Abstract
Bladder cancer is one of the most common causes of death in industrialized countries. New tumor markers and therapeutic approaches are still needed to improve management of bladder cancer patients. Choline Kinase alpha (ChoKα) is a metabolic enzyme that has a role in cell proliferation and transformation. Inhibitors of ChoKα display antitumoral activity and are expected to be soon in clinical trials. This study is aimed to asses whether ChoKα plays a role in the aggressiveness of bladder tumors and constitute a new approach for bladder cancer treatment. We demonstrate here that ChoKα is constitutively altered in human bladder tumor cells. Furthermore, in vivo murine models including an orthotopic model to mimic as much as possible the physiological conditions, revealed that increased levels of ChoKα potentiates both tumor formation (p≤0.0001) and aggressiveness of the disease over different endpoints (p=0.011). Accordingly, increased levels of ChoKα significantly reduces survival of mice with bladder cancer (p=0.05). Finally, treatment with ChoKα specific inhibitor resulted in a significant inhibition of tumor growth (p=0.02) and in a relevant increase in survival (p=0.03).
Keywords: Bladder cancer, Choline Kinase, Therapeutic target, Tumor promoter
INTRODUCTION
Bladder cancer is the fifth leading cause of cancer in industrialized countries and one of the most expensive for health care systems (Jemal et al., 2007). At diagnosis, most of the patients (70–75%) with urothelial bladder carcinoma present with superficial tumors, 20% display muscle-invasive tumors, and only around 5% show metastatic tumors. However, more that 50% of these initially superficial tumors will eventually recur (Sanderson et al., 2007).
Experimental and clinical evidence indicate that urothelial carcinomas arise and progress through at least two independent mechanisms that result in two different molecular and phenotypic versions with different prognosis (Wu, 2005). In addition, several markers have been evaluated as potential tools for risk estimation of tumor progression with promising results (Cordon-Cardo et al., 1997; Hernando et al., 2001; Lu et al., 2002; Quek et al., 2005; Wu, 2005). Beyond the clinico-pathological factors, molecular features associated with a greater risk of disease progression have been found such as multiple aneuploid cells, nuclear p53 overexpression, and expression of the Lewis-x blood group antigen (Wu, 2005). However, the biology of bladder carcinoma is highly complex, still resulting in an almost unpredictable clinical behaviour. Thus, additional markers for a more accurate patient classification, diagnosis and prognosis are still needed for a better management of bladder cancer.
Choline Kinase (ChoK) is a metabolic enzyme responsible for the generation of phosphorylcholine (PCho) in eukaryotic cells. In mammals, two different proteins have been identified, ChoKα and ChoKβ, encoded by two different genes located at 11q13 and 22q13 loci respectively (Aoyama et al., 2004). In the past years, it has been found that besides its structural function, the alpha isoform (ChoKα) is closely related to the regulation of cell growth, playing a relevant role in cell transformation and in the carcinogenic process (Lacal, 2001; Nakagami et al., 1999; Ramirez de Molina et al., 2005; Ramirez de Molina et al., 2002a; Ramirez de Molina et al., 2002b). Furthermore, a recent study performed in early-stage non-small cell lung cancer patients, points at ChoKα as a new relevant factor useful to identify patients with poor prognosis that could be susceptible of a different strategy of treatment (Ramirez de Molina et al., 2007). By contrast, recent evidences demonstrate that ChoKβ is not able to induce cell transformation due to a differential behaviour in phospholipids metabolism under in vivo conditions (Gallego-Ortega et al., 2009). Finally, specific inhibitors of ChoKα have been generated with proven antiproliferative and antitumoral activity both in vitro and in vivo that are expected to be soon under clinical trials (Al-Saffar et al., 2006; Gallego-Ortega et al., 2009; Hernandez-Alcoceba et al., 1999; Hernandez-Alcoceba et al., 1997; Lacal, 2001; Ramirez de Molina et al., 2004; Rodriguez-Gonzalez et al., 2005; Rodriguez-Gonzalez et al., 2004; Rodriguez-Gonzalez et al., 2003). Furthermore, it has been demonstrated that the specific ChoKα inhibitor MN58b displays a dramatically differential effect on tumoral cells which die by apoptosis, with respect to the equivalent normal cells that are reversibly arrested at G0/G1 phase of the cell cycle, due to a differential mechanism of action involving ceramide production (Al-Saffar et al., 2006; Rodriguez-Gonzalez et al., 2005; Rodriguez-Gonzalez et al., 2004; Rodriguez-Gonzalez et al., 2003).
In the present report, we analyze whether ChoKα activation contributes to the initiation and progression of bladder cancer both in vitro and in vivo. Experimental evidence presented here suggests that this lipid-related kinase is actually an important regulator of the pathogenesis of bladder cancer.
RESULTS
Human bladder cancer cell lines display constitutive ChoKα activation
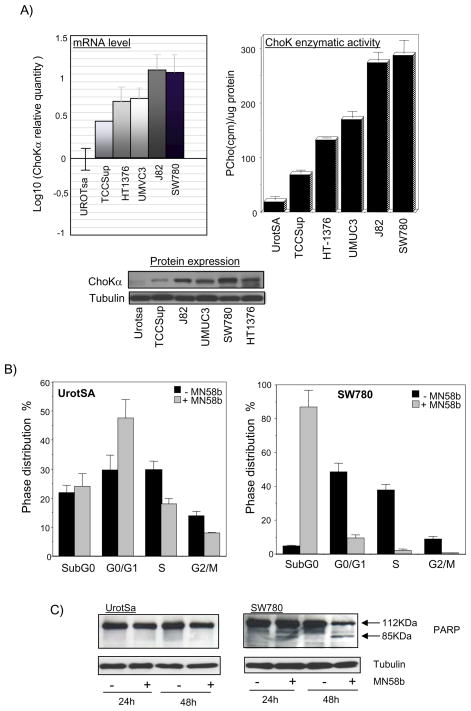
In order to analyze the relevance of ChoKα in bladder tumorigenesis, the expression and activity of this enzyme were assessed in a panel of human bladder cancer cell lines compared to non-transformed immortalized urothelial cells (UROtsa). As shown in Figure 1A, all tumoral cells display increased levels of ChoKα expression and activity with both of them oscillating between 3.6 and 15 fold with respect to control UROTsa cells. Of note, an excellent correlation between the number of mRNA copies, protein expression levels, and choline kinase enzymatic activity was observed.
Figure 1. ChoKα is constitutively over-expressed in a panel of human bladder cancer cells and might constitute a new antitumoral target for these tumors.
A) qRT-PCR for mRNA levels of ChoKα in five different human bladder cancer cells with respect to non-transformed immortalized UrotSa cells (black line of the left), correlates with ChoK enzymatic activity and ChoKα protein expression determined by western blot. Increase in ChoKα expression was found significant (p≤0.01) in all tumoral cell lines with respect to non-transformed UrotSa cells. B) ChoKα specific inhibition with MN58b induces cell death in bladder tumoral cells (p=0.001) whereas non-transformed cells are arrested in G1 (p=0.035). Cell cycle distribution analyzed by propidium iodide staining of DNA content of SW780 bladder tumoral cells treated with MN58b (right panel) compared to non-transformed UrotSA cells (left panel) is shown. C) MN58b-induced apoptosis in human bladder cancer cells determined by PARP processing. Three independent experiments in triplicate were performed in each case with similar results.
Results shown above indicate that ChoKα is constitutively overexpressed in human bladder cancer cells. To analyze whether these tumoral cells might be then appropriate candidates for an antitumoral treatment based on ChoKα inhibition, the effects of MN58b were assayed in human bladder cancer cells compared to control UROTsa cells. As shown in Figure 1B, MN58b treatment for 48h induces cell death in tumor cells (86,88 % cells subG0) whereas immortalized non-transformed cells were arrested in G0/G1. In addition, specific induction of apoptosis by MN58b in tumor cells was verified by monitoring poly-ADP-ribose polymerase (PARP) processing (Figure 1C).
ChoKα over-expression potentiates the tumorigenic properties of human bladder cancer cells
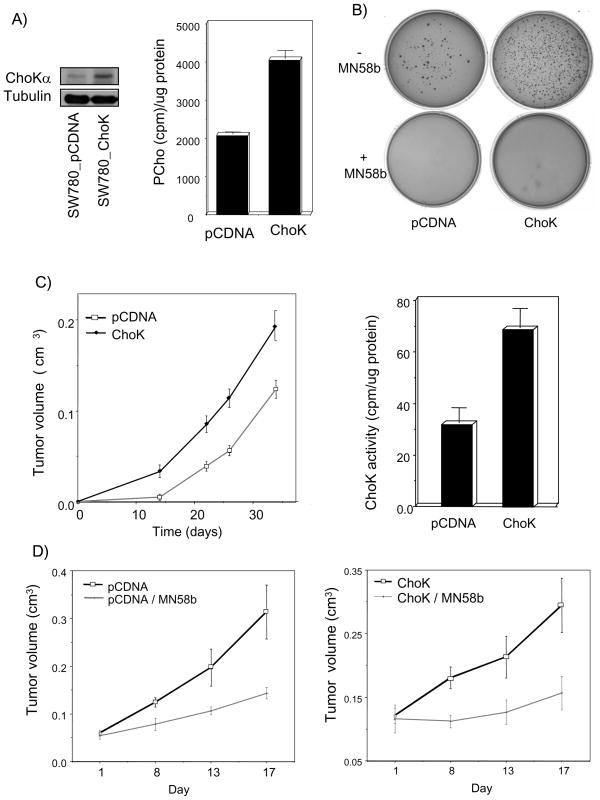
With the aim of analyzing if ChoKα overexpression is modulating the incidence and/or aggressiveness of bladder tumors, and to verify the hypothesis of ChoKα as a new therapeutic target in bladder cancer, several in vivo experiments were carried out. To that end, human bladder cancer-derived SW780 cells were stably transfected with an expression vector encoding ChoKα (SW780_ChoK), or with the pCDNA empty vector as control (SW780_pCDNA), and the functional over-expression of ChoKα in these cells was verified (Figure 2A). The effects of ChoKα on the oncogenic behavior of human SW780 bladder tumor cells were analyzed by two alternative methods. First, cells transfected either with the empty vector or the vector encoding for ChoKα were plated on soft agar in the presence or absence of MN58b. As shown in Figure 2B, ChoKα over-expression efficiently enhanced the transforming properties of SW780 bladder cancer cells as determined by the number of colonies. Moreover, addition of MN58b completely abrogated the anchorage-independent growth ability of these cells.
Figure 2. ChoKα overexpression potentiates the aggressiveness of bladder tumors. Antitumoral effect of ChoKα inhibition in bladder xenografts.
A) Human bladder tumoral SW780 cells were stably transfected with expression vector encoding for ChoKα or with pCDNA empty vector as control. Western blot analysis (left) and ChoK enzymatic activity (right) are shown. B) ChoKα over-expression efficiently potentiates achorage-independent growth of these cells. Addition of a few drops of new fresh media with MN58b (25 μM) five days after cells were seeded is sufficient to efficiently abrogate human bladder cancer cells transforming properties. Representative figures of one experiment of two independent experiments each performed in triplicate are shown. C) ChoKα significantly potentiates tumorogenesis in vivo. Both tumor volume and growth rate of SW780_ChoK were significantly higher in ChoKα over-expressing tumors than that observed in the control group (p=0.022). Right panel: ChoK enzymatic activity in generated tumors. D) Human bladder cancer xenografts are susceptible to growth inhibition upon treatment with MN58b. Results obtained for SW780_pCDNA (left) and SW780_ChoK (right) inoculated mice are shown (p≤0.001).
In addition, twenty-four mice were subcutaneously injected with SW780-pCDNA and other 24 with SW780_ChoK, and tumor growth was monitored every three days. Initially, whereas control SW780_pCDNA cells induced detectable tumors only in a small percentage of recipient animals (29%), SW780_ChoK cells developed tumors in almost all of injected mice (83%) approximately 10 to 15 d after injection (p≤0.0001) (Table I). Furthermore, both tumor volume and growth rate of SW780_ChoK cells were statistically significantly higher (p=0.011) than that displayed by SW780_pCDNA-derived tumors (Figure 2C). In addition, the overall tumor incidence at the end of the experiment (47 days after implantation) was statistically significantly higher for ChoKα over-expressing cells (p=0.023) than that observed for control cells (Table I). Accordingly, a complete necropsy of four mice from each group was performed, and retroperitoneal lymph node metastasis was found only in one ChoK-overexpressing mouse (data not shown). Finally, in order to verify that this oncogene was determinant for the enhanced tumorigenic behaviour of these cells, ChoKα over-expression was confirmed in the generated tumors (Figure 2C). These results suggest that ChoKα over-expression in human bladder cancer cells potentiates both tumor formation and aggressiveness.
Table I.
ChoKα potentiates tumorogenesis of human bladder cancer cells in vivo
| Mice | Early tumor incidence (day 14) | p | Final incidence of tumorigenesis (day 40) | P | |
|---|---|---|---|---|---|
| SW780_pCDNA | 24 | 7 (29%) | ≤ 0.0001 | 16 (67%) | 0.023 |
| SW780_ChoK | 24 | 20 (83%) | 23 (96%) |
Next, the sensitivity to ChoKα inhibition was also determined by treatment with MN58b. When tumors reached a mean volume of 0.05–0.1 cm3, mice were randomized in treated (intraperitoneally injected with MN58b, 5 mg/kg) or control groups (injected with vehicle). In keeping with previous results, a significant decrease (p=0,02) of 65,1% in tumor growth was observed on SW780_pCDNA injected mice upon treatment with this ChoKα specific inhibitor (Figure 2D). Furthermore, this effect was more noticeable in mice inoculated with ChoKα over-expressing cells (p=0.019), with 77% of tumor growth inhibition.
ChoKα promotes an aggressive and invasive phenotype of bladder tumors: orthotopic model
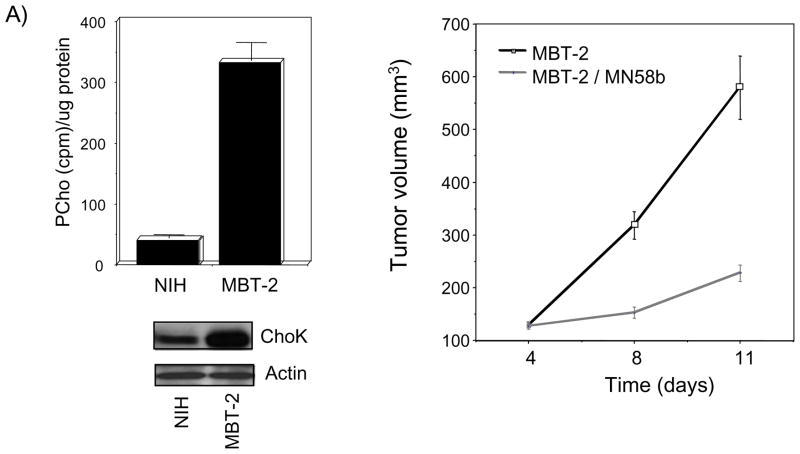
Novel animal models that parallel human disease have been recently developed. In the case of bladder cancer, the establishment of orthotopic bladder tumors has been carried out with promising results (Kikuchi et al., 2004; Mullerad et al., 2005). MBT-2 (murine bladder tumor) is a well-established model for in vivo experimental analysis of urothelial carcinoma in immunocompetent mice (Oliveira et al., 2006). Therefore, an orthotopic bladder cancer model of ChoKα over-expression was set up to provide definitive evidence for a role of ChoKα in bladder tumorogenesis. In addition, the effectiveness of an anti-bladder cancer therapy based on ChoKα specific inhibition has also been evaluated under these conditions that mimic the physiological process. In order to verify whether the MBT-2 model was comparable to that of human SW780 bladder cancer cells, some preliminary experiments were carried out. Firstly, we determined that MBT-2 cells displayed increased levels of ChoKα expression and activity when compared to non-transformed murine fibroblasts NIH3T3 (Figure 3A). In addition, the effect of MN58b on the oncogenic behavior of MBT-2 was also analyzed by anchorage-independent growth, observing that as expected, addition of MN58b completely abrogated their ability to form colonies in soft agar (data not shown). Furthermore, forty mice were injected subcutaneously with MBT-2 cells and once tumor volume reached 0.1 cm3, twenty of them were kept as controls, and the remaining twenty mice were treated intraperitoneally with MN58b. As it is shown in the figure, treatment with MN58b resulted in a statistically significant inhibition of tumor growth (77,6%) (p≤0.0001).
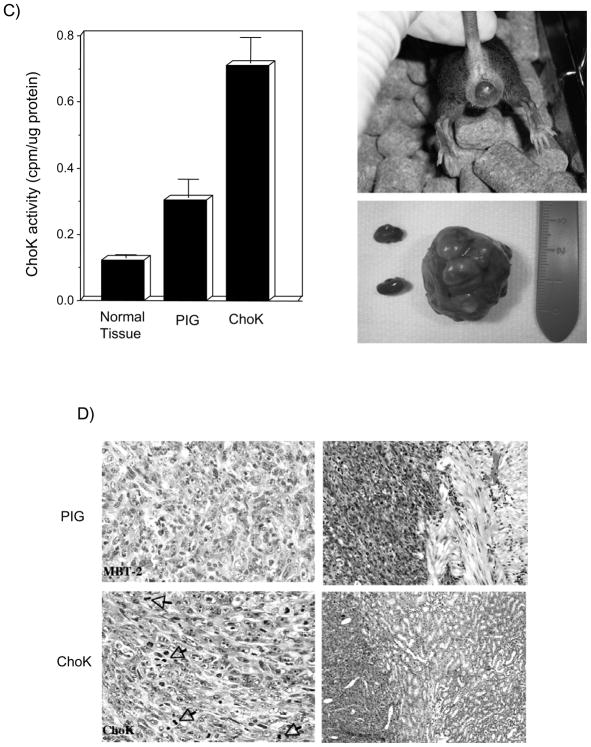
Figure 3. ChoKα acts a relevant promoter and new target in an orthotopic model of bladder tumorigenesis.
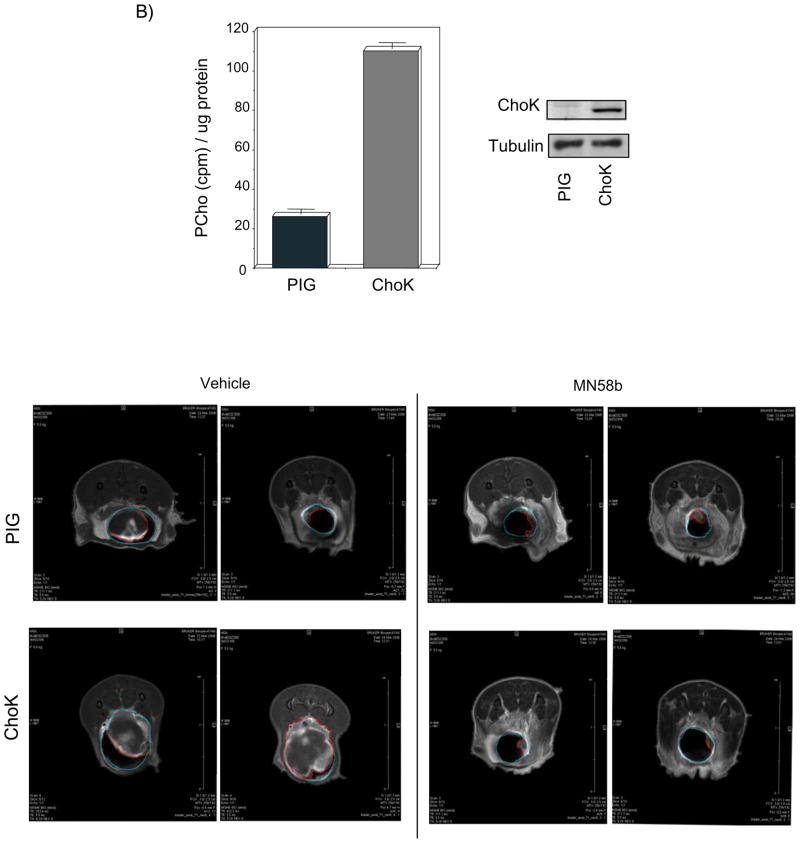
A) Basal levels of ChoKα determined by protein expression (lower panel) and ChoK enzymatic activity (upper panel), are increased in MBT-2 cells with respect to non-transformed murine NIH-3T3 cells. Tumor growth after injection of these MBT-2 cells with or without treatment with MN58b is shown (right panel) (p<0.001). B) MBT-2 cells were transduced either with empty PIG vector as control or with vector encoding for ChoKα (both protein expression determined by western blot analysis and ChoK enzymatic activity are shown in the upper panel). Control and ChoKα over-expressing cells were inoculated transurethrally directly into the mice bladders, and mice were scanned for analysis of the generated tumors by magnetic resonance imaging using Gd as contrast agent. Representative images are shown (lower panel). C) Right: surgically extracted tumors generated by ChoKα overexpression displayed increased ChoK activity (p=0.0025). Left: representative picture of the tumor size reached in some of the tumors over-expressing ChoKα protuberating outside of the bladder. D) Representative histological stainings (H&E) of ChoKα generated tumors in the bladders (upper right panel) and kidneys (lower right panel).
The effect of ChoKα on the establishment of orthotopic bladder tumors, and the in vivo response of these tumors to ChoKα inhibition, were next investigated in an orthotopic model. MBT-2 cells were permanently transduced either with a retroviral vector encoding for human ChoKα or with the corresponding empty vector (PIG) as control (Figure 3B), and after antibiotic selection they were inoculated trans-urethrally directly into the mouse bladder. Five days after instillation, every set of mice (PIG and ChoKα) was randomly separated into two groups, and treated or not with MN58b.
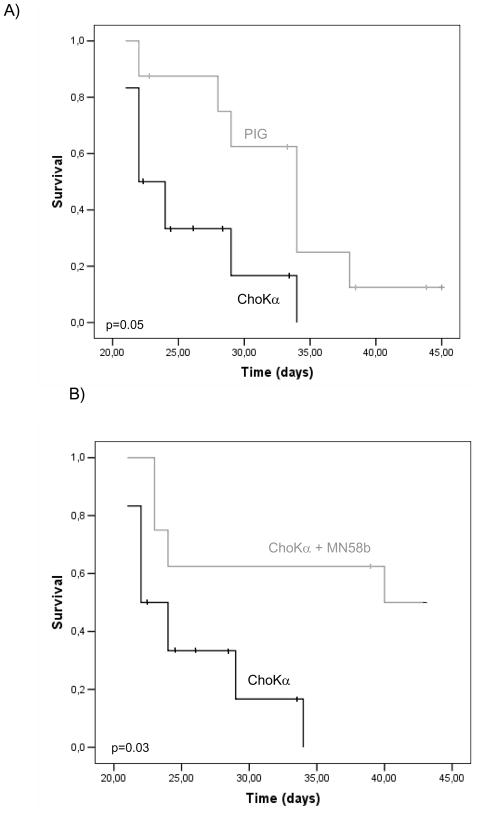
The feasibility of magnetic resonance imaging (MRI) for detection of early stage orthotopic murine bladder (MBT-2) tumor implants has been previously demonstrated (Chin et al., 1991; Kikuchi et al., 2003). This method provides an effective non-invasive system for the early detection and exact measurement of the tumors generated. 20 days after instillation, mice were monitored by MRI to obtain images of the generated tumors. A representative picture of the observed images is shown in Figure 3B. Mean tumor size of tumors found at that time in the control mice was 0.55 cm3 whereas for ChoKα over-expressing tumors was of 0.82 cm3, 50% bigger. Moreover, some of the ChoKα over-expressing tumors reached volumes that made them protrude outside of the bladder (Figure 3C). In addition, when comparing the mortality rate at 28 days after instillation, it was statistically significantly higher (p=0.031) for those mice with ChoKα over-expression (Table II). Furthermore, at the endpoint of the experiment, the presence of established MBT-2 intravesical tumors was confirmed by gross pathology. The histological examination revealed a higher incidence of kidney lesions (indicative of tumor dissemination) in mice with ChoKα over-expression (Table II). In order to verify that ChoK activation was present all over the process, ChoK activity was analyzed in all the extracted tumors at the end of the experiment (Figure 3C). MBT-2 induced tumors displayed a constitutive increase in the basal levels of ChoK activity when compared with normal tissues, which was significantly higher in tumors generated by ChoKα over-expressing cells. Histological analysis of orthotopic tumors was also performed, revealing an increased number of mitotic figures in ChoKα over-expressing than in vector-transduced MBT-2 tumors (Figure 3D). Consequently, all the ChoKα-mediated effects resulted, even in this particularly fast and aggressive bladder cancer model, in a significantly worse outcome of mice over-expressing this oncogene as it can be observed in Figure 4A (p=0.05). Thus, whereas median survival time of control mice was 32 days, this was reduced in ChoKα over-expressing mice to 22 days, a 36% reduction. Finally, the in vivo response of MBT-2 tumors to ChoKα inhibition was also evaluated in this orthotopic model. According to results shown above, ChoKα over-expressing tumors were more sensitive to the antitumoral effect of MN58b. In this group, mean tumor size of treated tumors was significantly reduced from 0.82 to 0.22 cm3, and importantly, the tumor incidence was also reduced in 43% in treated mice with respect to control (Table II). Furthermore, generated tumors displayed a retardation in tumor growth that resulted in increased median survival time from 34 to 42 days in mice inoculated with MBT-2 vector-transduced cells, more apparent in MBT-2 cells over-expressing ChoKα (from 22 to 40 days; p=0.03) (Figure 4B). Overall, these results are indicative of a remarkable oncogenic effect of ChoKα in the bladder.
Table II.
ChoKα enhances the aggressiveness of bladder cancer cells: orthotopic model
| Tumor incidence and mortality (day 28) |
|||
|---|---|---|---|
| Mice | Tumorogenesis | Early Mortality | |
| MBT-2/PIG | 10 | 7 (70%) | 1 (14,3%) |
| MBT-2/ChoK | 10 | 7 (70%) | 5 (71,4%) |
| MBT-2/PIG/MN58b | 9 | 7 (77%) | 1 (14,3%) |
| MBT-2/ChoK/MN58b | 8 | 3 (37,5%) | 2 (66,7%) |
|
T4 adquisition: kidney failure (endpoint) |
|||
| Mice | Bladder tumor | Kidney Tumor | |
| MBT-2/PIG | 10 | 7 (70%) | 2 (28,6%) |
| MBT-2/ChoK | 10 | 7 (70%) | 4 (57,1%) |
| MBT-2/PIG/MN58b | 9 | 7 (77%) | 1 (14,3%) |
| MBT-2/ChoK/MN58b | 8 | 3 (37,5%) | 0 (0%) |
Figure 4. ChoKα expression strongly falls in survival of mice in an orthotopic model of bladder tumorigenesis.
A) Over-expression of ChoKα is significantly associated with survival as depicted by the Kaplan-Meier curve of the survival analysis of control mice and mice over-expressing ChoK. B) Survival from bladder cancer of mice over-expressing ChoKα is significantly increased after treatment with its inhibitor MN58b.
DISCUSSION
ChoKα has been recently described as a novel human oncogene with clinical relevance as a prognostic factor in early-stage NSCLC (Glunde and Bhujwalla, 2007; Ramirez de Molina et al., 2005; Ramirez de Molina et al., 2007). Furthermore, specific inhibitors of ChoKα display antitumoral activity in vivo and are expected to be soon under clinical trials. Here, we have investigated the putative role of ChoKα in bladder tumors following complementary approaches. ChoKα expression and activity are increased in all (five) human bladder cancer cell lines analyzed with respect to the corresponding human non-transformed immortalized bladder cells. Interestingly, the lowest value of ChoK activation corresponds to TCCSup, a superficial non-tumorigenic cell line (ATCC, our unpublished results), whereas the highest values correspond to invasive tumorigenic cells, suggesting that ChoKα activation could contribute to the different in vivo growth properties of bladder cancer cells. Following this observation, we have evaluated the in vivo effect of ChoKα over-expression in human bladder tumor cells. The results indicate that ChoKα is not only contributing to transformation, but also promotes tumor progression since increased ChoKα activity in the tumor also significantly enhances the aggressiveness of the disease. Finally, to provide definitive evidence of the involvement of ChoKα in the genesis and progression of bladder cancer, an orthotopic model was developed and non-invasively monitored by MRI. Regardless of the intrinsic accelerated evolution of the MBT-2 orthotopic model, ChoKα over-expression was able to increase tumor size by 50%, and dissemination to the kidney by 200%. In addition, early mortality rate was significantly affected by the over-expression of this oncogene, resulting in a significantly worse overall outcome. These results strongly demonstrate for the first time that ChoKα is indeed a relevant molecule in bladder carcinogenesis and that it is involved in tumor generation, progression, and disease outcome.
Since ChoKα plays an important role in bladder tumor generation and evolution, the specific inhibition of this enzyme was evaluated as a potential novel antitumoral strategy against bladder cancer. To that end we used MN58b, a highly selective ChoKα inhibitor (Hernandez-Alcoceba et al., 1999; Hernandez-Alcoceba et al., 1997; Rodriguez-Gonzalez et al., 2005; Rodriguez-Gonzalez et al., 2004; Rodriguez-Gonzalez et al., 2003) whose specificity has been recently further demonstrated in vivo by Magnetic Resonance Spectroscopy (MRS) (Al-Saffar et al., 2006), and validated both in vitro and in vivo by RNA interference (Glunde et al., 2005). Bladder cancer cells massively undergo apoptosis after MN58b treatment, an effect that has been evaluated in three different cell lines: SW780 (Fig 2B and C), J82 and UMUC3 (data not shown). By contrast, non-transformed bladder cells remain arrested in G1 phase of the cell cycle, revealing the potential of ChoKα inhibition as a specific therapeutic strategy against bladder cancer. In addition, the inhibition of ChoKα showed an efficient antitumoral activity against human bladder cancer xenografts. Finally, when analyzing the in vivo response to ChoKα inhibition in a more physiological orthotopic model, a remarkable inhibition of tumor growth was noticed resulting in increased overall survival. Consistently, ChoKα over-expressing tumors were more sensitive to the antitumoral effect of MN58b, and tumor incidence was also reduced in this group by 43% in treated mice with respect to control. Thus, since only tumor cells die by apoptosis after MN58b treatment, and ChoKα inhibition has an efficient in vivo effect against bladder cancer, a new antitumoral therapy based on ChoKα inhibition seems an optimal approach for treatment of bladder cancer patients. The exquisite dependence of certain tumors for a specific catalytic activity or pathway for survival, named ‘oncogene addiction’, is being recently exploited for targeted therapy (Garraway and Sellers, 2006; Jonkers and Berns, 2004; Weinstein and Joe, 2006). The strict dependence of bladder cancer cells for ChoKα activity unravels a new therapeutically appealing molecular target for treatment of these tumors.
Urothelial carcinoma is one of the most common cancers worldwide. The current elevated morbidity associated to bladder cancer demands a better knowledge of the molecular mechanisms underlying bladder tumorigenesis and progression. Furthermore, new molecular biomarkers are needed for a more accurate patient classification and diagnosis of bladder cancer patients that allows a rational application of multimodality strategies that improve overall survival. Among the genetic alterations that characterize urothelial tumorigenesis, defects of chromosome 11, where ChoKα is localized, are frequent and have been often associated with tumor progression and invasiveness (Cordon-Cardo et al., 1997). Thus, though further studies are needed to determine the mechanism of ChoKα upregulation in bladder cancer, the results shown here demonstrate that this enzyme displays a significant role in the development and invasive behavior of these tumors and points out at this oncogene as a target for future studies focused on determining its relevance as a putative marker and therapeutic target for bladder cancer patients.
MATERIALS AND METHODS
Cell culture and transfections
All cell lines used in this study were maintained under standard conditions of temperature (37°C), humidity (95%), and carbon dioxide (5%). Human non-transformed UROTsa and human bladder tumoral cells (ATCC), were maintained in MEM (Gibco ref. 31095-029) supplemented with non-essential aminoacids (Gibco ref. 11140), 200mM glutamine (Gibco ref. 25030) and 10% Fetal Bovine Serum (FBS) (Gibco. Ref. 10106-169). Murine MBT-2 were grown in RPMI supplemented with 10% FBS and NIH3T3 cells were maintained in DMEM supplemented with 10% Newborn Calf Serum (NCS). Stable transfection of human SW780 cells was carried out using the Lipofectamine Plus Reagent from Gibco BRL as described by the manufactures, and 48h post-transfection selection with gentamicine was added. Human ChoKα cDNA was also subcloned into the retroviral vector MSCV-PIG. MBT-2 cells were transduced by three rounds of infection with fresh retroviral supernatants from MSCV or MSCV-Chokα transfected ecothropic Phoenix cells, filtered through 0.45um and supplemented with polybrene (4ug/ml). Transduced cells were selected 48h post-infection by adding puromycine (2 ug/ml).
Analysis of protein levels by Western-blotting
Cells were lysed in ice-cold lysis buffer (50 mM Tris, pH 7.4, 0.25% NP-40, 0.25% SDS, 150 mM NaCl, 15 mM b-Glycerophosphate, 10 mM Na PPi, 50 mM NaF, 10 mg/ml aprotinin, 1 mM PMSF). Nuclei and detergent-insoluble material were removed by centrifugation at 13,000 rpm for 20 minutes at 4°C. Western blot analysis of equal amounts of cell lysates (30 μg) was performed using each correspondent antibody. ChoKα monoclonal antibody was obtained as described in 26. When analyzing ChoKα expression in murine cells, a monoclonal antibody against this enzyme recognizing human and murine ChoK was used (Gallego-Ortega et al., 2006). Antibodies against PARP (SC-7150), tubulin (T9026) and actin (A2066) were purchased from Santa Cruz Biotechnologies and Sigma respectively.
Choline Kinase enzymatic assays
Choline kinase enzymatic assays were performed with cell extracts in buffer containing 100 mM Tris-HCl pH 8.0, 100 mM MgCl2 and 10 mM ATP, using as substrate the physiological choline concentration (200 μM) in presence of methyl[14C]-choline chloride (50–60μCi/mmol, Amersham International). Reactions were performed at 37°C for 30 minutes and stopped with ice cold trichloracetic acid (TCA) at a final concentration of 16%. After washing with water-saturated ethylether, samples were lyophilyzed. Hydrophilic derivatives of choline were resolved in TLC plates (60 A silica gel, Whatman, Clifton, NJ), using as liquid phase 0.9% NaCl:methanol:ammonium hydroxide (50:70:5; V:V:V). Radioactivity/radioactive signal corresponding to PCho was automatically quantified by an electronic radiography system (Instantimager; Packard, Meriden, CT).
Flow Cytometry Analysis
When indicated, cells were treated for 48h with MN58b (25 μM), and flow cytometry analysis was carried out. To that end, cells were trypsinized, centrifuged and diluted in 1 ml PBS, and 9 ml of ethanol 70% were added while vortexing. After 24 h at −20°C, cells were washed with PBS and resuspended in 1 mL of PBS buffer containing 2,5 mM C6H5Na3O7, 0,01% TX 100 and 20 μg propidium iodide. FACScan analysis for relative DNA content based on fluorescence was carried out. Cell cycle analysis was then performed by flow cytometry using a commercially available software package FACs SCAN Beckton Dickinson.
Anchorage-independent growth in soft agar
Anchorage independent growth assay was performed as previously described (Ramirez de Molina et al., 2005) by plating 50000 cells/60-mm dish. Five days after cells were seeded, a few drops of new fresh media with or without the presence of MN58b (25uM) were added every three days. After five weeks of incubation, medium was absorbed, 500 μL of 0.005% crystal violet was added and incubated for 1h at 37°C. Plates were then washed once with 1X phosphated-buffered saline and visualized under a microscope.
In vivo subcutaneous tumorigenic assay and ChoKα inhibitor treatment
Indicated cells were resuspended in DMEM just before inoculation (106 cells/0.1 ml), and injected subcutaneously in nu/nu immunosuppressed mice. Mice were kept in a pathogen-free housing under guidelines of the Spanish and MSKCC institutional Animal Care and Use Committee and Research Animal Resource Center. When tumors reached a mean volume of 0.1 cm3, mice were randomized to control and treated groups. Treatment with MN58b (and vehicle for control mice) was performed i.p. with a schedule of daily consecutive doses of 5 mg/Kg for 5 days, with a 9 days interval. Tumors were monitored at least twice a week by measuring the major (D) and minor (d) diameters, and tumor volume was calculated by V=(D*d2)/2.
In vivo orthotopic model
Experiments were approved by the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center. Eight-week-old female C3H/HeJ mice (Jackson Laboratory, Bar Harbor, Maine) were maintained in pathogen-free, isolated barrier facilities. For tumor implantation, animals were anesthetized with an intraperitoneal injection of ketamine (Fort Dodge Animal Health, Fort Dodge, Iowa) (100 mg/kg) and xylazine (Lloyd Laboratories, Shenandoah, Iowa) (20 mg/kg). A 22 gauge catheter was inserted into the bladder transurethrally and the urethra was temporarily ligated with a 3-zero silk suture that was mantained for 3 hours. Subsequently, 2×106 MBT-2 cells transduced either with empty PIG vector or vector encoding for ChoKα were instilled directly into the bladder.
Histopathological analysis
During the necropsy of the animals, a tissue portion was snap frozen in liquid nitrogen to assess Chokα levels. Another piece of tumor and kidney tissues was formalin fixed and embedded in paraffin for histological analyses.
Magnetic Resonance Imaging
All MR images were acquired on a Bruker 4.7T Biospec scanner (Bruker Biospin Inc., Billerica, Massachusetts) with a console running ParaVision 3.0. A 32 mm quadrature birdcage volume coil (Stark Contrast MRI Coils Research, Erlangen, Germany) was used for signal detection in all measurements. The animals were catheterized under anesthesia with 1.5% isofluorane and their bladders were emptied and then injected with a contrast medium comprised of 50% Magnevist (Berlex, Montville, New Jersey) and 50% air. The animals were kept under anesthesia during MR scan. Initial scout images along the 3 orthogonal orientations were acquired using a fast-spin-echo (RARE) sequence to locate the bladder position. A T-weighted spin-echo method (MSME) was used for bladder imaging, with a 30 degree flip angle, TR=211 ms, TE=5.5 ms and NEX=8. The axial image slice thickness is 1 mm with a 0.3 mm gap, FOV is 30 × 25 mm and a in-plane resolution of 117 × 130 μm. The imaging time for the axial scan is 5 minutes. Image post-processing was done using the Xtip graphics software suite on a Linux workstation (Bruker Biospin MRI, Erlangen, Germany). For each slice the tumor outline was manually traced and the area computed. The tumor volume was calculated from the sum of all the areas of the tumor and multiplied by the total slice separation.
Statistical analysis
The Kaplan-Meier method was used to estimate bladder cancer specific survival. The univariate association between ChoKα expression and tumour behaviour was analyzed using Fisher’s exact test. Continuous variables with non-normal distribution were compared by using Kruskal–Wallis and Mann–Whitney U tests and those with normal distribution were compared by means of Student’s t-test. All reported P-values are two-sided. Statistical significance was defined as P<0.05. The statistical analyses were performed using SPSS software, version 13.0 (Inc, Chicago, Illinois).
Acknowledgments
We gratefully thank E. Charytonowicz for technical assistance during the intravesical instillations; Mihaela Lupu and Cornelia Mateia for MRI monitoring; Miguel Angel García-Cabezas for helping with the histopathological analysis. This work has been supported by a grant to ARM from Fundación Mutua Madrileña (2006), and grants to JCL from Comunidad de Madrid (S-BIO/0280/2006), Ministerio de Educación y Ciencia (SAF2004-0577 and SAF2005-06195-C02-01), EU grant (LSHG-CT-2006-037278), Ministerio de Sanidad (RD06/0020/0016), Fundación Mutua Madrileña (2006), Acción transversal del Cáncer (FIS, Instituto de Salud Carlos III) and NIH 1R24CA83084, NIH 1R24CA83084. TCD Pharma (Madrid, Spain) provided ChoKα monoclonal antibody and MN58b free of charge.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Al-Saffar NM, Troy H, Ramirez de Molina A, Jackson LE, Madhu B, Griffiths JR, et al. Noninvasive magnetic resonance spectroscopic pharmacodynamic markers of the choline kinase inhibitor MN58b in human carcinoma models. Cancer Res. 2006;66:427–34. doi: 10.1158/0008-5472.CAN-05-1338. [DOI] [PubMed] [Google Scholar]
- Aoyama C, Liao H, Ishidate K. Structure and function of choline kinase isoforms in mammalian cells. Prog Lipid Res. 2004;43:266–81. doi: 10.1016/j.plipres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, Zhang ZF, Dalbagni G, Drobnjak M, Charytonowicz E, Hu SX, et al. Cooperative effects of p53 and pRB alterations in primary superficial bladder tumors. Cancer Res. 1997;57:1217–21. [PubMed] [Google Scholar]
- Chin J, Kadhim S, Garcia B, Kim YS, Karlik S. Magnetic resonance imaging for detecting and treatment monitoring of orthotopic murine bladder tumor implants. J Urol. 1991;145:1297–301. doi: 10.1016/s0022-5347(17)38618-4. [DOI] [PubMed] [Google Scholar]
- Gallego-Ortega D, Ramirez De Molina A, Gutierrez R, Ramos MA, Sarmentero J, Cejas P, et al. Generation and characterization of monoclonal antibodies against choline kinase alpha and their potential use as diagnostic tools in cancer. Int J Oncol. 2006;29:335–40. [PubMed] [Google Scholar]
- Gallego-Ortega D, Ramirez de Molina A, Ramos MA, Valdes-Mora F, Barderas MG, Sarmentero-Estrada J, et al. Differential role of human choline kinase alpha and beta enzymes in lipid metabolism: potential implications in cancer onset and treatment. 2009. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Sellers WR. Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer. 2006;6:593–602. doi: 10.1038/nrc1947. [DOI] [PubMed] [Google Scholar]
- Glunde K, Bhujwalla ZM. Choline kinase alpha in cancer prognosis and treatment. Lancet Oncol. 2007;8:855–7. doi: 10.1016/S1470-2045(07)70289-9. [DOI] [PubMed] [Google Scholar]
- Glunde K, Raman V, Mori N, Bhujwalla ZM. RNA interference-mediated choline kinase suppression in breast cancer cells induces differentiation and reduces proliferation. Cancer Res. 2005;65:11034–43. doi: 10.1158/0008-5472.CAN-05-1807. [DOI] [PubMed] [Google Scholar]
- Hernandez-Alcoceba R, Fernandez F, Lacal JC. In vivo antitumor activity of choline kinase inhibitors: a novel target for anticancer drug discovery. Cancer Res. 1999;59:3112–8. [PubMed] [Google Scholar]
- Hernandez-Alcoceba R, Saniger L, Campos J, Nunez MC, Khaless F, Gallo MA, et al. Choline kinase inhibitors as a novel approach for antiproliferative drug design. Oncogene. 1997;15:2289–301. doi: 10.1038/sj.onc.1201414. [DOI] [PubMed] [Google Scholar]
- Hernando E, Orlow I, Liberal V, Nohales G, Benezra R, Cordon-Cardo C. Molecular analyses of the mitotic checkpoint components hsMAD2, hBUB1 and hBUB3 in human cancer. Int J Cancer. 2001;95:223–7. doi: 10.1002/1097-0215(20010720)95:4<223::aid-ijc1038>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Berns A. Oncogene addiction: sometimes a temporary slavery. Cancer Cell. 2004;6:535–8. doi: 10.1016/j.ccr.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Kikuchi E, Menendez S, Ohori M, Cordon-Cardo C, Kasahara N, Bochner BH. Inhibition of orthotopic human bladder tumor growth by lentiviral gene transfer of endostatin. Clin Cancer Res. 2004;10:1835–42. doi: 10.1158/1078-0432.ccr-03-0099. [DOI] [PubMed] [Google Scholar]
- Kikuchi E, Xu S, Ohori M, Matei C, Lupu M, Menendez S, et al. Detection and quantitative analysis of early stage orthotopic murine bladder tumor using in vivo magnetic resonance imaging. J Urol. 2003;170:1375–8. doi: 10.1097/01.ju.0000075504.13456.41. [DOI] [PubMed] [Google Scholar]
- Lacal JC. Choline kinase: a novel target for antitumor drugs. IDrugs. 2001;4:419–26. [PubMed] [Google Scholar]
- Lu ML, Wikman F, Orntoft TF, Charytonowicz E, Rabbani F, Zhang Z, et al. Impact of alterations affecting the p53 pathway in bladder cancer on clinical outcome, assessed by conventional and array-based methods. Clin Cancer Res. 2002;8:171–9. [PubMed] [Google Scholar]
- Mullerad M, Bochner BH, Adusumilli PS, Bhargava A, Kikuchi E, Hui-Ni C, et al. Herpes simplex virus based gene therapy enhances the efficacy of mitomycin C for the treatment of human bladder transitional cell carcinoma. J Urol. 2005;174:741–6. doi: 10.1097/01.ju.0000164730.38431.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami K, Uchida T, Ohwada S, Koibuchi Y, Suda Y, Sekine T, et al. Increased choline kinase activity and elevated phosphocholine levels in human colon cancer. Jpn J Cancer Res. 1999;90:419–24. doi: 10.1111/j.1349-7006.1999.tb00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira PA, Colaco A, De la Cruz PL, Lopes C. Experimental bladder carcinogenesis-rodent models. Exp Oncol. 2006;28:2–11. [PubMed] [Google Scholar]
- Quek ML, Stein JP, Nichols PW, Cai J, Miranda G, Groshen S, et al. Prognostic significance of lymphovascular invasion of bladder cancer treated with radical cystectomy. J Urol. 2005;174:103–6. doi: 10.1097/01.ju.0000163267.93769.d8. [DOI] [PubMed] [Google Scholar]
- Ramirez de Molina A, Banez-Coronel M, Gutierrez R, Rodriguez-Gonzalez A, Olmeda D, Megias D, et al. Choline kinase activation is a critical requirement for the proliferation of primary human mammary epithelial cells and breast tumor progression. Cancer Res. 2004;64:6732–9. doi: 10.1158/0008-5472.CAN-04-0489. [DOI] [PubMed] [Google Scholar]
- Ramirez de Molina A, Gallego-Ortega D, Sarmentero J, Banez-Coronel M, Martin-Cantalejo Y, Lacal JC. Choline kinase is a novel oncogene that potentiates RhoA-induced carcinogenesis. Cancer Res. 2005;65:5647–53. doi: 10.1158/0008-5472.CAN-04-4416. [DOI] [PubMed] [Google Scholar]
- Ramirez de Molina A, Penalva V, Lucas L, Lacal JC. Regulation of choline kinase activity by Ras proteins involves Ral-GDS and PI3K. Oncogene. 2002a;21:937–46. doi: 10.1038/sj.onc.1205144. [DOI] [PubMed] [Google Scholar]
- Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, Martinez-Pineiro L, Sanchez J, Bonilla F, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun. 2002b;296:580–3. doi: 10.1016/s0006-291x(02)00920-8. [DOI] [PubMed] [Google Scholar]
- Ramirez de Molina A, Sarmentero-Estrada J, Belda-Iniesta C, Taron M, Ramirez de Molina V, Cejas P, et al. Expression of choline kinase alpha to predict outcome in patients with early-stage non-small-cell lung cancer: a retrospective study. Lancet Oncol. 2007;8:889–97. doi: 10.1016/S1470-2045(07)70279-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gonzalez A, Ramirez de Molina A, Banez-Coronel M, Megias D, Lacal JC. Inhibition of choline kinase renders a highly selective cytotoxic effect in tumour cells through a mitochondrial independent mechanism. Int J Oncol. 2005;26:999–1008. [PubMed] [Google Scholar]
- Rodriguez-Gonzalez A, Ramirez de Molina A, Fernandez F, Lacal JC. Choline kinase inhibition induces the increase in ceramides resulting in a highly specific and selective cytotoxic antitumoral strategy as a potential mechanism of action. Oncogene. 2004;23:8247–59. doi: 10.1038/sj.onc.1208045. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gonzalez A, Ramirez de Molina A, Fernandez F, Ramos MA, del Carmen Nunez M, Campos J, et al. Inhibition of choline kinase as a specific cytotoxic strategy in oncogene-transformed cells. Oncogene. 2003;22:8803–12. doi: 10.1038/sj.onc.1207062. [DOI] [PubMed] [Google Scholar]
- Sanderson KM, Cai J, Miranda G, Skinner DG, Stein JP. Upper tract urothelial recurrence following radical cystectomy for transitional cell carcinoma of the bladder: an analysis of 1,069 patients with 10-year followup. J Urol. 2007;177:2088–94. doi: 10.1016/j.juro.2007.01.133. [DOI] [PubMed] [Google Scholar]
- Weinstein IB, Joe AK. Mechanisms of disease: Oncogene addiction--a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006;3:448–57. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]