Abstract
Several methods have been developed to label peptides with fluorine-18. However, in general these are laborious and require a multistep synthesis. We present a facile method based on the chelation of [18F]aluminum fluoride (“Al18F”) by NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid). The method is characterized by labeling NOTA-octreotide (IMP466) with 18F.
Methods
Octreotide was conjugated with the NOTA chelate and was labeled with 18F in a two-step, one-pot method. The labeling procedure was optimized with regard to the labeling buffer, peptide, and aluminum concentration. Radiochemical yield, specific activity, in vitro stability, and receptor affinity were determined. Biodistribution of 18F-IMP466 was studied in AR42J tumor-bearing mice and compared to that of 68Ga-labeled IMP466. In addition, microPET/CT images were acquired.
Results
IMP466 was labeled with “Al18F” in a single step with 50% yield. The labeled product was purified by HPLC to remove unbound “Al18F” and unlabeled peptide. The radiolabeling, including purification, was performed in 45 min. The specific activity was 45,000 GBq/mmol and the peptide was stable in serum for 4 h at 37° C. Labeling was performed at pH 4.1 in sodium citrate, sodium acetate, HEPES and MES buffer and was optimal in sodium acetate buffer. The apparent IC50 of the 19F-labeled IMP466 determined on AR42J cells was 3.6 nM. Biodistribution studies at 2 h p.i. showed a high tumor uptake of 18F-IMP466 (28.3 ± 5.2 %ID/g, tumor-to-blood ratio: 300 ± 90), which could be blocked by an excess of unlabeled peptide (8.6 ± 0.7%ID/g), indicating that the accumulation in the tumor was receptor-mediated. Biodistribution of 68Ga-IMP466 was similar to that of 18F-IMP466. 18F-IMP466 was stable in vivo, since bone uptake was only 0.4 ± 0.2 %ID/g, whereas free “Al18F” accumulated rapidly in the bone (36.9 ± 5.0 %ID/g at 2 h p.i.). MicroPET/CT scans showed excellent tumor delineation and high preferential accumulation in the tumor.
Conclusions
NOTA-octreotide could be labeled rapidly and efficiently with 18F using a two-step, one-pot, method. The compound was stable in vivo and showed rapid accretion in SSTR2-receptor expressing AR42J tumors in nude mice. This method can be used to label other NOTA-conjugated compounds with 18F.
Keywords: octreotide, radiofluorination, NOTA, peptide, PET, aluminum fluoride
INTRODUCTION
During the past decade, radiolabeled receptor-binding peptides have emerged as an important class of radiopharmaceuticals that have changed radionuclide imaging. Peptides have been labeled with 111In and 99mTc for SPECT imaging and, more recently, with positron emitters like 68Ga, 64Cu, 86Y and 18F for PET imaging. 18F is the most widely used radionuclide in PET and has excellent characteristics for peptide-based imaging: the half-life (110 min) matches the pharmacokinetics of most peptides and the low positron energy of 635 keV results in short ranges in tissue and excellent preclinical imaging resolution (< 2 mm) (1). A wide range of methods to label peptides with 18F have been investigated. In general, an 18F-labeled synthon is produced by nucleophilic substitution, which is subsequently reacted with the functionalized peptide. One of the first generally applicable methods is based on conjugation of the synthon, N-succinimidyl-4-[18F]fluorobenzoate to a primary amino group of the peptide (2). Although widely used, the method requires a multi-step synthesis and therefore is time-consuming and laborious. Wester et al. have developed an improved 18F-labeling method by reacting [18F]fluorobenzaldehyde with an aminooxy-derivatized peptide forming an oxime bond (3). Alternatively, [18F]fluorobenzaldehyde was reacted with hydrazine-nicotinamide-conjugated peptides. However, this method resulted in increased lipophilicity of the peptide that may lead to unfavorable characteristics in vivo (4). Carbohydration of the peptide may counteract the enhanced lipophilicity (5). More recently [18F]FDG was explored for labeling of aminooxy-derivatized peptides (6), (7). This approach requires the use of carrier-free [18F]FDG, necessitating HPLC purification of [18F]FDG before conjugation with the functionalized peptide. Furthermore, methods based on the Huisgen cycloaddition of alkynes and azides were explored for the fluorination of peptides (8), (9), (10), (11). Recently, silicon-based building blocks were used to fluorinate bombesin peptides functionalized with two tertiary butyl groups. However, this method also resulted in a lipophilic 18F-peptide and loss of tumor targeting (12), (13).
We reported recently that a NOTA-conjugated pretargeting peptide could be labeled directly with 18F (14). To demonstrate that this two-step, one-pot, method can be applied to other peptides, we report a new approach to label the somatostatin analogue, NOTA-octreotide with 18F. Radiolabeled somatostatin analogues, such as DTPA-octreotide, DOTA-Tyr3-octreotide, NOTA-octreotide and DOTA-Tyr3-octreotate, can be used to image SSTR2-receptor-expressing tumors. With this two-step one-pot fluorination method, the peptide could be stably-labeled with a 50% radiochemical yield at a high specific activity within 45 min.
MATERIALS AND METHODS
General
The octreotide peptide analog, NOTA-D-Phe-cyclo[Cys-Phe-D-Trp-Lys-Thr-Cys]-Throl (MH+ 1305), designated IMP466, was synthesized using standard Fmoc-based solid phase peptide synthesis. After cleavage from the resin, the peptide was cyclicized by incubation with DMSO overnight. The Throl resin and the protected amino acids were purchased from CreoSalus Inc. (Louisville, KY). The bis-t-butyl NOTA ligand was provided by Immunomedics, Inc. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). All buffers used for the radiolabeling procedures were metal-free.
Radiolabeling
18F-labeling
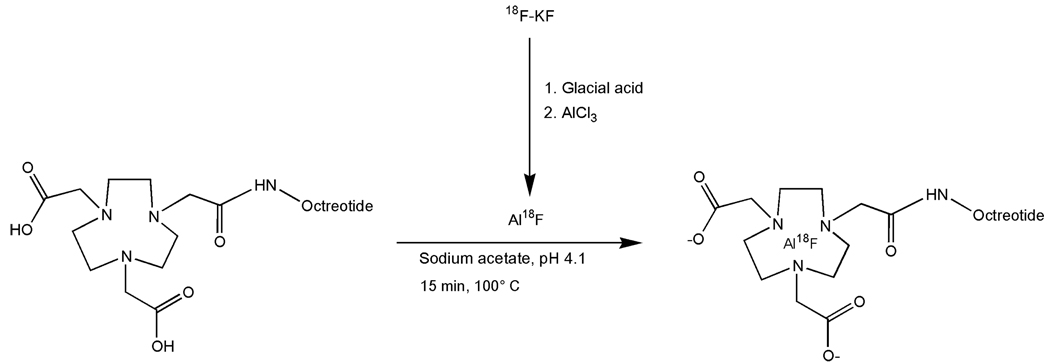
The reaction is summarized in Figure 1. A QMA SepPak light cartridge (Waters, Milford, MA) with 2–6 GBq 18F (BV Cyclotron VU, Amsterdam, The Netherlands) was washed with 3 mL metal-free water. 18F was eluted from the cartridge with 0.4 M KHCO3 and fractions of 200 µL were collected. The pH of the fractions was adjusted to pH 4, with 10 µL metal-free glacial acetic acid. Three µL of 2 mM AlCl3 in 0.1 M sodium acetate buffer, pH 4 were added. Then, 10–50 µL IMP466 (10 mg/mL) were added in 0.5 M sodium acetate, pH 4.1. The reaction mixture was incubated at 100° C for 15 min unless stated otherwise. The radiolabeled peptide was purified on RP-HPLC as described below. The 18F-IMP466-containing fractions were collected and diluted two-fold with H2O and purified on a 1-cc Oasis HLB cartridge (Waters, Milford, MA) to remove acetonitrile and TFA. In brief, the fraction was applied on the cartridge and the cartridge was washed with 3 mL H2O. The radiolabeled peptide was then eluted with 2 × 200 µL 50% ethanol. Upon injection in mice, the peptide was diluted with 0.9% NaCl.
Figure 1.
Preparation of “Al18F” and chelation with NOTA-octreotide.
Effect of buffer
The effect of the buffer on the labeling efficiency of IMP466 with 18F− was investigated (n=3 for each buffer). IMP466 was dissolved in sodium citrate buffer, sodium acetate buffer, 2-(N-morpholino)ethanesulfonic acid (MES) or 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer at 10 mg/mL (7.7 mM). The molarity of all buffers was 1 M and the pH was 4.1. To 200 µg (153 nmol) of IMP466, 100 µL “Al18F” (pH 4) were added and incubated at 100 °C for 15 min. Radiolabeling yield and specific activity were determined with RP-HPLC as described below.
68Ga-labeling
IMP466 was labeled with 68GaCl3 eluted from a TiO2-based 1,110 MBq 68Ge/68Ga generator (Cyclotron Co. Ltd., Obninsk, Russia) using 0.1 M HCl (Ultrapure, J.T. Baker, Deventer, The Netherlands). Five 1-ml fractions were collected and an aliquot of the second fraction was used for labeling the peptide. IMP466 (4 µg) was dissolved in 2.5 M HEPES buffer, pH 7.0. 68Ga eluate (120–240 MBq, 4 times the volume of the peptide) was added and the mixture was heated at 95° C for 20 min. Then 50 mM EDTA was added to a final concentration of 5 mM to complex the non-incorporated 68Ga3+. The 68Ga-labeled IMP466 was purified on a 1-cc Oasis HLB cartridge and eluted with 200 µL 50% ethanol. Specific activity of 68Ga-IMP466 was 20,000 GBq/mmol at time of injection.
HPLC analysis
The radiolabeled preparations were analyzed by RP-HPLC on an Agilent 1200 system (Agilent Technologies, Palo Alto, CA, USA). A C18 column (Onyx monolithic, 4.6 × 100 mm, Phenomenex, Torrance, CA, USA) was used at a flow rate of 2 mL/min with the following buffer system: Buffer A: 0.1% v/v TFA in water; Buffer B: 0.1% v/v TFA in acetonitrile; Gradient: 0–5 min 97% buffer A, 5–35 min 80% buffer A to 75% buffer A. The radioactivity of the eluate was monitored using an in-line NaI radiodetector (Raytest GmbH, Straubenhardt, Germany). Elution profiles were analyzed using Gina-star software (version 2.18, Raytest GmbH, Straubenhardt, Germany). Specific activity was determined by HPLC using calibration curves based on the UV signal.
Octanol-water partition coefficient (log Poctanol/water)
To determine the lipophilicity of the radiolabeled peptides, approximately 50,000 cpm of the radiolabeled peptide were diluted in 0.5 mL phosphate-buffered saline (PBS). An equal volume of 1-octanol was added to obtain a binary phase system. After vortexing the mixture for 2 min, the two layers were separated by centrifugation (100 × g, 5 min). Three 100-µL samples were taken from each layer and radioactivity was measured in a well-type gamma counter (Wallac Wizard 3”, Perkin-Elmer, Waltham, MA).
Stability
Ten µL of the 18F-labeled IMP466 were incubated in 500 µL of freshly collected human serum and incubated for 4 h at 37° C. An equal volume of acetonitrile was added and the mixture was vortexed followed by centrifugation at 1000 × g for 5 min to pellet the precipitated serum proteins. The supernatant was analyzed on RP-HPLC as described above.
The in vivo stability of 18F-IMP466 was examined by injecting 18.5 MBq of 18F-IMP466 in a BALB/c nude mouse. After 30 min, the mouse was euthanized, blood and urine were collected, and analyzed by HPLC.
Cell culture
The AR42J rat pancreatic tumor cell line was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco Life Technologies, Gaithersburg, MD, USA) supplemented with 4500 mg/L D-glucose, 10% (v/v) fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin. Cells were cultured at 37° C in a humidified atmosphere with 5% CO2.
IC50 determination
The apparent 50% inhibitory concentration (IC50) for binding the somatostatin receptors on AR42J cells was determined in a competitive binding assay using 19F-IMP466, 69Ga-IMP466 or 115In-DTPA-octreotide to compete for the binding of 111In-DTPA-octreotide.
19F-IMP466 was formed by mixing an aluminium fluoride (AlF) solution (0.02 M AlCl3 in 0.5 M NaAc, pH 4, with 0.1 M NaF in 0.5 M NaAc, pH 4) with IMP466 and heating at 100° C for 15 min. The reaction mixture was purified by RP-HPLC on a C-18 column (30 × 150 mm, Sunfire, Waters, Milford, MA), as described above.
69Ga-IMP466 was prepared by dissolving gallium nitrate (2.3×10−8 mol) in 30 µL mixed with 20 µL IMP466 (1 mg/mL) in 10 mM NaAc, pH 5.5, and heated at 90° C for 15 min. Samples of the mixture were used without further purification.
115In-DTPA-octreotide was made by mixing indium chloride (1×10−5 mol) with 10 µL DTPA-octreotide (1 mg/mL) in 50 mM NaAc, pH 5.5, and incubated at room temperature (RT) for 15 min. This sample was used without further purification. 111In-DTPA-octreotide (OctreoScan®) was radiolabeled according to the manufacturer’s protocol.
AR42J cells were grown to confluency in 12-well plates and washed twice with binding buffer (DMEM with 0.5% bovine serum albumin). After 10 min incubation at RT in binding buffer, 19F-IMP466, 69Ga-IMP466 or 115In-DTPA-octreotide was added at a final concentration ranging from 0.1–1000 nM, together with a trace amount (10,000 cpm) of 111In-DTPA-octreotide (radiochemical purity >95%). After incubation at RT for 3 h, the cells were washed twice with ice-cold PBS. Cells were scraped and cell-associated radioactivity was determined. Under these conditions, a limited extent of internalization may occcur. We therefore describe the results of this competitive binding assay as “apparent IC50” values rather than IC50. The apparent IC50 was defined as the peptide concentration at which 50% of binding without competitor was reached. Apparent IC50 values were calculated using GraphPad Prism software (Version 4.00 for Windows, GraphPad Software, San Diego CA, USA).
Biodistribution studies
Male nude BALB/c mice (6–8 weeks old) were injected subcutaneously in the right flank with 0.2 mL AR42J cell suspension of 1 × 107 cells/mL. Approximately two weeks after inoculation, when tumors were 5–8 mm in diameter, 370 kBq 18F-labeled or 68Ga-labeled IMP466 (both 0.2 nmol) were administered intravenously (n=5). Separate groups of mice (n=5) were co-injected with a 1,000-fold molar excess of unlabeled IMP466. One group of three mice was injected with unchelated “Al18F”. All mice were killed by CO2/O2 asphyxiation 2 h post injection (p.i.). Tissues of interest were dissected, weighed and counted in a gamma counter. The percentage of the injected dose per gram tissue (%ID/g) was calculated for each tissue based on a dilution of the product for injection. The animal experiments were approved by the local animal welfare committee and performed according to national regulations.
PET/CT imaging
Mice with s.c. AR42J tumors were injected intravenously with 10 MBq 18F-IMP466 or 68Ga-IMP466 (both 0.7 nmol) per mouse. One and two hours after the injection of peptide, mice were scanned on an animal PET/CT scanner (Inveon®, Siemens Preclinical Solutions, Knoxville, TN) with an intrinsic spatial resolution of 1.5 mm (1). The animals were placed in a supine position in the scanner. PET-emission scans were acquired over 15 min, followed by a CT scan for anatomical reference (spatial resolution 113 µm, 80 kV, 500 µA). Scans were reconstructed using Inveon Acquisition Workplace software version 1.2 (Siemens Preclinical Solutions, Knoxville, TN), using an ordered set expectation maximization-3D/maximum a posteriori (OSEM3D/MAP) algorithm with the following parameters: matrix 256 × 256 × 159, pixel size 0.43 × 0.43 × 0.8 mm3 and a beta-value of 0.1.
Statistical analysis
All mean values are given ± standard deviation (S.D.). Statistical analysis was performed using a Welch’s corrected unpaired Student’s t-test or one-way analysis of variance using GraphPad InStat software (version 3.06, GraphPad Software). The level of significance was set at P<0.05.
RESULTS
18F-labeling procedure
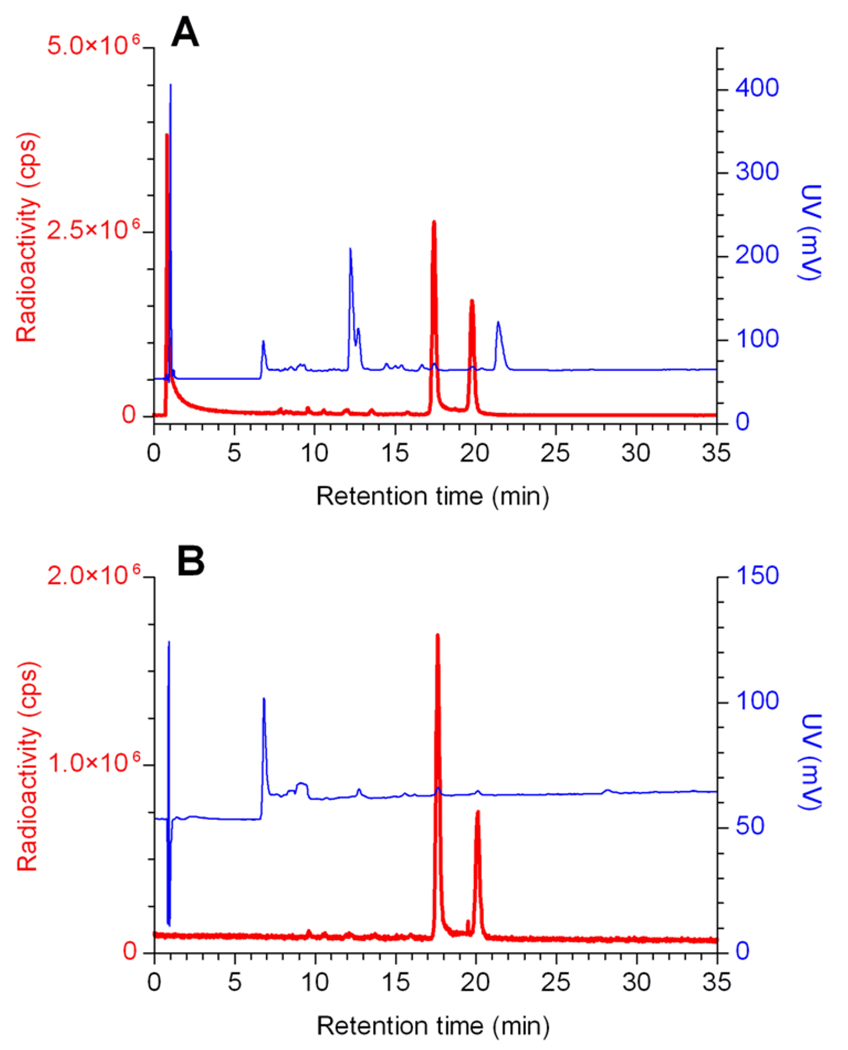
HPLC analysis of the IMP466 labeling mixture (Figure 2) showed the presence of unbound “Al18F” (Rt 0.8 min) and two radioactive peptide peaks (Rt 17.4 and 19.8 min), indicating the formation of two 18F-IMP466 stereoisomers. Moreover, a UV peak of unlabeled IMP466 is present (Rt 21.4 min). After HPLC and HLB purification, both the unbound “Al18F” and the unlabeled IMP466 UV peaks disappeared (Figure 2).
Figure 2.
RP-HPLC chromatograms of the IMP466 18F-labeling mix (panel A) and the purified 18F-IMP466 (panel B). Red traces represents radioactivity(left y-axis) and blue traces represents UV signal (right y-axis). In the HPLC chromatogram of the crude mixture, unbound “Al18F” eluted with the void volume (Rt = 0.8 min). Two radioactive peaks correspond to the stereoisomers of radiolabeled peptide times (Rt = 17.4 and Rt = 19.8 min). Finally, the unlabeled IMP466 was present in the UV channel (Rt = 21.4 min). After purification, only two radioactive peptide peaks are observed, indicating the formation of two stereoisomers.
Effect of buffer
When using sodium acetate, MES, or HEPES, radiolabeling yield was 49 ± 2%, 46 ± 2%, and 48 ± 3%, respectively (n=3 for each buffer). In sodium citrate, no labeling was observed. When the labeling reaction was carried out in sodium acetate buffer, the specific activity was 32,000 ± 17,000 GBq/mmol, whereas in MES and HEPES buffers specific activities of 29,000 ± 14,000 and 31,000 ± 23,000 GBq/mmol respectively. were obtained.
Effect of peptide concentration
The effect of peptide concentration on the labeling efficiency also was investigated. IMP466 was dissolved in sodium acetate buffer, pH 4.1, at a concentration of 7.7 mM (10 mg/mL). Either 38, 153, or 363 nmol of IMP466 were added to 200 µL “Al18F” (581–603 MBq) to yield final IMP466 concentrations of 190, 765, and 1815 µM, respectively. The radiolabeling yield increased with increasing amounts of peptide. At a concentration of 190 µM, radiolabeling yield was 8 ± 1 %, at 765 µM, the yield increased to 42 ± 3 %, and at the highest concentration the radiolabeling yield was 50 ± 2%. Specific activity of the products obtained at each concentration was 48,000 GBq/mmol.
Effect of AlCl3 concentration
Since AlCl3 is used to form “Al18F”, the added amount of AlCl3 is critical in the labeling procedure. Five stock solutions with various AlCl3 concentrations were prepared: 0.2, 0.5, 1.0, 2.0 and 20 mM. From these solutions in sodium acetate, 3 µL was added to 200 µL of [18F]-fluoride, pH 4 to form [18F]aluminum fluoride, resulting in final amounts of AlCl3 added of 0.6, 1.5, 3.0, 6.0 and 60 nmol, respectively. To these samples, 153 nmol of IMP466 (final concentration 765 µM) were added and incubated for 15 min at 100 °C. Radiolabeling yield was optimal (50 ± 2%, n=5) after incubation with 6 nmol AlCl3. Lowering the AlCl3 concentration resulted in reduced yields, ranging from 42% at 3 nmol to 10% at 0.6 nmol AlCl3. Increasing the amount lead to a similar effect. Incubation with 60 nmol AlCl3 resulted in a radiolabeling yield of only 6%.
Octanol-water partition coefficient
To determine the lipophilicity of the 18F- and 68Ga-labeled IMP466, the octanol-water partition coefficients were determined. The log Poctanol/water value for the 18F-IMP466 was −2.44 ± 0.12 and that of 68Ga-IMP466 was −3.79 ± 0.07, indicating that the 18F-IMP466 was slightly less hydrophilic than 68Ga-IMP466.
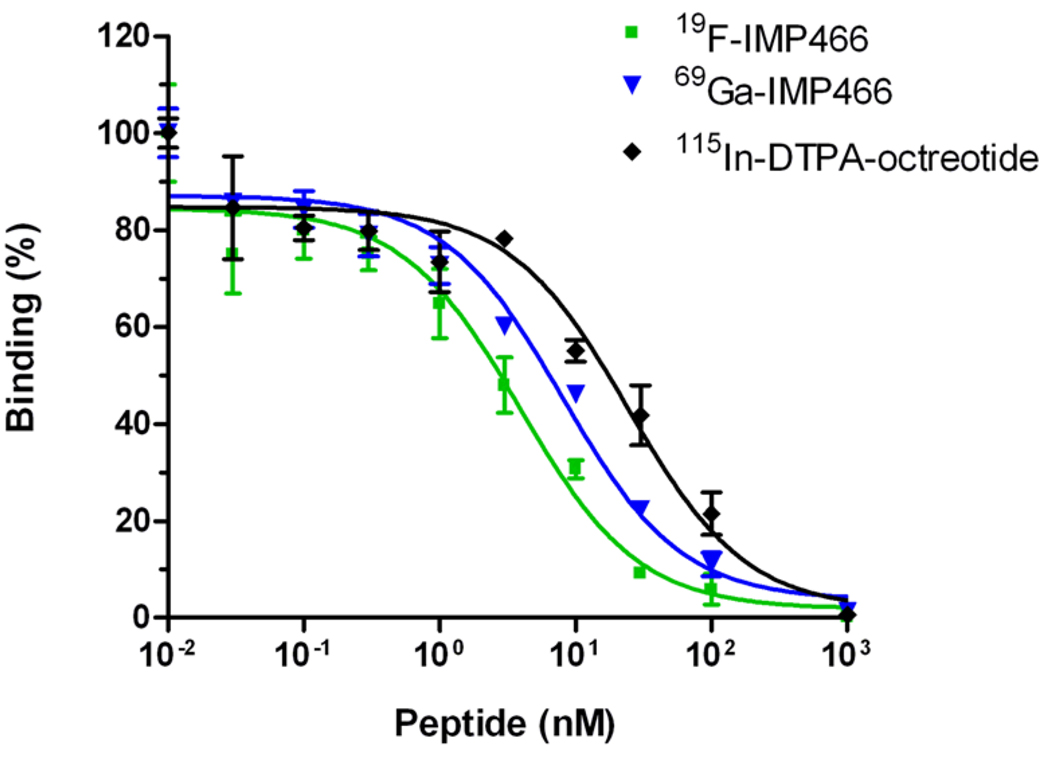
IC50 determination
The affinity profiles are shown in Figure 3. The apparent IC50 of Al19F-labeled IMP466 was 3.6 ± 0.6 nM, whereas that for 69Ga-labeled IMP466 was 13 ± 3 nM. The apparent IC50 of the reference peptide, 115In-DTPA-octeotride (OctreoScan®), was 6.3 ± 0.9 nM.
Figure 3.
Competitive binding assay (apparent IC50) of 19F-IMP466, 69Ga-IMP466 and 115In-DTPA-octreotide determined on AR42J tumor cells. Values on the y-axis represent binding expressed as a precentage of the binding without competitor.
Stability
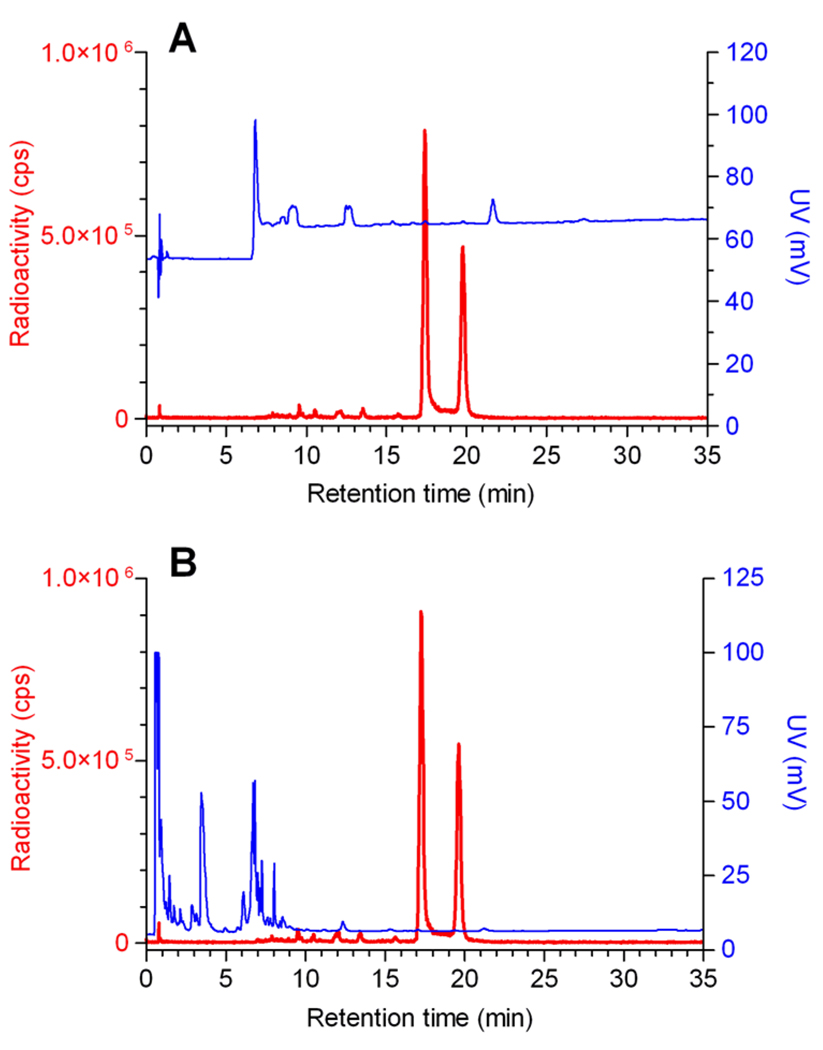
18F-labeled IMP466 did not release “Al18F” after incubation in human serum at 37° C for 4 h, indicating excellent stability of the “Al18F”-NOTA-octreotide. These findings were confirmed in vivo. After 30 min, only intact radiolabeled peptide was found in urine (Figure 4) and no free fluorine-18. In addition, PET/CT scans did not reveal any bone uptake, indicating the absence of free 18F-fluoride or “Al18F”.
Figure 4.
HPLC chromatograms of purified 18F-IMP466 before injection (panel A) and a urine sample 30 min p.i. (panel B). Red traces represents radioactivity(left y-axis) and blue traces represents UV signal (right y-axis). The HPLC traces of the two samples are very similar, indicating that the excreted product is intact 18F-IMP466.
Biodistribution studies
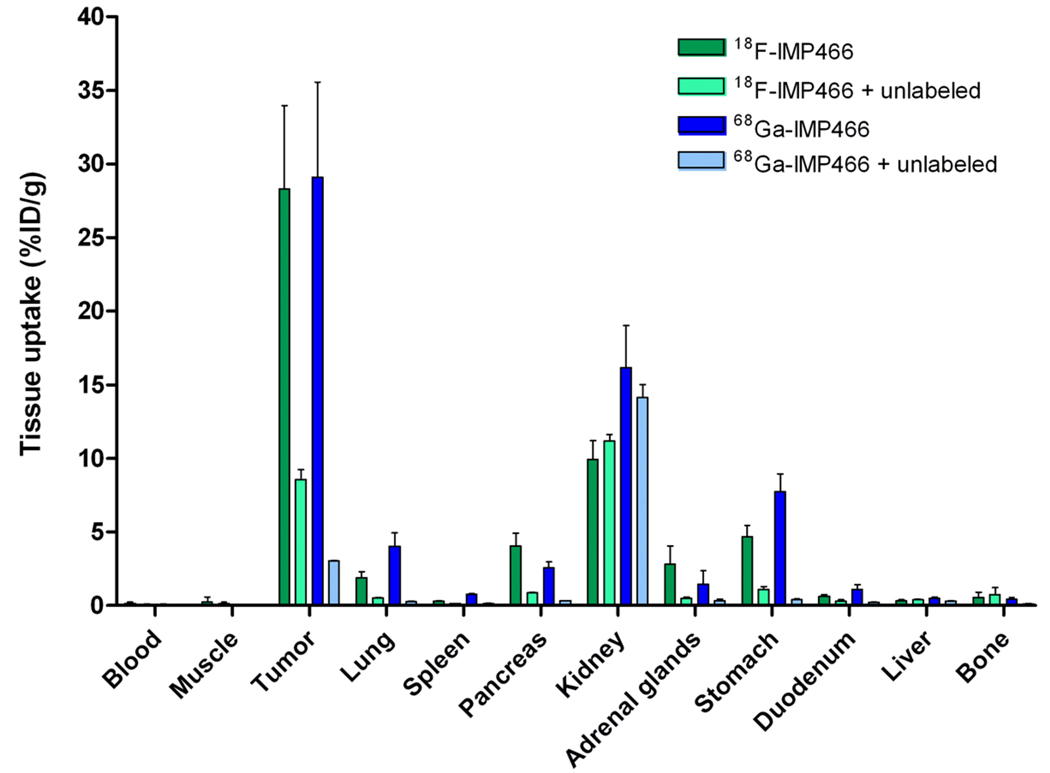
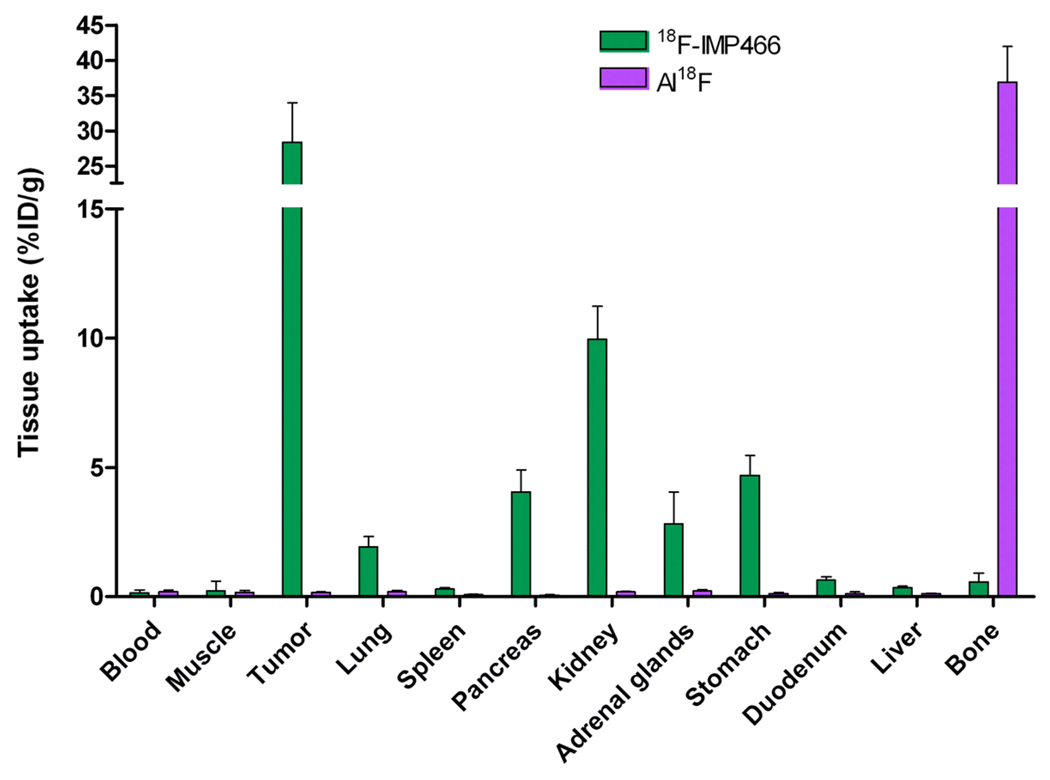
The biodistribution of both 18F-IMP466 and 68Ga-IMP466 in BALB/c nude mice with s.c. AR42J tumors at 2 h p.i. is summarized in Figures 5 and 6. Unchelated “Al18F” was included as a control. Tumor uptake of 18F-IMP466 was 28.3 ± 5.7% ID/g at 2 h p.i., and reduced in the presence of an excess of unlabeled IMP466 to 8.6 ± 0.7% ID/g, indicating that uptake was receptor-mediated. Non-specific uptake of 18F-IMP466 was somewhat higher than that of 68Ga-IMP466, as is illustrated by the less efficient blocking of the tumor uptake of 18F-IMP466. Blood levels were very low (0.10 ± 0.07% ID/g, 2 h p.i.), resulting in a tumor-to-blood ratio of 300 ± 90. Uptake in normal tissues, except in the kidneys, was low, with specific uptake in SST2 receptor-expressing tissues, such as adrenal glands, pancreas and stomach. Bone uptake of 18F-IMP466 was negligible as compared to uptake after injection of non-chelated “Al18F” (0.33 ± 0.07 vs. 36.9 ± 5.0% ID/g at 2 h p.i., respectively; P< 0.001), indicating good in vivo stability of the 18F-IMP466.
Figure 5.
Biodistribution of 18F-IMP466 and 68Ga-IMP466 at 2 h p.i. in AR42J tumor-bearing mice (n=5/group). As a control, mice in separate groups (n=3/group) received an excess of unlabeled octreotide to demonstrate receptor specificity. Tumors weighed 0.04 – 0.33 g.
Figure 6.
Biodistribution of 18F-IMP466 and unbound “Al18F” at 2 h p.i. in AR42J tumor-bearing mice (n=5/group). Tumors weighed 0.07 – 0.36 g.
Tumor uptake of 68Ga-IMP466 (29.2 ± 0.5% ID/g, 2 h p.i.) was similar to that of 18F-IMP466 (P = 0.7). Lung uptake of 68Ga-IMP466 was two-fold higher than that of 18F-IMP466 (4.0 ± 0.9% ID/g vs. 1.9 ± 0.4% ID/g, respectively). In addition, kidney retention of 68Ga-IMP466 was significantly higher than that of 18F-IMP466 (16.2 ± 2.86% ID/g vs. 10.0 ± 1.3% ID/g, respectively; P< 0.01).
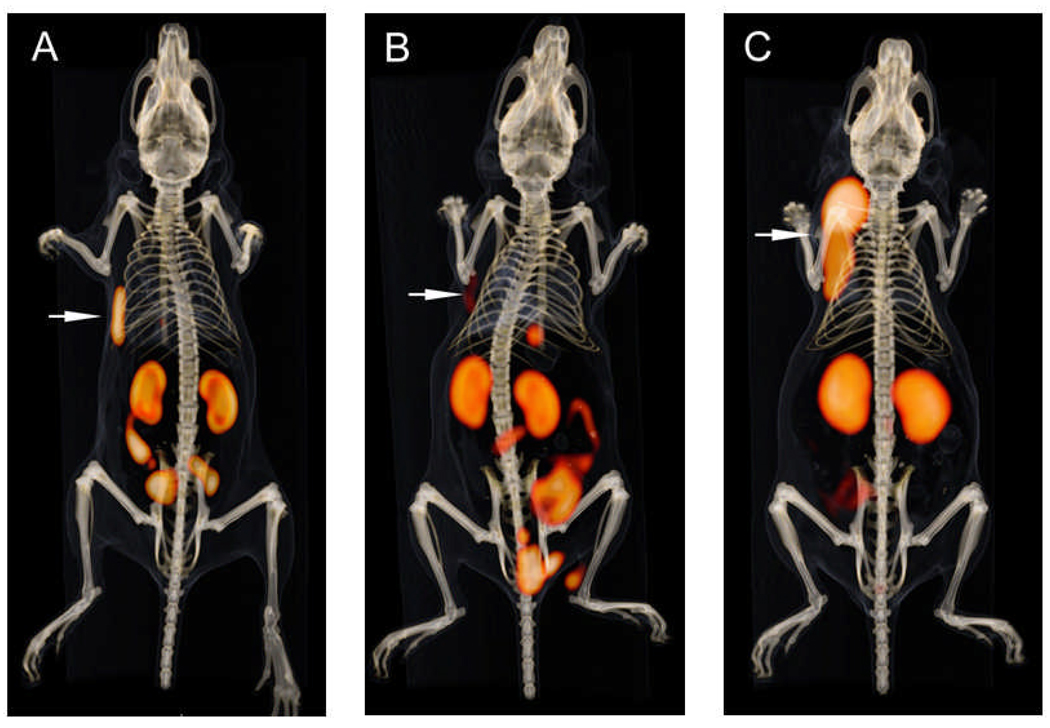
Fused PET and CT scans are shown in Figure 7. PET scans largely corroborated the biodistribution data. Both 18F-IMP466 and 68Ga-IMP466 showed high uptake in the tumor and high retention in the kidneys. The activity in the kidneys was localized mainly in the renal cortex. Some intestinal uptake was observed on the scans of 18F-IMP466. The PET/CT scans also demonstrated that the “Al18F” was stably-chelated by the NOTA chelator, since no bone uptake was observed.
Figure 7.
Anterior 3D volume-rendering projections of fused PET and CT scans of mice with a s.c. AR42J tumor on the right flank injected with 18F-IMP466 (A), 18F-IMP466 in the presence of an excess of unlabeled IMP466 (B), and 68Ga-IMP466 (C). Arrows indicate tumors. Scans were recorded at 2 h p.i.
DISCUSSION
Radiolabeling of peptides with fluorine-18 involves laborious and time-consuming procedures and first requires the synthesis of a 18F-labeled synthon. We showed recently that a NOTA-conjugated pretargeting peptide (IMP449) could be labeled with 18F in a two-step, one-pot, reaction by creating [18F]aluminumfluoride (14). Here, we describe the optimized 18F-labeling of NOTA-conjugated octreotide (IMP466) using the same approach. The biodistribution of the 18F-NOTA-octreotide was compared to that of 68Ga-NOTA-octreotide. We demonstrated that the affinity of 18F-NOTA-octreotide was at least as good as that of 111In-DTPA-octreotide, and was comparable with values reported in literature for DOTA-octreotate and DOTA-TOC (15).
In the present study, we used the directly-coupled NOTA derivative rather than the NCS-derivatized NOTA to diminish the effect of the phenyl group on the lipophilicity of the peptide. For 68Ga labeling it has been demonstrated that this NOTA variant is as good as the NCS-activated NOTA (16). After preparing the “Al18F” complex, the chelation of this complex by NOTA was investigated in various buffers. We showed that chelation of “Al18F” could be carried out in sodium acetate, MES and HEPES buffer but, remarkably, the chelation failed in sodium citrate buffer. The labeling reaction requires fairly high NOTA-peptide concentrations. At the concentrations studied, the reaction was most efficient at an IMP466 concentration of 1.8 mM. This optimal concentration is in the same range as reported for other peptide fluorination methods (3), (4), but lower than the optimal concentration required in the ‘classical’ SFB method (2).
Subsequently, we investigated the effect of the amount of Al3+ on the radiolabeling yield. The Al3+ concentration greatly affects the labeling yield. First, the 18F− complexes with the Al3+, then the “Al18F” complex is chelated by NOTA. After incubation at elevated temperature, the reaction mixture is purified using HPLC to separate the “Al18F”-labeled IMP466 from: i) unchelated “Al18F”, ii) Al3+-labeled IMP466 and iii) unlabeled IMP466. We showed that the optimal concentration of AlCl3 was 2 mM, whereas both at lower and at higher concentrations, the radiolabeling yield decreased. These data indicate that there is a delicate balance between 18F− and Al3+. Lowering the amount of Al3+ will lead to less 18F associated with Al3+. In contrast, a higher amount will yield more undesired NOTA-peptide labeled with Al3+, rather than labeled with “Al18F”. Since the formation of “Al18F” is carried out at pH 4.1, we believe that the form of the Al in sodium acetate buffer is probably Al3+(AcO−)3F−. In any case, the mole ratio of Al to F-18 is such that it is unlikely that any AlF2 or AlF3 is formed (17). We attempted to isolate the two isomers, but observed that there was a rapid equilibrium between them. Therefore, no further studies of separate isomers were possible.
The lipophilicity of both the 18F-labeled and 68Ga-labeled IMP466 was in the same range as reported for other 111In-labeled octreotide analogues (18). Other 18F-labeled octreotide analogues, such as the [18F]fluoropropionyl octreotide (log P −0.07 ± 0.01) and Nα-(1-deoxy-D-fructosyl)-Nε-(2-[18F]fluoropropionyl)-Lys0-Tyr3octreotate ([18F]FP-Gluc-TOCA) (log P −1.70 ± 0.02), displayed a higher lipophilicity (19). Biodistribution of [18F]FP-Gluc-TOCA was similar to the biodistribution of 18F-IMP466, but tumor uptake of the latter compound was two-fold higher than that of the carbohydrate analogue.
The biodistribution of 18F-IMP466 was very similar to that of 68Ga-IMP466, indicating that the “Al18F” complex did not affect the in vivo characteristics of octreotide. Both peptides showed an equally high tumor uptake at 2 h p.i., with lower uptake in all other organs. The in vivo studies also showed the excellent stability of the “Al18F”-NOTA complex, since no significant bone uptake could be measured, and the intact product was isolated in the urine.
The current method can be performed in one pot, is fast (45 min), yields carrier-free fluorinated peptide, and does not affect the pharmacokinetics of octreotide. In most 18F-labeling strategies for peptides and proteins, a fluorinated synthon needs to be synthesized first. In general, this fluorination is based on a nucleophilic substitution which requires laborious azeotropic drying of the [18F]fluoride/kryptofix complex. Examples of such a synthon are succinimidyl-[18F]fluorobenzoate (2), 4-[18F]fluorobenzaldehyde (3), and 2-[18F]fluoropropionic acid 4-nitrophenyl ester (20). Subsequently, the synthon is reacted with the (functionalized) peptide, leading to longer synthesis times and lower overall yields. In addition, these techniques also lead to increased lipophilicity, because most synthons contain aromatic groups and this might affect the biodistribution profile. It has been reported that this effect can be counteracted by carbohydration (5). However, this requires considerable peptide modification.
More recently, a method based on Si-F has been published, in which the 18F is bound to a silicon-containing building block in a single step (12), (13). Although somewhat similar to our approach, the Si-18F initially proved to be unstable, but could be stabilized by the addition of tertiary butyl groups. This, however, lead to a strong increase in lipohilicity (log P: 1.3 ± 0.1).
Finally, ‘click’ chemistry has been explored for the radiofluorination of peptides (8), (9), (10). Although the yield of these click chemistry-based labeling procedures based on the alkyne-azide cycloaddition is excellent (>80%), the method starts with the fluorination of an azide or alkyne, such as fluoro(ethyl)azide or a fluoroalkyne. This requires azeotropic drying of the fluoride, resulting in a time-consuming multistep procedure.
Compared to a 68Ga labeling, the “AlF” method is easy and versatile, mainly due to the fact that both methods are based on a chelator-derivatized peptide. One of the advantages of the AlF method is the longer half-life of 18F, allowing PET scanning at later time-points after injection of the tracer.
CONCLUSION
In conclusion, our new approach combines the ease of chelator-based radiolabeling methods with the advantages of 18F (i.e., half-life, availability and positron energy). The “Al18F”-labeled NOTA-octreotide could be synthesized in <45 min without the need to synthesize an 18F synthon. Moreover, the fluorinated peptide was stable in vitro and in vivo and has excellent tumor targeting properties. Therefore, this fluorination method is a promising facile and versatile fluorination procedure.
ACKNOWLEDGEMENTS
We thank Maarten Brom, Jonathan Disselhorst and Bianca Lemmers - de Weem for technical assistance. This work was funded in part by NIH grant 1R43 EB003751-01A1 from the National Institute of Biomedical Imaging and Bioengineering, Bethesda, MD.
Footnotes
Disclosure: WJM and DMG are employed or have financial interest in Immunomedics, Inc. PL, RMS, WJGO and OCB declare no conflicts.
REFERENCES
- 1.Visser EP, Disselhorst JA, Brom M, et al. Spatial resolution and sensitivity of the Inveon small-animal PET scanner. J Nucl Med. 2009;50:139–147. doi: 10.2967/jnumed.108.055152. [DOI] [PubMed] [Google Scholar]
- 2.Lang L, Eckelman WC. One-step synthesis of 18F labeled [18F]-N-succinimidyl 4-(fluoromethyl)benzoate for protein labeling. Appl Radiat Isot. 1994;45:1155–1163. doi: 10.1016/0969-8043(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 3.Poethko T, Schottelius M, Thumshirn G, et al. Two-step methodology for high-yield routine radiohalogenation of peptides: (18)F-labeled RGD and octreotide analogs. J Nucl Med. 2004;45:892–902. [PubMed] [Google Scholar]
- 4.Rennen HJ, Laverman P, van Eerd JE, Oyen WJ, Corstens FH, Boerman OC. PET imaging of infection with a HYNIC-conjugated LTB4 antagonist labeled with F-18 via hydrazone formation. Nucl Med Biol. 2007;34:691–695. doi: 10.1016/j.nucmedbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Schottelius M, Poethko T, Herz M, et al. First (18)F-labeled tracer suitable for routine clinical imaging of sst receptor-expressing tumors using positron emission tomography. Clin Cancer Res. 2004;10:3593–3606. doi: 10.1158/1078-0432.CCR-03-0359. [DOI] [PubMed] [Google Scholar]
- 6.Hultsch C, Schottelius M, Auernheimer J, Alke A, Wester HJ. (18)F-Fluoroglucosylation of peptides, exemplified on cyclo(RGDfK) Eur J Nucl Med Mol Imaging. 2009;36:653–658. doi: 10.1007/s00259-009-1122-0. [DOI] [PubMed] [Google Scholar]
- 7.Namavari M, Cheng Z, Zhang R, et al. A Novel Method for Direct Site-Specific Radiolabeling of Peptides Using [(18)F]FDG. Bioconjug Chem. 2009 Feb 18; doi: 10.1021/bc800422b. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaser M, Arstad E. "Click labeling" with 2-[18f]fluoroethylazide for positron emission tomography. Bioconjug Chem. 2007;18:989–993. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 9.Hausner SH, Marik J, Gagnon MK, Sutcliffe JL. In vivo positron emission tomography (PET) imaging with an alphavbeta6 specific peptide radiolabeled using 18F-"click" chemistry: evaluation and comparison with the corresponding 4-[18F]fluorobenzoyl- and 2-[18F]fluoropropionyl-peptides. J Med Chem. 2008;51:5901–5904. doi: 10.1021/jm800608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marik J, Sutcliffe JL. Click for PET: rapid preparation of [F-18]fluoropeptides using Cu-I catalyzed 1,3-dipolar cycloaddition. Tetrahedron Letters. 2006;47:6681–6684. [Google Scholar]
- 11.Li ZB, Wu Z, Chen K, Chin FT, Chen X. Click chemistry for (18)F-labeling of RGD peptides and microPET imaging of tumor integrin alphavbeta3 expression. Bioconjug Chem. 2007;18:1987–1994. doi: 10.1021/bc700226v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohne A, Mu L, Honer M, et al. Synthesis, 18F-labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconjug Chem. 2008;19:1871–1879. doi: 10.1021/bc800157h. [DOI] [PubMed] [Google Scholar]
- 13.Mu L, Hohne A, Schubiger PA, et al. Silicon-based building blocks for one-step 18F-radiolabeling of peptides for PET imaging. Angew Chem Int Ed Engl. 2008;47:4922–4925. doi: 10.1002/anie.200705854. [DOI] [PubMed] [Google Scholar]
- 14.McBride WJ, Sharkey RM, Karacay H, et al. A novel method of 18F radiolabeling for PET. J Nucl Med. 2009;50:991–998. doi: 10.2967/jnumed.108.060418. [DOI] [PubMed] [Google Scholar]
- 15.Reubi JC, Schar JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 16.Velikyan I, Maecke H, Langstrom B. Convenient preparation of 68Ga-based PET-radiopharmaceuticals at room temperature. Bioconjug Chem. 2008;19:569–573. doi: 10.1021/bc700341x. [DOI] [PubMed] [Google Scholar]
- 17.Martin RB. Ternary hydroxide complexes in neutral solutions of Al3+ and F−. Biochem Biophys Res Commun. 1988;155:1194–1200. doi: 10.1016/s0006-291x(88)81266-x. [DOI] [PubMed] [Google Scholar]
- 18.Antunes P, Ginj M, Walter MA, Chen J, Reubi JC, Maecke HR. Influence of different spacers on the biological profile of a DOTA-somatostatin analogue. Bioconjug Chem. 2007;18:84–92. doi: 10.1021/bc0601673. [DOI] [PubMed] [Google Scholar]
- 19.Wester HJ, Schottelius M, Scheidhauer K, et al. PET imaging of somatostatin receptors: design, synthesis and preclinical evaluation of a novel 18F-labelled, carbohydrated analogue of octreotide. Eur J Nucl Med Mol Imaging. 2003;30:117–122. doi: 10.1007/s00259-002-1012-1. [DOI] [PubMed] [Google Scholar]
- 20.Guhlke S, Wester HJ, Bruns C, Stocklin G. (2-[18F]fluoropropionyl-(D)phe1)-octreotide, a potential radiopharmaceutical for quantitative somatostatin receptor imaging with PET: synthesis, radiolabeling, in vitro validation and biodistribution in mice. Nucl Med Biol. 1994;21:819–825. doi: 10.1016/0969-8051(94)90161-9. [DOI] [PubMed] [Google Scholar]