Abstract
There is growing evidence that positive affect may influence health and immune function, although few studies have examined links between positive affect and immune processes in clinical populations. The purpose of this study was to examine whether positive affect is associated with changes in proinflammatory cytokines in cancer patients undergoing radiation treatment. Subjects were 50 individuals with early-stage breast and prostate cancer who completed psychosocial questionnaires and provided blood samples at seven time points before, during, and after radiation treatment. Positive affect was assessed before treatment onset using the CES-D (Center for Epidemiological Studies Depression Scale). Blood samples were assayed for serum levels of proinflammatory cytokines IL-1β and IL-6. Patients with higher levels of positive affect before treatment exhibited higher mean levels of IL-1β and IL-6 during radiation treatment (all ps < .05). Results suggest that positive affect enhances the acute inflammatory response to radiation treatment, perhaps facilitating tissue repair processes.
Keywords: Positive affect, Cytokines, Interleukin-6, Interleukin-1β, Breast cancer, Prostate cancer, Radiation treatment
1. Introduction
There is compelling evidence that emotions may influence immune system function and thus susceptibility to and severity of immune-related diseases (Glaser and Kiecolt-Glaser, 2005). This body of research has traditionally focused on negative affective states–such as depression, anxiety, and anger (Kiecolt-Glaser et al., 2002; Raison et al., 2006). However, there is growing interest in how positive psychological factors–such as positive affect, optimism, and benefit finding–affect health (Pressman and Cohen, 2005; Bower et al., 2008b) and the immunological pathways through which they exert their effects (Marsland et al., 2007).
To date, positive affect is the most commonly studied positive psychological factor relating to health and immune outcomes. For example, research has shown that positive affect predicts lower risk of HIV-related mortality (Moskowitz, 2003), enhanced antibody response to Hepatitis B vaccination (Marsland et al., 2006), decreased susceptibility to experimentally-exposed rhinovirus/influenza A virus (Cohen et al., 2006), fewer objective and subjective signs of illness following viral exposure (Doyle et al., 2006), and faster skin wound healing (Robles et al., 2009). In the context of viral challenge, higher levels of positive affect are associated with lower levels of nasal proinflammatory cytokines, which appear to mediate effects on illness symptoms (Doyle et al., 2006; Janicki-Deverts et al., 2007). Effects of positive affect on these outcomes appear to be independent of, and in some cases, stronger than effects of negative affect (Cohen et al., 2006; Janicki-Deverts et al., 2007; Prather et al., 2007; Robles et al., 2009).
Naturalistic studies have shown more equivocal associations between positive affect and inflammatory markers. One such study found no association between positive affect and the soluble IL-6 receptor (sIL-6r) in healthy older women, but did find a positive association between eudemonic well-being (i.e. purpose in life) and sIL-6r (Ryff et al., 2004). A large longitudinal study of 2873 healthy adults found that positive affect was associated with lower circulating levels of C-reactive protein (CRP) and IL-6 for women, but not for men (Steptoe et al., 2008). Another study also found a negative association between positive affect and stimulated production of IL-6 and IL-10, but not IL-1β or TNF-α (Prather et al., 2007).
To date, studies of positive affect and inflammation have primarily focused on healthy populations. However, inflammatory processes may have particular relevance in the context of cancer, as inflammation is increasingly recognized as a contributor to cancer development and progression (Coussens and Werb, 2002; Shacter and Weitzman, 2002) and may also play a role in cancer-related symptoms such as fatigue (Bower, 2008). In women with advanced ovarian cancer, positive psychological factors such as social support have been linked to lower levels of circulating IL-6 and VEGF, a cytokine involved with tumor angiogenesis (Costanzo et al., 2004; Lutgendorf et al., 2002). In contrast, depression is associated with elevations in proinflammatory cytokines in cancer populations (Jehn et al., 2006; Lutgendorf et al., 2008; Musselman et al., 2001).
To our knowledge, positive affect has not been examined in relation to inflammatory cytokines in cancer patients. We choose to examine this relationship among breast and prostate cancer patients undergoing radiation therapy. Radiation is a mainstay of cancer treatment, and works by interfering with tumor growth and metastasis by damaging the DNA of malignant cancer cells. Radiation therapy activates proinflammatory cytokine production as part of a coordinated response designed to control damage and promote tissue repair (Petrini et al., 1992; Barcellos-Hoff, 1998; Stone et al., 2003; Okunieff et al., 2008). To the extent that proinflammatory cytokines facilitate tissue recovery, increases in cytokine concentrations during treatment should have beneficial effects for patients undergoing radiation therapy. Indeed, acute wound healing studies have found that higher levels of IL-1β and IL-6 at the wound site are associated with faster wound healing (Kiecolt-Glaser et al., 2005). However, radiation-induced elevations in proinflammatory cytokines may also have detrimental effects. For example, elevations in circulating levels of IL-6 predicted the development of radiation pneumonitis in lung cancer patients and acute proctitis in prostate cancer patients (Arpin et al., 2005; Hartsell et al., 2007; Christiansen et al., 2007). Cytokine activation might be particularly problematic if it persists beyond treatment completion, suggesting more chronic inflammation.
The current study was designed to test the association between positive affect and inflammation among breast and prostate cancer patients undergoing radiation treatment. Primary analyses focused on two key proinflammatory cytokines–IL-1β and IL-6–that are known to be elevated during radiation therapy and have previously been associated with positive affective processes (Ryff et al., 2004; Prather et al., 2007; Steptoe et al., 2008). To investigate the impact of positive affect on inflammation, we examined whether individuals who reported higher levels of positive affect prior to treatment onset showed a differential cytokine response to treatment. To clarify the clinical significance of this response, we examined treatment-related side effects and followed patients after treatment completion.
2. Methods
2.1. Participants
Participants were breast and prostate cancer patients scheduled to undergo external beam radiation treatment at UCLA. They were recruited from the UCLA Radiation Oncology Clinic between January 2001 and September 2003. Eligibility criteria for participation in this study were as follows: (1) age 25–75; (2) newly diagnosed with localized breast cancer (stage 0–II) or prostate cancer (T1–T3, N0 and M0); (3) external beam radiation therapy as part of the primary treatment plan; (4) completion of definitive primary surgery (for breast cancer patients); and (5) ability to read and write English. Exclusion criteria included: (1) recurrent cancer; (2) prior or planned treatment with chemotherapy; and (3) regular use of immunosuppressive medication or tobacco.
A total of 107 patients were screened for study eligibility. Forty-one patients were not eligible due to medical conditions (e.g., previous cancer treatment) or use of tobacco, and 15 were eligible but refused participation due to concerns about blood draws, time demands, or general lack of interest. A total of 51 patients were enrolled in the study and completed the baseline questionnaire. One prostate cancer patient withdrew immediately after treatment onset due to concerns about blood draws and was not included in analyses. The final sample included 50 patients (n = 28 breast cancer patients, n = 22 prostate cancer patients). The UCLA Institutional Review Board approved the study procedures and written informed consent was obtained from all participants.
2.2. Procedures
Potential participants were screened for eligibility during consultations at the UCLA Radiation Oncology Clinic. After determination of eligibility, subjects completed a baseline assessment prior to starting treatment. Patients with localized breast and prostate cancer typically receive daily radiation therapy, Monday through Friday, for a 6–8 week course of treatment. Study assessments occurred prior to treatment (Baseline), after 5 days of treatment (Treatment week 1), after 10 days of treatment (Treatment week 2), after 20 days of treatment (Treatment week 4), during the final week of treatment (Treatment week 6/8), and at two regularly scheduled follow-up visits targeted at 2 weeks and 2 months after treatment completion. Assessments were scheduled to coincide with treatment appointments and thus did not occur at the same time of day for all participants. However, appointments for individual participants typically did occur at the same time of day (e.g., some participants were routinely seen at 9AM, while others were routinely seen at 10AM). The majority of appointments were conducted in the morning (before noon), and all were completed by 3PM. Subjects completed self-report questionnaires and provided blood samples for immune analysis at each assessment. As part of the questionnaire, subjects indicated if they had experienced an illness, infection, or injury in the past week. If so, blood samples were not collected at that assessment to avoid confounding effects on cytokine levels.
2.3. Measures
Demographic and health information was collected at baseline, and presence of acute illnesses or infections was determined at each assessment by self-report questionnaire. Cancer and treatment-related information (e.g., cancer stage) was determined from chart reviews.
2.3.1. Positive affect
Positive affect was assessed at each assessment using the CES-D (Center for Epidemiological Studies Depression Scale), a 20-item measure with excellent reliability and validity (Radloff, 1977). This questionnaire assesses affect and symptoms experienced during the past week, and responses are on a scale from 0 = “rarely or none of the time” to 3 = “most or all of the time.” Previous factor analyses have indicated a four-factor structure to the CES-D that includes subscales for positive affect, negative affect, somatic, and interpersonal symptoms (Sheehan et al., 1995; Knight et al., 1997; Moskowitz, 2003; Bower et al., 2005). The positive affect subscale includes four items: “I felt hopeful about the future,” “I felt I was just as good as other people,” “I was happy,” “I enjoyed life.”
Each patient’s positive affect score was computed solely from their baseline (pre-treatment) time point in order to minimize any confounds with direction of causality during radiation treatment (i.e. whether positive affect influences inflammation or vice-versa). Baseline positive affect was significantly correlated with positive affect at all subsequent time points (r’s = .908–.961, all p’s < .01). The single measures interclass correlation coefficient also indicated high reliability of positive affect over time (ICC = .882, p < .001). These results provide justification that the baseline measure represents “trait” positive affect, rather than a “state” measure of how patients were feeling at the time.
Breast cancer patients had a mean baseline positive affect of 2.69 (range: 1.5–3.0), and prostate cancer patients had a mean baseline positive affect of 2.69 (range: 1.25–3.0). The distribution of positive affect was positively skewed, indicating that the majority of patients experienced positive affect “most of the time.”
2.3.2. Treatment-related side effects
At each assessment, patients completed a symptom checklist to evaluate the presence of treatment-related symptoms. We focused here on reddening or irritation of skin in the treatment area, a common side effect of radiation exposure. Patients rated the extent to which they had experienced this symptom on a scale from 0 = “not at all” to 4 = “extremely.”
2.3.3. Proinflammatory cytokines
Serum samples were separated according to standard procedures and stored at −70 °C for subsequent batch testing. Analyses focused on serum concentrations of the proinflammatory cytokines IL-1β and IL-6, which were measured using Quantikine High Sensitivity Immunoassay kits (R&D Systems, Minneapolis, MN). The measurement of cytokine levels was performed according to the manufacturer’s instructions. Quality control procedures for our laboratory were conducted in the manner reported by Aziz et al. (1998, 1999). The intra-assay precision of all tests was less than or equal to 15% for in-house quality control samples. All samples for a given participant were run in parallel to minimize inter-assay variability.
The proinflammatory cytokine assays had a lower limit of .02 pg/ml for IL-1β and .025 pg/ml for IL-6. Of the 271 samples collected, 34 (10.5%) fell below the lower limit of detection for IL-1β, and no samples fell below the lower limit of detection for IL-6. These low value samples occurred with similar frequency at each assessment point. For these samples, a below detection level value was inserted (defined as 50% of the lowest level of IL-1β assay sensitivity, i.e. 0.01 pg/ml). IL-1β and IL-6 data were positively skewed, and so were transformed prior to analyses, using a log (1 + x) transformation, in order to normalize the distributions and retain positive values.
2.4. Statistical analyses
Descriptive analyses were conducted with SPSS version 16.0. Multilevel models were used to evaluate the association between positive affect and proinflammatory cytokines over the assessment period. Multilevel models are best suited to evaluate these questions because they account for the repeated measures, and any missing data in longitudinal analyses. Multilevel modeling was conducted using Hierarchical Linear Modeling (HLM), version 6.06.
Preliminary analyses indicated no moderating effect of gender/cancer type, and so breast and prostate cancer patients were combined in order to maximize statistical power. In the hierarchical linear models, the inflammatory markers (i.e. IL-1β and IL-6) were treated as (level 1) outcome variables. Positive affect was the (level 2) predictor variable. Gender, age and BMI were also included as (level 2) control variables, in order minimize potential confounding effects on the inflammatory markers.
Post-hoc analyses were conducted to determine whether the association between positive affect and inflammation differed depending on treatment phase. The five on-treatment assessment points were coded as “on treatment”, as was the 2-week post-treatment assessment, which falls within the acute phase of treatment response. The 2-month post-treatment assessment was coded as “off treatment”.
Although positive affect was treated as a continuous variable for statistical analyses, this variable was dichotomized in the figures for visual clarity. Subjects were divided into “high” (n = 32) and “low” (n = 18) positive affect groups using an approximate median split in the positive affect scale. High positive affect individuals endorsed all four positive affect items “most or all of the time” (the highest possible response), while low positive affect subjects endorsed these items rarely, some of the time, or occasionally.
3. Results
3.1. Demographic and control variables
Demographic characteristics of study participants are reported in Table 1. Patients in this sample tended to be Caucasian, well-educated, and married, reflecting the characteristics of patients treated at UCLA.
Table 1.
Demographic characteristics of study participants.
| Prostate cancer | Breast cancer | |
|---|---|---|
| Number of subjects | 22 | 28 |
| Age (range) | 70.4 (54.2–79.3) | 57.1 (29.2–75.4) |
| Body Mass Index–BMI (range) | 27.0 (22.0–32.7) | 23.8 (17.4–31.5) |
| Ethnicity | ||
| Caucasian | 61.9% | 71.4% |
| Hispanic | 9.5% | 3.6% |
| African-American | 19.0% | 7.1% |
| Asian | 9.5% | 10.7% |
| Other | 0% | 7.1% |
| Married | 76.2% | 57.1% |
| Education (college grad or higher) | 57.1% | 67.9% |
| Employment (at least part-time work or school) | 38.1% | 57.1% |
| Income ($100,000 or higher) | 35.0% | 53.8% |
| Positive affect (range) | 2.69 (1.25–3.0) | 2.69 (1.5–3.0) |
Positive affect was not correlated with gender, BMI, or with baseline levels of IL-1β or IL-6. The association between positive affect and age approached significance (r = .258, p = .07). Baseline cytokine levels did not correlate with cancer stage or time since surgery completion.
3.2. Analyses of proinflammatory cytokines
Higher levels of positive affect at baseline were associated with higher levels of IL-1β (β = .059, p = .033) and IL-6 (β = .072, p = .042) across the assessment period. As illustrated in Figs. 1 and 2, patients with higher positive affect exhibited higher levels of IL-1β and IL-6 than lower positive affect patients during the acute treatment phase, though this difference was minimized by 2 months post-treatment. We conducted additional analyses to confirm that that association between positive affect and proinflammatory cytokines was restricted to the acute phase of radiation treatment. Indeed, patients who were higher in positive affect had significantly higher levels of IL-1β (β = .070, p = .031) and IL-6 (β = .096, p = .015) than patients who were lower in positive affect during the acute treatment phase, but not at 2 months post-treatment. This suggests that positive affect is associated with a greater acute increase in circulating cytokines during radiation treatment, which normalizes by post-treatment.
Fig. 1.
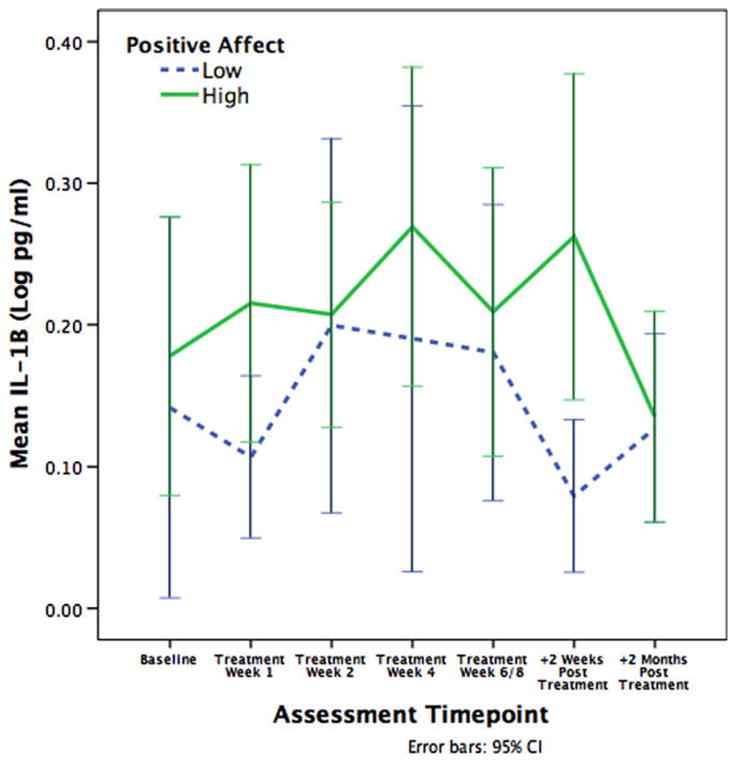
Mean levels of IL-1β across radiation treatment. Patients higher in positive affect exhibited significantly higher serum levels of IL-1β than patients lower in positive affect.
Fig. 2.
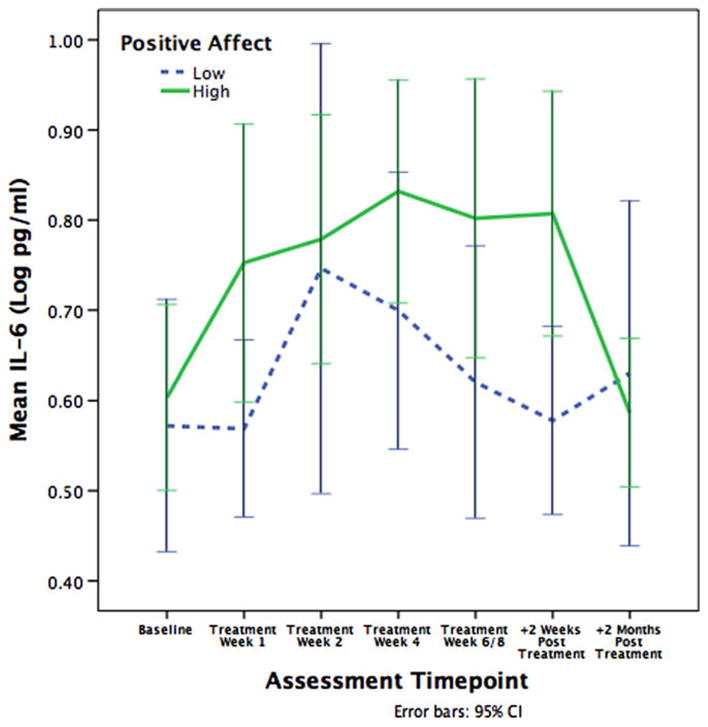
Mean levels of IL-6 across radiation treatment. Patients higher in positive affect exhibited significantly higher serum levels of IL-6 than patients lower in positive affect.
To clarify the clinical significance of cytokine elevations, additional analyses were conducted to determine whether increases in IL-6 and IL-1β were associated with skin toxicity, a common side effect of radiation therapy. Results showed no association between cytokine levels and self-reported skin reddening or irritation in the treatment area (ps > .33). Analyses were also conducted to test whether cytokine levels returned to baseline after treatment completion. We found no significant difference between baseline and 2-month post-treatment levels of IL-1β or IL-6 (ps > .45; see Table 2 for median cytokine levels at each assessment point).
Table 2.
Serum cytokine levels over the assessment period.
| Baseline | Treatment week 1 | Treatment week 2 | Treatment week 4 | Treatment week 6/8 | +2 weeks post-treatment | +2 months post-treatment | |
|---|---|---|---|---|---|---|---|
| IL-1β (pg/ml) median | 0.15 | 0.17 | 0.23 | 0.20 | 0.26 | 0.18 | 0.21 |
| IL-6 (pg/ml) median | 2.66 | 2.94 | 3.65 | 5.10 | 3.68 | 3.53 | 2.70 |
4. Discussion
This study is the first to identify a relationship between positive affect and treatment-related changes in proinflammatory cytokines among early-stage breast or prostate cancer patients undergoing radiation therapy. In particular, we found that positive affect was associated with higher circulating levels of IL-1β and IL-6 during radiation treatment in breast and prostate cancer patients.
This finding may seem counterintuitive, since previous studies conducted with healthy individuals have shown either no relationship or an inverse relationship between positive affect and markers of inflammation (Ryff et al., 2004; Prather et al., 2007; Steptoe et al., 2008). In these populations, inflammation is generally considered to have negative consequences for future health, given its association with cardiovascular disease (Cesari et al., 2003), type-2 diabetes (Pradhan et al., 2001), and all-cause mortality (Harris et al., 1999). However, among cancer patients undergoing radiation therapy, the health implications of cytokine elevations are unclear. Radiation activates proinflammatory cytokine production as part of a coordinated response designed to control damage and promote tissue repair. Thus, increases in cytokine levels during treatment may have beneficial effects on tissue recovery, similar to effects seen in acute wound healing studies (Kiecolt-Glaser et al., 2005).
Ultimately, we hypothesize that radiation-induced elevations in proinflammatory cytokines may be a double-edged sword, with effects depending on the timing and level of their expression (Wyss-Coray and Mucke, 2002; Hagemann et al., 2007). Inflammatory responses may be adaptive if they are circumscribed and time-limited, but may have detrimental effects if they are too large, occur too frequently, or remain elevated over time. In one study with lung cancer patients, development of radiation toxicity was predicted by mean baseline IL-6 levels of 15.7 pg/ml (vs. 7.6 pg/ml in patients who did not) (Arpin et al., 2005). Baseline IL-6 levels in our sample (mean = 4.03 pg/ml, median = 2.66 pg/ml) fell well below this range, and subsequent elevations in IL-6 levels returned to baseline levels after completion of treatment. Further, increases in IL-1β and IL-6 were not associated with skin toxicity, a common side effect of radiation exposure in breast and prostate cancer patients. These results suggest that the circumscribed elevations in proinflammatory cytokines associated with positive affect in this study are unlikely to have detrimental effects on health, and may instead facilitate the tissue healing process.
Our study was not designed to identify the mechanisms through which positive affect is associated with inflammation, and thus pathways remain speculative. It was notable that the majority of our participants reported feeling happy, hopeful, and enjoying life “most or all of the time” at treatment onset, suggesting that high levels of positive affect were the norm in this sample. Similar high levels of positive affect on the CES-D have been observed in other, larger samples (Moskowitz et al., 2008). If high positive affect is indeed normative, another interpretation of our results is that positive affect does not necessarily enhance the inflammatory response to radiation treatment, but rather that a deficiency in positive affect may dampen an otherwise healthy response. This hypothesis is in line with research that suggests that positive affect is associated with enhanced allostasis–buffering against the negative effects of stress and promoting restorative physiological systems (Bower et al., 2008). In the context of radiation therapy, positive affect may buffer against cancer and treatment-related stressors that dysregulate normal inflammatory response.
The primary limitation of this study was the small sample size. Given variability in cytokine levels during treatment, future studies may need larger sample sizes to increase the reliability of findings. In addition, although our assessment schedule was relatively intensive, more frequent sampling of cytokine levels may be required to fully capture the dynamic changes in cytokine production that occur during radiation therapy and to more accurately characterize the timing and duration of cytokine elevations. We also did not precisely control for time of blood draws, although the majority of samples were collected in the morning. Furthermore, a longer post-treatment follow-up would provide critical information about the association between positive affect and persistent inflammation. Finally, because we did not utilize an experimental design, we cannot draw conclusions about direction of causality. However, since positive affect was assessed before the onset of radiation therapy, it is unlikely that radiation-induced cytokine elevations drove positive affect, rather than vice versa.
Our results, though preliminary, suggest that positive affect is associated with changes in the inflammatory response to radiation treatment. These findings may have relevance for tissue repair processes. The importance of positive affect for inflammation, both in acute and chronic settings, merits focused attention in future research.
Acknowledgments
This research was supported by career development awards from the National Cancer Institute (K07 CA90407) and the University of California Breast Cancer Research Program to Dr. Bower, as well as support from the UCLA Clinical Immunology Research Laboratory. Saviz Sepah was supported in part by Eugene V. Cota-Robles and NSF Graduate Research Fellowships.
Footnotes
Please see Brief Commentary by Susan Lutgendorf on page 1066 of this issue.
References
- Arpin D, et al. Early variations of circulating interleukin-6 and interleukin-10 levels during thoracic radiotherapy are predictive for radiation pneumonitis. J Clin Oncol. 2005;23:8748–8756. doi: 10.1200/JCO.2005.01.7145. [DOI] [PubMed] [Google Scholar]
- Aziz N, Nishanian P, Fahey JL. Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures. Clin Diagn Lab Immunol. 1998;5:755–761. doi: 10.1128/cdli.5.6.755-761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diagn Lab Immunol. 1999;6:89–95. doi: 10.1128/cdli.6.1.89-95.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH. How do tissues respond to damage at the cellular level? The role of cytokines in irradiated tissues. Radiat Res. 1998;150:S109–S120. [PubMed] [Google Scholar]
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Low CA, Moskowitz JT, Sepah S, Epel E. Benefit finding and physical health: positive psychological changes and enhanced allostasis. Soc Pers Psychol Compass. 2008;2:223–244. [Google Scholar]
- Bower JE, Meyerowitz BE, Desmond KA, et al. Perceptions of positive meaning and vulnerability following breast cancer: predictors and outcomes among long-term breast cancer survivors. Ann Behav Med. 2005;29:236–245. doi: 10.1207/s15324796abm2903_10. [DOI] [PubMed] [Google Scholar]
- Cesari M, et al. Inflammatory markers and onset of cardiovascular events. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Christiansen H, Saile B, Hermann RM, Rave-Frank M, Hille A, Schmidberger H, Hess CF, Ramadori G. Increase of hepcidin plasma and urine levels is associated with acute proctitis and changes in hemoglobin levels in primary radiotherapy for prostate cancer. J Cancer Res Clin Oncol. 2007;133:297–304. doi: 10.1007/s00432-006-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Alper M, Doyle WJ, Treanor JJ, Turner RB. Positive emotional style predicts resistance to illness after experimental exposure to rhinovirus or influenza A virus. Psychosom Med. 2006;68:809–815. doi: 10.1097/01.psy.0000245867.92364.3c. [DOI] [PubMed] [Google Scholar]
- Costanzo ES, Lutgendorf SK, Kohut ML, Nisly N, Rozeboom K, Spooner S, Benda J, McElhaney JE. Mood and cytokine response to influenza virus in older adults. J Gerontol. 2004;12:1328–1333. doi: 10.1093/gerona/59.12.1328. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WJ, Gentile DA, Cohen S. Emotional style, nasal cytokines, and illness expression after experimental rhinovirus exposure. Brain Behav Immun. 2006;20:175–181. doi: 10.1016/j.bbi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Balkwell F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell. 2007;12:300–301. doi: 10.1016/j.ccr.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hartsell WF, Scott CB, Dundas GS, Mohiuddin M, Meredith RF, Rubin P, et al. Can serum markers be used to predict acute and late toxicity in patients with lung cancer? Analysis of RTOG 91-03. Am J Clin Oncol. 2007;30:368–376. doi: 10.1097/01.coc.0000260950.44761.74. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Doyle WJ, Turner RB, Treanor JJ. Infection-induced proinflammatory cytokines are associated with decreases in positive affect, but not increases in negative affect. Brain Behav Immun. 2007;21:301–307. doi: 10.1016/j.bbi.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, Schmid P, Possinger K, Flath BC. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Knight RG, William S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behav Res Ther. 1997;35:373–380. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Johnson EL, Cooper B, Anderson B, Sorosky JI, Buller RE, Sood AK. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, Henderson PJ, Sephton SE, Rohleder N, Lucci JA, Cole S, Sood AK, Lubaroff DM. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26:4820–4827. doi: 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Cohen S, Rabin BS, Manuck SB. Trait positive affect and antibody response to hepatitis B vaccination. Brain Behav Immun. 2006;20:261–269. doi: 10.1016/j.bbi.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Pressman S, Cohen S. Positive affect and immune function. In: Ader R, editor. Psychoneuroimmunology. 4. Vol. 2. Elsevier; San Diego, CA: 2007. pp. 761–779. [Google Scholar]
- Moskowitz JT. Positive affect predicts lower risk of AIDS Mortality. Psychosom Med. 2003;65:620–626. doi: 10.1097/01.psy.0000073873.74829.23. [DOI] [PubMed] [Google Scholar]
- Moskowitz JT, Epel ES, Acree M. Positive affect uniquely predicts lower risk of mortality in people with diabetes. Health Psychol. 2008;27:S73–S82. doi: 10.1037/0278-6133.27.1.S73. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Okunieff P, Chen Y, Maguire DJ, Huser AJ. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev. 2008;27:363–374. doi: 10.1007/s10555-008-9138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini B, Andersson B, Strannegard O, Wasserman J, Blomgren H, Glas U. Monocyte release and plasma levels of interleukin-6 in patients irradiated for cancer. In Vivo. 1992;6:531–534. [PubMed] [Google Scholar]
- Pradhan AD, Manson JM, Rifai N, Buring J, Ridker P. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Prather AA, Marsland AL, Muldoon MF, Manuck SB. Positive affective style covaries with stimulated IL-6 and IL-10 production in a middle-aged community sample. Brain Behav Immun. 2007;21:1033–1037. doi: 10.1016/j.bbi.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. J Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Brooks KP, Pressman SD. Trait positive affect buffers the effects of acute stress on skin barrier recovery. Health Psychol. 2009;28:373–378. doi: 10.1037/a0014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff CD, Singer BH, Dienerg Love G. Positive health: connecting well-being with biology. Philos Trans R Soc Lond B Biol Sci. 2004;359:1383–1394. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–229. [PubMed] [Google Scholar]
- Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression Scale. J Pers Assess. 1995;64:507–521. doi: 10.1207/s15327752jpa6403_9. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O’Donnell K, Badrick E, Kumari M, Marmot M. Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women: the Whitehall II study. Am J Epidemiol. 2008;167:96–102. doi: 10.1093/aje/kwm252. [DOI] [PubMed] [Google Scholar]
- Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease–a double edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]


