Abstract
Efforts are currently underway to develop a vaccine against Clostridium difficile infection (CDI). We developed two decision analytic Monte Carlo computer simulation models: (1) an Initial Prevention Model depicting the decision whether to administer C. difficile vaccine to patients at-risk for CDI and (2) a Recurrence Prevention Model depicting the decision whether to administer C. difficile vaccine to prevent CDI recurrence. Our results suggest that a C. difficile vaccine could be cost-effective over a wide range of C. difficile risk, vaccine costs, and vaccine efficacies especially when being used post-CDI treatment to prevent recurrent disease.
Keywords: Clostridium difficile, vaccine, economics, computer simulation
1. INTRODUCTION
Efforts are currently underway to develop a vaccine against Clostridium difficile infection (CDI), a major and potentially growing cause of substantial morbidity, costs, and mortality throughout the developed world [1-9]. Although numerous interventions have been implemented to control the spread of Clostridium difficile (C. difficile) in hospitals, the bacterial pathogen remains established in many locations and continues to spread to others. CDI can result in longer hospital length of stay, necessitate antibiotic use that may lead to more antibiotic-resistant bacteria, and even require surgical procedures. A significant percentage of treated patients may experience relapse of disease, in some cases multiple relapses [10]. Moreover, the recent emergence of more virulent strains may make combating the nosocomial pathogen even more difficult [11].
Candidate C. difficile vaccines currently are in pre-clinical and early clinical development and show promise as options for both preventing and treating CDI. A potential vaccine containing C. difficile toxoids A and B has been shown to induce immune response in healthy adults [12]. Antibody levels measured from study participants exceeded the level previously shown to be associated with CDI prevention [9]. There is also evidence that such a vaccine could effectively treat recurrent infections, particularly those that other methods have failed to remedy [8].
Constructing economic models early in a vaccine's development can help identify appropriate target populations, establish vaccine efficacy targets, assist in pricing and reimbursement decisions, and help determine the investment that should be made into developing the vaccine when substantial changes are still possible. A number of vaccines have faced challenges when economic modeling occurred too late in the vaccine timeline to make necessary changes [13]. To answer such questions regarding C. difficile vaccine, we constructed computer models to simulate the decision of whether to administer C. difficile vaccine to patients. One model simulated the choice of whether to perform universal vaccination on at-risk patients. A second model simulated the option of vaccinating those currently with CDI and undergoing antibiotic treatment to prevent recurrence. Sensitivity analyses explored how the economic value of the vaccine varied with CDI risk, vaccine cost, and vaccine efficacy.
2. METHODS
Using TreeAge Pro 2009 (TreeAge Software, Williamstown, MA), we developed two decision analytic Monte Carlo computer simulation models:
Initial Prevention Model: depicting the decision whether to administer C. difficile vaccine to patients at-risk for CDI.
Recurrence Prevention Model: depicting the decision whether to administer C. difficile vaccine to patients currently with CDI to prevent CDI recurrence.
The model assumed the societal, hospital, and third party payer perspectives and simulated the potential consequences of the decision.
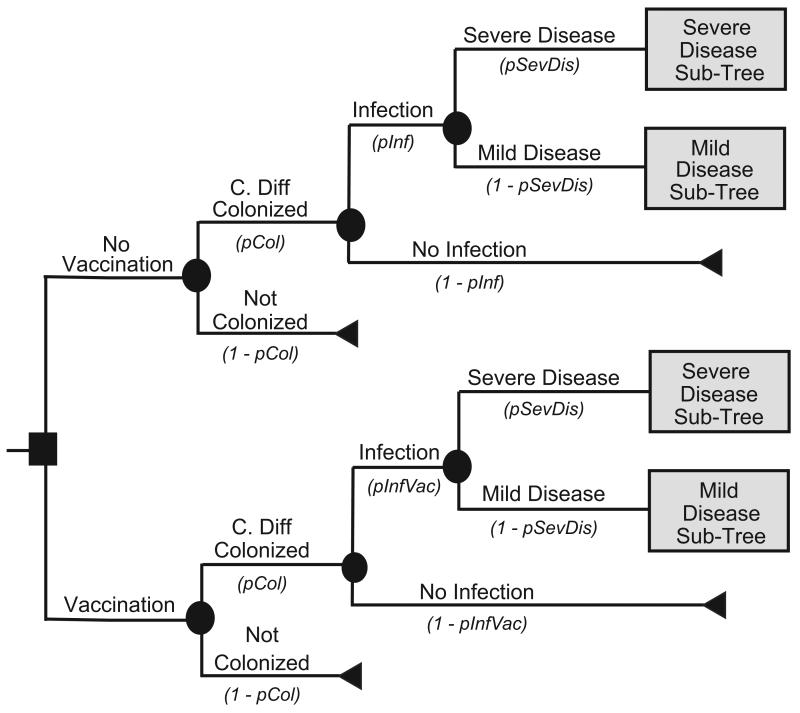
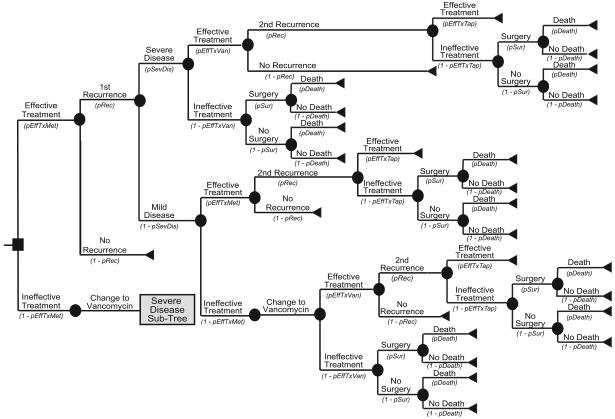
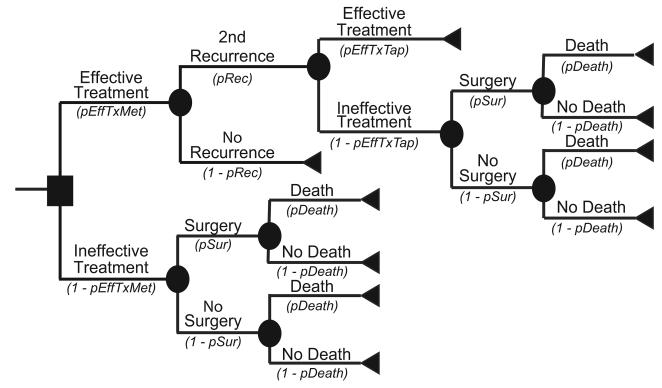
Figure 1a illustrates the Initial Prevention Model structure. Each patient had a risk of C. difficile colonization based on the local C. difficile prevalence. Figures 1 and 2 showdifferent variable names as the probabilities of moving down each branch. These variable names correspond to the variable names in the second column of Table 1. For example, the variable pInf represents the probability of infection; its complement 1-pInf calculates the probability of no infection. The variable pInf draws from the distribution with the parameters indicated in Table 1. The median age of a patient was 71 years, the median age of patients discharged with a diagnosis of C. difficile from the 2007 National Inpatient Survey from the Healthcare Cost and Utilization Project [14]. Each colonized patient then entered into a C. difficile outcomes sub-tree. Colonized patients had probabilities of remaining as asymptomatic carriers or progressing to CDI. Figures 1b and 1c show the CDI outcome models for mild and severe CDI, respectively. Both mild and severe CDI required antibiotic treatment, which had probabilities of being effective. Ineffective treatment allowed progression to more severe infections, requiring surgery and potentially leading to death. Patients successfully treated with antibiotics could either remain free of disease or suffer a CDI recurrence, i.e., reappearance of CDI within three months of successful treatment. Those who had a successfully treated first recurrence could then have a second recurrence. Patients who suffered two or more recurrences that were unsuccessfully treated had a probability of progressing to a severe disease state requiring surgery.
FIGURE 1.
FIGURE 1a: Initial Prevention Main Model Structure
FIGURE 1b: Initial Prevention Mild Disease Outcomes Sub-Tree Structure
FIGURE 1c: Initial Prevention Severe Disease Outcomes Sub-Tree Structure
FIGURE 2.
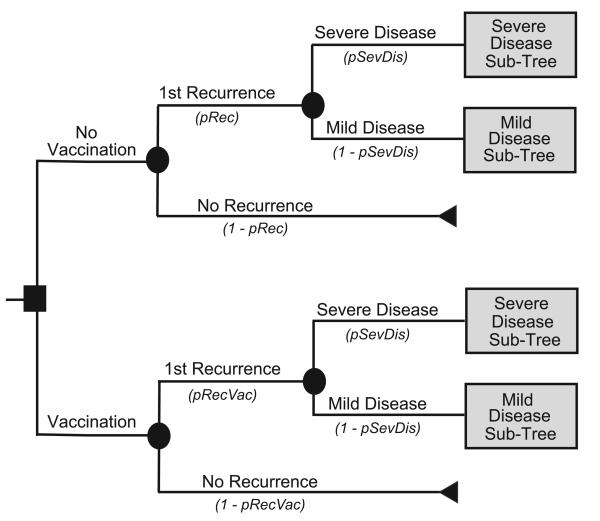
FIGURE 2a: Recurrence Prevention Main Model Structure
FIGURE 2b: Recurrence Prevention Mild Disease Outcomes Sub-Tree Structure
FIGURE 2c: Recurrence Prevention Severe Disease Outcomes Sub-Tree Structure
TABLE 1.
Data Inputs for Model Variables
| Description (units) | Variable Name |
Median | Upper Limit (or SDa) |
Lower Limit | Source |
|---|---|---|---|---|---|
| COSTS ($US) | |||||
| Abdominal CT Scan | 1535.58 | 1013.48 | 2057.68 | 23 | |
| Hospitalization | 7517.54 | 242.95a | 14 | ||
| Metronidazole (IV) | 107.86 | 10.28a | 24 | ||
| Metronidazole (PO) | 60.90 | 39.28a | 24 | ||
| Peripheral Intravenous Line Insertion | 146.25 | 96.52 | 195.98 | 25 | |
| Surgery (Colectomy) | 15995.10 | 5412.71a | 14 | ||
| Vancomycin | 1184.90 | 782.03 | 1587.77 | 24 | |
| Vancomycin (Tapered) | 2221.70 | 1466.32 | 2977.07 | 24 | |
| UTILITIES (DALYs) | |||||
| 1st Recurrence | −0.0029 | −0.0039 | −0.0019 | 18 | |
| 2nd+ Recurrences | −0.0043 | −0.0058 | −0.0029 | 18 | |
| C. difficile Infection | −0.0014 | −0.0019 | −0.0009 | 18 | |
| Mortality | −14.00 | −18.76 | −9.24 | 18 | |
| PROBABILITIES (%) | |||||
| Given C. difficile colonization: | |||||
| Developing C. difficile infection | pInf | 26.15 | 25.69a | 26-29 | |
| Given C. difficile infection: | |||||
| Mortality | pDeath | 2.66 | 2.13a | 14, 30-32 | |
| Recurrence | pRec | 20.00 | 6.77a | 33-35 | |
| Severe Disease | pSevDis | 8.09 | 13.05a | 30, 36, 37 | |
| Mild Disease | 91.91b | b | 30, 36, 37 | ||
| Surgery (Colectomy) | pSur | 1.20 | 2.51a | 19, 30, 34, 38-42 |
|
| C. difficile treatment efficacies: | |||||
| Metronidazole | pEffTxMet | 90.41 | 9.28a | 43-50 | |
| Vancomycin | pEffTxVan | 98.18 | 7.64a | 45, 46, 48-53 |
|
| Vancomycin (Tapered) | pEffTxTap | 68.97 | 45.52 | 92.41 | 54 |
Standard deviation.
Probability of mild disease is calculated as 1 - pSevDis
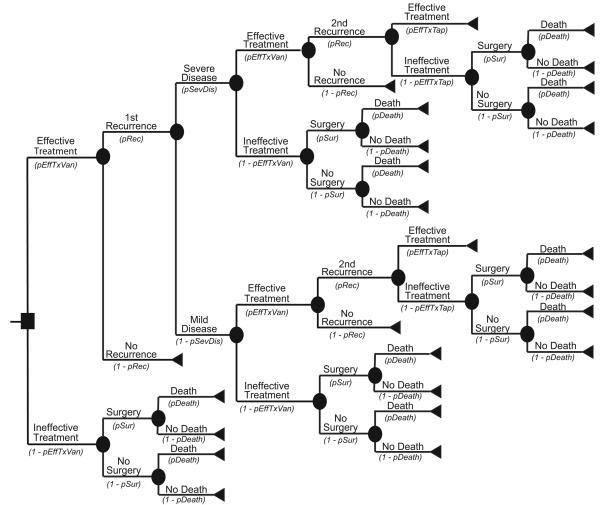
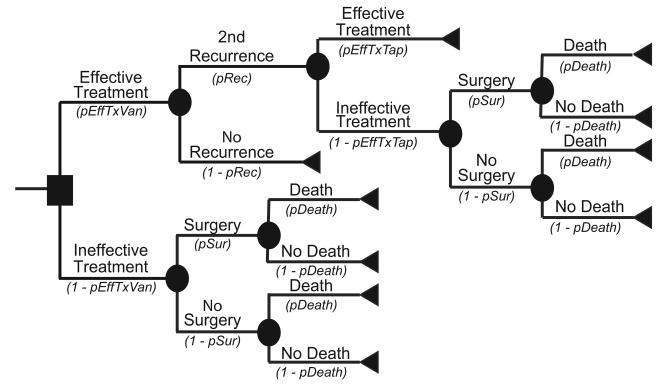
Figure 2a depicts the main decision model for the Recurrence Prevention Model. For this model, the median patient age was also 71 years. Each patient began with successfully treated CDI and then had a probability of experiencing recurrent CDI. All recurrences had probabilities of progressing either to mild or severe disease. Figure 2b represents the outcome model for mild CDI, while Figure 2c represents the outcome model for severe CDI, with both forms of CDI requiring antibiotic treatment. An effectively treated patient could then suffer a second recurrence. Ineffective treatments at any point in the model allowed progression to more serious CDI that required surgery and could result in death.
Treatment options depended on the disease severity, prior treatments, and number of recurrences. For mild disease, metronidazole was the first-line antibiotic treatment, and vancomycin was the second-line. For severe disease, the treatment of choice was vancomycin along with intravenous metronidazole prior to surgery. When patients suffered a recurrence, the first-line treatment was the same antibiotic that worked for the initial CDI episode (e.g. a patient who relapsed after being successfully treated with metronidazole then received metronidazole again). Patients suffering two or more recurrences received a tapered course of vancomycin. Additionally, patients with severe disease required peripheral intravenous line insertion as well as an abdominal computerized tomography.
Our model measures effectiveness in disability-adjusted life years (DALYs) prevented using the following formula:
For each simulation run, we determined the incremental cost effectiveness ratio (ICER) of C. difficile vaccination as defined as:
Vaccination was considered to be cost-effective if the ICER fell below three times the per capita gross domestic product (GDP) or $80,412/DALY prevented for the United States, a frequently cited threshold for cost-effectiveness [15, 16].
2.1 Data Inputs
Table 1 lists the input parameters for the model, including variable names (featured in the Figures), probabilities, costs, and utilities, as well as the distribution parameters for each variable. Probabilities assumed beta distributions, except for the efficacy of tapered vancomycin treatment (triangular distribution), as well as the probability of colonization and the probability of successful isolation, which were fixed values during each simulation. All costs were in 2009 U.S. dollars. A 3% discount rate adjusted all costs to 2009 dollars.
We used two separate approaches to determine the costs associated with CDI:
Health Care Resource Use: A first approach involved identifying the procedure and hospitalization costs associated with different CDI conditions. Costs drew from triangular distributions, with several exceptions: costs of hospitalization, metronidazole [intravenous (IV) and oral (PO)], and surgery assumed gamma distributions, while the cost of in-hospital death was fixed at $5000.
Opportunity Cost of Lost Bed-Days: An alternative approach re-conducted our analyses using a method described by Graves to ascribe economic costs to hospital infections [17]. When CDI caused a patient to occupy a bed for a longer period of time, the hospital lost revenue because the bed could have been filled by another patient. The Graves method involved valuing the opportunity cost of a lost bed day and then multiplying it by the extended hospital length-of-stay caused by the ensuing type of CDI.
Disability weights corresponding to diarrheal disease came from the World Health Organization's Global Burden of Disease [18]. CDI, CDI recurrences, and death each resulted in corresponding DALY increments (Table 1). The length of disease was six days, the median length of hospitalization for a 71-year-old patient with CDI.
2.2 Sensitivity Analyses
Because C. difficile risk may differ significantly from hospital-to-hospital, sensitivity analyses systematically varied the risk of C. difficile from 0.1% to 90%. Additional sensitivity analyses ranged vaccine efficacy from 25% to 100%. Because one of our goals is to estimate the cost thresholds under which a vaccine would remain cost-effective, we started vaccine cost at $25 for the Initial Prevention Model and $100 for the Recurrence Prevention Model. We then systematically increased the cost of the vaccine until it was no longer cost-effective. (Consequently, sensitivity analyses ranged vaccine cost from $25 to $100 in the Initial Prevention Model and from $100 to $1600 in the Recurrence Prevention Model.) Furthermore, probabilistic (Monte Carlo) sensitivity analyses simultaneously varied the values of each parameter throughout the ranges shown in Table 1. Another set of sensitivity analyses varied the probability of undergoing colectomy due to severe CDI from its baseline probability distribution listed in Table 1 down to 0.6% and 0.2%.
3. RESULTS
3.1 Health Care Resource Use Approach
Each simulation run comprised of a cohort of 5,000 patients, each travelling 5,000 times through the model for a total of 2,500,000 simulated trials. Table 2 displays the results from the Initial Prevention Model when varying C. difficile risk, vaccine efficacy, and vaccine cost. Shaded cells correspond to situations where vaccination is not cost-effective. Vaccination was economically dominant (both less costly and more effective) over no vaccination when C. difficile risk was at least 5% at almost every vaccine efficacy and cost combination, except when vaccine efficacy dropped to 25% and vaccine cost was at least $50 and when vaccine efficacy was 50% and cost was equal to $100. A $25 vaccine was dominant as long as C. difficile risk was at least 2.5%.
TABLE 2.
Incremental Cost-Effectiveness Ratio ($US/DALY prevented) of Vaccination for Initial Prevention of Clostridium difficile infection (CDI) using the Health Care Resource Use Approach.
| Vaccine Efficacy |
Clostridium difficile Risk | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.1% | 1.0% | 2.5% | 5.0% | 10.0% | 15.0% | 20.0% | 25.0% | |
| Cost of Vaccination = $25 | ||||||||
| 25% | 12,697,913 | 4,189,295 | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant |
| 50% | 11,832,473 | 1,320,623 | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant |
| 75% | 5,429,310 | 445,746 | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant |
| 100% | 1,420,236 | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant |
| Cost of Vaccination = $50 | ||||||||
| 25% | 28,084,233 | 4,329,877 | 2,176,586 | 727,421 | Dominant | Dominant | Dominant | Dominant |
| 50% | 26,917,206 | 4,074,365 | 522,459 | Dominant | Dominant | Dominant | Dominant | Dominant |
| 75% | 21,388,859 | 2,256,369 | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant |
| 100% | 12,064,352 | 887,840 | Dominant | Dominant | Dominant | Dominant | Dominant | Dominant |
| Cost of Vaccination = $100 | ||||||||
| 25% | 117,638,162 | 29,845,039 | 4,258,047 | 3,664,621 | 751,912 | 2,169 | Dominant | Dominant |
| 50% | 66,314,756 | 7,378,601 | 1,783,465 | 478,200 | Dominant | Dominant | Dominant | Dominant |
| 75% | 59,743,367 | 4,459,509 | 1,646,619 | Dominant | Dominant | Dominant | Dominant | Dominant |
| 100% | 41,956,115 | 3,483,069 | 539,666 | Dominant | Dominant | Dominant | Dominant | Dominant |
NOTE: Dominant = Vaccination is the dominant strategy (i.e., less costly and more effective)
Table 3 shows results from the Recurrence Prevention Model at different vaccine efficacies and costs. Vaccination was cost-effective, and frequently economically dominant, in most situations. An $800 vaccine was dominant as long as vaccine efficacy was at least 50%. Even at a cost of $1600, a 75% efficacious vaccination was cost-effective.
TABLE 3.
Incremental Cost-Effectiveness Ratio ($US/DALY prevented) of Vaccination for Clostridium difficile infection (CDI) Recurrence Prevention of using the Health Care Resource Use Approach.
| Vaccine Efficacy |
Cost of Vaccination | ||||
|---|---|---|---|---|---|
| $1600 | $800 | $400 | $200 | $100 | |
| 25% | 354,064 | 92,691 | Dominant | Dominant | Dominant |
| 50% | 94,054 | Dominant | Dominant | Dominant | Dominant |
| 75% | 5,081 | Dominant | Dominant | Dominant | Dominant |
| 100% | Dominant | Dominant | Dominant | Dominant | Dominant |
NOTE: Dominant = Vaccination is the dominant strategy (i.e., less costly and more effective)
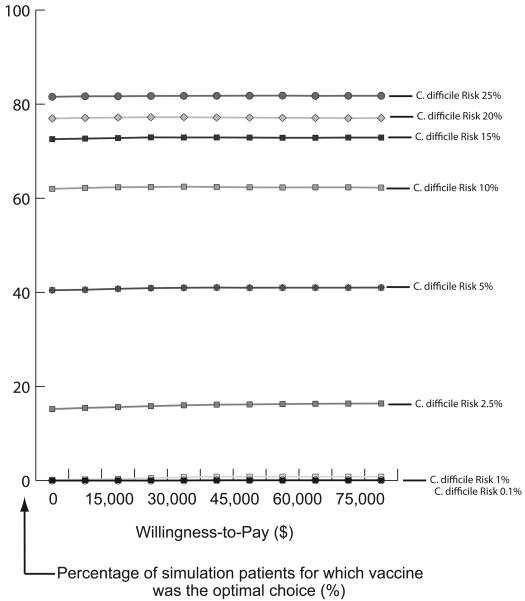
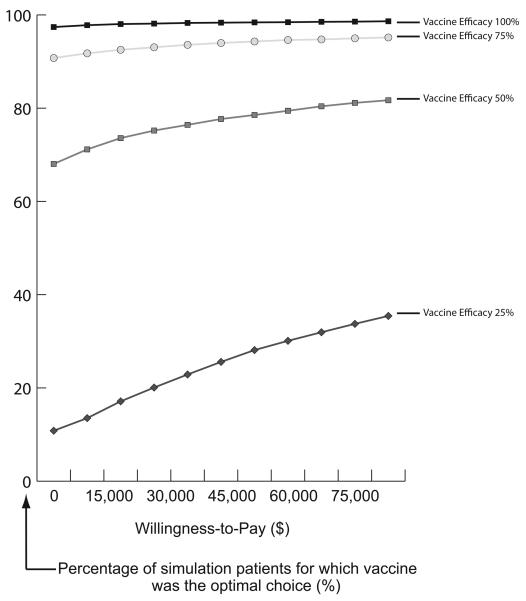
Figure 3 displays acceptability curves at different prevalence levels with vaccine efficacy equal to 75% and cost of vaccine equal to $100 for the Initial Prevention Model. Each curve represents the proportion of patients per simulation for which vaccination was a more cost-effective strategy (optimal choice) over no vaccination at various willingness-to-pay (WTP) thresholds. For example, when C. difficile risk was 5%, vaccination was the optimal strategy for over 40% of patients regardless of the WTP threshold. When risk was 10%, vaccination was the optimal choice for over 60% of patients at all WTP thresholds. Figure 4 displays acceptability curves for the Recurrence Prevention Model at varying vaccine efficacy rates when the cost of vaccine was $800. When vaccine efficacy was 50%, vaccination was the optimal choice for 68% of patients at a WTP threshold of $0. When vaccine efficacy was 75%, vaccination was the optimal selection for over 90% of patients regardless of the WTP threshold. All results remained robust to (i.e., were not affected by) varying the probability of severe disease necessitating colectomy.
FIGURE 3.
Acceptability Curves at Different C. difficile Risk for Vaccine Efficacy of 75% and Cost of Vaccination of $100 for Initial Prevention
FIGURE 4.
Acceptability Curves at Different C. difficile Vaccine Efficacy for Cost of Vaccination of $800 for Recurrence Prevention
3.2 Opportunity Cost of Lost Bed-Day Approach
Each simulation run comprised of a cohort of 5,000 patients, each travelling 5,000 times through the model for a total of 2,500,000 simulated trials. Table 4 presents the results for the Initial Prevention Model at varying C. difficile risk, vaccine efficacies, and vaccine costs. Vaccination was cost-effective when vaccine cost was $25 and C. difficile risk was equal to or greater than 10%, except when efficacy was 25% and C. difficile risk was less than or equal to 15%.
TABLE 4.
Incremental Cost-Effectiveness Ratio ($US/DALY prevented) of Vaccination for Initial Prevention of Clostridium difficile infection (CDI) using the Opportunity Cost of Lost Bed-Days Approach.
| Vaccine Efficacy |
Clostridium difficile Risk | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.1% | 1.0% | 2.5% | 5.0% | 10.0% | 15.0% | 20.0% | 25.0% | |
| Cost of Vaccination = $25 | ||||||||
| 25% | 102,512,396 | 5,802,049 | 3,150,365 | 670,243 | 219,928 | 159,819 | 51,886 | 9,750 |
| 50% | 7,835,447 | 1,774,240 | 1,120,416 | 235,026 | 46,099 | Dominant | Dominant | Dominant |
| 75% | 9,242,325 | 1,135,269 | 437,496 | 125,266 | Dominant | Dominant | Dominant | Dominant |
| 100% | 13,588,967 | 1,114,979 | 300,389 | 56,679 | Dominant | Dominant | Dominant | Dominant |
| Cost of Vaccination = $50 | ||||||||
| 25% | 355,495,778 | 9,495,689 | 4,775,304 | 1,901,144 | 697,507 | 419,099 | 312,448 | 189,174 |
| 50% | 62,561,269 | 3,928,690 | 2,619,269 | 674,142 | 260,932 | 130,529 | 54,358 | 5,540 |
| 75% | 38,053,933 | 3,606,097 | 1,274,169 | 448,297 | 134,118 | 29,308 | Dominant | Dominant |
| 100% | 31,053,420 | 2,377,616 | 755,625 | 271,070 | 46,872 | Dominant | Dominant | Dominant |
| Cost of Vaccination = $100 | ||||||||
| 25% | 397,800,954 | 14,296,136 | 13,956,184 | 2,151,773 | 1,241,526 | 1,089,628 | 878,411 | 949,495 |
| 50% | 121,217,228 | 7,336,363 | 2,797,316 | 1,645,005 | 885,973 | 436,334 | 277,371 | 196,372 |
| 75% | 99,166,559 | 6,559,099 | 2,093,538 | 994,250 | 421,389 | 227,919 | 143,188 | 68,418 |
| 100% | 34,978,919 | 6,098,157 | 1,590,522 | 695,307 | 285,349 | 147,266 | 54,144 | 10,528 |
NOTE: Dominant = Vaccination is the dominant strategy (i.e., less costly and more effective)
Table 5 lists the cost-effectiveness of vaccination for the Recurrence Prevention Model. Vaccination was cost-effective at all vaccine efficacy and cost combinations except when cost was equal to $800 and efficacy was equal to or less than 50% and when cost was equal to $400 and efficacy was equal to 25%. Vaccination became dominant when the cost of vaccine was $200 or less, except when efficacy dropped to 50% at vaccine cost $200 and 25% at vaccine cost $100. All results remained robust to varying the probability of colectomy.
TABLE 5.
Incremental Cost-Effectiveness Ratio ($US/DALY prevented) of Vaccination for Clostridium difficile infection (CDI) Recurrence Prevention using the Opportunity Cost of Lost Bed-Days Approach.
| Vaccine Efficacy |
Cost of Vaccination | ||||
|---|---|---|---|---|---|
| $800 | $400 | $200 | $100 | $50 | |
| 25% | 243,994 | 107,576 | 42,704 | 9,247 | Dominant |
| 50% | 111,054 | 42,259 | 9,653 | Dominant | Dominant |
| 75% | 66,756 | 21,121 | Dominant | Dominant | Dominant |
| 100% | 43,500 | 8,919 | Dominant | Dominant | Dominant |
NOTE: Dominant = Vaccination is the dominant strategy (i.e., less costly and more effective)
4. DISCUSSION
C. difficile vaccination appears to be cost-effective for a wide range of C. difficile risk, vaccine efficacies, and vaccine costs. In fact, vaccination quickly becomes economically dominant as C. difficile risk and vaccine efficacy increase, suggesting that vaccination in some settings could actually save society, third party payers, and hospitals money while preventing morbidity and mortality. Economically dominant interventions are not common in health care, as many measures require some cost to prevent morbidity and mortality. Therefore, finding an intervention to be cost saving in addition to beneficial to health strongly supports its implementation. So, while the risk of C. difficile may vary significantly from health care facility-to-health care facility and patient-to-patient, vaccination may be favorable in many circumstances. This is compelling evidence for policymakers and researchers to further invest in the development of C. difficile vaccine.
If and when a C. difficile vaccine reaches the market, choosing an appropriate target population will be important. Even effective vaccines such as the Lyme disease vaccine have struggled when target populations were not selected carefully [13]. Our results suggest that preventing CDI recurrence may be good initial indication for the vaccine. This initial indication could support even higher vaccine prices, which may provide further motivation for manufacturers to bring the vaccine to market. As expected, broader use for the initial prevention of CDI may not support as high prices, but increased volume may compensate for lower prices. Our results also outlined possible effects of using various C. difficile risk thresholds if vaccination is to be restricted to higher-risk individuals.
The potential value of the vaccine stems from the heavy burden of CDI. Even mild disease such as diarrhea, abdominal pain, and nausea can add to costs and lengthen hospital stay. More severe conditions such as fever, severe shock or sepsis, and toxic megacolon can result in expensive procedures and may even lead to death. The biology of C. difficile makes it a difficult pathogen to control; C. difficile spores are resistant to heat, ethanol-based hand sanitizers, and quaternary ammonium disinfectants and can survive for months without proper disinfection [1]. In fact, C. difficile may be a growing problem. From 1993 to 2003, both the number of C. difficile cases and deaths more than doubled in the United States (cases went from 261 to 546 cases and deaths went from 20.3 to 50.2 per 100,000 discharged patients) [19]. Moreover, recent years have seen the emergence of a hypervirulent C. difficile strain [11].
Certainly, developing a functional C. difficile vaccine faces some technological challenges [20-22]. A parenteral or intravenous vaccine candidate may stimulate the production of circulating antibodies in the bloodstream but not adequately protect the gastrointestinal mucosa, where the pathogen inflicts most of its damage. Inducing complete mucosal protection (e.g., stimulating gut-associated lymphoid tissue) may require the stimulation of all immune system arms, including mucosal secretory IgA, functional serum IgG antibodies, and systemic and local cell-mediated immune responses. Direct local delivery of an adequate dose to the gastrointestinal mucosa may be possible but not necessarily easy. Achieving adequate protection may require an initial priming dose and then subsequent booster doses. Nonetheless encouraging advances in mucosal immunization have occurred over the past decade. Flumist (a live attenuated influenza vaccine), the Sabin oral polio vaccine, Ty21a (for typhoid fever), CVD 103-HgR (for cholera), and RotaTeq (for rotavirus infection) are examples of licensed and effective mucosally administered vaccines.
Our intent was to be conservative and err on the side of underestimating the benefits of a C. difficile vaccine. Our model included only the more common CDI sequelae and, when choices were available, the less expensive procedures. It also excluded chronic disease exacerbations that CDI may induce (e.g., dehydration leading to diabetic ketoacidosis in a diabetic) and relatively rare C. difficile complications. In addition, our model did not incorporate how vaccination could prevent C. difficile transmission or reduce the selection pressure for antibiotic-resistant bacteria (such as vancomycin-resistant Staphylococcus aureus) by minimizing the use of antibiotics to treat CDI. Moreover, combining vaccine with other infection control measures (e.g., hand hygiene and cohorting) could have compounding effects in controlling C. difficile spread.
4.1 Limitations
All computer models are simplifications of real life and cannot completely represent every possible C. difficile and CDI-associated factor, event, and outcome. Computer models also cannot fully represent the full spectrum of socio-demographic and clinical heterogeneity among hospital patients and hospitals. Additionally, as stated earlier, our model focused on more common clinical outcomes for which data were available and did not incorporate all of the potential benefits of vaccination. Finally, the data inputs for our model derived from different studies of varying quality.
4.2 Conclusions
Once developed, a C. difficile vaccine could be cost-effective over a wide range of C. difficile risk, vaccine costs, and vaccine efficacies. The vaccine could be particularly valuable for patients currently treated for CDI to prevent recurrent disease. Our study results support further investment into developing a C. difficile vaccine and suggest that vaccine efficacy targets do not necessarily have to be exceptionally high for the vaccine to have value. Our results also identified possible price points for the vaccine to assist manufacturers, third party payers, and other potential purchasers.
ACKNOWLEDGEMENT
This study was supported by the National Institute of General Medical Sciences Models of Infectious Agent Study (MIDAS) grant 1U54GM088491-0109, the Pennsylvania Department of Health, and the Vaccine Modeling Initiative (VMI), funded by the Bill and Melinda Gates Foundation. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hull MW, Beck PL. Clostridium difficile-associated colitis. Can Fam Physician. 2004 Nov 50;:1536–40. 43-5. [PMC free article] [PubMed] [Google Scholar]
- 2.McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol. 2008 Jan;5(1):40–8. doi: 10.1038/ncpgasthep1029. [DOI] [PubMed] [Google Scholar]
- 3.Gardiner DF, Rosenberg T, Zaharatos J, Franco D, Ho DD. A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A. Vaccine. 2009 Jun 2;27(27):3598–604. doi: 10.1016/j.vaccine.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brun P, Scarpa M, Grillo A, Palu G, Mengoli C, Zecconi A, et al. Clostridium difficile TxAC314 and SLP-36kDa enhance the immune response toward a co-administered antigen. J Med Microbiol. 2008 Jun;57(Pt 6):725–31. doi: 10.1099/jmm.0.47736-0. [DOI] [PubMed] [Google Scholar]
- 5.Ni Eidhin DB, O'Brien JB, McCabe MS, Athie-Morales V, Kelleher DP. Active immunization of hamsters against Clostridium difficile infection using surface-layer protein. FEMS Immunol Med Microbiol. 2008 Mar;52(2):207–18. doi: 10.1111/j.1574-695X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 6.Pechine S, Janoir C, Boureau H, Gleizes A, Tsapis N, Hoys S, et al. Diminished intestinal colonization by Clostridium difficile and immune response in mice after mucosal immunization with surface proteins of Clostridium difficile. Vaccine. 2007 May 16;25(20):3946–54. doi: 10.1016/j.vaccine.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 7.Ghose C, Kalsy A, Sheikh A, Rollenhagen J, John M, Young J, et al. Transcutaneous immunization with Clostridium difficile toxoid A induces systemic and mucosal immune responses and toxin A-neutralizing antibodies in mice. Infect Immun. 2007 Jun;75(6):2826–32. doi: 10.1128/IAI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sougioultzis S, Kyne L, Drudy D, Keates S, Maroo S, Pothoulakis C, et al. Clostridium difficile toxoid vaccine in recurrent C. difficile-associated diarrhea. Gastroenterology. 2005 Mar;128(3):764–70. doi: 10.1053/j.gastro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Aboudola S, Kotloff KL, Kyne L, Warny M, Kelly EC, Sougioultzis S, et al. Clostridium difficile vaccine and serum immunoglobulin G antibody response to toxin A. Infect Immun. 2003 Mar;71(3):1608–10. doi: 10.1128/IAI.71.3.1608-1610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008 Oct 30;359(18):1932–40. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 11.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005 Sep 24-30;366(9491):1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 12.Kotloff KL, Wasserman SS, Losonsky GA, Thomas W, Jr., Nichols R, Edelman R, et al. Safety and immunogenicity of increasing doses of a Clostridium difficile toxoid vaccine administered to healthy adults. Infect Immun. 2001 Feb;69(2):988–95. doi: 10.1128/IAI.69.2.988-995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2009 Sep 25; doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. HCUP.net. Healthcare Cost and Utilization Project 2007 [cited 2009 June 15]; Available from: http://www.ahrq.gov/HCUPnet/
- 15.Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006 Sep;21(5):402–8. doi: 10.1093/heapol/czl018. [DOI] [PubMed] [Google Scholar]
- 16.Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003 Dec 19;1(1):8. doi: 10.1186/1478-7547-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graves N, Halton K, Lairson D. Economics and preventing hospital-acquired infection: broadening the perspective. Infect Control Hosp Epidemiol. 2007 Feb;28(2):178–84. doi: 10.1086/510787. [DOI] [PubMed] [Google Scholar]
- 18.Murray CJL, Lopez AD, Harvard School of Public Health. World Health Organization. World Bank . Published by the Harvard School of Public Health on behalf of the World Health Organization and the World Bank ; Distributed by Harvard University Press; Cambridge, MA: 1996. The global burden of disease : a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. [Google Scholar]
- 19.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007 Jul;142(7):624–31. doi: 10.1001/archsurg.142.7.624. discussion 31. [DOI] [PubMed] [Google Scholar]
- 20.Cross AS, Chen WH, Levine MM. A case for immunization against nosocomial infections. J Leukoc Biol. 2008 Mar;83(3):483–8. doi: 10.1189/jlb.0607379. [DOI] [PubMed] [Google Scholar]
- 21.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005 Apr;11(4 Suppl):S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 22.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006 Feb;6(2):148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 23.East Ohio Regional Hospital Patient Price Information List. 2006 [cited 2009 August 5]; Available from: http://www.eastohioregionalhospital.com/pricelist.asp.
- 24.Red Book 2009: Pharmacy's Fundamental Reference. 113 ed. Thomson Reuters; New York: 2009. [Google Scholar]
- 25.Schwengel DA, McGready J, Berenholtz SM, Kozlowski LJ, Nichols DG, Yaster M. Peripherally inserted central catheters: a randomized, controlled, prospective trial in pediatric surgical patients. Anesth Analg. 2004 Oct;99(4):1038–43. doi: 10.1213/01.ANE.0000132547.39180.88. table of contents. [DOI] [PubMed] [Google Scholar]
- 26.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000 Feb 10;342(6):390–7. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 27.Johnson S, Clabots CR, Linn FV, Olson MM, Peterson LR, Gerding DN. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990 Jul 14;336(8707):97–100. doi: 10.1016/0140-6736(90)91605-a. [DOI] [PubMed] [Google Scholar]
- 28.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989 Jan 26;320(4):204–10. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 29.Shim JK, Johnson S, Samore MH, Bliss DZ, Gerding DN. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. Lancet. 1998 Feb 28;351(9103):633–6. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 30.Dallal RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, Lee KK, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002 Mar;235(3):363–72. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubberke ER, Butler AM, Reske KA, Agniel D, Olsen MA, D'Angelo G, et al. Attributable outcomes of endemic Clostridium difficile-associated disease in nonsurgical patients. Emerg Infect Dis. 2008 Jul;14(7):1031–8. doi: 10.3201/eid1407.070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MA, Hyland M, Ofner-Agostini M, Gourdeau M, Ishak M. Morbidity, mortality, and healthcare burden of nosocomial Clostridium difficile-associated diarrhea in Canadian hospitals. Infect Control Hosp Epidemiol. 2002 Mar;23(3):137–40. doi: 10.1086/502023. [DOI] [PubMed] [Google Scholar]
- 33.Barbut F, Richard A, Hamadi K, Chomette V, Burghoffer B, Petit JC. Epidemiology of recurrences or reinfections of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000 Jun;38(6):2386–8. doi: 10.1093/gao/9781884446054.article.t031141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbut F, Gariazzo B, Bonne L, Lalande V, Burghoffer B, Luiuz R, et al. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect Control Hosp Epidemiol. 2007 Feb;28(2):131–9. doi: 10.1086/511794. [DOI] [PubMed] [Google Scholar]
- 35.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997 Mar;24(3):324–33. doi: 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 36.Pepin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004 Aug 31;171(5):466–72. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin MS, Bodenstein LE, Kent KC. Severe Clostridium difficile colitis. Dis Colon Rectum. 1995 Apr;38(4):350–4. doi: 10.1007/BF02054220. [DOI] [PubMed] [Google Scholar]
- 38.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008 Feb;143(2):150–4. doi: 10.1001/archsurg.2007.46. discussion 5. [DOI] [PubMed] [Google Scholar]
- 39.Hermsen JL, Dobrescu C, Kudsk KA. Clostridium difficile infection: a surgical disease in evolution. J Gastrointest Surg. 2008 Sep;12(9):1512–7. doi: 10.1007/s11605-008-0569-9. [DOI] [PubMed] [Google Scholar]
- 40.Jobe BA, Grasley A, Deveney KE, Deveney CW, Sheppard BC. Clostridium difficile colitis: an increasing hospital-acquired illness. Am J Surg. 1995 May;169(5):480–3. doi: 10.1016/S0002-9610(99)80199-8. [DOI] [PubMed] [Google Scholar]
- 41.Koss K, Clark MA, Sanders DS, Morton D, Keighley MR, Goh J. The outcome of surgery in fulminant Clostridium difficile colitis. Colorectal Dis. 2006 Feb;8(2):149–54. doi: 10.1111/j.1463-1318.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 42.Morris AM, Jobe BA, Stoney M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002 Oct;137(10):1096–100. doi: 10.1001/archsurg.137.10.1096. [DOI] [PubMed] [Google Scholar]
- 43.Belmares J, Gerding DN, Parada JP, Miskevics S, Weaver F, Johnson S. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007 Dec;55(6):495–501. doi: 10.1016/j.jinf.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005 Jun 1;40(11):1586–90. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 45.Nair S, Yadav D, Corpuz M, Pitchumoni CS. Clostridium difficile colitis: factors influencing treatment failure and relapse--a prospective evaluation. Am J Gastroenterol. 1998 Oct;93(10):1873–6. doi: 10.1111/j.1572-0241.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 46.Olson MM, Shanholtzer CJ, Lee JT, Jr., Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991. Infect Control Hosp Epidemiol. 1994 Jun;15(6):371–81. doi: 10.1086/646934. [DOI] [PubMed] [Google Scholar]
- 47.Pepin J, Alary ME, Valiquette L, Raiche E, Ruel J, Fulop K, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005 Jun 1;40(11):1591–7. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 48.Teasley DG, Gerding DN, Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983 Nov 5;2(8358):1043–6. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- 49.Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996 May;22(5):813–8. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 50.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007 Aug 1;45(3):302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 51.de Lalla F, Nicolin R, Rinaldi E, Scarpellini P, Rigoli R, Manfrin V, et al. Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother. 1992 Oct;36(10):2192–6. doi: 10.1128/aac.36.10.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudley MN, McLaughlin JC, Carrington G, Frick J, Nightingale CH, Quintiliani R. Oral bacitracin vs vancomycin therapy for Clostridium difficile-induced diarrhea. A randomized double-blind trial. Arch Intern Med. 1986 Jun;146(6):1101–4. [PubMed] [Google Scholar]
- 53.Young GP, Ward PB, Bayley N, Gordon D, Higgins G, Trapani JA, et al. Antibiotic-associated colitis due to Clostridium difficile: double-blind comparison of vancomycin with bacitracin. Gastroenterology. 1985 Nov;89(5):1038–45. doi: 10.1016/0016-5085(85)90206-9. [DOI] [PubMed] [Google Scholar]
- 54.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002 Jul;97(7):1769–75. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]