Abstract
Introduction
Microbial translocation has been implicated as a contributing factor to the heightened immune activation observed during HIV-1 disease progression. When examined in a longitudinal study of HIV-1 seroconverters in Rakai, Uganda microbial translocation was not associated with HIV-1 disease progression. However, the role of general immune activation in HIV disease progression in this population was not fully examined.
Methods
Longitudinal serum samples of HIV-1 seroconverters in three HIV-1 disease progression groups [long-term nonprogressors (LTNP), standard progressors (SP), and rapid progressors (RP)] from Rakai, Uganda, were tested for levels of C-reactive protein (CRP), a marker for immune activation.
Results
CRP levels significantly increased in the SP group (p<0.0001), but not in the RP group or the LTNP group. CRP levels during the first year post-HIV-seroconversion in the RP group were significantly higher than those observed in the LTNP group (p<0.05). For the entire population CRP levels negatively correlated with Lipopolysaccharide levels (p<0.05) and were not associated with endotoxin antibody levels.
Conclusions
This study suggests that in this population increased immune activation is significantly associated with HIV-1 disease progression, but not microbial translocation.
Introduction
Immune activation has been demonstrated in multiple studies to be a significant contributing factor to HIV-1 disease progression1-4. In a cross-sectional analysis of subjects in the US, it was observed that this immune activation was associated with increased levels of the bacterial components in the blood, which was hypothesized to be due to increased microbial translocation from the lumen of the gastrointestinal tract5. This increase in microbial translocation was hypothesized to contribute to HIV-1 disease progression5, 6. When this theory was examined in a longitudinal study of HIV-1 seroconverters with known progression outcomes from Rakai, Uganda, it was found that microbial translocation or its subsequent innate cytokine response had no association with HIV-1 disease progression7. However, this study did not measure general immune activation specifically. CRP is an indicator of immune activation in response to inflammatory damage or infection and has been shown to increase in HIV-1 infected individuals8-11. Therefore, we examined C-reactive protein (CRP) levels in these subjects to determine if immune activation was associated with disease progression in this population.
Methods
The study population has been described in detail previously7. Briefly, serum samples of HIV-1 seroconverters from Rakai, Uganda were tested for levels of CRP by ELISA using manufacturer's recommendations (Invitrogen, Carlsbad CA; or Biovender Laboratory Medicine, Czech Republic for samples that were out of range on the Invitrogen assay). Longitudinal samples from pre-infection throughout disease progression were tested, and the subjects were divided into three progression groups [long-term nonprogressors (LTNP, CD4 count >600 cells/μL at 7+ years after seroconversion; n=27), standard progressors (SP, death>5 years but <9 years after seroconversion; n=41), and rapid progressors (RP, death <4 years after seroconversion; n=39)]. The change in CRP level by progression group was then examined using a linear mixed effects model, and the slopes (mg/year, i.e. the change of CRP along time) were tested and compared between progression groups at significance level of α=0.057, 8. CRP levels at the first year post-seroconversion were compared using an ANOVA on ranks test, and Dunn's method for pairwise comparisons (p<0.05). Due to the variability observed in the longitudinal CRP levels the population was divided into those that showed signs of consistent immune activation (serially increasing CRP levels in 80% of subsequent timepoints, n=39) and inconsistent activators (all other subjects, n=68). CRP levels were compared to indicators of microbial translocation tested previously [lipopolysaccharide (LPS), endotoxin core antibody (EndoCab), LPS-binding protein (LBP), soluble CD14 (sCD14)] with a Spearman correlation7. The cohort was approved by Institutional Review Boards (IRBs) in Uganda (the Uganda Virus Research Institute's Science and Ethics Committee and the Uganda National Council for Science and Technology) and from the Institution Review Boards (IRBs) of collaborating US institutions (Walter Reed Army Institute of Research, Columbia University and Johns Hopkins University).
Results
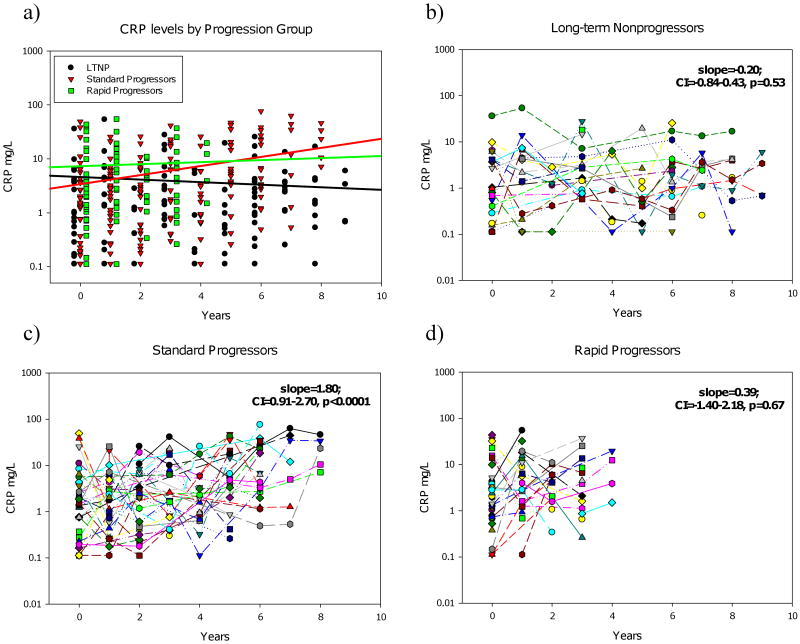
It was observed that CRP levels significantly increased in the SP group (slope=1.80mg/year, p<0.0001), but not in the RP group (slope=0.39, p=0.67) or the LTNP group (slope=-0.20, p=0.53) (Figure 1). The increase in CRP levels observed in the SP group was significantly higher than in the LTNP group (p=0.0005)(Figure 1A). The slope of the increase in CRP levels in the SP group is similar to levels observed by Lau et al in US subjects who progress to AIDS (slope=1.39)8. CRP levels during the first year post-seroconversion in the RP group (median=4.76mg/L, IQR=1.37-13.8) were significantly higher than those observed in the LTNP group (median=0.57; IQR=0.21-4.21) (p<0.05), but not the SP group (median=2.55, IQR=0.65-5.08).
Figure 1.
Longitudinal changes of CRP levels according to disease progression groups. The last HIV-negative time point for each patient (year 0) and all subsequent years post-seroconversion are shown together (A) and for each progression group [LTNP (B) (circles, n=27), SP (C) (inverted triangles, n=41), and RP (D) (squares, n=39)]. Longitudinal variation in patient levels was determined using linear multilevel modeling fit with slopes and 95% confidence intervals shown for each group. The resulting regression line for each group is shown in the respective color (A).
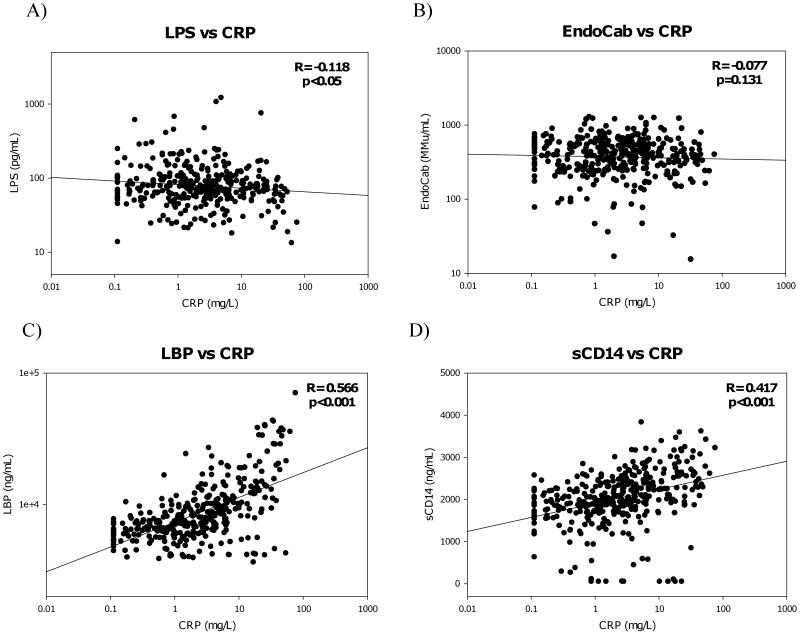
CRP levels were slightly negatively correlated with LPS (r=-0.12, p<0.05), but were significantly correlated to LBP (r=0.57, p<0.001), and the monocyte activation marker sCD14 (r=0.42, p<0.001) (Figure 2A,C,&D). There was no correlation between CRP and EndoCab levels (Figure 2B). When these parameters were examined according to progression group consistent patterns were seen for LBP and sCD14, but LPS was negatively correlated to CRP in the SP group only (Table 1). CRP did not correlate with LPS in those subjects with consistent immune activation (r=-0.08, p=0.325), but was negatively correlated in those individuals with inconsistent activation (r=-0.204, p<0.01).
Figure 2.
Comparisons of CRP and LPS (A), EndoCab (B), LBP (C), and sCD14 (D) levels for the total population were calculated using Spearman Rank order analysis. Correlation coefficients (R) for all cytokines and microbial translocation markers are shown with corresponding p-values (significance was determined to be p<0.05).
Table 1.
Spearman correlations of CRP and microbial translocation markers according to disease progression groups.
| Progression Group | LPS | EndoCab | LBP | sCD14 |
|---|---|---|---|---|
| LTNP | -0.050 (p=0.73) | -0.033 (p=0.73) | 0.255 (p<0.01) | 0.265 (p<0.01) |
| Standard Progressors | -0.228 (p<0.01) | -0.069 (p=0.38) | 0.737 (p<0.001) | 0.585 (p<0.001) |
| Rapid Progressors | -0.008 (p=0.94) | -0.039 (p=0.73) | 0.601 (p<0.001) | 0.287 (p<0.01) |
Correlation coefficients and corresponding p values are shown with significant correlations shown in bold.
Discussion
The similarity between these results and those seen in US based studies would suggest that at least in the case of longitudinal CRP levels the two populations are similar8. This is surprising since the previous findings found significant differences in a variety of markers for immune activation in HIV-uninfected individuals7. Additionally, it is interesting that CRP levels did not significantly increase in the RP group, but this may be due to the higher initial levels of CRP seen in this group. The short lifespan of the RP group also may not provide enough time for the CRP levels to noticeably increase. It is also possible that the higher initial level of immune activation seen in the RP group contributes to rapid disease progression. The absence of change in the LTNP group was expected since lower rates of CRP increase have been seen in individuals who do not progress to AIDS8. These results are also somewhat divergent from a study done in Zambia that found that increased CRP levels only associated with HIV-1 disease progression in patients who had diarrhea, although that was a cross sectional study and therefore is not strictly comparable to the current longitudinal study10. It has previously been speculated that CRP levels may be used as a marker for disease progression in resource-poor settings, but the variability observed in individual CRP trajectories overtime most likely limits its clinical utility12.
The negative correlation of CRP with LPS levels in the total population and the SP group, as well as the lack of any significant correlation in those individuals that show consistent immune activation, suggests that the activation observed in this population is not caused by an increase in microbial translocation, even though CRP correlated with other markers for immune activation (LBP and sCD14) that have been linked to microbial translocation5. In conclusion, these data suggest that in this Ugandan population immune activation is associated with HIV-1 disease progression in the absence of microbial translocation.
Acknowledgments
The authors would like to thank all the participants of the Rakai cohort, and the staff of the Rakai health science program. This study was supported in part by funding from the Division of Intramural Research, NIAID, NIH; NIAID (grants R01 A134826 and R01 A134265); NICHD (grant 5P30HD06826); the World Bank STI Project, Uganda; the Henry M. Jackson Foundation; the Fogarty Foundation (grant 5D43TW00010); and the Bill and Melinda Gates Institute for Population and Reproductive Health at JHU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995 Apr;16(4):187–191. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 2.Rizzardini G, Piconi S, Ruzzante S, et al. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. Aids. 1996 Nov;10(13):1535–1542. doi: 10.1097/00002030-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 3.McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001 Apr 19;410(6831):974–979. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 4.Douek DC. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 2003 Jul-Sep;5(3):172–177. [PubMed] [Google Scholar]
- 5.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006 Mar;7(3):235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 7.Redd AD, Dabitao D, Bream JH, et al. Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6718–6723. doi: 10.1073/pnas.0901983106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau B, Sharrett AR, Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006 Jan 9;166(1):64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 9.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003 Jun;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zulu I, Hassan G, Njobvu RNL, Dhaliwal W, Sianongo S, Kelly P. Cytokine activation is predictive of mortality in Zambian patients with AIDS-related diarrhoea. BMC Infect Dis. 2008;8:156. doi: 10.1186/1471-2334-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melchior JC, Niyongabo T, Henzel D, Durack-Bown I, Henri SC, Boulier A. Malnutrition and wasting, immunodepression, and chronic inflammation as independent predictors of survival in HIV-infected patients. Nutrition. 1999 Nov-Dec;15(11-12):865–869. [PubMed] [Google Scholar]
- 12.Drain PK, Kupka R, Msamanga GI, Urassa W, Mugusi F, Fawzi WW. C-reactive protein independently predicts HIV-related outcomes among women and children in a resource-poor setting. AIDS. 2007 Oct 1;21(15):2067–2075. doi: 10.1097/QAD.0b013e32826fb6c7. [DOI] [PMC free article] [PubMed] [Google Scholar]