Abstract
One of the most provocative and exciting issues in cognitive science is how neural specificity for semantic categories of common objects arises in the functional architecture of the brain. More than two decades of research on the neuropsychological phenomenon of category-specific semantic deficits has generated detailed claims about the organization and representation of conceptual knowledge. More recently, researchers have sought to test hypotheses developed on the basis of neuropsychological evidence with functional imaging. From those two fields, the empirical generalization emerges that object domain and sensory modality jointly constrain the organization of knowledge in the brain. At the same time, research within the embodied cognition framework has highlighted the need to articulate how information is communicated between the sensory and motor systems, and processes that represent and generalize abstract information. Those developments point toward a new approach for understanding category specificity in terms of the coordinated influences of diverse regions and cognitive systems.
Keywords: category-specific semantic deficits, apraxia, semantic organization, domain specific, sensory motor
INTRODUCTION
The scientific study of how concepts are represented in the mind/brain extends to all disciplines within cognitive science. Within the psychological and brain sciences, research has focused on studying how the perceptual, motor, and conceptual attributes of common objects are represented and organized in the brain. Theories of conceptual representation must therefore explain not only how conceptual content itself is represented and organized, but also the role played by conceptual content in orchestrating perceptual and motor processes.
Cognitive neuropsychological studies of brain-damaged patients provide strong evidence about the representation of conceptual knowledge and the relationship between conceptual knowledge and perceptual and motor processes. The cognitive neuropsychological approach ultimately seeks to evaluate models of cognitive processing through the proximate goal of explaining the profile of behavioral performance observed in brain-damaged patients. In the measure to which it is possible to establish the functional locus of impairment in a patient within a given model of cognitive functioning, then it is possible to test other assumptions of that model through further experiments with that patient. Dissociations of abilities in patients (and of processes in models) are central to the neuropsychological approach. This is because if a given behavior/process X can be impaired while another behavior/process Y is preserved, then one may conclude that the former process is not causally involved in the latter process. Another important source of evidence from neuropsychology are aspects of cognitive functioning that are observed to be systematically impaired or spared together (for discussion of methodological issues in cognitive neuropsychology, see Caramazza 1986, 1992; Shallice 1988).
Scope of the Review
The modern study of the representation of concepts in the brain was initiated by a series of papers by Elizabeth Warrington, Tim Shallice, and Rosaleen McCarthy. Those authors described patients with disproportionate semantic impairments for one, or several, categories of objects compared to other categories (see Hécaen & De Ajuriaguerra 1956 for earlier work). Since those initial investigations, a great deal has been learned about the causes of category-specific semantic deficits, and by extension, the organization of object knowledge in the brain.
The focus of this review is on neuropsychological research, and in particular, on the phenomenon of category-specific semantic deficits. Evidence from other fields within cognitive science and neuroscience and functional neuroimaging is reviewed as it bears on the theoretical positions that emerge from the study of category-specific semantic deficits. In particular, we highlight findings in functional neuroimaging related to the representation of different semantic categories in the brain. We also discuss the degree to which conceptual representations are grounded in sensory and motor processes, and the critical role that neuropsychological studies of patients with impairments to sensory and motor knowledge can play in constraining theories of semantic representation.
CATEGORY-SPECIFIC SEMANTIC DEFICITS: INTRODUCTION TO THE PHENOMENON
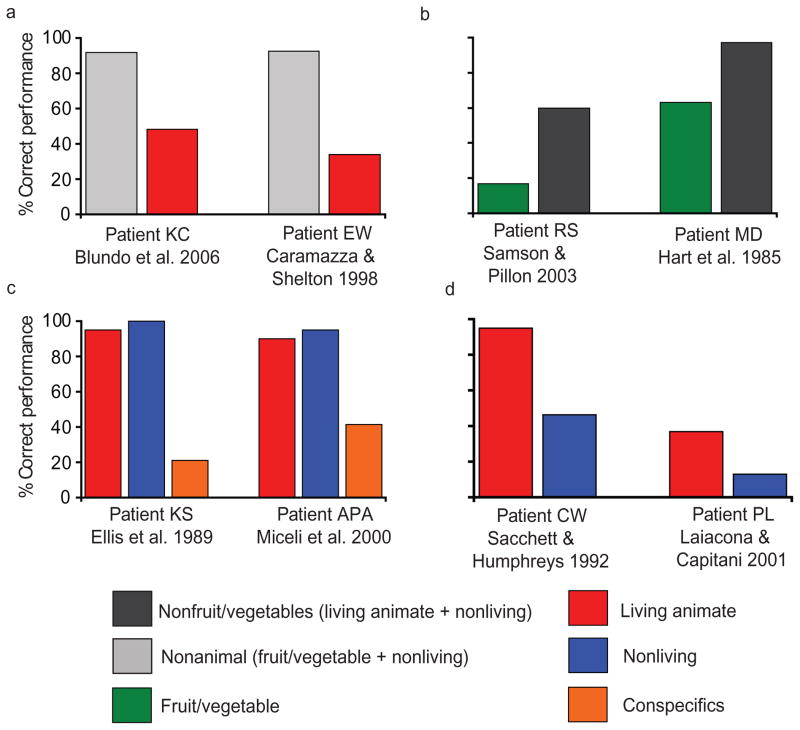
Patients with category-specific semantic deficits present with disproportionate or even selective impairments for one semantic category compared to other semantic categories. Figure 1 illustrates cases of disproportionate impairment for animals (upper left; Blundo et al. 2006, Caramazza & Shelton 1998), fruit/vegetables (upper right; Hart et al. 1985, Samson & Pillon 2003), conspecifics (lower left; Miceli et al. 2000, Ellis et al. 1989), and non-living things (lower right; Laiacona & Capitani 2001, Sacchett & Humphreys 1992). More than one hundred cases of category-specific semantic impairment have been reported (for review and discussion, see Capitani et al. 2003, Hart et al. 2007, Humphreys & Forde 2001, Tyler & Moss 2001). The majority of reported patients have disproportionate impairments for living things compared to nonliving things (Capitani et al. 2003).
Figure 1.
Representative picture naming performance of patients with category-specific semantic deficits for (a) living animate things, (b) fruit/vegetables, (c) conspecifics, and (d) nonliving.
One important aspect of the performance profile of patients with category-specific semantic impairment is that the impairment is to conceptual knowledge and not (only) to modality-specific input or output representations. The evidence for locating the deficit at a conceptual level is that the category-specific deficit does not depend on stimuli being presented or that patients respond in only one modality of input or output. For instance, patients KC and EW (Figure 1a) were impaired for naming living animate things compared to nonliving things and fruit/vegetables. Both patients were also impaired for answering questions about living animate things, such as “Does a whale have legs” or “Are dogs domestic animals,” but were unimpaired for the same types of questions about nonanimals (Figure 2a, for data from EW and other representative patients).
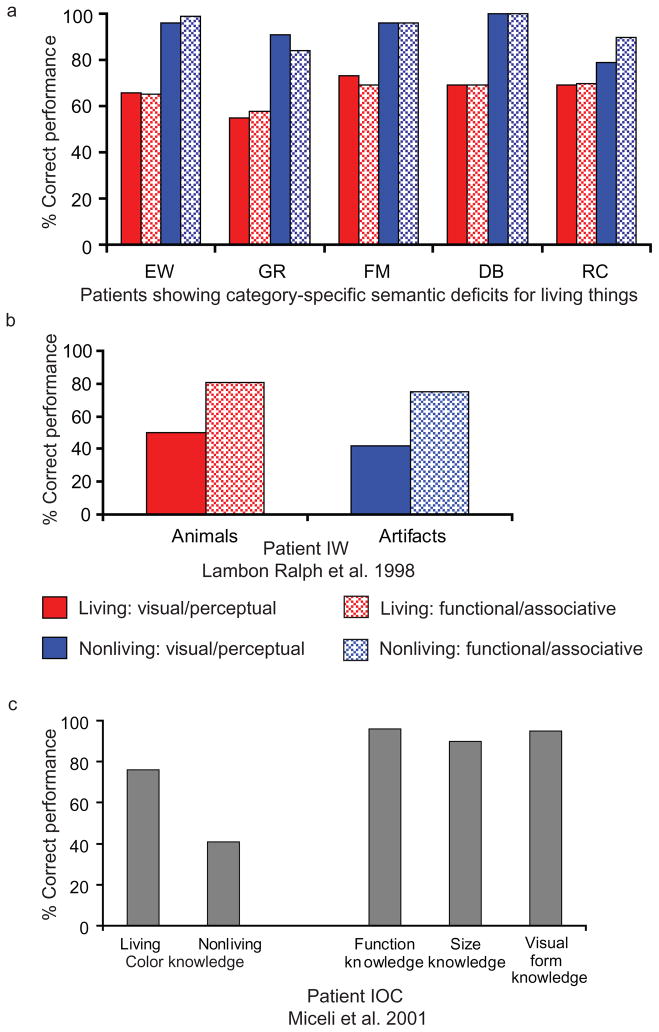
Figure 2.
Relation between impairments for a type or modality of knowledge and category-specific semantic deficits. These data show that (a) category-specific semantic impairments are associated with impairments for all types of knowledge about the impaired category, (b) differential impairments for visual/perceptual knowledge can be associated with (if anything) a disproportionate impairment for nonliving things compared to living things, and (c) selective impairment for knowledge of object color is not associated with a corresponding disproportionate deficit for fruit/vegetables. Data for EW from Caramazza & Shelton 1998; GR and FM from Laiacona et al. 1993; DB from Lambon Ralph et al. 1998; and RC from Moss et al. 1998.
Patients with category-specific semantic deficits may have additional, and also category-specific, deficits at presemantic levels of processing. For instance, patient EW was impaired for judging whether pictures depicted real or unreal animals but was unimpaired for the same task over nonanimal stimuli. The ability to make such decisions is assumed to index the integrity of the visual structural description system, a presemantic stage of object recognition (Humphreys et al. 1988). In contrast, patient KC was relatively unimpaired on an object decision task, even for the category of items (living animate) that the patient was unable to name. A similar pattern to that observed in patient KC was present in patient APA (Miceli et al. 2000). Patient APA was selectively impaired for conceptual knowledge of people (see Figure 1c). Despite a severe impairment for naming famous people, APA did not have a deficit at the level of face recognition (prosopagnosia).
Another important aspect of patients with category-specific semantic impairments is that they have difficulty distinguishing among basic-level items within the impaired category but do not necessarily have problems assigning items they cannot identify to the correct superordinate level category (e.g., they may know that a picture of a dog is an animal, but do not know which animal) (see Humphreys & Forde 2005 for discussion of a patient with greater difficulty at a superordinate than a basic level across all semantic categories).
A number of studies have now documented that variables such as lexical frequency, concept familiarity, and visual complexity may be unbalanced if items are sampled randomly from different semantic categories (Cree & McRae 2003, Funnell & Sheridan 1992, Stewart et al. 1992). In addition, Laiacona and colleagues (Barbarotto et al. 2002, Laiacona et al. 1998) have highlighted the need to control for gender-specific effects on variables such as concept familiarity (for discussion of differences between males and females in the incidence of category-specific semantic deficits for different categories, see Laiacona et al. 2006). However, the existence of category-specific semantic deficits is not an artifact of such stimulus-specific attributes. Clear cases have been reported for which stimulus-specific variables have been carefully controlled, and double dissociations have been reported using the same materials (e.g., Hillis & Caramazza 1991; see also the separate case reports in Barbarotto et al. 1995 and Laiacona & Capitani 2001).
OVERVIEW OF THEORETICAL EXPLANATIONS OF THE CAUSES OF CATEGORY-SPECIFIC SEMANTIC DEFICITS
Theories developed in order to explain category-specific semantic deficits fall into two broad groups (Caramazza 1998). Theories within the first group, based on the neural structure principle, assume dissociable neural substrates are differentially (or exclusively) involved in representing different semantic categories. Theories within the second group, based on the correlated structure principle, assume that conceptual knowledge of items from different semantic categories is not represented in functionally dissociable regions of the brain.
According to theories based on the neural structure principle, category-specific semantic deficits are due to differential or selective damage to the neural substrate upon which the impaired category of items depends. Two broad classes of theories based on the neural structure principle are the sensory/functional theory (Warrington & McCarthy 1983, 1987; Warrington & Shallice 1984) and the domain-specific hypothesis (Caramazza & Shelton 1998).
The sensory/functional theory is composed of two assumptions. The first—the multiple semantics assumption—is that conceptual knowledge is organized into subsystems that parallel the sensory and motor modalities of input and output. The second assumption is that the critical semantic attributes of items from different categories of objects are represented in different modality-specific semantic subsystems.
The domain-specific hypothesis assumes that the first-order constraint on the organization of conceptual knowledge is object domain, with the possible domains restricted to those that could have had an evolutionarily relevant history—living animate, living inanimate, con-specifics, and tools.
Theories based on the correlated structure principle model semantic memory as a system that represents statistical regularities in the co-occurrence of object properties in the world (Caramazza et al. 1990, Devlin et al. 1998, McClelland & Rogers 2003, Tyler & Moss 2001). This class of models has been instrumental in motivating large-scale empirical investigations of how different types of features are distributed and correlated for different semantic categories. Several theories based on the correlated structure principle have been developed in order to explain the causes of category-specific semantic deficits (Caramazza et al. 1990, Devlin et al. 1998, Tyler & Moss 2001).
This review is organized to reflect the role that different theoretical assumptions have played in motivating empirical research. Initial hypotheses that were developed in order to explain category-specific semantic deficits appealed to a single principle of organization (modality specificity, domain specificity, or correlated structure). The current state of the field of category-specific semantic deficits is characterized by complex models that integrate assumptions from multiple theoretical frameworks (for discussion, see Caramazza & Mahon 2003).
THE NEURAL STRUCTURE PRINCIPLE
The Multiple Semantics Assumption
Beauvois initially proposed that the organization of the semantic system follows the organization of the various input and output modalities to and from the semantic system (Beauvois 1982, Beauvois et al. 1978). The original motivation for the assumption of multiple semantics was the phenomenon of optic aphasia (e.g., Lhermitte & Beavuois 1973; for review, see Plaut 2002). Patients with optic aphasia present with impaired naming of visually presented objects but relatively (or completely) spared naming of the same objects when presented through the tactile modality (e.g., Hillis & Caramazza 1995). The fact that optic aphasic patients can name objects presented through the tactile modality indicates that the naming impairment to visual presentation is not due to a deficit at the level of retrieving the correct names. In contrast to patients with visual agnosia (e.g., Milner et al. 1991), patients with optic aphasia can recognize, at a visual level of processing, the stimuli they cannot name. Evidence for this is provided by the fact that some optic aphasic patients can demonstrate the correct use of objects that they cannot name (e.g., Coslett & Saffran 1992, Lhermitte & Beauvois 1973; for discussion, see Plaut 2002). Beauvois (1982) explained the performance of optic aphasic patients by assuming that the conceptual system is functionally organized into visual and verbal semantics and that optic aphasia is due to a disconnection between the two semantic systems.
Along with reporting the first cases of category-specific semantic deficit, Warrington and her collaborators (Warrington & McCarthy 1983, Warrington & Shallice 1984) developed an influential explanation of the phenomenon that built on the proposal of Beauvois (1982). Warrington and colleagues argued that category-specific semantic deficits are due to differential damage to a modality-specific semantic subsystem that is not itself organized by semantic category. Specifically, those authors noted that the patients they had reported with impairments for living things also had impairments for foods, plants, and precious stones (Warrington & Shallice 1984); in contrast, a patient with an impairment for nonliving things (Warrington & McCarthy 1983) was spared for living things, food, and plant life. Warrington and her collaborators reasoned that the association of impaired and spared categories was meaningfully related to the degree to which identification of items from those categories depends on sensory or functional knowledge. Specifically, they argued that the ability to identify living things differentially depends on sensory knowledge, whereas the ability to identify nonliving things differentially depends on functional knowledge.
Farah & McClelland (1991) implemented the theory of Warrington and colleagues in a connectionist framework. Three predictions follow from the computational model of Farah & McClelland (1991; for discussion, see Caramazza & Shelton 1998). All three of those predictions have now been tested. The first prediction is that the grain of category-specific semantic deficits should not be finer than living versus nonliving. This prediction follows from the assumption that all living things differentially depend on visual knowledge. However, as represented in Figure 1, patients have been reported with selective semantic impairments for fruit/vegetables (e.g., Hart et al. 1985, Laiacona et al. 2005, Samson & Pillon 2003) and animals (e.g., Blundo et al. 2006, Caramazza & Shelton 1998). The second prediction is that an impairment for a given category of knowledge will be associated with a disproportionate impairment for the modality of knowledge that is critical for that category. At variance with this prediction, it is now known that category-specific semantic deficits are associated with impairments for all types of knowledge (sensory and functional) about items from the impaired category (Figure 2a; e.g., Blundo et al. 2006, Caramazza & Shelton 1998, Laiacona & Capitani 2001, Laiacona et al. 1993, Lambon Ralph et al. 1998, Moss et al. 1998). The third prediction is that impairments for a type of knowledge will necessarily be associated with differential impairments for the category that depends on that knowledge type. Patients exhibiting patterns of impairment contrary to this prediction have been reported. For instance, Figure 2b shows the profile of a patient who was (a) more impaired for visual compared to functional knowledge, and (b) if anything, more impaired for nonliving things than living things (Lambon Ralph et al. 1998; see also Figure 2c, Figure 4, and discussion below).
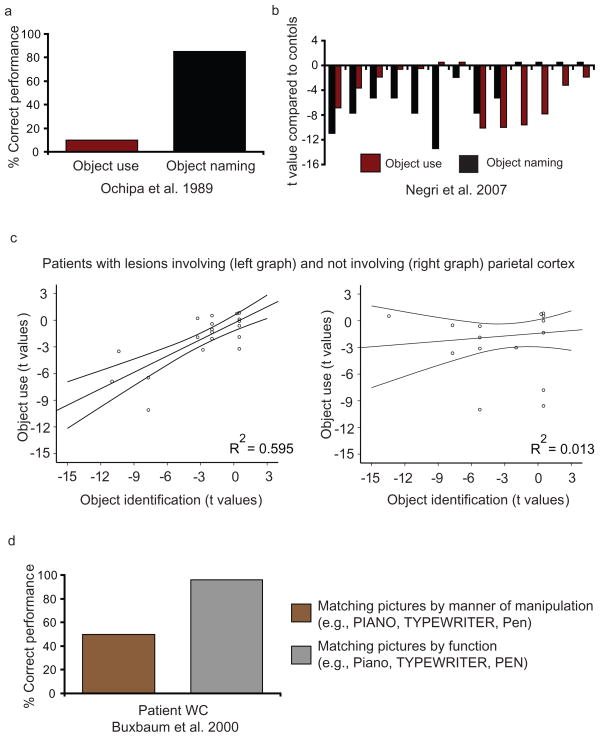
Figure 4.
Relation between knowledge of how to manipulate tools and other knowledge of tools. (a) Ochipa and colleagues (1989) reported a patient with a severe impairment for manipulating objects but relatively preserved naming of the same objects. (b) A multiple single-case study of unselected unilateral stroke patients asked patients to use and identify the same set of objects (Negri et al. 2007). Performance of the patients is plotted as t values (Crawford & Garthwaite 2006) compared to control (n = 25) performance. (c) Lesions to parietal cortex, in the context of lesions to lateral temporal and frontal regions, can be instrumental in modulating the relationship between performance in object identification and object use, at the group level (see Mahon et al. 2007, figure 7, for details and lesion overlap analyses). Each circle in the plots represents the performance of a single patient in object identification and object use. The 95% confidence intervals around the regression lines are shown. Reproduced with permission from Mahon and colleagues (2007). (d) Patient WC (Buxbaum et al. 2000) was impaired for matching pictures based on how objects are manipulated but was spared for matching pictures based on the function of the objects.
Second Generation Sensory/Functional Theories
The original formulation of the sensory/functional theory was based on a simple division between visual/perceptual knowledge and functional/associative knowledge. Warrington & McCarthy (1987; see also Crutch & Warrington 2003) suggested, however, that knowledge of object color is differentially important for fruit/vegetables compared to animals. Since Warrington and McCarthy’s proposal, further sensory- and motor-based dimensions that may be important for distinguishing between semantic categories have been articulated (e.g., Cree & McRae 2003, Vinson et al. 2003).
Cree & McRae (2003) used a feature-listing task to study the types of information that normal subjects spontaneously associate with different semantic categories. The semantic features were then classified into nine knowledge types: color, visual parts and surface properties, visual motion, smell, sound, tactile, taste, function, and encyclopedic (see Vinson et al. 2003 for a slightly different classification). Hierarchical cluster analyses were used to determine which semantic categories differentially loaded on which feature types. The results of those analyses indicated that (a) visual motion and function information were the two most important knowledge types for distinguishing living animate things (high on visual motion information) from nonliving things (high on function information), (b) living animate things were weighted lower on color information than fruit/vegetables, but higher on this knowledge type than nonliving things, and (c) fruit/vegetables were distinguished from living animate and nonliving things by being weighted the highest on both color and taste information.
Cree and McRae’s analyses support the claim that the taxonomy of nine knowledge types is effective in distinguishing between the domains living animate, fruit/vegetables, and nonliving. Those analyses do not demonstrate, however, that the nine knowledge types are critical for distinguishing between items within the respective categories. As noted above, patients with category-specific semantic impairments do not necessarily have difficulty distinguishing between different domains (i.e., they might know it is an animal but cannot say which one). It is therefore not obvious that Cree and McRae’s analyses support the claim that category-specific semantic deficits may be explained by assuming damage to one (or more) of the nine knowledge types.
At a more general level, the open empirical question is whether the additional knowledge types and the corresponding further functional divisions that are introduced into the semantic system can account for the neuropsychological evidence. Clearly, if fruit/vegetables and animals are assumed to differentially depend on different types of information (and by inference, different semantic subsystems), it is in principle possible to account for the tripartite distinction between animals, fruit/vegetables, and nonliving. As for the original formulation of the sensory/functional theory, the question is whether fine-grained category-specific semantic impairments are associated with impairments for the type of knowledge upon which items from the impaired category putatively depend. However, patients have been reported with category-specific semantic impairments for fruit/vegetables, without disproportionate impairments for color knowledge (e.g., Samson & Pillon 2003). Patients have also been reported with impairment for knowledge of object color without a disproportionate impairment for fruit/vegetables compared to other categories of objects (see Figure 2c; Luzzatti & Davidoff 1994, Miceli et al. 2001).
Another way in which investigators have sought to provide support for the sensory/functional theory is to study the semantic categories that are systematically impaired together. As noted above, one profile of the first reported cases that motivated the development of the sensory/functional theory (Warrington & Shallice 1984) was that the categories of animals, plants, and foods tended to be impaired or spared together. Those associations of impairing and sparing of categories made sense if all of those categories depended on the same modality-specific system for their identification. Following the same logic, it was argued that musical instruments patterned with living things (because of the importance of sensory attributes) (see Dixon et al. 2000 for relevant data), and that body parts patterned with nonliving things (because of the importance of functional attributes associated with object usage (e.g., Warrington & McCarthy 1987). However, as was the case for the dissociation between living animate things (animals) and living inanimate things (e.g., plants), it is now known that musical instruments dissociate from living things and that body parts dissociate from nonliving things (Caramazza & Shelton 1998, Laiacona & Capitani 2001, Shelton et al. 1998, Silveri et al. 1997, Turnbull & Laws 2000; for review and discussion, see Capitani et al. 2003).
More recently, Borgo & Shallice (2001, 2003) have argued that sensory-quality categories, such as materials, edible substances, and drinks are similar to animals in that they depend on sensory information for their identification. Those authors reported that impairment for living things was associated with impairments for sensory-quality categories. However, Laiacona and colleagues (2003) reported a patient who was impaired for living things but spared for sensory-quality categories (for further discussion, see Carroll & Garrard 2005).
Another dimension that has been argued to be instrumental in accounting for category-specific semantic deficits is differential similarity in the visual structure of items from different categories. Humphreys & Forde (2001; see also Tranel et al. 1997) argued that living things tend to be more structurally similar than nonliving things. If that were the case, then it could be argued that damage to a system not organized by object category will result in disproportionate disruption of items that are more “confusable” (see also Lambon Ralph et al. 2007, Rogers et al. 2004). Within Humphreys and Forde’s framework, it is also assumed that activation dynamically cascades from visual object recognition processes through to lexical access. Thus, perturbation of visual recognition processes could trickle through the system to disrupt the normal functioning of subsequent processes, resulting in a naming deficit (see Humphreys et al. 1988). Laws and colleagues (Laws & Gale 2002, Laws & Neve 1999) also argued for the critical role of similarity in visual structure for explaining category-specific semantic deficits. However, in contrast to Humphreys and Forde (see also Tranel et al. 1997), Laws and colleagues argued that nonliving things tend to be more similar than living things.
Clearly, much work remains to be done in order to understand the role that visual similarity, and the consequent crowding (Humphreys & Forde 2001) of visual representations, has in explaining category-specific semantic deficits. On the one hand, there is no consensus regarding the relevant object properties over which similarity should be calculated or how such a similarity metric should be calculated. On the other hand, assuming an agreed-upon means for determining similarity in visual shape, the question remains open as to the role that such a factor might play in explaining the facts of category-specific semantic deficits.
The Domain-Specific Hypothesis
The domain-specific hypothesis of the organization of conceptual knowledge in the brain (Caramazza & Shelton 1998) assumes that the first-order constraint on the organization of information within the conceptual system is object domain. The semantic categories that may be organized by domain-specific constraints are limited to those that could have had an evolu-tionarily relevant history: living animate, living inanimate, conspecifics, and tools. On this proposal, the phenomenon of category-specific semantic deficit reflects differential or selective damage to the neural substrates that support one or another domain of knowledge. Research from developmental psychology converges with the assumption that conceptual knowledge is organized, in part, by innately specified constraints on object knowledge (e.g., Baillargeon 1998, Carey & Spelke 1994, Gallistel 1990, Gelman 1990, Keil 1981, Spelke et al. 1992, Wellman & Gelman 1992; for a review, see Santos & Caramazza 2002; see, e.g., Kiani et al. 2007 for convergent findings using neurophysiological methods with nonhuman primates). Research in developmental psychology has also highlighted other domains of knowledge beyond those motivated by neuropsychological research on patients with category-specific deficits, such as number and geometric/spatial reasoning (e.g., JF Cantlon, M Platt, & EM Brannon, manuscript under review; Feigenson et al. 2004; Hermer & Spelke 1994).
Unique predictions are generated by the original formulation of the domain-specific hypothesis as it was articulated in the context of category-specific semantic deficits. One prediction is that the grain of category-specific semantic deficits will reflect the grain of those categories that could plausibly have had an evolutionarily relevant history (see Figure 1). Another prediction is that category-specific semantic impairments will be associated with impairments for all types of knowledge about the impaired category (see Figure 2a). A third prediction made by the domain-specific hypothesis is that it should be possible to observe category-specific impairments that result from early damage to the brain. Evidence in line with this expectation is provided by the case of Adam (Farah & Rabinowitz 2003). Patient Adam, who was 16 at the time of testing, suffered a stroke at one day of age. Adam failed to acquire knowledge of living things, despite normal levels of knowledge about nonliving things. As would be expected within the framework of the domain-specific hypothesis, Adam was impaired for both visual and nonvisual knowledge of living things (Farah & Rabinowitz 2003).
The Distributed Domain-Specific Hypothesis
The original formulation of the domain-specific hypothesis (Caramazza & Shelton 1998) anticipated the possibility of other dimensions of organization beyond object domain. It was proposed that correlational structure plays an important role in determining the organization of knowledge within domains. More important in the present context, it was also proposed that domain specificity would be found at both conceptual and perceptual levels of processing. We have since attempted to develop this account and have explored a model in which (a) object domain and sensory, motor, and emotional properties jointly constrain the organization of conceptual knowledge, and (b) object domain is a constraint on the organization of information at both a conceptual level as well as at the level of modality-specific visual input representations (Caramazza & Mahon 2003, 2006; Mahon & Caramazza 2003, 2008). We refer to this framework as the distributed domain-specific hypothesis in order to capture the idea that both object domain and a distributed network of modality-specific representations constrain the organization of conceptual knowledge of objects (we previously referred to this view as the domain-specific sensory-motor hypothesis; Mahon & Caramazza 2008). The central idea of this proposal is that domain-specific organization within a particular region is driven, in part, by the functional connectivity of the brain. That is, domain specificity is determined not only by the specific characteristics of processing within a given region, but also by how information in that region relates to information that is computed elsewhere and which is salient for the domain. The grain of that functional connectivity, according to the hypothesis, should reflect those object domains with evolutionarily important histories.
One expectation on the distributed domain-specific hypothesis is that impairments to abstract conceptual knowledge will dissociate from category-specific impairments at the level of object recognition. Consistent with this, and as noted above, impairments to conceptual knowledge are not necessarily associated with impairments at a modality-specific input level of visual processing. Some patients with conceptual-level impairments do have associated impairments for recognizing visually presented items (e.g., Caramazza & Shelton 1998), whereas other patients do not (e.g., Blundo et al. 2006; for a review, see Capitani et al. 2003).
Further convergent evidence is provided by the study of prosopagnosia. Patients with prosopagnosia have a deficit for recognizing visually presented faces but do not have difficulties retrieving other knowledge about the people they cannot recognize. For instance, such patients may be able to recognize the same people by the sound of their voice. The reverse dissociation—sparing of face recognition compared to recognition of other categories—has also been reported (Moscovitch et al. 1997).
Patients with prosopagnosia also constitute the other side of a double dissociation with patients such as APA, discussed above (see Figure 1c; Miceli et al. 2000). Patients such as APA are impaired for conceptual knowledge of conspecifics but are not necessarily prosopagnosic. Thus, within the domain of conspecifics, category-specific deficits at a modality-specific level of visual recognition dissociate from impairments to more abstract knowledge of conspecifics. In addition, prosopagnosia can arise developmentally, suggesting that the constraints that drive neural specificity for face perception are, in part, innately specified (Duchaine et al. 2006, Nunn et al. 2001; for a comprehensive review of acquired and developmental prosopagnosia, see Duchaine & Yovel 2008).
THE CORRELATED STRUCTURE PRINCIPLE
Theories based on the correlated structure principle assume that the conceptual system has no structure that is specifically reflected in functional neuroanatomy. For instance, the organized unitary content hypothesis (OUCH) (Caramazza et al. 1990) was initially formulated as an explanation of optic aphasia that did not invoke the assumption of multiple semantics. Caramazza and colleagues (1990; see also Riddoch et al. 1988) argued that there are privileged relationships between certain types of input representations (e.g., visual form) and certain types of output representations (e.g., knowledge of object manipulation), thus explaining how optic aphasic patients might be spared for gesturing to objects while impaired for naming them.
Other researchers subsequently developed highly specified proposals based on the correlated structure principle, all of which build on the idea that different types of features are differentially correlated across different semantic categories (Devlin et al. 1998, Rogers et al. 2004, Tyler & Moss 2001). Those models of semantic memory have been implemented computationally, with simulated damage, in order to provide existence proofs that a system with no explicit functional organization may be damaged so as to produce category-specific semantic deficits. Because theories based on the correlated structure principle do not assume that the conceptual system has structure at the level of functional neuroanatomy, they are best suited to modeling the patterns of progressive loss of conceptual knowledge observed in neurodegenerative diseases, such as dementia of the Alzheimer type and semantic dementia. The type of damage in such patients is diffuse and widespread and can be modeled in connectionist architectures by removing, to varying degrees, randomly selected components of the network.
One important proposal is the conceptual-structure account of Tyler, Moss, and colleagues (Bright et al. 2005, Tyler & Moss 2001). That proposal assumes that living things have more shared features, whereas nonliving things have more distinctive features. The model further assumes that the shared features of living things are highly correlated (has eyes/can see), whereas for nonliving things, distinctive features are highly correlated (used for spearing/has tines). If distinctive features are critical for identification, and if greater correlation confers resilience to damage, then an interaction between the severity of overall impairment and the direction of category-specific semantic deficit is predicted. Mild levels of impairments should produce disproportionate impairments for living things compared to nonliving things. At more severe levels of impairments, the distinctive features of nonliving things will be lost and a disproportionate impairment for this category will be observed. The opposite prediction regarding the severity of overall impairment and the direction of category-specific impairment is predicted by the account of Devlin and colleagues (1998) because it is assumed that as damage becomes severe, whole sets of intercorrelated features will be lost, resulting in a disproportionate impairment for living things. However, it is now known that neither prediction finds clear empirical support (Garrard et al. 1998, Zannino et al. 2002; see also Laiacona & Capitani 2001 for discussion within the context of focal lesions; for further discussion and theoretical developments, see Cree & McRae 2003, Vinson et al. 2003).
One issue that is not resolved is whether correlations between different features should be calculated in a concept-dependent or concept-independent manner (Zannino et al. 2006). For instance, although the (distinctive) information “has tines” is highly correlated with the function “used for spearing” in the concept FORK (correlated as concept dependent), the co-occurrence of those properties in the world is relatively low (concept independent). Sartori, Lombardi, and colleagues (Sartori & Lombardi 2004, Sartori et al. 2005) have addressed a similar issue by developing the construct of “semantic relevance,” which is computed through a nonlinear combination of the frequency with which particular features are produced for an item and the distinctiveness of that feature for all concepts in the database. Those authors have shown that living things tend to be lower, on average, than nonliving things in terms of their relevance, thus making living things on average “harder” than nonliving things. As is the case for other accounts of category-specific semantic deficits that are based on differences across categories along a single dimension, the existence of disproportionate deficits for the relatively “easy” category (nonliving things) is difficult to accommodate (see e.g., Hillis & Caramazza 1991, Laiacona & Capitani 2001; Figure 1d). Nevertheless, the theoretical proposal of Sartori and colleagues highlights the critical and unresolved issue of how to determine the psychologically relevant metric for determining feature correlations.
Another unresolved issue is whether high correlations between features will provide resilience to damage for those features, or will rather make damage contagious among them. It is often assumed that high correlation confers resilience to, or insulation from, damage; however, our understanding of how damage to one part of the brain affects other regions of the brain remains poorly developed. It is also not obvious that understanding the behavior of connectionist architectures constitutes the needed motivation for deciding whether greater correlation confers greater resilience to damage. In fact, theoretical differences about the role of correlations in conferring resilience to damage are in part responsible for the contrasting predictions that follow from the models of Tyler and colleagues (Tyler & Moss 2001) and Devlin and colleagues (1998) (for discussion, see Zannino et al. 2006).
Another example that illustrates our current lack of understanding of the role of feature correlation in determining patterns of impairment is provided by dissociations between sensory, motor, and conceptual knowledge. For instance, the visual structure of objects is highly correlated with more abstract knowledge of the conceptual features of objects. Even so, patients with impairments to abstract conceptual features of objects do not necessarily have corresponding impairments to object recognition processes (see above and Capitani et al. 2003 for review). Similarly, although manipulation knowledge (“how to” knowledge) is correlated with functional knowledge (“what for” knowledge), damage to the former does not imply damage to the latter (see Buxbaum et al. 2000, Figure 4d, and discussion below).
Theories based on the correlated structure principle are presented as alternatives to proposals that assume neural structure within the conceptual system. The implicit assumption in that argument is that the theoretical construct of a semantic feature offers a means for reducing different categories to a common set of elements (see Rogers et al. 2004 for an alternative proposal). However, no semantic features have been described that are shared across semantic categories, aside from very abstract features such as “has mass.” In other words, in the measure to which semantic features are the substance of conceptual representations, different semantic categories would be represented by nonoverlapping sets of features. Thus, and as has been proposed on the basis of functional neuroimaging data (see, e.g., Haxby et al. 2001 and discussion below), it may be the case that regions of high feature correlation (e.g., within semantic category correlations in visual structure) are reflected in the functional neuroanatomy of the brain (see also Devlin et al. 1998 for a hybrid model in which both focal and diffuse lesions can produce category-specific effects and Caramazza et al. 1990 for an earlier proposal along those lines).
THE ANATOMY OF CATEGORY-SPECIFICITY
An important development in cognitive neuroscience that has paralleled the articulation of theories of semantic organization is the discovery of multiple channels of visual processing (Goodale & Milner 1992, Ungerleider & Miskin 1982). It is now known that visual processing bifurcates into two independent but interconnected streams (for discussion of how best to characterize the two streams, see Pisella et al. 2006). The ventral visual object–processing stream projects from V1 through ventral occipital and temporal cortices, terminating in anterior regions of the temporal lobe, and subserves visual object identification. The dorsal object–processing stream projects from V1 through dorsal occipital cortex to posterior parietal cortex and subserves object-directed action and spatial analysis for the purposes of object-directed grasping. The two-visual systems hypothesis has played a central role in understanding the neuroanatomy of category specificity.
Lesion Analyses
A natural issue to arise in neuropsychological research concerns which brain regions tend to be lesioned in association with category-specific deficits. The first study to address this issue systematically was by H. Damasio and colleagues (1996). Those authors found that name-retrieval deficits for pictures of famous people were associated with left temporal pole lesions, a result confirmed by other investigators (see Lyons et al. 2006 for an overview). Damasio and colleagues also found that deficits for naming animals were associated with (more posterior) lesions of anterior left ventral temporal cortex. Subsequent research has confirmed that deficits for naming animals are associated with lesions to anterior regions of temporal cortex (e.g., Brambati et al. 2006). Damasio and collaborators also found that deficits for naming tools were associated with lesions to posterior and lateral temporal areas, overlapping the left posterior middle gyrus. The critical role of the left posterior middle temporal gyrus for knowing about tools has also since been confirmed by other lesion studies (e.g., Brambati et al. 2006).
A subsequent report by Damasio and colleagues (2004) demonstrated that the same regions were also reliably damaged in patients with impairments for recognizing stimuli from those three categories. In addition, Damasio and colleagues (2004) found that deficits for naming tools, as well as fruit/vegetables, were associated with lesions to the inferior pre-and postcentral gyri as well as the insula. Consensus about the association of lesions to the regions discussed above with category-specific deficits is provided by Gainotti’s analyses (e.g., Gainotti 2000) of published reports of patients with category-specific semantic deficits.
A number of investigators have interpreted the differential role of anterior mesial aspects of ventral temporal cortex in the processing of living things to reflect the fact that living things have more shared properties than non-living things, such that more fine-grained discriminations are required to name them (Bright et al. 2005, Damasio et al. 2004, Simmons & Barsalou 2003; see also Humphreys & Forde 2001). Within this framework, the association of deficits to unique person knowledge and lesions to the most anterior aspects of the temporal lobe is assumed to reflect the greater discrimination that is required for distinguishing among conspecifics compared to animals (less) and nonliving things (even less).
Functional Imaging
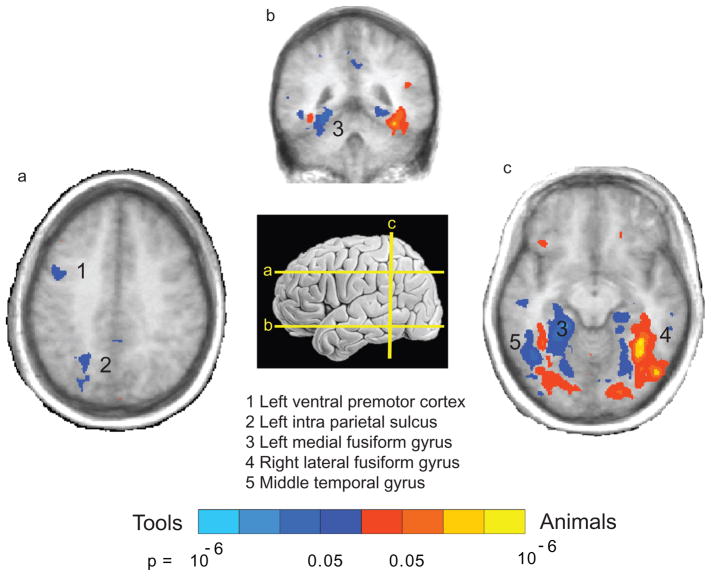
Data from functional imaging, and in particular fMRI, have added in important ways to our understanding of how different semantic categories are processed in the healthy brain. In particular, although the lesion overlap approach is powerful in detecting brain regions that are critical for performing a given task, functional imaging has the advantage of detecting regions that are critical as well as regions that are automatically engaged by the mere presentation of a certain type of stimulus. Thus, in line with the lesion evidence described above, nonliving things, and in particular tools, differentially activate the left middle temporal gyrus (Figure 3, e.g., Martin et al. 1996, Thompson-Schill et al. 1999; see Devlin et al. 2002 for a review). Other imaging data indicate that this region plays an important role in processing the semantics of actions (e.g., Kable et al. 2002, Kemmerer et al. 2008, Martin et al. 1995) as well as mechanical (i.e., unarticulated) motion (Beauchamp et al. 2002, 2003; Martin & Weisberg 2003).
Figure 3.
Category-specific patterns of blood oxygen–level dependent (BOLD) responses in the healthy brain. A network of regions that are differentially activated for living animate things is in red; a network of regions that are differentially activated for nonliving things is in blue. Data from Chao et al. (2002); graphics provided by Alex Martin.
In contrast, and not as apparent in lesion studies, tools differentially activate dorsal stream regions that mediate object-directed action. The activation of some of those regions is independent of whether action information is necessary in order to perform the task in which participants are engaged (e.g., picture naming). For instance, regions within dorsal occipital cortex, posterior parietal cortex, through to the anterior intraparietal sulcus, are automatically activated when participants observe manipulable objects (e.g., Chao & Martin 2000, Fang & He 2005). Those regions are important for determining volumetric and spatial information about objects as well as shaping and transporting the hand for object grasping (Binkofski et al. 1998, Culham et al. 2003, Frey et al. 2005). However, those dorsal occipital and posterior parietal regions are not thought to be critical for object identification or naming (e.g., Goodale & Milner 1992). Naming tools also differentially activates the left inferior parietal lobule (e.g., Mahon et al. 2007, Rumiati et al. 2004), a region that is important for representing complex object-associated manipulations (e.g., for review, see Johnson-Frey 2004, Lewis 2006).
One clear way in which functional imaging data have contributed beyond lesion evidence to our understanding of category specificity in the brain is the description of highly consistent topographic biases by semantic categories in the ventral object–processing stream (see Figure 3b,c; for reviews, see Bookheimer 2002, Gerlach 2007, Grill-Spector & Malach 2004, Op de Beeck et al. 2008, Thompson-Schill 2003). As opposed to the anterior-posterior mapping of semantic categories within the ventral stream described by the lesion evidence (e.g., Damasio et al. 1996), there is also a lateral-to-medial organization. The fusiform gyrus on the ventral surface of temporal-occipital cortex is critical for representing object color and form (e.g., Martin 2007, Miceli et al. 2001). Living animate things such as faces and animals elicit differential neural responses in the lateral fusiform gyrus, whereas nonliving things (tools, vehicles) elicit differential neural responses in the medial fusiform gyrus (e.g., Chao et al. 1999, Mahon et al. 2007, Noppeney et al. 2006). Stimuli that are highly definable in terms of their spatial context, such as houses and scenes, differentially activate regions anterior to these fusiform regions, in the vicinity of parahip-pocampal cortex (e.g., Bar & Aminoff 2003, Epstein & Kanwisher 1998). Other visual stimuli also elicit consistent topographical biases in the ventral stream, such as written words (see Dehaene et al. 2005 for discussion) and images of body parts (e.g., Downing et al. 2001).
HOW DOES THE ANATOMY OF CATEGORY SPECIFICITY INFORM COGNITIVE THEORY?
The existence of category specificity in the normal brain reduces confidence in theories that reject the neural structure principle. However, those functional imaging data are also relevant for adjudicating among theories based on the neural structure principle, in that different aspects of the functional imaging evidence sit more or less naturally with the distributed domain–specific hypothesis and the sensory/functional theory.
Tool Stimuli Differentially Activate Dorsal Stream Structures Involved in Object-Directed Action
The activation by tool stimuli of regions of the brain that mediate object-directed action has been argued to follow naturally from the sensory/functional theory. On that theory, the activation of dorsal structures by tool stimuli indexes the critical role of function knowledge in the recognition of nonliving things (e.g., Boronat et al. 2004, Kellenbach et al. 2003, Martin et al. 2000, Noppeney et al. 2006, Simmons & Barsalou 2003). That argument is weakened, however, in the measure to which it is demonstrated that the integrity of action knowledge is not necessary in order to have other types of knowledge about tools, such as knowledge of their function.
The neuropsychological phenomenon of apraxia offers a way of testing whether action knowledge is critical for supporting conceptual processing of tools. Apraxia refers to an impairment for using objects that cannot be explained by a deficit in visual object recognition or an impairment to low-level motor processes themselves. Figure 4a summarizes the performance profile of a patient (reported by Ochipa et al. 1989) who was impaired for using objects but was relatively preserved for naming the same objects [see Figure 4b for similar dissociations in a series of single case analyses (Negri et al. 2007); see also Rosci et al. (2003); for clear cases studies, see Moreaud et al. (1998), Rapcsak et al. (2005), Rumiati et al. (2001); see Rothi et al. (1991) for an influential cognitive model; for review, see Johnson-Frey (2004), Mahon & Caramazza (2005)]. Apraxic deficits for using objects are often observed subsequent to lesions in the regions of the dorsal stream (in particular, the left inferior parietal lobule), reviewed above, that are automatically activated when participants name tools. The fact that apraxic patients may be able to name objects that they cannot use indicates that the activation of those regions during naming tasks is not, in and of itself, necessary for successful completion of the task. At the same time, lesions to parietal cortex, in the context of lesions to the middle temporal gyrus and frontal regions, do modulate performance in object identification. In a recent analysis (Mahon et al. 2007), a group of unilateral stroke patients was separated into two groups according to the anatomical criterion of having lesions involving (Figure 4c, middle left) or not involving parietal cortex (Figure 4c, middle right). There was a relationship between performance in object identification and object use at the group level only in patients with lesions involving parietal cortex, a finding that suggests that action knowledge associated with objects is relevant for successful identification.
Other neuropsychological data indicate that the integrity of action knowledge is not necessary in order for patients to have accurate knowledge of object function. Figure 4d depicts the performance of patient WC (Buxbaum et al. 2000) on two picture-matching tasks. In a picture-matching task that required knowledge of object manipulation, performance was impaired; however, in a picture-matching task that required knowledge of object function, performance was spared. Functional imaging studies (Boronat et al. 2004, Canessa et al. 2008, Kellenbach et al. 2003) converge with those neuropsychological data in showing that manipulation but not function knowledge modulates neural responses in the inferior parietal lobule. There is also evidence, from both functional neuroimaging (e.g., Canessa et al. 2008) and neuropsychology (e.g., Sirigu et al. 1991), that temporal and not parietal cortex may be involved in the representation of function knowledge of objects.
The convergence between the neuropsychological evidence from apraxia and the functional-imaging evidence indicates that although a dedicated system exists for knowledge of object manipulation, that system is not critically involved in representing knowledge of object function. This suggests that the automatic engagement of action processing by manipulable objects, as observed in neuroimaging, may have consequences for a theory of pragmatics and/or action, but not necessarily for a theory of semantics (Goodale & Milner 1992, Jeannerod & Jacob 2005). This in turn weakens the claim that automatic activation of dorsal stream structures by manipulable objects is evidence for the sensory/functional theory.
Category Specificity Within the Ventral Stream
One finding from functional neuroimaging that sits more naturally with the distributed domain–specific hypothesis than the sensory/functional theory is the fact that ventral temporal cortex shows topographic biases by semantic category. In order to explain those data within the context of the sensory/functional theory, further assumptions are necessary about why there would be an organization by semantic category within the (putative) visual modality. In short, a hybrid model is required that combines the assumption of multiple semantics with some claim about how information would come to be topographically segregated by semantic category. A number of such proposals have been advanced, although not always in the context of the sensory/functional theory or more generally within the context of theories that emerge from category-specific semantic deficits (see, e.g., Gauthier et al. 2000, Haxby et al. 2001, Ishai et al. 1999, Levy et al. 2001, Mechelli et al. 2006, Rogers et al. 2005). All of those proposals share the view that dimensions defined over visual information generate the observed effects of category specificity in the ventral stream.
An alternative framework (see Mahon et al. 2007) is that category specificity in the ventral stream is not the result of only bottom-up processes operating locally over visual information. Rather, the organization of the ventral stream is just one manifestation of a network that includes many other regions. Such a connectivity-constrained account (Riesenhuber 2007) of category specificity is theoretically neutral regarding the issue of whether or not innately specified constraints determine such connectivity. For instance, although there is neural specificity for both written words and faces in regions of the ventral stream, face recognition—but not reading—could have a direct evolutionarily relevant history. Yet, it may be that neural specificity for written words in the ventral stream is driven by functional connectivity that relates visual processing to phonological processing (see Buchel et al. 1998 for relevant findings, and Dehaene et al. 2005 and Martin 2006 for discussion).
A connectivity-constrained account would offer a natural explanation for how category effects within ventral temporal cortex could be driven by nonvisual properties of the stimuli. For instance, Martin & Weisberg (2003) showed that different types of motion carried by the same geometrical shapes can drive responses in a category-specific manner in ventral temporal cortex. These findings are surprising because ventral temporal-occipital cortex is not itself motion sensitive (Beauchamp et al. 2003). In the same line, Mahon and colleagues (2007) found that neural responses for manipulable objects in the medial fusiform gyrus are driven by action-related properties of objects even though action knowledge is not itself represented in the fusiform gyrus. A connectivity constrained account also offers a natural account for why structures involved in affective processing and mental state attribution should be part of the network that is activated when information about living animate things is processed (for early discussion, see Caramazza & Shelton 1998; for findings from fMRI, see e.g. Martin & Weisberg 2003, Mitchell et al. 2002, Morris et al. 1999, Pasley et al. 2004).
It is unlikely that a single dimension will explain all aspects of the organization of the ventral object–processing stream. In particular, it may be the case that neural specificity for some stimulus types will be determined by qualitatively different types of constraints than neural specificity for other stimulus types. A recent study (Polk et al. 2007) investigated this issue by studying the similarity in neural responses to faces, houses, pseudowords, and chairs in monozygotic and dizygotic twins and in unrelated participants. The authors found that face-and place-related responses within face- and place-selective regions, respectively, were significantly more similar for monozygotic than for dizygotic twins. However, there was no difference between the two twin groups for written words in regions that responded selectively to written words. Those data demonstrate innate constraints on the patterns of neural responses to faces and places in regions of the ventral stream selective for those categories. Future research is required to address how innate factors influence neural specificity within the ventral object–processing stream and the organization of object knowledge in the brain more generally.
THE RELATION BETWEEN SENSORY, MOTOR, AND CONCEPTUAL KNOWLEDGE
Early formulations of the sensory/functional theory assumed that conceptual content, although tied in important ways to the sensory and motor systems, was more abstract than the token-based information contained within the sensory and motor systems (Warrington & McCarthy 1983, 1987; Warrington & Shallice 1984; see also Crutch & Warrington 2003). More recent formulations of the multiple-semantics approach have argued, within the embodied cognition framework, that conceptual content can be reductively grounded in sensory and motor processes (e.g., Barsalou 1999, 2008; H. Damasio et al. 2004; Gallese & Lakoff 2005; Prinz 2002; Pulvermüller 2005; Zwaan 2004; see also Patterson et al. 2007).
The first detailed articulation of the embodied cognition framework was by Allen Allport (1985), who proposed that conceptual knowledge is organized according to sensory and motor modalities and that the information represented within different modalities was format specific:
The essential idea is that the same neural elements that are involved in coding the sensory attributes of a (possibly unknown) object presented to eye or hand or ear also make up the elements of the auto-associated activity-patterns that represent familiar object-concepts in “semantic memory.” This model is, of course, in radical opposition to the view, apparently held by many psychologists, that “semantic memory” is represented in some abstract, modality-independent, “conceptual” domain remote from the mechanisms of perception and motor organization. (Allport 1985, p. 53; emphasis in original)
One type of evidence, discussed above, that has been argued to support an embodied representation of object concepts is the observation that regions of the brain that directly mediate object-directed action are automatically activated when participants observe manipulable objects. However, the available neuropsychological evidence (Figure 4) reduces confidence in the claim that action knowledge plays a critical role in grounding the diverse types of knowledge that we have about tools. The strongest evidence for the relevance of motor and perceptual processes to conceptual processing is provided by demonstrations that the sensory and motor systems are automatically engaged by linguistic stimuli that imply action (e.g., Boulenger et al. 2006, Buccino et al. 2005, Glenberg & Kaschak 2002, Oliveri et al. 2004). It has also been demonstrated that activation of the motor system automatically spreads to conceptual and perceptual levels of processing (e.g., Pulvermüller et al. 2005).
The embodied cognition hypothesis makes strong predictions about the integrity of conceptual processes after damage to sensory and motor processes. It predicts, necessarily, and as Allport wrote, that “ … the loss of particular attribute information in semantic memory should be accompanied by a corresponding perceptual (agnostic) deficit.” (1985, p. 55; emphasis in original). Although there are long traditions within neuropsychology of studying patients with deficits for sensory and/or motor knowledge, only recently have those deficits been of such clear theoretical relevance to hypotheses about the nature of semantic memory. Systematic and theoretically informed studies of such patients will play a pivotal role in evaluating the relation between sensory, motor, and conceptual knowledge. Central to that enterprise will be to specify how information is dynamically exchanged between systems in the context of specific task requirements. This will be important for determining the degree to which sensory and motor activation is in fact a critical component of conceptual processing (see Machery 2007, Mahon & Caramazza 2008 for discussion). It is theoretically possible (and in our view, likely) that although concepts are not exhausted by sensory and motor information, the organization of abstract concepts is nonetheless shaped in important ways by the structure of the sensory and motor systems. It is also likely, in our view, that processing of such abstract conceptual content is heavily interlaced with activation of the sensory and motor systems.
TOWARD A SYNTHESIS
We have organized this review around theoretical explanations of category specificity in the human brain. One theme that emerges is the historical progression from theories based on a single principle of organization to theories that integrate multiple dimensions of organization. This progression is due to the broad recognition in the field that a single dimension will not be sufficient to explain all aspects of the organization of object knowledge in the brain. However, not every dimension or principle of organization is of equal importance because not all dimensions have the same explanatory scope. A relative hierarchy of principles is therefore necessary to determine which of the many known facts are theoretically important and which are of only marginal significance.
Two broad findings emerge from cognitive neuropsychological research. First, patients have been reported with disproportionate impairments for a modality or type of knowledge (e.g., visual/perceptual knowledge, Figure 2b; manipulation knowledge, Figure 4). Second, category-specific semantic deficits are associated with impairments for all types of knowledge about the impaired category (Figure 2a). Analogues to those two facts are also found in functional neuroimaging. First, the attributes of some categories of objects (e.g., tools) are differentially represented in modality-specific systems (i.e., motor systems). Second, within a given modality-specific system (e.g., ventral visual pathway), there is functional organization by semantic category (e.g., living animate versus nonliving) (see Figure 3 for an overview). Thus, across both neuropsychological studies and functional imaging studies, the broad empirical generalization emerges that there are two, orthogonal, constraints on the organization of object knowledge: object domain and sensory/motor modality. This empirical generalization is neutral with respect to how one explains the causes of category-specific effects in both functional neuroimaging and neuropsychology.
Many theoretical proposals of the causes of category specificity articulate dimensions along which semantic categories differ (e.g., Cree & McRae 2003, Devlin et al. 1998, Gauthier et al. 2000, Haxby et al. 2001, Humphreys & Forde 2001, Laws & Gale 2002, Levy et al. 2001, Mechelli et al. 2006, Op de Beeck et al. 2008, Rogers et al. 2004, Sartori & Lombardi 2004, Simmons & Barsalou 2003, Tranel et al. 1997, Tyler & Moss 2001, Warrington & Shallice 1984, Zannino et al. 2006). Understanding the role that such dimensions play in the genesis of category specificity in a particular part of the brain, or a particular component of a cognitive model, will be central to characterizing the functioning of that component of the system. However, progress in understanding the causes of category specificity in one region of the brain, or one functional component of a cognitive model, will require an understanding of how category specificity is realized throughout the whole brain and throughout the whole cognitive model.
All current theories of the organization of conceptual knowledge assume that a concept is composed of distinct types of information. This shared assumption permits an explanation of how thinking about a single concept (e.g., hammer) can engage different regions of the brain that process distinct types of information (e.g., sensory versus motor). It also allows for an account of how patients may present with impairments for a type or modality of knowledge (e.g., know what a hammer looks like but not know how to use it). However, that assumption begs the question of how the different types of information that constitute a given concept are functionally unified. A central theoretical issue to be addressed by the field is to understand the nature of the mechanisms that unify different types of knowledge about the same entity in the world and that give rise to a functionally unitary concept of that entity.
Our own view—the distributed domain-specific hypothesis—assumes that the first-order principle of organization is object domain. Within any given domain of knowledge, there will be functional and neural specialization according to types or modalities of knowledge. For instance, visual motion properties of living animate things are represented in a different region/system than are visual form properties of living animate things. In addition, affective properties of living animate things may be represented by other, functionally and neuroanatomically, distinct systems. However, all of those types of information constitute the domain “living animate.” For that reason, it is critical to specify the nature of the functional connectivity that relates processing across distinct subsystems specialized for different types of information. The basic expectation of the distributed domain-specific hypothesis is that the functional connectivity that relates processing across distinct types of information (e.g., emotional value versus visual form) will be concentrated around those domains that have had evolutionarily important histories. The strong prediction that follows from that view is that such neural circuits are the same circuits that are damaged in patients with category-specific semantic deficits.
Independently of whether the distributed domain-specific hypothesis is empirically confirmed, it serves to highlight two key aspects of human conceptual processing. First, humans do not have systems that support rich conceptual knowledge of objects in order to carry out only explicit knowledge tasks, such as object naming or similarity judgments. We have those systems because they serve action and ultimately have been in the service of survival. An understanding of the architecture of the conceptual system must therefore be situated in the context of the real-world computational problems that the conceptual system is structured to support. Second, human behavior arises due to the integration of multiple cognitive processes that individually operate over distinct types of knowledge. In contrast to the view that domain specificity implies modularity, we have emphasized the distributed nature of domain-specific neural circuits. On the distributed domain-specific hypothesis, the distinct (and potentially modular) processes within the sensory, motor, and affective systems are components of broader structures within the mind/brain. This framework thus emphasizes the need to understand how different types of cognitive processes, operating over different types of information, work in concert to orchestrate behavior.
In the more than 25 years since Warrington and colleagues’ first detailed reports of patients with category-specific semantic deficits, new fields of investigation have emerged around the study of the organization and representation of conceptual knowledge. Despite that progress, the theoretical questions that currently occupy researchers are the same as those that were initially framed and debated two decades ago: What are the principles of neural organization that give rise to effects of category specificity? Are different types of information involved in processing different semantic categories and, if so, what distinguishes those different types of information? Future research will undoubtedly build upon the currently available theories as well as redeploy their individual assumptions within new theoretical frameworks.
FUTURE DIRECTIONS.
To what degree do sensory and motor processes participate in higher cognitive function? The available evidence from neuropsychology places a clear upper limit on the degree to which conceptual knowledge can be assumed to be “embodied.” However, equally compelling findings from functional neuroimaging demonstrate that the sensory and motor systems are automatically engaged during conceptual processing. It will be important to develop articulated models of the dynamics of activation flow among concepts and the sensory and motor systems in order to test hypotheses about the causes of sensory and motor activation during conceptual processing.
Are different domains of knowledge represented differently in males and females? Some researchers have highlighted the fact that patients with disproportionate semantic impairments for fruit/vegetables are male. This pattern remains even after controlling for gender-specific familiarity among items from different categories. Those data raise the question of whether early (and culturally influenced) differences in experience can qualitatively shape the functional architecture of the conceptual system.
How does damage to one region of the brain affect processing in other regions of the brain? Little is currently known about how damage to distinct regions within a network affects processing in other regions of the network. Detailed cognitive and anatomical studies of patients with semantic deficits will aide in understanding the dynamics of brain damage and the implications for cognitive models of conceptual processing.
Acknowledgments
Preparation of this article was supported in part by a National Science Foundation Graduate Research Fellowship to BZM, National Institutes of Health grant DC04542 to AC, and by a grant from the Fondazione Cassa di Risparmio di Trento e Rovereto. The authors are grateful to Erminio Capitani, Marcella Laiacona, Alex Martin, and Daniel Schacter for their comments on an earlier draft.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
Contributor Information
Bradford Z. Mahon, Email: Mahon@fas.harvard.edu.
Alfonso Caramazza, Email: Caram@wjh.harvard.edu.
LITERATURE CITED
- Allport DA. Distributed memory, modular subsystems and dysphasia. In: Newman SK, Epstein R, editors. Current Perspectives in Dysphasia. New York: Churchill Livingstone; 1985. pp. 207–44. [Google Scholar]
- Baillargeon R. Infants’ understanding of the physical world. In: Sabourin M, Craik F, Robert M, editors. Advances in Psychological Science: 2. Biological and Cognitive Aspects. London: Psychol. Press; 1998. pp. 503–29. [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–58. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Barbarotto R, Capitani E, Spinnler H, Trivelli C. Slowly progressive semantic impairment with category specificity. Neurocase. 1995;1:107–19. [Google Scholar]
- Barbarotto R, Laiacona M, Macchi V, Capitani E. Picture reality decision, semantic categories, and gender: a new set of pictures, with norms and an experimental study. Neuropsychologia. 2002;40:1637–53. doi: 10.1016/s0028-3932(02)00029-5. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Perceptual symbol systems. Behav Brain Sci. 1999;22:637–60. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annu Rev Psychol. 2008;59:617–45. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;24:149–59. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. fMRI responses to video and point-light displays of moving humans and manipulable objects. J Cogn Neurosci. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Beauvois M-F. Optic aphasia: a process of interaction between vision and language. Philos Trans R Soc Lond B. 1982;298:35–47. doi: 10.1098/rstb.1982.0070. [DOI] [PubMed] [Google Scholar]
- Beauvois M-F, Saillant B, Mhninger V, Llermitte F. Bilateral tactile aphasia: a tacto-verbal dysfunction. Brain. 1978;101:381–401. doi: 10.1093/brain/101.3.381. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, et al. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253–59. doi: 10.1212/wnl.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Blundo C, Ricci M, Miller L. Category-specific knowledge deficit for animals in a patient with herpes simplex encephalitis. Cogn Neuropsychol. 2006;23:1248–68. doi: 10.1080/02643290600896449. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Borgo F, Shallice T. When living things and other “sensory-quality” categories behave in the same fashion: a novel category-specific effect. Neurocase. 2001;7:201–20. doi: 10.1093/neucas/7.3.201. [DOI] [PubMed] [Google Scholar]
- Borgo F, Shallice T. Category specificity and feature knowledge: evidence from new sensory-quality categories. Cogn Neuropsychol. 2003;20:327–53. doi: 10.1080/02643290244000310. [DOI] [PubMed] [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, et al. Distinctions between manipulation and function knowledge of objects: evidence from functional magnetic resonance imaging. Cogn Brain Res. 2004;23:361–73. doi: 10.1016/j.cogbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Roy AC, Paulignan Y, Deprez V, Jeannerod M, Nazir TA. Cross-talk between language processes and overt motor behavior in the first 200 msec of processing. J Cogn Neurosci. 2006;18:1607–15. doi: 10.1162/jocn.2006.18.10.1607. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Myers D, Wilson A, Rankin KP, Allison SC, et al. The anatomy of category-specific object naming in neurodegenerative diseases. J Cogn Neurosci. 2006;18:1644–53. doi: 10.1162/jocn.2006.18.10.1644. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss HE, Stamatakis EA, Tyler LK. The anatomy of object processing: the role of anteromedial temporal cortex. Q J Exp Psychol B. 2005;58:361–77. doi: 10.1080/02724990544000013. [DOI] [PubMed] [Google Scholar]
- Buccino G, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action related sentences modulates the activity of the motor system: a combined TMS and behavioral study. Cogn Brain Res. 2005;24:355–63. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Buchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998;394:274–77. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Veramonti T, Schwartz MF. Function and manipulation tool knowledge in apraxia: knowing “what for” but not “how. Neurocase. 2000;6:83–97. [Google Scholar]
- Canessa N, Borgo F, Cappa SF, Perani D, Falini A, et al. The different neural correlates of action and functional knowledge in semantic memory: an fMRI study. Cereb Cortex. 2008;18:740–51. doi: 10.1093/cercor/bhm110. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Platt M, Brannon EM. Beyond the number domain. Trends Cogn Sci. 2008 doi: 10.1016/j.tics.2008.11.007. Manuscr. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Mahon B, Caramazza A. What are the facts of category-specific deficits? A critical review of the clinical evidence. Cogn Neuropsychol. 2003;20:213–62. doi: 10.1080/02643290244000266. [DOI] [PubMed] [Google Scholar]
- Caramazza A. On drawing inferences about the structure of normal cognitive systems from the analysis of patterns of impaired performance: the case for single-patient studies. Brain Cogn. 1986;5:41–66. doi: 10.1016/0278-2626(86)90061-8. [DOI] [PubMed] [Google Scholar]
- Caramazza A. Is cognitive neuropsychology possible? J Cogn Neurosci. 1992;4:80–95. doi: 10.1162/jocn.1992.4.1.80. [DOI] [PubMed] [Google Scholar]
- Caramazza A. The interpretation of semantic category-specific deficits: What do they reveal about the organization of conceptual knowledge in the brain? Neurocase. 1998;4:265–72. [Google Scholar]
- Caramazza A, Hillis AE, Rapp BC, Romani C. The multiple semantics hypothesis: multiple confusions? Cogn Neuropsychol. 1990;7:161–89. [Google Scholar]
- Caramazza A, Mahon BZ. The organization of conceptual knowledge: the evidence from category-specific semantic deficits. Trends Cogn Sci. 2003;7:354–61. doi: 10.1016/s1364-6613(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Mahon BZ. The organisation of conceptual knowledge in the brain: the future’s past and some future directions. Cogn Neuropsychol. 2006;23:13–38. doi: 10.1080/02643290542000021. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. Domain specific knowledge systems in the brain: the animate-inanimate distinction. J Cogn Neurosci. 1998;10:1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- Carey S, Spelke ES. Domain specific knowledge and conceptual change. In: Hirschfeld L, Gelman S, editors. Mapping the Mind: Domain Specificity in Cognition and Culture. London: Cambridge Univ. Press; 1994. pp. 169–200. [Google Scholar]
- Carroll E, Garrard P. Knowledge of living, nonliving and “sensory quality” categories in semantic dementia. Neurocase. 2005;11:338–50. doi: 10.1080/13554790591006339. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in posterior temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–19. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–84. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chao LL, Weisberg J, Martin A. Experience-dependent modulation of category related cortical activity. Cereb Cortex. 2002;12:545–51. doi: 10.1093/cercor/12.5.545. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Saffran EM. Optic aphasia and the right hemisphere: a replication and extension. Brain Lang. 1992;43:148–61. doi: 10.1016/0093-934x(92)90026-b. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH. Methods of testing for a deficit in single case studies: evaluation of statistical power by Monte Carlo simulation. Cogn Neuropsychol. 2006;23:877–904. doi: 10.1080/02643290500538372. [DOI] [PubMed] [Google Scholar]
- Cree GS, McRae K. Analyzing the factors underlying the structure and computation of the meaning of chipmunk, cherry, chisel, cheese, and cello and many other such concrete nouns. J Exp Psychol Gen. 2003;132:163–201. doi: 10.1037/0096-3445.132.2.163. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. The selective impairment of fruit and vegetable knowledge: a multiple processing channels account of fine-grain category specificity. Cogn Neuropsychol. 2003;20:355–72. doi: 10.1080/02643290244000220. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSourza JFX, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res. 2003;153:180–89. doi: 10.1007/s00221-003-1591-5. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends Cogn Sci. 2005;9:335–41. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Devlin J, Gonnerman L, Andersen E, Seidenberg M. Category-specific semantic deficits in focal and widespread brain damage: a computational account. J Cogn Neurosci. 1998;10:77–94. doi: 10.1162/089892998563798. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Moore CJ, Mummery CJ, Gorno-Tempini ML, Phillips JA, et al. Anatomic constraints on cognitive theories of category-specificity. Neuroimage. 2002;15:675–85. doi: 10.1006/nimg.2001.1002. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Piskopos M, Schweizer TA. Musical instrument naming impairments: the crucial exception to the living/nonliving dichotomy in category-specific agnosia. Brain Cogn. 2000;43:158–64. [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–73. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Yovel G. Face recognition. In: Basbaum A, Hoy R, Kaneko A, Shepherd G, Westheimer G, editors. The Senses: A Comprehensive Reference. Amsterdam: Elsevier; 2008. pp. 329–58. [Google Scholar]
- Duchaine BC, Yovel G, Butterworth EJ, Nakayama K. Prosopagnosia as an impairment to face-specific mechanisms: elimination of the alternative hypotheses in a developmental case. Cogn Neuropsychol. 2006;23:714–47. doi: 10.1080/02643290500441296. [DOI] [PubMed] [Google Scholar]
- Ellis AW, Young AW, Critchley AMR. Loss of memory for people following temporal lobe damage. Brain. 1989;112:1469–83. doi: 10.1093/brain/112.6.1469. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat Neurosci. 2005;8:1380–85. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- Farah M, McClelland J. A computational model of semantic memory impairment: modality specificity and emergent category specificity. J Exp Psychol Gen. 1991;120:339–57. [PubMed] [Google Scholar]
- Farah MJ, Rabinowitz C. Genetic and environmental influences on the organization of semantic memory in the brain: Is “living things” an innate category? Cogn Neuropsychol. 2003;20:401–8. doi: 10.1080/02643290244000293. [DOI] [PubMed] [Google Scholar]
- Feigenson L, Dehaene S, Spelke ES. Core systems of number. Trends Cogn Sci. 2004;8:307–14. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Frey SH, Vinton D, Norlund R, Grafton ST. Cortical topography of human anterior intraparietal cortex active during visually guided grasping. Cogn Brain Res. 1995;23:397–405. doi: 10.1016/j.cogbrainres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Funnell E, Sheridan J. Categories of knowledge: unfamiliar aspects of living and nonliving things. Cogn Neuropsychol. 1992;9:135–53. [Google Scholar]
- Gainotti G. What the locus of brain lesion tells us about the nature of the cognitive defect underlying category-specific disorders: a review. Cortex. 2000;36:539–59. doi: 10.1016/s0010-9452(08)70537-9. [DOI] [PubMed] [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: the role of the sensory motor system in reason and language. Cogn Neuropsychol. 2005;22:455–79. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The Organization of Learning. Cambridge, MA: Bradford/MIT Press; 1990. [Google Scholar]
- Garrard P, Patterson K, Watson PC, Hodges JR. Category-specific semantic loss in dementia of Alzheimer’s type: functional-anatomical correlations from cross-sectional analyses. Brain. 1998;121:633–46. doi: 10.1093/brain/121.4.633. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–97. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gelman R. First principles organize attention to and learning about relevant data: number and the animate-inanimate distinction as examples. Cogn Sci. 1990;14:79–106. [Google Scholar]
- Gerlach C. A review of functional imaging studies on category specificity. J Cogn Neurosci. 2007;19:296–314. doi: 10.1162/jocn.2007.19.2.296. [DOI] [PubMed] [Google Scholar]
- Glenberg AM, Kaschak MP. Grounding language in action. Psychon Bull Rev. 2002;9:558–65. doi: 10.3758/bf03196313. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. The human visual cortex. Annu Rev Neurosci. 2004;27:649–77. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- Hart J, Anand R, Zoccoli S, Maguire M, Gamino J, et al. Neural substrates of semantic memory. J Int Neuropsychol Soc. 2007;13:865–80. doi: 10.1017/S135561770707110X. [DOI] [PubMed] [Google Scholar]
- Hart J, Jr, Berndt RS, Caramazza A. Category-specific naming deficit following cerebral infarction. Nature. 1985;316:439–40. doi: 10.1038/316439a0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–30. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hécaen H, De Ajuriaguerra J. Agnosie visuelle pour les objets inanimées par lésion unilatérale gauche. Révue Neurol. 1956;94:222–33. [PubMed] [Google Scholar]
- Hermer L, Spelke ES. A geometric process for spatial reorientation in young children. Nature. 1994;370:57–59. doi: 10.1038/370057a0. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. Category-specific naming and comprehension impairment: a double dissociation. Brain. 1991;114:2081–94. doi: 10.1093/brain/114.5.2081. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. Cognitive and neural mechanisms underlying visual and semantic processing: Implications from “optic aphasia. J Cogn Neurosci. 1995;7:457–78. doi: 10.1162/jocn.1995.7.4.457. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Forde EME. Hierarchies, similarity, and interactivity in object recognition: “category-specific” neuropsychological deficits. Behav Brain Sci. 2001;24:453–75. [PubMed] [Google Scholar]
- Humphreys GW, Forde EME. Naming a giraffe but not an animal: base-level but not superordinate naming in a patient with impaired semantics. Cogn Neuropsychol. 2005;22:539–58. doi: 10.1080/02643290442000176. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Riddoch MJ, Quinlan PT. Cascade processes in picture identification. Cogn Neuropsychol. 1988;5:67–103. [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schourten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci USA. 1999;96:9379–84. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M, Jacob P. Visual cognition: a new look at the two-visual systems model. Neuropsychologia. 2005;43:301–12. doi: 10.1016/j.neuropsychologia.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH. The neural bases of complex tool use in humans. Trends Cogn Sci. 2004;8:71–78. doi: 10.1016/j.tics.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kable JW, Lease-Spellmeyer J, Chatterjee A. Neural substrates of action event knowledge. J Cogn Neurosci. 2002;14:795–805. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- Keil FC. Constraints on knowledge and cognitive development. Psychol Rev. 1981;88:197–227. [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Actions speak louder than functions: the importance of manipulability and action in tool representation. J Cogn Neurosci. 2003;15:20–46. doi: 10.1162/089892903321107800. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Gonzalez Castillo J, Talavage T, Patterson S, Wiley C. Neuroanatomical distribution of five semantic components of verbs: evidence from fMRI. Brain Lang. 2008 doi: 10.1016/j.bandl.2007.09.003. In press. [DOI] [PubMed] [Google Scholar]
- Kiani R, Esteky H, Mirpour K, Tanaka K. Object category structure in response patterns of neuronal population in monkey inferior temporal cortex. J Neurophysiol. 2007;97:4296–309. doi: 10.1152/jn.00024.2007. [DOI] [PubMed] [Google Scholar]
- Laiacona M, Barbarotto R, Capitani E. Perceptual and associative knowledge in category specific impairment of semantic memory: a study of two cases. Cortex. 1993;29:727–40. doi: 10.1016/s0010-9452(13)80293-6. [DOI] [PubMed] [Google Scholar]
- Laiacona M, Barbarotto R, Capitani E. Semantic category dissociation in naming: Is there a gender effect in Alzheimer disease? Neuropsychologia. 1998;36:407–19. doi: 10.1016/s0028-3932(97)00125-5. [DOI] [PubMed] [Google Scholar]
- Laiacona M, Barbarotto R, Capitani E. Animals recover but plant life knowledge is still impaired 10 years after herpetic encephalitis: the long-term follow-up of a patient. Cogn Neuropsychol. 2005;22:78–94. doi: 10.1080/02643290442000004. [DOI] [PubMed] [Google Scholar]
- Laiacona M, Barbarotto R, Capitani E. Human evolution and the brain representation of semantic knowledge: Is there a role for sex differences? Evol Hum Behav. 2006;27:158–68. [Google Scholar]
- Laiacona M, Capitani E. A case of prevailing deficit on nonliving categories or a case of prevailing sparing of living categories? Cogn Neuropsychol. 2001;18:39–70. doi: 10.1080/02643290042000035. [DOI] [PubMed] [Google Scholar]
- Laiacona M, Capitani E, Caramazza A. Category-specific semantic deficits do not reflect the sensory-functional organisation of the brain: a test of the “sensory-quality” hypothesis. Neurocase. 2003;9:3221–31. doi: 10.1076/neur.9.3.221.15562. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Howard D, Nightingale G, Ellis AW. Are living and nonliving category-specific deficits causally linked to impaired perceptual or associative knowledge? Evidence from a category-specific double dissociation. Neurocase. 1998;4:311–38. [Google Scholar]
- Lambon Ralph MA, Lowe C, Rogers TT. Neural basis of category-specific semantic deficits for living things: evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130:1127–37. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Laws KR, Gale TM. Category-specific naming and the “visual” characteristics of line drawn stimuli. Cortex. 2002;38:7–21. doi: 10.1016/s0010-9452(08)70635-x. [DOI] [PubMed] [Google Scholar]
- Laws KR, Neve C. A “normal” category-specific advantage for naming living things. Neuropsychologia. 1999;37:1263–69. doi: 10.1016/s0028-3932(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–39. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Lewis JW. Cortical networks related to human use of tools. Neuroscientist. 2006;12:211–31. doi: 10.1177/1073858406288327. [DOI] [PubMed] [Google Scholar]
- Lhermitte F, Beauvois M-F. A visual speech disconnection syndrome: report of a case with optic aphasia, agnosic alexia and color agnosia. Brain. 1973;96:695–714. doi: 10.1093/brain/96.4.695. [DOI] [PubMed] [Google Scholar]
- Luzzatti C, Davidoff J. Impaired retrieval of object-color knowledge with preserved color naming. Neuropsychologia. 1994;32:1–18. doi: 10.1016/0028-3932(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Lyons F, Kay J, Hanley JR, Haslam C. Selective preservation of memory for people in the context of semantic memory disorder: patterns of association and dissociation. Neuropsychologia. 2006;44:2887–98. doi: 10.1016/j.neuropsychologia.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Machery E. Concept empiricism: a methodological critique. Cognition. 2007;104:19–46. doi: 10.1016/j.cognition.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. Constraining questions about the organisation and representation of conceptual knowledge. Cogn Neuropsychol. 2003;20:433–50. doi: 10.1080/02643290342000014. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. The orchestration of the sensory-motor systems: clues from neuropsychology. Cogn Neuropsychol. 2005;22:480–94. doi: 10.1080/02643290442000446. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J Physiol Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Milleville S, Negri GAL, Rumiati RI, Martin A, Caramazza A. Action-related properties of objects shape object representations in the ventral stream. Neuron. 2007;55:507–20. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. Shades of Déjerine—forging a causal link between the visual word form area and reading. Neuron. 2006;50:173–75. doi: 10.1016/j.neuron.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–5. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Martin A, Ungerleider LG, Haxby JV. Category specificity and the brain: the sensory/motor model of semantic representations of objects. In: Gazzaniga MS, editor. Higher Cognitive Functions: The New Cognitive Neurosciences. Cambridge, MA: MIT Press; 2000. pp. 1023–36. [Google Scholar]
- Martin A, Weisberg J. Neural foundations for understanding social and mechanical concepts. Cogn Neuropsychol. 2003;20:575–87. doi: 10.1080/02643290342000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–52. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci. 2003;4:310–22. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Sartori G, Orlandi P, Price CJ. Semantic relevance explains category effects in medial fusiform gyri. Neuroimage. 2006;3:992–1002. doi: 10.1016/j.neuroimage.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Miceli G, Capasso R, Daniele A, Esposito T, Magarelli M, Tomaiuolo F. Selective deficit for people’s names following left temporal damage: an impairment of domain-specific conceptual knowledge. Cogn Neuropsychol. 2000;17:489–516. doi: 10.1080/02643290050110629. [DOI] [PubMed] [Google Scholar]
- Miceli G, Fouch E, Capasso R, Shelton JR, Tamaiuolo F, Caramazza A. The dissociation of color from form and function knowledge. Nat Neurosci. 2001;4:662–67. doi: 10.1038/88497. [DOI] [PubMed] [Google Scholar]
- Milner AD, Perrett DI, Johnson RS, Benson OJ, Jordan TR, et al. Perception and action “visual form agnosia. Brain. 1991;114:405–28. doi: 10.1093/brain/114.1.405. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA. 2002;99:15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreaud O, Charnallet A, Pellat J. Identification without manipulation: a study of the relations between object use and semantic memory. Neuropsychologia. 1998;36:1295–301. doi: 10.1016/s0028-3932(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA. 1999;96:1680–85. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G, Behrmann M. What is special about face recognition? Nineteen experiments on a person with visual object agnosia and dyslexia but with normal face recognition. J Cogn Neurosci. 1997;9:555–604. doi: 10.1162/jocn.1997.9.5.555. [DOI] [PubMed] [Google Scholar]
- Moss HE, Tyler LK, Durrant-Peatfield M, Bunn EM. “Two eyes of a see-through”: impaired and intact semantic knowledge in a case of selective deficit for living things. Neurocase. 1998;4:291–310. [Google Scholar]
- Negri GAL, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cogn Neuropsychol. 2007;24:795–816. doi: 10.1080/02643290701707412. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, Friston KJ. Two distinct neural mechanisms for category-selective responses. Cereb Cortex. 2006;16:437–45. doi: 10.1093/cercor/bhi123. [DOI] [PubMed] [Google Scholar]
- Nunn JA, Postma P, Pearson R. Developmental prosopagnosia: Should it be taken at face value? Neurocase. 2001;7:15–27. doi: 10.1093/neucas/7.1.15. [DOI] [PubMed] [Google Scholar]
- Ochipa C, Rothi LJG, Heilman KM. Ideational apraxia: a deficit in tool selection and use. Ann Neurol. 1989;25:190–93. doi: 10.1002/ana.410250214. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Finocchiaro C, Shapiro K, Gangitano M, Caramazza A, Pascual-Leone A. All talk and no action: a transcranial magnetic stimulation study of motor cortex activation during action word production. J Cogn Neurosci. 2004;16:374–81. doi: 10.1162/089892904322926719. [DOI] [PubMed] [Google Scholar]
- Op de Beeck HP, Haushofer J, Kanwisher NG. Interpreting fMRI data: maps, modules, and dimensions. Nat Rev Neurosci. 2008;9:123–35. doi: 10.1038/nrn2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, Mayes LC, Schultz RT. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron. 2004;42:163–72. doi: 10.1016/s0896-6273(04)00155-2. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pisella L, Binkofski BF, Lasek K, Toni I, Rossetti Y. No double-dissociation between optic ataxia and visual agnosia: multiple substreams for multiple visuo-manual integrations. Neuropsychologia. 2006;44:2734–48. doi: 10.1016/j.neuropsychologia.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Plaut DC. Graded modality-specific specialization in semantics: a computational account of optic aphasia. Cogn Neuropsychol. 2002;19:603–39. doi: 10.1080/02643290244000112. [DOI] [PubMed] [Google Scholar]
- Polk TA, Park J, Smith MR, Park DC. Nature versus nurture in ventral visual cortex: a functional magnetic resonance imaging study of twins. J Neurosci. 2007;27:13921–25. doi: 10.1523/JNEUROSCI.4001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz JJ. Furnishing the Mind. Concepts and Their Perceptual Basis. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nat Rev Neurosci. 2005;6:576–82. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O, Nikolin VV, Ilmoniemi RJ. Functional links between language and motor systems. Eur J Neurosci. 2005;21:793–97. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Ochipa C, Anderson KC, Poizner H. Progressive ideomotor apraxia: evidence for a selective impairment in the action production system. Brain Cogn. 1995;27:213–36. doi: 10.1006/brcg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Riddoch MJ, Humphreys GW, Coltheart M, Funnell E. Semantic systems or system? Neuropsychological evidence re-examined. Cogn Neuropsychol. 1988;5:3–25. [Google Scholar]
- Riesenhuber M. Appearance isn’t everything: news on object representation in cortex. Neuron. 2007;55:341–44. doi: 10.1016/j.neuron.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Mechelli A, Patterson K, Price CJ. Fusiform activation to animals is driven by the process, not the stimulus. J Cogn Neurosci. 2005;17:434–45. doi: 10.1162/0898929053279531. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–35. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rosci C, Chiesa V, Laiacona M, Capitani E. Apraxia is not associated to a disproportionate naming impairment for manipulable objects. Brain Cogn. 2003;53:412–15. doi: 10.1016/s0278-2626(03)00156-8. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis. Cogn Neuropsychol. 1991;8:443–58. [Google Scholar]
- Rumiati Rl, Zanini S, Vorano L. A form of ideational apraxia as a selective deficit of contention scheduling. Cogn Neuropsychol. 2001;18:617–42. doi: 10.1080/02643290126375. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, et al. The neural basis of pantomiming the use of visually presented objects. Neuroimage. 2004;21:1224–31. doi: 10.1016/j.neuroimage.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Sacchett C, Humphreys GW. Calling a squirrel a squirrel but a canoe a wigwam: a category-specific deficit for artifactual objects and body parts. Cogn Neuropsychol. 1992;9:73–86. [Google Scholar]
- Samson D, Pillon A. A case of impaired knowledge for fruit and vegetables. Cogn Neuropsychol. 2003;20:373–400. doi: 10.1080/02643290244000329. [DOI] [PubMed] [Google Scholar]
- Santos LR, Caramazza A. The domain-specific hypothesis: a developmental and comparative perspective on category-specific deficits. In: Forde EME, Humphreys GW, editors. Category-Specificity in the Brain and Mind. New York: Psychol. Press; 2002. pp. 1–23. [Google Scholar]
- Sartori G, Lombardi L. Semantic relevance and semantic disorders. J Cogn Neurosci. 2004;16:439–52. doi: 10.1162/089892904322926773. [DOI] [PubMed] [Google Scholar]
- Sartori G, Lombardi L, Mattiuzzi L. Semantic relevance best predicts normal and abnormal name retrieval. Neuropsychologia. 2005;43:754–70. doi: 10.1016/j.neuropsychologia.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Shallice T. From Neuropsychology to Mental Structure. London: Cambridge Univ. Press; 1988. [Google Scholar]
- Shelton JR, Fouch E, Caramazza A. The selective sparing of body part knowledge: a case study. Neurocase. 1998;4:339–51. [Google Scholar]
- Silveri MC, Gainotti G, Perani D, Cappelletti JY, Carbone G, Fazio F. Naming deficit for nonliving items: neuropsychological and PET study. Neuropsychologia. 1997;35:359–67. doi: 10.1016/s0028-3932(96)00084-x. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Barsalou LW. The similarity-in-topography principle: reconciling theories of conceptual deficits. Cogn Neuropsychol. 2003;20:451–86. doi: 10.1080/02643290342000032. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel J, Poncet M. The role of sensorimotor experience in object recognition. Brain. 1991;114:2555–73. doi: 10.1093/brain/114.6.2555. [DOI] [PubMed] [Google Scholar]
- Spelke ES, Breinlinger K, Macomber J, Jacobson K. Origins of knowledge. Psychol Rev. 1992;99:605–32. doi: 10.1037/0033-295x.99.4.605. [DOI] [PubMed] [Google Scholar]
- Stewart F, Parkin AJ, Hunkin NM. Naming impairments following recovery from herpes simplex encephalitis. Q J Exp Psychol A. 1992;44:261–84. doi: 10.1080/02724989243000037. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where. Neuropsychologia. 2003;41:280–92. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Aguirre GK, D’Esposito M, Farah MJ. A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia. 1999;37:671–76. doi: 10.1016/s0028-3932(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Tranel D, Logan CG, Frank RJ, Damasio AR. Explaining category-related effects in the retrieval of conceptual and lexical knowledge of concrete entities: operationalization and analysis of factor. Neuropsychologia. 1997;35:1329–39. doi: 10.1016/s0028-3932(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Turnbull OH, Laws KR. Loss of stored knowledge of object structure: implication for “category-specific” deficits. Cogn Neuropsychol. 2000;17:365–89. doi: 10.1080/026432900380445. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Moss HE. Towards a distributed account of conceptual knowledge. Trends Cogn Sci. 2001;5:244–52. doi: 10.1016/s1364-6613(00)01651-x. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–86. [Google Scholar]
- Vinson DP, Vigliocco G, Cappa S, Siri S. The breakdown of semantic knowledge: insights from a statistical model of meaning representation. Brain Lang. 2003;86:347–65. doi: 10.1016/s0093-934x(03)00144-5. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Category specific access dysphasia. Brain. 1983;106:859–78. doi: 10.1093/brain/106.4.859. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Categories of knowledge: further fractionations and an attempted integration. Brain. 1987;110:1273–96. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairment. Brain. 1984;107:829–54. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Gelman SA. Cognitive development: foundational theories of core domains. Annu Rev Psychol. 1992;43:337–75. doi: 10.1146/annurev.ps.43.020192.002005. [DOI] [PubMed] [Google Scholar]
- Zannino GD, Perri R, Carlesimo GA, Pasqualetti P, Caltagirone C. Category-specific impairment in patients with Alzheimer’s disease as a function of disease severity: a cross-sectional investigation. Neuropsychologia. 2002;40:2268–79. doi: 10.1016/s0028-3932(02)00110-0. [DOI] [PubMed] [Google Scholar]
- Zannino GD, Perri R, Pasqualetti P, Caltagirone C, Carlesimo GA. Analysis of the semantic representations of living and nonliving concepts: a normative study. Cogn Neuropsychol. 2006;23:515–40. doi: 10.1080/02643290542000067. [DOI] [PubMed] [Google Scholar]
- Zwaan RA. The immersed experiencer: toward an embodied theory of language comprehension. In: Ross BH, editor. The Psychology of Learning and Motivation. New York: Academic; 2004. pp. 35–62. [Google Scholar]