Abstract
Thiazide diuretics can impair glucose metabolism and increase new onset diabetes. Adding an angiotensin receptor blocker to diuretics may protect against these metabolic effects; however, the mechanism of this protection is unclear. To explore potential mechanisms, a 16-week multicenter trial was conducted to ascertain the relative glucose metabolism effects of combined hydrochlorothiazide and angiotensin receptor blocker (valsartan) therapy compared to hydrochlorothiazide and calcium channel blocker (amlodipine) treatment in 412 centrally obese hypertensive subjects (BMI = 35±7 kg/m2, seated BP = 159±8/94±8 mmHg, and mean age 56 years). Subjects were randomized to valsartan/hydrochlorothiazide, with force-titration to 320/25 mg or amlodipine plus hydrochlorothiazide titrated to 10 mg 25 mg, respectively. Changes from baseline to Week 16 in fasting and 2-hour postprandial glucose and insulin levels after an oral glucose load were measured. At Week 16, clinic blood pressure reductions were similar (P>0.05) in both groups. Fasting and 2-hour glucose levels increased (P<0.05) with the amlodipine combination but not with the valsartan combination. In concert with these glucose responses, postprandial insulin increases from baseline were substantially greater with valsartan than with amlodipine plus hydrochlorothiazide group (P=0.001). The glucose responses were inversely related to insulin responses at the study conclusion. The novel observation of this investigation was that the combination of valsartan and hydrochlorothiazide was associated with greater glucose-stimulated insulin secretory and lesser glycemic excursion responses than the amlodipine combination group. Thus, this data suggests that adding an angiotensin receptor blocker attenuates the negative effects of thiazides on pancreatic beta-cell glucose induced insulin secretion.
Keywords: Valsartan, hydrochlorothiazide, hypertension, insulin secretion, obesity, metabolic syndrome
Introduction
Although diuretics are effective antihypertensive agents with documented benefits in reducing cardiovascular morbidity and mortality [1], there is increasing concern about the adverse metabolic and inflammatory effects of thiazide diurectics [2–10]. Mechanisms related to impaired glucose metabolism with thiazide diuretic therapy have been primarily attributed to impaired insulin metabolic signaling which, in turn, is thought to be associated, in part, with reductions in serum potassium levels [3,4]. The negative metabolic effects of thiazide diuretics are, however, multifactorial and their actions to activate inflammation, the sympathetic and the renin-angiotensin system (RAS), may account for potential negative effects on pancreatic insulin secretory capacity, as well as skeletal muscle, liver and fat insulin metabolic signaling pathways [10].
Observational studies have reported an increase in the incidence of new-onset diabetes mellitus with thiazide diuretics compared to RAS blockers or calcium channel blockers [6–8]. This effect is especially pronounced in hypertensive patients with prediabetes/and or the metabolic syndrome [8–10]. A recent report [9] indicated that antihypertensive therapy with hydrochlorothiazide (HCTZ) alone or, in conjunction with a beta-blocker, was associated with a significant increase in impaired glucose metabolism in hypertensive patients with abdominal obesity. Thus, it may be appropriate to use thiazide diuretics with caution and at lower doses in patients with abdominal obesity and/or metabolic syndrome, even though this conservative approach may still not prevent the negative effects of diuretic therapy [8–10].
The addition of an ACE inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) to a thiazide diuretic has been suggested as a strategy to prevent or reduce new onset diabetes [7–9]. A potential mechanism by which inhibition of the RAS can improve glucose tolerance or insulin sensitivity is that of amelioration of the diuretic-induced hypokalemia; however, studies have demonstrated that diuretics can impair peripheral insulin sensitivity independent of lowering serum potassium levels [2,7,11–13]. Combination RAS blockade (ACE-I or ARB) with diuretic therapy has been reported to cause fewer adverse effects on glucose metabolism than diuretic therapy alone [13–15]; however, in some of these studies, the negative metabolic effects of diuretics were not completely ameliorated by RAS blockade [8,11,16–17].
The purpose of the current investigation was to a) more closely examine the glucose metabolism effects, and potential salutary mechanisms, of combined treatment with the ARB, valsartan, and HCTZ, in comparison to combined diuretic and amlodipine (metabolically neutral agent added for last eight weeks of study to minimize BP differences) therapy, and b) further dissect the mechanisms by which the addition of an ARB to diuretic therapy mitigates the diuretic-induced negative metabolic effects. This investigation focused on the impact of these two antihypertensive therapies on pancreatic insulin secretion as well as systemic glucose metabolism [9].
Methods
The Valsartan and Hydrochlorothiazide In HyperTensive Abdominally ObEse (VITAE) trial was a 16-week, double-blind, randomized, forced-titration study, in non-diabetic subjects with hypertension and abdominal obesity. This study was conducted according to the Declaration of Helsinki. The study protocol was approved by the institutional review board at each site and all patients provided informed written consent. Patients meeting the eligibility criteria required that they have a complete withdrawal of their antihypertensive medication (i.e. washout period). The washout period could not exceed 28 days.
Subjects
After screening 790 subjects in total, 412 male or female subjects with central obesity and hypertension were enrolled in the study. Inclusion criteria were as follows: outpatients ≥40 years of age, central obesity defined in men as waist circumference >40 inches (>35 inches in Asian Americans), and in women as waist circumference >35 inches (>31 inches in Asian Americans), and hypertension defined as a mean of three sitting BP measurements: mean sitting systolic BP ≥ 150 mm Hg but <180 mm Hg and mean sitting diastolic BP <110 mm Hg following a maximum four week washout period.
Patients were excluded if they were taking >3 antihypertensive medications at the time of screening, were unable to discontinue all prior antihypertensive medications safely during the washout period, experienced weight loss >10 pounds (4.5 kg) during the screening/washout period, or had a documented history of Type 1 or 2 diabetes or fasting plasma glucose ≥7.0 mmol/L or a serum potassium level <3.5 mEq/L or >5.5 mEq/L at screening.
Study Design
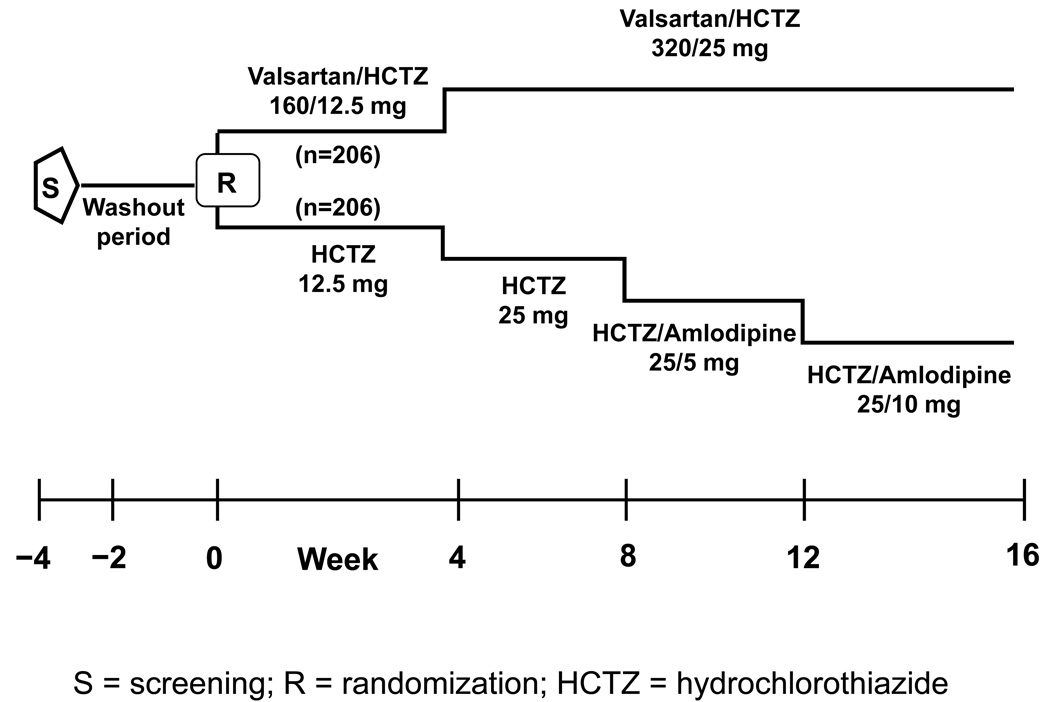
A schematic overview of the study design is provided in Figure 1. After screening, antihypertensive medication was stopped and subjects entered a washout period lasting for up to four weeks. Eligible patients were then randomized to either once-daily valsartan/HCTZ 160/12.5 mg or HCTZ 12.5 mg alone. At Week 4, doses were force-titrated to valsartan/HCTZ 320/25 mg and HCTZ 25 mg in their respective groups. At Weeks 8 and 12, patients in the valsartan/HCTZ group remained at the same dose (320/25 mg), while all patients in the HCTZ group received add-on amlodipine 5 mg and 10 mg at Weeks 8 and 12 respectively, in order to minimize differences in BP between the two groups by the end of the study. Downward titration of study drug doses was not permitted. All medications were taken once daily at the same time each morning.
Figure 1. Study design.
Randomization occurred at baseline (Week 0), followed by forced titration of doses at Week 4, 8, and 12. HCTZ=hydrochlorothiazide; R=randomization; S=screening.
STUDY POPULATION CLINICAL CHARACTERISICS
BP was measured to the nearest millimeter of mercury using a sphygmomanometer and an arm cuff with dimensions adjusted according to arm circumference. The measurements were done at trough, i.e. 24 hours after the last administration of the study drug and with subjects sitting for ≥5 min. BP was recorded three times, ≥2 min apart, and the average was used for analyses. The arm with the highest BP at enrollment was used for all subsequent measures. Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. Waist circumference was measured by placing the measuring tape snug (but not compressing the skin) around the abdomen at the level of the umbilicus just above the upper most lateral border of the right iliac crest and at normal minimal respiration with the patient in the standing position and his/her hands by their side.
Oral Glucose Tolerance Test (OGTT)
An oral glucose tolerance test (OGTT) was performed at baseline and Week 16 after an overnight fast to assess fasting and postprandial glucose and insulin. For the OGTT, plasma samples were measured at 0, 30, 60, 90, and 120 min after a 75 g glucose load was given in a fluid volume of 250–300 mL and was orally consumed within a 5 min period. The homeostasis model assessment of insulin resistance (HOMA-IR) [18] a measure of peripheral insulin sensitivity, and the insulinogenic index [19], a measure of early insulin response (30 min), were determined at baseline and at the end of the study. The percentage of patients with the metabolic syndrome was identified at baseline. High-sensitivity C-reactive protein [hs-CRP] was assessed at baseline and Week 16. Mean sitting systolic and diastolic BP were measured at baseline and every four weeks throughout the study.
Biochemical and Hormonal Assay
Serum insulin was measured by radio-immunoassay (RIA) (Immulite®, Diagnostic Products Corporation, Los Angeles, CA) and plasma glucose was determined by the hexokinase method (Roche Diagnostics, Indianapolis, IN). Serum hsCRP was measured by immunoturbidimetry (Roche Diagnostics, Indianapolis, IN).
Safety and Tolerability Assessments
Physical examinations were performed at screening, baseline, Week 4, Week 8, Week 12, and study end (Week 16 or discontinuation). All observed or volunteered adverse events (AEs), including serious AEs, were recorded throughout the study period.
Statistical Analysis
The primary intent of this study was to examine the fasting and postprandial glucose and insulin responses between the two treatments after 16 weeks of therapy. Efficacy variables were evaluated using last observation carried forward (LOCF) approach with the intent to treat (ITT) population. Assessments of metabolic measures were made at baseline and at the end of the study only. Descriptive and inferential analyses were performed for baseline demographics; change in BP, and for change in metabolic measures. Baseline demographic characteristics were summarized. Two-sample t-test for continuous variables and chi-squared test for categorical variables were used to test for homogeneity between the two treatments at baseline. All statistical tests were conducted under a two-sided alternative hypothesis, employing a significance level of 0.05.
To compare the change in a dependent variable (e.g. BP) between the two treatment groups, an analysis of covariance (ANCOVA) was used with baseline assessment as a covariate and treatment as factor in the model. Postprandial glucose and insulin after the OGTT challenge were also analyzed using area under the curve (AUC) analyses for the 0–120 min time period. Pearson product correlation analyses were also performed between changes in serum potassium, changes in fasting glucose and postprandial insulin, and between postprandial insulin and glucose and insulinogenic levels in the two treatment cohorts.
Results
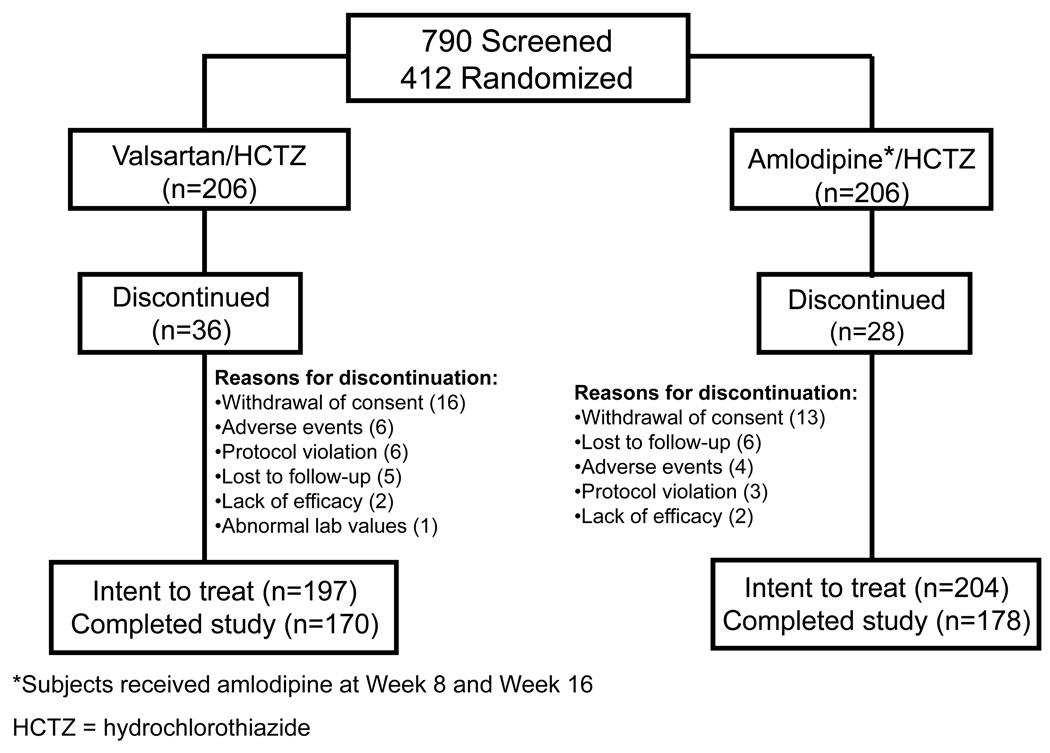
Among the 412 subjects randomly assigned to valsartan/HCTZ (n=206) or amlodipine/HCTZ therapy (n=206), 401 patients (valsartan/HCTZ=197, amlodipine/HCTZ=204) received at least one dose of study medication and had at least one valid post-baseline efficacy assessment, and were thus included in the ITT population. Of the randomized patients, 348 (84.5%) [170 (82.5%) in the valsartan/HCTZ group and 178 (86.4%) in the amlodipine/HCTZ group] completed the study. Reasons for discontinuation were withdrawal of consent (n = 29) or lost-to-follow up (n = 11). Discontinuation due to adverse events occurred in 10 patients; six in the valsartan/HCTZ arm and four in the amlodipine/HCTZ therapy arm.
The demographic and baseline characteristics of subjects were comparable between the two treatment groups (Table 1). The mean age was 56.0 years and 85.4% of the patients were <65 years old. The proportion of women was higher (P<0.05) in the amlodipine/HCTZ group (72%) than in the valsartan/HCTZ group (60%). Overall, 71% of subjects had the metabolic syndrome and 47% were prediabetic (fasting plasma glucose ≥5.5 and <7.0 mmol/L). There were a total of 18 (4.5%) subjects in the two groups who were pre-diabetic prior to randomization but had fasting plasma glucose values in the diabetic range at the randomization visit. Approximately 70% of patients were on prior antihypertensive medication (55% ACE inhibitor or angiotensin II receptor blocker, 21% diuretic, 9% calcium channel blocker, 9% beta-blocker) with both treatments having similar distribution. At the end of the study patients in both groups reduced their body weight by −0.4±3 kg in valsartan/HCTZ and −0.8±3 kg in amlodipine/HCTZ.
Table 1.
Demographic and Baseline Characteristics of Trial Subjects
| Valsartan/HCTZ (n=206) |
Amlodipine/HCTZ* (n=206) |
|
|---|---|---|
| Age, y | ||
| Mean ± SD, y | 56.5±8.6 | 55.4±8.5 |
| Range | 40–85 | 40–82 |
| <65 y, n (%) | 172(83.5) | 180(87.4) |
| ≥65 y, n (%) | 34(16.5) | 26 (12.6) |
| Sex, n (%) | ||
| Men | 82 (39.8) | 58 (28.2) |
| Women | 124 (60.2) | 148(71.8) |
| Race, n (%) | ||
| White | 103 (50.0) | 109 (52.9) |
| Black | 67 (32.5) | 59 (28.6) |
| Hispanic | 28(13.6) | 34(16.5) |
| Other (including Asian) | 8(3.9) | 4(1.9) |
| Prior antihypertensive**, n(%) | 144 (69.9) | 146 (70.9) |
| Diabetes status, n (%)– based on fasting plasma glucose | ||
| Normoglycemia¶ | 94 (47.7) | 93 (45.6) |
| Pre diabetes§ | 93 (47.2) | 99 (48.5) |
| Diabetes‡ | 8(4.1) | 10(4.9) |
| Metabolic syndrome† n (%) | 144 (69.9) | 150(72.8) |
| Estimated GFR, mean ± SD, mL/min/1.73 m2 | 72.3±13.8 | 73.4±13.1 |
| BMI, mean ± SD, kg/m2 | 34.8±6.9 | 35.2±7.3 |
| Waist circumference, mean ± SD, cm | 109.6±13.3 | 109.0±15.7 |
| MSSBP, mean ± SD, mmHg | 159.7±7.9 | 158.9±7.6 |
| MSDBP, mean ± SD, mmHg | 94.9±7.9 | 93.6±8.1 |
| Standing SBP, mean ± SD, mmHg | 158.6±12.2 | 158.4±11.2 |
| Standing DBP, mean ± SD, mmHg | 97.2±9.3 | 96.0±9.4 |
| HDL-C, mean ± SD, mmol/L | 1.3±0.4 | 1.3±0.3 |
| LDL-C, mean ± SD, mmol/L | 2.7±0.7 | 2.9±0.8 |
| Triglycerides, mean ± SD, mmol/L | 1.7±1.0 | 1.6±0.8 |
GFR=glomerular filtration rate; BMI=body mass index; MSSBP=mean sitting systolic blood pressure MSDBP=mean sitting diastolic blood pressure; HDL-C=high density lipoprotein cholesterol; LDL-C=low density lipoprotein cholesterol
Subjects received amlodipine at Week 8 and Week 12
Use of antihypertensive medication during the 30 days prior to screening.
Fasting plasma glucose <5.5 mmol/L at baseline.
Fasting plasma glucose ≥5.5 mmol/Land <7 mmol/L at baseline
Fasting plasma glucose ≥7 mmol/L at baseline. At the time of randomization, these patients were prediabetic and enrolled in the study. Immediately after randomization, baseline measures were determined when these subjects had a fasting plasma glucose ≥7 mmol/L
Subjects were hypertensive and obese, hence only one or more of the following criteria were required to be diagnosed with the metabolic syndrome: fasting plasma glucose ≥5.5 mmol/L, high density lipoprotein cholesterol <1 mmol/L in men or < 1.3 mmol/L in women, and fasting triglycerides ≥1.7 mmol/L.
Efficacy
Glucose
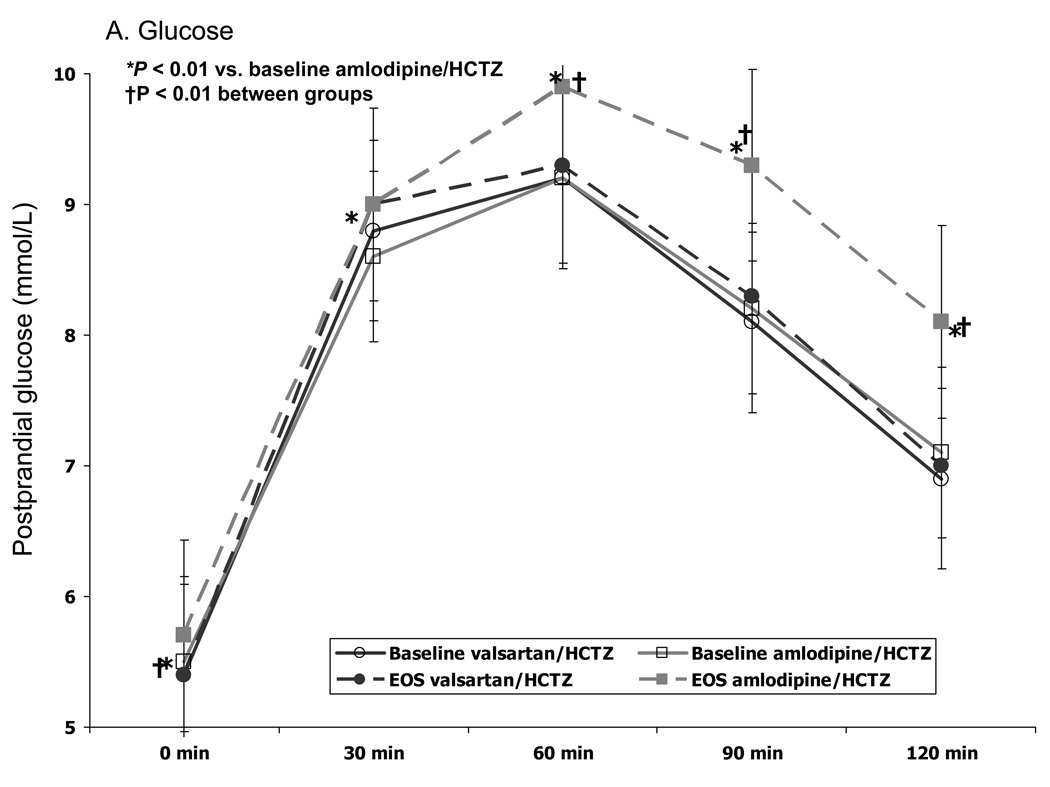
From baseline to the end of the study, fasting plasma glucose levels increased significantly with amlodipine/HCTZ (P=0.01) but not with valsartan/HCTZ therapy (P=0.66) (Table 2). A similar trend, however, was not observed for hemoglobin A1c as both groups reported a small increase (0.12–0.15%) from baseline (Table 2). Treatment with amlodipine/HCTZ was associated with a greater increase (P=0.0001) in postprandial glucose from baseline [as assessed by AUC from 0 to 120 min] than valsartan/HCTZ (79 mmol/L/min vs 6.2 mmol/L/min, P=0.0001) after 16 weeks of therapy. This increase in postprandial glucose after OGTT was greater (P<0.01) compared with the baseline response at all time points with amlodipine/HCTZ (Fig 2A). In contrast, valsartan/HCTZ therapy did not alter the glucose response to the OGTT after 16 weeks. The difference between the two groups was significant (P<0.01) at 0, 60, 90, and 120 min after OGTT.
Table 2.
Metabolic Measures and Plasma Inflammatory Markers, at Baseline and End of Study and Change From Baseline
| Valsartan/HC TZ (n=197) |
Amlodipine/HCTZ (n=204) |
P value between groups |
|
|---|---|---|---|
| Fasting plasma glucose | |||
| Baseline, mmol/L | 5.4±0.9 | 5.5±1.1 | |
| EOS, mmol/L | 5.4±0.9 | 5.7±1.0 | |
| Change, mmol/L | 0.0±0.8 | 0.2±1.0* | 0.005 |
| Fasting plasma insulin | |||
| Baseline, µU/mL | 19.7±20.4 | 20.4±20.6 | |
| EOS, µU/mL | 23.4±26.0 | 23.6±19.3 | |
| Change, µU/mL | 3.4±28.4 | 3.7±19.2* | 0.88 |
| Hemoglobin A1c | |||
| Baseline, % | 5.85±0.42 | 5.86±0.39 | |
| EOS, % | 5.96±0.42 | 6.01±0.44 | |
| Change, % | 0.12±0.22* | 0.15±0.25* | 0.12 |
| HOMA-IR | |||
| Baseline | 5.1±7 | 5.3±7 | |
| EOS | 6.0±7 | 6.4±6 | |
| Change | 0.7±8 | 1.2±6* | 0.49 |
| Insulinogenic index | |||
| Baseline, (µU/mL)/(mmol/L) | 27.5±22 | 28.2±32 | |
| EOS, (µU/mL)/(mmol/L) | 40.8±45 | 26.8±31 | |
| Change, (µU/mL)/(mmmol/L) | 14.6±45* | 1.5±32 | 0.002 |
| hsCRP | |||
| Baseline, median, mg/L | 2.48 | 2.52 | |
| EOS, mg/L | 2.9 | 3.0 | |
| Change, mg/L | 0.30* | 0.38* | 0.38 |
| Serum potassium | |||
| Baseline, mmol/L | 4.24±0.4 | 4.30±0.4 | |
| EOS, mmol/L | 3.99±0.4 | 3.83±0.5 | |
| Change, mmol/L | −0.25±0.4* | −0.47±0.5* | 0.001 |
| ALT | = | ||
| Baseline, U/L | 16.4±7.7 | 15.8±8.4 | |
| EOS, U/L | 17.1±10.5 | 17.9±12.5 | |
| Change, U/L | 0.6±7.4 | 2.0±9.8* | 0.14 |
| AST | |||
| Baseline, U/L | 17.2±4.7 | 16.9±4.8 | |
| EOS, U/L | 17.4±6.4 | 18.5±9.9 | |
| Change, U/L | 0.1±5.1 | 1.6±9.4* | 0.06 |
| GGT | |||
| Baseline, U/L | 20.1±11.2 | 18.4±9.9 | |
| EOS, U/L | 20.8±11.8 | 21.0±15.4 | |
| Change, U/L | 0.8±7.6 | 2.6±10.6* | 0.07 |
EOS = end of study
HCTZ=hydrochlorothiazide; HOMA-IR=homeostasis model assessment of insulin resistance (fasting glucose[mmol/L] X fasting insulin[µU/mL]/22.5); Insulinogenic indext=(30-min insulin level-fasting insulin)/(30-min plasma glucose – fasting plasma glucose); hsCRP=high sensitivity C-reactive protein; ALT (SGPT)=alanine aminotransferase; AST (SGOT)= aspartate aminotransferase, GGT = gamma-glutamyl transpeptidase
All values represent mean or mean change from baseline, except hsCRP, which is reported as median and median change from baseline.
Significant change from baseline (P<0.05).
Figure 2. Patient Disposition.
Postprandial glucose (A) and insulin (B) after OGTT challenge at baseline and end of study. EOS=end of study
Insulin
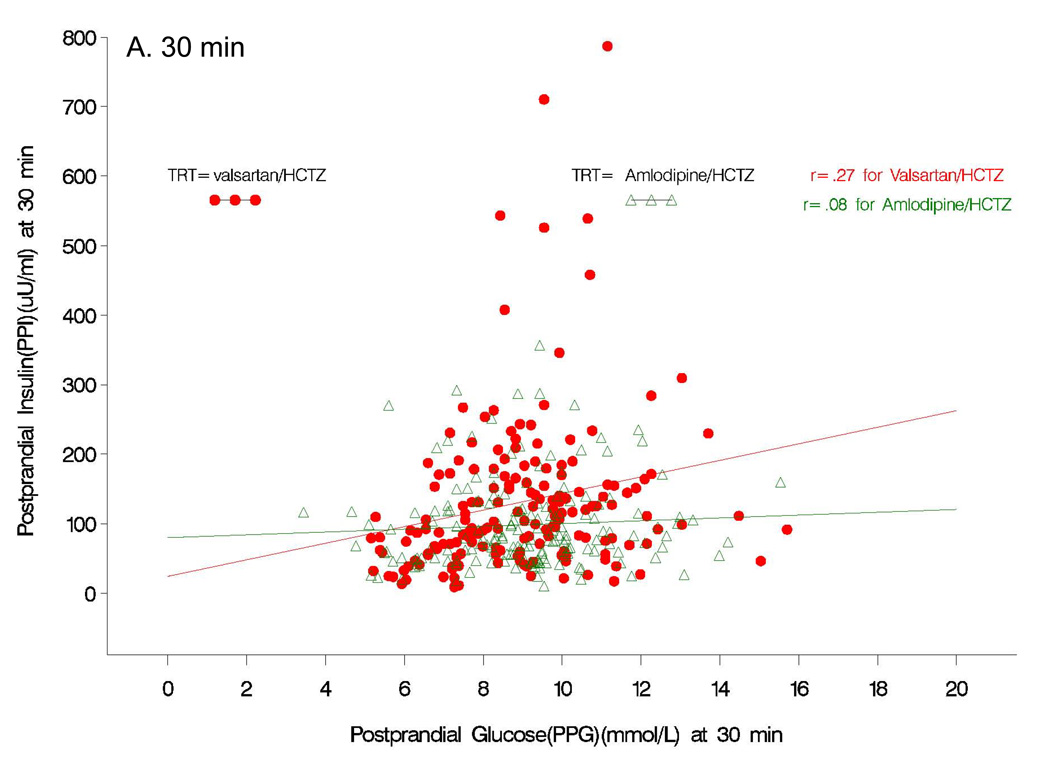
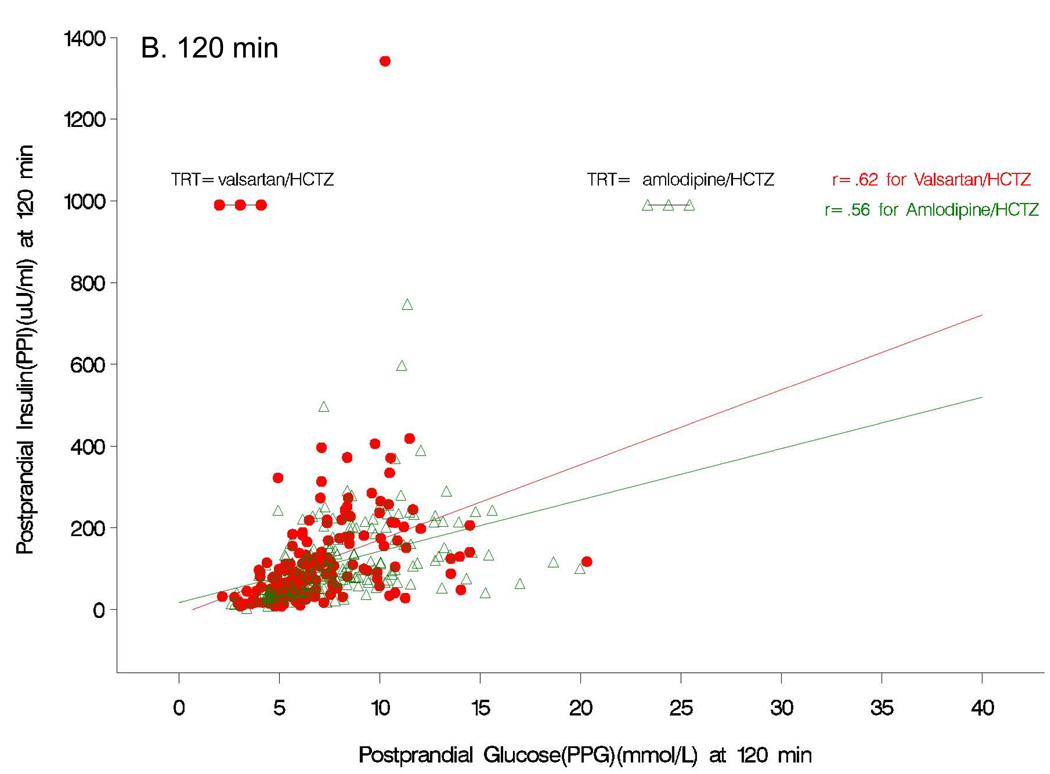
Although fasting insulin increased from baseline values with both amlodipine/HCTZ (+3.7 uU/ml; P=0.01) and valsartan/HCTZ (+3.4 uU/ml; P=0.12) therapy at the end of study (Table 2), the fasting levels were not different between the two treatment groups (P=0.88). In contrast to the results observed for postprandial glucose, subjects receiving valsartan/HCTZ had a significantly greater increase in insulin response to a glucose load (P<0.05) than those receiving amlodipine/HCTZ at 30, 60, and 90 min (Fig 2B). The AUC for postprandial insulin change from baseline was greater (P=0.001) in the valsartan/HCTZ group compared to the amlodipine/HCTZ group (4006 µU/mL/min vs. 1608 µU/mL/min, P=0.0012). There was also a greater increase in the insulinogenic index (i.e. early insulin response) from baseline in subjects receiving valsartan/HCTZ than for the subjects on amlodipine/HCTZ (Table 2). At the end of the study, correlational analyses between postprandial insulin and postprandial glucose at the 30 min time point showed no relation to the amlodipine/HCTZ group (r=0.08, P=0.3); whereas, in the valsartan/HCTZ group there was a positive relationship (r=0.27, P<0.0005) (Fig 3A). Correlational analyses between postprandial insulin and postprandial glucose at 120 min at the end of the study, was positive for both amlodipine/HCTZ (r=0.56, P<0.0001) and valsartan/HCTZ (r=0.62, P<0.0001) (Fig 3B).
Figure 3.
Pearson product correlations between postprandial insulin and postprandial glucose at 30 (A) and 120 (B) min at the end of the study.
Other laboratory measures
HOMA-IR increased significantly from baseline with amlodipine/HCTZ, but the mean change was not significantly different between groups (Table 2). Plasma hsCRP levels did not change significantly (P>0.05) in either group from baseline levels (Table 2). Liver enzyme tests for alanine transferase (ALT), aspartate aminotransferase (AST) and gamma-glutamyl transpeptidase (GGT) showed increases (P<0.05) from baseline in amlodipine/HCTZ but not in valsartan/HCTZ (Table 2).
Serum potassium declined significantly from baseline in both groups (P<0.05) but smaller reductions were observed with valsartan/HCTZ (3.99±0.4 mmol/L; −0.25 mmol/L) than with amlodipine/HCTZ (3.83±0.5 mmol/L; −0.47 mmol/L, P<0.001) (Table 2). Hypokalemia, defined as a serum potassium decrease >20% from baseline, occurred in 20 % of those in the amlodipine/HCTZ group compared with 8.1% in the valsartan/HCTZ group. Correlations between change in serum potassium and fasting and postprandial glucose (r=−0.06, r=−0.08, respectively), between potassium and fasting and postprandial insulin (r=0.015, r=0.015, respectively) and between potassium and the insulinogenic index (r=0.01) were non-significant. It was observed that the insulin responses were not normally distributed so correlations were retested between the change in serum potassium and the log transformed fasting and postprandial insulin, however, correlations were still non-significant (p>0.05).
BP efficacy
At the end of the study (Week 16), treatment with both valsartan/HCTZ and amlodipine/HCTZ was associated with significant decreases in mean sitting SBP (−30.6±15.5 mmHg vs. −28.3±12.6 mmHg, respectively) and mean sitting DBP (−14.0±9.9 mmHg vs. −12.7±8.3 mmHg, respectively) from baseline. The magnitude of the reductions was not significantly different between the two treatment groups for both SBP (P=0.15) and DBP (P=0.4).
Safety
Both treatment regimens were safe and well tolerated. The overall incidence of AEs (including type, causality, and severity) was comparable between the two treatments. Overall, 187 (45.4%) patients experienced at least one AE (39.3% with valsartan/HCTZ and 51.5% with amlodipine/HCTZ) that was mild to moderate in severity. The most common AEs reported by > 5% of patients in either group were peripheral edema (1.5% valsartan/HCTZ vs 9.7% amlodipine/HCTZ), upper respiratory tract infection (2.9% valsartan/HCTZ vs 6.8% amlodipine/HCTZ), and fatigue (5.3% valsartan/HCTZ vs 2.9% amlodipine/HCTZ). Hypokalemia was reported as an AE only in the amlodipine/HCTZ group (1.9%).
Discussion
The addition of the ARB, valsartan to HCTZ in obese, hypertensive, non-diabetic patients, minimized the reduction in both serum potassium and the dysglycemic response to an oral glucose load, when compared to the addition of amlodipine to HCTZ. Indeed, these differences in the metabolic response occurred despite similar reductions in BP between the two different combination treatment strategies. Substantially greater increases from baseline in the post-prandial glycemic excursion, as well as fasting glucose, were observed after treatment with amlodipine/HCTZ rather than with valsartan/HCTZ. The novel mechanistic observation was the substantially reduced glucose response to an oral load in the valsartan/HCTZ group achieved through a preserved glucose-stimulated insulin response, sufficient to overcome the negative beta cell and insulin resistance effects engendered by HCTZ. This notion is supported by the correlational analyses indicating that the ARB modulation of the HCTZ related increases in postprandial glucose excursions was due to improved insulin secretion in response to a glucose load.
Previous studies with combined RAS/HCTZ therapy have reported a mitigation of the fasting hyperglycemia response to diuretic treatment, but the mechanism of this benefit is unclear [15,16]. There is good evidence to suggest that RAAS blockade can prevent the deterioration of glucose tolerance by reducing pancreatic oxidative stress and inflammation and improve pancreatic islet blood flow and islet morphology, thus enhancing beta-cell function [13,20,21]. Recent studies have also demonstrated a role for RAAS blockade in improving the early phase beta-cell insulin response to a glucose challenge [22,23]. In the current study, correlational analyses supports greater insulin responsiveness to ambient glucose levels in the valsartan/HCTZ group compared to amlodipine/HCTZ (Fig 3A). The preserved early insulin-glucose response in these patients with obesity and insulin resistance suggests that RAS blockade corrects the beta cell impairment induced by diuretic therapy. The mechanisms involved in this beta-cell protection may be related to reductions in Ang II and aldosterone effects, and reduced inflammation and oxidative stress engendered by HCTZ therapy [10,13].
The adverse metabolic effects of HCTZ, particularly the inhibition of beta-cell stimulated insulin release, have generally been associated with reductions in serum potassium [3,4,24]. In this study, correlation analysis between changes in serum potassium and fasting insulin (r=0.015) and postprandial insulin (r=0.015) revealed no significant correlation (P>0.05). Even though serum potassium may not be a sensitive marker of total body potassium, the findings suggest an influence of factors other than hypokalemia alone in the differing metabolic response observed between the two groups. Several prior studies have reported persistent negative metabolic effects of combined ACE-inhibitor or ARB and HCTZ therapy on peripheral insulin sensitivity despite no changes in serum potassium levels [11,16,17]. In the Study of Trandolapril/Verapamil SR And Insulin Resistance (STAR), the combination of losartan/HCTZ resulted in an elevated glycemic response to an oral glucose load in patients who had a serum potassium level (K+ ~4.0 mmol/L) close to normal [9]. These data are in concert with a prior report that the RAAS blocker may not completely overcome the negative metabolic effects of the diuretic caused by factors independent of the serum potassium level [9].
A recent report suggested that peripheral insulin resistance associated with HCTZ therapy may be linked to liver fat accumulation, with associated increases in liver enzymes, and a heightened plasma inflammatory response (e.g. increases in hsCRP) [2,5]. Interestingly, in our study there were significant increases in the liver enzymes, ALT, AST and GGT, from baseline for patients in the amlodipine/HCTZ group but not in valsartan/HCTZ group. Diuretic therapy has been shown to affect basal hepatic glucose production through inhibition of hepatic insulin sensitivity and this may be an important effect to link to the observed increase in fatty liver in obese patients with HCTZ therapy [12]. Despite evidence for a heightened inflammatory response to diuretic therapy and a reduced inflammatory response with added ARB, as reported in previous studies [2,15], we did not find a significant difference in the plasma hsCRP response between valsartan/HCTZ and amlodipine/HCTZ. Thus, the HCTZ induced inflammation does not appear to be influenced by the addition of an ARB [5].
There is accumulating experimental [10,23–25] and clinical [2,11,27–29] evidence suggesting that blockade of the RAS improves insulin sensitivity and glucose metabolism. Cardiovascular outcome studies, including the VALUE study with valsartan, have demonstrated that ARB therapy may delay the onset of new-incidence diabetes in hypertensive patients [27–29]. In the VALUE study, valsartan was more effective than the calcium-channel antagonist, amlodipine, in the prevention of new-onset of diabetes [29]. This benefit of valsartan was also observed in patients receiving concomitant diuretics/beta-blockers. Moreover, therapy with valsartan not only reduced the development of diabetes, but also reduced the number of patients progressing from normal fasting glucose to impaired fasting glucose compared to amlodipine [29]. Perhaps the mechanism for ARBs to improve metabolic function may involve effects RAS blockade related to pancreatic, hepatic and peripheral insulin action, all contributing to a delay in the progression of normoglycemia to impaired glucose metabolism and new onset diabetes [9]. In our study, the addition of the ARB valsartan to HCTZ was able to restore beta-cell insulin secretory responsiveness to an oral glucose load but not to totally overcome the negative effects of the diuretic on peripheral insulin sensitivity.
Perspectives
In the current study, conducted in non-diabetic, obese patients with hypertension, there was a reduced glycemic response to a glucose challenge in the group of patients treated with valsartan/HCTZ, as opposed to the group of patients treated with combination amlodipine/HCTZ. The novel observation in this investigation was that treatment with an ARB, but not a dyhydropyridine calcium antagonist, in addition to HCTZ reduced the glycemic load in response to a glucose challenge through a preserved glucose-stimulated insulin release. The study was designed to examine the metabolic effects of combining the RAS blocker, with HCTZ and to compare it to HCTZ therapy. In order to limit differences in BP between the two groups, the metabolically neutral drug amlodipine [27] was used in the comparative treatment regimen. It should also be noted that our study was of a short-duration (16 weeks), and dictates further long-term evaluation of the metabolic effects of HCTZ and ARB therapy on both insulin secretion and sensitivity.
We conclude that adding a RAS blocker is an important strategy to limit the negative metabolic effects of diuretic therapy, particularly in patients susceptible to the development of diabetes, such as the obese hypertensive [9–10]. Future research should focus on how diuretic therapy chronically affects pancreatic beta cell function, as well as insulin metabolic signaling in order to better understand the mechanisms by which ARBs improve insulin secretion in this setting.
Acknowledgments
The authors express their appreciation to Susan Ritter, M.S. of Novartis Pharmaceuticals Corporation for expert assistance in project management. (ClinicalTrials.gov identifier: NCT00439738). The authors thank Brenda Hunter for providing editorial support for styling the manuscript to the journal requirements.
All authors approved the final version of the manuscript submitted for publication.
Source of Funding
This study was funded by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial number: NCT00439738
Conflict(s) of Interest/Disclosure(s)
JRS has NIH and VA funding and has served as a consultant for Novartis Pharmaceuticals Corporation and Forest Pharmaceuticals. Research funding grants were provided to University of Missouri by Novartis Pharmaceuticals Corporation and Forest Pharmaceuticals.
LR has served as a consultant and speaker for Novartis Pharmaceuticals Corporation.
IJ has served as a consultant for Novartis Pharmaceuticals Corporation.
BE has served as a consultant and speaker for Novartis Pharmaceuticals Corporation, Pfizer Inc. and GlaxoSmithKline. Research support received from Novartis Pharmaceuticals Corporation and AstraZeneca Pharmaceuticals.
EO has served as a consultant and speaker for Novartis Pharmaceuticals Corporation. She has served as a consultant for Bristol-Myers Squibb, Nitromed and Sanofi-Aventis and speaker for Merck Pharmaceuticals.
PCD has served as a consultant and speaker for Novartis Pharmaceuticals Corporation, Forest, GlaxoSmithKline and Pfizer. Research support received from Novartis Pharmaceuticals Corporation and AstraZeneca Pharmaceuticals.
RS, DZ and DP are employees at Novartis Pharmaceuticals Corporation
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]; Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson JW, Jansson PA, Carlberg B, Hagg A, Kurland L, Svensson MK, et al. Hydrochlorothiazide, but not candesartan, aggravates insulin resistance and causes visceral and hepatic fat accumulation: the mechanisms for the diabetes preventing effect of candesartan (MEDICA) study. Hypertension. 2008;52(6):1030–1037. doi: 10.1161/HYPERTENSIONAHA.108.119404. [DOI] [PubMed] [Google Scholar]
- 3.Zillich AJ, Garg J, Basu S, Bakris GL, Carter BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. 2006;48(2):219–224. doi: 10.1161/01.HYP.0000231552.10054.aa. [DOI] [PubMed] [Google Scholar]
- 4.Dronavalli S, Bakris GL. Mechanistic insights into diuretic-induced insulin resistance. Hypertension. 2008;52(6):1009–1011. doi: 10.1161/HYPERTENSIONAHA.108.120923. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Danielson E, Rifai N, Glynn RJ. Valsartan, blood pressure reduction, and C-reactive protein: primary report of the Val-MARC trial. Hypertension. 2006;48(1):73–79. doi: 10.1161/01.HYP.0000226046.58883.32. [DOI] [PubMed] [Google Scholar]
- 6.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 7.Barzilay JI, Davis BR, Cutler JA, Pressel SL, Whelton PK, Basile J, et al. Fasting glucose levels and incident diabetes mellitus in older nondiabetic adults randomized to receive 3 different classes of antihypertensive treatment: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2006;166:2191–2201. doi: 10.1001/archinte.166.20.2191. [DOI] [PubMed] [Google Scholar]
- 8.Bakris G, Molitch M, Hewkin A, Kipnes M, Sarafidis P, Fakouhi K, et al. Differences in glucose tolerance between fixed-dose antihypertensive drug combinations in people with metabolic syndrome. Diabetes Care. 2006;29(12):2592–2597. doi: 10.2337/dc06-1373. [DOI] [PubMed] [Google Scholar]
- 9.Cooper-DeHoff RM, Wen S, Bietelshees AL, Zineh I, Gums JG, Turner ST, et al. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension. 2009;54 doi: 10.1161/HYPERTENSIONAHA.109.139592. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manrique C, Johnson M, Sowers JR. Thiazide diuretics alone or with beta-blockers impair glucose metabolism in hypertensive patients with abdominal obesity. Hypertension. 2010;55:15–17. doi: 10.1161/HYPERTENSIONAHA.109.142620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin DM, Atkinson AB, Ennis CN, Browne J, Hunter SJ, Sheridan B, Bell PM. Comparison of effects of combined ACE inhibitor and low-dose thiazide diuretic with ACE inhibitor alone on insulin action in patients with hypertension and Type 2 diabetes: a double-blind crossover study. Diabet Med. 2008;25(5):631–634. doi: 10.1111/j.1464-5491.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 12.Klauser R, Prager R, Gaube S, Gisinger C, Schnack C, Küenburg E, Schernthaner G. Metabolic effects of isradipine versus hydrochlorothiazide in diabetes mellitus. Hypertension. 1991;17(1):15–21. doi: 10.1161/01.hyp.17.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Sowers JR, Whaley-Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150(11):776–783. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middeke M, Richter WO, Schwandt P, Holzgreve H. The effects of antihypertensive combination therapy on lipid and glucose metabolism: hydrochlorothiazide plus sotalol vs. hydrochlorothiazide plus captopril. Int J Clin Pharmacol Ther. 1997;35(6):231–234. [PubMed] [Google Scholar]
- 15.Zappe DH, Sowers JR, Hsueh WA, Haffner SM, Deedwania PC, Fonseca VA, et al. Metabolic and antihypertensive effects of combined angiotensin receptor blocker and diuretic therapy in prediabetic hypertensive patients with the cardiometabolic syndrome. J Clin Hypertens (Greenwich) 2008;10(12):894–903. doi: 10.1111/j.1751-7176.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter SJ, Harper R, Ennis CN, Crothers E, Sheridan B, Johnston GD, et al. Effects of combination therapy with an angiotensin converting enzyme inhibitor and thiazide diuretic on insulin action in essential hypertension. J Hypertens. 1998;16(1):103–109. doi: 10.1097/00004872-199816010-00015. [DOI] [PubMed] [Google Scholar]
- 17.Hunter SJ, Wiggam MI, Ennis CN, Whitehead HM, Sheridan B, Atkinson AB, Bell PM. Comparison of effects of captopril used either alone or in combination with a thiazide diuretic on insulin action in hypertensive Type 2 diabetic patients: a double-blind crossover study. Diabet Med. 1999;16(6):482–487. doi: 10.1046/j.1464-5491.1999.00010.x. [DOI] [PubMed] [Google Scholar]
- 18.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997;20(7):1087–1092. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 20.Hayden MR, Sowers JR. Pancreatic renin-angiotensin-aldosterone system in the cardiometabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2008;3(3):129–131. doi: 10.1111/j.1559-4572.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- 21.Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin- angiotensin system in the ZDF rat. Diabetes. 2004;53(4):989–997. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Nakagawa O, Aizawa Y. Improved early-phase insulin response after candesartan treatment in hypertensive patients with impaired glucose tolerance. Clin Exp Hypertens. 2008;30(5):309–314. doi: 10.1080/10641960802269927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281(46):35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 24.Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, et al. Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab. 2005;288(2):E353–E359. doi: 10.1152/ajpendo.00402.2004. [DOI] [PubMed] [Google Scholar]
- 25.Habibi J, Whaley-Connell A, Hayden MR, DeMarco VG, Schneider R, Sowers SD, et al. Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic Ren2 rat. Endocrinology. 2008;149(11):5643–5653. doi: 10.1210/en.2008-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lastra G, Habibi J, Whaley-Connell AT, Manrique C, Hayden MR, Rehmer J, et al. Direct renin inhibition improves systemic insulin resistance and skeletal muscle glucose transport in a transgenic rodent model of tissue renin overexpression. Endocrinology. 2009;150:2561–2568. doi: 10.1210/en.2008-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 28.Abuissa H, O'Keefe J., Jr The role of renin-angiotensin-aldosterone system-based therapy in diabetes prevention and cardiovascular and renal protection. Diabetes Obes Metab. 2008;10(12):1157–1166. doi: 10.1111/j.1463-1326.2008.00898.x. [DOI] [PubMed] [Google Scholar]
- 29.Kjeldsen SE, Julius S, Mancia G, McInnes GT, Hua T, Weber MA, et al. Effects of valsartan compared to amlodipine on preventing type 2 diabetes in high-risk hypertensive patients: the VALUE trial. J Hypertens. 2006;24(7):1405–1412. doi: 10.1097/01.hjh.0000234122.55895.5b. [DOI] [PubMed] [Google Scholar]