Abstract
Declines in immune function have been associated with declines in the function of naïve CD4 T cells. In vitro studies of naïve CD4 T cells in TCR-specific transgenic AND mice have shown age-related defects in immunosynapse formation, activation, proliferation and cytokine production. Previous work has also documented age-related alteration in the glycosylation of surface proteins involved in TCR signaling, and shown that enzymatic treatments to remove specific surface glycoproteins can restore in vitro function in CD4 cells from aged mice. Here an adoptive transfer system shows that a large percentage of naïve CD4 T cells from old mice fail to express CD69 and expand in antigen primed mice, but these declines in CD69 and expansion can be restored by ex-vivo pretreatment of the T cells with the bacterial enzyme O-sialoglycoprotein endopeptidase (OSGE). OSGE treatment also repairs the age-dependent loss of CD69 expression after in vivo activation.
Keywords: T cells, Aging, Lymphocytes, TCR signaling, glycoproteins
Introduction
Age-related declines in immune function can be attributed to defects in CD4 T cell function (Miller et al. 2005), including defects in signal transduction in the first minutes of the activation process, diminished proliferation (Miller et al. 1997) and declines in production of cytokines (Ernst et al. 1990, Hobbs et al. 1991, Bining & Miller 1997). It has been suggested that the decline with age in the ratio of naïve to memory T cells (Ernst et al. 1990) may explain some of the age-related differences in protective immunity. However, studies using antigen-specific TCR transgenic mice, in which most of the CD4 cells remain in naïve state throughout life, have pointed to intrinsic age-related defects in the function of naïve CD4 T cells (Linton et al. 1996). Using one such system, the AND mouse, whose CD4+ Vβ3-TCR+ (Trg+) recognize amino acids 88 to 103 of the pigeon cytochrome C (PCC) protein, we have shown in vitro age-related defects in immunosynapse (Garcia & Miller 2001), exclusion of CD43 from the site of synapse formation (Garcia & Miller 2003), TCR signaling (Miller et al. 2005), and the expression of CD69 and CD25 markers (Garcia & Miller 2003). Other groups have also shown age-related defects in cytokine production including IL-2, INFγ and IL-4 in the AND system (Linton et al. 1996). In addition, using adoptive transfer of Trg+ cells into syngeneic CD4KO mice, Haynes et al. (Haynes et al. 2004) found that old donors cells did not proliferate well in the early phase of expansion and have a reduction in their in vivo cognate helper function, leading to declines in B cell expansion and IgG production (Eaton et al. 2004). These data have suggested intrinsic age-related defects in the activation and proliferation of naïve CD4 cells from old mice, but have provided only limited insights into which age-related changes in the surface of CD4 cells could be involved in the declines of immune synapse formation and T cell function, or how these might be reversed.
Trg+ cells from old AND donors exhibit in vitro defects in the very earliest phase of interaction with APC (Garcia & Miller 2001, 2002, 2003). We hypothesized that age-dependent changes in glycosylation of T cell proteins (Garcia et al. 2005) might contribute to derangements of TCR-MHC interactions and immunosynapse formation. These age-associated changes in glycosylation include increases in levels of (α2,3) sialic acid and declines in (α2,6) sialic acid residues of glycoproteins, but it is unclear to what extent specific polysaccharide changes contribute to declines in TCR signaling. To test the functional implications of altered protein glycosylation, we evaluated the effects of a bacterial enzyme, O-sialoglycoprotein endopeptidase (OSGE), which digests segments of extracellular proteins containing O-linked glycans bearing terminal sialic acid residues. We found that OSGE treatment could restore in vitro many of the biological functions of CD4 cells from old donors, including synapse formation, expression of CD25, cytokine production, and cytotoxic function (Garcia & Miller 2003, Berger et al. 2005, Berger et al. 2006, Sadighi Akha et al. 2006). Here we report experiments designed to test whether a similar approach could improve the function of T cells in vivo after adoptive transfer of CD4 cells to antigen-primed host.
Material and Methods
Animals and cell culture
H-2(k/k) TCR-Vα11Vβ3 CD4+ mice (AND) on the B10.BR background were bred in our facilities from stock generously provided by Susan Swain and Laura Haynes. Specific-pathogen free B10.BR mice were purchased from the Charles River Laboratories (Kingston, NJ). The mice were housed at the University of Michigan and were given free access to food and water. Sentinel animals were examined quarterly for serological evidence of viral infection; all such tests were negative during the course of these studies. Mice that were found to have splenomegaly or macroscopically visible tumors at the time of sacrifice were not used for experiments. AND mice used were at 6–8 (young) or 15–18 (old) months of age, and the B10.BR adoptive host mice were 2–4 months of age.
Antibodies, reagents, cell preparation
Pigeon Cytochrome C (PCC) was purchased from Sigma (www.sigmaldrich.com). CFSE labeling kits were purchased from Invitrogen (www.invitrogen.com). CD69 Pacific Blue was purchased from Biolegends (www.biolegends.com), and other antibodies for flow cytometric analysis were purchased from Becton Dickson (www.bdbiosciences.com), including CD4PerCP, PE- TCRVβ3 or biotin-TCRVβ3/streptavin-Alexa700. CD4 cells from spleen and lymph nodes were obtained by negative selection as previously described (Garcia & Miller 1998). Flow-cytometric analysis of a typical preparation showed it to be 90 % positive for both CD3 and CD4.
Priming, tail vain injection and preparation of lymph node and spleen cells from B10.BR adoptive host mice
24 hours prior to adoptive transfer the B10.BR host mice (2–4 months of age) were primed with 10 μg of PCC protein in PBS by subcutaneous injection in the flank of the left leg. CD4 cells from young and old AND mice were purified by negative selection (Garcia & Miller 2001) and labeled with CFSE for 10 min following the manufacturer’s suggested protocol. Each cell preparation was then divided into two aliquots; one was left untreated and the other treated with OSGE (20 μg/ml) for 1 h at 37°C as described (Garcia & Miller 2003). The cells were then resuspended at 40×106 cell/ml in PBS, and 7–8 × 106 cells in 200 μl were injected into the tail vain of a primed B10.BR recipient. Host mice were injected subcutaneously with an additional 10 μg of PCC, and given a third injection 24 hr after receipt of the donor cells. Lymph nodes (pooled cervical, axillary, brachial and inguinal nodes) and spleen of each individual mouse were harvested at the specific times indicated in the text. CD4 cells were then purified by negative selection (Garcia & Miller 1998), and stained for CD69, CD4 and Vβ3. Analysis was performed using a Canto-II flow Cytometer, gating for expression of CD4+ and Vβ3 as described in the text.
Results
In vivo, the number of antigen-specific CD4 cells that can enter cell division declines with age, but this defect can be reversed by treatment with OSGE
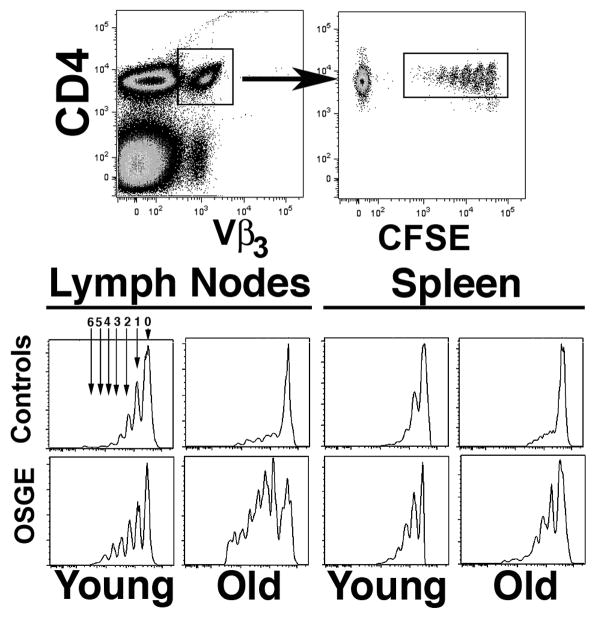
To test if the age-related declines in proliferation of AND Trg+ cells (Linton et al. 1996, Linton et al. 1997) can also be observed in vivo we used an adoptive transfer model. Purified CD4 cells from young or old AND mice were labeled with CFSE and transferred to PCC primed syngenetic young B10.BR host mice. After 72 hours, T cells from draining lymph nodes and from spleen were harvested and CD4 cells purified by negative selection, then stained for CD4 and TCR-Vβ3 and analyzed for distribution of CFSE levels. Most Trg+ cells from AND mice remain in a naïve stage until late in life, but there is an age-related decline in the number of cells of the TCR-Vβ 3 Vα11 population and in the level of TRC expression (Linton et al. 1996, Linton et al. 1997). We therefore performed all CFSE analyses on cells gated for expression of both Vβ3+ and CD4+. The dot-plot on the left of Figure 1 shows the gating for CD4+ and Vβ3high (or Trg+ cells) and the dot-plot at right shows distribution of CFSE among the gated CD4 cells. Histograms for CFSE expression are shown in the bottom panels of Figure 1, showing both young and old donors with and without OSGE pre-treatment. In each histogram, the peak labeled “0” shows cells that have not divided in vivo, and peaks 1–6 represent cells that have undergone the indicated number of cell divisions after adoptive transfer. In this particular experiment, about 60% of the Trg+ lymph node cells (LN) from recipients of untreated young donor cells had progressed to various stages of cell division, and only 40% of the cells remained in the non-proliferative stage (peak 0). In contrast, the proportion of non-proliferating (peak 0) cells from recipients of Trg+ cells from the old donor was much larger. The spleen data show a similar pattern, although the number of cells from young donors than have entered division is lower than that seen in LN. Cells transferred into unprimed B10.BR host mice showed no proliferation of the CFSE+ Trg+ (not shown), and identical results were found if a different protein was used to prime the B10.BR host mice (in this case OVA, data not shown), suggesting that stimulation of cell division requires encounter with PCC antigenic determinants.
Figure 1. Flow cytometric analysis of in vivo proliferation of young and old naïve Trg+ from AND mice.
Untreated (controls) or ex-vivo OSGE treated (OSGE) Trg+ cells were labeled with CFSE+ and transferred to primed B10.BR hosts. After 72 hours, the LN and spleen were collected, and the CD4 cells purified and stained for Vβ3 and CD4. Cell divisions were analyzed by the level of CFSE. The top panels show the gating procedure used to select cells for histogram analyses (lower panels). Arrows on the histogram indicate populations that represent different numbers of cell division cycles in vivo, from 0 to 6 cell divisions.
We have recently shown that treatments with OSGE reverse the age-related decline of T cell function in several in vitro assays using antibody stimulation (Garcia & Miller 2003, Berger et al. 2005). To test if OSGE treatment can also restore in vivo proliferation of Trg+ from old donors, we treated aliquots of CD4 cells from young and old AND mice with OSGE prior to adoptive transfer. The bottom panels in Figure 1 show CFSE distributions, from the same donors, using cells that had been treated with OSGE prior to transfer. OSGE increased the proportion of Trg+ cells from young donors entering cell division in both spleen and LN. The effect of OSGE on Trg+ cells from old donors was more dramatic, leading to levels of T cell proliferation similar to that seen in the young cells.
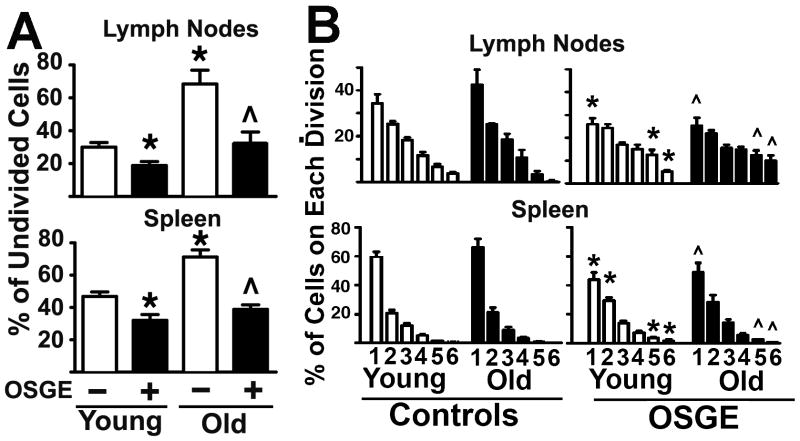
Figure 2-A shows results of a set of 6 such experiments, each of which involved at least 2 young mice with 1 old mouse. Each bar shows the number of cells that have not entered cell division (peak 0) after 72 hours of in vivo stimulation, as a percentage of the total number of CFSE+ cells recovered. In LN approximately 30 % of untreated Trg+ cells from young AND donors fail to undergo cell division, compared to 70 % of Trg+ cells from old donors (p=0.001) An age-related decline in the numbers of CFSE+ cells that do not divide is also seen in the spleen (p=0.003), although the proportion of cells that fail to divide is higher in spleen than in LN, perhaps reflecting lower stimulation capability of the spleen environment, or differences in the proportion of locally stimulated as opposed to migratory cells.
Figure 2. The number of cells that can enter cell cycle division declines with age, but does not affect the rate of cell division among cells that do enter the mitotic cycle, while ex-vivo OSGE treatments can reverse this defect.
A- The untreated and OSGE treated Trg+ cells corresponding to the peak 0 of cell division were quantified from matched sets of 3 young (open bars) and 1 old (filled bars) in each of 5 experiments. Each bar shows mean percentage ± SEM. The * indicates statistical significance with respect to young cells not exposed to OSGE, and the ^ represents significant differences with respect to old cells not exposed to OSGE. B-Untreated (−) and OSGE treated (+) Trg+ cells corresponding to peaks 1 to 6 of the CFSE cell division profile were counted from matched sets of at least 2 young (open bars) and 1 old (filled bars) in each of 7 experiments. The bars show mean percentage ± SEM for each peak in the CFSE cell division pattern. The * indicates significant differences with respect to untreated cells from young mice, and the ^ represents significant differences with respect to untreated of old mice (control) from the same division peak.
Figure 2-A also shows that OSGE treatment diminishes significantly the proportion of CD4 cells that remain in the undivided state. This OSGE effect is seen in recipients of both young and old cells, and seen in both spleen and LN, but is larger in magnitude for cells from aged donors. There is no difference between cells from young and old donors that have been OSGE treated prior to transfer. Overall, these results suggest that with age there is a significant decline in the number of naïve CD4 cells capable of entering cell division after transfer into antigen-primed hosts, and that this number can be restored to the level seen in the young by removing specific surface glycosylation proteins using OSGE.
In addition to evaluating the effects of age and OSGE on the proportion of Trg+ cells that can enter cell cycle in vivo, we also calculated, for each adoptive recipient, the distribution of cells that had undergone 1, 2, 3, or more in vivo divisions. To do this, we expressed the number of cells in each CFSE population as a proportion of the total cells that had undergone at least 1 division. Figure 2-B shows the result of this calculation from 6 experiments, each of which used at least 2 young donors and 1 old donor. In the lymph nodes, as well as spleen, comparisons between untreated young and old donors showed no age effects (by t-test) for any of the six discernable cell division peaks. This result suggests that those Trg+ cells from old mice that can enter cell division proceed to proliferate to the same extent as naïve CD4 cells from young donors.
To see if treatment with OSGE can increase the rate or extend of proliferation among cells that enter the cell cycle, we used t-tests to compare untreated and OSGE treated cells from young and old donors. For young mice, OSGE treatment led to a small, but statistically significant decline (p= 0.01) in the proportion of cells that are in division 1. This change was accompanied by significant increases in the proportion of cells that have the highest numbers of divisions (peaks 5 and 6). OSGE also had a similar significant effect on distribution of cells among peaks in recipients of old CD4 cells. Thus the data suggest that with age there is a significant decline in the number of cells that can enter cell division (Figures 1 and 2-A), but little effect on the proliferative potential of cells that do enter the cell division cycle (Figure 2-B). In addition, the removal of specific surface proteins bearing O-linked glycans from CD4 cells can increase the number of cells that proliferate, overcoming the effect of age, and also increase slightly the fraction of proliferating cells that go beyond the first cell division cycle for both young and old donors.
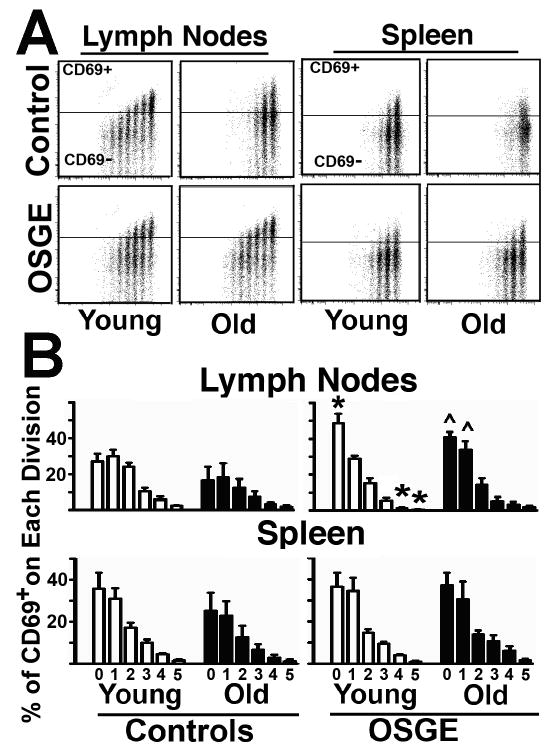
The data presented above suggest that aging diminishes the number of Trg+ cells that can enter cell division in vivo. But there is evidence, from tests of cognate helper function of CD4 cells from old mice (Eaton et al. 2004), that after cell division the later stages of activation could be also affected by age. To test this hypothesis, we performed a similar series of adoptive transfer experiments using CFSE to track cell division and CD69 to monitor later stages of activation. CD69 is expressed approximately 16 hours after antigen encounter (see Fig 4 for examples) and then declines in expression by the time cells enter division roughly 48 hours after antigen encounter, (Ziegler et al. 1994a, Ziegler et al. 1994b). Figure 3-A shows a typical set of dot-plots for CFSE+ and CD69 expression in young and old CD4 from lymph nodes and spleen, gated using the same methodology described in Figure 1. The horizontal line in each figure shows the level of intensity used to discriminate CD69-positive from CD69-negative cells, as defined by an isotype-matched control antibody used in place of the anti-CD69 antibody. As expected, most of the lymph node CD69+ cells derived from young control Trg+ cells are found in the peak of undivided cells, and cell division is associated with progressively lower levels of CD69 expression. In the spleen, young control cells show lower levels of CD69 expression (compared to LN cells), even in cells that fail to divide (peak 0); whether this represents a failure to activate CD69 expression, or a failure to divide by cells that have activated and then repressed CD69 levels, cannot be determined (early time points for a similar analysis are shown in Fig 4). Similarly, in vivo activation of untreated Trg+ cells from old donors shows relatively low levels of CD69 expression in the LN, even in cells that have not divided (peak 0), when compared to Trg+ from young donors. Consistent with the results presented above, treatment with OSGE increases the number of cells entering cell division, particularly in cells from old donors. In addition, OSGE appears to increase the proportion of CD69+ cells within some of the CFSE-defined populations. To evaluate this point quantitatively, we calculated the ratio of CD69+ cells in each peak to the total number of cells in that peak. Figure 3-B shows mean ± SEM for CD69+ cells in a set of 5 experiments, each using at least 2 young and one old donor. In untreated control cells (left panels), we found that when CD4 cells from old mice enter cell division there is no significant effect of age on the levels of CD69 expression for any CFSE peak. In cells pre-treated with OSGE prior to adoptive transfer, there is a trend towards increased CD69 expression for both young and old T cells in both lymph nodes and spleen. However, the effects are small and only reach statistical significance for lymph node cells in the peaks corresponding to relatively few cell divisions. These results suggest that a small population of CD4 cells from old donors can divided, express CD69 and induce a later decline in the CD69 expression that are not different that CD4 cells from young mice.
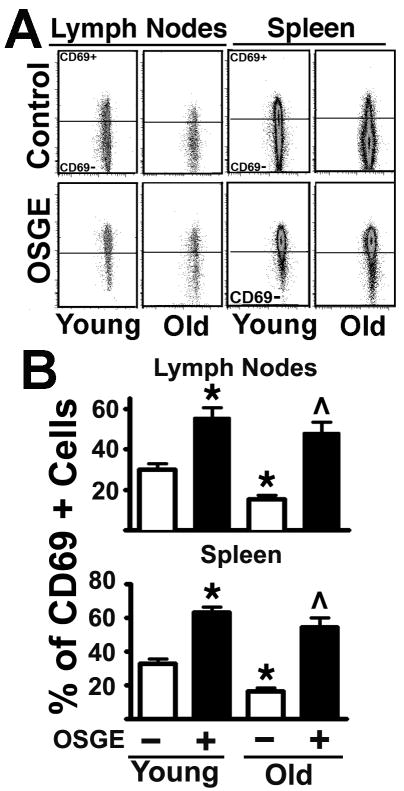
Figure 4. Analysis of the early expression of the CD69 activation marker in Tgr+ cells from young and old AND mice shows that age and OSGE affect the early expression of the CD69 activation marker in cells that have not divided.
A- 16 hours after adoptive transfer, untreated and OSGE-treated Trg+ cells were gated and analyzed as described in Figure 1, but with the addition of CD69 staining and analysis. No cell division was found using the CFSE+ as a marker of proliferation. The crossbar in each dot-plot represents the threshold used to discriminate CD69+ from CD69 negative cells in each peak defined by CFSE expression. B-The untreated and OSGE treated Trg+ cells corresponding to undivided cells were quantified from matched sets of 2 young (open bars) and 1 old (filled bars) in each of 4 experiments. Each bar shows mean percentage ± SEM. The * indicates statistical significance with respect to young cells not exposed to OSGE, and the ^ represents significant differences with respect to old cells not exposed to OSGE.
Figure 3. Analysis of CD69 expression in Tgr+ cells from young and old AND mice show that OSGE, but not age, affects the late decline in CD69 in cells that have divided.
A- 72 hours after adoptive transfer, untreated and OSGE-treated Trg+ cells were gated and analyzed as described in Figure 1, but with the addition of CD69 staining and analysis. The crossbar in each dot-plot represents the threshold used to discriminate CD69+ from CD69 negative cells in each peak defined by CFSE expression. B-Matched sets of at least 2 young (open bars) and 1 old (filled bars) in each of 5 experiments were quantified for CD69 expression. The panel shows the mean percentage of CD69+ cells ± SEM for each cell division peak delineated by CFSE level. No statistical differences were found between untreated young and old controls samples. The * indicates statistical significant differences relevant to young untreated mice (control), while the ^ represents significant differences to old untreated (control) from the same CFSE peak.
Age leads to a decline in early expression of CD69 in CD4 cells, which can be reversed by OSGE treatment
The data presented Fig 2 suggest that aging diminishes the number of Trg+ cells that can enter cell division, but cells that do progress in to the cell division seems to have a pattern of CD69 expression that is not affected by age. These studies did not determine whether CD4 cells from old mice show defects in the very early activation, i.e. before cell division take place. Activation of CD4 cells is a complex process that leads to many changes, including the rapid early expression of CD69 (Ziegler et al. 1994a, Ziegler et al. 1994b). Therefore, measuring CD69 before cell division may represent a good indicator of early stages in CD4 cell activation. To test how age affects the early expression of CD69, we performed a similar series of experiments using the method presented in Figure 1, but harvesting lymph nodes and spleen at 16 hours after adoptive transfer. After staining for CD69, CD4 and Vβ3 we measured CD69 expression following gating protocols described in Fig 1 and 3. Figure 4-A shows a typical dot-plot of CFSE versus CD69 expression in lymph nodes and spleen of the Trg+ cells from young and old mice after 16 hours of in vivo stimulation. In all cases, the analysis of CFSE reveals a single population that has not yet divided. The CD69 levels on untreated CFSE+ cells (control) found in lymph nodes and spleen (Fig 4-A, top panels) showed that approximately 30 % of the Trg+ cells from young mice express this early activation marker. In contrast, only 5–10 % of the Trg+ cells from old mice show expression of CD69. Treatment with OSGE can dramatically enhance the number of CD4 cells from young and old mice expressing CD69 (Fig. 4-A, bottom panels). For quantification we performed a series of 4 experiments, using 8 young and 4 old mice, measuring CD69 expression at 16 hours. The Figure 4-B shows the results: 30 % of the Trg+ cells from lymph nodes and spleen of young mice express CD69. In contrast lymph nodes the Trg+ cells from old donors show a statistical significant 50 % decline in the number of cells expressing CD69 (p=0.0005). A similar age-dependent decline was found in cells isolated from the spleen (p=0.001). When Trg+ cells from young donors were treated with OSGE there was a 50 % increase in the numbers of cells expressing CD69 in the lymph nodes (p=0.0001) and spleen (p=0.0001). OSGE treatments in Trg+ cells from old mice also led to increases in the numbers of cells expressing CD69 (in lymph nodes p=0.0001 and in spleen p=0.0001). There were no significant differences in CD69 expression between young and old mice after OSGE treatments. These results show that aging impairs early activation of CD4 cells in vivo and that removing surface glycoproteins by OSGE treatments can restore and enhance not only in vivo cell expansion but also early activation.
Discussion
During aging there is a significant shift from the naïve phenotype to a memory phenotype (Ernst et al. 1990, Miller 1997), which can complicate the study of age effects on T cell function. In AND mice, most of the Trg+ cells remain in a naïve state in late life (Linton et al. 1996, Linton et al. 1997). This model has thus been helpful for analyses of age-related changes in naïve T cell function. Using this model we have previously shown, using in vitro activation, that naïve Trg+ cells from old mice exhibit significant declines in immunosynapse formation and defects in the early expression of CD69 (Garcia et al. 2005). In addition, other groups have shown age-related declines in proliferation and cytokine production by AND CD4 cells (Linton et al. 1996, Haynes et al. 2004). The in vitro data suggest that defects in activation and proliferation are the results of defects in the early TCR signaling.
We have recently found that CD4 cells from old mice show alteration in the pattern of polysaccharide branching of several surface glycoproteins that can negatively regulate TCR signals, including CD43 and CD45 (Garcia et al. 2005). We also found that CD4 cells from old mice show increases in (α2,3)-sialic acid residues, accompanied by declines in (α2,6)-sialic acids in surface glycoproteins (Garcia & Miller 2003, Garcia et al. 2005). We hypothesized that those glycosylation changes may interfere with immunosynapse formation and TCR signaling.
Enzymes that remove polysaccharide moieties from glycoproteins, or which, like OSGE, cleave glycosylated segments of proteins, can enhance in vitro T cell function in CD4 T cells from young mice and overcome age-related declines in CD4 T cell function (Garcia & Miller 2003, Berger et al. 2005, Berger et al. 2006). Although these data suggest that surface glycosylation plays a role in age-dependent decline of T cell function, the relevance of these in vitro phenomena to immune responses in vivo has been untested. Our new results clarify this situation by using adoptive transfer of naïve Trg+ cells to syngeneic young B10.BR hosts. In contrast to some models in which endogenous CD4 cells are depleted prior to introduction of donor cells (Eaton et al. 2004), our approach evaluates the function of transferred T cells in the context of an intact endogenous immune system. As shown in Figure 1 and 2, after 72 hr the newly adopted Trg+ cells from young donors proliferate in LN and, to a lesser degree, in the spleen. However, Trg+ cells from old donors show a significant decline in the number of cells able to proliferate. Treating the cells with OSGE prior to adoptive transfer can restore proliferation of Trg+ cells from aged donors. In addition, we found no effect of age or OSGE in the homing of Trg+ cells to the different lymph organs (data not shown), suggesting that the OSGE effects are not related to differences in cell migration. In general, these in vivo results are consistent with the prior data on cultured cells, suggesting that age-related changes in surface glycosylation can block immunosynapse formation and TCR signaling leading to defects in T cell function (Garcia & Miller 2003, Garcia et al. 2005).
In addition to defects in TCR signaling, CD4 cells from old AND mice may have deficits in subsequent stages of the activation process. Of those cells from aged mice that do form immune synapses, about 50% have defects in CD43 exclusion (Garcia & Miller 2003) and NFAT translocation (Garcia & Miller 2001). This result suggested that CD4 cells from old mice that could enter the cell cycle and express CD69 in response to antigenic encounter in vivo might have subsequent deficiencies at later stages, such as the loss of CD69 surface marker expression after cell division. To test this, we evaluated loss of expression of CD69 after cell division of CD4 cells from young and old mice (Figure 3). We found no age effect on the proportion of cells that lose CD69 expression, suggesting that in this model system CD4 cell from old donors that progress through cell division may not show additional defects in function. These results correspond well with our previous analysis of immune synapse formation and lammellopodia formation suggesting the presence of two distinct CD4 cell populations in the old, one that responds well to stimulation and another that does not respond at all (Garcia & Miller 2002, 2003).
Studies by Laura Haynes and Susan Swain (Haynes et al. 2005, Clise-Dwyer et al. 2007) have shown that naïve peripheral CD4 cells recently arriving from the thymus in old donors can respond to antigen stimulation and proliferate as well as T cells from young mice. It is possible that the small percentage of Trg+ cells from old donors able to proliferate well to antigen stimulation may correspond to recent thymic immigrants, and that T cells produced in the periphery, or at earlier ages, may have developed defects in TCR signaling and proliferation, perhaps as a result of alteration in surface glycosylation (Garcia et al. 2005). In this context OSGE is known to be able to cleave external domains of CD44 (Sutherland et al. 1992) and other surface molecules that can negatively regulated TCR signaling, suggesting that changes in their glycosylation status and/or protein expression may be involved in the age-related decline of CD4 function.
In vitro studies of naïve CD4 cell function have shown that IL-2, but not other common γ-chain-binding cytokines, can reverse or overcome the defect in generation of CD4 effectors T cells from aged mice (Haynes et al. 1999). However, cytokine treatments require multiple rounds of proliferation to promote expansion of the original T cell population, and it is unclear how well CD4 cells expanded in this way would respond in vivo, in the presence of normal numbers of non-responding T cells, after encounter with complementary antigenic peptides. Our protocol shows that ex-vivo treatment for 1 hour with OSGE can fully restore in vivo the defects in T cell proliferation from old mice, and in addition can enhance proliferation of T cells from young mice. This simple methodology may allow further studies, in vivo, of age-related declines of T cell function, could help to identify the surface molecules responsible for the decline of T cell function, and may eventually lead to approaches to improve immune function in the elderly.
Acknowledgments
We wish to thank Lynn Winkelman, Maggie Lauderdale, Jessica Sewald, Bill Kohler, Dustyn Wright and Melissa Han for technical assistance. This work was supported by NIH grants AG019619 and AG030828.
Abbreviations
- Trg+
Vβ3-TCR+ transgenic CD4+ cells
- SNA-I
Sambucus nigra type I lectin
- CFSE
carboxyfluerescein diacetate, succinimidyl ester
- OSGE
O-sialoglycoprotein endopeptidase
Footnotes
Conflict of Interest: None of the authors have any financial or commercial conflicts of interest with this research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berger SB, Sadighi Akha AA, Miller RA. A glycoprotein endopeptidase enhances calcium influx and cytokine production by CD4+ T cells of old and young mice. Int Immunol. 2005;17:983–991. doi: 10.1093/intimm/dxh279. [DOI] [PubMed] [Google Scholar]
- Berger SB, Sadighi Akha AA, Miller RA, Garcia GG. CD43-independent augmentation of mouse T-cell function by glycoprotein cleaving enzymes. Immunology. 2006;119:178–186. doi: 10.1111/j.1365-2567.2006.02419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bining N, Miller RA. Cytokine production by subsets of CD4 memory T cells differing in P-glycoprotein expression: effects of aging. J Gerontol A Biol Sci Med Sci. 1997;52:B137–B145. doi: 10.1093/gerona/52a.3.b137. [DOI] [PubMed] [Google Scholar]
- Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, Hayakawa K, Hardy RR, Weigle WO. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- Garcia GG, Berger SB, Sadighi Akha AA, Miller RA. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 2005;35:622–631. doi: 10.1002/eji.200425538. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Increased Zap-70 association with CD3zeta in CD4 T cells from old mice. Cell Immunol. 1998;190:91–100. doi: 10.1006/cimm.1998.1394. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33:3464–3472. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs MV, Ernst DN, Torbett BE, Glasebrook AL, Rehse MA, McQuitty DN, Thoman ML, Bottomly K, Rothermel AL, Noonan DJ. Cell proliferation and cytokine production by CD4+ cells from old mice. J Cell Biochem. 1991;46:312–320. doi: 10.1002/jcb.240460406. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Haynes L, Klinman NR, Swain SL. Antigen-independent changes in naive CD4 T cells with aging. J Exp Med. 1996;184:1891–1900. doi: 10.1084/jem.184.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ, Haynes L, Tsui L, Zhang X, Swain S. From naive to effector--alterations with aging. Immunol Rev. 1997;160:9–18. doi: 10.1111/j.1600-065x.1997.tb01023.x. [DOI] [PubMed] [Google Scholar]
- Miller RA. Age-related changes in T cell surface markers: a longitudinal analysis in genetically heterogeneous mice. Mech Ageing Dev. 1997;96:181–196. doi: 10.1016/s0047-6374(97)01893-9. [DOI] [PubMed] [Google Scholar]
- Miller RA, Berger SB, Burke DT, Galecki A, Garcia GG, Harper JM, Sadighi Akha AA. T cells in aging mice: genetic, developmental, and biochemical analyses. Immunol Rev. 2005;205:94–103. doi: 10.1111/j.0105-2896.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Sadighi Akha AA, Berger SB, Miller RA. Enhancement of CD8 T-cell function through modifying surface glycoproteins in young and old mice. Immunology. 2006;119:187–194. doi: 10.1111/j.1365-2567.2006.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland DR, Abdullah KM, Cyopick P, Mellors A. Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. J Immunol. 1992;148:1458–1464. [PubMed] [Google Scholar]
- Ziegler SF, Levin SD, Johnson L, Copeland NG, Gilbert DJ, Jenkins NA, Baker E, Sutherland GR, Feldhaus AL, Ramsdell F. The mouse CD69 gene. Structure, expression, and mapping to the NK gene complex. J Immunol. 1994a;152:1228–1236. [PubMed] [Google Scholar]
- Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994b;12:456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]