Abstract
The pancreatic islet displays diverse patterns of endocrine cell arrangement. The prototypic islet, with insulin-secreting β-cells forming the core surrounded by other endocrine cells in the periphery, is largely based on studies of normal rodent islets. Recent reports on large animals, including humans, show a difference in islet architecture, in which the endocrine cells are randomly distributed throughout the islet. This particular species difference has raised concerns regarding the interpretation of data based on rodent studies to humans. On the other hand, further variations have been reported in marsupials and some nonhuman primates, which possess an inverted ratio of β-cells to other endocrine cells. This review discusses the striking plasticity of islet architecture and cellular composition among various species including changes in response to metabolic states within a single species. We propose that this plasticity reflects evolutionary acquired adaptation induced by altered physiological conditions, rather than inherent disparities between species.
Keywords: pancreatic β-cell, α-cell, δ-cell, PP-cell, islet of Langerhans, islet architecture
Introduction
Pancreatic islets are highly-vascularized micro-organs1,2 which consist of several endocrine cell types that function together to maintain glucose homeostasis. The major hormone-secreting cell types include β-cells (insulin), α-cells (glucagon),3–5 δ-cells (somatostatin),3,6 PP-cells (pancreatic-polypeptide)7,8 and ε-cells (ghrelin).9,10 Murine islets are the most well studied and are often described as having a highly ordered structure that is composed primarily of β-cells clustered in a central core, surrounded by smaller numbers of α-cells, δ-cells, PP-cells and ε-cells in the periphery.3,11,12 Current islet studies, however, starkly distinguish human islet architecture from that of rodents.13–16 Unlike the defined β-cell core and α-cell mantle characteristic of rodent islets, human islets have been described as maintaining a more scattered organization of endocrine cells and a higher percentage of α-cells.13,14 Differences in islet structure have additionally been noted in studies of more distantly related organisms as well. The expanded presence of α-cells in marsupial islets17–19 and δ-cells in bird islets,16 and the presence of scattered endocrine cells throughout the pancreatic tissue and absence of distinct islets in some reptiles20 are only a few such examples.
We have recently shown that islet composition varies not only between species, but also within species.16 Murine islets display a wide array of morphologies under various physiological conditions, including pregnancy, obesity and diabetes. In db/db mice, which are obese and diabetic due to a mutation in the leptin receptor, islet architecture and cellular composition resemble that of humans. Here we review pancreatic islet architecture and endocrine cell composition from fish to humans.
Rodent
Laboratory mice (Mus musculus) and rats (Rattus norvegicus) have played a central role in studies of islet biology. Their islets have a well-defined structure with a central core of β-cells representing 60–80% of the cells of the islet and a layer of other endocrine cells surrounding the core including α-cells (15–20% of the cells of the islet), δ-cells (<10% of islet cells) and PP-cells (<1% of cells).13–16,21,22 The α-cells appear in many tissue sections to form a continuous mantle around the β-cell core. However, three-dimensional analysis of their distribution shows that the α-cells form a non-continuous mantle.15 The size of mouse and rat islets can vary considerably, from ten or fewer cells to thousands of cells.16,22
In the adult guinea pig (Cavia porcellus), the islets are composed primarily of β-cells that are distributed throughout the islet in large groups.23,24 The α-cells are primarily located in the periphery, with a few scattered α-cells located in the interior. The δ-cells are often centrally located within the islet whereas PP-cells are peripherally located, either singly or in groups of two to three cells. In the African ice rat (Otomys sloggetti robertsi), the β-cells form a central core surrounded by two to three layers of α-cells.25 This species displays a greater proportion of α-cells and δ-cells than other species of rats and mice. The β-cells are also centrally located in the islets of the common vole (Microtus arvalis).26
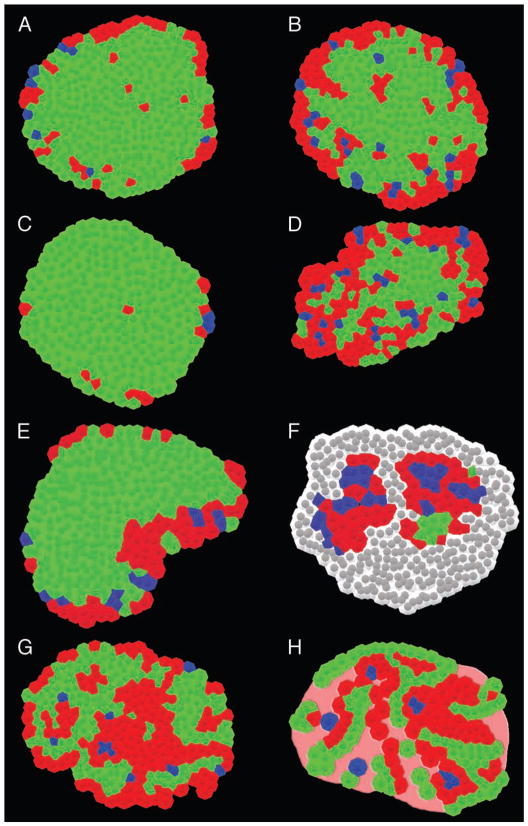
Our studies of mouse islets in different physiological states including diabetes, pregnancy and obesity have revealed a high degree of structural plasticity.16 The islets from diabetic db/db leptin receptor mutant mice display a random internal organization with a higher ratio of α-cells similar to that seen in humans, while average islet size is similar to normal mice.16 They also show an increased ratio of δ-cells that are present all throughout the islet. Pregnant mouse islets maintain a cellular arrangement similar to the one observed in normal mice, except for a small population of α-cells occasionally appearing in islet centers and a slightly increased ratio of α-cells especially in small islets.15,16 The islets of obese ob/ob mice who have a mutation in leptin are nearly entirely composed of β-cells with α-cells scattered along the periphery.16 Diabetic non-obese diabetic (NOD) mice have decreased numbers of islets and those islets that are present have severely reduced number of β-cell and elevated numbers of α-cells, δ-cells and PP-cells.27–29 Studies in obesity-prone yellow Avy/− mice have shown that increased β-cell mass precedes the onset of obesity.30
Zucker fatty rats who like db/db mice have a mutation in the leptin receptor are insulin-resistant, although not overtly diabetic. In these animals, β-cell mass begins to increase after six weeks of age, peaking at nineteen weeks; over this time, the pancreatic volume density of β-cells increases from 2 to 4%.31
There may also be regional differences in islet distribution in the rodent pancreas. Some studies suggest that there is a higher number of islets in the tail of the pancreas compared to the head.11 Our studies suggest that islet size and distribution is similar in different regions of the adult mouse pancreas.16 PP-cells are more common in the head region of the pancreas, while α-cells are more common in the tail.32,33
We have examined the formation of mouse islets during development. Our studies suggest that islets form through a process of fission that occurs in the first two weeks of life.34 The endocrine cells in newborn mice are present in cord-like structures with α-cells peripherally located around a core of β-cells. These cord-like structures then divide into discrete islets. The β-cells and α-cells continue to divide with β-cell replication outpacing that of α-cells and, the percentage of β-cells increases from ~60% to greater than 90%.
Human
Human islets tend to contain fewer β-cells and more α-cells compared to rodent islets.13–16,35 The endocrine cells in human islets also do not have a distinct distribution as in rat islets where β-cells form the core of the islet and there is a mantle of other endocrine cells. In human islets, the α-, β- and δ-cells appear to be randomly distributed throughout the islet.13,16,34 The adult human islet is about ~50% β-cells, ~40% α-cells, 10% δ-cells and few PP-cells.13,14 Cabrera reported little difference in the proportion of endocrine cells in islets from different regions of the pancreas although Brissova and Stefan described greater numbers of PP-cells in the head and greater numbers of α-cells and β-cells in the neck, body and tail.13,14,36 Small islets appear to be composed of mainly β-cells, whereas large islets have intermingled α- and β-cells.16 It is worth noting that diabetic mouse islets show a similar intermingling of α- and β-cells, suggesting a high degree of plasticity in islet morphology in response to changing physiological conditions.16
Studies of islet structure during human development suggest that the organization of endocrine cells in islets in human embryos is similar to those of adult mice.37 In 12–14 week gestation human pancreas, the endocrine cells are located in clusters, in which β-cells form a core with a periphery of α-cells and δ-cells similar to mouse islets. However, by 18-weeks of gestation, the peripheral α-cells and δ-cells dissociate from the β-cell core to form juxtaposed homogenous islets. Presumably, these homogenous islets would reintegrate after week 22 to form the configuration seen in adult islets. Previous studies present a similar observation in islet plasticity; while the amount of insulin content increases with age, the amount of glucagon peaks in the fetus (at ~20 wk), decreases in infants, and then increases in adults.38
A recent study has suggested that β-cell replication is the major mechanism of β-cell mass expansion in early childhood as it is in rodents.39 This β-cell growth occurs most rapidly in infants, prior to the age of five. β-cell proliferation does not increase during adolescence.
In obese humans, β-cells increase in number to compensate for increased insulin demand.40,41 In patients with type 2 diabetes, there are reduced numbers of β-cells due to increased apoptosis; however, islet morphology is not significantly different between type 2 diabetic and non-diabetic subjects except for the presence of amyoloid deposits in the islets of type 2 diabetic subjects.41 In subjects with type 1 diabetes, β-cell mass is drastically reduced, and 50–70% of the islet is composed of α-cells.42
Nonhuman Primate
β-cells are the predominant cell type in the islets of nonhuman primates, as they are in mice.43,44 However, unlike murine islets, the α-cells are located in the interior and the β-cells peripherally.22,43
β-cells are arranged in an irregular mantle around the islet or in single cells or clusters on the exterior of the islet in some species, such as the crab-eating macaque (Macaca fascicularis).22 In Platyrrhini monkeys, which include the common marmoset (Callithrix jacchus), the squirrel monkey (Saimiri boliviensis), the owl monkey (Aotus azarae) and the brown capuchin monkey (Cebus apella), β-cells are located in a peripheral mantle or in polar regions, although in some islets β-cells are found in the interior.44 β-cells comprise 60–90% of islet cells in Platyrrhini monkeys.44
The α-cells are typically located centrally in the islet, although they are occasionally found in the periphery.22,43 In Platyrrhini monkeys, the α-cells are found in ribbons running through the core region, or in the polar regions of islets.44 α-cells comprise 20% of islet cells in Platyrrhini monkeys.44 In the Chacma baboon (Papio ursinus), α-cells are most commonly found in islets from the tail of the pancreas.45
Depending on the species, the δ-cells are located in the islet core (as in the bonnet macaque, Macaca radiata), distributed throughout the islet (as in Macaca fascicularis), or in the periphery (as in Platyrrhini monkeys).22,43 The δ-cells comprise ~5% of islet cells.44
PP-cells are rare and primarily located in the islet periphery in Macaca fascicularis.22 In Papio ursinus, PP-cells are found in clumps in islets from the uncinate process of the head of the pancreas. 45 Co-localization of glucagon and pancreatic polypeptide has also been noted in islets of the Vervet monkey (Chlorocebus pygerythrus), both during development and in the adult.47
The islets of streptozotocin-induced diabetic rhesus monkeys (Macaca mulatta) have decreased numbers of β-cells and a relative increase in the proportion of α- and δ-cells.46
The architecture of islets from the type 2 diabetic baboon (Papio hamadryas), is similar to those humans with type 2 diabetes. 48 Amyloid deposits are spread throughout the islet, with increased numbers of α-cells present in the islet core and fewer β-cells throughout the islet, all of which are arranged in a manner that resembles type 2 diabetic human islets.
Domestic Cat
The islets of the domestic cat (Felis domesticus) are composed mainly of β-cells with a few scattered α- and δ-cells.49 Some islets are found in the interlobular connective tissue. These islets have a centralized area of α-cells with an equal number of surrounding β-cells and a few solitary δ-cells. α-, β- and δ-cells have also been observed in pancreatic ducts and nerves, and in ganglia of ductules near interlobular pancreatic ducts.
The relative proportion of α, β and δ-cells is similar to human islets.49 Thirty percent of islet cells are α-cells in all regions of the pancreas except in the head.50 PP-cells are numerous in the body and tail of the pancreas, but present single cells or in small groups in the head.51
Domestic Dog
The islets of dogs (Canis lupus familiaris) at 6-mo of age are composed of a core of β-cells (>50% of the islet) and are generally surrounded by peripherally located α-cells (<30%).52 However, α-cells can occasionally be found in the interior of the islet and β-cells are sometimes found on the periphery.22,53 About 75% of the β-cells are present in the islets, with the other 25% being located in the exocrine tissues near the acinar glands.54 The δ-cells tend to be randomly distributed through the islet.52,53
The size and cellular composition of canine islets varies according to their location in the pancreas. The tail of the pancreas tends to contain islets that are large and compact, while the head contains smaller islets and single cells. The α-, β- and δ-cells tend to congregate in organized islets in the tail lobe.22,54 The δ-cells comprise <15% of islet cells in the tail lobe, and very few α-cells are found in the head.52,53 PP-cells comprise <4% of all pancreatic endocrine cells, and tend to predominate in the head of the pancreas.55,56 The α-, β- and δ-cells are found in very small numbers in the head of the pancreas.22 Canine islets typically contain 20–30 cells, although clusters of a few cells are found in connective tissues adjacent to the exocrine ducts.22,53
Studies have been performed on dogs with diabetes or chronic pancreatic insufficiency. The degenerative condition of chronic pancreatic insufficiency primarily affects the exocrine tissues, but dogs affected by the disorder are found to have dispersed islets with irregular islet morphology.57 The β-cells in these islets are speculated to be due to β-cell regeneration. In a line of diabetic Keeshond dogs aged from birth to 6-months of age, β-cells are congenitally absent.54
Fur Seal
The most abundant endocrine cell type found in seal (Arctocephalus pusillus) islets are β-cells, which are confined to the core, followed by α-, δ- and PP-cells that are localized in the periphery.58 While α and δ-cells are randomly distributed around the periphery, PP-cells are typically restricted to one pole. Variation in the shape and distribution of endocrine cells in the mantles is similar to that of other carnivores, particularly cats and dogs. There are fewer but larger, elongated islets in the head of the pancreas, as opposed to smaller and more circular ones in the body and tail. Overall, seal islets show considerable heterogeneity in shape.
Fruit Bat
Endocrine tissue comprises ~10% of the volume of the pancreas in the Egyptian fruit bat (Rousettus aegyptiacus).59 The islets are large and irregularly shaped. β-cells are found throughout the islet and comprise ~50% of islet cells. α-cells comprise ~30% of islet cells and are typically found toward the periphery of the islet without forming a complete mantle around it, but can also be found within the interior. δ-cells comprise ~10% of islet cells and can be found individually or in small clusters within the islet but generally in the periphery. PP-cells consist of ~15% of islet cells and are scattered throughout the islet, with most in the periphery.
Horse
β-cells comprise the majority of the endocrine cell population of islets and form a near continuous mantle around the islet in horses (Equus ferus caballus). A central mass of α-cells is observed, almost entirely surrounded by a mantle of β-cells.60,61 δ-cells are found discontinuously in the islet periphery, either in the outermost periphery with β-cells or between the α- and β-cell groups.62,63 PP-cells are found sporadically in the periphery as well.60 As has been noted with other species, islet composition and size vary between the pancreatic regions. The duodenal region of the pancreatic body has a greatly reduced α-cell population (0.1% compared to ~30% in the other regions), while the concentration of PP-cells is greatly increased (12% compared to ~1% in the other regions).60,61 In the head, β-cells comprise the majority of islets, with δ-cells located among β-cells singly or in small clusters, and PP-cells located in the periphery of islets, either in clusters or in a layer.60,62 Islets are also smaller in the head, with an average diameter of about half of that in the other regions.60
Cattle
Cattle (Bos taurus) islets exhibit a central mass of β-cells, which comprise the primary endocrine cell population.64–66 This central mass is partially surrounded by a mantle of α-cells, although β-cells sometimes extend into the periphery. δ-cells are also located primarily in the periphery of the islet, sometimes extending into the central portion, while PP-cells are located in a dense region in the periphery of the islet. Two populations of islets are observed: small islets, 25–200 μm in diameter, and larger islets.64 The relative population of large islets decreases as the animal ages, with the number of large islets becoming negligible in the adult.64 Two types of islet are reported: islets with high numbers of α-cells and islets with high numbers of PP-cells (and β-cells).67 In addition, there are more δ-cells in islets with higher proportions of α-cells, and more PP-cells in islets with lower proportions of α-cells.
Studies of the endocrine cell development in fetal calves show that α-cells, β-cells, δ-cells and PP-cells appear before the 100th day of gestation.68 The fetal islets are organized with a central core of β-cells surrounded by α-cells in the periphery.
Domestic Goat
β-cells are the primary endocrine cell population in goat (Capra aegagrus hircus) islets.69 The β-cells are centrally located and α-cells are typically located in the periphery. δ-cells are arranged in ribbons that run throughout the islet, and are present in both the interior and periphery. PP-cells are located in a dense segment within the islet.69
Domestic Sheep
Fetal endocrine cell development and islet formation has been studied in domestic sheep (Ovis aries).70 The β-cells are present as both clusters (i.e., islets) and as single cells near ducts from 60–80 days gestation. From 80 days, nearly all β-cells are identified as part of a developing islet. The α-cells have a distribution similar to β-cells, although they are less numerous and concentrated peripherally in the islet. They are represent a greater proportion of endocrine cells in islets >80 days gestation. At 40 days gestation, PP-cells are rare and only found as single cells, but as the fetus develops, the number of PP-cells increases. The PP-cells are located peripherally, and occasionally in the islet core. The islet composition of δ-cells mirrors that of PP-cells, with a very small proportion of single δ-cells early on that located peripherally and centrally with the developing islet and higher numbers at later stages of fetal development.
Domestic Pig
β-cells are found as single cells or grouped together to form the core of islets at all ages in domestic pigs (Sus domesticus).71 Some α-cells are located in the center of islets, but most reside in the periphery, along with a smaller number of δ-cells and an even smaller number of PP-cells.72 The number of δ-cells and PP-cells in islets decrease as animals age. PP-cells are more abundant in the head, as opposed to the tail area of the pancreas.71 Pig islets are smaller than 250 μm in diameter at 5-wk gestation, but become larger than 250 μm at 12 and 24-wk.71 Though the number of larger islets increases with gestational age, the percentage volume density of β-cells does not.
In pigs at 8-months of age, there are differences in the cellular composition of islets from different regions of the pancreas with islets in the head being rich in PP-cells and poor in α-cells, while islets in the tail are rich in α-cells and poor in PP-cells.73
Dromedary Camel
β-cells form the primary endocrine cell type and are concentrated in the core of the islet in dromedary camels (Camelus dromedarius).74 They are surrounded by a mantle of α-cells with a small number of δ-cells. PP-cells are detected within the islet, as well as in the exocrine tissue. The endocrine cells are found in roughly equal quantities and proportions in various regions of the pancreas.
Rabbit
In adult rabbits (Oryctolagus cuniculus), β-cells comprise more than two thirds of islet endocrine cells.75 Islets are composed either entirely of β-cells, or of several cell types, with the majority being β-cells. α-cells are the next most populous cell type, comprising ~25% of endocrine cells, and can typically be found forming a mantle around a β-cell core.16,75 The δ-cells are found in lower numbers than α-cells, and tend to form clusters within the islet.
The rabbit pancreas is characterized as a mesenterial-type, which means that only the tail portion of the pancreas is a compact organ.75 There are minor differences in the distribution of α- and δ-cells between neonatal and adult pancreata. There are also cells that appear to express both glucagon and PP.75 Rabbit islets are especially heterogeneous in with respect to endocrine cell composition. There are islets with a high percentage of β-cells, then α-cells, and a few δ-cells, islets containing more δ- than α-cells, islets with α- and δ-cells both located at only one pole, and islets composed entirely of β-cells. There are islets with β-cells in the core and α- and δ-cells in the periphery, and there are also islets with a more random distribution of endocrine cells.75
Striped Hyena
β-cells are found both in the core and periphery of the islet in striped hyenas (Hyaena hyaena).76 Some β-cells are scattered among the exocrine tissues. α-cells are only located peripherally, forming an incomplete mantle. δ-cells and PP-cells are fewer in number; δ-cells are located evenly throughout the entire islet, while PP-cells are seen only in the periphery of islets.
Water Buffalo
Pancreatic islets can be divided into three types in water buffalos (Bubalus bubalis).77 Large islets (~500 μm in diameter) are composed primarily of β-cells, occasionally with interspersed α-cells, δ-cells and PP-cells arranged singly. Smaller islets (~150 μm in diameter) contain all four endocrine cell types, with a core of β-cells surrounded by α-cells that can form ribbons across the periphery. β-cells consist of the majority of cells, and δ-cells and PP-cells form a mantle around the periphery of this islet type. In these islets, PP-cells are the least common cell type. Small islets (~10 μm in diameter), the third islet type, are composed primarily of PP-cells, which aggregate at one pole of the islet (in a few cases comprising nearly the entirety of the islet). The α-, β- and δ-cells can also be found in these islets.
When neonatal and early juvenile specimens are compared to adults, the relative percentage of β-cells and PP-cells are observed to decrease during development, while the percentages of α-cells and δ-cells increase.77 Nonetheless, β-cells maintain a majority throughout development.
Beluga Whale
Islets contain both α- and β-cells, though some islets are exclusively composed of one or the other endocrine cell type in beluga whales (Delphinapterus leucas).78 While most α-cells are located in the periphery and β-cells in the core, the relative numbers of α- and β-cells are found to vary considerably between individual whales. The average percentage of α-cells is ~40% but the range is 3–70%. This variation is not associated with age, gender or body mass.
African Elephant
In African elephants (Loxodonta africana), β-cells are located in the islet center while α and δ-cells are mainly located in the periphery.79 There is a higher percentage of islets in the body and tail of the pancreas than in the head. A few α and δ-cells are found in the ductal epithelium, though with no difference between the head and tail regions.79 A few single PP-cells and numerous slender neuropeptide Y (NPY)-cells are also found in islets.79
Three-Toed Sloth
β-cells are typically located in the islet core, and are most numerous in the head region of the pancreas in three-toed sloths (Bradypus variegatus).80 The α-cells reside in the islet periphery and are slightly more numerous than β-cells. The α-cells are more abundant in the body and tail of the pancreas and largely absent from the head. α-cells are also found in the vicinity of capillaries. δ-cells are few in number scattered throughout the islet. PP-cells are not detected.
Marsupial
Islet structure has been studied in a large number of marsupials. β-cells represent only 8% of islet endocrine cells in red kangaroos (Macropus rufus, formerly Megaleia rufa) and 15% in grey kangaroos (Macropus giganteus). The α-cells are the predominant endocrine cells comprising up to 70% of the islet cells, depending on the species.17 A predominance of α-cells has also been reported in opossum species, including the Virginia opossum (Didelphis virginiana) and the common brushtail opossum (Trichosurus vulpecula; β-cells and α-cells, ~50% and ~40% of endocrine cells, respectively).17–19
The distribution of endocrine cells within the islets differs among species. In the kangaroo species examined, the β-cells are found in peripheral clusters, with occasional β-cells in the islet interior which is composed of α-cells.17 In Trichosurus vulpecula, β-cells are found in the periphery and α-cells in the islet center, except where they reached the periphery through β-cell clusters.17,19 δ-cells are reportedly found throughout the islet.17 In Didelphis virginiana and the Australian fat-tailed dunnart (Sminthopsis crassicaudata), β-cells are located in the center of the islet, surrounded by a mantle of α-, δ- and PP-cells.18,81 δ-cells are typically located two or three cell layers beneath the islet surface, and α-cells are found to form knot-like structures and occur individually.18 In Trichosurus vulpecula, some islets are composed of predominantly PP-cells.19 Islets are found to contain a mean of ~33 endocrine cells in grey kangaroos, ~43 cells in red kangaroos, and ~48 cells in Trichosurus vulpecula.18
Pancreatic development studies have been conducted in Didelphis virginiana. Endocrine cells are found in high concentrations in newborn animals, with α-cells and PP-cells predominating. Islets are often poorly defined. The relative abundance of endocrine cells decreases as the animals grow, primarily due to exocrine tissue expansion rather than reduction of endocrine tissue.18
Echidna
Islets are uniformly distributed through all regions of the pancreas in echidnas (Tachyglossus aculeatus).82 Islets are grouped into two categories: those producing either insulin or pancreatic polypeptide. These islet types are found in different pancreatic lobes, with PP islets present in the head region. Insulin-producing islets contain α-, β-, δ- and PP-cells, with β-cells being the most numerous. PP-cell islets are primarily composed of PP-cells, with few or no other endocrine cells present.
Bird
Three different types of islets have been observed in birds, primarily studies of the white-crowned sparrow (Zonotrichia leucophrys gambelii), the mallard (Anas platyrhynchos) and the chicken (Gallus gallus).83,84 They include: (1) Dark-staining islets consisting of α- and δ-cells and a few isolated β-cells; (2) Light-staining islets consisting of pericapillary β-cells with a thin layer of δ-cells at the periphery; and (3) Mixed islets consisting of α-, δ- and β-cells. The proportions of α-, δ- and β-cells are similar and do not vary seasonally, by photostimulation, or by the amount of fat deposition in Zonotrichia leucophrys gambelii.83 Some species, such as the Australian wedge-tailed eagle (Aquila audax), have mixed islets of random distribution, rather than light and dark islets.85
In the rufous-collared sparrow (Zonotrichia capensis subtorquata), β-cells are located predominantly in the center of the islet. The α- and δ-cells, though mainly present in the periphery, are also found in the interior of islets. PP-cells are exclusively in the periphery of islets and peptide YY ( PYY)-containing cells are rarely seen.86 β-cells are present in all four pancreatic lobes (i.e., dorsal, ventral, third and splenic) of the rose-ringed parakeet (Psittacula krameri) and α-cells only in the splenic and third lobes.87 In pancreatic islets of the Houbara bustard (Chlamydotis undulata), β-cells are located centrally and represent 70% of the endocrine cells, α-cells are found in the periphery at 15%, δ-cells are found in both central and peripheral regions at 22%, PP-cells are also in the periphery at 11%, and there is 20% overlap between α- and PP-cells.88 In some birds, such as the zebra finch (Taeniopygia guttata), β-cells are rare and δ-cells are more common.16
In chickens (Gallus gallus domesticus), most α-cells, as well as other endocrine cells, are concentrated in the tail region of the pancreas, while PP-cells are nearly absent in this region and found [indicate region] in both adults and chicks.89
Reptile
The α- and β-cells are the most common endocrine cell types in the islets of most species of reptiles.20,90 Among snakes, β-cells can compose between 27% of islet cells as in the viperine snake (Natrix maura) and 40% as in the common garter snake (Thamnophis sirtalis). The α-cells comprise 35% of islet cells in Thamnophis sirtalis and 56% in Natrix maura.90 In islets, β-cells tend to be centrally located and α-cells are peripheral to the β-cells.90,91 In Natrix maura and the European asp (Vipera aspis), α-cells are located in the interior of the islet, but remain peripheral to β-cells.90 In Thamnophis sirtalis, α-cells and β-cells are intermingled.92 The δ-cells are nearly as abundant as α- and β-cells in the spectacled caiman (Caiman fuscus), and are located peripherally.20,90–92 PP-cells can be located singly or in small groups.20,91,93 In the spur-thighed tortoise (Testudo graeca), PP-cells are not associated with islets.93 In Caiman fuscus, PP-cells can be occasionally immunoreactive to glucagon.91 In some snake species, such as Vipera aspis and Natrix maura, PP-cells are not detected; however, they are reported in Thamnophis sirtalis and in the Italian wall lizard (Podarcis sicula).90,92,94
Islet shape and organization is highly variable. Islets can be round or elongated, as in Vipera aspis and Natrix maura;90 they can be linked into a continuous structure, as in the ocellated skink (Chalcides ocellatus);91 or endocrine cells may be diffuse, forming no true islet at all, as in Caiman fuscus.20 In species such as the ocellated skink (Chalcides ocellatus) and Testudo graeca, islets tend to predominate in the tail lobe of the pancreas.91,93
Amphibian
β-cells comprise ~40% of islet endocrine cells in the oriental firebellied toad (Bombina orientalis) and occurs mainly as clusters of cells centralized in islets as well as single cells.95,96 Similarly, α-cells which comprise ~20% of islet endocrine cells are found as clusters of cells, both within the core of as well as dispersed throughout the islet, and as single cells.95 δ-cells (~10% of endocrine cells) are found mainly as single or small clusters of cells situated in the exterior area of islets. PP-cells (<10% of cells) are randomly located as single cells in the islet.
The endocrine tissue of the eastern newt (Notophthalmus viridescens) constitutes ~10% of the pancreas.97 As in many mammals, newt islets are mostly composed of centrally located β-cells, with a small portion of α- and δ-cells found predominantly in the periphery.
About 2% of the pancreas of the northern leopard frog (Rana pipiens) consists of endocrine cells, which are typically arranged into islets.98 However, these cells can also be found individually and in clusters of a single cell type within the exocrine tissue. Islets contain a core of β-cells nearly surrounded by a mantle of α- and δ-cells, which can be several cells thick. The α- and δ-cells can also be found in clusters near ribbons of β-cells. Glucagon and pancreatic polypeptide are also found colocalized, with cells only occasionally expressing one or the other.
Fish
Fish display a wide variety of islet architecture and morphology. In the European river lamprey (Lampetra fluviatilis), islets contain a core of β-cells, but appear to lack α-cells.99
In many teleosts, islets can be divided into principal and secondary islets.100 Principal islets (also known as Brockman bodies) are large and few in number, and tend to be arranged as a core of β-cells surrounded by a mantle of α-cells.99–106 In some species, β-cells can occasionally be found in the mantle layer.103 Depending on the species, δ-cells can be found in the core, as in zebrafish (Danio rerio) and carp (Cyprinus carpio), in the mantle, as in the golden mandarin fish (Siniperca scherzeri), or occasionally distributed throughout the islet.99–105 PP-cells can be found in small numbers in the periphery of principal islets (and occasionally in the core in some species, such as Cyprinu carpio), but can make up the majority of cells in secondary islets.101,103,105 Typically, all endocrine cell types can also be found in the exocrine tissue. In the European sea bass (Dicentrarchus labrax), the primary islet has a cord-like structure surrounded by a thin layer of endocrine cells; however, the architecture is otherwise typical, with β-cells located in the interior of these cords.105 Teleosts also produce two or more types of somatostatin, often produced by different populations of δ-cells.107
Studies in zebrafish embryos suggest that insulin-expressing cells begin forming in the bilateral pancreatic anlages even before these structures join together to form a single organ.106 These cells are initially somewhat dispersed, but aggregate in the head region roughly 24 hr after fertilization. However, studies in carp embryos show that endocrine cell clusters steadily increase in size and proliferate along the veins of the pancreas.108
Conclusion
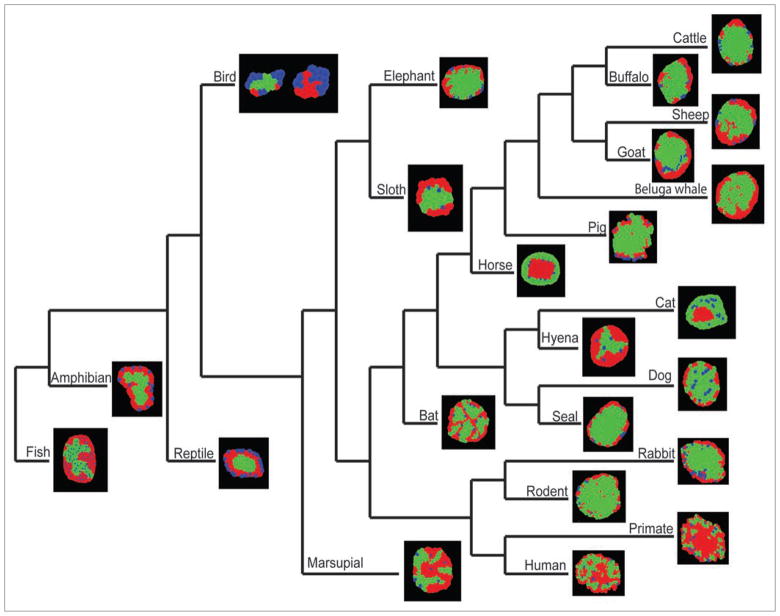
There is great diversity of islet structure among vertebrates, from the principal islet in many species of fish to the individual islets that characterize mice and humans (Table 1, Fig. 1). Moreover, islet structure is not static but changes in response to normal physiology and pathophysiology such as diabetes mellitus (Fig. 2). This diversity suggests that there may be different mechanisms of islet formation between species. In this regard, studies in zebrafish embryos have indicated islet formation by fusion,106 whereas our studies in mice and studies in carp and humans suggest formation by fission.16 As technology advances and more species are analyzed by 3D imaging techniques, more detailed information should be available.
Table 1.
Islet endocrine cell arrangement by species
| Species | α-cells | β-cells | δ-cells | PP-cells | References |
|---|---|---|---|---|---|
| Rodent | Periphery; 7% | Core; 87% | Periphery; 5% | Periphery; rare | 11, 13–16, 21–34 |
| Human | Core + periphery; 36% | Core + periphery; 54% | Core + periphery; 10% | Core + periphery; <5% | 13–16, 34–42 |
| Nonhuman primate | Core (periphery in some species) | Periphery | Core + periphery; random | Periphery; random (rare) | 22, 43–48 |
| Cat | Core + periphery; clusters + single cells; ~30% | Core; ~60% | Core; clusters; ~4% | Periphery; ~1% | 49–51 |
| Dog | Periphery; <30% | Core; >50% | Core + periphery; <15% | <4% in head lobe | 22, 52–57 |
| Fur seal | Periphery; random | Core; highest % | Periphery | Periphery (one pole) | 58 |
| Bat | Periphery, radiating inward; 30% | Core + periphery; 50% | Periphery; single cell + clusters; 10% | Periphery, radiating inward; 15% | 59 |
| Horse | Core | Periphery; continuous mantle | Periphery, outermost or between α- and β-cells | Periphery; sporadic | 60–63 |
| Cow | Periphery; few in core | Core | Core + periphery; ribbon-like pattern | Periphery | 64–68 |
| Goat | Periphery | Core | Core + periphery; “ribbon-like” | Dense region in islet | 69 |
| Sheep | Core + periphery; Random | Periphery; clusters | P40—single cells; P80—clusters | P40—single cells; P80—clusters | 70 |
| Pig | Periphery; some core | Core | Periphery | Periphery; very rare | 67–73 |
| Camel | Periphery | Core | Few | Few | 74 |
| Rabbit | Periphery | Core + periphery | Single cell + clusters | 0.5–1% | 75 |
| Hyena | Periphery | Core + periphery | Core + periphery | Periphery | 76 |
| Water buffalo | Periphery; form cord-like structures | Core | Periphery | Periphery | 77 |
| Beluga whale | Periphery | Core | No data | No data | 78 |
| Elephant | Periphery | Core | Periphery | Single cells; rare | 79 |
| Three-toed sloth | Periphery; highest % | Core | Core + periphery | Not detected | 80 |
| Marsupial | Core + periphery; highest % | Core + periphery | Periphery; few in core | Periphery or scattered | 17–19, 81 |
| Echidna | Periphery | Core | Periphery | Core + periphery | 82 |
| Bird | Core, 15% in dark-staining islets | Core, 70% in light-staining islets | Periphery; light and dark islets; 22% | Periphery; overlap with α-cells; 11% | 83–89 |
| Reptile | Periphery | Core | Periphery | Rare | 20, 90–94 |
| Amphibian | Core + periphery; multiple or singular layers; 20% | Core; 40% | Periphery; 10% | Periphery; random; <10% | 95–98 |
| Fish | Periphery | Core | Core + periphery | Periphery | 99–108 |
Figure 1.
Multi-species comparison of islet structure and composition. Islets from various species are organized into a phylogenetic tree. Representative islets are pseudo-colored models of actual islets based on immunohistochemical images composed of α-cells (red), β-cells (green) and δ-cells (blue). The following species are shown: mouse (Mus musculus); human (Homo sapiens sapiens); rhesus macaque (Macaca mulatta); cat (Felis domesticus); dog (Canis lupus familiaris); fur seal (Arctocephalus pusillus); Egyptian fruit bat (Rousettus aegyptiacus); horse (Equus ferus caballus); cattle (Bos taurus); domestic goat (Capra aegagrus hircus); domestic sheep (Ovis aries); domestic pig (Sus domesticus); European rabbit (Oryctolagus cuniculus); striped hyena (Hyaena hyaena); water buffalo (Bubalus bubalis); beluga whale (Delphinapterus leucas); African elephant (Loxodonta africana); three-toed sloth (Bradypus variegatus); common brushtail opossum (Trichosurus vulpecula); zebra finch (Taeniopygia guttata); European asp (Vipera aspis); northern leopard frog (Rana pipiens) and rainbow trout (Salmo gairdneri). Note that high-quality images were not available for the echidna and camel. No information on δ-cells was available for the beluga whale. The phylogenetic tree was derived from work by Miller et al.109
Figure 2.
Comparison of mouse and human islets under various pathophysiological conditions. Mouse and human islets under various pathophysiological conditions are shown; α-cells (red), β-cells (green), and δ-cells (blue) based on immunohistochemical images. (A) Wild-type mouse (6 mo). (B) Pregnant mouse (3 mo). (C) ob/ob mouse (15 wk). (D) db/db mouse (15 wk). (E) Neonatal mouse (P14). (F) NOD mouse (40 wk). Note that lymphocytes (shown in gray) infiltrate the islet replacing the majority of the endocrine cells.15 (G) Normal human (41 yr).16 (H) Type-2 diabetic human.40 Note that islets from patients with type 2 diabetes often contain acellular amyloid deposits as shown in pink.
The variation in islet structure between species may result from variation in developmental mechanisms. However, we suspect that differing metabolic requirements and physiological conditions play a more prominent role in determining islet structure. We have previously shown in mice that conditions such as pregnancy and obesity result in increased β-cell mass and islet size accompanied with architectural changes.15,16 This adaptability, along with comparable islet size distributions between human and mouse islets, suggests that their structural differences may be more due to different metabolic requirements and conditions rather than to intrinsic differences in islet development between the species.
Studies of islet structure and formation in diverse species under normal and pathophysiological conditions, such as pregnancy, diabetes and obesity, provide a foundation for examining the changes in the organization of alpha- and beta-cells within the islets. Together with lineage tracing, they may provide insight into how these structural changes occur, whether migration of peripheral cells into the islet core or trans differentiation of cells. Comparative studies may also provide insight into the cellular signals, both intrinsic and extrinsic, regulating endocrine cell proliferation and function. This understanding may lead to new approaches for treating diabetes including cell-based therapies to replace pancreatic β-cell lost as a consequence of autoimmune attack or other mechanisms.
Acknowledgments
The authors thank Dr. Graeme Bell for his helpful discussions. The study was supported by US Public Health Service Grants DK-081527 and DK-072473, and DK-020595 to the University of Chicago Diabetes Research and Training Center (Animal Models Core), as well as a gift from the Kovler Family Foundation.
References
- 1.Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes. 1988;37:616–21. doi: 10.2337/diab.37.5.616. [DOI] [PubMed] [Google Scholar]
- 2.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120:59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 3.Samols E, Bonner-Weir S, Weir GC. Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab. 1986;15:33–58. doi: 10.1016/s0300-595x(86)80041-x. [DOI] [PubMed] [Google Scholar]
- 4.Weir GC, Bonner-Weir S. Islets of Langerhans: the puzzle of intraislet interactions and their relevance to diabetes. J Clin Invest. 1990;85:983–7. doi: 10.1172/JCI114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 6.Weir GC, Bonner-Weir S. Pancreatic somatostatin. Adv Exp Med Biol. 1985;188:403–23. doi: 10.1007/978-1-4615-7886-4_22. [DOI] [PubMed] [Google Scholar]
- 7.Lonovics J, Devitt P, Watson LC, Rayford PL, Thompson JC. Pancreatic polypeptide. A review. Arch Surg. 1981;116:1256–64. doi: 10.1001/archsurg.1981.01380220010002. [DOI] [PubMed] [Google Scholar]
- 8.Floyd JC., Jr Pancreatic polypeptide. Clin Gastroenterol. 1980;9:657–78. [PubMed] [Google Scholar]
- 9.Broglio F, Prodam F, Riganti F, Muccioli G, Ghigo E. Ghrelin: from somatotrope secretion to new perspectives in the regulation of peripheral metabolic functions. Front Horm Res. 2006;35:102–14. doi: 10.1159/000094313. [DOI] [PubMed] [Google Scholar]
- 10.Hill JT, Mastracci TL, Vinton C, Doyle ML, Anderson KR, Loomis ZL, et al. Ghrelin is dispensable for embryonic pancreatic islet development and differentiation. Regul Pept. 2009;157:51–6. doi: 10.1016/j.regpep.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186:629–37. [PMC free article] [PubMed] [Google Scholar]
- 12.Orci L, Unger RH. Functional subdivision of islets of Langerhans and possible role of D Cells. Lancet. 1975;2:1243–4. doi: 10.1016/s0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- 13.Cabrera O, Berman M, Kenyon NS, Ricordi C, Berggren P, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. PNAS. 2006;103:2334–9. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberb B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–97. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 15.Kharouta M, Miller K, Kim A, Wojcik P, Kilimnik G, Dey A, et al. No mantle formation in rodent islets—the prototype of islet revisited. Diabetes Research and Clinical Practice. 2009;85:252–7. doi: 10.1016/j.diabres.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim A, Miller K, Jo J, Kilimnik G, Wojcik P, Hara M. Islet architecture: a comparative study. Islets. 2009;1:129–36. doi: 10.4161/isl.1.2.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White AW, Harrop CJF. The islets of Langerhans of the pancreas of macropodid marsupials: a comparison with eutherian species. Aust J Zool. 1975;23:309–19. [Google Scholar]
- 18.Krause WJ, Cutts JH, 3rd, Cutts JH, Yamada J. Immunohistochemical study of the developing endocrine pancreas of the opossum (Didelphis virginiana) Acta Anat. 1989;135:84–96. doi: 10.1159/000146727. [DOI] [PubMed] [Google Scholar]
- 19.Reddy S, Bibby NJ, Fisher SL, Elliott RB. Immunolocalization of insulin, glucagon, pancreatic polypeptide and somatostatin in the pancreatic islets of the possum, Trichosurus vulpecula. Gen Comp Endocrinol. 1986;64:157–62. doi: 10.1016/0016-6480(86)90042-0. [DOI] [PubMed] [Google Scholar]
- 20.Rhoten WB. Somatostatin-containing and other endocrine cells in the pancreas of the spectacled caiman. Acta Anat. 1987;129:257–61. doi: 10.1159/000146411. [DOI] [PubMed] [Google Scholar]
- 21.Quesada I, Tudurí E, Ripoll C, Nadal Á. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. Journal of Endocrinology. 2008;199:5–19. doi: 10.1677/JOE-08-0290. [DOI] [PubMed] [Google Scholar]
- 22.Wieczorek G, Pospischil A, Perentes E. A comparative immunohistochemical study of pancreatic islets in laboratory animals (rats, dogs, minipigs, nonhuman primates) Exp Toxicol Pathol. 1998;50:151–72. doi: 10.1016/S0940-2993(98)80078-X. [DOI] [PubMed] [Google Scholar]
- 23.Reddy S, Bibby NJ, Elliott RB. Cellular distribution of insulin, glucagon, pancreatic polypeptide hormone and somatostatin in the fetal and adult pancreas of the guinea pig: a comparative immunohistochemical study. Eur J Cell Biol. 1985;38:301–5. [PubMed] [Google Scholar]
- 24.Baskin DG, Gorray KC, Fujimoto WY. Immunocytochemical identification of cells containing insulin, glucagon, somatostatin and pancreatic polypeptide in the islets of Langerhans of the guinea pig pancreas with light and electron microscopy. Anat Rec. 1984;208:567–78. doi: 10.1002/ar.1092080412. [DOI] [PubMed] [Google Scholar]
- 25.Gustavsen CR, Pillay N, Heller RS. An immunohistochemical study of the endocrine pancreas of the Africa ice rat, Otomys sloggetti robertsi. Acta Histochemica. 2008;110:294–301. doi: 10.1016/j.acthis.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki M, Arai T, Komeda K. An ultrastructural study on the pancreatic islet B cells in diabetic herbivorous voles. Vet Res Commun. 1991;15:205–10. doi: 10.1007/BF00343225. [DOI] [PubMed] [Google Scholar]
- 27.Gómez Dumm CLA, Cónsole GM, Luna GC, Dardenne M, Goya RG. Quantitative immunohistochemical changes in the endocrine pancreas of nonobese diabetic (NOD) mice. Pancreas. 1995;11:396–401. doi: 10.1097/00006676-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Sreenan S, Pick AJ, Levisetti M, Baldwin AC, Pugh W, Polonsky KS. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes. 1999;48:989–96. doi: 10.2337/diabetes.48.5.989. [DOI] [PubMed] [Google Scholar]
- 29.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–45. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- 30.Warbritton A, Gill AM, Yen TT, Bucci T, Wolff GL. Pancrratic islet cells in preobese yellow Avy/− mice: relation to adult hyperinsulinemi and obesity. Proc Soc Ex Biol Med. 1994;206:145–51. doi: 10.3181/00379727-206-43733. [DOI] [PubMed] [Google Scholar]
- 31.Augstein P, Salzsieder E. Morphology of pancreatic islets: a time course of pre-diabetes in Zucker fatty rats. Methods Mol Biol. 2009;560:159–89. doi: 10.1007/978-1-59745-448-3_12. [DOI] [PubMed] [Google Scholar]
- 32.Park I, Bendayan M. Coexistence of glucagon and pancreatic polypeptide in human and rat pancreatic endocrine cells. Endocrine Pathology. 1992;3:134–43. doi: 10.1007/BF02921354. [DOI] [PubMed] [Google Scholar]
- 33.Ku SK, Lee HS. An immunohistochemical study of the pancreatic endocrine cells of the nude mouse, Balb/c-nu/nu. Eur J Histochem. 2006;50:61–8. [PubMed] [Google Scholar]
- 34.Miller K, Kim A, Kilimnik G, Jo J, Moka U, Periwal V, Hara M. Islet formation during the neonatal development in mice. PLoS ONE. 2009;4:7739. doi: 10.1371/journal.pone.0007739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grube D, Bohn R. The microanatomy of human islets of Langerhans, with special reference to somatostatin (D−) cells. Arch Histol Jpn. 1983;46:327–53. doi: 10.1679/aohc.46.327. [DOI] [PubMed] [Google Scholar]
- 36.Stefan Y, Grasso S, Perrelet A, Orci L. The pancreatic polypeptide-rich lobe of the human pancreas: definitive identification of its derivation from the ventral pancreatic primordium. Diabetologia. 1982;23:141–2. doi: 10.1007/BF01271177. [DOI] [PubMed] [Google Scholar]
- 37.Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem. 2009;57:811–24. doi: 10.1369/jhc.2009.953307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefan Y, Grasso S, Perrelet A, Orci L. A quantitative immunofluorescent study of the endocrine cell populations in the developing human pancreas. Diabetes. 1983;32:293–301. doi: 10.2337/diab.32.4.293. [DOI] [PubMed] [Google Scholar]
- 39.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, et al. B-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008;57:1584–94. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type II diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 41.Bonner-Weir S, O’Brien TD. Islets in type 2 diabetes: in honor of Dr. Robert C. Turner Diabetes. 2008;57:2899–904. doi: 10.2337/db07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanafusa T, Miyazaki A, Miyagawa J, Tamura S, Inada M, Yamada K, et al. Examination of islets in the pancreas biopsy specimens from newly diagnosed type 1 (insulin-dependent) diabetic patients. Diabetologia. 1990;33:105–11. doi: 10.1007/BF00401048. [DOI] [PubMed] [Google Scholar]
- 43.Sujatha SR, Pulimood A, Gunasekaran S. Comparative immunocytochemistry of isolated rat & monkey pancreatic islets cell types. Indian J Med Res. 2004;119:38–44. [PubMed] [Google Scholar]
- 44.Sánchez A, Celani S, Lawzewitsch IV. Pancreatic islets in Platyrrhini Monkeys: Callithrix jacchus, Saimiri boliviensis, Aotus azarae and Cebus apalla. A cytological and immunocytochemical study. Primates. 1991;32:93–103. [Google Scholar]
- 45.Wolfe-Coote SA, Du Toit DF. Distribution of cell types of the islets of Langerhans throughout the pancreas of the Chacma baboon. Anat Rec. 1987;217:172–7. doi: 10.1002/ar.1092170209. [DOI] [PubMed] [Google Scholar]
- 46.Jones CW, Reynolds WA, Hoganson GE. Streptozotocin diabetes in the monkey: plasma levels of glucose, insulin, glucagon and somatostatin, with corresponding morphometric analysis of islet endocrine cells. Diabetes. 1980;29:536–46. doi: 10.2337/diab.29.7.536. [DOI] [PubMed] [Google Scholar]
- 47.Wolfe-Coote S, Louw J, Woodroof C, du Toit DF. Development, differentiation and regeneration potential of the Vervet monkey endocrine pancreas. Microsc Res Tech. 1998;43:322–31. doi: 10.1002/(SICI)1097-0029(19981115)43:4<322::AID-JEMT6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, Majluf-Cruz A, et al. Pancreatic islet amyloidosis, β-cell apoptosis, and α-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. PNAS. 2009;106:13992–7. doi: 10.1073/pnas.0906471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien TD, Hayden DW, Johnson KH, Fletcher TF. Immunohistochemical morphometry of pancreatic endocrine cells in diabetic, normoglycaemic glucose-intolerant and normal cats. J Comp Pathol. 1986;96:357–69. doi: 10.1016/0021-9975(86)90031-9. [DOI] [PubMed] [Google Scholar]
- 50.Furuzawa Y, Ohmori Y, Watanabe T. Immunohistochemical morphometry of pancreatic islets in the cat. J Vet Med Sci. 1992;54:1165–73. doi: 10.1292/jvms.54.1165. [DOI] [PubMed] [Google Scholar]
- 51.Larsson LI, Sundler F, Håkanson R. Pancreatic polypeptide— a postulated new hormone: identification of its cellular storage site by light and electron microscopic immunocytochemistry. Diabetologia. 1976;12:211–26. doi: 10.1007/BF00422088. [DOI] [PubMed] [Google Scholar]
- 52.Muranishi T, Takehana K, Hiratsuka T, Kobayashi A, Eerdunchaolu, Iwasa K, et al. An investigation of the relationship between duct system and A cell-rich and PP cell-rich pancreatic islets in the canine pancreas. J Vet Med Sci. 1999;61:737–42. doi: 10.1292/jvms.61.737. [DOI] [PubMed] [Google Scholar]
- 53.Hawkins KL, Summers BA, Kuhajda FP, Smith CA. Immunocytochemistry of normal pancreatic islets and spontaneous islet cell tumors in dogs. Vet Pathol. 1987;24:170–9. doi: 10.1177/030098588702400211. [DOI] [PubMed] [Google Scholar]
- 54.Kramer JW, Nottingham S, Robinette J, Lenz G, Sylvester S, Dessouky MI. Inherited, early onset, insulin-requiring diabetes mellitus of Keeshond dogs. Diabetes. 1980;29:558–65. doi: 10.2337/diab.29.7.558. [DOI] [PubMed] [Google Scholar]
- 55.Greider MH, Gersell DJ, Gingerich RL. Ultrastructural localization of pancreatic polypeptide in the F cell of the dog pancreas. J Histochem Cytochem. 1978;26:1103–8. doi: 10.1177/26.12.366015. [DOI] [PubMed] [Google Scholar]
- 56.Forssmann WG, Helmstaedter V, Metz J, Greenberg J, Chance RE. The identification of the F-cell in the dog pancreas as the pancreatic polypeptide producing cell. Histochemistry. 1977;50:281–90. doi: 10.1007/BF00507121. [DOI] [PubMed] [Google Scholar]
- 57.Pfister K, Rossi GL, Freudiger U, Bigler B. Morphological studies in dogs with chronic pancreatic insufficiency. Virchows Arch A Pathol Anat Histol. 1980;386:91–105. doi: 10.1007/BF00432647. [DOI] [PubMed] [Google Scholar]
- 58.Erasmus CP, Van Aswegen G. The endocrine pancreas of the Cape fur seal, Arctocephalus pusillus (Schreber, 1776): an immunocytochemical study. Onderstepoort J Vet Res. 1997;64:239–42. [PubMed] [Google Scholar]
- 59.Michelmore AJ, Keegan DJ, Kramer B. Immunocytochemical identification of endocrine cells in the pancreas of the fruit bat, Rousettus aegyptiacus. Gen Comp Endocrinol. 1998;110:319–25. doi: 10.1006/gcen.1998.7077. [DOI] [PubMed] [Google Scholar]
- 60.Furuoka H, Ito H, Hamada M, Suwa T, Satoh H, Itakura C. Immunocytochemical component of endocrine cells in pancreatic islets of horses. Jpn J Vet Sci. 1989;51:35–43. doi: 10.1292/jvms1939.51.35. [DOI] [PubMed] [Google Scholar]
- 61.Helmstaedter V, Feurle GE, Forssmann WG. Insulin-, glucagon- and somatostatin-immunoreactive endocrine cells in the equine pancreas. Cell Tiss Res. 1976;172:447–54. doi: 10.1007/BF00220331. [DOI] [PubMed] [Google Scholar]
- 62.Ito S, Yamada Y, Hayashi M, Matsubara Y. Somatostatin containing cells in the rat and horse pancreatic islets. Tohoku J exp Med. 1978;124:57–64. doi: 10.1620/tjem.124.57. [DOI] [PubMed] [Google Scholar]
- 63.Forssmann A. The ultrastructure of the cell types in the endocrine pancreas of the horse. Cell Tiss Res. 1976;167:179–95. doi: 10.1007/BF00224326. [DOI] [PubMed] [Google Scholar]
- 64.Bonner-Weir S, Like AA. A dual population of islets of Langerhans in bovine pancreas. Cell Tiss Res. 1980;206:157–70. doi: 10.1007/BF00233616. [DOI] [PubMed] [Google Scholar]
- 65.Marchetti P, Giannarelli R, Cosimi S, Masiello P, Coppelli A, Paolo V, et al. Massive isolation, morphological and functional characterization, and xenotransplantation of bovine pancreatic islets. Diabetes. 1995;44:375–81. doi: 10.2337/diab.44.4.375. [DOI] [PubMed] [Google Scholar]
- 66.Nakajima S, Kitamura N, Yamada J, Yamashita T, Watanabe T. Immunohistochemical study on the endocrine pancreas of cattle with special reference to coexistence of serotonin and glucagon or bovine pancreatic polypeptide. Acta Anat. 1988;131:235–40. doi: 10.1159/000146522. [DOI] [PubMed] [Google Scholar]
- 67.Hiratsuka T, Abe M, Takehana K, Iwasa K, Hiraga T, Kobayashi A. Immunohistochemical analysis of the endocrine cells in the pancreatic islets of cattle. Okajimas Folia Anat Jpn. 1996;72:285–95. doi: 10.2535/ofaj1936.72.6_285. [DOI] [PubMed] [Google Scholar]
- 68.Reddy SN, Elliot RB. Ontogeny of cells containing insulin, glucagon, pancreatic polypeptide hormone and somatostatin in the bovine pancreas. Aust J Biol Sci. 1985;83:237–43. doi: 10.1071/bi9850237. [DOI] [PubMed] [Google Scholar]
- 69.Reddy S, Elliot RB. Insulin, glucagon, pancreatic polypeptide hormone and somatostatin in the goat pancreas: Demonstration by immunocytochemistry. Aust J Biol Sci. 1985;38:59–66. [PubMed] [Google Scholar]
- 70.Reddy S, Bibby NJ, Elliott RB. An immunofluorescent study of insulin-, glucagon-, pancreatic polypeptide- and somatostatin-containing cells in the early ovine fetal pancreas. Q J Exp Physiol. 1988;73:225–32. doi: 10.1113/expphysiol.1988.sp003135. [DOI] [PubMed] [Google Scholar]
- 71.Jay TR, Heald KA, Carless NJ, Topham DE, Downing R. The distribution of porcine pancreatic beta-cells at ages 5, 12 and 24 weeks. Xenotransplantation. 1999;6:131–40. doi: 10.1034/j.1399-3089.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 72.Crowther NJ, Gotfredsen CF, Moody AJ, Green IC. Porcine islet isolation, cellular composition and secretory response. Horm Metab Res. 1989;21:590–5. doi: 10.1055/s-2007-1009296. [DOI] [PubMed] [Google Scholar]
- 73.Marchetti P, Giannarelli R, di Carlo A, Vassalle A, Picaro L, Gattai V, et al. Morphometrical and immunocytochemical characterization of the porcine endocrine pancreas. Transplant Proc. 1990;22:727–8. [PubMed] [Google Scholar]
- 74.Khatim MS, Gumaa KA, Petersson B, Lundqvist G, Grimelius L, Hellerström C. The structure and hormone content of the endocrine pancreas of the one-humped camel (Camelus dromedarius) Anat Anz. 1985;159:181–6. [PubMed] [Google Scholar]
- 75.Jörns A, Barklage E, Grube D. Heterogeneities of the islets in the rabbit pancreas and the problem of “paracrine” regulation of islet cells. Anat Embryol. 1988;178:297–307. doi: 10.1007/BF00698661. [DOI] [PubMed] [Google Scholar]
- 76.Endo H, Kusanagi A, Kurohmaru M, Hayashi Y, Sakamoto K, Kimura J. Pancreas morphology of the striped hyena (Hyena hyena) J Vet Med Sci. 1997;59:635–40. doi: 10.1292/jvms.59.635. [DOI] [PubMed] [Google Scholar]
- 77.Lucini C, Castaldo L, Lai O, De Vico G. Ontogeny, postnatal development and ageing of endocrine pancreas in Bubalus bubalis. J Anat. 1998;192:417–24. doi: 10.1046/j.1469-7580.1998.19230417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Quay WB. Pancreatic Weight and Histology in the White Whale. J Mammal. 1957;38:185–92. [Google Scholar]
- 79.Van Aswegen G, Van Noorden S, Kotze SH, De Vos V, Schoeman JH. The intestine and endocrine pancreas of the African elephant: a histological immunocytochemical and immunofluorescence study. Onderstepoort J Vet Res. 1996;63:335–40. [PubMed] [Google Scholar]
- 80.da Mota DL, Yamada J, Gerge LL, Pinheiro PBN. An immunohistochemical study on the pancreatic endocrine cells of the three-toed sloth, Bradypus variegatus. Arch Histol Cytol. 1992;55:203–9. doi: 10.1679/aohc.55.203. [DOI] [PubMed] [Google Scholar]
- 81.Edwin N, Yamada J, Leigh CM. A light-microscopic immunocytochemical study of the endocrine pancreas in the Australian fat-tailed dunnart (Sminthopsis crassicaudata) Singapore Med J. 1992;33:260–1. [PubMed] [Google Scholar]
- 82.Yamada J, Krause WJ, Edwin N, Mochizuki T, Yanaihara N. A survey of endocrine cells in the pancreas of the echidna (Tachyglossus aculeatus) with special reference to pancreatic motilin cells. J Anat. 1990;171:223–31. [PMC free article] [PubMed] [Google Scholar]
- 83.Epple A, Farner D. The pancreatic islets of the white-crowned sparrow Zonotrichia leucophrys gambelii, during its annual cycle and under experimental conditions. Zeitschrift für Zellforschung. 1967;79:185–97. doi: 10.1007/BF00369284. [DOI] [PubMed] [Google Scholar]
- 84.Do Prado ML, Campos MN, Ricciardi Cruz AR. Distribution of A, B and mixed pancreatic islets in two bird species (Anas platyrhincus Gallus gallus, Linné, 1758)—a morphometric study. Gegenbaurs Morphol Jahrb. 1989;135:379–84. [PubMed] [Google Scholar]
- 85.Edwin N, Leigh CM. The endocrine pancreas in the Australian wedge-tailed eagle (Aquila audax)—an immunocytochemical study. Eur J Histochem. 1993;37:219–24. [PubMed] [Google Scholar]
- 86.Nascimento AA, Sales A, Cardoso TRD, Pinheiro NL, Mendes RMM. Immunocytomechical study of the distribution of endocrine cells in the pancreas of the Brazillian sparrow species Zonotrichia Capensis Subtorquata. Braz J Biol. 2007;67:735–40. doi: 10.1590/s1519-69842007000400021. [DOI] [PubMed] [Google Scholar]
- 87.Gupta YK, Kumar S. Islets of Langerhans in the parakeet, Psittacula krameri. Anat Anz. 1980;147:51–9. [PubMed] [Google Scholar]
- 88.Mensah-Brown EP, Bailey TA, Pallot DJ, Garner A. Peptidergic hormones and neuropeptides, and aminergic neurotransmitters of the pancreatic islets of the Houbara bustard (Chlamydotis undulata) J Anat. 2000;196:233–41. doi: 10.1046/j.1469-7580.2000.19620233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tomita T, Doull V, Pollack HG, Kimmell JR. Regional distribution of pancreatic polypeptide and other hormones in chicken pancreas: reciprocal relationship between pancreatic polypeptide and glucagon. General and Comparative Endocrinology. 1985;58:303–10. doi: 10.1016/0016-6480(85)90346-6. [DOI] [PubMed] [Google Scholar]
- 90.Masini MA. Immunocytochemical localization of peptides in the endocrine pancreas of the snakes Vipera aspis and Natrix maura. Acta Histochem. 1988;84:111–9. doi: 10.1016/S0065-1281(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 91.El-Salhy M, Abu-Sinna G, Wilander E. The endocrine pancreas of a squamate reptile, the desert lizard (Chalcides ocellatus). A histological and immunocytochemical investigation. Histochemistry. 1983;78:391–7. doi: 10.1007/BF00496625. [DOI] [PubMed] [Google Scholar]
- 92.Rhoten WB. Immunocytochemmcal localization of four hormones in the garter snake, Thamnophis sirtalis. The Anatomical Record. 1984;208:233–42. doi: 10.1002/ar.1092080210. [DOI] [PubMed] [Google Scholar]
- 93.Ayala AG, Lozano MT, Agulleiro B. Endocrine pancreas of Testudo graeca L. (Chelonia) in summer and winter: an immunocytochemical and ultrastructural study. Gen Comp Endocrinol. 1987;68:235–48. doi: 10.1016/0016-6480(87)90035-9. [DOI] [PubMed] [Google Scholar]
- 94.Putti R, Varano L, Cavagnuolo A, Laforgia V. The endocrine pancreas of Podarcis s. sicula Raf: an immunocytochemical study at light and electron microscopic levels. Eur J Basic Appl Histochem. 1991;35:145–59. [PubMed] [Google Scholar]
- 95.Lee J, Ku S, Lee H, Kitagawa H. An immunohistochemical study of endocrine cells in the pancreas of the Red-bellied frog (Bombina orintalis) Eur J Histo. 2003;47:165–72. doi: 10.4081/823. [DOI] [PubMed] [Google Scholar]
- 96.Putti R, Della Rossa A, Maglio M, Tagliafierro G. Islets and diffuse endocrine component in the pancreas of three red frogs species: relationships between endocrine and exocrine tissue. Tissue Cell. 1997;29:355–63. doi: 10.1016/s0040-8166(97)80011-0. [DOI] [PubMed] [Google Scholar]
- 97.Foty RA, Lai-Fook JE, Liversage RA. Localization of insulin, glucagon and somatostatin in the pancreas of the adult newt, Notophthalmus viridescens. Tissue Cell. 1989;21:1–10. doi: 10.1016/0040-8166(89)90015-3. [DOI] [PubMed] [Google Scholar]
- 98.Kaung HC, Elde RP. Distribution and morphometric quantitation of pancreatic endocrine cell types in the frog, Rana pipiens. Anat Rec. 1980;196:173–81. doi: 10.1002/ar.1091960208. [DOI] [PubMed] [Google Scholar]
- 99.Van Noorden S, Pearse AG. Immunoreactive polypeptide hormones in the pancreas and gut of the lamprey. Gen Comp Endocrinol. 1974;23:11–24. doi: 10.1016/0016-6480(74)90075-6. [DOI] [PubMed] [Google Scholar]
- 100.Youson JH, Al-Mahrouki AA, Amemiya Y, Graham LC, Montpetit CJ, Irwin DM. The fish endocrine pancreas: review, new data, and future research directions in ontogeny and phylogeny. Gen Comp Endocrinol. 2006;148:105–15. doi: 10.1016/j.ygcen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 101.Lee JH, Ku SK, Park KD, Lee HS. Comparative study of endocrine cells in the principal pancreatic islets of two teleosts, Silurus asotus (Siluridae) and Siniperca scherzeri (Centropomidae) J Vet Sci. 2001;2:75–80. [PubMed] [Google Scholar]
- 102.Argenton F, Zecchin E, Bortolussi M. Early appearance of pancreatic hormone-expressing cells in the zebrafish embryo. Mech Dev. 1999;87:217–21. doi: 10.1016/s0925-4773(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 103.Kong HS, Lee JH, Park KD, Ku SK, Lee HS. Immunohistochemical study of the endocrine cells in the pancreas of the carp, Cyprinus carpio (Cyprinidae) J Vet Sci. 2002;3:303–14. [PubMed] [Google Scholar]
- 104.Hansen GN, Hansen BL, Jørgensen PN. Insulin-, glucagon- and somatostatin-like immunoreactivity in the endocrine pancreas of the lungfish, Neoceratodus forsteri. Cell Tissue Res. 1987;248:181–5. doi: 10.1007/BF01239979. [DOI] [PubMed] [Google Scholar]
- 105.Lozano MT, Ayala AG, Abad ME, Aguilleiro B. Pancreatic Endocrine Cells in Sea Bass (Dicentrarchus labrax L) Gen Comp Endo. 1991;81:198–206. doi: 10.1016/0016-6480(91)90004-p. [DOI] [PubMed] [Google Scholar]
- 106.Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- 107.Nozaki M, Miyata K, Oota Y, Gorbman A, Plisetskaya EM. Different cellular distributions of two somatostatins in brain and pancreas of salmonids, and their associations with insulin- and glucagon-secreting cells. Gen Comp Endocrinol. 1988;69:267–80. doi: 10.1016/0016-6480(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 108.Fishelson L, Becker K. Development and aging of the liver and pancreas in the domestic carp, Cyprinus carpio: from embryogenesis to 15-year-old fish. Environ Biol Fish. 2001;61:85–99. [Google Scholar]
- 109.Miller W, Rosenbloom K, Hardison RC, Hou M, Taylor J, Raney B, et al. 28-way vertebrate alignment and conservation track in the UCSC genome browser. Genome Res. 2007;17:1797–808. doi: 10.1101/gr.6761107. [DOI] [PMC free article] [PubMed] [Google Scholar]