Abstract
Deep Brain Stimulation (DBS) provides therapeutic benefit for several neuropathologies including Parkinson’s disease (PD), epilepsy, chronic pain, and depression. Despite well established clinical efficacy, the mechanism(s) of DBS remains poorly understood. In this review we begin by summarizing the current understanding of the DBS mechanism. Using this knowledge as a framework, we then explore a specific hypothesis regarding DBS of the subthalamic nucleus (STN) for the treatment of PD. This hypothesis states that therapeutic benefit is provided, at least in part, by activation of surviving nigrostriatal dopaminergic neurons, subsequent striatal dopamine release, and resumption of striatal target cell control by dopamine. While highly controversial, we present preliminary data that are consistent with specific predications testing this hypothesis. We additionally propose that developing new technologies, e.g., human electrometer and closed-loop smart devices, for monitoring dopaminergic neurotransmission during STN DBS will further advance this treatment approach.
Keywords: Deep brain stimulation, Parkinson’s disease, subthalamic nucleus, voltammetry, wireless integrated circuit
The neurosurgical approach of Deep Brain Stimulation (DBS) is an established restorative therapy for Parkinson’s disease (PD) (1,2), tremor (3), and dystonia (4). In addition, DBS has also been investigated as a viable treatment option for other neurological and psychiatric disorders such as epilepsy (5–7) chronic pain (8,9), Tourette's syndrome (10), obsessive-compulsive disorder (11,12), and depression (13–15).
Conventional DBS systems use a high frequency (100–250 Hz) pulse train applied continuously to a surgically implanted stimulating electrode (16). Optimal clinical benefit of DBS is by programming the stimulation parameters (e.g., amplitude, frequency, pulse width, and active electrode contact) post-operatively. This trial-and-error process typically requires many hours of neurologist and patient time, and is ultimately limited by the placement of the stimulation electrode, which is fixed at this critical therapeutic juncture. Conventional DBS also operates in an open-loop mode, which means that the neurologist must occasionally adjust stimulation parameters to obtain the best therapeutic response in the patient and parameters are fixed in between adjustments. Simply stated, electrodes are implanted in what are assumed to be optimal sites within brain nuclei and the stimulator is turned on without any form of continuous regulatory feedback.
Despite the fact that more than 40,000 people have been implanted with DBS devices, the DBS neurosurgical approach would benefit from significant improvements. A major challenge is the incomplete understanding of the central mechanisms of action of DBS, especially why certain stimulation parameters and stimulation of specific targets are effective. However, there are now active research programs to understand these central mechanisms so that future DBS devices and electrode implants can be intelligently engineered to work in a closed-loop feedback manner. Understanding the mechanisms of action of DBS would provide the essential framework for the development of next-generation “smart” DBS devices that significantly improve clinical outcomes for a broad range of neurological and psychiatric conditions. In theory, novel DBS devices based on continuous or intermittent monitoring of electrophysiological or neurochemical phenomena at the site of DBS or distal nuclei could improve targeting of electrode implants, as well as a means of regulating brain neurotransmitter levels that may be critical to the therapeutic effectiveness of DBS (17).
Translational research of the mechanisms of DBS directed at developing superior DBS systems is currently being conducted using state-of-the-art electrophysiological, neurochemical (amperometry and voltammetry), wireless application-specific integrated circuits, and functional stereotactic neurosurgical techniques. Success in this research will likely open a new era in medical diagnosis, intervention, and treatment of neuropsychiatric disorders by applying a new therapeutic strategy of feedback-loop monitoring and regulation of human neurotransmitter systems. Here, we review the current state of knowledge on DBS central mechanisms as it relates to a “dopamine hypothesis” of the actions of DBS in the subthalamic nucleus (STN) for the treatment of PD, outline potential applications of such research, and describe our vision of future DBS systems and neurosurgical procedures where state-of-the-art neurochemical recording techniques may play a key role in their development.
Hypotheses of DBS Mechanisms for Parkinson’s Disease
Despite acceptance of STN DBS as a therapeutic tool in the treatment of PD, the precise mechanisms of action are currently unknown. Because the therapeutic effects of DBS are similar to those of a lesion (i.e., subthalamotomy), DBS has been traditionally thought to silence pathologically hyperactive neurons at the site of stimulation (depolarization block) (18–21). However, emerging evidence from a number of basic and clinical studies implicates additional mechanisms, besides a [1] silencing hypothesis, that involve [2] activation of local neuronal terminals that inhibit and/or excite efferent outputs (synaptic modulation). In turn, this has been postulated to either [3] deplete (synaptic depression) or [4] enhance (synaptic facilitation) efferent neurotransmission that ultimately normalizes activity (network jamming or modulation) within structures of the basal ganglia complex (1,17,22–27).
A number of pre-clinical animal studies have utilized a range of stimulation frequencies (referred to as High Frequency Stimulation or HFS) with constant voltage or constant current pulses in an attempt to mimic stimulation parameters typically utilized in human DBS treatment (17). These pre-clinical in vivo and in vitro studies with mice, rats and non-human primates have provided important insights into the central mechanisms of action of this restorative neurosurgical technique. For example, several in vitro electrophysiological studies support hypothesis 1 of direct inhibition of spontaneous activity in STN cells via depolarization block of action potential generation. Indeed, these intracellular and extracellular studies (28,29), including our own (30), have shown that STN neurons fire at high rates initially in response to HFS but are silenced with continued stimulation. Recent studies examining an activation-inhibition mechanism however, offer an alternative for this hypothesis (31,32). In this multicompartment model continuous STN stimulation may generate efferent output at the stimulus frequency in the axon despite suppression of spontaneous cell body firing. These investigators argued that cell body firing does not accurately reflect the efferent output of neurons stimulated with high frequency extracellular pulses, and that decoupling of somatic and axonal activity may permit modulatory outflow of information from the site of DBS to, for example, SNc dopaminergic cells in the midbrain. Suprathreshold stimulation caused suppression of intrinsic spontaneous firing in the cell body, but generated efferent output locked to the stimulus frequency in its axon. This independence of cell body and axon firing may resolve the apparent contradictory results on the effects of HFS of the STN, notably depolarization block (hypothesis 1) versus local synaptic modulation (hypothesis 2) and synaptic depression and/or facilitation (hypothesis 3 and 4) of neurotransmitter release at the site of stimulation, and distally at efferent target nuclei.
While hypotheses 1 has enjoyed popular support, hypothesis 2 and corresponding hypotheses 3 and 4 have undergone less scrutiny and remain the most controversial of the possible mechanisms of STN DBS (17). The notion that inactivation of STN neurons by DBS may not underlie all therapeutic actions of STN DBS leaves open a number of compelling questions: [1] does the release of excitatory neurotransmitters such as glutamate locally and in afferent targets in the basal ganglia complex have the ability to activate a severely compromised nigrostriatal dopaminergic system that leads to functional striatal dopamine release and [2] if the therapeutic efficacy of DBS is directly correlated to the level of evoked local glutamate and striatal dopamine release can continuous monitoring of these neurochemical phenomena serve as feedback to regulate DBS devices for improved therapeutic efficacy? These questions as they pertain to dopamine release in the basal ganglia and glutamate release at the site of STN stimulation will be examined here.
The Dopamine Release Hypotheses of DBS
In this section we explore the hypothesis that DBS of the STN contributes to symptom relief in PD by activation of surviving nigrostriatal dopaminergic neurons, subsequent striatal dopamine release, and resumption of striatal target cell control by dopamine. The “dopamine release” hypothesis, as defined here, is clearly controversial. While supporting evidence is available, most basic and clinical studies are in strong opposition. We contend that some inconsistencies in the literature may be due to technical difficulties encountered when measuring striatal dopamine release in animal models of PD and in PD patients. We additionally provide preliminary pre-clinical data supporting specific predictions testing the dopamine release hypothesis. Thus, these studies serve as a starting point to address some of the critical questions noted above regarding the mechanism of STN DBS. As described in the next section, these basic studies serve as a framework for the development of next generation approaches that can detect and modulate neurotransmitter release for more effective DBS treatment of neurological and psychiatric disorders.
Bilateral STN DBS reverses the three cardinal motor symptoms in PD patients, akinesia, rigidity, and tremor (33,34), and decreases or eliminates the need for L-DOPA (35,36). Whether these observations support the dopamine release hypothesis is equivocal. On the one hand, cardinal symptoms of PD are associated with severe nigrostriatal dopaminergic denervation (37) and L-DOPA, the primary pharmacological treatment for PD and a biochemical precursor to dopamine is thought to act by increasing endogenous dopamine synthesis and release (38–40). On the other hand, STN DBS could be altering neuronal circuits downstream of striatal dopamine release to provide therapeutic benefit. However, DBS of the STN is most effective in PD patients who respond well to L-DOPA (41) and contraindicated for those who do not (42), suggesting that effective DBS requires endogenous dopamine production. Also consistent with activation of surviving nigrostriatal dopaminergic neurons are the observations that DBS elicits dyskinesias that resemble those seen when excess L-DOPA is given (43) and like L-DOPA, contributes to impulsivity, a behavior thought to be dopamine-mediated (43).
Two major lines of reasoning clearly oppose the dopamine release hypothesis. First, most basic studies using in vivo microdialysis do not report an increase in striatal dopamine release during HFS of the STN in intact or 6-hydroxydopamine (6-OHDA)-lesioned rats (44–47), a widely used animal model of PD in which SNc dopaminergic neurons are selectively destroyed. In vivo microdialysis is a monitoring technique that uses a relatively small dialysis probe (typically 0.15 mm o.d. × 1–2 mm length) to sample the concentration of neurotransmitters in brain extracellular fluid for subsequent chemical analysis ex vivo using, for example, high performance liquid chromatography with electrochemical detection. Second, STN DBS does not increase striatal dopamine release in PD patients as measured by PET imaging (48–50), which assesses brain extracellular dopamine levels indirectly by measuring the displacement by endogenous dopamine of an exogenously applied dopamine receptor ligand that has been radio-labeled, such as [11C] raclopride. However, PET scanning with raclopride has relatively poor temporal resolution and requires an increase of greater than 90% of basal dopamine levels in order to detect a significant change in the PET signal (49,51). As well, adaptive changes in dopamine receptor populations, such as D2 receptor internalization and/or recycling occurring over long-term STN stimulation, has been suggested to interfere with PET quantification of dopamine release in these patients (52). Thus, whether STN DBS improves PD symptoms via the release of dopamine remains an important question that is of paramount importance and the focus of this review.
Anatomical Basis for the Dopamine Release Hypothesis
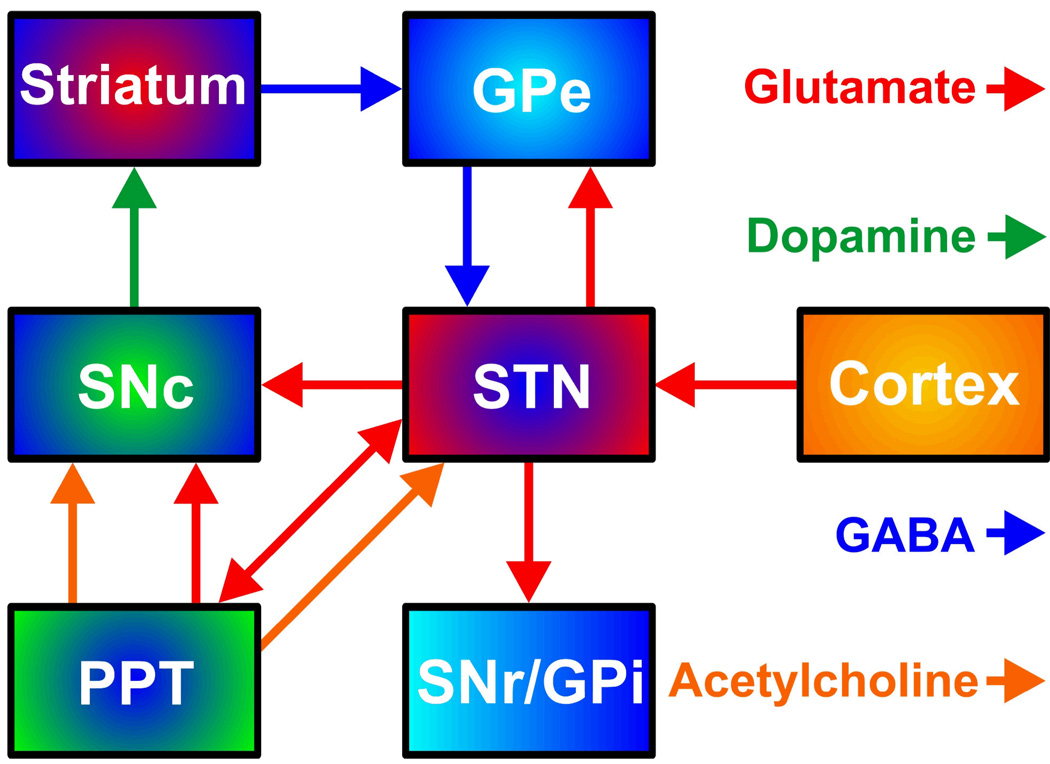
The STN occupies a central position in the indirect pathway of the basal ganglia by linking the external segment of the globus pallidus (GPe) to the two output nuclei, internal segment of the globus pallidus (GPi) and substantia nigra reticulata (SNr) (53,54) (Fig. 1). The STN is additionally innervated by the cortex directly, is reciprocally connected to both the GPe and the pedunculopontine tegmental nucleus (PPT) in the pons, and it projects to the SNc. Several neurotransmitters are utilized in the basal ganglia circuitry. The STN and PPT are the only nuclei in the basal ganglia complex whose neurons containing the excitatory neurotransmitter glutamate directly innervate nigrostriatal dopaminergic neurons (55,56). In addition to glutamate, the PPT provides the only known excitatory cholinergic projection to SNc dopaminergic cells in the SNc (57). The other major neurotransmitter is GABA, which is inhibitory. The SNr, via GABAergic collaterals, inhibit dopaminergic neurons originating in the SNc (58,59). Diverse neuropeptides also co-localize with other neurotransmitters in several basal ganglia neuronal pathways, particularly the GABAergic efferents of the striatum (60).
FIGURE 1.
Neurochemical interconnectivity within the basal ganglia complex. STN, subthalamic nucleus; SNc and SNr, substantia nigra compacta and reticulata, respectively; PPT, pedunculopontine nucleus; GPe and GPi, globus pallidus external and internal, respectively.
DBS of the STN could elicit striatal dopamine release by at least four possible routes. First, stimulation of the glutamatergic innervations originating from the STN and terminating in the SNc would activate nigrostriatal dopaminergic neurons directly (61). Second, stimulation of STN glutamatergic neurons targeting the PPT would activate nigrostriatal dopaminergic neurons both by the reciprocal excitatory innervation and subsequent activation of the SNc by STN glutamatergic efferents, and indirectly by activating PPT cholinergic and glutamatergic inputs to the SNc (62–64). Third, because of the reciprocal innervation between the STN and GPe, STN stimulation would activate the GPe inhibition of STN glutamatergic afferents to the SNr, releasing the GABAergic inhibition of the SNr on the SNc (65–66). Fourth and similarly, GABAergic pathways descending from the striatum and GPe and traversing near the STN would be activated directly and also relieve inhibition of the SNc by the SNr (67).
Predictions of the Dopamine Release Hypothesis
The strength of a scientific hypothesis is related to its ability to predict outcomes successfully. In this regard, the dopamine release hypothesis of STN DBS for the treatment of PD lends itself to several important predictions that can be tested experimentally and clinically. While all of these predications may not be definitive, in the sense that if proven false the hypothesis would be rejected, these predictions if proven true would lend support to the dopamine release hypothesis. As described next we have recently obtained preliminary data in experimental animals to test some of these predictions related to STN DBS excitation of local neurons, activation of dopaminergic neurons in the SNc, and increased striatal dopamine release. We also will identify technical approaches suitable for testing other predictions. In the last section of this review, we describe the development of new technologies to test predictions clinically and to advance the DBS approach for the treatment of neuropathologies.
Prediction 1. HFS of the STN Elicits Local Glutamate Release
As described above one general mechanism of DBS is activation of local neuronal terminals that inhibit and/or excite efferent outputs (synaptic modulation). This mechanism is critical to the dopamine release hypothesis if chemical activation of STN efferents is the first step by which STN DBS ultimately increases striatal dopamine release. Recall from Fig. 1 that activation of STN excitatory efferents would stimulate nigrostriatal dopaminergic neurons by various pathways involving the GPe, PPT, and SNr, and the neurotransmitters glutamate, acetylcholine, and GABA. While disparate results have been reported (e.g., 29), work from several groups including our own have demonstrated that alteration of STN neuronal activity in rat brain slices by HFS of the STN is mediated by neurotransmitter release, because these effects are blocked by receptor antagonists for GABA and glutamate (68–69). The local release of glutamate by HFS of the STN, which would then chemically excite STN afferents, is therefore an important prediction for the dopamine release hypothesis.
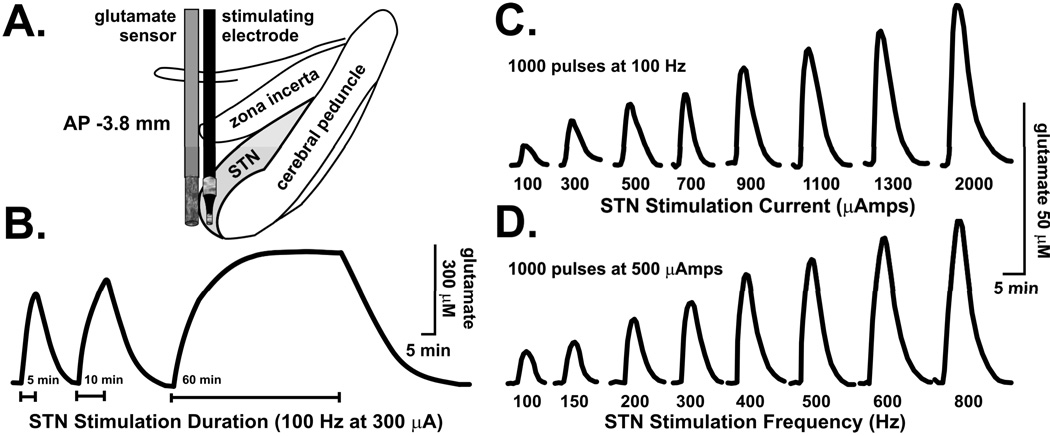
We tested the local glutamate release prediction using a biosensor approach in vivo. Glutamate is not electroactive, which means that this neurotransmitter cannot be monitored directly by electrochemical techniques. Rather, a “biological recognition element” in the form of glutamate oxidase is first used to act on glutamate and generate an electroactive product, hydrogen peroxide, which is measured electrochemically by the sensor using the technique of amperometry. Co-implanting an enzyme-linked biosensor for glutamate adjacent to a stimulating electrode in the STN of an anesthetized rat resulted in local glutamate release that was reproducible and dependent on stimulus duration, current intensity, and frequency (70) (Fig. 2). Taken together, these results strongly suggest that local glutamate release, and subsequent excitation of STN neuronal efferents, is potentially a key initial mechanism by which STN DBS ultimately increases striatal dopamine release.
FIGURE 2.
Positioning of a glutamate sensor adjacent to a bipolar stimulating electrode in the subthalamic nucleus (STN) (A) permitted glutamate release to be recorded at the site of stimulation in response to different durations (B), current intensities (C), and frequencies (D) of electrical stimulation in the STN of anesthetized rats. Modified from ref. (70).
A similar approach was used to establish local glutamate release from the ferret brain slice. Although circuits are not intact as in vivo, the in vitro preparation enjoys several experimental advantages, including fine manipulation of the extracellular milieu (71). In preliminary studies we exploited this feature to investigate the cellular source of glutamate release elicited by HFS of the STN. Interestingly, HFS-induced glutamate release was unchanged in the presence of the Na+ channel blocker, tetrodotoxin, but was eliminated with the vesicular H+-ATPase inhibitor, bafilomycin A1, and the calcium chelator, BAPTA-AM (data not shown). These early results suggest that glutamate release is derived from some non-neuronal, but exocytotic origin (i.e., calcium-dependent vesicular release). The source of this glutamate is likely from glia, which constitute the majority of cells in the brain. To date, research has generally ignored the effects of DBS on glia, with the prevailing point of view that DBS only affects neuronal populations to achieve clinical benefits.
Using the same type of biosensor applied in vivo (see Fig. 2) and in our brain slice preparation, additional preliminary studies examined HFS evoked glutamate release in non-neuronal cultures of primary astrocytes, a pure source of glia (72). Indeed, electrical stimulation was able to evoke glutamate release that was quantitatively similar to glutamate release from these two preparations. Moreover, HFS also evoked intracellular calcium transients in these cultured astrocytes, as determined by the calcium dye (Fluo-4) and an imaging system (2-photon confocal microscopy), and blocking these transients with application of BAPTA-AM additionally inhibited glutamate release (73). Together, these results suggest that vesicular release of glutamate from astrocytes represents an important cellular mechanism by which STN DBS is able to alter neuronal activity at the site of stimulation.
Prediction 2. HFS of the STN Elicits EPSPs and Action Potentials in Nigrostriatal Dopaminergic Neurons
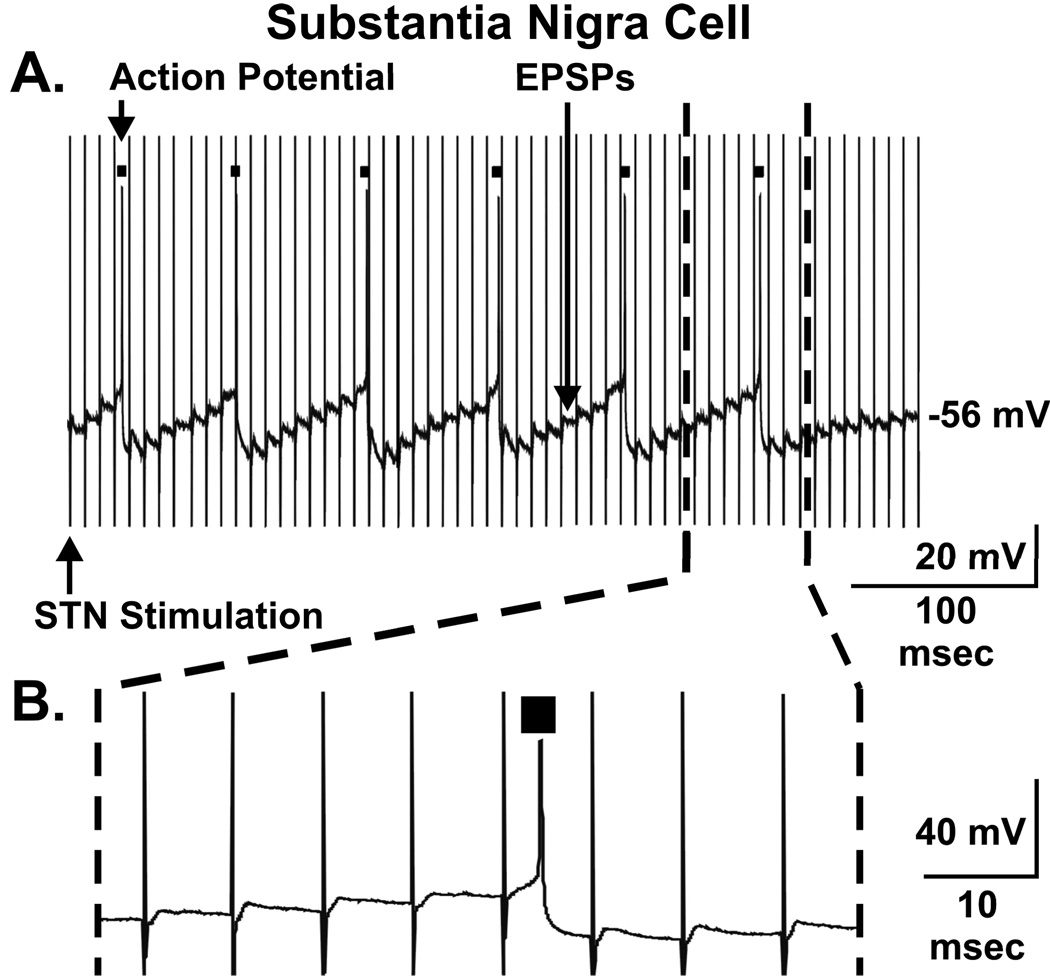
A second important prediction of the dopamine release hypothesis is that STN DBS activates dopaminergic neurons in the SNc. Fortunately, there is ample experimental evidence to test this key prediction, as in vivo studies in anesthetized rats clearly demonstrate that HFS of the STN results in increased firing of SNc neurons recorded either extracellularly (74–76) or intracellularly (77). This prediction can also be tested in vitro, because a parasagittal slice of the brain can be sectioned that includes intact connections between the STN and SNc. Using intracellular recording technique and the parasagittal slice, we have recently reported that HFS of the STN elicited EPSPs in SNc dopaminergic neurons resulting in action potential generation (Fig. 3 A–B) (69). These studies indicate that HFS does not lead to depletion of glutamate in glutamatergic terminals within the SNc (hypothesis 3 synaptic depression), rather that sustained STN stimulation can enhance nigrostriatal dopaminergic cell activity. Additional work using a similar approach has demonstrated that this STN evoked intracellular activity in SNc dopaminergic neurons is blocked by the glutamate receptor antagonist, 6-cyano-7-nitroquinoxalene-2,3-dione (67). Thus, these electrophysiological findings suggest that increased firing of STN neurons evoked by STN DBS could result in terminal release of an excitatory neurotransmitter onto dopaminergic cell bodies in the SNc and subsequent neuronal activation.
FIGURE 3.
Electrical stimulation of the subthalamic nucleus (STN) in rat brain slices results in excitatory postsynaptic potentials (EPSPs) and action potential generation (black boxes) in putative dopaminergic neurons in the substantia nigra pars compacta (A). Blow up from A of a portion of the stimulated response (dashed lines) (B). Modified from ref. (69).
Prediction 3. HFS of the STN Elicits Striatal Dopamine Release
Although an increased firing rate of dopaminergic neurons in the SNc elicited by STN DBS is consistent with the dopamine release hypothesis, actual measurements of striatal dopamine release during HFS of the STN in animals, and in particular human DBS, are required to test this hypothesis more completely. In fact, there are dissociations reported between dopaminergic neuron firing rate and dopamine release (78), which prevents a direct extrapolation between the two processes. Also, if STN DBS does indeed elicit striatal dopamine release, it is critical to determine how much dopamine is released, where in the striatum this release occurs, and its pattern of release, in order to fully appreciate the contribution of the dopamine release hypothesis to the mechanism of STN DBS.
As mentioned earlier, most previous attempts at determining dopamine release during HFS of the STN used the technique of microdialysis. To test the prediction that STN DBS elicits striatal dopamine release, we used another general monitoring approach called in vivo voltammetry to measure extracellular dopamine levels. A voltammetric microsensor is similar to the glutamate enzyme-linked biosensor used to establish local glutamate release during HFS of the STN above, except that, unlike glutamate, dopamine is directly electroactive. Consequently, no biological recognition element is needed to detect dopamine electrochemically. Voltammetry enjoys several analytical advantages over microdialysis including fast measurements (subsecond versus minutes) at a smaller probe (microns versus hundreds of microns), although microdialysis is considered to exhibit better sensitivity and selectivity.
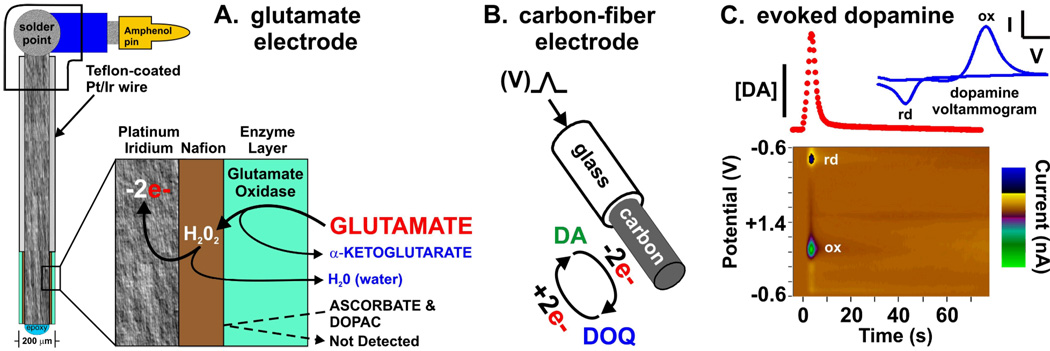
Two types of voltammetry are described here (Fig. 4). The first type, amperometry, was also used in conjunction with glutamate oxidase to create the glutamate biosensor. In amperometry, the potential of the sensor is fixed at a level sufficient to oxidize (remove electrons from) the analyte (i.e., an electroactive compound such as dopamine). Because of this simple measurement scheme, amperometry is noted for its fast temporal resolution (even down to microseconds) and kinetically pure recordings. The second type, fast-scan cyclic voltammetry, involves scanning the potential to a level sufficient for oxidation and reversing the direction of the scan to return to the baseline potential, which then may cause reduction. Though more technically involved, exhibiting poorer temporal resolution, and more prone to kinetic distortion and ionic artifacts compared to amperometry, fast-scan cyclic voltammetry enjoys the key analytical attribute of determining a chemical signature in the form of a voltammogram to identify the species detected. The voltammogram is a plot of the oxidation and reduction current as a function of an applied voltage. As described below, we have made use of the advantages of both amperometry and fast-scan cyclic voltammetry to test the prediction that HFS of the STN elicits striatal dopamine release.
FIGURE 4.
Glutamate recording electrode (A) utilizing an enzyme (glutamate oxidase) bound to an anion exchange resin (Nafion) that in turn is bound to a platinum/iridium (Pt/Ir) surface. Peroxide (H2O2) formed by the conversion of endogenous glutamate to α-ketoglutarate is oxidized at the Pt/Ir surface resulting in water as a byproduct and recorded oxidation current (−2e-). In (B) a triangular wave potential (V) is applied to a carbon-fiber electrode that results in a voltammogram (blue line) of the oxidation (ox) of dopamine (DA) and subsequent reduction (rd) of the electroformed dopamine-ortho-quinone (DOQ) back to dopamine during the negative scan. A plot of the oxidation current peaks of these voltammograms during electrical stimulation of dopaminergic fibers yields a temporal plot of stimulation evoked dopamine release (red dots). These voltammetric peaks when plotted sequentially (C) with applied potential and current intensity form a pseudo-color plot (x axis: time, y axis: potential, z axis: current) establishing dopamine as the source of the signal.
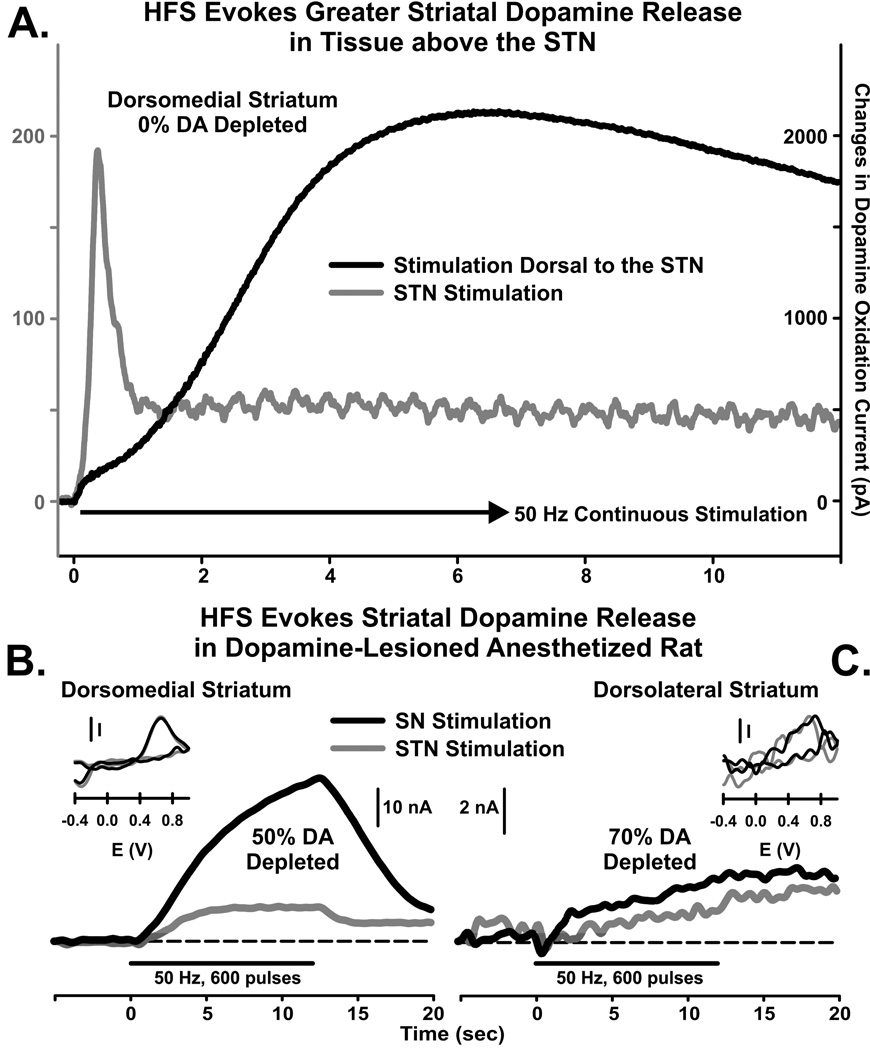
Our first test of the prediction that HFS of the STN elicits striatal dopamine release used amperometry in the anesthetized intact rat to compare the effects of medial forebrain bundle (MFB) and STN stimulation (30). Dopaminergic fibers originating in the SNc ascend via the MFB before terminating in the striatum. As shown in Fig. 5 A, stimulation of either the MFB or STN increased striatal dopamine release coincident with the applied pulse train. However, both the magnitude and the dynamics of dopamine release varied according to the region of stimulation. The magnitude of dopamine release was substantially higher with MFB than STN stimulation, by about 11 fold. HFS of the MFB also elicited dopamine release that increased to a plateau followed by a gradual decline. In marked contrast, STN stimulation caused an immediate transient increase that gave way to a low-level steady-state response. Different dynamics suggest different mechanisms underlying dopamine release, for example, direct axonal activation followed by fatigue with MFB stimulation and monosynaptic activation followed by postsynaptic receptor desensitization with STN stimulation (30). Nevertheless, these preliminary results strongly suggest that STN DBS is capable of activating nigrostriatal dopaminergic neurons sufficiently to elicit measurable dopamine release in the striatum as quantified by voltammetry.
FIGURE 5.
Continuous high frequency stimulation (HFS) of the subthalamic nucleus (STN) evoked a transient release of dopamine in the dorsomedial striatum that peaked within 20 applied pulses at 50 Hz (A, gray line), as compared to an 11-fold greater and more sustained dopamine release to stimulation of dopaminergic axons dorsal to the STN (A, black line; note the right y-axis is 10 × greater than the left y-axis). Despite a 50% (B) to 70% (C) loss in striatal dopamine, HFS of the substantia nigra (SN) or STN (black and gray lines, respectively) evoked reliable and quantifiable release of striatal dopamine in an awake parkinsonian rat model. (A) modified from ref. (30).
While studies in the intact rat are promising, it is imperative to establish striatal dopamine release evoked by HFS of the STN in the dopamine-depleted brain mimicking PD. For these studies, we used fast-scan cyclic voltammetry in the anesthetized rat lesioned by 6-OHDA. Fast-scan cyclic voltammetry was deemed critical for these measurements, because the voltammogram is necessary for identifying dopamine from the small signals expected in the denervated striatum. The 6-OHDA-depleting procedure denervated dopaminergic terminals in the medial aspect of the striatum to a greater degree than the lateral aspect, providing a single animal with differential lesion degrees (79,80).
As shown in Fig. 5 B, stimulation of the substantia nigra (SN) elicited greater increases in dopamine release in the medial versus lateral striatum, which was consistent with the different denervation levels, approximately 50 and 70%, respectively, in these two striatal regions. While 50% denervation would represent the preclinical phase of PD, the 70% level is more consistent with the beginning of symptomatic PD. More importantly, STN stimulation elicited dopamine release in both the medial and lateral striatum to similar levels (Fig. 5 C), where all signals evoked by SN and STN stimulation were identified as dopamine by their voltammograms (INSETS, I vs E). These results not only suggest that STN stimulation elicits measurable dopamine release in the dopamine-depleted striatum resembling PD, but that this release is also disproportionately higher than expected based on denervation degree.
To our knowledge, the recordings shown in Fig. 5 C are the first demonstrating that striatal dopamine release can be measured during HFS of the STN in a parkinsonian-like brain. Most microdialysis studies have not been able to detect dopamine release evoked by HFS of the STN in either the intact or 6-OHDA-lesioned striatum (45–47), with Bruet et al., (44) as one exception. In this study, measurements in 6-OHDA-lesioned rats were collected in the striatum in the location between the denervated lateral region (∼80%) and the non-denervated medial region, suggesting that the actual lesion degree in the sampled tissue was more reflective of the preclinical phase of PD.
The difficulty microdialysis has with detecting striatal dopamine release during HFS of the STN may be due to large probe size (∼300 µm o.d. in the cited studies). The relatively large size of microdialysis probes typically used in these analyses have been shown to disrupt tissue in the immediate vicinity of the probe leading to an increase in non-exocytotic dopamine release, indicative of significant neuronal damage (81,82). Indeed, a voltammetric microsensor (∼10 µm diameter) positioned immediately adjacent to the dialysis probe is unable to detect MFB-stimulated dopamine release in the striatum, yet another sensor positioned at a distance of 1 mm away concurrently measured ∼10 µM dopamine (83). Several microdialysis studies have also shown an increase in c-fos expression only in a zone of striatal tissue within 600 µm from the surface of the implanted dialysis probe following monoamine oxidase or dopamine reuptake inhibition, suggesting an altered function of dopamine terminals related to the presence of the dialysis probe in tissue (84,85). These studies suggest that dialysis probe implantation results in changes in the reactivity of the implanted region of tissue that alters responsivity to dopaminergic agents, as well as under-estimations of extracellular dopamine levels compared to voltammetry (83,86,87). In contrast, ultra-structural analysis of brain tissue surrounding a carbon-fiber microelectrode utilized in voltammetry reveal evidence of only minor tissue reactions within 6.5 µm radius of the surface of the fiber (88). Altogether, these results suggest that the voltammetric microsensor approach may be more suitable for characterizing striatal dopamine during STN DBS than the technique of microdialysis.
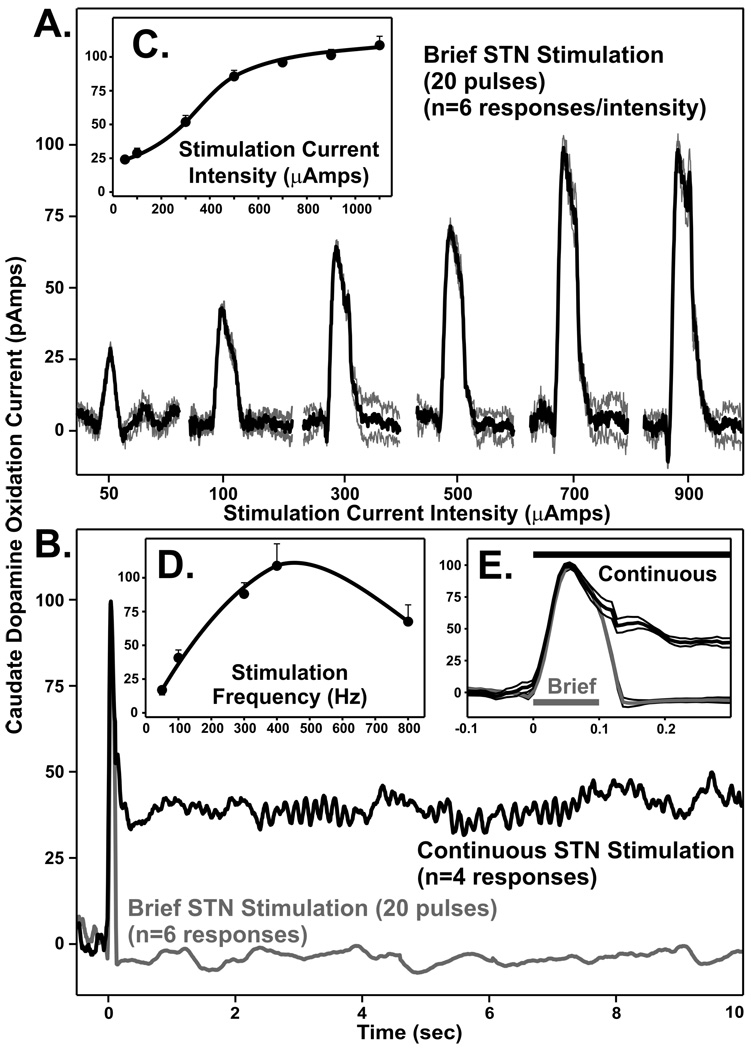
Studies in the rat provide strong support for the hypothesis that STN DBS evokes striatal dopamine release. However, it was important to demonstrate a similar phenomenon in a brain more resembling the human, such as the non-human primate. As shown in Fig. 6, we have obtained preliminary data describing striatal dopamine release in response to HFS of the STN in the awake intact adult male rhesus monkeys (macaca mulatta) (17). These measurements, collected by amperometry with a micro-carbon sensor placed stereotactically into the caudate nucleus (striatum), establish reproducible striatal dopamine release with STN stimulation that is sensitive to current intensity and train duration. Moreover, a pattern of dopamine release similar to the rat was observed during continuous HFS of the STN (i.e., transient peak followed by a sustained steady-state response). In combination with rat studies, these non-human primate measurements suggest that STN DBS as used in PD patients may derive its benefit by a mechanism involving DBS-evoked release of striatal dopamine.
FIGURE 6.
Subthalamic nucleus (STN) stimulation (20 pulses; 200 Hz) in awake monkey evoked striatal dopamine release over a range of current intensities (A), and in response to brief and continuous STN stimulation (B). Black lines in A and black and gray lines in B correspond to mean evoked responses (n=4–6) with light gray lines in A and thin black lines in E the S.E.M. for each stimulation. Inset (C and D): Mean±S.E.M. evoked increase in striatal dopamine over 50–1100 µAmps and 50–800 Hz of STN stimulation, respectively. Inset (E): Expanded time frame for the initial responses to continuous and brief STN stimulation.
Prediction 4. Striatal Dopamine Release Elicited by HFS of the STN is Functionally Relevant
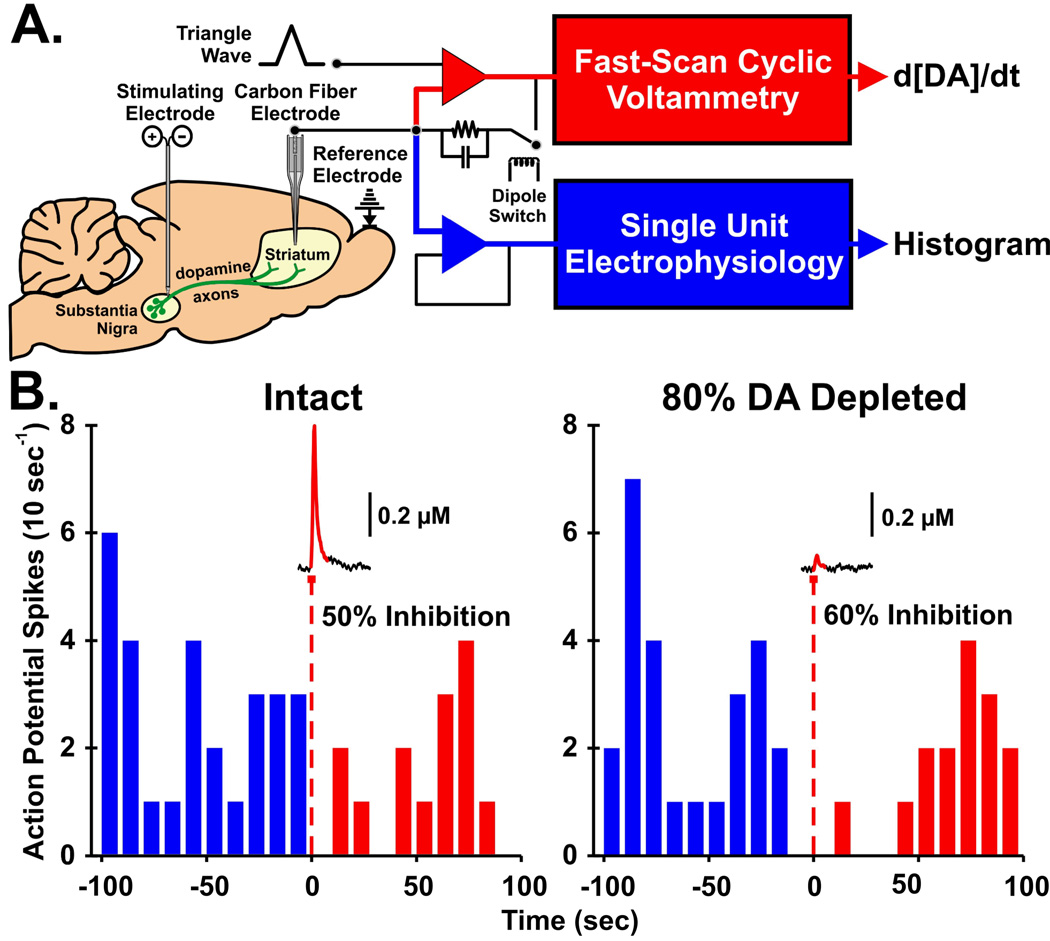
Demonstrating striatal dopamine release with HFS of the STN is an important milestone, but establishing whether this released dopamine is functionally relevant in the striatum is requisite for more fully realizing the dopamine release hypothesis of DBS. Here we describe an experimental approach called quasi-simultaneous voltammetry and electrophysiology that can be used to test this prediction directly and elegantly. We also present preliminary data consistent with the notion that electrically evoked dopamine release in the parkinsonian-like rat striatum is sufficient to alter postsynaptic target cell activity.
The technique of quasi-simultaneous voltammetry and electrophysiology (89) is conceptually described in Fig. 7 A. By switching the microsensor between electrical circuits for fast-scan cyclic voltammetry and extracellular electrophysiology on a sub-second time scale, it is possible to record dopamine levels and the single-unit target-cell response to these neurotransmitter levels in the “same” space and time domains. We have recently demonstrated that dopamine release in the rat striatum denervated to mimic the symptomatic phase of PD (>80%) is not only capable of inhibiting spontaneous target cell activity, it is also more effective than dopamine released in the intact striatum (Fig. 7 B). It is important to note, that although electrically evoked dopamine levels are markedly lower in the denervated striatum as expected, a similar (∼55%) inhibition is produced. The enhanced responsivity of striatal cells to dopamine following degeneration of nigrostriatal dopaminergic neurons is well known as denervation supersensitivity (90,91). These post-synaptic compensatory adaptations in striatal cell sensitivity to dopamine would be predicted to enhance the functional efficacy of dopamine released in the striatum by STN DBS for the treatment of PD. Taken together, these results suggest that surviving nigrostriatal dopaminergic neurons are capable of generating functionally relevant dopamine levels in the striatum even when denervation is reminiscent of PD and that this released dopamine is highly efficacious at regulating target cell activity.
FIGURE 7.
For the quasi-simultaneous technique, the carbon fiber microelectrode is switched in time between a current-to-voltage transducer circuit (red op-amp) for fast-scan cyclic voltammetry and a voltage-follower circuit (blue op-amp) for electrophysiology (A). Out of each 200 ms epoch, ∼20 ms is used for electrochemistry and circuit relaxation, with the remaining ∼180 ms available for bioelectric recording. Raw voltammetry data are analyzed to determine the change in dopamine concentration with time (d[DA]/dt), and raw histogram of post-synaptic single unit responses per time. Histograms of single unit activity are plotted with the corresponding changes in extracellular dopamine levels evoked by electrical stimulation (60 Hz, 30 pulses, 300 µA) (B). Data from the left panel were collected in the striatum of an intact rat, whereas data in the right panel are from a 6-OHDA lesioned rat with the striatum depleted of dopamine to ∼80%. Percent inhibition of post-synaptic unit activity was calculated by dividing the number of spikes post-stimulation (0 to 100 sec) by pre-stimulation (−100 to 0 sec). Although severely dopamine-depleted to mimic PD, surviving dopamine neurons terminating in the striatum are still capable of sustaining small amounts of dopamine release evoked by medial forebrain bundle stimulation. Despite this reduced level of evoked dopamine, the degree of post-synaptic inhibition was similar, indicative of the phenomenon of dopamine denervation supersensitivity.
Prediction 5. HFS of the STN Elicits Striatal Dopamine Release by Direct Activation of Dopaminergic Fibers
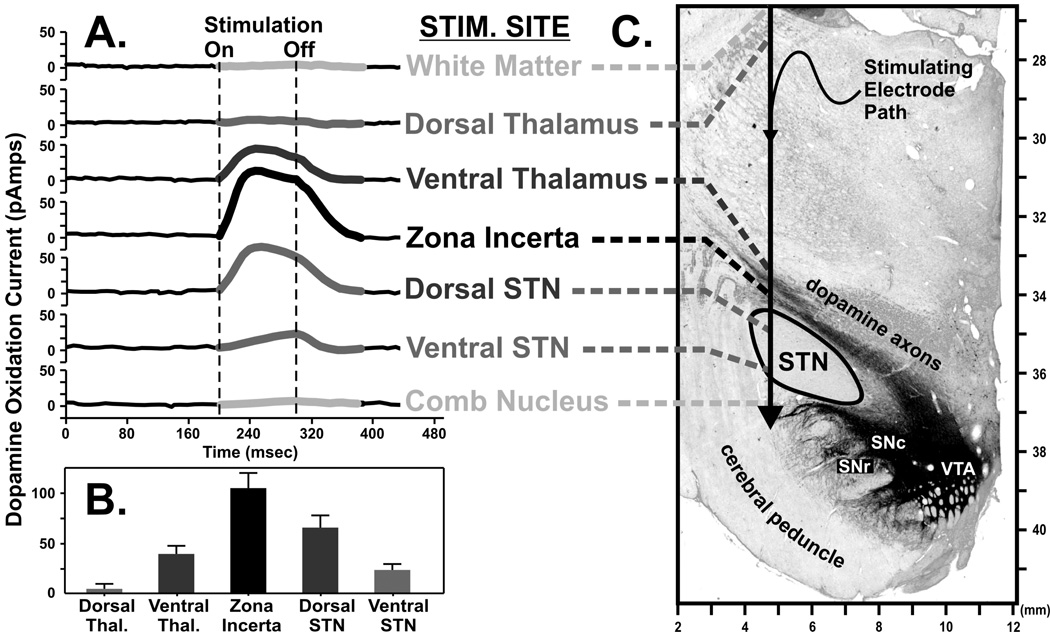
As part of our preliminary study using amperometry to record dopamine release during HFS of the STN in the intact rat (Fig. 8) we also performed post mortem histological analysis of the brain for tyrosine hydroxylase, the rate limiting enzyme for dopamine production. This procedure revealed dopaminergic fibers coursing along the dorsal edge of the STN (30) and suggested yet another mechanism by which STN DBS may elicit striatal dopamine release, direct activation of dopaminergic axons. As shown in Fig. 8 A–B, we have tested this new prediction in the awake monkey preparation. Using amperometry to record dopamine release in the dorsomedial tail of the caudate nucleus, pulse trains were applied to a stimulating electrode at various locations along a dorsoventral trajectory aimed towards that STN. The stimulation electrode path, traversing dopaminergic axons just dorsal to this region and between the ventral thalamus and zona incerta, is shown in the tyrosine hydroxylase-stained micrograph Fig. 8 C. Interestingly, not only was striatal dopamine release observed with STN stimulation, but also when the stimulating electrode was positioned in the ventral thalamus and zona incerta. In fact, the greatest dopamine release was observed with zona incerta stimulation, in the immediate vicinity of ascending dopaminergic fibers.
FIGURE 8.
Dopamine release measured in the dorsomedial tail of the caudate nucleus of the awake monkey (A) in response to electrical stimulation applied to various anatomical locations in and around the subthalamic nucleus (STN) as depicted in a representative coronal section of the monkey midbrain. Graph of the relationship of maximal increases in dopamine release with respect to the location of the stimulating electrode (B). Tyrosine hydroxylase-immunostaining highlights catecholaminergic axons originating in the dopamine-containing cell body regions of the substantia nigra pars compacta and reticulata (SNc and SNr, respectively) and ventral tegmental area (VTA) and can be seen coursing dorsally and through the STN on their way to the caudate (C).
Interestingly, in some PD patients the best symptom improvement has been reported to result from placement of the active electrode within white matter dorsal to the STN (92,93), including the dorsolateral border of the STN (94). Thus, it is reasonable to consider the possibility that dopaminergic fibers are directly activated during DBS and that this activation contributes to symptom relief. These clinical data suggest that striatal release of dopamine elicited by stimulating dopaminergic fibers just dorsal to the STN potentially has great implications for the DBS approach and treatment of PD, and possibly for other neuropsychiatric disorders. It is intriguing to consider the possibility that even in STN DBS locations that provide benefit to PD patients, improved outcomes may be provided by subtle changes in electrode location. For example, positioning the stimulating electrode within the zona incerta and dorsal STN, rather than ventral STN, which collectively elicit a greater degree of dopamine release in the primate caudate nucleus, may provide an enhancement of therapeutic benefits as well. Thus, as we discuss in the next section, measuring dopamine release intraoperatively during DBS surgery could provide a new procedure to guide electrode placement very precisely to improve symptom relief.
Prediction 6. Striatal Dopamine Release Elicited by HFS of the STN Reverses Behavioral Deficits
The ultimate prediction for the dopamine release hypothesis of DBS in animal studies is that striatal dopamine release elicited by HFS of the STN reverses behavioral deficits caused by the loss of nigrostriatal dopaminergic neurons. Several studies have now established that HFS is effective at ameliorating behavioral deficits in the 6-OHDA-lesioned rat, as indexed by assessments such as treadmill location, limb use asymmetry, reaction time, and sensorimotor integration (for review, see 95). However, to the best of our knowledge, concomitant measurements of dopamine release and behavior during HFS of the STN are not available. Therefore, there is a great need to test the prediction that striatal dopamine release elicited by HFS of the STN is correlated with a reversal of behavioral deficits using a combined dopamine measurement-behavior approach. While these pre-clinical studies have yet to be performed, we describe here a technical approach that should be well suited for testing this prediction.
As shown in Fig. 9 B, using a technique coupling fast-scan cyclic voltammetry with electrical stimulation in the awake 6-OHDA-lesioned rat elicited quantifiable dopamine release in the striatum. The voltammogram (Fig. 9 A, red line) clearly indicates that the evoked signal measured in the striatum denervated to ∼60% arises from dopamine. Incidentally, the stimulation parameters eliciting dopamine release are behaviorally relevant, as rats will work (lever press) to obtain these trains in the classic paradigm of intracranial self-stimulation (78,89,96–98).
FIGURE 9.
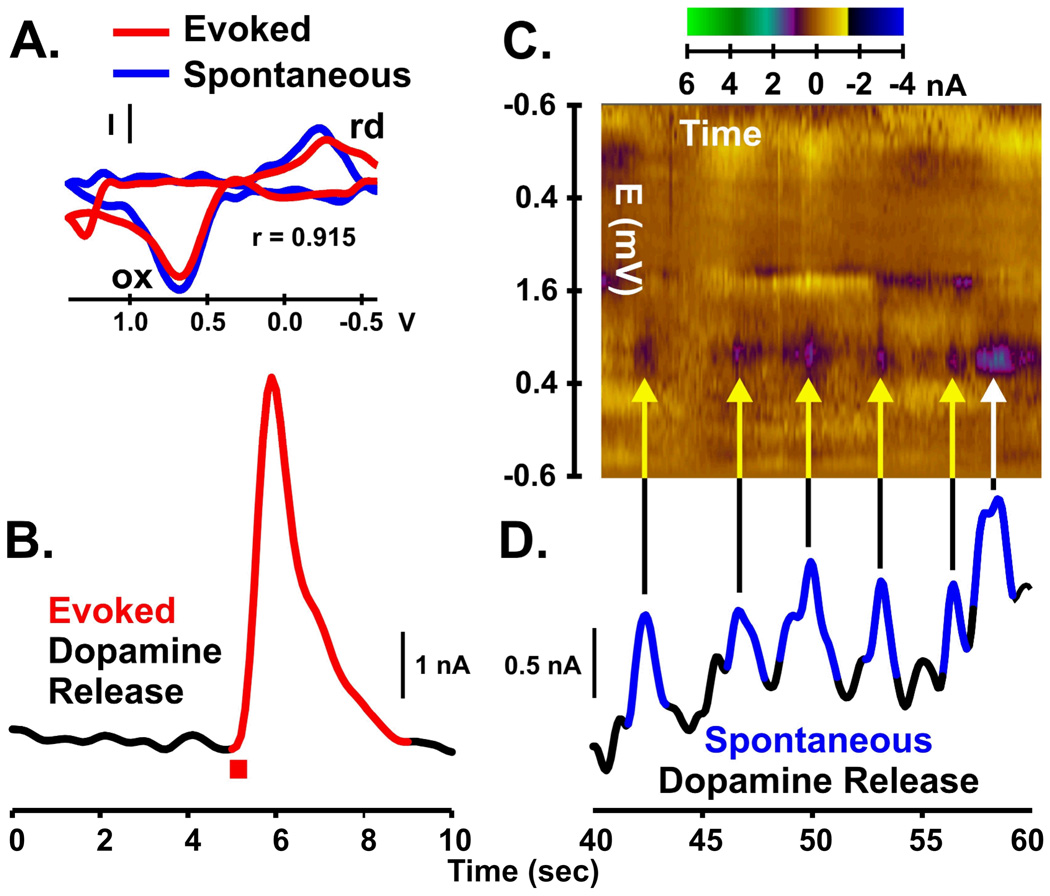
Electrically evoked (A–B) and spontaneous (C–D) dopamine release measured in the striatum of a freely moving, 6-OHDA lesioned rat. Electrical stimulation of the medial forebrain bundle (60 Hz, 24 pulses, 125 µA; B, red bar) was used to evoke dopamine release. Background subtracted cyclic voltammogram (A, red line) for the electrically evoked signal (B, red line) is indicative of maximal dopamine oxidative current. Several spontaneous (non-electrically evoked) dopamine transients (D, blue lines) are shown in the same recording at a later time where voltammograms are plotted with respect to time in the form of a pseudo-color plot (C, time, x-axis; voltage, y-axis; current, z-axis). The predominant brown color represents baseline current levels, which is a “zeroing” produced by the subtraction procedure. Note that each spontaneous peak in trace D has corresponding features in the pseudo-color plot C, coincident in time (yellow arrows). These colors reflect current due to oxidation (ox) of dopamine to a quinone (purple) and reduction (rd) of the electroformed quinone back to dopamine (yellow). These colors also correspond to the ox and rd peaks of the single voltammogram (A, blue line) for the peak response of the last peak (white arrow) with excellent statistical similarity (r = 0.915) with the voltammogram for the evoked dopamine response. Collectively, the voltammogram defines the chemical signature of the transient and demonstrates that its origin is indeed dopamine.
As shown in Fig. 9 D, this in vivo electrochemical technique also permits the quantification of spontaneous non-electrically mediated changes in dopamine release. In this case, spontaneous dopamine release was augmented by administration of the cannabinoid CB1 agonist WIN55,212-2 known to increase phasic activity of dopaminergic neurons (99). These rapid concentration spikes, termed spontaneous “dopamine transients”, are the terminal field manifestation of synchronized burst firing in dopaminergic cell bodies. Phasic dopaminergic signaling is thought to respond to primary rewards and the cues that predict these rewards (100–102). Each dopamine transient is characterized by a dopamine voltammogram, whose peak response is shown by the sequential plotting of voltammograms in the pseudo-color graph above the recording (Fig. 9 C, yellow and white arrows and purple-blue features) and the single voltammogram shown in Fig. 9 A (blue line). Thus, the capability to monitor electrically evoked dopamine levels and dopamine transients in the awake parkinsonian rat model bodes well for future studies directly testing the prediction that striatal dopamine release elicited by STN DBS reverses behavioral motoric deficits.
Overall, these preliminary data obtained from electrophysiological and neurochemical studies in experimental animals support the hypothesis that activation of surviving nigrostriatal dopaminergic neurons, subsequent striatal dopamine release, and resumption of striatal target cell control by dopamine, contribute to the therapeutic benefit of STN DBS for the treatment of PD. In terms of future animal studies, several important issues remain to be resolved. One critical issue is identifying suitable stimulation parameters and stimulating electrode designs consistent with DBS in humans. The criterion of highest priority is the demonstration of the reversal of behavioral deficits in the animal model of PD under study (95). Once established, this stimulation procedure, together with analysis of glutamate release at the site of stimulation, can be used to test the predictions that neurotransmitter release elicited by STN DBS is functionally relevant and reverses behavioral deficits. Our data also suggest that the techniques of amperometry in the intact awake non-human primate and quasi-simultaneous fast-scan cyclic voltammetry and electrophysiology in the parkinsonian rat are well suited for testing these predictions.
Future Directions: The Next Generation of DBS Systems
Although studies using animal models have proven to be invaluable for characterizing DBS mechanism and will continue to play an important role in future studies assessing this mechanism further, for clinicians and patients the eventual focus must be the human application and ultimately, increased therapeutic benefit. In this section we describe new technological innovations that will not only permit examination of the dopamine release hypothesis of STN DBS in humans, but will also advance the DBS approach to improve efficacy for the treatment of PD and other neuropathologies for which this neurosurgical procedure is prescribed. Our neurochemical recordings (Fig. 5 and 8) collected in the primate and rat brain indicate that striatal dopamine release is highly sensitive to placement of the stimulating electrode along a dorsoventral trajectory aimed at the STN and glutamate at the site of stimulation may contribute to this outcome. Assuming that striatal dopamine and local glutamate release contributes to the therapeutic benefit of STN DBS, then concurrent dopamine and glutamate measurements could be used to guide optimal placement of the stimulating electrode during surgery. To realize electrochemical recordings as an adjunct in the DBS surgical procedure, technological innovation is needed on two fronts. First, to the best of our knowledge, no FDA-approved potentiostat (electrometer), the instrument controlling neurochemical (voltammetric and amperometric) measurements of dopamine and glutamate, is available. Second, a suitable FDA-approved dopamine/glutamate sensor would also need to be identified or developed.
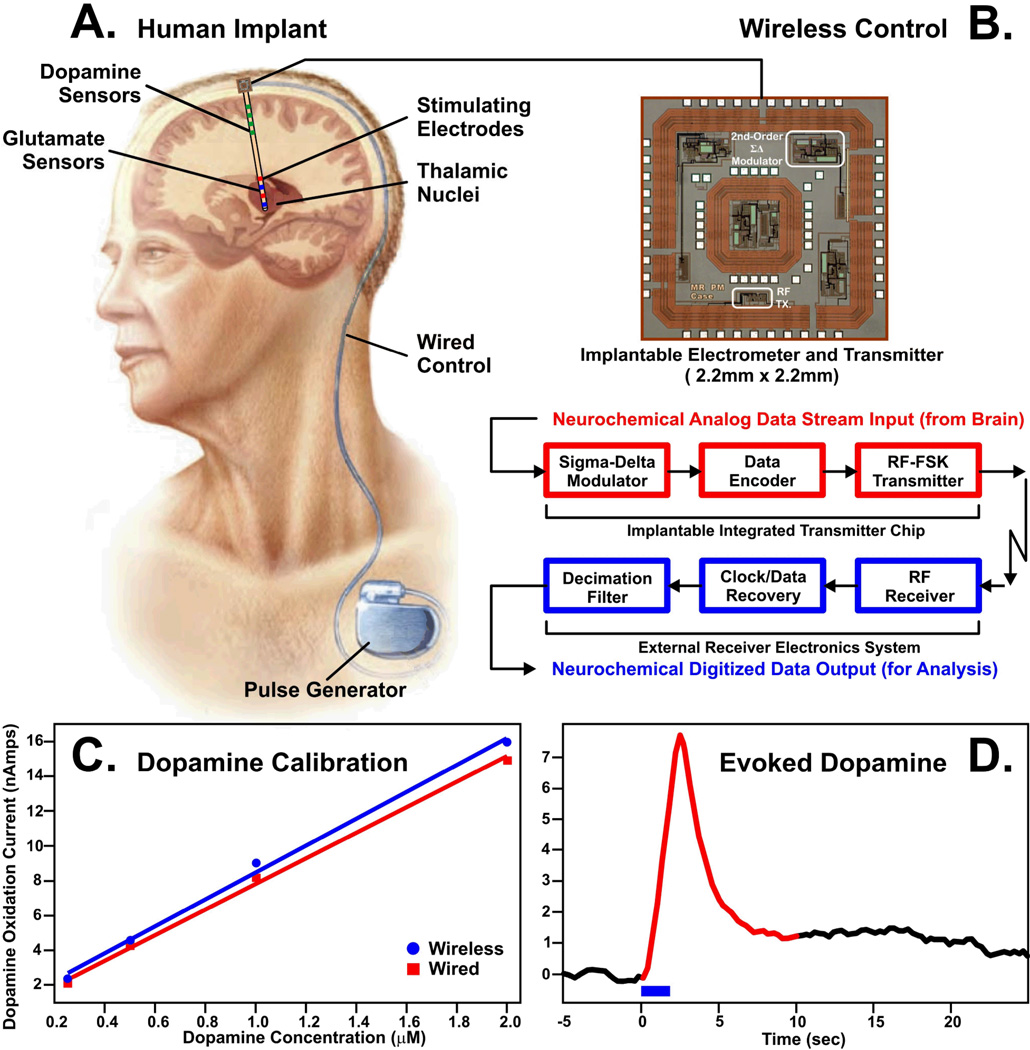
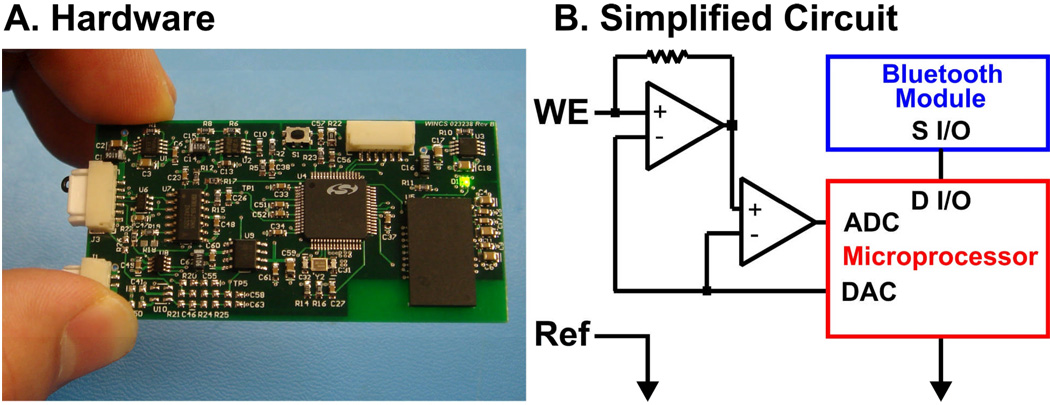
To address the potentiostat issue, we are developing a novel instrument we have dubbed Wireless Instantaneous Neurotransmitter Concentration Sensor (WINCS) to support electrochemical dopamine and glutamate measurements during STN DBS surgery (Fig. 11). Based on technology for fast-scan cyclic voltammetry applied to experimental animals (103, 104), the battery-powered WINCS patient module comprises three major elements: analog front-end circuitry to impose the desired sensor potential and to convert sensor current to voltage; a microcontroller to produce the applied voltammetric waveform (or constant amperometric potential) and to perform analog-to-digital conversion of the Faradaic sensor current; and a Bluetooth® radio to transmit the data wirelessly to a base station receiver. The base station can be located some distance away, at any convenient location in the operating room. In addition to recording the raw data, the base station can display various representations of the amperometric or voltammetric data in near real time. The WINCS patient module is packaged in a small, sterilizable enclosure that can be unobtrusively mounted on the microdrive that controls the depth positioning of the electrode during surgery. WINCS has been designed with patient safety in mind. The battery’s low terminal voltage (∼3.7 V) is within the range of DBS stimulation. Battery power and wireless data communication together afford isolation from extraneous fault currents. Once positioned by coordinates obtained typically by intra-operative magnetic resonance imaging (MRI), WINCS could fine-tune the final placement of the stimulation electrode guided by optimal neurotransmitter release. Thus, speed and accuracy would be improved by this innovative approach.
FIGURE 11.
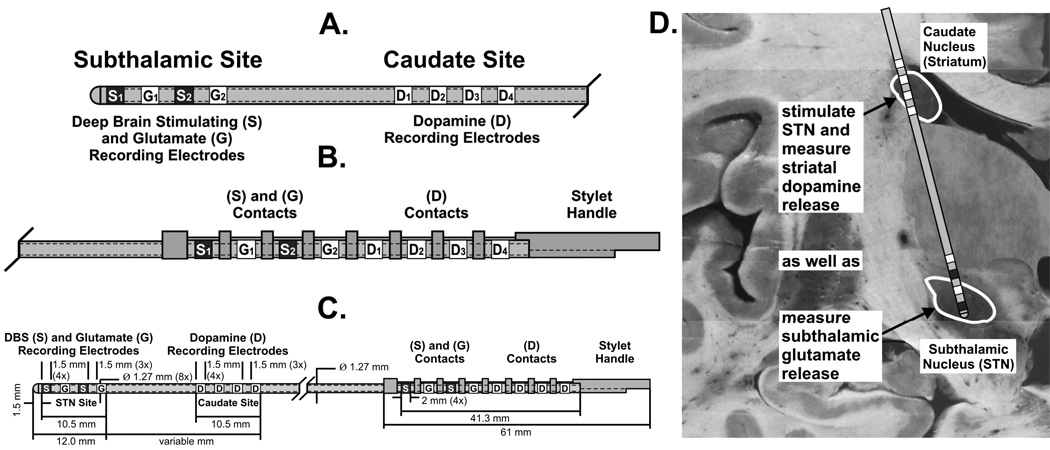
Schematic of a probe comprised of an array of glutamate sensors (G1–2) positioned between subthalamic nucleus stimulating electrodes (S1–2) at the tip and a distal array of four sensors for dopamine (D1–4) (A–C). By taking advantage of the neuroanatomy of the striatum and subthalamic nucleus, this newly designed probe can be implanted in both nuclei such that simultaneous stimulation and neurochemical recording of both transmitters can be achieved with a single probe as depicted to scale in a representative coronal section of the human brain (D).
We have also begun to address the issue of a suitable sensor/stimulator to support neurotransmitter measurements and electrical stimulation during STN DBS surgery. A straightforward approach is a side-by-side neurochemical probe and conventional DBS electrode configuration for initial short-term, intraoperative monitoring. This is a similar approach we have used to monitor local electrophysiological activity evoked by DBS during implantation of the stimulating electrode in patients (105). Eventual long-term post-operative recordings are a goal as well. One such strategy under consideration, a multifunctional neuromodulation-neuromonitoring electrode array, is illustrated in Fig. 11 A–C. At the distal end are alternative sites for electrical stimulation and glutamate biosensing, using the enzyme-linked detection scheme described previously. Dopamine recording sites, based on fast-scan cyclic voltammetry, are located more proximally. Other configurations are possible, including substitution of electrophysiological recording sites as well as incorporating the quasi-simultaneous voltammetry and electrophysiology dual sensing scheme at single recording sites.
The multifunctional probe architecture shown described in Fig. 11 A–C was designed to exploit the neuroanatomical trajectory shown in Fig. 11 D, with glutamate recording and electrical stimulation in the STN and dopamine recording dorsally in the caudate nucleus along the shaft of a single probe. The probe will also be compatible with WINCS, as amperometry will be supported in addition to fast-scan cyclic voltammetry. Future WINCS models are planned to incorporate electrophysiology and combined electrical and chemical measurements at a single recording site, realizing the full potential of the multifunctional probe. Merging multifunctional probe and WINCS technologies will support directed placement of the stimulating electrode during DBS surgery and afford the opportunity to test several predictions of the dopamine/glutamate release hypothesis in one individual simultaneously, including correlating DBS-evoked dopamine/glutamate levels with symptom relief. Thus, engineering of human-compatible monitoring instruments and probes represents an essential starting point in the effort to establish the potential use of resultant neurochemical readings as a means of improving clinical outcomes of DBS.
Closed-loop Smart DBS Devices
Intraoperative and post-operative neurochemical monitoring is projected to advance the DBS approach by neurochemical feedback guidance of surgical placement of the stimulating electrode and by providing the technical approaches for testing critical predictions of the neurotransmitter release (synaptic modulation) hypothesis of STN DBS. Implementation should be relatively straightforward, as existing methodologies, established in other research areas, are applied to DBS in humans. A more long-term goal is developing radically new technology and approaches to advance DBS on other fronts. One such strategy is a closed-loop smart device supporting all-in- one neuromonitoring and neuromodulation. The instrument rationale is built around neurochemical sensing feedback to maintain neurotransmitters at optimal levels for therapeutic efficacy. Conceivably, a neuroprosthesis supporting instantaneous chemical sensing, feedback, and response (to stimulation) is superior to drug treatments such as L-DOPA that entail onset and offset effects.
As shown in Fig. 12, we have initiated work towards the ultimate realization of a closed-loop smart DBS device. One critical component is an ultra-small, low-power integrated circuit supporting wireless neurochemical monitoring. By using very-large-scale-integration (VLSI) techniques in standard complementary-metal-oxide-semiconductor (CMOS) technology, we have been able to fabricate a wireless device, with dimensions of 2.2 mm × 2.2 mm, supporting single-channel fast-scan cyclic voltammetry. This device compared favorably to a conventional hardwired system in calibration tests in vitro and for measuring MFB-evoked dopamine release in the anesthetized rat (106).
FIGURE 12.
Conceptual illustration of wireless transmission of neurochemical data from a dual probe comprised of dopamine and glutamate sensors and stimulating electrodes positioned in thalamic nuclei of a human patient (A). The brain-implantable CMOS chip (2.2mm × 2.2mm) containing an electrometer to conduct in vivo neurochemistry (B) will record and wirelessly transmit to the outside world the electrochemical signals corresponding to extracellular concentration variations of distal dopamine and local glutamate levels elicited by deep brain stimulation of thalamic nuclei in human patients. These neurochemical responses will be used as feedback to modulate the stimulation parameters in the chest-implanted pulse generator. Typical dopamine calibration curves generated by flow injection analysis for hardwired (red line) and wireless (blue line) fast-scan cyclic voltammetry recording systems (C). Example of electrically (blue bar) evoked dopamine release measured wirelessly by the chip in the striatum of an anesthetized rat (D). (B–D) modified from ref. (106)
CONCLUSION
As our understanding of the DBS mechanisms of action continues to expand, it is likely that such advances will enable advances in the DBS approach by supporting improvements in the surgical technique and the development of next-generation “smart” DBS devices. The hope of significant improvements will also result in broadening the range of neurological and psychiatric conditions that can be treated by DBS. Whether next-generation “smart” DBS devices will utilize continuous neurochemical sensing feedback to regulate brain neurotransmitter levels is unknown, but such technologies will undoubtedly have a profound impact on both basic and clinical neuroscience. In addition, advances in integrated circuit design and fabrication will undoubtedly enable the development of ultra-small-scale DBS systems incorporating both conventional stimulus pulse generation and novel in vivo monitoring of neurochemical activity during intra- and post-operation periods.
FIGURE 10.
WINCS hardware (A) enabling wireless monitoring of dopamine and glutamate release from implanted electrodes during deep brain stimulation in human PD patients. WINCS simplified circuit (B) utilizing Bluetooth technology for instantaneous transmission of deep brain stimulation evoked changes in neurotransmitter release at electrochemical sensors (WE, working [recording] electrodes).
Footnotes
Conflict of Interest Statement:
Kendall H. Lee, MD, PhD, and Charles D. Blaha, PhD, have a patent pending on a smart DBS system. No other conflict of Interest was reported.
REFERENCES
- 1.Benabid AL. Deep brain stimulation for Parkinson's disease. Cur Opin Neurobio. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 3.Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson's disease, essential tremor and extra-pyramidal dyskinesias. Acta Neurochir Suppl (Wien) 1993;58:39–44. doi: 10.1007/978-3-7091-9297-9_8. [DOI] [PubMed] [Google Scholar]
- 4.Greene P. Deep-brain stimulation for generalized dystonia. N Engl J Med. 2005;352:498–500. doi: 10.1056/NEJMe048333. [DOI] [PubMed] [Google Scholar]
- 5.Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43:603–608. doi: 10.1046/j.1528-1157.2002.26001.x. [DOI] [PubMed] [Google Scholar]
- 6.Boon P, Vonck K, De HV, Van Dycke A, Goethals M, Goossens L, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- 7.Vonck K, Boon P, Van Roost D. Anatomical and physiological basis and mechanism of action of neurostimulation for epilepsy. Acta Neurochir Suppl. 2007;97:321–328. doi: 10.1007/978-3-211-33081-4_35. [DOI] [PubMed] [Google Scholar]
- 8.Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, Aziz TZ. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515–519. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21:E8. doi: 10.3171/foc.2006.21.6.10. [DOI] [PubMed] [Google Scholar]
- 10.Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107:1004–1014. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 12.Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery. 2007;61:1–11. doi: 10.1227/01.neu.0000279719.75403.f7. [DOI] [PubMed] [Google Scholar]
- 13.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Hardesty DE, Sackeim HA. Deep brain stimulation in movement and psychiatric disorders. Biol Psychiatry. 2007;61:831–835. doi: 10.1016/j.biopsych.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 15.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 16.McIntyre CC, Butson CR, Maks CB, Noecker AM. Optimizing deep brain stimulation parameter selection with detailed models of the electrode-tissue interface. Conf Proc IEEE Eng Med Biol Soc. 2006;115:893–895. doi: 10.1109/IEMBS.2006.260844. [DOI] [PubMed] [Google Scholar]
- 17.Lee KH, Blaha CD, Bledsoe JM. Mechanisms of action of deep brain stimulation: a review. In: Krames E, Peckham H, Rezai A, editors. A Textbook of Neuromodulation. Amsterdam: Elsevier; 2008. pp. 1–25. [Google Scholar]
- 18.Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 19.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 20.Benazzouz A, Piallat B, Pollak P, Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett. 1995;189:77–80. doi: 10.1016/0304-3940(95)11455-6. [DOI] [PubMed] [Google Scholar]
- 21.Patel NK, Heywood P, O’Sullivan K, McCarter R, Love S, Gill SS. Unilateral subthalamotomy in the treatment of Parkinson’s disease. Brain. 2003;126:1136–1145. doi: 10.1093/brain/awg111. [DOI] [PubMed] [Google Scholar]
- 22.Benazzouz A, Hallett M. Mechanism of action of deep brain stimulation. Neurology. 2000;55:S13–S16. [PubMed] [Google Scholar]
- 23.McIntyre CC, Thakor NV. Uncovering the mechanisms of deep brain stimulation for Parkinson's disease through functional imaging, neural recording, and neural modeling. Crit Rev Biomed Eng. 2002;30:249–281. doi: 10.1615/critrevbiomedeng.v30.i456.20. [DOI] [PubMed] [Google Scholar]
- 24.Lozano AM, Eltahawy H. How does DBS work? Suppl Clin Neurophysiol. 2004;57:733–736. doi: 10.1016/s1567-424x(09)70414-3. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Perlmutter JS, Mink JW. Deep Brain Stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uc EY, Follett KA. Deep brain stimulation in movement disorders. Semin Neurol. 2007;27:170–182. doi: 10.1055/s-2007-971175. [DOI] [PubMed] [Google Scholar]
- 28.Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol. 2001;85:1351–1356. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- 29.Magarinos-Ascone C, Pazo JH, Macadar O, Buno W. High-frequency stimulation of the subthalamic nucleus silences subthalamic neurons: a possible cellular mechanism in Parkinson's disease. Neuroscience. 2002;115:1109–1117. doi: 10.1016/s0306-4522(02)00538-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee KH, Blaha CD, Harris BT, Cooper S, Hitti FL, Leiter JC, et al. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson’s disease. Eur J Neurosci. 2006;23:1005–1014. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- 31.McIntyre CC, Gril WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre CC, Savasta M, Walter BL, Vitek JL. How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol. 2004;21:40–50. doi: 10.1097/00004691-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 34.Volkmann J. Deep brain stimulation for the treatment of Parkinson's disease. J Clin Neurophysiol. 2004;21:6–17. doi: 10.1097/00004691-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- 36.Molinuevo JL, Valldeoriola F, Tolosa E, Rumia J, Valls-Sole J, Roldan H, Ferrer E. Levodopa withdrawal after bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch Neurol. 2000;57:983–988. doi: 10.1001/archneur.57.7.983. [DOI] [PubMed] [Google Scholar]
- 37.Agid Y. Parkinson's disease: pathophysiology. Lancet. 1991;337:1321–1324. doi: 10.1016/0140-6736(91)92989-f. [DOI] [PubMed] [Google Scholar]
- 38.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1153. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 39.Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 40.Gerlach M, van den Buuse M, Blaha CD, Bremen D, Riederer P. Entacapone increases and prolongs the central effects of L-DOPA in the 6-hyroxydopamine-lesioned rat. Naunyn-Schmied Arch Pharmac. 2004;370:388–394. doi: 10.1007/s00210-004-0984-8. [DOI] [PubMed] [Google Scholar]
- 41.Breit S, Schulz JB, Benabid AL. Deep brain stimulation. Cell Tissue Res. 2004;318:275–288. doi: 10.1007/s00441-004-0936-0. [DOI] [PubMed] [Google Scholar]
- 42.Kern DS, Kumar R. Deep brain stimulation. Neurologist. 2007;13:237–252. doi: 10.1097/NRL.0b013e3181492c48. [DOI] [PubMed] [Google Scholar]
- 43.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 44.Bruet N, Windels F, Bertrand A, Feuerstein C, Poupard A, Savasta M. High frequency stimulation of the subthalamic nucleus increases the extracellular contents of striatal dopamine in normal and partially dopaminergic denervated rats. J Neuropathol Exp Neurol. 2001;60:15–24. doi: 10.1093/jnen/60.1.15. [DOI] [PubMed] [Google Scholar]
- 45.Paul G, Reum T, Meissner W, Marburger A, Sohr R, Morgenstern R, Kupsch A. High frequency stimulation of the subthalamic nucleus influences striatal dopaminergic metabolism in the naive rat. Neuroreport. 2000;11:441–444. doi: 10.1097/00001756-200002280-00003. [DOI] [PubMed] [Google Scholar]
- 46.Meissner W, Reum T, Paul G, Harnack D, Sohr R, Morgenstern R, Kupsch A. Striatal dopaminergic metabolism is increased by deep brain stimulation of the subthalamic nucleus in 6-hydroxydopamine lesioned rats. Neurosci Lett. 2001;303:165–168. doi: 10.1016/s0304-3940(01)01758-x. [DOI] [PubMed] [Google Scholar]
- 47.Meissner W, Harnack D, Paul G, Reum T, Sohr R, Morgenstern R, Kupsch A. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neurosci Lett. 2002;328:105–108. doi: 10.1016/s0304-3940(02)00463-9. [DOI] [PubMed] [Google Scholar]
- 48.Abosch A, Kapur S, Lang AE, Hussey D, Sime E, Miyasaki J, et al. Stimulation of the subthalamic nucleus in Parkinson's disease does not produce striatal dopamine release. Neurosurgery. 2003;53:1095–1102. doi: 10.1227/01.neu.0000088662.69419.1b. [DOI] [PubMed] [Google Scholar]
- 49.Hilker R, Voges J, Ghaemi M, Lehrke R, Rudolf J, Koulousakis A, et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord. 2003;18:41–48. doi: 10.1002/mds.10297. [DOI] [PubMed] [Google Scholar]
- 50.Thobois S, Fraix V, Savasta M, Costes N, Pollak P, Mertens P, et al. Chronic subthalamic nucleus stimulation and striatal D2 dopamine receptors in Parkinson's disease--A [(11)C]-raclopride PET study. J Neurol. 2003;250:219–223. doi: 10.1007/s00415-003-0188-z. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Fowler JS, Wang GJ, Dewey SL, Schlyer D, MacGregor R, et al. Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain. J Nucl Med. 1993;34:609–613. [PubMed] [Google Scholar]
- 52.Laruelle M, Slifstein M, Huang Y. Positron emission tomography: imaging and quantification of neurotransporter availability. Methods. 2002;27:287–299. doi: 10.1016/s1046-2023(02)00085-3. [DOI] [PubMed] [Google Scholar]
- 53.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 54.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 55.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 56.Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- 57.Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- 58.Garzon M, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter. J Comp Neurol. 1999;410:197–210. doi: 10.1002/(sici)1096-9861(19990726)410:2<197::aid-cne3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 59.Grillner P, Mercuri NB. Intrinsic membrane properties and synaptic inputs regulating the firing activity of the dopamine neurons. Behav Brain Res. 2002;130:149–169. doi: 10.1016/s0166-4328(01)00418-1. [DOI] [PubMed] [Google Scholar]
- 60.McGinty JF. Co-localization of GABA with other neuroactive substances in the basal ganglia. Prog Brain Res. 2007;160:273–284. doi: 10.1016/S0079-6123(06)60016-2. [DOI] [PubMed] [Google Scholar]
- 61.Meltzer LT, Christoffersen CL, Serpa KA. Modulation of dopamine neuronal activity by glutamate receptor subtypes. Neurosci Biobehav Rev. 1997;21:511–518. doi: 10.1016/s0149-7634(96)00030-9. [DOI] [PubMed] [Google Scholar]
- 62.Blaha CD, Winn P. Modulation of dopamine efflux in the striatum following cholinergic stimulation of the substantia nigra in intact and pedunculopontine tegmental nucleus-lesioned rats. J Neurosci. 1993;13:1035–1044. doi: 10.1523/JNEUROSCI.13-03-01035.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci. 2003;17:751–762. doi: 10.1046/j.1460-9568.2003.02511.x. [DOI] [PubMed] [Google Scholar]
- 65.Paladini CA, Celada P, Tepper JM. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience. 1999;89:799–812. doi: 10.1016/s0306-4522(98)00355-8. [DOI] [PubMed] [Google Scholar]
- 66.Celada P, Paladini CA, Tepper JM. GABAergic control of nigra dopamine neurons: role of globus pallidus and nigra reticulata. Neuroscience. 1999;89:813–825. doi: 10.1016/s0306-4522(98)00356-x. [DOI] [PubMed] [Google Scholar]
- 67.Iribe Y, Moore K, Pang KC, Tepper JM. Subthalamic stimulation-induced synaptic responses in substantia nigra pars compacta dopaminergic neurons in vitro. J Neurophys. 1999;82:925–933. doi: 10.1152/jn.1999.82.2.925. [DOI] [PubMed] [Google Scholar]
- 68.Shen KZ, Zhu ZT, Munhall A, Johnson SW. Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse. 2003;50:314–319. doi: 10.1002/syn.10274. [DOI] [PubMed] [Google Scholar]
- 69.Lee KH, Chang SY, Roberts DW, Kim U. Neurotransmitter release from high-frequency stimulation of the subthalamic nucleus. J Neurosurg. 2004;101:511–517. doi: 10.3171/jns.2004.101.3.0511. [DOI] [PubMed] [Google Scholar]
- 70.Lee KH, Kristic K, van Hoff R, Hitti FL, Blaha CD, Harris B, et al. High frequency stimulation of the subthalamic nucleus increases glutamate in the subthalamic nucleus of rats as demonstrated by in vivo enzyme-linked glutamate sensor. Brain Res. 2007;1162:121–129. doi: 10.1016/j.brainres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 71.Lee KH, Hitti FL, Shalinsky MH, Kim U, Leiter JC, Roberts DW. Abolition of spindle oscillations and 3-Hz absence seizurelike activity in the thalamus by using high-frequency stimulation: potential mechanism of action. J Neurosurg. 2005;103:538–545. doi: 10.3171/jns.2005.103.3.0538. [DOI] [PubMed] [Google Scholar]
- 72.Vernadakis A, Lee K, Kentroti S, Brodie C. Role of astrocytes in aging: late passage primary mouse brain astrocytes and C-6 glial cells as models. Prog Brain Res. 1992;94:391–409. doi: 10.1016/s0079-6123(08)61767-7. [DOI] [PubMed] [Google Scholar]
- 73.Lee KH, Hitti FL, Tawfik VL, Kristic KJ, Harris BT, Leiter JC, Roberts DW. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. High frequency stimulation abolishes network oscillations by astrocytic glutamate release. Program No. 898.2. [Google Scholar]
- 74.Benazzouz A, Gao D, Ni Z, Benabid AL. High frequency stimulation of the STN influences the activity of dopamine neurons in the rat. Neuroreport. 2000;11:1593–1596. [PubMed] [Google Scholar]
- 75.Benazzouz A, Piallat B, Ni Z, Koudsie G, Pollak A, Benabid AL. Implication of the subthalamic nucleus in the pathophysiology and pathogenesis of Parkinson's disease. Cell Transplant. 2000;9:215–221. doi: 10.1177/096368970000900207. [DOI] [PubMed] [Google Scholar]
- 76.Hammond C, Deniau JM, Rizk A, Feger J. Electrophysiological demonstration of an excitatory subthalamonigral pathway in the rat. Brain Res. 1978;151:235–244. doi: 10.1016/0006-8993(78)90881-8. [DOI] [PubMed] [Google Scholar]
- 77.Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12:287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- 78.Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- 79.Bergstrom BP, Garris PA. Utility of a tripolar stimulating electrode for eliciting dopamine release in the rat striatum. J Neurosci Meth. 1999;87:201–208. doi: 10.1016/s0165-0270(99)00009-6. [DOI] [PubMed] [Google Scholar]
- 80.Bergstrom BP, Garris PA. 'Passive stabilization' of striatal extracellular dopamine across the lesion spectrum encompassing the presymptomatic phase of Parkinson's disease: a voltammetric study in the 6-OHDA-lesioned rat. J Neurochem. 2003;87:1224–1236. doi: 10.1046/j.1471-4159.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- 81.Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Meth. 1999;90:129–142. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- 82.Tang A, Bungay PM, Gonzales RA. Characterization of probe and tissue factors that influence interpretation of quantitative microdialysis experiments for dopamine. J Neurosci Meth. 2003;126:1–11. doi: 10.1016/s0165-0270(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 83.Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Meth. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Morelli M, Carboni E, Cozzolino A, Tanda GL, Pinna A, Di Chiara G. Combined microdialysis and fos immunohistochemistry for the estimation of dopamine neurotransmission in the rat caudate-putamen. J Neurochem. 1992;59:1158–1160. doi: 10.1111/j.1471-4159.1992.tb08359.x. [DOI] [PubMed] [Google Scholar]
- 85.Di Chiara G, Carboni E, Morelli M, Cozzolino A, Tanda GL, Pinna A, et al. Stimulation of dopamine transmission in the dorsal caudate nucleus by pargyline as demonstrated by dopamine and acetylcholine microdialysis and Fos immunohistochemistry. Neuroscience. 1993;55:451–456. doi: 10.1016/0306-4522(93)90514-g. [DOI] [PubMed] [Google Scholar]
- 86.Blaha CD, Phillips AG. A critical assessment of electrochemical procedures applied to the measurement of dopamine and its metabolites during drug-induced and species-typical behaviours. Behav Pharmacol. 1996;7:675–708. [PubMed] [Google Scholar]
- 87.Blaha CD, Coury A, Phillips AG. Does monoamine oxidase inhibition by pargyline increase extracellular dopamine concentrations in the striatum? Neuroscience. 1996;75:543–550. doi: 10.1016/0306-4522(96)00289-8. [DOI] [PubMed] [Google Scholar]
- 88.Peters JL, Miner LH, Michael AC, Sesack SR. Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. J Neurosci Meth. 2004;137:9–23. doi: 10.1016/j.jneumeth.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Cheer JF, Heien ML, Garris PA, Carelli RM, Wightman RM. Simultaneous electrochemical and single-unit recordings in the nucleus accumbens reveal GABA-mediated responses: implications for intracranial self-stimulation. Proc Natl Acad Sci USA. 2005;102:19150–19155. doi: 10.1073/pnas.0509607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- 91.Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson's disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- 92.Saint-Cyr JA, Hoque T, Pereira LC, Dostrovsky JO, Hutchison WD, Mikulis DJ, et al. Localization of clinically effective stimulating electrodes in the human subthalamic nucleus on magnetic resonance imaging. J Neurosurg. 2002;97:1152–1166. doi: 10.3171/jns.2002.97.5.1152. [DOI] [PubMed] [Google Scholar]
- 93.Voges J, Volkmann J, Allert N, Lehrke R, Koulousakis A, Freund HJ, Sturm V. Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: correlation of therapeutic effect with anatomical electrode position. J Neurosurg. 2002;96:269–279. doi: 10.3171/jns.2002.96.2.0269. [DOI] [PubMed] [Google Scholar]
- 94.Herzog J, Fietzek U, Hamel W, Morsnowski A, Steigerwald F, Schrader B, et al. Most effective stimulation site in subthalamic deep brain stimulation for Parkinson's disease. Mov Disord. 2004;19:1050–1054. doi: 10.1002/mds.20056. [DOI] [PubMed] [Google Scholar]
- 95.Chang J-Y, Shi L-H, Luo F, Zhang W-M, Woodward DJ. Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson’s disease. Neurosci Biobehav Rev. 2007;31:643–657. doi: 10.1016/j.neubiorev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 96.Blaha CD, Phillips AG. Application of in vivo electrochemistry to the measurement of changes in dopamine release during intracranial self-stimulation. J Neurosci Meth. 1990;34:125–133. doi: 10.1016/0165-0270(90)90050-p. [DOI] [PubMed] [Google Scholar]
- 97.Kilpatrick MR, Rooney MB, Michael DJ, Wightman RM. Extracellular dopamine dynamics in rat caudate-putamen during experimenter-delivered and intracranial self-stimulation. Neuroscience. 2000;96:697–706. doi: 10.1016/s0306-4522(99)00578-3. [DOI] [PubMed] [Google Scholar]
- 98.Yavich L, Tiihonen J. In vivo voltammetry with removable carbon fibre electrodes in freely-moving mice: dopamine release during intracranial self-stimulation. J Neurosci Meth. 2000;104:55–63. doi: 10.1016/s0165-0270(00)00321-6. [DOI] [PubMed] [Google Scholar]
- 99.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 101.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007a;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 102.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 103.Garris PA, Ensman R, Poehlman J, Alexander A, Langley PE, Sandberg SG, et al. Wireless transmission of fast-scan cyclic voltammetry at a carbon-fiber microelectrode: proof of principle. J Neurosci Meth. 2004;140:103–115. doi: 10.1016/j.jneumeth.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 104.Garris PA, Greco PG, Sandberg SG, Howes G, Pongmayteegul S, Heidenreich BA, et al. In vivo voltammetry with telemetry. In: Michael AC, Borland LM, editors. Electrochemical Methods for Neuroscience. Boca Raton: CRC Press; 2006. pp. 233–259. [Google Scholar]
- 105.Agnesi F, Tye SJ, Blaha CD, Garris PA, Lin J, Goerss S, et al. Complex electrophysiological correlates to deep brain stimulation in patients with Parkinson’s disease, dystonia, and essential tremor. Proc Nat Acad Sci. 2008 submitted. [Google Scholar]
- 106.Roham M, Daberkow DP, Ramsson ES, Covey DP, Pakdeeronachit S, Garris PA, et al. A wireless IC for wide-range neurochemical monitoring using amperometry and fast-scan cyclic voltammetry. IEEE Trans Biomed Circuits and Systems. 2008;2:3–9. doi: 10.1109/TBCAS.2008.918282. [DOI] [PubMed] [Google Scholar]