Abstract
Background
This study was performed to evaluate the effectiveness of intravenous low dose ketamine for reducing the incidence and severity of postoperative sore throat (POST).
Methods
This was a prospective, randomized, double-blind clinical trial. The study population consisted of 70 patients between 20 and 70 years old who were classified as American Society of Anesthesiologists I-II and were scheduled for elective laparoscopic cholecystectomy. The patients were divided randomly into two groups. Patients in the ketamine group received an intravenous injection of 0.5 mg/kg of ketamine just before induction, followed by 10 µg/kg/min throughout the operation. Patients in the control group received intravenous saline instead of ketamine. The patients were interviewed 1, 6, and 24 h after the operation. The incidence and severity of POST were recorded.
Results
No significant differences in the incidence and severity of POST during the 24 h after the operation were found between the two groups (21/31 in the ketamine group vs. 26/34 in the control group, P = 0.398).
Conclusions
Intravenous injection of low dose ketamine was not effective for reducing POST.
Keywords: Complications, Intubation, Ketamine
Introduction
Protection of sensory neurons against central sensitization may offer relief from pain occurring after injury or surgery [1]. Based on this theory, preemptive analgesia has been advocated as an effective tool to manage postoperative pain [2,3]. The mechanisms involved in preemptive analgesia may include intercepting nociceptive input, increasing the threshold for nociception, and blocking N-methyl-D-aspartate (NMDA) receptor activation. Therefore, NMDA receptor antagonists may improve preemptive analgesia. Ketamine is the most potent of all NMDA antagonists currently available for use in humans [4]. It binds noncompetitively to the NMDA receptors and inhibits the NMDA receptor-mediated central sensitization, potentially reducing postoperative pain [5,6]. Although the role of ketamine in the treatment of postoperative pain remains controversial [7,8], optimal regimens of ketamine should manage acute pain while minimizing the adverse effects. Recent trials have demonstrated that, when added as an adjunct to general anesthesia, intravenous sub-anesthetic low dose ketamine reduces postoperative pain and opioid requirements without adverse effects [9-13].
Many patients complain of postoperative sore throat (POST) after tracheal intubation. Although POST is not a major complication and is self-limiting, it causes a great deal of patient dissatisfaction. Therefore, it would be useful if the intravenous ketamine as an analgesic adjunct to general anesthesia could also reduce POST. In this study, we evaluated the effect of intravenous low dose ketamine on POST.
Materials and Methods
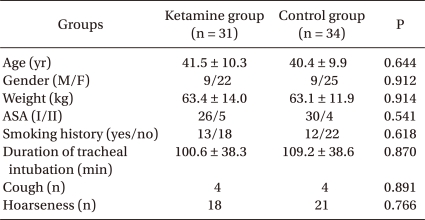
This was a prospective, randomized, double-blind clinical trial, and it was approved by the institutional review board (SCHBC_IRB_08). After obtaining written informed consent, patients scheduled for an elective laparoscopic cholecystectomy were enrolled between April and July, 2008. Patients were between 20 and 70 years of age and had American Society of Anesthesiologists (ASA) physical status I-II. Both groups were similar with respect to age, gender, weight, ASA status, smoking history, and duration of tracheal intubation (Table 1).
Table 1.
Patient Characteristics
Data are given as the mean ± SD or absolute numbers. ASA: American Society of Anesthesiologists.
Exclusion criteria included a history of recent respiratory tract infection or sore throat and preoperative use of analgesics such as non-steroidal anti-inflammatory drugs or opioids. Patients who required more than one attempt for tracheal intubation, had a nasogastric tube, or had a duration of tracheal intubation of <60 min or >300 min were eliminated from the analysis.
All patients were premedicated with an intramuscular injection of 0.2 mg glycopyrrolate 30 min before their arrival in the operating room. Monitoring in the operating room consisted of three-lead electrocardiography, noninvasive arterial blood pressure, pulse oximetry, and end-tidal CO2. Before surgery, the patients were divided randomly into two groups using a computer-generated random number table and the sealed envelope method. Patients in the ketamine group were injected with 0.5 mg/kg ketamine (5 mg/ml) just before induction, followed by an infusion of 10 µg/kg/min throughout the operation. Patients in the control group received the same volume of saline. The study drug was prepared in a syringe labeled with the patient number, but without a drug name, by one nurse who knew the group allocation. All anesthetic procedures were performed by experienced anesthesiologists who were blinded to the group allocation. The investigators who collected data and interviewed patients did not perform any of the procedures, and they were blinded to the group allocation. All patients were blinded to the group allocation.
Induction was accomplished with 100 µg fentanyl and propofol (2 mg/kg), followed by rocuronium (0.6 mg/kg). A direct laryngoscopy with a Macintosh #3 blade and tracheal intubation were performed 2 min after the rocuronium injection. The trachea was intubated with a high volume/low pressure cuff endotracheal tube (Euromedical; Kedah, Malaysia) with an internal diameter of 8.0 or 7.0 mm for male or female patients, respectively. Immediately after intubation, the tracheal tube cuff was inflated with room air until no air leakage could be heard at a peak airway pressure of 20 cmH2O. Then, the cuff pressure was adjusted to 10-20 cmH2O using a handheld pressure gauge (Portex Cuff Inflator/Pressure Gauge, SIMS Portex, Hythe, Kent, UK). No humidifier or heat and moisture exchangers were used in either group. Anesthesia was maintained with 50% O2 in air, 7-8 vol% desflurane, and rocuronium. The end-tidal CO2 was maintained at 35-40 mmHg.
At the end of surgery, the patients were given 10 mg pyridostigmine and 0.2 mg glycopyrrolate intravenously, and the lungs were ventilated with 100% O2 until the patient was fully awake and had recovered from the muscle relaxation. After gently aspirating the oral secretions, the cuff was deflated fully, and the tracheal tube was removed. Ketamine infusion was discontinued after extubation, and the duration of the tracheal intubation was recorded.
A single dose of intravenous ketorolac 30 mg was given to all the patients in the postanesthesia care unit, and additional fentanyl was administered according to the degree of postoperative complaints about wound pains. No other analgesics were used. The total dose of fentanyl administered during induction and within 24 hours after the operation was recorded.
The incidences of POST, cough, and hoarseness were measured using direct questions at 1, 6, and 24 hours after the operation. The visual analog scale (VAS) scores of POST and wound pain were also recorded at the same times.
Statistical analyses were performed using SPSS (ver. 14.0, SPSS Inc., Chicago, IL, USA). The unpaired t-test was used for comparisons between groups in age, weight, duration of tracheal intubation, VAS scores of POST and wound pain, and the total dose of fentanyl administered during induction and 24 hours after the operation. Between-group differences in gender, ASA status, smoking history, and the incidence of POST, cough, and hoarseness 24 hours after the operation were analyzed using the chi-square and Fisher's exact tests, as appropriate. The results are expressed as the mean ± SD or absolute number. P < 0.05 was considered significant.
The primary outcome variable was the incidence of POST during the 24 hours after the operation. According to a previous study, we presumed the incidence of POST to be 65% [14]. The sample size was estimated from the assumption that a 50% reduction in the incidence of POST would be clinically relevant. Power analysis suggested that a minimum of 31 patients in each group would be needed for β = 0.20 and α = 0.05. To compensate for potential dropouts, 70 patients were enrolled.
Results
Of the 70 patients enrolled, two patients required more than one attempt for intubation, and the duration of intubation was <60 min in one patient and >300 min in two patients, so they were excluded from the analysis. Of the remaining 65 patients, 31 were in the ketamine group and 34 in the control group.
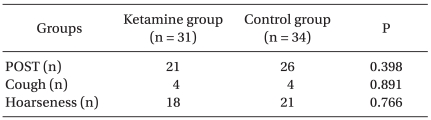
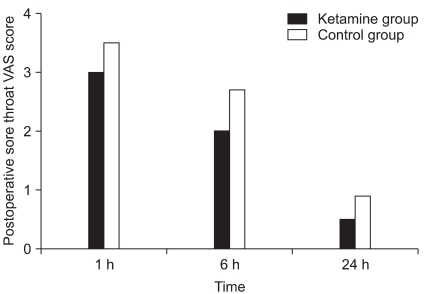
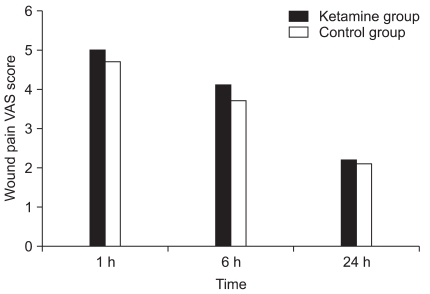
During the 24 hours after the operation, no significant difference in the incidence of POST or the incidence of cough and hoarseness was observed between the two groups (Table 2). The VAS scores of POST and wound pain were not significantly different between the two groups (Fig. 1, 2). The total doses of fentanyl administered during induction and for 24 hours after the operation were 32.3 ± 47.4 µg in the ketamine group and 40.3 ± 43.6 µg in the control group (P = 0.483).
Table 2.
The Incidence of POST, Cough, and Hoarseness
Data are given as the absolute numbers. No significant differences are found between the groups. POST: postoperative sore throat.
Fig. 1.
The visual analog scale (VAS) scores of postoperative sore throat. No significant differences are found between the groups.
Fig. 2.
The visual analog scale (VAS) scores of wound pain. No significant differences are found between the groups.
Discussion
We infused low dose ketamine intravenously throughout the period of endotracheal intubation under the hypothesis that preemptive ketamine would reduce POST. However, intravenous low dose ketamine had no effect on the incidence and severity of POST.
Ketamine is in the middle of the affinity range of uncompetitive NMDA antagonists. Preemptive administration of ketamine before and during surgery could block the development of central sensitization in the postoperative period and thus maximize their analgesic efficacy [1]. A number of controlled studies have demonstrated the benefit of this approach [10,11,15]. In the study of Menigaux et al. [9], intravenous injection of small dose ketamine after the induction of anesthesia improved postoperative analgesia. Kwok et al. [10] concluded that small preoperative dose of ketamine produced preemptive analgesia. Argiriadou et al. [13] presented that preincisional and repeated intraoperative small-dose S(+)-ketamine improves postoperative pain relief. We expected that the preemptive effect of ketamine could also reduce POST.
Nociceptive and inflammatory signals are generated throughout surgery and after the procedure. Therefore, a single administration of a short-acting drug such as ketamine either before or after a procedure will not provide analgesia that lasts far into the postoperative period. To prevent pathologic pain, ketamine needs to be applied at least throughout the operation [16], and onset can be delayed if a loading dose is not administered [17]. Therefore, we infused ketamine continuously throughout the period of endotracheal intubation, with a preinduction bolus.
When ketamine is administered systemically, it may cause adverse cardiovascular or respiratory effects and sedation, dreams, hallucinations, and other psychomimetic adverse reactions. Therefore, we used low-dose ketamine, which does not cause respiratory depression [7] and only minimal changes in heart rate and blood pressure [17]. A sub-anesthetic dose of ketamine did not increase the incidence of postoperative adverse psychic effects, sedation, or nausea and vomiting [9,10,18]. Schmid et al. [13] defined low dose ketamine as a bolus dose less than 1 mg/kg and continuous administration at a rate of ≤ 20 µg/kg/min.
In a long-term outcome trial on adenocarcinoma surgery with general or epidural anesthesia, ketamine injected as a 0.5 mg/kg pre-incisional bolus followed by an intraoperative infusion of 250 µg/kg/h reduced postoperative morphine needs and the incidence of residual pain [9]. Kararmaz et al. [12] demonstrated that intravenous ketamine had an improved analgesic or opioid-sparing effect when it was combined with epidural bupivacaine and morphine after an intraoperative ketamine infusion of 500 µg/kg/h preceded by a pre-incisional 0.5 mg/kg bolus. Fu et al. [19] reported preemptive ketamine decreases postoperative opioid requirement when given 0.5 mg/kg before surgical incision followed by an infusion of 10 µg/kg/min. Based on these results, we injected ketamine as a 0.5 mg/kg preinduction bolus followed by a 10 µg/kg/min intraoperative infusion. This dosing schedule is similar to or greater than that used in previous studies and the recommended dose for painful procedures by Himmelseher et al. [16] (0.5 mg/kg preincisional bolus followed by an intraoperative infusion of 500 µg/kg/hr). We expected that this dose of ketamine might affect POST, but it did not. We did not observe an effect on POST and did not detect a difference in postoperative pain VAS or the doses of fentanyl administered. Therefore, it may be an insufficient dose or a third dose range in which ketamine has no analgesic potency on its own [17,20,21]; this is a possible explanation for our negative result.
Canbay et al. [14] found that a ketamine gargle (40 mg ketamine in saline 30 ml; gargled for 30 s 5 min before induction) reduced the incidence and severity of POST in patients undergoing septorhinoplasty under general anesthesia with endotracheal intubation, potentially because of local anti-inflammatory and anti-hyperalgesic effect of ketamine. However, because Canbay et al. did not measure plasma ketamine levels, they could not rule out a systemic effect of ketamine. Our results suggest that topical application, and not systemic ketamine, may influence POST, reduce local inflammation, and mediate the peripheral anti-nociceptive effect.
The incidence of POST in our study was higher than expected. Women tend to have a higher incidence of postoperative sore throat than men [22,23], and our study population included more women then men, which might cause the high incidence of POST. The interview method could also affect the results, as direct questioning can increase the perceived incidence of sore throat [22,24].
In conclusion, low dose ketamine did not affect POST as a 0.5 mg/kg preinduction bolus followed by an intraoperative infusion of 10 µg/kg/min. However, we did not compare the effects of various dose regimens. Therefore, we cannot reach a firm conclusion about the effect of low-dose ketamine on POST.
References
- 1.Wall PD. The prevention of postoperative pain. Pain. 1988;33:289–290. doi: 10.1016/0304-3959(88)90286-2. [DOI] [PubMed] [Google Scholar]
- 2.Katz J, Kavanagh BP, Sandler AN, Nierenberg H, Boylan JF, Friedlander M, et al. Preemptive analgesia. clinical evidence of neuroplasticity contributing to postoperative pain. Anesthesiology. 1992;77:439–446. doi: 10.1097/00000542-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ, Chong MS. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 4.Chizh BA. Low dose ketamine: a therapeutic and research tool to explore N-methyl-D-aspartate (NMDA) receptor-mediated plasticity in pain pathways. J Psychopharmacol. 2007;21:259–271. doi: 10.1177/0269881105062484. [DOI] [PubMed] [Google Scholar]
- 5.Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- 6.Orser BA, Pennefather PS, MacDonald JF. Multiple mechanisms of ketamine blockade of N-methyl-D-spartate receptors. Anesthesiology. 1997;86:903–917. doi: 10.1097/00000542-199704000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Edwards ND, Fletcher A, Cole JR, Peacock JE. Combined infusions of morphine and ketamine for postoperative pain in elderly patients. Anaesthesia. 1993;48:124–127. doi: 10.1111/j.1365-2044.1993.tb06849.x. [DOI] [PubMed] [Google Scholar]
- 8.Jaksch W, Lang S, Reichhalter R, Raab G, Dann K, Fitzal S. Perioperative small-dose S(+)-ketamine has no incremental beneficial effects on postoperative pain when standard-practice opioid infusions are used. Anesth Analg. 2002;94:981–986. doi: 10.1097/00000539-200204000-00038. [DOI] [PubMed] [Google Scholar]
- 9.Menigaux C, Guignard B, Fletcher D, Sessler DI, Dupont X, Chauvin M. Intraoperative small-dose ketamine enhances analgesia after outpatient knee arthroscopy. Anesth Analg. 2001;93:606–612. doi: 10.1097/00000539-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Kwok RF, Lim J, Chan MT, Gin T, Chiu WK. Preoperative ketamine improves postoperative analgesia after gynecologic laparoscopic surgery. Anesth Analg. 2004;98:1044–1049. doi: 10.1213/01.ANE.0000105911.66089.59. [DOI] [PubMed] [Google Scholar]
- 11.De Kock M, Lavand'homme P, Waterloos H. 'Balanced analgesia' in the perioperative period: is there a place for ketamine? Pain. 2001;92:373–380. doi: 10.1016/S0304-3959(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 12.Kararmaz A, Kaya S, Karaman H, Turhanoglu S, Ozyilmaz MA. Intraoperative intravenous ketamine in combination with epidural analgesia: postoperative analgesia after renal surgery. Anesth Analg. 2003;97:1092–1096. doi: 10.1213/01.ANE.0000080205.24285.36. [DOI] [PubMed] [Google Scholar]
- 13.Argiriadou H, Himmelseher S, Papagiannopoulou P, Georgiou M, Kanakoudis F, Giala M, et al. Improvement of pain treatment after major abdominal surgery by intravenous S+-ketamine. Anesth Analg. 2004;98:1413–1418. doi: 10.1213/01.ane.0000111204.31815.2d. [DOI] [PubMed] [Google Scholar]
- 14.Canbay O, Celebi N, Sahin A, Celiker V, Ozgen S, Aypar U. Ketamine gargle for attenuating postoperative sore throat. Br J Anaesth. 2008;100:490–493. doi: 10.1093/bja/aen023. [DOI] [PubMed] [Google Scholar]
- 15.Aida S, Yamakura T, Baba H, Taga K, Fukuda S, Shimoji K. Preemptive analgesia by intravenous low-dose ketamine and epidural morphine in gastrectomy: a randomized double-blind study. Anesthesiology. 2000;92:1624–1630. doi: 10.1097/00000542-200006000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102:211–220. doi: 10.1097/00000542-200501000-00030. [DOI] [PubMed] [Google Scholar]
- 17.Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82:111–125. doi: 10.1016/S0304-3959(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 18.Dahl V, Ernoe PE, Steen T, Raeder JC, White PF. Does ketamine have preemptive effects in women undergoing abdominal hysterectomy procedures? Anesth Analg. 2000;90:1419–1422. doi: 10.1097/00000539-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 19.Fu ES, Miguel R, Scharf JE. Preemptive ketamine decreases postoperative narcotic requirements in patients undergoing abdominal surgery. Anesth Analg. 1997;84:1086–1090. doi: 10.1097/00000539-199705000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Chapman V, Dickenson AH. The combination of NMDA antagonism and morphine produces profound antinociception in the rat dorsal horn. Brain Res. 1992;573:321–323. doi: 10.1016/0006-8993(92)90780-d. [DOI] [PubMed] [Google Scholar]
- 21.Dickenson AH. Combination therapy in analgesia; seeking synergy. Curr Opin Anaesthesiol. 1993;6:861–865. [Google Scholar]
- 22.Kim WJ, Oh HK. A Clinical study of sore throat after endotracheal intubation. Korean J Anestheisol. 1977;10:41–45. [Google Scholar]
- 23.Maruyama K, Sakai H, Miyazawa H, Toda N, Iinuma Y, Mochizuki N, et al. Sore throat and hoarseness after total intravenous anaesthesia. Br J Anaesth. 2004;92:541–543. doi: 10.1093/bja/aeh098. [DOI] [PubMed] [Google Scholar]
- 24.Harding CJ, McVey FK. Interview method affects incidence of postoperative sore throat. Anaesthesia. 1987;42:1104–1107. doi: 10.1111/j.1365-2044.1987.tb05179.x. [DOI] [PubMed] [Google Scholar]