Summary
There have been substantial recent changes in the global distribution and nature of bluetongue virus (BTV) infection of ungulates, perhaps as a result of climate change. To evaluate the epidemiology of BTV infection in California, an area historically endemic for the virus, we monitored newborn dairy calves at different sites for one year for the presence of BTV RNA and virus-specific antibodies. The data confirm both localized, vector-mediated, seasonal transmission of BTV as well as dissemination of BTV and/or viral nucleic acid to newborn calves following ingestion of colostrum.
Keywords: Bluetongue Virus, Sentinel, Dairy Calves, Colostrum
Introduction
Bluetongue virus (BTV) is the cause of bluetongue (BT), a re-emerging arboviral disease of ruminants transmitted by various species of hematophagous Culicoides insects. BT was first described in South Africa, and BTV has subsequently been identified on all continents except Antarctica (Spreull, 1905; Verwoerd et al., 2004). There has been a profound recent alteration in the global distribution of BTV (Maclachlan, 2010). Since 1998, at least 8 different BTV serotypes have invaded extensive portions of Europe precipitating an economically devastating epidemic, and BTV is now widely dispersed throughout much of Europe and spread by species of Culicoides midges that were not proven previously to be vectors of the virus (Gomez-Trejedor, 2004; Meiswinkel et al., 2008; Mellor et al., 2008; Wilson et al., 2008; Saegerman et al., 2008). Coincidentally, some 10 novel BTV serotypes have been identified since 1998 in the southeastern United States (serotypes 1,2,5,6,9,12,14,19,22,24) and 2 previously exotic BTV serotypes have recently been isolated in northern Australia (serotypes 2,23) (Johnson, 2007; Ostlund, 2009; Maclachlan et al., 2010). Climate change has been incriminated in the remarkable recent expansion of the global distribution of BTV infection (Purse et al., 2005; Purse et al., 2008; Maclachlan, 2010; Maclachlan et al., 2010).
BTV infection of ruminants traditionally has been regarded as “non contagious” and exclusively transmitted by infected vector Culicoides insects (Erasmus, 1985; Verwoerd et al., 2004; Maclachlan et al., 2009). Although transplacental infection is a property of certain strains of BTV, such as those propagated in cell culture and the strain of BTV serotype 8 currently circulating in Europe (MacLachlan et al., 2000; De Clercq et al., 2008; Menzies et al., 2008; Backx et al., 2009; Darpel et al., 2009; Maclachlan et al., 2009; Worwa et al., 2009; Saegerman et al., 2010), suggestions of persistent infection of ruminants following vertical transmission of the virus have been discredited (Maclachlan et al., 2000; Maclachlan et al., 2009). However, it was shown as early as 1965 that calves could be infected after oral ingestion of BTV(Jochim et al., 1965), consistent with findings among calves in the ongoing European epidemic (De Clercq et al., 2008; Backx et al., 2009; Saegerman et al., 2010). The objective of the present study was to re-evaluate the epidemiology of BTV infection in California where BTV has been endemic for more than 50 years (McKercher et al., 1953; Osburn et al., 1981; Metcalf et al., 1981; Stott et al., 1985).
Materials and Methods
The study began in January, 2009, with enrollment of 123 newborn heifer calves on 10 commercial dairy farms located in 5 different regions of California, including northwestern California that has historically been free of BTV infection ( Metcalf et al., 1981). Only calves born during the winter months (January to March) were included to preclude vector transmission of BTV, which is highly seasonal (typically August to December) in California (Osburn et al., 1981; Stott et al., 1985). Cattle in California are not vaccinated against BTV infection. Serum and blood were collected from each calf within 1–3 days of birth, and at monthly intervals thereafter for approximately one year. Sera and whole blood were respectively analyzed for the presence of antibodies and viral RNA by BTV-specific competitive ELISA (cELISA; VMRD Inc., Pullman, WA) and quantitative PCR (qRT-PCR) assays (Ortega et al., 2010). Dams of calves that were viral RNA positive by qRT-PCR assay at initial sampling were also evaluated by cELISA for serological evidence of BTV infection (seroconversion). Prevalence proportions and 95% confidence intervals (CI) were utilized to characterize the serologic and qRT-PCR positive status among sentinel calves within all sites.
Virus isolation was performed on individual blood samples that were qRT-PCR positive, essentially as previously described but using bovine pulmonary artery endothelial (bPAEC) cells (DeMaula et al., 2001; Bonneau et al., 2002). Virus isolates were confirmed as BTV by indirect immunofluorescent staining of infected bPAEC monolayers grown on chamber slides, using a monoclonal antibody to BTV core protein VP7 (Whetter et al., 1989). Virus isolates were serotyped by virus neutralization assays utilizing serotype - specific monoclonal and polyclonal antisera to BTV serotypes 10, 11, 13, and 17, which are the only BTV serotypes known to occur in California (Stott et al., 1985; Rossitto et al., 1992). The S10 gene of viral nucleic in qRT-PCR-positive blood samples was directly amplified for sequence and topotype analysis, as previously described (Balasuriya et al., 2008).
Results
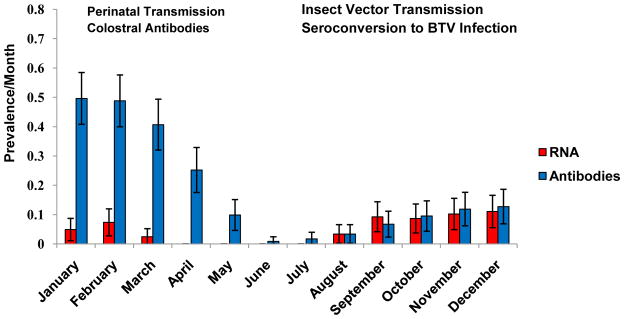
Approximately 50% of the sentinel calves (61/123) were seropositive at first sampling at 1 – 3 days of age. The initial sampling occurred after the calves had all ingested colostrum, which typically was pooled colostrum that was collected and stored on each individual farm. BTV-specific antibody persisted in these calves until 4–5 months of age, as expected for the decay of colostral antibodies (Heinrichs et al., 2009). BTV RNA was detected by qRT-PCR assay in the blood of individual calves in two distinct time periods: first, in newborn calves during the putatively BTV transmission-free months of January – June (mean Ct 35.1; SD 5.90); and second, during the expected seasonal transmission period of July – December (mean Ct 29.1; SD 4.05) (Figures 1 and 2). The prevalence of BTV qRT-PCR positive calves varied among study sites and although surveillance for Culicoides vectors was not conducted during the present study; previous studies have shown that vector-mediated transmission of BTV is highly seasonal in California; specifically, vector-mediated transmission of BTV occurs during the late Summer and Autumn (Osburn et al., 1981; Loomis et al., 1985; Stott et al., 1985; Gerry et al., 2001).
Figure 1.
Prevalence during 2009 of BTV-specific RNA and antibodies amongst sentinel calves by month. Red bars show prevalence of viral RNA as determined by qRT-PCR, blue bars prevalence of BTV specific antibodies as determined by cELISA, and error bars indicate 95% confidence intervals.
Figure 2.
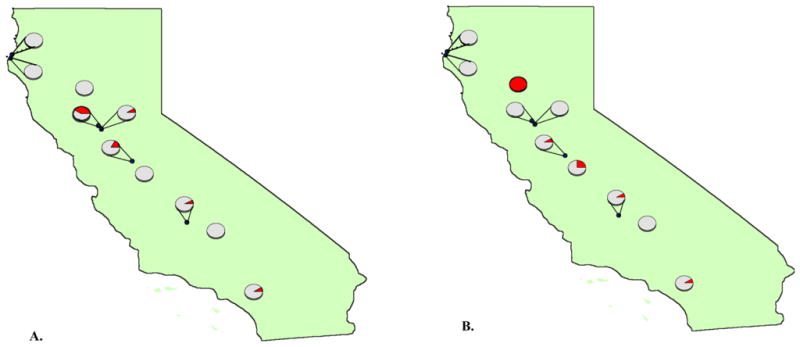
BTV infection of sentinel calves at different sites in California during 2009. Prevalence at each site of viral RNA positive animals as detected by qRT-PCR during A. January-June and B. July-December.
Sixteen calves tested positive (Ct≤ 35) for BTV RNA between July – December; these calves were 6–7 months of age when first positive. All of these calves seroconverted after becoming qRT-PCR positive confirming that they had been productively infected with BTV. Viral RNA persisted in the blood of individual calves for 3–4 months, and the prevalence of infected calves ranged from 0 – 100%, depending on site. Specifically, the prevalence of viral RNA positive calves was 100%, (95% CI, 74% to 100%) amongst calves from Orland in the Sacramento Valley, and ranged from 8.3% (95% CI, 0.2% to 38.5%) to 25% (95% CI, 5.5% to 57.2%) amongst calves on farms in Modesto, Merced, and Tulare in the San Joaquin Valley and Riverside in southern California (Figure 2). BTV (serotype 17) was isolated from the blood of individual calves at Orland and Merced.
Six calves were qRT-PCR positive immediately after birth and during the initial months of testing (January-June), when they were also all seropostive. Five of these calves remained positive by qRT-PCR (mean Ct 34.3; SD 1.11) from birth until 2 months of age when all 5 became negative, which they remained for the duration of the study. BTV was never isolated from these 5 calves, so the virus serotype was not determined. All of these transiently virus-positive calves became seronegative following loss of colostral antibody, indicating that they were not productively infected with BTV. In contrast, BTV (serotype 11) was isolated from the sixth calf and this animal remained seropositive for the duration of the study confirming that it had been productively infected; viral RNA (mean Ct 28.3; SD 5.52) was detected in the blood of this calf for 3 months after birth, after which the calf was consistently negative for viral RNA. The dams of all of the qRT-PCR-positive newborn calves that were available for sampling (5 of 6 animals) were seronegative to BTV, precluding the possibility of vertical BTV transmission and congenital infection. Thus, to determine if the pooled colostrum fed to these calves was responsible for their positive qRT-PCR status at first sampling, the pooled colostrum fed to the calves at one site (Davis) was evaluated by qRT-PCR assay and shown to contain BTV RNA. Sequence analysis of the S10 genes amplified from viral nucleic acid in colostrum (GenBank Accession GU954427) and the blood of one colostrum-fed calf (GenBank Accession GU954426) confirmed sequence homology (>99.75% nucleotide identity; > 99.5% amino acid identity) of amplicons from the two sources, indicating that viral nucleic acid in the colostrum was most likely responsible for the positive qRT-PCR status of the newborn calves. The S10 gene sequence of the virus isolated from the infected newborn calf in Modesto (GenBank Accession GU954425) was distinct from that of the genes amplified from colostrum and calf blood from the site at Davis, indicating that genetically distinct viruses were spread in colostrum at each farm.
Discussion
Data from this sentinel calf study in California confirm both localized, vector-mediated, seasonal transmission of BTV as well as dissemination of BTV and/or viral nucleic acid to newborn calves via colostrum. Specifically, seasonal transmission of BTV serotype 17 occurred at multiple sites although, for reasons undetermined at this time, at lower frequency than anticipated from the results of similar studies undertaken approximately 30 years previously. In these previous studies, the mean proportion of viremic ruminants was 4.5% and seroprevalence was 47.8% as determined respectively by virus isolation in embryonated chicken eggs and agar gel immunodiffusion test (Osburn et al., 1981; Stott et al., 1985). The cause of this apparent decrease in the prevalence of BTV infection of cattle in California is uncertain, but changes in husbandry practices along with climatic and environmental parameters might be responsible (Purse et al., 2008; Zimmer et al., 2010).
Unexpectedly, BTV nucleic acid also was detected in 6 colostrum-fed, newborn calves during the winter months when vector Culicoides insects are not active, and BTV serotype 11 was isolated from one of these calves. Sequence analysis confirmed colostrum to be the source of viral nucleic acid in the positive calves at Davis, and the dams of these five qRT-PCR-positive calves were all seronegative to BTV at least one year after parturition indicating that transplacental infection was not responsible for infection of these calves. However, the dam of other positive calf (Modesto 22) that was productively infected with BTV at first sampling after birth was not available for follow-up and, although unlikely, transplacental virus infection during late gestation cannot be definitively excluded in this calf.
The role of perinatal BTV infection in the epidemiology of BTV infection is uncertain, as viral nucleic acid was only transiently detected in the blood of individual calves and infectious virus was isolated from only a single calf. Indeed, the 5 calves that were transiently qRT-PCR assay positive but virus isolation negative following ingestion of colostrum did not seroconvert to BTV, indicating they were not productively infected. BTV nucleic acid can also persist for long periods in the blood of infected ruminants in the absence of infectious virus (Maclachlan et al., 1994; Bonneau et al., 2002; Maclachlan et al., 2009). Findings from the current study also are consistent with those of prior experimental studies in which oral transmission was demonstrated among calves following ingestion of colostrum that was spiked with wild type BTV serotype 8 (Backx et al., 2009). However, our study confirms for the first time the natural dissemination of BTV and/or BTV nucleic acid in colostrum, as determined by surveillance monitoring of a sentinel cohort of dairy calves on commercial farms.
Acknowledgments
We gratefully acknowledge the assistance of veterinarians at the California Department of Food and Agriculture for sample collection in addition to producers, staff, and herd managers of the farms from which the samples were obtained. We also thank Dr. Udeni Balasuriya for assistance with sequence and phylogenetic analyses.
These studies were supported by funds provided by the Center for Food Animal Health at the University of California-Davis, the U.S. Department of Agriculture under the Animal Health Act, 1977, Public Law 95–113, and grant number T32 AI074550-01A2 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH. This project was also supported by the Center for Equine Health with funds provided by the Bernice Barbour and Harriet E. Pfleger foundations.
References
- Backx A, Heutink R, van Rooij E, van Rijn P. Transplacental and oral transmission of wild-type bluetongue virus serotype 8 in cattle after experimental infection. Vet Microbiol. 2009;138:235–243. doi: 10.1016/j.vetmic.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Balasuriya UB, Nadler SA, Wilson WC, Pritchard LI, Smythe AB, Savini G, Monaco F, De Santis P, Zhang N, Tabachnick WJ. The NS3 proteins of global strains of bluetongue virus evolve into regional topotypes through negative (purifying) selection. Vet Microbiol. 2008;126:91–100. doi: 10.1016/j.vetmic.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Bonneau KR, DeMaula CD, Mullens BA, MacLachlan NJ. Duration of viraemia infectious to culicoides sonorensis in bluetongue virus-infected cattle and sheep. Vet Microbiol. 2002;88:115–125. doi: 10.1016/s0378-1135(02)00106-2. [DOI] [PubMed] [Google Scholar]
- Darpel KE, Batten CA, Veronesi E, Williamson S, Anderson P, Dennison M, Clifford S, Smith C, Philips L, Bidewell C. Transplacental transmission of bluetongue virus 8 in cattle UK. Emerg Infect Dis. 2009;15:2025–2028. doi: 10.3201/eid1512.090788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq K, Vandenbussche F, Vandemeulebroucke E, Vanbinst T, De Leeuw I, Verheyden B, Goris N, Mintiens K, Meroc E, Herr C. Transplacental bluetongue infection in cattle. Vet Rec. 2008;162:564. doi: 10.1136/vr.162.17.564. [DOI] [PubMed] [Google Scholar]
- DeMaula CD, Jutila MA, Wilson DW, MacLachlan NJ. Infection kinetics, prostacyclin release and cytokine-mediated modulation of the mechanism of cell death during bluetongue virus infection of cultured ovine and bovine pulmonary artery and lung microvascular endothelial cells. J Gen Virol. 2001;82:787–794. doi: 10.1099/0022-1317-82-4-787. [DOI] [PubMed] [Google Scholar]
- Erasmus BJ. The history of bluetongue. Prog Clin Biol Res. 1985;178:7–12. doi: 10.1016/b978-012369368-6.50006-x. [DOI] [PubMed] [Google Scholar]
- Gerry AC, Mullens BA, Maclachlan NJ, Mecham JO. Seasonal transmission of bluetongue virus by culicoides sonorensis (diptera:ceratopogonidae) at a southern California dairy and evaluation of vectorial capacity as a predictor of bluetongue virus transmission. J Med Entomol. 2001;38:197–209. doi: 10.1603/0022-2585-38.2.197. [DOI] [PubMed] [Google Scholar]
- Gomez-Trejedor C. Brief overview of bluetongue situation in Mediterranean Europe. Vet Italiana. 2004;40:57–60. [PubMed] [Google Scholar]
- Heinrichs AJ, Jones CM. In: Large Animal Internal Medicine. 4. Smith BP, editor. Mosby Elsevier; St. Louis, Missouri: 2009. pp. 367–369. [Google Scholar]
- Jochim MM, Luedke AJ, Bowne JG. The clinical and immunogenic response of sheep to oral and intradermal administrations of bluetongue virus. Am J Vet Res. 1965;26:1254–1260. [PubMed] [Google Scholar]
- Johnson DJ. Identification of new United States bluetongue types. Proc Ann Mtg US Anim Health Assoc. 2007;111:209–210. [Google Scholar]
- Loomis EC, Bushnell RB, Osburn BI. Epidemiologic study of bluetongue virus on the tejon ranch, California: vector, host, virus relationships. Prog Clin Biol Res. 1985;178:583–588. [PubMed] [Google Scholar]
- Maclachlan NJ. Global implications of the recent emergence of bluetongue virus in Europe. Vet Clin North Am Food Anim Pract. 2010;26:163–171. doi: 10.1016/j.cvfa.2009.10.012. [DOI] [PubMed] [Google Scholar]
- MacLachlan NJ, Conley AJ, Kennedy PC. Bluetongue and equine viral arteritis viruses as models of virus-induced fetal injury and abortion. Anim Reprod Sci. 2000;60–61:643–651. doi: 10.1016/s0378-4320(00)00105-6. [DOI] [PubMed] [Google Scholar]
- Maclachlan NJ, Drew CP, Darpel KE, Worwa G. The pathology and pathogenesis of bluetongue. J Comp Pathol. 2009;141:1–16. doi: 10.1016/j.jcpa.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Maclachlan NJ, Guthrie AJ. Re-emergence of bluetongue, african horse sickness, and other orbivirus diseases. Vet Res. 2010;41:35. doi: 10.1051/vetres/2010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclachlan NJ, Osburn BI. Induced brain lesions in calves infected with bluetongue virus. Vet Rec. 2008;162:490–491. doi: 10.1136/vr.162.15.490-b. [DOI] [PubMed] [Google Scholar]
- McKercher DG, McGowan B, Howarth JA, Saito JK. A preliminary report on the isolation and identification of the bluetongue virus from sheep in California. J Am Vet Med Assoc. 1953;122:300–301. [PubMed] [Google Scholar]
- Meiswinkel R, Baldet T, de Deken R, Takken W, Delecolle JC, Mellor PS. The 2006 outbreak of bluetongue in northern Europe-the entomological perspective. Prev Vet Med. 2008;87:55–63. doi: 10.1016/j.prevetmed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Mellor PS, Carpenter S, Harrup L, Baylis M, Mertens PP. Bluetongue in Europe and the mediterranean basin: history of occurrence prior to 2006. Prev Vet Med. 2008;87:4–20. doi: 10.1016/j.prevetmed.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Menzies FD, McCullough SJ, McKeown IM, Forster JL, Jess S, Batten C, Murchie AK, Gloster J, Fallows JG, Pelgrim W. Evidence for transplacental and contact transmission of bluetongue virus in cattle. Vet Rec. 2008;163:203–209. doi: 10.1136/vr.163.7.203. [DOI] [PubMed] [Google Scholar]
- Metcalf HE, Pearson JE, Klingsporn AL. Bluetongue in cattle: a serologic survey of slaughter cattle in the United States. Am J Vet Res. 1981;42:1057–1061. [PubMed] [Google Scholar]
- Ortega J, Crossley B, Dechant JE, Drew CP, Maclachlan NJ. Fatal bluetongue virus infection in an alpaca (vicugna pacos) in California. J Vet Diagn Invest. 2010;22:134–136. doi: 10.1177/104063871002200129. [DOI] [PubMed] [Google Scholar]
- Osburn BI, McGowan B, Heron B, Loomis E, Bushnell R, Stott J, Utterback W. Epizootiologic study of bluetongue: virologic and serologic results. Am J Vet Res. 1981;42:884–887. [PubMed] [Google Scholar]
- Ostlund EN. 2009: Update to the bluetongue and related orbiviruses committee. Proc Ann Mtg US Anim Health Assoc. in press. [Google Scholar]
- Purse BV, Brown HE, Harrup L, Mertens PP, Rogers DJ. Invasion of bluetongue and other orbivirus infections into Europe: the role of biological and climatic processes. Rev Sci Tech. 2008;27:427–442. [PubMed] [Google Scholar]
- Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PP, Baylis M. Climate change and the recent emergence of bluetongue in Europe. Nat Rev Microbiol. 2005;3:171–181. doi: 10.1038/nrmicro1090. [DOI] [PubMed] [Google Scholar]
- Rossitto PV, MacLachlan NJ. Neutralizing epitopes of the serotypes of bluetongue virus present in the United States. J Gen Virol. 1992;73:1947–1952. doi: 10.1099/0022-1317-73-8-1947. [DOI] [PubMed] [Google Scholar]
- Saegerman C, Berkvens D, Mellor P. Bluetongue epidemiology in the European union. Emerg Infect Dis. 2008;14:539–544. doi: 10.3201/eid1404.071441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegerman C, Bolkaerts B, Baricalla C, Raes M, Wiggers L, de Leeuw I, Vandenbussche F, Zimmer JY, Haubruge E, Cassart D. The impact of naturally-occurring, trans-placental bluetongue virus serotype-8 infection on reproductive performance in sheep. Vet J. 2010 doi: 10.1016/j.tvjl.2009.11.012. in press. [DOI] [PubMed] [Google Scholar]
- Spreull J. Malarial catarrhal fever (bluetongue) of sheep in South Africa. J Comp Pathol. 1905;18:321–337. [Google Scholar]
- Stott JL, Osburn BI, Bushell R, Loomis EC, Squire KRE. Epizootiological study of bluetongue virus infection in California livestock: an overview. Prog Clin Biol Res. 1985;178:571–582. [PubMed] [Google Scholar]
- Verwoerd D, Erasmus BJ. In: Infectious Diseases of Livestock. 2. Coetzer JAW, Tustin RC, editors. Oxford University Press; Cape Town, South Africa: 2004. pp. 1201–1230. [Google Scholar]
- Whetter LE, Maclachlan NJ, Gebhard DH, Heidner HW, Moore PF. Bluetongue virus infection of bovine monocytes. J Gen Virol. 1989;70:1663–1676. doi: 10.1099/0022-1317-70-7-1663. [DOI] [PubMed] [Google Scholar]
- Wilson A, Mellor P. Bluetongue in Europe: vectors, epidemiology and climate change. Parasitol Res. 2008;103:S69–77. doi: 10.1007/s00436-008-1053-x. [DOI] [PubMed] [Google Scholar]
- Worwa G, Hilbe M, Ehrensperger F, Chaignat V, Hofmann MA, Griot C, Maclachlan NJ, Thuer B. Experimental transplacental infection of sheep with bluetongue virus serotype 8. Vet Rec. 2009;164:499–500. doi: 10.1136/vr.164.16.499. [DOI] [PubMed] [Google Scholar]
- Zimmer JY, Saegerman C, Losson B, Haubruge E. Breeding sites of bluetongue virus vectors in cowshed, Belgium. Emerging Infect Dis. 2010;16:575–576. doi: 10.3201/eid1603.091311. [DOI] [PMC free article] [PubMed] [Google Scholar]