Red and white blood cell counts in the treated animals were also within normal ranges, indicating that Flt3L expression in the brain tumor does not substantially alter the levels of circulating immune cells.
HC-Ad-tetOn-Flt3L- and HC-Ad-TK (+GCV)-treated long-term survivors do not exhibit behavioral deficits
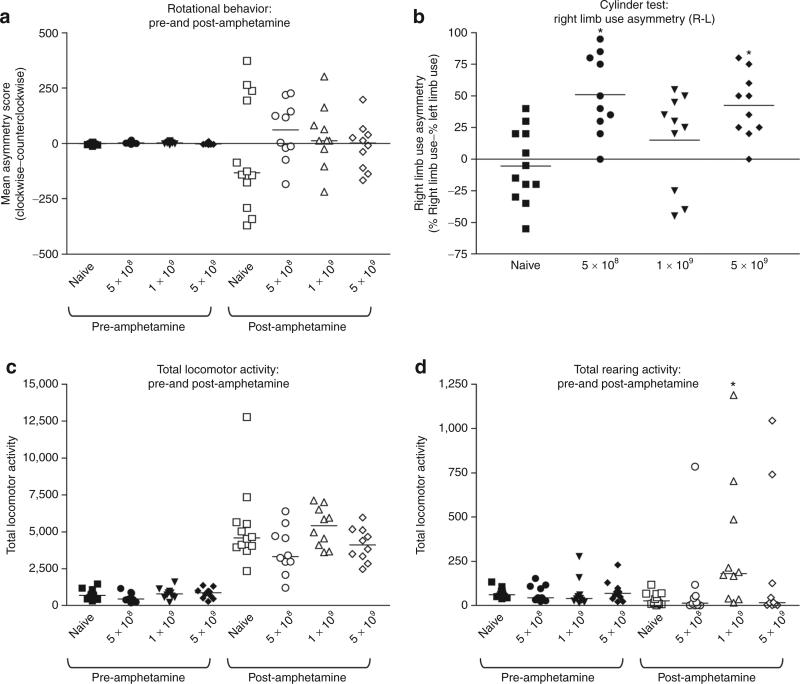
In order to rule out the occurrence of chronic neurological deficits, tumor-bearing animals treated with escalating doses of HC-Ad-TetOn-Flt3L and HC-Ad-TK long-term survivor animals were subjected to a panel of neurobehavioral tests 1 year after treatment. Analysis of amphetamine-induced rotational behavior and total locomotor activity failed to identify any behavioral abnormalities as compared with age-matched naive controls (Figure 6a,c). Abnormalities in limb-use asymmetry test and rearing activity test were detected in HC-Ad-treated long-term survivors after 1 year. However, these differences did not appear to be HC-Ad dose-dependent and are therefore probably a consequence of the regression of a very large brain tumor mass (Figure 6b,d).
Figure 6.
Behavioral assessment of long-term survivors 1 year after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK. Behavioral assessment was performed before and after amphetamine treatment in the animals that were long-term survivors, at 1 year after intratumoral administration of escalating doses of HC-Ad-TetOn-Flt3L and HC-Ad-TK. Naive, age-matched rats were used as controls. (a) Rotational behavior; (b) asymmetrical right-limb use; (c) spontaneous motor behavior; and (d) rearing behavior. Flt3L, fms-like tyrosine kinase ligand 3; HC-Ad, high-capacity adenovirus; TK, thymidine kinase.
HC-Ad-TetOn-Flt3L and HC-Ad-TK therapy improves survival of tumor-bearing animals having a preexisting anti-Ad immune response
Because most patients who undergo gene therapy clinical trials have previously been exposed to Ad in their lifetime,20 we assessed the efficacy of HC-Ad-TetOn-Flt3L and HC-Ad-TK (5 × 109 vp each) in a preclinical setting that models this likely clinical scenario (Figure 7a). Lewis rats were immunized with an intradermal injection of an Ad vector without a transgene (Ad0). Animals preimmunized against Ad displayed high levels of circulating neutralizing antibodies against Ad 2 weeks later, at the time of tumor implantation (Figure 7b). HC-Ad treatment led to the survival of >70% of the animals in both immunized and nonimmunized groups for at least 1 year (Figure 7b).
Figure 7.
HC-Ad-induced tumor regression and antiglioma immunological memory, despite active systemic anti-Ad immunity. (a) Illustration depicting the experimental design. Rats peripherally immunized against Ads (Ad0) were implanted with CNS-1 tumors in the brain and treated with 5 × 109 of each HC-Ad (n = 8–14/group), or saline (n = 6–7/group). (b) Kaplan–Meier survival curves show the survival of preimmunized and nonpreimmunized rats. *P < 0.05 vs. saline. Log-rank test. Scatter plot shows the titers of circulating anti-Ad NAB in each group. (c) Kaplan–Meier survival curves show the survival of preimmunized long-term survivors rechallenged in the contralateral striatum of the brain at 1 year after treatment. Naive animals were implanted with CNS-1 cells as controls. *P < 0.05 vs. naive. Log-rank test. DOX, doxycycline; GCV, ganciclovir; HC-Ad, high-capacity adenovirus; NAB, neutralizing antibodies.
Because recurrence of the tumor is one of the hallmarks of GBM, we sought to assess whether, despite anti-Ad immunity, HC-Ad treatment would induce anti-GBM immunological memory. For this purpose, we rechallenged preimmunized long-term survivor animals with a second tumor in the contralateral striatum (Figure 7c). Although no further treatment was administered, ~70% of the animals survived the rechallenge. These findings indicate that, despite the pre-existing anti-Ad immunity, treatment with HC-Ad-TetOn-Flt3L + HC-Ad-TK elicits immunological memory against GBM, and this is capable of preventing the progression of a recurrent brain tumor.
DISCUSSION
The hallmarks of GBM, such as multifocal and recurrent tumors infiltrating the normal brain tissue, make the treatment of this disease particularly challenging.2 We developed a gene therapy approach that combines the conditionally cytotoxic TK with the immunostimulatory Flt3L.12 Although the normal brain parenchyma exhibit a paucity of antigen-presenting cells, Ad-Flt3L delivered directly into the brain tumor elicits the recruitment of these cells into the GBM microenvironment. Ad-TK (+GCV) induces the killing of tumor cells, and the brain tumor antigen released in response to the killing is phago cytosed by dendritic cells.8 Dendritic cells loaded with brain tumor antigen migrate to the draining lymph node, where they present tumor antigen to naive T cells, thereby inducing a brain tumor–specific immune response8,9 and generating immunological memory that protects against recurrent brain tumors.8,14 We previously showed the therapeutic efficacy of this approach in syngeneic intracranial rat and mouse models of GBM;8,13 however, pre-existing systemic immunity against Ad is highly prevalent in humans20 and could hamper the efficacy of Ad-mediated gene therapy in this setting.21–23 In this study, therefore, we tested the hypothesis that HC-Ad vectors encoding TK and Flt3L would be efficacious and safe, even in the presence of anti-Ad immunity, as may occur in humans undergoing clinical trials.20
In anticipation of a phase I clinical trial for GBM using HC-Ad-TetOn-Flt3L + HC-Ad-TK, we performed a dose-escalation study and performed a comprehensive analysis of the efficacy and toxicity of this therapeutic approach in a syngeneic intracranial GBM model. To our knowledge, these data represent the first from an efficacy and toxicity study utilizing gutless, helper-dependent HC-Ad vectors in an animal model of brain cancer. In fact, the number of preclinical efficacy studies in animal models of disease remains surprisingly limited for such a promising gene delivery technology. The efficacy of HC-Ad vectors has been assessed in animal models of sensory neuronopathies, hemophilia, diabetic retinopathy, glycogen storage disease, monogenic hypoalphalipoproteinemia, and hypertension.32–38 Two of these studies involved the use of regulatable adenoviral vectors,35,38 which, given the large size of regulatable expression cassettes, can be engineered only on HC-Ad vector backbones.
Using escalating doses of combination HC-Ad-TetOn-Flt3L + HC-Ad-TK, we demonstrated high-therapeutic efficacy and concomitant reduction of the tumor mass within 30 days of treatment in ~70% of rats. Tumor regression occurs concomitantly with a reduction in the copies of vector genomes and expression of Flt3L protein. These data suggest that tumor cells transduced with HC-Ad vectors are rapidly eliminated upon treatment.
Our previous studies in normal brains of preimmunized animals demonstrated sustained levels of HC-Ad vector genomes, even in the presence of anti-Ad immunity.28,39 Although considerably reduced from their levels at day 5 after treatment, sustained levels of HC-Ad vector genomes persist in the brain for up to 1 year after treatment, suggesting that the cytotoxic effects of TK or the immunostimulatory effects of Flt3L can be “reactivated” with the readministration of GCV or DOX, respectively, if necessary.
The restoration of the normal brain architecture, the absence of severe long-term behavioral deficits 1 year after treatment, and the absence of significant chronic inflammation in the brain provide strong evidence of the safety of this combined approach. The decrease in the number of immune cells in the brain at 6 months after treatment may be attributed, at least partly, to the regulatable features engineered into HC-Ad-TetOn-Flt3L, which expresses Flt3L only in the presence of DOX. The absence of demyelination at all time points tested attests to the overall safety of HC-Ad-mediated delivery of TK and Flt3L. Importantly, the delivery of HC-Ads into the brains of tumor-bearing animals did not result in an alteration of the biochemical or hematological parameters. This was in contrast to the findings of a recent study that used a retargeted adenoviral vector encoding TK in an animal model of recurrent ovarian cancer.40
Importantly, our data, showing that the HC-Ad vector genomes are restricted to the injected brain hemisphere, demonstrate that HC-Ad vectors do not diffuse to other regions of the CNS or peripheral organs. This finding is in agreement with those of several preclinical studies that assessed the safety and biodistribution of first-generation and oncolytic adenoviral vectors after intracranial injection in naive rodents.41,42
The demonstration of therapeutic efficacy of HC-Ad-TetOn-Flt3L + HC-Ad-TK in a large brain tumor model in animals preimmunized against Ad highlights the high efficacy of HC-Ad vectors in delivering therapeutic genes at biologically appropriate levels in an animal model of GBM exhibiting anti-Ad systemic immunity. This is important because it mirrors the scenario likely to be encountered in a clinic setting.20 Most importantly, long-term immunological memory protects against tumor rechallenge, even at 1 year after treatment, which demonstrates the therapeutic efficacy of this approach in countering recurrences of GBM. The data reported represent the first experimental evidence to indicate the persistence of immunological memory that is sustained for up to 1 year after HC-Ad-mediated combined delivery of Flt3L and TK, even in the presence of a systemic anti-Ad immune response. We previously demonstrated that HC-Ad-TK alone is effective in eradicating intracranial brain tumors in preimmunized animals.23 However, when administered alone, it failed to induce anti-GBM immunological memory. This shows that TK alone cannot provide protection in cases of tumor recurrence as would be encountered in the clinic (data not shown). In summary, the efficacy and safety of this approach, as demonstrated in clinically relevant animal models, strongly support the further development of HC-Ad-TetOn-Flt3L + HC-Ad-TK as a second-generation delivery platform for implementation in phase I clinical trials for GBM.
METHODS
Please see Supplementary Materials and Methods S1 online for a more detailed description of materials and methods used.
Adenoviral vectors
We have previously described the molecular characterization, rescue, and amplification of HC-Ad-TK, HC-Ad-TetOn-Flt3L, and HC-Ad-TetOn-βgal and Ad0.29,31
In vitro characterization of HC-Ad vectors
CNS-1 cells and astrocytes were seeded (25,000/well) and infected 24 h later with either HC-Ad vector (2,000 vp/cell). Transgene expression was assessed using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) and immunocytochemistry.8,43 TK cytotoxicity was assessed by flow cytometric analysis of propidium iodide/Annexin V–stained cells.8,9,44
Brain tumor rodent models
Rat GBM CNS-1 cells (4,500 cells, 3 μl) were stereotactically implanted in the right striatum of the brain in syngeneic Lewis rats (220–250 g; Harlan, Indianapolis, IN) as previously described.9,45 Four days after cell implantation (2 days before HC-Ad administration), the rats were started on doxycycline-containing chow ad libitum (see Supplementary Materials and Methods S1 online). Six days after cell implantation (after 2 days on the doxycycline chow), the rats received an intratumoral injection of HC-Ad-TetOn-Flt3L + HC-Ad-TK, the control vector HC-Ad-TetOn-βgal, or saline. Starting at 24 h after treatment, the rats received GCV (25 mg/kg, intraperitoneal) twice daily for 10 days. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Biodistribution of vector genomes and quantification of Flt3L transgene copies in the brain
Analysis of the biodistribution of vector genomes was performed at 5 days, 30 days, 6 months, or 1 year after treatment. Striatal tissue (25 mg) samples were dissected from the brain tumor and the contralateral hemisphere, the cerebellum, and the brain stem. Tissue samples (25 mg) were also obtained from the spleen, liver, testes, gut, lung, heart, cervical draining lymph nodes, kidney, and lumbar spinal cord (Supplementary Figure S2 online). Total DNA was purified and used for the quantitation of vector genomes and Flt3L transgene copies by real-time quantitative PCR46 as described in Supplementary Materials and Methods S1 online. The sequence of HC-Ad primers and probe for detection of HC-Ad vector genomes are shown in Supplementary Figure S2 online. Vector genomes and Flt3L transgene copies are shown as ratios relative to 25 mg of tissue. Graphs show the values and means of n = 5 per group.
Neuropathological analysis
Neuropathological analysis of the brain was performed at 5 days, 30 days, 6 months, or 1 year after treatment. The analysis is described in Supplementary Materials and Methods S1 online.
Assessment of Flt3L expression in brain tissue
Analysis of Flt3L expression in the brain of treated animals was performed 5 days and 30 days after treatment (as described in Supplementary Materials and Methods S1 online), using an enzyme-linked immunosorbent assay kit (DFK00; R&D Systems) specific for human soluble Flt3L.
Analysis of blood biochemistry
At 5 days, 30 days, 6 months, and 1 year after treatment, blood was collected and a comprehensive panel of tests involving serum chemistry and hematologic parameters was performed by Antech Diagnostics (Irvine, CA). Normative reference values were established using blood from naive, age-matched animals. The median, minimum, and maximum values for each parameter are shown in Supplementary Tables S1–S4 online.
Behavioral analysis
The long-term behavioral impact of intratumoral delivery of HC-Ad was evaluated at 1 year after treatment by assessing amphetamine-induced rotational behavior, abnormalities in limb-use asymmetry test and spontaneous motor and rearing behavior as described elsewhere (see ref. 14 and Supplementary Materials and Methods S1 online). Naive, age-matched Lewis rats were used as controls.
Preimmunization and rechallenge studies
Anti-Ad immunization was performed by administering an intradermal injection in the back of the neck of the animal, with 1 × 109 infectious units of first-generation adenoviral vector Ad0, or saline as a control, 2 weeks before implantation of CNS-1 tumor cells. In order to confirm the presence of an anti-Ad immune response, blood was collected by retro-orbital bleeding during implantation of the CNS-1 tumors, and the circulating levels of anti-Ad neutralizing antibodies were assessed as described earlier (see ref. 23 and Supplementary Materials and Methods S1 online). Six days after tumor implantation, each rat received an intratumoral injection of 5 × 109 vp each of HC-Ad-TetOn-Flt3L + HC-Ad-TK or saline. At 1 year after treatment, long-term survivors were rechallenged with a second tumor in the contralateral striatum. No further treatment was given after the rechallenge.
Statistical analysis
Sample sizes were calculated in order to detect differences between groups with a power of 80% at a 0.05 significance level, using PASS 2008 (Power and Sample Size software; NCSS, Kaysville, UT). Data were analyzed using one-way analysis of variance followed by Tukey's post-test or two-tailed Student's t-test (NCSS). Where data were not normally distributed, they were either log-transformed or analyzed using a Mann–Whitney U nonparametric post-test. Kaplan–Meier survival curves were analyzed using the Mantel-log rank test (GraphPad Prism version 3.00; GraphPad Software, San Diego, CA). P values <0.05 were used to determine the null hypothesis to be invalid. The statistical tests used are indicated in the figure legends.
Supplementary Material
SUPPLEMENTAL MATERIALS AND METHODS
Adenoviral vectors
The molecular characterization, rescue, and amplification of HC-Ad-TK, HC-Ad-TetOn-Flt3L, and HC-Ad-TetOn-βgal were described by us previously (1, 2). Briefly, HC-Ad-TK encodes herpes simplex type 1 thymidine kinase (TK) constitutively expressed under the control of the powerful mCMV promoter (3). HC-Ad-TetOn-Flt3L or HC-Ad-TetOn-βgal encode either human soluble fms-like tyrosine kinase 3 ligand (Flt3L) or the reporter gene lacZ respectively under the control of the tightly regulated, mCMV-TetOn inducible expression system developed by us (2). All three HC-Ad vectors are completely devoid of all adenoviral encoding genes; HC-Ad stuffer sequences are derived from non-coding sequences from the human HPRT locus and cosmid 346 (4). All three vectors were scaled up and purified as described by us previously (5). An E1/E3 deleted first generation adenoviral vector without an expression cassette was scaled up and purified by as described previously (6).
In vitro characterization of HC-Ad vectors
CNS1 cells and astrocytes were seeded (25,000 per well) and infected 24 hours later with either HC-Ad-TetOn-Flt3L or HC-Ad-TK (2,000 vp/cell). Cells transduced with HC-Ad-TetOn-Flt3L were incubated with or without the inducer doxycycline (1 μg/mL). Cells transduced with HC-Ad-TK were incubated with or without gancyclovir (GCV, 25 μmol/L). We used an Flt3L specific ELISA (R&D Systems) to assess levels of Flt3L in 50 μL of cell culture supernatant 72 hours after infection (7). To assess levels of cell death in HC-Ad-TK (+GCV) transduced cells, cells were stained with propidium iodide (PI) and Annexin V 72 hours after incubation with GCV and analyzed by flow cytometry. For immunofluoresence analysis of transgene expression, monolayers of transduced cells were fixed in 4% paraformaldehyde, pH 7.4 (PFA). 24 hours after infection, labeled with custom made rabbit polyclonal antibodies against Flt3L (8) or TK (9), followed by an anti-rabbit secondary antibody conjugated to Alexa-488 (Invitrogen).
Brain tumor rodent models
4,500 rat GBM CNS1 cells (3 μl) were stereotactically implanted in the right striatum of syngeneic Lewis rats (220-250g, Harlan, Indianapolis, IN USA) as previously described (10, 11). CNS1 cells were grown in DMEM culture media (CellGro, Herndon, VA), supplemented with 10% fetal calf serum (FCS), 1% L-Glutamine, 1% Penicillin-Streptomycin, 1% non-essential aminoacids and passaged routinely. The day of surgery CNS1 cells were trypsinized, cells counted, resuspended in PBS and kept on ice for up to 2 hours. Rats were housed in pathogen free environment, humidity and temperature controlled vivarium on a 12:12 hour light/dark cycle with free access to food and water. All animal experiments were performed after prior approval by the Institutional Animal Care and Use Committee at Cedars Sinai Medical Center and conformed to the policies and procedures of the Comparative Medicine Department. After induction of anesthesia, animals were placed in a stereotactic apparatus and injected unilaterally into the right striatum. Rats were injected using a 10 μl Hamilton syringe (coordinates: 1 mm forward from bregma, 3.2 mm lateral and ventral 5 mm from the dura). Animals were allowed to recover and their health status was closely monitored. Six days after tumor implantation, rats received an intratumoral injection with either the combination therapy HC-Ad-TetOn-Flt3L + HC-Ad-TK, the control vector HC-Ad-TetOn-βgal, or saline. Treatment was performed at the dosage indicated in each figure, utilizing the same drill hole to inject saline or HC-Ad(s) in a volume of 3 μl (delivered in 3 locations ventral of the dura: 5.5, 5.0 and 4.5 mm) into the tumor mass. Starting 2 days before HC-Ads administration, rats were fed ad libitum a custom rodent chow (Purina Test Diet 5001) containing 2000 PPM doxycycline and a green dye (Newco Distributors, Rancho Cucamonga, CA). Before beginning long-term dose escalation studies, we first established the optimal regime for DOX administration to achieve Flt3L expression at therapeutically effective levels. To do so, tumor bearing animals were treated with an intratumoral injection of HC-Ad-TetOn-Flt3L and HC-Ad-TK (1×109 vp of each vector), HC-Ad-TetOn-β-gal (control vector, 2×109 vp), or saline. GCV was administered twice daily for ten days. Animals were fed rodent chow containing DOX ad libitum starting 2 days before HC-Ad injection and lasting for either two weeks or four weeks. Only animals receiving DOX chow for four weeks survived up to 90 days. All other animals succumbed to tumor burden within thirty days of tumor cell implantation (Supp. Figure 1). Thus, DOX chow was administered for 4 weeks for the remainder of the experiments.
Twenty-four hours after delivery of HC-Ads or saline, animals that were treated with GCV (25 mg/kg, i.p.), twice daily for 10 days. Animals were monitored daily and euthanized at the first signs of moribund behavior or at predetermined time points for analysis of biodistribution of vector genomes, neuropathology, Flt3L expression in the brain and serum, and a serum chemistry panel. Animals were euthanized according to the guidelines of the Institutional Animal Care and Use Committee at Cedars–Sinai Medical Center, by terminal perfusion with oxygenated Tyrode's solution (132 mM NaCl, 1.8 mM CaCl2, 0.32 mM NaH2PO4, 5.56 mM glucose, 11.6 mM NaHCO3, 2.68 mM KCl, and 100 USP/L heparin) under deep anesthesia. Animals undergoing neuropathological analysis were then perfused with 4% paraformaldehyde (PFA). Brains were removed and further fixed in 4% PFA for 3 days.
Biodistribution of vector genomes and quantification of Flt3L transgene copies in the brain
We assessed the biodistribution of HC-Ad vector genomes in the central nervous system and in peripheral organs over a period of one year post treatment using a qPCR method with a primer and probe set specific for sequences located within the HC-Ad backbone (Supp. Figure 2). Animals that were perfused without fixative 5 days, 30 days, 6 months, or 1 year post treatment were used for analysis of biodistribution of vector genomes. Brains were removed from the skull and a 25 mg sample of brain tissue immediately adjacent to the HC-Ad injection site was dissected using a razor blade and a rat brain matrix (Alto, Harvard Apparatus, Holliston, MA) as described by us previously (12, 13). 25 mg of striatal tissue was also dissected from the brain hemisphere contralateral to the injection site, the cerebellum, and the brain stem. 25 mg of tissue was also obtained from the spleen, liver, testes, gut, lung, heart, cervical draining lymph nodes, kidney, and lumbar spinal cord. Total DNA was purified from each 25 mg tissue sample using DNeasy Blood and Tissue Kit (Qiagen, Germantown, Md) and eluted in 150 μL. 5 μL of DNA was used to determine the DNA concentration by UV spectrophotometry. 5 μL of DNA was used for the quantitation of vector genomes by Real-Time quantitative PCR using a primer and probe specific for the cosmid sequences contained in the HC-Ad vector backbone as described by us previously (12). To quantify copies of Flt3L in the brain, qPCR analysis of DNA samples extracted from 25 mg of brain tissue was also performed. The standard plasmid (pSt-Flt3L, 3579bp) used to generate the standard curve was constructed by cloning the human soluble Flt3L cDNA into the plasmid pSP72. A set of primers and probe specific for the Flt3L transgene were designed (Primer Express software v2.0, Applied Biosystems) as follows: Flt3L-Forward primer, 5’ GGATGGAGCGGCTCAAGA 3’; Flt3L-Reverse primer, 5’ TCACGCGCTCCAGCAA 3’; Flt3L-Probe, 6~FAM 5’ TGTCGCTGGGTCCAAGAAGATGCAAGG 3’ TAMRA. The quantification of vector genomes and copies of Flt3L transgene are displayed as the average of triplicates for each DNA sample, and are shown as a ratio of vector genomes/25 mg of tissue. Based on previous studies, we considered the limit of detection to be 300 vector genomes (12). Results are based on n=5 per group.
Neuropathological analysis
Neuropahological analysis of treated tumor bearing animals was performed at 5 days, 30 days, 6 months, or 1 year post treatment. Following perfusion with Tyrode's solution and 4% PFA, brains were fixed in 4% PFA for 3 additional days. Sixty-micrometer serial coronal sections were cut through the striatum at the area immediately adjacent to the site of tumor cells’ injection and free-floating immunocytochemistry was performed as previously described (10, 14, 15) with markers for oligodendrocytes and myelin sheath (MBP), dopaminergic nerve terminals (TH), residual tumor cells or activated astrocytes (vimentin), CD8+ T cells (CD8), macrophages and microglia (ED1), macrophages, microglia and immune cells (MHC-II), or transgene expression (TK or Flt3L). Nissl staining was used to determine the histopathological features of the brains. Tissues were photographed with Carl Zeiss Optical Axioplan microscope using Axiovision Rel 4.6 and MOSAIX software (Carl Zeiss, Chester, VA, USA).
Assessment of Flt3L expression in brain tissue
Analysis of Flt3L expression in the brains of treated animals was performed 5 days or 30 days post treatment. Animals perfused without fixative were used for analysis of Flt3L expression in the brain. 50 mg of striatal brain tissue immediately adjacent to the HC-Ad injection site (ipsilateral) and from the striatum of the contralateral brain hemisphere was dissected. The tissue was snap frozen in an eppendorf tube by immersion in liquid N2 and stored at -80°C until processing. Brain tissue proteins were isolated on ice by adding 500 μl of 50mM TrisHCl buffer (pH 7.4) containing 1x EDTA free protease inhibitor cocktail (78415, Thermo Scientific), using a glass tissue grinder (258003, Wheaton, NJ). Tissue debris were remove by centrifugation at 13,000 rpm, 4°C and lysate was transferred to a fresh tube that was snap frozen, as described earlier. The protein concentration of the lysate was measured using the BCA protein assay kit (23225, Thermo Scientific). Human soluble Flt3L quantification was determined by utilizing an ELISA kit (DFK00, R&D Systems) following the manufacturer's instructions. Results are expressed as pg of Flt3L/ mg of total protein.
Analysis of clinical laboratory based toxicity
During euthanasia, blood was collected from each animal. A comprehensive panel of general hematologic and clinical chemistry parameters was performed by Antech Diagnostics (Irvine, CA) at 5 days, 30 days, 6 months, or 1 year post treatment. To establish normative, reference values, blood was collected from naïve, age-matched animals and general hematologic and clinical chemistry parameters was assessed. The mean, minimum, and maximum values for each parameter are shown. 50 μL of serum was also used undiluted to measure the level of circulating Flt3L in the serum at 5 days or 30 days post treatment using a Flt3L specific ELISA (R&D) as described by us previously (16).
Behavioral analysis
The long-term behavioral impact of intratumoral delivery of HC-Ad was assessed by analyzing amphetamine-induced rotational behavior, abnormalities in limb use asymmetry, and spontaneous motor and rearing behavior as described by us in detail (17). Animals were tested 1 year post intratumoral delivery of HC-Ad vectors. Naïve, age-matched Lewis rats were used as controls. Briefly, amphetamine-induced rotational behavior was measured using a RotoMax apparatus and software (AccuScan Instruments, Columbus, OH) for 90 minutes after s.c. injection of 1.5 mg/kg d-amphetamine sulfate (Sigma, St. Louis, MO). To measure forelimb use asymmetry, contacts made by each forepaw with the wall of a 20.3 cm wide clear cylinder were scored from videotape over a 10 minute period in slow motion by two independent, experimentally blinded observers. Forelimb contact with the walls of the cylinder was scored measuring only initial contact with the cylinder walls. Baseline spontaneous locomotor and rearing activity was recorded for thirty minutes in 40.6 × 40.6 cm × 38.1 cm enclosed box using photobeam breaks and optical sensors. Spontaneous locomotor and rearing activity was then monitored for 120 minutes after s.c. injection of 1.5 mg/kg d-amphetamine sulfate (Sigma, St. Louis, MO). Data was scored as total number of beam breaks summed over the observation period (17).
Preimmunization and rechallenge studies
To measure the effects of a pre-existing anti-adenoviral immune response, naïve Lewis rats were injected intradermally in the back of the neck with 1×109 infectious units of first-generation adenoviral vector Ad-0 in a volume of 50 μL two weeks before intrastriatal injections of CNS1 tumor cells. Animals were housed under previously described normal conditions for 2 weeks to generate an immune response to the recombinant adenoviral vector. As controls, naïve rats received an intradermal injection of 50 μL of saline. 2 weeks post immunization, animals were stereotactically injected with CNS1 tumor cells as described above and treated 6 days later with an intratumoral injection of 5×109 vp each of HC-Ad-TetOn-Flt3L + HC-Ad-TK, or saline as a control. Animals were monitored daily and euthanized at the first signs of moribund behavior. One year post treatment, long-term survivors were rechallenged with an injection of 5,000 CNS1 cells into the contralateral brain hemisphere. No further treatment was given. Animals were monitored for survival.
Neutralizing Antibodies
To confirm the presence of an anti-adenovirus immune response, blood was collected by retro-orbital bleeding during implantation of CNS1 tumor cells and the levels of adenovirus specific neutralizing antibodies was assessed as described by us previously (2, 15, 18). Briefly, serum samples were heat-inactivated at 56°C for 30 min and serially diluted twofold from 1:2 to 1:4,096. Each dilution was incubated with 3 × 105 iu of Ad-hCMV-β-Gal, added to a 96-well plate containing HEK 293 cells and incubated at 37°C for 20 h before fixing with 4% paraformaldehyde and staining with X-gal (Sigma). The neutralizing antibody titer for each animal is given as the reciprocal of the highest dilution of serum at which 50% of Ad-hCMV-β-Gal mediated transduction was inhibited.
References
(1) Candolfi, M. et al. Effective High-Capacity Gutless Adenoviral Vectors Mediate Transgene Expression in Human Glioma Cells. Mol Ther 14, 371-81 (2006).
(2) Xiong, W. et al. Regulatable Gutless Adenovirus Vectors Sustain Inducible Transgene Expression in the Brain in the Presence of an Immune Response against Adenoviruses. J Virol 80, 27-37 (2006).
(3) Gerdes, C.A., Castro, M.G. & Lowenstein, P.R. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol Ther 2, 330-8 (2000).
(4) Morsy, M.A. et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci U S A 95, 7866-71 (1998).
(5) Palmer, D. & Ng, P. Improved system for helper-dependent adenoviral vector production. Mol Ther 8, 846-52 (2003).
(6) Southgate, T., Kroeger, K.M., Liu, C., Lowenstein, P.R. & Castro, M.G. Gene transfer into neural cells in vitro using adenoviral vectors. Curr Protoc Neurosci Chapter 4, Unit 4 23 (2008).
(7) Curtin, J. et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Medicine 6, e10 (2009).
(8) Ali, S. et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther 10, 1071-84 (2004).
(9) Dewey, R.A. et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med 5, 1256-63 (1999).
(10) Candolfi, M. et al. Intracranial glioblastoma models in preclinical neurooncology: neuropathological characterization and tumor progression. J Neurooncol 85, 133-48 (2007).
(11) Candolfi, M. et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res 15, 4401-14 (2009).
(12) Puntel, M. et al. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum Gene Ther 17, 531-44 (2006).
(13) Thomas, C.E., Kroeger, K.M., Puntel, M., Sanderson, N.S.R., Castro, M.G., and Lowenstein, P.R. Gene transfer into rat brain using adenoviral vectors. In: Current Protocols in Neuroscience, Vol. 4.23.1-4.23.40 (ed. Gerfen, J.N., McKay, R., Rogawski, M.A., Sibley, D.R. and Skolnick, P.) 4.23.1-4.40 (John Wiley and Sons, New York, New York, NY, 2009).
(14) King, G.D. et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol 10, 19-31 (2008).
(15) King, G.D. et al. High-Capacity adenoviral vector-mediated anti-glioma gene therapy in the presence of systemic anti-adenovirus immunity. J Virol 82, 4680-4 (2008).
(16) Curtin, J.F. et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol 176, 3566-77 (2006).
(17) King, G.D. et al. Flt3L in combination with HSV1-TK mediated gene therapy reverses brain tumor induced behavioral deficits. Mol Ther 16, 682-90 (2008).
(18) Thomas, C.E., Schiedner, G., Kochanek, S., Castro, M.G. & Lowenstein, P.R. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci U S A 97, 7482-7 (2000).
Supplemental Figure 1. A regimen of four weeks of doxycycline in combination with intratumoral delivery of HC-Ads is required to improve long-term survival in tumor bearing rats. (A) 4,500 CNS1 cells were injected in the striatum of naive Lewis rats. Six days after tumor implantation, rats were injected with 1×109 vp each of HC-Ad-TetOn-Flt3L and HC-Ad-TK (n=18), 2×109 vp HC-Ad-TetOn-βgal (n=7) or saline alone (n=12). GCV (25mg/kg, i.p.) was administered twice a day for 10 days. To assess the optimal duration of doxycycline (DOX) administration to “turn on” therapeutically acceptable levels of transgene expression, rodent chow containing DOX was administered for either 2 weeks (n=6) or 4 weeks (n=12) ad libitum. Log-rank analysis of Kaplan-Meyer survival curves reveals only animals receiving HC-Ad-TetOn-Flt3L and HC-Ad-TK (+GCV) and DOX for 4 weeks survived long term (*p<0.05 vs. all other groups).
Supplemental Figure 2. Experimental design of biodistribution studies. (A) Illustration of a rat brain depicting the regions dissected for biodistribution analysis including the injection site in the striatum, the cerebellum, and the brain stem. (B) Illustration depicting the various peripheral organs dissected for biodistribution analysis including lumbar spinal cord, cervical draining lymph node, spleen, heart, gut, testes, lung, liver, and kidney. (C) The sequences of the HC-Ad specific primers and probe used for detection of HC-Ad vector genomes and for Flt3L transgene copies are shown. (D) Illustration of plasmid used to generate the standard curve for qPCR analysis of the biodistribution of HC-Ad vector genomes. The primers and probes used detect a region of the cosmid sequence in the HC-Ad vector genome. (E) A representative standard curve and (F) Amplification Plot used to quantitate levels of HC-Ad vector genomes by qPCR are shown.
Supplemental Figure 3. The biodistribution of vector genomes in tumor bearing animals is restricted to the site of the injection at thirty days, six months, and twelve months after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK. Tumor bearing Lewis rats were euthanized at specific time points after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK at (A-C) 5×108 vp, (D-F) 1×109 vp, and (G-I) 5×109 vp of each vector. Animals were euthanized at (A, D, E) 1 month, (B, E, H) 6 months, and (C, F, I) 12 months post-HC-Ad treatment. Thirteen tissues were harvested: spleen, liver, testes, gut, lung, heart, cervical draining lymph nodes, kidney, brain tumor injection side and brain contralateral side, brain stem, cerebellum, and lumbar spinal cord. DNA was isolated from 25mg of each tissue for each animal. Isolated DNA from each tissue was used for qPCR with a primer and probe set specific for the HC-Ad vector backbone to assess the biodistribution of the vector genomes. Vector genomes’ quantification results are the average of triplicates for each DNA sample, and are shown as a ratio of vector genomes/25 mg of tissue. Results are based on n=5 per group.
Supplemental Figure 4. HC-Ad treated, tumor bearing animals show a complete regression of the brain tumor mass and restoration of the normal brain architecture thirty days treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK. Tumor bearing Lewis rats surviving long term were euthanized one month after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK at 5×108 vp, 1×109 vp, and 5×109 vp of each vector (n= 5 animals/group), and perfused with fixative. Brains were sectioned (60 μm) and stained with Nissl for gross morphology, or assessed for evidence of inflammation and neurotoxicity by immunocytochemistry with markers for oligodendrocytes and myelin sheath (MBP), dopaminergic nerve terminals (TH), residual tumor cells or activated astrocytes (vimentin), CD8+ T cells (CD8), macrophages and microglia (ED1), macrophages, microglia and immune cells (MHC-II), or transgene expression (TK or Flt3L). Note that areas indicative of inflammation are restricted to the site of tumor implantation and vector delivery.
Supplemental Figure 5. Long-term survivors do not show evidence of severe inflammation six months after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK. Tumor bearing Lewis rats surviving long term were euthanized six months after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK at 5×108 vp, 1×109 vp, and 5×109 vp of each vector (n= 5 animals/group), and perfused with fixative. Brains were sectioned (60 μm) and stained with Nissl for gross morphology, or assessed for evidence of inflammation and neurotoxicity by immunocytochemistry with markers for oligodendrocytes and myelin sheath (MBP), dopaminergic nerve terminals (TH), residual tumor cells or activated astrocytes (vimentin), CD8+ T cells (CD8), macrophages and microglia (ED1), macrophages, microglia and immune cells (MHC-II), or transgene expression (TK or Flt3L). Note that areas indicative of inflammation are restricted to the site of tumor implantation and vector delivery.
Supplemental Table 1. Analysis of clinical hematological parameters of tumor bearing animals five days after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK.
Supplemental Table 2. Analysis of clinical hematological parameters of tumor bearing animals thirty days after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK.
Supplemental Table 3. Analysis of clinical hematological parameters of tumor bearing animals six months after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK.
Supplemental Table 4. Analysis of clinical hematological parameters of tumor bearing animals twelve months after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK.
ACKNOWLEDGMENTS
We thank S. Melmed, L. Fine, and Mark Greene for their support and John Young and his staff in the Department of Comparative Medicine, Cedars-Sinai Medical Center (CSMC). We also thank Jaime DeBuncyo, Maricel Gozo, Maksim Khayznikov, and Ali Zadmehr for technical assistance. This work was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) grant 1UO1 NS052465. The brain tumor program at our institute is funded by NIH/NINDS grants 1RO1-NS 057711, 1R21-NSO54143 (to M.G.C.), 1 RO1 NS 054193, and RO1 NS 061107 (to P.R.L.); the Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics to P.R.L. and M.G.C., respectively; the Drown Foundation; the Linda Tallen & David Paul Kane Foundation Annual Fellowship; and the Board of Governors at CSMC. M.C. is supported by NIH/NINDS 1F32 NS058156. D.F. was supported by the Irene & Eric Simon Brain Research Foundation.
Footnotes
The first two authors contributed equally to this work.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Central Nervous System Tumors (discussion) Central Nervous System Tumors Track.. ASCO Annual Meeting; Orlando, FL. 29 May–2 June, 2009. [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Grossman SA, et al. Current survival statistics for patients with newly diagnosed glioblastoma treated with radiation and temozolomide on research studies in the United States.. ASCO Annual Meeting; Orlando, FL. 29 May–2 June, 2009. [Google Scholar]
- 4.Stupp R, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups Changing paradigms–an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hottinger AF, van den Bent MJ, Dietrich PY, Brandes AA. Frequently asked questions in the medical management of high-grade glioma: a short guide with practical answers. Ann. Oncol. 2008;19(suppl. 7):vii209–vii216. doi: 10.1093/annonc/mdn474. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 7.Curtin JF, et al. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr. Top. Med. Chem. 2005;5:1151–1170. doi: 10.2174/156802605774370856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtin JF, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candolfi M, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin. Cancer Res. 2009;15:4401–4414. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Candolfi M, et al. Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety. Curr. Gene Ther. 2009;9:409–421. doi: 10.2174/156652309789753301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J, et al. Topotecan enhances immune clearance of gliomas. Cancer Immunol. Immunother. 2009;58:259–270. doi: 10.1007/s00262-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali S, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muhammad AKMG, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: Humoral and cellular immunity lead to tumor regression. Clin. Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King GD, et al. Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol. Ther. 2008;16:682–690. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King GD, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro-oncology. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulkkanen KJ, Yla-Herttuala S. Gene therapy for malignant glioma: current clinical status. Mol. Ther. 2005;12:585–598. doi: 10.1016/j.ymthe.2005.07.357. [DOI] [PubMed] [Google Scholar]
- 17.Immonen A, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol. Ther. 2004;10:967–972. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Sathornsumetee S, Rich JN. Designer therapies for glioblastoma multiforme. Ann. N. Y. Acad. Sci. 2008;1142:108–132. doi: 10.1196/annals.1444.009. [DOI] [PubMed] [Google Scholar]
- 19.Ark-Therapeutics Cerepro® Phase III trial update: Analyses strengthen as more patients reach endpoint. 2009 <http://investors.arktherapeutics.com/servlet/HsPublic?context=ir.access&ir_option=RNS_NEWS&item=126418067393456&ir_client_id=4553&transform=newsitem_new>
- 20.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 21.Thomas CE, Schiedner G, Kochanek S, Castro MG, Löwenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barcia C, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J. Exp. Med. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King GD, et al. High-capacity adenovirus vector-mediated anti-glioma gene therapy in the presence of systemic antiadenovirus immunity. J. Virol. 2008;82:4680–4684. doi: 10.1128/JVI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morral N, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Neal WK, et al. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol. Med. 2000;6:179–195. [PMC free article] [PubMed] [Google Scholar]
- 27.Maione D, et al. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barcia C, et al. Sustained, one year expression from high-capacity helper-dependent adenoviral vectors delivered to the brain of animals with a pre-existing systemic anti-adenoviral immune response: implications for clinical trials. Mol. Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Candolfi M, et al. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol. Ther. 2006;14:371–381. doi: 10.1016/j.ymthe.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candolfi M, et al. Optimization of adenoviral vector-mediated transgene expression in the canine brain in vivo, and in canine glioma cells in vitro. Neuro-oncology. 2007;9:245–258. doi: 10.1215/15228517-2007-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong W, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J. Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terashima T, Oka K, Kritz AB, Kojima H, Baker AH, Chan L. DRG-targeted helper-dependent adenoviruses mediate selective gene delivery for therapeutic rescue of sensory neuronopathies in mice. J. Clin. Invest. 2009;119:2100–2112. doi: 10.1172/JCI39038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunetti-Pierri N, et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum. Gene Ther. 2005;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- 34.McCormack WM, Jr, et al. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J. Thromb. Haemost. 2006;4:1218–1225. doi: 10.1111/j.1538-7836.2006.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamartina S, et al. Helper-dependent adenovirus for the gene therapy of proliferative retinopathies: stable gene transfer, regulated gene expression and therapeutic efficacy. J. Gene Med. 2007;9:862–874. doi: 10.1002/jgm.1083. [DOI] [PubMed] [Google Scholar]
- 36.Kiang A, et al. Fully deleted adenovirus persistently expressing GAA accomplishes long-term skeletal muscle glycogen correction in tolerant and nontolerant GSD-II mice. Mol. Ther. 2006;13:127–134. doi: 10.1016/j.ymthe.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Oka K, et al. Sustained phenotypic correction in a mouse model of hypoalphalipoproteinemia with a helper-dependent adenovirus vector. Gene Ther. 2007;14:191–202. doi: 10.1038/sj.gt.3302819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schillinger KJ, et al. Regulatable atrial natriuretic peptide gene therapy for hypertension. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13789–13794. doi: 10.1073/pnas.0506807102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 40.Matthews K, et al. Identifying the safety profile of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in anticipation of a phase I clinical trial in patients with recurrent ovarian cancer. Clin. Cancer Res. 2009;15:4131–4137. doi: 10.1158/1078-0432.CCR-08-3354. [DOI] [PubMed] [Google Scholar]
- 41.Langford G, Dayan A, Yla-Herttuala S, Eckland D. A preclinical assessment of the safety and biodistribution of an adenoviral vector containing the herpes simplex virus thymidine kinase gene (Cerepro) after intracerebral administration. J. Gene Med. 2009;11:468–476. doi: 10.1002/jgm.1328. [DOI] [PubMed] [Google Scholar]
- 42.Sonabend AM, et al. Biodistribution of an oncolytic adenovirus after intracranial injection in permissive animals: a comparative study of Syrian hamsters and cotton rats. Cancer Gene Ther. 2009;16:362–372. doi: 10.1038/cgt.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali S, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol. Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dewey RA, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat. Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 45.Candolfi M, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J. Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puntel M, et al. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum. Gene Ther. 2006;17:531–544. doi: 10.1089/hum.2006.17.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL MATERIALS AND METHODS
Adenoviral vectors
The molecular characterization, rescue, and amplification of HC-Ad-TK, HC-Ad-TetOn-Flt3L, and HC-Ad-TetOn-βgal were described by us previously (1, 2). Briefly, HC-Ad-TK encodes herpes simplex type 1 thymidine kinase (TK) constitutively expressed under the control of the powerful mCMV promoter (3). HC-Ad-TetOn-Flt3L or HC-Ad-TetOn-βgal encode either human soluble fms-like tyrosine kinase 3 ligand (Flt3L) or the reporter gene lacZ respectively under the control of the tightly regulated, mCMV-TetOn inducible expression system developed by us (2). All three HC-Ad vectors are completely devoid of all adenoviral encoding genes; HC-Ad stuffer sequences are derived from non-coding sequences from the human HPRT locus and cosmid 346 (4). All three vectors were scaled up and purified as described by us previously (5). An E1/E3 deleted first generation adenoviral vector without an expression cassette was scaled up and purified by as described previously (6).
In vitro characterization of HC-Ad vectors
CNS1 cells and astrocytes were seeded (25,000 per well) and infected 24 hours later with either HC-Ad-TetOn-Flt3L or HC-Ad-TK (2,000 vp/cell). Cells transduced with HC-Ad-TetOn-Flt3L were incubated with or without the inducer doxycycline (1 μg/mL). Cells transduced with HC-Ad-TK were incubated with or without gancyclovir (GCV, 25 μmol/L). We used an Flt3L specific ELISA (R&D Systems) to assess levels of Flt3L in 50 μL of cell culture supernatant 72 hours after infection (7). To assess levels of cell death in HC-Ad-TK (+GCV) transduced cells, cells were stained with propidium iodide (PI) and Annexin V 72 hours after incubation with GCV and analyzed by flow cytometry. For immunofluoresence analysis of transgene expression, monolayers of transduced cells were fixed in 4% paraformaldehyde, pH 7.4 (PFA). 24 hours after infection, labeled with custom made rabbit polyclonal antibodies against Flt3L (8) or TK (9), followed by an anti-rabbit secondary antibody conjugated to Alexa-488 (Invitrogen).
Brain tumor rodent models
4,500 rat GBM CNS1 cells (3 μl) were stereotactically implanted in the right striatum of syngeneic Lewis rats (220-250g, Harlan, Indianapolis, IN USA) as previously described (10, 11). CNS1 cells were grown in DMEM culture media (CellGro, Herndon, VA), supplemented with 10% fetal calf serum (FCS), 1% L-Glutamine, 1% Penicillin-Streptomycin, 1% non-essential aminoacids and passaged routinely. The day of surgery CNS1 cells were trypsinized, cells counted, resuspended in PBS and kept on ice for up to 2 hours. Rats were housed in pathogen free environment, humidity and temperature controlled vivarium on a 12:12 hour light/dark cycle with free access to food and water. All animal experiments were performed after prior approval by the Institutional Animal Care and Use Committee at Cedars Sinai Medical Center and conformed to the policies and procedures of the Comparative Medicine Department. After induction of anesthesia, animals were placed in a stereotactic apparatus and injected unilaterally into the right striatum. Rats were injected using a 10 μl Hamilton syringe (coordinates: 1 mm forward from bregma, 3.2 mm lateral and ventral 5 mm from the dura). Animals were allowed to recover and their health status was closely monitored. Six days after tumor implantation, rats received an intratumoral injection with either the combination therapy HC-Ad-TetOn-Flt3L + HC-Ad-TK, the control vector HC-Ad-TetOn-βgal, or saline. Treatment was performed at the dosage indicated in each figure, utilizing the same drill hole to inject saline or HC-Ad(s) in a volume of 3 μl (delivered in 3 locations ventral of the dura: 5.5, 5.0 and 4.5 mm) into the tumor mass. Starting 2 days before HC-Ads administration, rats were fed ad libitum a custom rodent chow (Purina Test Diet 5001) containing 2000 PPM doxycycline and a green dye (Newco Distributors, Rancho Cucamonga, CA). Before beginning long-term dose escalation studies, we first established the optimal regime for DOX administration to achieve Flt3L expression at therapeutically effective levels. To do so, tumor bearing animals were treated with an intratumoral injection of HC-Ad-TetOn-Flt3L and HC-Ad-TK (1×109 vp of each vector), HC-Ad-TetOn-β-gal (control vector, 2×109 vp), or saline. GCV was administered twice daily for ten days. Animals were fed rodent chow containing DOX ad libitum starting 2 days before HC-Ad injection and lasting for either two weeks or four weeks. Only animals receiving DOX chow for four weeks survived up to 90 days. All other animals succumbed to tumor burden within thirty days of tumor cell implantation (Supp. Figure 1). Thus, DOX chow was administered for 4 weeks for the remainder of the experiments.
Twenty-four hours after delivery of HC-Ads or saline, animals that were treated with GCV (25 mg/kg, i.p.), twice daily for 10 days. Animals were monitored daily and euthanized at the first signs of moribund behavior or at predetermined time points for analysis of biodistribution of vector genomes, neuropathology, Flt3L expression in the brain and serum, and a serum chemistry panel. Animals were euthanized according to the guidelines of the Institutional Animal Care and Use Committee at Cedars–Sinai Medical Center, by terminal perfusion with oxygenated Tyrode's solution (132 mM NaCl, 1.8 mM CaCl2, 0.32 mM NaH2PO4, 5.56 mM glucose, 11.6 mM NaHCO3, 2.68 mM KCl, and 100 USP/L heparin) under deep anesthesia. Animals undergoing neuropathological analysis were then perfused with 4% paraformaldehyde (PFA). Brains were removed and further fixed in 4% PFA for 3 days.
Biodistribution of vector genomes and quantification of Flt3L transgene copies in the brain
We assessed the biodistribution of HC-Ad vector genomes in the central nervous system and in peripheral organs over a period of one year post treatment using a qPCR method with a primer and probe set specific for sequences located within the HC-Ad backbone (Supp. Figure 2). Animals that were perfused without fixative 5 days, 30 days, 6 months, or 1 year post treatment were used for analysis of biodistribution of vector genomes. Brains were removed from the skull and a 25 mg sample of brain tissue immediately adjacent to the HC-Ad injection site was dissected using a razor blade and a rat brain matrix (Alto, Harvard Apparatus, Holliston, MA) as described by us previously (12, 13). 25 mg of striatal tissue was also dissected from the brain hemisphere contralateral to the injection site, the cerebellum, and the brain stem. 25 mg of tissue was also obtained from the spleen, liver, testes, gut, lung, heart, cervical draining lymph nodes, kidney, and lumbar spinal cord. Total DNA was purified from each 25 mg tissue sample using DNeasy Blood and Tissue Kit (Qiagen, Germantown, Md) and eluted in 150 μL. 5 μL of DNA was used to determine the DNA concentration by UV spectrophotometry. 5 μL of DNA was used for the quantitation of vector genomes by Real-Time quantitative PCR using a primer and probe specific for the cosmid sequences contained in the HC-Ad vector backbone as described by us previously (12). To quantify copies of Flt3L in the brain, qPCR analysis of DNA samples extracted from 25 mg of brain tissue was also performed. The standard plasmid (pSt-Flt3L, 3579bp) used to generate the standard curve was constructed by cloning the human soluble Flt3L cDNA into the plasmid pSP72. A set of primers and probe specific for the Flt3L transgene were designed (Primer Express software v2.0, Applied Biosystems) as follows: Flt3L-Forward primer, 5’ GGATGGAGCGGCTCAAGA 3’; Flt3L-Reverse primer, 5’ TCACGCGCTCCAGCAA 3’; Flt3L-Probe, 6~FAM 5’ TGTCGCTGGGTCCAAGAAGATGCAAGG 3’ TAMRA. The quantification of vector genomes and copies of Flt3L transgene are displayed as the average of triplicates for each DNA sample, and are shown as a ratio of vector genomes/25 mg of tissue. Based on previous studies, we considered the limit of detection to be 300 vector genomes (12). Results are based on n=5 per group.
Neuropathological analysis
Neuropahological analysis of treated tumor bearing animals was performed at 5 days, 30 days, 6 months, or 1 year post treatment. Following perfusion with Tyrode's solution and 4% PFA, brains were fixed in 4% PFA for 3 additional days. Sixty-micrometer serial coronal sections were cut through the striatum at the area immediately adjacent to the site of tumor cells’ injection and free-floating immunocytochemistry was performed as previously described (10, 14, 15) with markers for oligodendrocytes and myelin sheath (MBP), dopaminergic nerve terminals (TH), residual tumor cells or activated astrocytes (vimentin), CD8+ T cells (CD8), macrophages and microglia (ED1), macrophages, microglia and immune cells (MHC-II), or transgene expression (TK or Flt3L). Nissl staining was used to determine the histopathological features of the brains. Tissues were photographed with Carl Zeiss Optical Axioplan microscope using Axiovision Rel 4.6 and MOSAIX software (Carl Zeiss, Chester, VA, USA).
Assessment of Flt3L expression in brain tissue
Analysis of Flt3L expression in the brains of treated animals was performed 5 days or 30 days post treatment. Animals perfused without fixative were used for analysis of Flt3L expression in the brain. 50 mg of striatal brain tissue immediately adjacent to the HC-Ad injection site (ipsilateral) and from the striatum of the contralateral brain hemisphere was dissected. The tissue was snap frozen in an eppendorf tube by immersion in liquid N2 and stored at -80°C until processing. Brain tissue proteins were isolated on ice by adding 500 μl of 50mM TrisHCl buffer (pH 7.4) containing 1x EDTA free protease inhibitor cocktail (78415, Thermo Scientific), using a glass tissue grinder (258003, Wheaton, NJ). Tissue debris were remove by centrifugation at 13,000 rpm, 4°C and lysate was transferred to a fresh tube that was snap frozen, as described earlier. The protein concentration of the lysate was measured using the BCA protein assay kit (23225, Thermo Scientific). Human soluble Flt3L quantification was determined by utilizing an ELISA kit (DFK00, R&D Systems) following the manufacturer's instructions. Results are expressed as pg of Flt3L/ mg of total protein.
Analysis of clinical laboratory based toxicity
During euthanasia, blood was collected from each animal. A comprehensive panel of general hematologic and clinical chemistry parameters was performed by Antech Diagnostics (Irvine, CA) at 5 days, 30 days, 6 months, or 1 year post treatment. To establish normative, reference values, blood was collected from naïve, age-matched animals and general hematologic and clinical chemistry parameters was assessed. The mean, minimum, and maximum values for each parameter are shown. 50 μL of serum was also used undiluted to measure the level of circulating Flt3L in the serum at 5 days or 30 days post treatment using a Flt3L specific ELISA (R&D) as described by us previously (16).
Behavioral analysis
The long-term behavioral impact of intratumoral delivery of HC-Ad was assessed by analyzing amphetamine-induced rotational behavior, abnormalities in limb use asymmetry, and spontaneous motor and rearing behavior as described by us in detail (17). Animals were tested 1 year post intratumoral delivery of HC-Ad vectors. Naïve, age-matched Lewis rats were used as controls. Briefly, amphetamine-induced rotational behavior was measured using a RotoMax apparatus and software (AccuScan Instruments, Columbus, OH) for 90 minutes after s.c. injection of 1.5 mg/kg d-amphetamine sulfate (Sigma, St. Louis, MO). To measure forelimb use asymmetry, contacts made by each forepaw with the wall of a 20.3 cm wide clear cylinder were scored from videotape over a 10 minute period in slow motion by two independent, experimentally blinded observers. Forelimb contact with the walls of the cylinder was scored measuring only initial contact with the cylinder walls. Baseline spontaneous locomotor and rearing activity was recorded for thirty minutes in 40.6 × 40.6 cm × 38.1 cm enclosed box using photobeam breaks and optical sensors. Spontaneous locomotor and rearing activity was then monitored for 120 minutes after s.c. injection of 1.5 mg/kg d-amphetamine sulfate (Sigma, St. Louis, MO). Data was scored as total number of beam breaks summed over the observation period (17).
Preimmunization and rechallenge studies
To measure the effects of a pre-existing anti-adenoviral immune response, naïve Lewis rats were injected intradermally in the back of the neck with 1×109 infectious units of first-generation adenoviral vector Ad-0 in a volume of 50 μL two weeks before intrastriatal injections of CNS1 tumor cells. Animals were housed under previously described normal conditions for 2 weeks to generate an immune response to the recombinant adenoviral vector. As controls, naïve rats received an intradermal injection of 50 μL of saline. 2 weeks post immunization, animals were stereotactically injected with CNS1 tumor cells as described above and treated 6 days later with an intratumoral injection of 5×109 vp each of HC-Ad-TetOn-Flt3L + HC-Ad-TK, or saline as a control. Animals were monitored daily and euthanized at the first signs of moribund behavior. One year post treatment, long-term survivors were rechallenged with an injection of 5,000 CNS1 cells into the contralateral brain hemisphere. No further treatment was given. Animals were monitored for survival.
Neutralizing Antibodies
To confirm the presence of an anti-adenovirus immune response, blood was collected by retro-orbital bleeding during implantation of CNS1 tumor cells and the levels of adenovirus specific neutralizing antibodies was assessed as described by us previously (2, 15, 18). Briefly, serum samples were heat-inactivated at 56°C for 30 min and serially diluted twofold from 1:2 to 1:4,096. Each dilution was incubated with 3 × 105 iu of Ad-hCMV-β-Gal, added to a 96-well plate containing HEK 293 cells and incubated at 37°C for 20 h before fixing with 4% paraformaldehyde and staining with X-gal (Sigma). The neutralizing antibody titer for each animal is given as the reciprocal of the highest dilution of serum at which 50% of Ad-hCMV-β-Gal mediated transduction was inhibited.
References
(1) Candolfi, M. et al. Effective High-Capacity Gutless Adenoviral Vectors Mediate Transgene Expression in Human Glioma Cells. Mol Ther 14, 371-81 (2006).
(2) Xiong, W. et al. Regulatable Gutless Adenovirus Vectors Sustain Inducible Transgene Expression in the Brain in the Presence of an Immune Response against Adenoviruses. J Virol 80, 27-37 (2006).
(3) Gerdes, C.A., Castro, M.G. & Lowenstein, P.R. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol Ther 2, 330-8 (2000).
(4) Morsy, M.A. et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci U S A 95, 7866-71 (1998).
(5) Palmer, D. & Ng, P. Improved system for helper-dependent adenoviral vector production. Mol Ther 8, 846-52 (2003).
(6) Southgate, T., Kroeger, K.M., Liu, C., Lowenstein, P.R. & Castro, M.G. Gene transfer into neural cells in vitro using adenoviral vectors. Curr Protoc Neurosci Chapter 4, Unit 4 23 (2008).
(7) Curtin, J. et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Medicine 6, e10 (2009).
(8) Ali, S. et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther 10, 1071-84 (2004).
(9) Dewey, R.A. et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med 5, 1256-63 (1999).
(10) Candolfi, M. et al. Intracranial glioblastoma models in preclinical neurooncology: neuropathological characterization and tumor progression. J Neurooncol 85, 133-48 (2007).
(11) Candolfi, M. et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res 15, 4401-14 (2009).
(12) Puntel, M. et al. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum Gene Ther 17, 531-44 (2006).
(13) Thomas, C.E., Kroeger, K.M., Puntel, M., Sanderson, N.S.R., Castro, M.G., and Lowenstein, P.R. Gene transfer into rat brain using adenoviral vectors. In: Current Protocols in Neuroscience, Vol. 4.23.1-4.23.40 (ed. Gerfen, J.N., McKay, R., Rogawski, M.A., Sibley, D.R. and Skolnick, P.) 4.23.1-4.40 (John Wiley and Sons, New York, New York, NY, 2009).
(14) King, G.D. et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol 10, 19-31 (2008).
(15) King, G.D. et al. High-Capacity adenoviral vector-mediated anti-glioma gene therapy in the presence of systemic anti-adenovirus immunity. J Virol 82, 4680-4 (2008).
(16) Curtin, J.F. et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol 176, 3566-77 (2006).
(17) King, G.D. et al. Flt3L in combination with HSV1-TK mediated gene therapy reverses brain tumor induced behavioral deficits. Mol Ther 16, 682-90 (2008).
(18) Thomas, C.E., Schiedner, G., Kochanek, S., Castro, M.G. & Lowenstein, P.R. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci U S A 97, 7482-7 (2000).
Supplemental Figure 1. A regimen of four weeks of doxycycline in combination with intratumoral delivery of HC-Ads is required to improve long-term survival in tumor bearing rats. (A) 4,500 CNS1 cells were injected in the striatum of naive Lewis rats. Six days after tumor implantation, rats were injected with 1×109 vp each of HC-Ad-TetOn-Flt3L and HC-Ad-TK (n=18), 2×109 vp HC-Ad-TetOn-βgal (n=7) or saline alone (n=12). GCV (25mg/kg, i.p.) was administered twice a day for 10 days. To assess the optimal duration of doxycycline (DOX) administration to “turn on” therapeutically acceptable levels of transgene expression, rodent chow containing DOX was administered for either 2 weeks (n=6) or 4 weeks (n=12) ad libitum. Log-rank analysis of Kaplan-Meyer survival curves reveals only animals receiving HC-Ad-TetOn-Flt3L and HC-Ad-TK (+GCV) and DOX for 4 weeks survived long term (*p<0.05 vs. all other groups).
Supplemental Figure 2. Experimental design of biodistribution studies. (A) Illustration of a rat brain depicting the regions dissected for biodistribution analysis including the injection site in the striatum, the cerebellum, and the brain stem. (B) Illustration depicting the various peripheral organs dissected for biodistribution analysis including lumbar spinal cord, cervical draining lymph node, spleen, heart, gut, testes, lung, liver, and kidney. (C) The sequences of the HC-Ad specific primers and probe used for detection of HC-Ad vector genomes and for Flt3L transgene copies are shown. (D) Illustration of plasmid used to generate the standard curve for qPCR analysis of the biodistribution of HC-Ad vector genomes. The primers and probes used detect a region of the cosmid sequence in the HC-Ad vector genome. (E) A representative standard curve and (F) Amplification Plot used to quantitate levels of HC-Ad vector genomes by qPCR are shown.
Supplemental Figure 3. The biodistribution of vector genomes in tumor bearing animals is restricted to the site of the injection at thirty days, six months, and twelve months after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK. Tumor bearing Lewis rats were euthanized at specific time points after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK at (A-C) 5×108 vp, (D-F) 1×109 vp, and (G-I) 5×109 vp of each vector. Animals were euthanized at (A, D, E) 1 month, (B, E, H) 6 months, and (C, F, I) 12 months post-HC-Ad treatment. Thirteen tissues were harvested: spleen, liver, testes, gut, lung, heart, cervical draining lymph nodes, kidney, brain tumor injection side and brain contralateral side, brain stem, cerebellum, and lumbar spinal cord. DNA was isolated from 25mg of each tissue for each animal. Isolated DNA from each tissue was used for qPCR with a primer and probe set specific for the HC-Ad vector backbone to assess the biodistribution of the vector genomes. Vector genomes’ quantification results are the average of triplicates for each DNA sample, and are shown as a ratio of vector genomes/25 mg of tissue. Results are based on n=5 per group.
Supplemental Figure 4. HC-Ad treated, tumor bearing animals show a complete regression of the brain tumor mass and restoration of the normal brain architecture thirty days treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK. Tumor bearing Lewis rats surviving long term were euthanized one month after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK at 5×108 vp, 1×109 vp, and 5×109 vp of each vector (n= 5 animals/group), and perfused with fixative. Brains were sectioned (60 μm) and stained with Nissl for gross morphology, or assessed for evidence of inflammation and neurotoxicity by immunocytochemistry with markers for oligodendrocytes and myelin sheath (MBP), dopaminergic nerve terminals (TH), residual tumor cells or activated astrocytes (vimentin), CD8+ T cells (CD8), macrophages and microglia (ED1), macrophages, microglia and immune cells (MHC-II), or transgene expression (TK or Flt3L). Note that areas indicative of inflammation are restricted to the site of tumor implantation and vector delivery.
Supplemental Figure 5. Long-term survivors do not show evidence of severe inflammation six months after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK. Tumor bearing Lewis rats surviving long term were euthanized six months after treatment with HC-Ad-TetOn-Flt3L and HC-Ad-TK at 5×108 vp, 1×109 vp, and 5×109 vp of each vector (n= 5 animals/group), and perfused with fixative. Brains were sectioned (60 μm) and stained with Nissl for gross morphology, or assessed for evidence of inflammation and neurotoxicity by immunocytochemistry with markers for oligodendrocytes and myelin sheath (MBP), dopaminergic nerve terminals (TH), residual tumor cells or activated astrocytes (vimentin), CD8+ T cells (CD8), macrophages and microglia (ED1), macrophages, microglia and immune cells (MHC-II), or transgene expression (TK or Flt3L). Note that areas indicative of inflammation are restricted to the site of tumor implantation and vector delivery.
Supplemental Table 1. Analysis of clinical hematological parameters of tumor bearing animals five days after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK.
Supplemental Table 2. Analysis of clinical hematological parameters of tumor bearing animals thirty days after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK.
Supplemental Table 3. Analysis of clinical hematological parameters of tumor bearing animals six months after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK.
Supplemental Table 4. Analysis of clinical hematological parameters of tumor bearing animals twelve months after treatment with HC-Ad-TetOnFlt3L and HC-Ad-TK.