Abstract
Ricin toxin is a CDC level B biothreat. We have developed a ricin vaccine, RiVax, which is a recombinant mutant of ricin A chain. RiVax is safe, immunogenic and protective in mice when administered intramuscularly (IM). We have now attempted to increase the utility and immunogenicity of RiVax by administering it intradermally (ID) with or without alum. Without alum, Rivax administered by the ID and IM routes was equally immunogenic and protective. With alum, ID vaccinations were more immunogenic and protective against both systemic and mucosal challenge with ricin and superior in protecting animals from ricin-induced lung damage.
Keywords: intradermal, vaccine, ricin
Introduction
Ricin is a heterodimeric toxin which inactivates ribosomes. It can be easily isolated from the beans of the castor bean plant. It consists of two disulfide linked subunits, an A chain (RTA) and a B chain (RTB), each with a molecular weight of 30–32 kDa. The RTB facilitates cell entry and intracellular routing. Once the holotoxin is internalized, the RTA translocates into the cytosol and catalytically inactivates ribosomes [1]. Symptoms of ricin intoxication depend upon the route of exposure, i.e. ingestion, inhalation or injection. Due to its prevalence, ease of production, and toxicity, ricin has been classified as a level B biothreat by the Centers for Disease Control [2]. Therefore, protection from ricin toxicity would be most important for military personnel and first responders.
Attempts to treat and/or protect animals from ricin intoxication have been numerous and varied and fall into three categories: (1) post exposure passive immunization, (2) post exposure treatment with small molecules, and (3) prophylactic immunization. Post-exposure administration of anti-ricin antibody is highly effective but it must be given within hours of exposure, before there are symptoms of intoxication [3–5]. Unfortunately these symptoms mimic those of many other diseases and therefore would not be easily recognized in the setting of bioterrorism. Inexpensively manufactured small molecule inhibitors of ricin have also been studied and are promising when tested in vitro. However, these inhibitors will also require administration soon after exposure. In addition, the majority of them have not yet been studied in animals, and those that have do not confer 100% survival [6–7]. Prophylactic immunization provides the most reliable method for inducing protection against ricin toxicity. Several approaches have been taken to develop an effective vaccine. Vaccination with formaldehyde treated ricin holotoxin, both with and without adjuvant, provides good protection, but this toxoid can retain or develop some toxicity during storage [8]. Vaccination with deglycosylated RTA (dgRTA) with adjuvant protected animals from death, but did not ameliorate lung damage following aerosol challenge [9]. DgRTA is also expensive and dangerous to produce. The use of recombinant RTA containing a 25 amino acid inactivating insertion plus alum was immunogenic and protective in mice, but residual catalytic activity makes it an unlikely candidate for a human vaccine [10]. Another vaccine developed by United States Army Medical Research Institute for infectious diseases (USAMRIID) utilizes a truncated RTA, which is structurally very stable, immunogenic and is a highly promising vaccine [11]. However, none of these vaccines eliminates the vascular leak-inducing site found in RTA and therefore, immunization could cause local tissue damage.[12].
We have developed a recombinant mutant RTA vaccine called RiVax that contains two mutations, Y80A and V76M. These mutations inactivate both the ribotoxic site and the motif responsible for causing vascular leak syndrome [13–14]. RiVax retains its native structure as determined by X ray crystallography [15] and is both safe and highly immunogenic in mice, rabbits and humans [14]. Three doses of 3.3 μg each, administered IM, at 28 day intervals protect 100% of the animals from a challenge with a 10 X LD50 dose of ricin administered by intraperitoneal (IP) injection, gastric gavage or aerosol [16]. When sera from immunized rabbits or humans were passively transferred to naïve mice, the mice were protected from injected ricin. [14–17]. Most recently, we have tested the stability of lyophilized RiVax and shown that it remains active for at least 1 year following storage at either 4°C or 25°C [18].
In addition to the use of an adjuvant, such as alum, dose sparing regimens and simplified methods of administration would further improve the utility of RiVax. In general, dose sparing methods, be it administration of smaller doses or administration of fewer doses, is advantageous especially when vaccine stocks may be scarce and/or during an emergency. Additionally, ease of administration will increase the chance that a vaccine is used properly. One approach to improving the immunogenicity and reducing the dose of RiVax would be to alter the route of administration. While mucosal vaccine administration has been used to deliver other protein vaccines, it often requires specialized adjuvants in order to facilitate uptake and avoid degradation in the harsh mucosal environment [19]. ID and transcutaneous administration of vaccines have also been studied as alternatives to the more common IM route of administration [20]. ID administration of protein vaccines has been used to reduce the dose of some vaccines since the skin is thought to be a more immunogenic route of administration than the muscle [21–23]. Dose sparing and enhancing immunogenicity are particularly important in patient populations who are difficult to immunize such as the elderly and hemodialysis patients [24–25]. Furthermore, the ID vaccination route is also attractive because it can be carried out rapidly using a needle-free ID gun, thus eliminating the risk and cost of needle disposal. Based on the advantages of ID vaccination with other protein vaccines, we hypothesized that lower doses of RiVax would also be effective if administered via the ID route in the presence of alum.
In this study we have compared the efficacy of RiVax administered via the ID or IM routes both with and without alum. Our results demonstrate that both ID and IM vaccination with RiVax elicit similar antibody titers and confer protective responses both systemically and at mucosal sites. Importantly, as compared to the IM route, when RiVax is administered with alum via the ID route, less vaccine is required to elicit protective antibody responses regardless of whether the mice are challenged with ricin by IP injection, gastric gavage or aerosol. Finally, ID administration is effective at decreasing lung damage as well as protecting mice against the lethality of aerosolized toxin. Hence the ID route has numerous advantages and clearly shows improved protection against mucosal ricin intoxication.
Materials and Methods
Experimental design
Swiss Webster mice (Taconic, Hudson, NY) were injected either ID or IM with RiVax, prepared as previously described [13, 14, 18]. The vaccine formulation consisted of 0.2 mg/mL RiVax in 20% trehalose (Sigma, St. Louis, NJ) and 0.04% Tween 80 (Fischer, Fair Lawn, NJ). This was then lyophilized [18] and stored at 4°C. Reconstituted and diluted vaccine was administered in a volume of 50 μL either with or without 1 mg/mL alum (Alhydrogel 1.3%, Brenntag Biosector, Denmark) at one of three dose levels. RiVax with alum was administered at 1.0, 0.1 and 0.01 μg per dose; RiVax without alum was administered at 10, 1.0 and 0.1 μg per dose. Control mice were injected with formulation alone or formulation plus alum. Vaccine was administered on days 0, 28, and 56. Two weeks following the last injection, mice were bled to determine serum antibody titers and then challenged with a previously determined 10 X LD50 dose of ricin by one of three routes (100 μg/kg by gastric gavage, 100 μg/kg by IP injection, and 40 μg/kg by aerosol) [16]. Weights and survival of all mice were followed for 14 days following challenge. Animals receiving aerosol exposure underwent lung function assessment by plethysmography on days 0, 1, 2, 3, 5, 7, 10, 14.
Radioimmunoassay (RIA) to determine RiVax-specific antibody titers
RIAs were carried out using ninety-six well, U- bottom, vinyl plates (Thermo, Millford, MA) coated with 100 μL of RiVax in phosphate buffered saline (PBS) overnight (ON) at 4°C. Plates were washed and blocked with 10% fetal calf serum (FCS) (HyClone, Logan, UT), 0.05% sodium azide in PBS for 2 hours at room temperature (RT) and then frozen until use. Plates were thawed, washed and coated with 100 μL of a known amount of affinity purified mouse anti-RiVax (1–1000 ng/mL) or test serum serially diluted in 10% FCS, 0.05% sodium azide in PBS, incubated ON at 4°C, washed and incubated with 125I-labeled rabbit anti-mouse IgG (105 cpm/100 μL per well). Plates were incubated for 2 hours at RT and washed 5 times with distilled water. The wells of the plates were cut out, individually placed into 12 × 75mm glass tubes and the radioactivity in each tube was measured on a Wizard 1470 Automatic Gamma Counter (Perkin Elmer, Waltham, MA). Select serum samples were also assayed on ricin-coated plates and in these assays all samples and buffers contained 0.1 M galactose. The titers on the RiVax-coated plates were approximately 2-fold higher that those obtained on the ricin coated plates. We do not find this to be surprising since some of the epitopes on the ricin molecule might be obscured by the presence of the B chain or the fact that ricin A and B chains, but not RiVax are glycosylated.
Vaccination and ricin challenge
Groups of 8 female Swiss Webster mice, age 6–8 weeks old, were injected either ID or IM, with RiVax either with or without alum. IM vaccinations were administered in the left flank. The belly was prepped with an alcohol pad prior to ID vaccination in order to see the skin; the formation of a ‘blister’ after injection confirmed ID delivery of the vaccine. Following challenge by either IP injection, gastric gavage or aerosol, mice were euthanized if moribund or after having lost >25% of their pre-challenge body weight.
Aerosol challenge
Mice were exposed to aerosolized ricin in a nose-only exposure chamber (InTox, Moriarty, NM) as previously described [16]. Lung function was measured using a 12 chamber whole body plethysmograph (Buxco, Wilmington, NC). The higher the Penh the more labored the breathing.
Gavage challenge
Mice were challenged by gastric gavage as previously described [16]. Briefly, mice were fasted and moved to a clean cage 20 hours before challenge. They were dosed with 100 μg/kg ricin in a volume equal to 1% of their body weight in PBS using a feeding needle delivered into their stomach while being restrained by hand. Mice were then fasted for an additional 4 hours. They were monitored for 14 days for weight loss and survival.
IP challenge
Mice were injected IP with 100 μL ricin at 100 μg/kg in PBS and weight loss and survival were monitored for 14 days.
Statistical Analysis
Statistical significance was considered to be < 0.05 in all cases. In the gavage and IP challenge models, duplicate experiments of 8 mice each were carried out and data for 16 mice were combined for analysis. In the aerosol challenge model, 4 experiments of 4 mice each were carried out, data were combined and then analyzed. Titer data in Figure 1 represent 6 experiments of 8 mice in each dose group, combined into groups of 48 mice each for analysis. To determine significant difference in survival, the Log-rank test was used. To determine significant difference in titer data and Penh levels, the Student’s t test was used. All error bars represent 1 standard deviation from the mean.
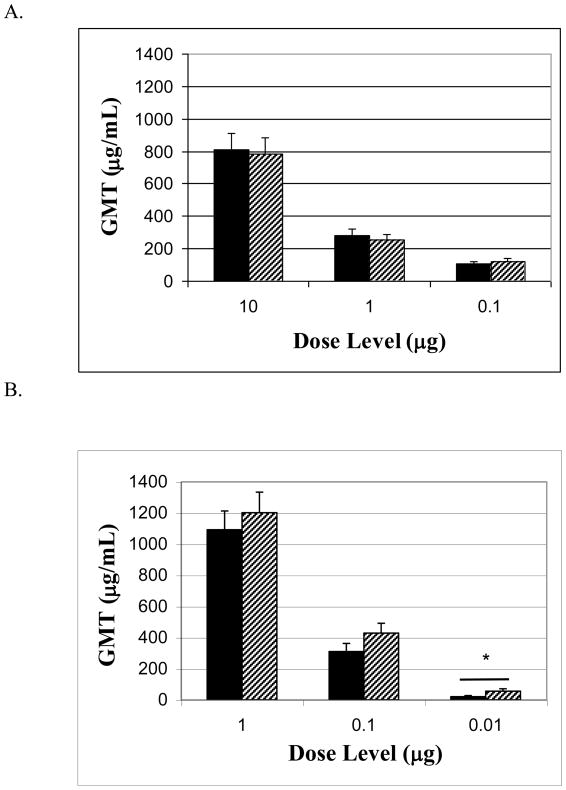
Figure 1. Serum antibody titers.
Mice were vaccinated three times at 4 week intervals. Two weeks after the last vaccination mice were bled before ricin challenge and sera were analyzed for RiVax-specific antibodies, black bars = IM, hatched bars = ID; All data represent 6 experiments of 8 mice each, compiled into groups of 48 mice for analysis. (A) without alum, P > 0.05 when comparing ID and IM in each dose level (B) with alum, 0.01 μg IM and 0.01 μg ID, P < 0.05, indicated with a bar and asterisk.
Results
RiVax-specific antibodies
The ability to elicit high levels of circulating, antigen specific antibodies is an important indicator of an effective vaccine. In order to compare the ability of ID vs. IM vaccinations to induce specific antibodies, RiVax was administered to mice as described in the Methods. Mice were bled one day prior to challenge with ricin to measure the titers of anti-RiVax antibodies. As shown in Figure 1, the geometric mean titers (GMT) of RiVax-specific antibodies in mice vaccinated with RiVax in the absence of alum (Figure 1A) were dose dependent; the addition of alum to the vaccine significantly increased these titers (Figure 1B). In comparing titers following vaccination by either the ID or IM route, we observed significantly higher titers in ID vaccinated mice at the 0.01 μg dose level (p <0.01). Comparisons between ID and IM at all other dose levels showed that the ID vaccination route was as effective as the IM route in inducing RiVax-specific antibodies. Overall, at low doses the ID vaccination route is significantly better than the IM route in inducing RiVax-specific antibodies.
Post challenge survival after ID or IM vaccination with RiVax
While levels of circulating antibodies are good predictors of vaccine efficacy, these antibodies must protect animals. In order to determine the ability of ID vs. IM vaccinations to protect mice against ricin intoxication, we carried out a series of experiments where mice were vaccinated with RiVax as described in the Methods. Two weeks after the last vaccination mice were challenged with a 10 X LD50 dose of ricin either by IP injection, gastric gavage or aerosol.
As shown in Figure 2, following IP challenge, 100% of mice vaccinated either by the ID or IM routes at the 10 and 1.0 μg dose levels, survived. At the 0.1 μg dose level, 88% of IM vaccinated mice and 56% of ID vaccinated mice survived, demonstrating that protective effects of ID vaccination were not significantly different from those observed with IM vaccination. (p > 0.05 by Log-rank) (Figure 2A).
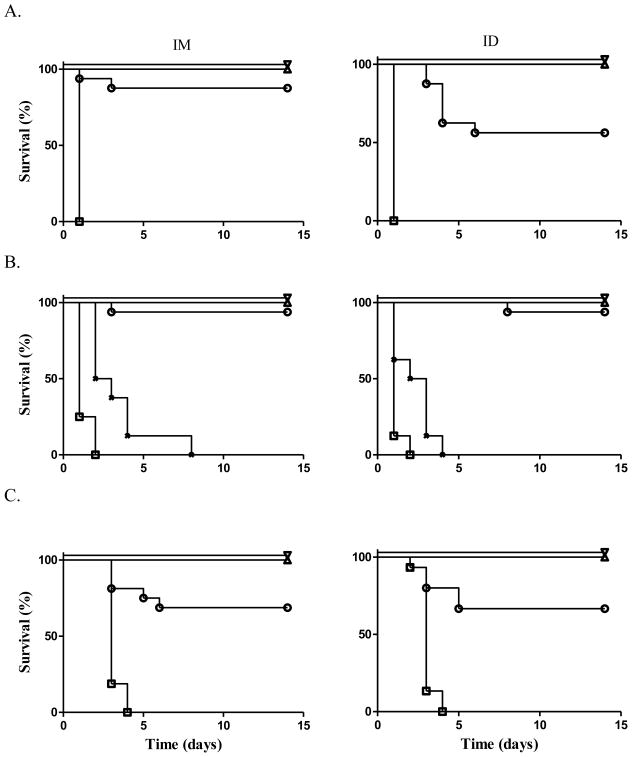
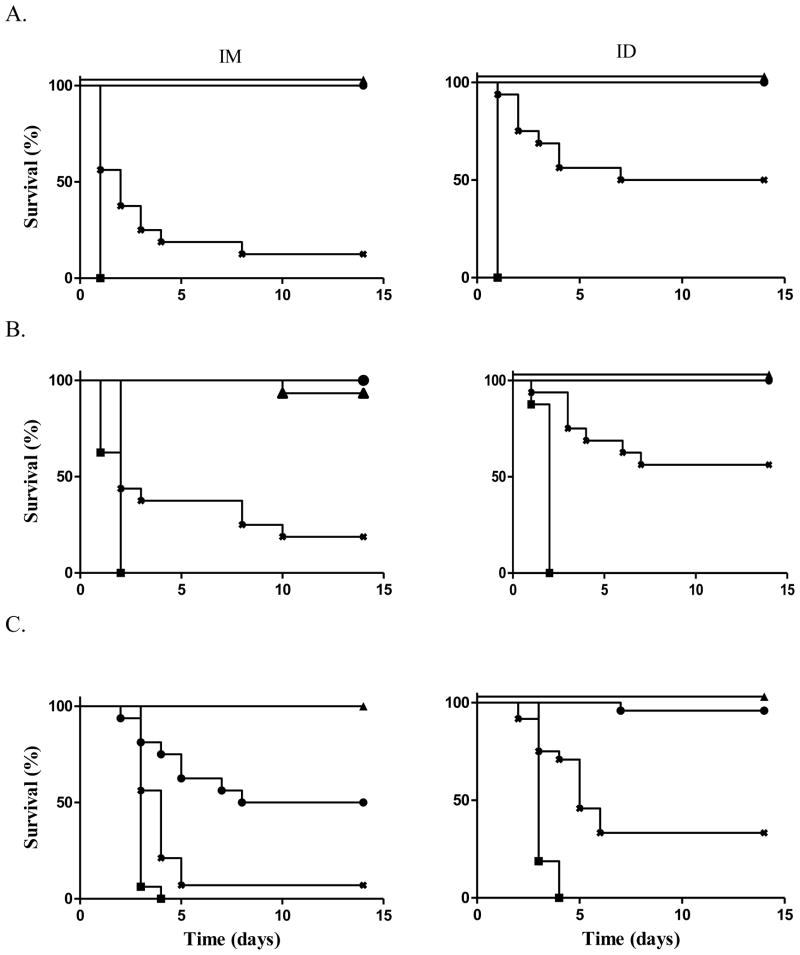
Figure 2. Ricin challenge after vaccination with RiVax without alum.
Mice were vaccinated three times at 4 week intervals. Two weeks after the last vaccination mice were challenged with ricin. P values compare survival of ID vs. IM vaccinated mice within a dose level; ∇ = 10 μg, △ = 1.0 μg, ○ = 0.1 μg, X = 0.01 μg, □ = 0 μg; challenge via (A) IP injection, at the 0.1 μg dose level, P = 0.078, (B) gastric gavage, at the 0.01 μg dose level, P = 0.218, (C) aerosol, at the 0.1 μg dose level, P = 0.848. (A) and (B) represents 2 experiments of 8 mice each combined into groups of 16 mice for analysis; (C) represents 4 experiments of 4 animals each combined into groups of 16 mice for analysis.
All of the mice that received 3 doses of 10, 1.0 or 0.1 μg of vaccine doses survived a gavage challenge with ricin regardless of the vaccination route. In order to find a dose level that might demonstrate a dose-related difference in potency between the two different vaccination routes, we repeated the experiment at the 1.0, 0.1, and 0.01 μg dose levels. All of the mice at the 1.0 μg and 88% (7/8) at the 0.1 μg dose levels survived. In contrast, ricin was lethal to all the mice at the 0.01 μg dose level. Overall, the percent survival following either vaccination route was identical. The combined results of the two experiments demonstrated that all the mice vaccinated with three doses of 10 and 1.0 μg each, survived oral ricin challenge. In addition 94% (15/16 mice) receiving 3 doses of 0.1 μg survived. In contrast, none of the mice receiving 3 doses of 0.01 μg survived (Figure 2B).
We also investigated the survival of mice vaccinated via the ID or IM routes followed by a challenge with aerosolized ricin. At the 10 and 1.0 μg dose levels 100% of the mice in both groups survived. At the 0.1 μg dose level, 69% (5/16 mice) vaccinated via the IM route and 66% (5/15 mice) vaccinated via the ID route survived (Figure 2C). Hence, vaccination via the ID and IM route with RiVax alone protected animals from death when ricin was administered systemically, by gavage or by aerosol.
Post challenge survival after ID or IM vaccination with RiVax plus alum
Despite the fact that survival following vaccination with RiVax was equivalent following vaccination by either the IM or ID routes, several findings led us to postulate that ID vaccination would be advantageous when RiVax was administered with alum: (1) recent work by others suggested that alum works by enhancing activation of dendritic cells (DCs) [26]; (2) DCs (Langerhan’s cells) are highly prevalent in the skin; (3) our data presented in Figure 1B which demonstrate that ID administration with alum is superior to IM administration with alum in eliciting specific antibodies. To test this hypothesis, mice were vaccinated either ID or IM with various doses of RiVax in 1 mg/mL alum. Two weeks after the last vaccination, mice were challenged with ricin administered by IP injection, gastric gavage or aerosol.
As shown in Figure 3A, when challenged by IP injection 100% of animals vaccinated with 3 doses of 1.0 and 0.1 μg each survived, regardless of the vaccination route. 13% of mice receiving 3 doses of 0.01 μg RiVax by the IM route, and 50% of mice vaccinated by the ID route survived.
Figure 3. Ricin challenge after vaccination with RiVax + alum.
Mice were vaccinated three times at 4 week intervals with RiVax in 1 mg/mL alum. Two weeks after the last vaccination mice were challenged with ricin. P values compare survival of ID vs. IM vaccinated mice within a dose level; ▲ = 1.0 μg, ● = 0.1 μg, X = 0.01 μg, ■ = 0 μg. Challenge via (A) IP injection, at the 0.01 μg dose level, P < 0.05, (B) gastric gavage, at the 0.01 μg dose level, P < 0.05, (C) aerosol, at the 0.1 μg dose level, P < 0.05, at the 0.01 μg dose level P < 0.05. (A) and (B) represent 2 experiments of 8 mice each combined into groups of 16 mice for analysis; (C) represents 4 experiments of 4 animals each combined into groups of 16 mice for analysis
As shown in Figure 3B, when challenged by gastric gavage, > 90% of the mice receiving 3 doses of 1.0 and 0.1 μg each, survived regardless of the route of administration. At the low dose level ID administration of RiVax proved superior with 19% and 56% survival using the IM and ID vaccination routes, respectively.
Finally, vaccinated animals were challenged with aerosolized ricin. At the high dose level of 3 doses of 1.0 μg each, 100% of mice vaccinated by either the ID or IM routes survived. At the middle dose level, 93% of the mice vaccinated via the ID route survived, while only 50% of the mice vaccinated via the IM route survived. At the low dose level, vaccination via the ID vs. IM routes was again significantly better at protecting animals with 25% vs. 13% survival, respectively (Figure 3C). Hence, RiVax administered in alum via the ID route was superior to administration via the IM route in protecting animals against the systemic, gut-mucosal and respiratory toxicity of ricin.
The relationship between survival and specific antibody titers
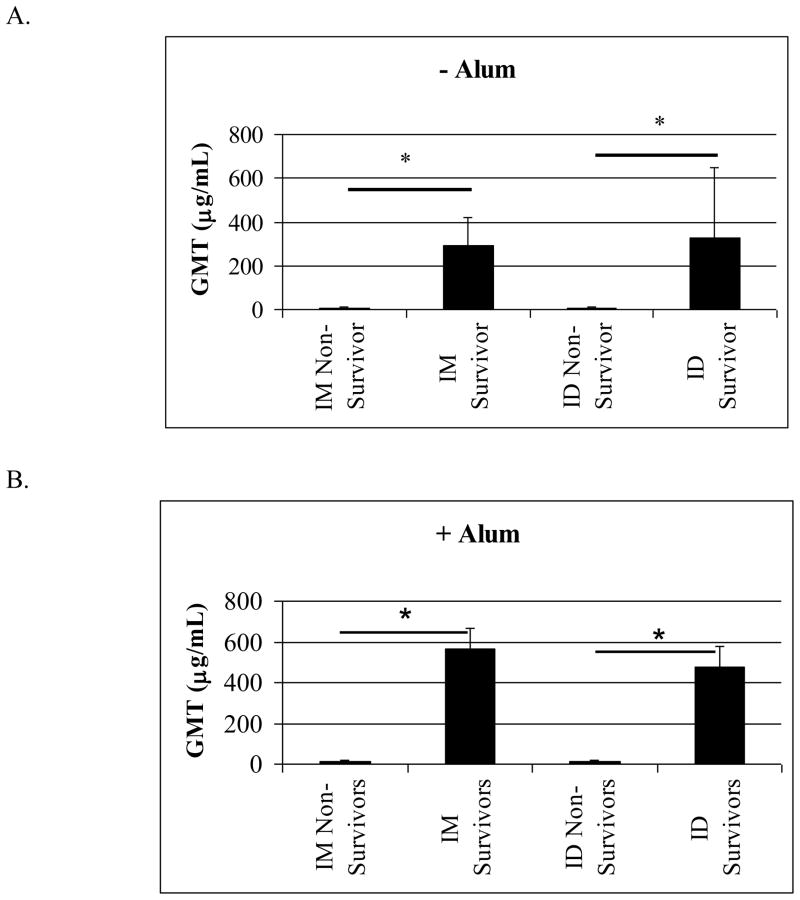
Effective vaccination induces significant levels of protective antigen-specific antibody. To validate this in our model, we compared the GMT of RiVax-specific antibody in surviving vs. non-surviving animals from all challenge groups. We also compared titers according to vaccination route to determine if surviving mice vaccinated via the ID route had significantly higher antibody titers than surviving mice vaccinated via the IM route. As expected, when RiVax was administered without adjuvant, the survivors show significantly higher antibody titers than the non-survivors following vaccination via both the ID and IM routes. In comparing surviving mice vaccinated via either the ID or IM routes, there were no significant differences in their antibody levels nor were there differences when comparing ID and IM vaccinated non-survivors (Figure 4A). The results of this comparison were similar when RiVax was administered with alum (Figure 4B). While more mice vaccinated with RiVax plus alum via the ID vs. the IM route survived, the survivors in both groups had similar antibody titers. These data support the observation that higher levels of RiVax-specific antibodies in the blood correlate with better protection.
Figure 4. Levels of RiVax specific antibodies vs. survival after ricin challenge.
Titers from animals at all dose levels were compiled, then grouped to compare non-survivors and survivors, (A) without alum, IM non-survivors, n = 38; IM survivors, n = 118; ID non-survivors, n = 37; ID survivors, n = 110, (B) with alum, IM non-survivors, n = 68; IM survivors, n = 94; ID non-survivors, n = 42; ID survivors, n = 109 In all cases, * indicates P < 0.05.
Lung function in mice exposed to aerosolized ricin
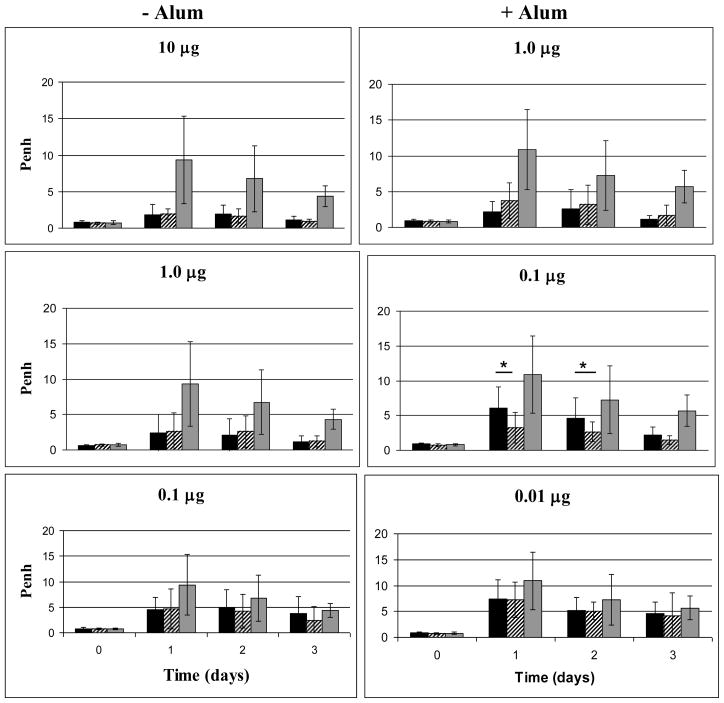
We were interested in determining whether ID vaccination would not only increase survival, but also result in less lung damage following exposure to aerosolized ricin. There was no reason to expect this unless the higher levels of antibody induced by ID administration exudated into the lungs. To this end we monitored lung function in mice vaccinated with RiVax, by both routes with or without alum, followed by an aerosol challenge with ricin. Lung function was assessed on days 0, 1, 2, 3, 5, 7, 10, and 14 post-challenge by whole-body plethysmography. Many mice receiving the lowest doses of vaccine died by day 3 so the most relevant comparisons are prior to that time point (with alum, 60% of the low dose group was surviving on day 3; without alum 87% of the low dose group was surviving on day 3. In addition, data from mice surviving 5–14 days post challenge showed no significant difference related to the route of vaccination and therefore are not shown. Overall, both ID and IM administration of RiVax conferred significant protection for ricin related lung damage as compared to controls. When comparing ID vs. IM vaccinated mice within a dose level and adjuvant group, both were equally protected from ricin-related lung damage. The only exception was at the 0.1 μg dose level, where vaccination by the ID route with alum was significantly better at protecting mice from lung damage (Figure 5). Our data demonstrate that protection of the lungs following IM vs. ID vaccination is equivalent at higher dose levels of vaccine, but that when low doses on alum are given, ID vaccination is more effective than IM vaccination.
Figure 5. Lungs function following aerosol challenge with ricin.
Mice were vaccinated three times at 4 week intervals with the doses of RiVax indicated in the panels. Two weeks after the last vaccination, mice were challenged with aerosolized ricin and lung function was measured as the Penh on days 0, 1, 2, 3, 5, 7, 9, 10, 14. Penh values for days 0, 1, 2, 3 are shown. Black bars = IM, hatched bars = ID, grey bars = control vaccinated mice. * P < 0.05. All data represent 4 experiments of 4 mice each.
Survival and lung function
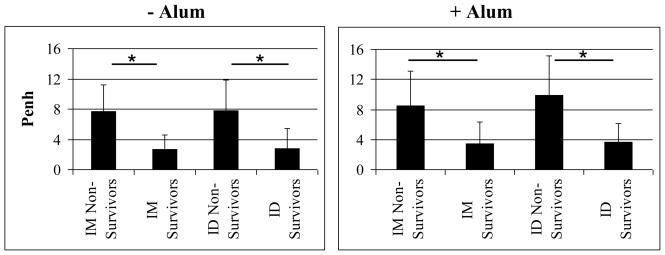
Although the specific mechanisms responsible for death by ricin intoxication are not clearly understood and are probably multifactorial, in the case of exposure to aerosolized ricin, it is presumed that lung damage plays a significant role [2]. In order to determine if this was the case in our experiments, we compared the day 1 post-challenge Penh levels in non-surviving and surviving mice. In making this comparison we found that regardless of the vaccination route or the presence of alum in the formulation, mice that survived had significantly better lung function at day 1 than mice that subsequently died (Figure 6). In general, all mice that survived to day 14 post challenge had low Penh values that reflected normal lung function indicating that much of the lung damage had already resolved as we observed histologically in past experiments [16]. While significantly more mice vaccinated via the ID route survived, the Penh levels in surviving mice vaccinated by either route were not significantly different; the same was true in comparing non-surviving mice vaccinated by the ID vs. IM route. These results suggest that if ricin-induced lung damage can be minimized, the chances of survival may also improve
Figure 6. Lung function compared to RiVax-specific antibody and survival.
Mice were vaccinated three times at 4 week intervals. Two weeks after the last vaccination, mice were challenged with aerosolized ricin and lung function was measured as the Penh on days 0, 1, 2, 3, 5, 7, 10, 14. Penh values from animals at all dose levels were compiled, then grouped to compare non-survivors and survivors; left graph: without alum, IM non-survivors, n = 21; IM survivors, n = 41; ID non-survivors, n = 21; ID survivors, n = 41; right graph: with alum, IM non-survivors, n = 38; IM survivors, n = 25; ID non-survivors, n = 29; ID survivors, n = 35. * indicates P < 0.05.
Discussion
We have previously described a ricin vaccine, RiVax, composed of a recombinant ricin A chain containing two site mutations to eliminate all known toxicities [13]. We have demonstrated that when RiVax is administered to mice by IM injection without alum it was entirely safe and protected mice from ricin-induced death following delivery of the toxin by IP injection, gastric gavage, or aerosol [16]. RiVax was safe and immunogenic in mice, rabbits and humans [14]. Adoptive transfer of human anti-RiVax antibody (at levels found in human serum) into mice protected them from ricin intoxication [17].
Thus far all of our studies have been carried out using IM vaccination of RiVax with or without alum. Several published studies with other vaccines have reported that small doses might be more effective if administered via the ID vaccination route. Therefore, the purpose of this study was to determine whether RiVax administered by ID injection would be more immunogenic and protective at lower doses [27–29]. ID vaccination has several practical advantages in protecting humans due to the ease of administration, especially when using an ID gun, thereby eliminating the need for needles and subsequent needle disposal. In some cases lower doses have been reported to be as effective as higher doses administered by the IM route [27–29]. It was, however, unclear whether ID vaccination would induce antibodies that would protect mucosal surfaces more effectively than we have already reported using IM vaccination [16]. Hence, in this study, we have compared ID and IM vaccination with or without alum at several dose levels and determined the levels of anti-RiVax antibodies generated in serum as well as the ability of the vaccine to protect mice against ricin intoxication following systemic, gastric gavage or aerosol challenges, the latter two being the most likely routes of exposure.
The major findings to emerge from this study are as follows: (1) ID vs. IM administration of RiVax without alum conferred equal protection; (2) RiVax on alum was significantly better than RiVax alone in eliciting specific antibodies and both systemic and mucosal protection when 90–99% less vaccine was used. This was consistent with our previous report using an IP challenge with ricin [18]; (3) vaccination with RiVax on alum via the ID route is significantly better than vaccination via the IM route at protecting animals from ricin challenge, hence, smaller doses of vaccine are required when ID vaccination is used; (4) in comparing IM vs. ID vaccination with RiVax on alum, at low doses, the latter was more effective at protecting mice from ricin-induced lung damage; (5) RiVax-specific antibody levels correlated with post challenge survival.
Extensive studies in our laboratory have shown RiVax to be highly protective when administered via IM injection without alum [14–16]. In this study, we observed that RiVax alone delivered by the ID or IM routes was equally effective at protecting mice from aerosolized, gavaged, or IP injected ricin. This result is consistent with published reports demonstrating that ID vs. IM administration of other protein vaccines confers similar protection in the absence of adjuvant [30].
Administration of vaccines on alum as a method of augmenting immunogenicity has been standard practice for decades. For nearly 70 years alum has been reported as effective in increasing antigen specific antibody levels, seroconversion rates, and the longevity of these responses [31]. Here we compared RiVax administration alone and with alum and found that our results are comparable to reports using other vaccines and to our previous findings following IP ricin challenge [18]. While RiVax alone was very effective in inducing seroconversion, we found that the addition of alum significantly increased specific antibody levels while also improving protection. The addition of alum to RiVax decreases the dose needed for protection by at least 10 fold.
ID administration of RiVax on alum is significantly more protective than IM administration of the same formulation. Most importantly, we observed that ID administration of RiVax on alum significantly improved protection against ricin delivered by gavage or aerosol. This finding is significant for two main reasons: (1) the most likely route of ricin intoxication would be via aerosolized liquid or powder, or contamination of food or water sources; (2) most pathogens and toxins enter the body through mucosal surfaces making it important to find novel ways to improve mucosal protection. Hence, research has focused on novel adjuvants and administration routes to improve mucosal protection. In our studies we have observed that a protein vaccine, such as RiVax, administered with alum via the ID route protects both gut and respiratory mucosa as well as or better than an equivalent dose administered by the IM route. While IM administration of RiVax on alum was superior to RiVax alone, the improvement observed when the vaccine on alum was administered via the ID route was even greater. This is not surprising for several reasons: (1) ID administration of RiVax on alum induced significantly higher antibody titers; (2) past studies in humans comparing ID vs. IM administration of vaccines on alum have shown that the ID route is 5-fold more effective at inducing antigen specific antibodies [27, 29]; (3) recent findings have demonstrated that alum enhances both activation and antigen presentation by DCs [26]. Since DCs initiate adaptive immune responses to vaccines, the large numbers of such cells in the skin would suggest that ID administration of a vaccine would be advantageous.
In our study, we investigated three doses levels of vaccine over a two log range. This has given us enough evidence to suggest that ID is superior to IM vaccination at lower dose levels. Due to the wide range of doses that we tested, we were unable to identify the minimum dose that could be administered ID in order to achieve protection which was equivalent to that of IM administration. However, this will be an important avenue of research for future studies. Prior studies comparing seroconversion following ID vs. IM administration of adjuvented protein vaccines have revealed that approximately a 20% dose given ID is equivalent to a 100% dose given IM [27, 29, 18]. Therefore, we were not surprised that our low ID dose levels did not confer protection that was equal to our middle and high IM dose levels since each dose level was one log different from the next.
As compared to the IM route, administration of RiVax with alum via the ID route was also superior in minimizing lung damage following exposure to aerosolized ricin. While immunization with RiVax protected mice from ricin-mediated death, it was also important to determine whether it prevented organ damage since this could result in significant downstream morbidity and mortality. Mucosal protection is mediated primarily by IgG and sIgA. sIgA is produced locally and most of the IgG is exudated into the mucosa by diffusion [32]. Local IgA works primarily by preventing a toxin or pathogen from breaching the epithelium. IgG can neutralize and opsonize toxins in mucosal sites as well [33]. Although we did not study antibody isotypes at mucosal sites in this study, this will be the subject of future experiments.
Previous in vitro studies have demonstrated that subsets of dermal DCs induce humoral immune responses including the production of IgG and IgA [34]. It has also been shown that transcutaneous vaccination stimulates antibody production at mucosal surfaces [35]. Taken together with the current findings that ID vaccination is more effective at maintaining normal lung function following challenge with aerosolized ricin it can be concluded that ID vaccination may be superior at providing mucosal protection against toxin exposure and perhaps pathogen exposure as well.
Lastly, we found that levels of RiVax-specific antibodies in the serum correlated with survival. Serum antibody levels have long been accepted as an indicator of vaccine efficacy especially in human studies where challenges with toxins or pathogens are not possible. In our animal models of ricin toxicity, we were able to clearly show a correlation between specific antibody levels in the serum and survival. In comparing the antibody levels in ID and IM vaccinated mice, we observed that regardless of the route of vaccine administration, the level of specific antibodies correlated with survival. This will be important in evaluating future studies of RiVax in primates and humans.
There have been many attempts to develop a prophylactic ricin vaccine, using different preparations of the ricin holotoxin with or without various adjuvants [8–10]. But none of these has been as extensively studied as RiVax and none have looked at the ID vaccination route. Since it is likely that a ricin vaccine would be most useful for military personnel, the ease of ID vaccination with lower doses of vaccine is important.
Clearly these studies must be extended to non-human primates and eventually humans, but it is likely that similar observations will be made. This, combined with the fact that RiVax is stable for at least a year as a lyophilized formulation [18], indicates that we have now improved the conditions for storage and utilization of this vaccine.
Acknowledgments
Supported by NIH grant AI-056372 and NIH Grant 5T32GM008203
We would like to thank Ms. Phyllis Barron for expert assistance with the mice; Ms. Theresa Vo for carrying out RIAs and Ms. Kelly Mapes and Dr. John Gu for providing reagents and advice. We thank Ms. Jue Yang for assistance with the aerosol challenge model; and Ms. Linda Berry for administrative assistance. We thank Drs. John Schindler and Xiaoyun Liu and Pavitra Chakravarty for helpful comments concerning the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994;8(2):201–8. [PubMed] [Google Scholar]
- 2.Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005;294(18):2342–51. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 3.Foxwell BM, Detre SI, Donovan TA, Thorpe PE. The use of anti-ricin antibodies to protect mice intoxicated with ricin. Toxicology. 1985;34(1):79–88. doi: 10.1016/0300-483x(85)90080-0. [DOI] [PubMed] [Google Scholar]
- 4.Lemley PV, Wright DC. Mice are actively immunized after passive monoclonal antibody prophylaxis and ricin toxin challenge. Immunology. 1992;76(3):511–3. [PMC free article] [PubMed] [Google Scholar]
- 5.Roche JK, Stone MK, Gross LK, Lindner M, Seaner R, Pincus SH, et al. Post-exposure targeting of specific epitopes on ricin toxin abrogates toxin-induced hypoglycemia, hepatic injury, and lethality in a mouse model. Lab Invest. 2008;88(11):1178–91. doi: 10.1038/labinvest.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller DJ, Ravikumar K, Shen H, Suh JK, Kerwin SM, Robertus JD. Structure-based design and characterization of novel platforms for ricin and shiga toxin inhibition. J Med Chem. 2002;45(1):90–8. doi: 10.1021/jm010186s. [DOI] [PubMed] [Google Scholar]
- 7.Mabley JG, Pacher P, Szabo C. Activation of the cholinergic antiinflammatory pathway reduces ricin-induced mortality and organ failure in mice. Mol Med. 2009;15(5–6):166–72. doi: 10.2119/molmed.2008.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths GD, Phillips GJ, Holley J. Inhalation toxicology of ricin preparations: animal models, prophylactic and therapeutic approaches to protection. Inhal Toxicol. 2007;19(10):873–87. doi: 10.1080/08958370701432124. [DOI] [PubMed] [Google Scholar]
- 9.Kende M, Del Giudice G, Rivera N, Hewetson J. Enhancement of intranasal vaccination in mice with deglycosylated chain A ricin by LTR72, a novel mucosal adjuvant. Vaccine. 2006;24(12):2213–21. doi: 10.1016/j.vaccine.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Marsden CJ, Knight S, Smith DC, Day PJ, Roberts LM, Phillips GJ, et al. Insertional mutagenesis of ricin A chain: a novel route to an anti-ricin vaccine. Vaccine. 2004;22(21–22):2800–5. doi: 10.1016/j.vaccine.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Olson MA, Carra JH, Roxas-Duncan V, Wannemacher RW, Smith LA, Millard CB. Finding a new vaccine in the ricin protein fold. Protein Eng Des Sel. 2004;17(4):391–7. doi: 10.1093/protein/gzh043. [DOI] [PubMed] [Google Scholar]
- 12.Baluna R, Rizo J, Gordon BE, Ghetie V, Vitetta ES. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci U S A. 1999;96(7):3957–62. doi: 10.1073/pnas.96.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smallshaw JE, Firan A, Fulmer JR, Ruback SL, Ghetie V, Vitetta ES. A novel recombinant vaccine which protects mice against ricin intoxication. Vaccine. 2002;20(27–28):3422–7. doi: 10.1016/s0264-410x(02)00312-2. [DOI] [PubMed] [Google Scholar]
- 14.Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine. 2005;23(39):4775–84. doi: 10.1016/j.vaccine.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Legler PM, Brey RN, Vitteta ES, Millard CB. Chain A, Structure Of Rivax: A Human Ricin Vaccine. Protein Database: Molecule 3BJG_A. 2007 [Google Scholar]
- 16.Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007;25(42):7459–69. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, et al. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A. 2006;103(7):2268–73. doi: 10.1073/pnas.0510893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smallshaw JE, Vitetta ES. A lyophilized formulation of RiVax, a recombinant ricin subunit vaccine, retains immunogenicity. Vaccine. 2010;28:2428–2435. doi: 10.1016/j.vaccine.2009.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker RI. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12(5):387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 20.Giudice EL, Campbell JD. Needle-free vaccine delivery. Adv Drug Deliv Rev. 2006;58(1):68–89. doi: 10.1016/j.addr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Patel SM, Atmar RL, Sahly HE, Cate TR, Keitel WA. A phase I evaluation of inactivated influenza A/H5N1 vaccine administered by the intradermal or the intramuscular route. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frosner G, Steffen R, Herzog C. Virosomal hepatitis a vaccine: comparing intradermal and subcutaneous with intramuscular administration. J Travel Med. 2009;16(6):413–9. doi: 10.1111/j.1708-8305.2009.00351.x. [DOI] [PubMed] [Google Scholar]
- 23.Wahl M, Hermodsson S. Intradermal, subcutaneous or intramuscular administration of hepatitis B vaccine: side effects and antibody response. Scand J Infect Dis. 1987;19(6):617–21. doi: 10.3109/00365548709117195. [DOI] [PubMed] [Google Scholar]
- 24.Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27(52):7304–12. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Propst T, Propst A, Lhotta K, Vogel W, Konig P. Reinforced intradermal hepatitis B vaccination in hemodialysis patients is superior in antibody response to intramuscular or subcutaneous vaccination. Am J Kidney Dis. 1998;32(6):1041–5. doi: 10.1016/s0272-6386(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 26.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21(1):23–9. doi: 10.1016/j.coi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Chiu SS, Peiris JS, Chan KH, Wong WH, Lau YL. Immunogenicity and safety of intradermal influenza immunization at a reduced dose in healthy children. Pediatrics. 2007;119(6):1076–82. doi: 10.1542/peds.2006-3176. [DOI] [PubMed] [Google Scholar]
- 28.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351(22):2286–94. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 29.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351(22):2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 30.Hauge S, Madhun A, Cox RJ, Haaheim LR. Quality and kinetics of the antibody response in mice after three different low-dose influenza virus vaccination strategies. Clin Vaccine Immunol. 2007;14(8):978–83. doi: 10.1128/CVI.00033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baylor NW, Egan W, Richman P. Aluminum salts in vaccines--US perspective. Vaccine. 2002;20(Suppl 3):S18–23. doi: 10.1016/s0264-410x(02)00166-4. [DOI] [PubMed] [Google Scholar]
- 32.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25(30):5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Janeway . Immunobiology: The Immune System in Health and Disease. 6. Garland Science; 2005. [Google Scholar]
- 34.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang SY, Cha HR, Igarashi O, Rennert PD, Kissenpfennig A, Malissen B, et al. Cutting edge: Langerin+ dendritic cells in the mesenteric lymph node set the stage for skin and gut immune system cross-talk. J Immunol. 2008;180(7):4361–5. doi: 10.4049/jimmunol.180.7.4361. [DOI] [PubMed] [Google Scholar]