Abstract
Background
A recent case report suggested the presence of asymmetrical lateral ventricular enlargement associated with motor asymmetry in Parkinson’s disease (PD). The current study explored these associations further.
Methods
Magnetic resonance imaging (3T) scans were obtained on 17 PD and 15 healthy Control subjects at baseline and 12–30 months later. Baseline and longitudinal lateral ventricular volumetric changes were compared between contralateral and ipsilateral ventricles in PD subjects relative to symptom onset side and in Controls relative to their dominant hand. Correlations between changes in ventricular volume and United Parkinson’s Disease Rating Scale motor scores (UPDRS-III) while on medication were determined.
Results
The lateral ventricle contralateral to symptom onset side displayed a faster rate of enlargement compared to the ipsilateral (p=0.004) in PD subjects, with no such asymmetry detected (p=0.312) in Controls. There was a positive correlation between ventricular enlargement and worsening motor function assessed by UPDRS-III scores (r=0.96, p<0.001).
Discussion
There is asymmetrical lateral ventricular enlargement that is associated with PD motor asymmetry and progression. Further studies are warranted to investigate the underlying mechanism(s), as well as the potential of using volumetric measurements as a marker for PD progression.
Keywords: Structural magnetic resonance imaging, semi-automatic segmentation, lateral ventricular volume, motor asymmetry, Parkinson’s disease
Background
Parkinson’s disease (PD) is marked clinically by asymmetrical presentation of motor dysfunction. Among other changes, PD is characterized pathologically by loss of dopamine neurons in the substantia nigra par compacta (SNc) of the basal ganglia (BG), and often is accompanied by cognitive deficits related to frontal lobe dysfunction. Like other neurodegenerative disorders [1,2], accelerated generalized/selective brain atrophy may occur and be useful to reflect cell loss in PD during its progression. Previous studies of brain structural changes, however, have yielded inconsistent and/or nonspecific findings in PD [1–4].
As the site of primary PD pathology [5], the SN has been intensively studied using many imaging technologies, including most recently transcranial ultrasound [6]. Several lines of evidence, however, suggest that structural changes at the SN level will not be very useful to reflect PD-specific cell loss during disease progression. First, it is difficult to define the SN boundary precisely using current imaging technology [7,8]. Second, the volume of the SN is so small that it accounts for only 0.5–0.6% of the total brain volume. Lastly, there is a long asymptomatic period before PD is manifested clinically, by which time patients may have lost 50%–80% of their dopamine neurons [9,10].
Although nigral dopamine neuron loss could have far reaching effects on the downstream structures of the striatum (caudate, putamen), most studies on striatal volumetric changes have yielded inconsistent results [3,7,11,12]. For example, some studies have reported caudate atrophy in PD compared to control subjects [1,11], whereas others have reported no change [3,7,12,13]. Similarly, there have been reports of putamen atrophy in PD compared to control subjects [7,11,14], but others that have reported no distinction between the groups [13]. These inconsistent findings may be due to small sample sizes, cross sectional design, and/or variability in imaging analysis methods that are highly rater-dependant [1–4].
The lateral ventricles surround both the BG and its downstream (e.g. frontal cortex) structures that may be influenced by PD. The lateral ventricles are large compared to the SN and striatal structures, and the segmentation method is relatively mature, straightforward, and has good reliability [15]. These factors, coupled with recent knowledge of the diffuse nature of the PD process [16], suggested that lateral ventricular volume enlargement may be sensitively associated with PD-related cell loss. Previous results on lateral ventricle volume changes in PD have, again, been equivocal, with some studies reporting increased lateral ventricle volume in PD [1], and others no change [3,4]. Some studies [17,18] have associated lateral ventricular changes with cognitive decline in PD, but not with PD-specific motor changes. One recent case report [19] suggested the possibility of the association of asymmetrical lateral ventricular enlargement with PD-specific motor asymmetry. In order to explore this association further, we collected longitudinal structural MRI data on 17 PD and 15 control subjects to determine whether changes in lateral ventricle volume may encode PD-specific information (i.e., asymmetry and motor progression).
Methods
Study subjects
Seventeen early-stage PD patients [Hoehn and Yahr (HY) stage I–II] [20] and 15 healthy control subjects participated in the study (see Table 1 for detailed demographic information). The shortest follow up visit for PD and control subjects occurred at 12.4 and 11.8 months after baseline, respectively, and the longest at 30.5 and 42.8 months, respectively. The mean time (SD) between scans was 20.3 (5.4) months for PD and 21.0 (8.4) months for control subjects. The time from diagnosis of PD to initial study time had a range of 0–95.8 months. The average UPDRS score for subjects at baseline was 9.4 and that at follow up was 10.8. All PD patients received medical treatment for their PD, which was optimized by a movement disorder specialist (X.H.) during the course of the study. PD subjects were identified through a tertiary-care movement disorders clinic, and all PD subjects met published diagnostic criteria for PD [21]. The study protocol received Institution Review Board approval, complied with all tenets of the Helsinki accords, and written informed consent was obtained from all subjects.
Table 1.
Demographic information for study subjects
| Controls (N=15) | PD (N=17) | P* | |
|---|---|---|---|
| Mean age at Baseline, yr (SD) | 58.2 (12.3) | 59.9 (13.0) | 0.748 |
| Male, n (%) | 11 (73%) | 10 (59%) | 0.259 |
| Right Handedness, n (%) | 14 (93%) | 16 (94%) | 0.274 |
| Right Side PD Symptom, n (%) | n.a. | 11 (65%) | n.a. |
| Mean duration of illness, mo (SD) | n.a | 30.0 (29.3) | n.a |
P-values by Wilcoxon test for baseline age, and Fisher’s exact test for gender and handedness.
All subjects were screened to be free from other major medical illnesses, as well as liver, kidney, or electrolyte abnormality, or B12 or folate deficiency. Mini-Mental Status Exam (MMSE) scores of all study subjects were above 27 at the baseline of the study.
Structural MRI acquisition
The structural MRI for subjects were acquired on a 3.0 T Siemens scanner (Siemens, Erlangen, Germany) with a birdcage type standard quadrature head coil and an advanced nuclear magnetic resonance echoplanar system. The imaging protocol was comprised of a high-resolution T1 weighted anatomical image (3D SPGR, TR=14 ms, TE=7.7 ms, flip angle=25°, voxel dimensions 1.0 × 1.0 × 1.0 mm, 176 × 256 voxels, 160 slices) for each subject.
Image Processing
All MRI analyses were performed at the University of North Carolina Neuro Image Analysis Laboratory (NIAL, UNC-Chapel Hill, NC). All datasets were first processed by a rater-independent, fully-automatic tissue-segmentation method [22] that generates detailed maps of gray matter, white matter, and cerebrospinal fluid (CSF). This processing step includes a correction for mainly RF coil induced intensity inhomogeneities, as well as skull stripping and intensity calibration. A semi-automatic, rater-initialized method was employed to segment the lateral ventricles based on the probabilistic CSF map [23]. The lateral ventricular volume consisted of the lateral ventricles surrounding both the BG and its downstream (e.g. frontal cortex) structures, excluding the temporal horn, third, and fourth ventricles. A detailed protocol is accessible at http://www.ia.unc.edu/dev/tutorials/Documents/UNC-NeuroimagingLab-Manual.pdf. All scans (both baseline and follow up for each subject) were coded with a random number that was not shared with the personnel performing the analysis. As such, personnel were blinded to the time of subjects’ scans (baseline or follow up) or any other personal information concerning the subjects. The coefficient of variation (CV) from this study was less than 1% for the measurements [24]. This level of reliability has not been achieved via pure manual segmentations of brain structures, with the latter generally yielding considerably larger CVs (4–5 times larger than the automated method) [25].
Motor function evaluation for PD subjects
The side of symptom onset was determined by patients’ reports that were all corroborated by motor exam (the symptom onset side remained the more symptomatic side during the course of PD [26]). The motor function in PD subjects was quantified by the United Parkinson’s Disease Rating Scale motor part (UPDRS-III) score that was obtained while the PD patients were on medication. This provided a clinical estimation for the severity of motor function deficits that are not alleviated by medication, a factor that might have relevance to intrinsic brain structural atrophy. The UPDRS was administered to all subjects by the same movement disorders specialist (XH) who also had optimized PD subjects’ medications. All authors involved in volumetric calculations were blind to subject identity until final statistical analyses were performed.
Statistical analysis
Single-point analysis comparing the 17 PD or 15 control subjects at either baseline or follow up was done using t-tests. Longitudinal comparison of the age trajectories for PD subjects was completed using the quadratic growth curve model (PROC MIXED). We then compared the longitudinal trend of lateral ventricle volume changes in 17 PD subjects during disease progression between the contralateral and ipsilateral side relative to PD symptom onset. A random coefficient growth curve model (PROC MIXED) was used to fit the evolving trends with duration of disease. Lateral ventricle volume (in mm3) was entered as the dependent variable, years since diagnosis, quadratic term of years since diagnosis, side (contralateral vs. ipsilateral), and the interaction of time since diagnosis and side were entered as fixed effects. To account for heterogeneity between subjects, the model included a subject-specific intercept and years since diagnosis as random effects with variance-covariance structure of compound symmetry for multiple observations within subjects. A similar analysis was completed for the 15 control subjects with contralateral defined as the side opposite their dominant hand and without the inclusion of the PD-specific terms.
The positive finding of asymmetric atrophy trajectories was followed by ad hoc paired t-tests between contralateral and ipsilateral ventricle volumes at baseline and follow up. Pearson correlation coefficient (r) was calculated for the association between annual change rates in total lateral ventricle volume and rates in UPDRS-III scores. A conservative robust correlation procedure also was employed to evaluate the association while constraining the impact of an influence data point. Statistical analyses were performed with SAS v.9.1 (SAS Institute Inc., Cary, NC).
Results
For control subjects, single-time-point analysis revealed no significant difference between their contralateral and ipsilateral ventricle volumes at either baseline or follow up (see Table 2). In addition, there were no significant differences in the rate of lateral ventricular volumetric changes in normal subjects between ventricles ipsilateral and contralateral to the dominant hand (p=0.312).
Table 2.
Mean volumes for the contralateral and ipsilateral lateral ventricles at baseline and follow up.
| Subjects | Timepoint | Contralateral (mm3)* Mean (SEM) |
Ipsilateral (mm3) Mean (SEM) |
P value** |
|---|---|---|---|---|
| Healthy Controls (N=15) | Baseline | 9393 (1288) | 9071 (1283) | 0.537 |
| Follow up | 9886 (1388) | 9511 (1386) | 0.446 | |
| Parkinson’s disease (N=17) | Baseline | 10530 (1393) | 9277 (1023) | 0.070 |
| Follow up | 11480 (1678) | 9984 (1296) | 0.048 |
For control subjects, contralateral was defined as the side opposite the dominant hand; for PD subjects it was the side opposite to symptom onset side.
P value is based on two tailed paired t-tests.
In contrast, the single-time-point analysis at baseline and follow up visits in PD subjects showed an asymmetric enlargement in lateral ventricles between the contralateral and ipsilateral ventricles relative to side of symptom onset. The paired t-test showed the lateral ventricle on the contralateral side was 1253 (2659.6) mm3 [mean (SEM)] larger than the ipsilateral side (t(16)=1.94, p=0.070) at study baseline. At follow up, the difference increased to 1496 (2875.7) mm3 [mean (SEM), t(16)=2.14, p=0.048; Table 2)].
Analysis of the longitudinal trajectories revealed that PD subjects showed a significantly more rapid ventricular increase contralateral to symptom side onset compared to the ipsilateral side of the brain [F(3, 46.6)=5.00, p=0.004)]. As shown in Figure 1, the enlargement in the lateral ventricle contralateral to symptom side started right after disease diagnosis and continued almost linearly afterwards. The enlargement in the lateral ventricle ipsilateral to symptom side, on the other hand, progressed much slower, with no obvious change in the first 3 years but gradually picked up speed and reached a similar level after 8 years.
Figure 1.
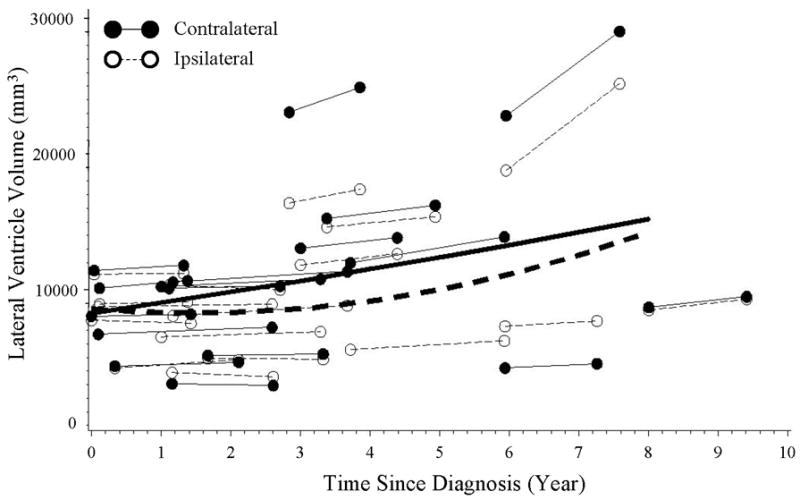
The longitudinal course of lateralized volumetric changes in ipsilateral (open circles, dashed lines) and contralateral (closed circles, solid lines) lateral ventricles relative to side of symptom onset in PD subjects. The equations for the lines are as follows (where DA = duration of illness in years):
Lateral ventricle volumecontra = 8253.6 + 795.78*DA + 13.8*DA2;
Lateral ventricleipsi = 8571.0 − 421.5*DA + 141.2*DA2.
The annual rate of ventricular enlargement was associated with the rate of worsening motor function (while “on” medication), as measured by the UPDRS-III score. The scatter plot of this association is shown in Figure 2, and follows a linear trend with faster ventricle enlargement associated with significantly hastening deterioration in the UPDRS-III score (r=0.96, p<0.001). Even with the exclusion of an influential data point at the far end of the distribution, a conservative robust correlation procedure replicated the significant association with r=0.77, p<0.001.
Figure 2.
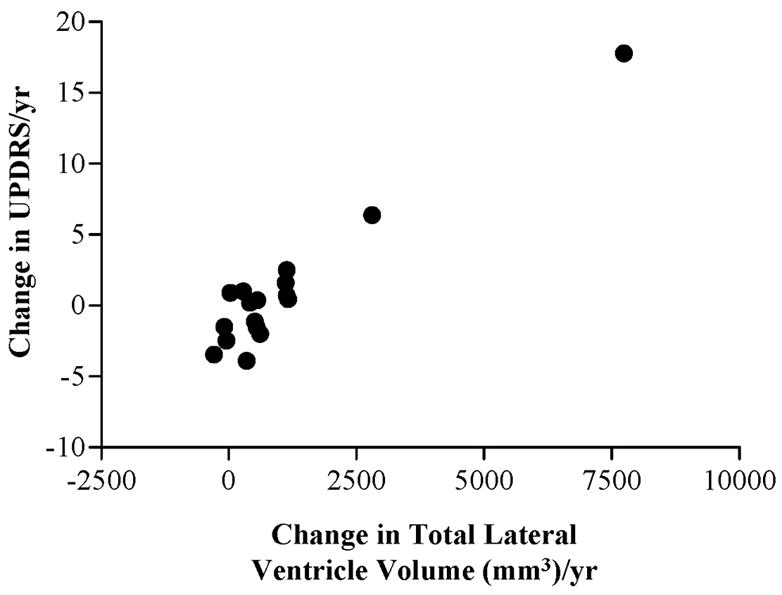
Scatter plot of the association between changes in total ventricular volume and changes in UPDRS-III scores in PD subjects.
Discussion
In this longitudinal structural MRI study we found asymmetrical lateral ventricular enlargement in PD subjects, with faster lateral ventricular enlargement on the contralateral side relative to symptom onset. Such a change was not seen in healthy Control subjects. PD is characterized as an asymmetrical disease, with one side (the side on which symptoms start) always affected more than the other [26,27]. The finding of asymmetrical lateral ventricular enlargement following the same pattern as motor asymmetry in PD is consistent with a prior case report of asymmetrical lateral ventricular enlargement in a pair of discordant monozygotic PD twins [19].
The strength of the study includes longitudinal design, semi-automatic segmentation methods, and using the less affected side of the PD patients as a reference. The results raise interesting questions about the neuroanatomical and pathological mechanisms underlying asymmetric ventricular increase in PD. Moreover, it also raises the possibility of using lateral ventricle volumetric measurements as a possible marker of disease progression.
Ventricular enlargement is often considered a non-specific marker for neurodegeneration and has only been associated with cognitive deficits in PD in the past [18,28–30]. Changes in surrounding structures may contribute to this ventricular enlargement, as some studies have demonstrated decreased volume in hippocampus [2,31], caudate [1,11], putamen [11,32], thalamus [11], and cortex [11,33] in PD subjects, whereas other studies have failed to detect any differences [3,7,12,13]. Neuropathologically, there is clear evidence of a loss of projections to the caudate and putamen in PD [34]. In addition, in PD there is decreased serotonergic, noradrenergic, and cholinergic content [35–38], such that the dysfunction of these neurotransmitters and their associated neuromodulators (trophic factors or peptides) also may cause atrophic changes in regions that surround the lateral ventricles. Although the current study could not address the exact mechanism(s) that contribute to ventricular enlargement, the asymmetry of lateral ventricular enlargement supports the idea that lateral ventricular volume may encode PD-specific information. Ideally, PD subjects would be “off” medication when we assessed the structural lateralization and the lateralized motor subscores. This was not done, however, in the current study for practical reasons, especially the ethical issues in withholding effective medical treatment for long-periods of time. In addition, there is evidence that a true “off” state cannot be achieved with an acceptable period [39].
Instead, the motor end point (UPDRS-III) was obtained with subjects on optimized dopaminergic drug therapy that is known to modulate the lateralized subscores of the UPDRS-III (tremor, rigidity, finger dexterity) [40]. Future studies that record the lateralized motor score in the “off” drug state will be useful to clarify these issues further. Nevertheless, we found a significant association between changes in total lateral ventricular volume and PD motor progression as reflected by changes of total UPDRS motor scores while subjects were “on” medication. Because residual UPDRS-III scores that we measured may reflect a non-dopamine-responsive component of motor dysfunction, this finding suggests that overall lateral ventricular volume changes also may be related to an extranigral, non-dopaminergic-related PD process. This is consistent with a previous report of an association of axial signs of PD motor dysfunction with ventricular volume [4].
Although motor asymmetry is a well-known clinical phenomenon, cognitive asymmetry also has been described in PD. For example, Tomer et al. [41] demonstrated that only PD subjects whose symptoms started on the left, not the right, displayed decreases on the Wisconsin Card Sorting Test. In addition, PD subjects with initial left side symptoms were more impaired on spontaneous flexibility tests (assessed by the Alternate Uses test), whereas initial right side subjects commit more reversal errors. In a study on the effect of left vs. right subthalamic nucleus deep brain stimulation (DBS) on speech, those subjects requiring left side DBS had reduced articulatory accuracy and speaking rates at baseline compared to subjects needing right side DBS [42]. Future studies to correlate the progressions of these non-motor asymmetry symptoms with localized brain atrophy will be especially usefully to guide our understanding of the neuroanatomical and pathological mechanisms underlying the asymmetric ventricular increase in PD.
Structural measures that could reflect disease-specific information may have utility in following disease progression in vivo. First, structural measurements may reveal directly the consequence of cell loss that is not easily modulated by symptomatic treatments. Second, PD is known to have diffuse cell loss beyond the nigrostriatal dopamine system [16] that may not be reflected adequately by limited clinical evaluations focused on one or a few aspects of the disease, or by the simple measurement of nigrostriatal dopaminergic terminal integrity. For these reasons, future studies on the structural substrates contributing to the lateralized ventricular enlargement and their correlation to PD-specific functional changes (motor and non-motor measurements) appear warranted.
In summary, this longitudinal MRI study supports the hypothesis that there are PD-specific structural changes that may be reflected by lateralized ventricular volumetric changes in PD. Further studies (e.g., correlating the structural changes with both motor and non-motor functions in both the “on” and “off” drug states, in both early and later stage PD patients) are warranted to investigate the underlying mechanism(s) of these relationships, as well as the potential of using volumetric measurements as a marker for PD progression.
Acknowledgments
Grant Support: This work was supported in part by NIH grants AG021491 (XH), the UNC GCRCR (RR000046), and the UNC Neurodevelopmental Disorders Research Center (HD003110).
We greatly appreciate the support of the all subjects that consented to participate in this study.
Footnotes
Disclosure: The authors report no conflicts of interest.
Statistical Analysis: Performed by Hongbin Gu4, Ph.D., Hongtu Zhu5, Ph.D., and Yimei Li5, M.S.
REFERENCE LIST
- 1.Brenneis C, Seppi K, Schocke MF, Muller J, Luginger E, Bosch S, et al. Voxel-based morphometry detects cortical atrophy in the Parkinson variant of multiple system atrophy. Mov Disord. 2003 Oct;18(10):1132–8. doi: 10.1002/mds.10502. [DOI] [PubMed] [Google Scholar]
- 2.Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, et al. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996 Mar;46(3):678–81. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 3.Cordato NJ, Pantelis C, Halliday GM, Velakoulis D, Wood SJ, Stuart GW, et al. Frontal atrophy correlates with behavioural changes in progressive supranuclear palsy. Brain. 2002 Apr;125(Pt 4):789–800. doi: 10.1093/brain/awf082. [DOI] [PubMed] [Google Scholar]
- 4.Acharya HJ, Bouchard TP, Emery DJ, Camicioli RM. Axial signs and magnetic resonance imaging correlates in Parkinson’s disease. Can J Neurol Sci. 2007 Feb;34(1):56–61. doi: 10.1017/s0317167100005795. [DOI] [PubMed] [Google Scholar]
- 5.Oppenheimer DR. Diseases of the basal ganglia, cerebellum and motor system. In: Adams JH, Corsellis JAN, Duchen LW, editors. Greenfield’s Neuropathology. 4. New York: Wiley; 1984. [Google Scholar]
- 6.Berg D, Hochstrasser H, Schweitzer KJ, Riess O. Disturbance of iron metabolism in Parkinson’s disease -- ultrasonography as a biomarker. Neurotox Res. 2006 Jan;9(1):1–13. doi: 10.1007/BF03033302. [DOI] [PubMed] [Google Scholar]
- 7.Geng DY, Li YX, Zee CS. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson’s disease. Neurosurgery. 2006 Feb;58(2):256–62. doi: 10.1227/01.NEU.0000194845.19462.7B. [DOI] [PubMed] [Google Scholar]
- 8.Seppi K, Schocke MF, Esterhammer R, Kremser C, Brenneis C, Mueller J, et al. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the parkinson variant of multiple system atrophy. Neurology. 2003 Mar 25;60(6):922–7. doi: 10.1212/01.wnl.0000049911.91657.9d. [DOI] [PubMed] [Google Scholar]
- 9.Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, et al. [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology. 1996 Jan;46(1):231–7. doi: 10.1212/wnl.46.1.231. [DOI] [PubMed] [Google Scholar]
- 10.Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen AG, van Royen EA, et al. Iodine-123-N-omega-fluoropropyl-2beta-carbomethoxy-3beta-(4-iod ophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease. J Nucl Med. 1998 Jul;39(7):1143–8. [PubMed] [Google Scholar]
- 11.Lisanby SH, McDonald WM, Massey EW, Doraiswamy PM, Rozear M, Boyko OB, et al. Diminished subcortical nuclei volumes in Parkinson’s disease by MR imaging. J Neural Transm Suppl. 1993;40:13–21. [PubMed] [Google Scholar]
- 12.Almeida OP, Burton EJ, McKeith I, Gholkar A, Burn D, O’Brien JT. MRI study of caudate nucleus volume in Parkinson’s disease with and without dementia with Lewy bodies and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16(2):57–63. doi: 10.1159/000070676. [DOI] [PubMed] [Google Scholar]
- 13.Schulz JB, Skalej M, Wedekind D, Luft AR, Abele M, Voigt K, et al. Magnetic resonance imaging-based volumetry differentiates idiopathic Parkinson’s syndrome from multiple system atrophy and progressive supranuclear palsy. Ann Neurol. 1999 Jan;45(1):65–74. [PubMed] [Google Scholar]
- 14.Krabbe K, Karlsborg M, Hansen A, Werdelin L, Mehlsen J, Larsson HB, et al. Increased intracranial volume in Parkinson’s disease. J Neurol Sci. 2005 Dec 15;239(1):45–52. doi: 10.1016/j.jns.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Gouttard S, Styner M, Joshi S, Smith RG, Hazlett HC, Gerig G. Subcortical structure segmentation using probabilistic atlas priors. 2007. pp. 37–46. [Google Scholar]
- 16.Lang AE. The progression of Parkinson disease: a hypothesis. Neurology. 2007 Mar 20;68(12):948–52. doi: 10.1212/01.wnl.0000257110.91041.5d. [DOI] [PubMed] [Google Scholar]
- 17.Huber SJ, Shuttleworth EC, Christy JA, Chakeres DW, Curtin A, Paulson GW. Magnetic resonance imaging in dementia of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1989 Nov;52(11):1221–7. doi: 10.1136/jnnp.52.11.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu MT, White SJ, Chaudhuri KR, Morris RG, Bydder GM, Brooks DJ. Correlating rates of cerebral atrophy in Parkinson’s disease with measures of cognitive decline. J Neural Transm. 2001;108(5):571–80. doi: 10.1007/s007020170057. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Lee Y, Mckeown M, Gerig G, Gu H, Lin W, et al. Asymmetrical ventricular enlargement in Parkinson’s disease. Mov Disord. 2007;22(11):1657–60. doi: 10.1002/mds.21626. [DOI] [PubMed] [Google Scholar]
- 20.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 21.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson’s disease. Adv Neurol. 1990;53:245–9. [PubMed] [Google Scholar]
- 22.Van LK, Maes F, Vandermeulen D, Suetens P. Automated model-based bias field correction of MR images of the brain. IEEE Trans Med Imaging. 1999 Oct;18(10):885–96. doi: 10.1109/42.811268. [DOI] [PubMed] [Google Scholar]
- 23.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006 Jul 1;31(3):1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Gouttard S, Styner M, Joshi S, Smith RG, Cody H, Gerig G. Subcortical structure segmentation using probabilistic atlas priors. SPIE Medical Imaging. 2007 In press. [Google Scholar]
- 25.Styner M, Charles C, Park J, Gerig G. Multisite validation of image analysis methods -Assessing intra and inter site variability. Proc SPIE Medical Imaging. 2002;4684:278–86. [Google Scholar]
- 26.Lee CS, Schulzer M, Mak E, Hammerstad JP, Calne S, Calne DB. Patterns of asymmetry do not change over the course of idiopathic parkinsonism: implications for pathogenesis. Neurology. 1995 Mar;45(3Pt 1):435–9. doi: 10.1212/wnl.45.3.435. [DOI] [PubMed] [Google Scholar]
- 27.Lee CS, Schulzer M, de l F-F, Mak E, Kuramoto L, Sossi V, et al. Lack of regional selectivity during the progression of Parkinson disease: implications for pathogenesis. Arch Neurol. 2004 Dec;61(12):1920–5. doi: 10.1001/archneur.61.12.1920. [DOI] [PubMed] [Google Scholar]
- 28.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007 Mar;78(3):254–9. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton EJ, McKeith IG, Burn DJ, Williams ED, OBrien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004 Apr;127(Pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 30.Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005 Jan 25;64(2):224–9. doi: 10.1212/01.WNL.0000149510.41793.50. [DOI] [PubMed] [Google Scholar]
- 31.Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson’s disease is associated with hippocampal atrophy. Mov Disord. 2003 Jul;18(7):784–90. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- 32.Atasoy HT, Nuyan O, Tunc T, Yorubulut M, Unal AE, Inan LE. T2-weighted MRI in Parkinson’s disease; substantia nigra pars compacta hypointensity correlates with the clinical scores. Neurol India. 2004 Sep;52(3):332–7. [PubMed] [Google Scholar]
- 33.O’Neill J, Schuff N, Marks WJ, Jr, Feiwell R, Aminoff MJ, Weiner MW. Quantitative 1H magnetic resonance spectroscopy and MRI of Parkinson’s disease. Mov Disord. 2002 Sep;17(5):917–27. doi: 10.1002/mds.10214. [DOI] [PubMed] [Google Scholar]
- 34.Waters CM, Peck R, Rossor M, Reynolds GP, Hunt SP. Immunocytochemical studies on the basal ganglia and substantia nigra in Parkinson’s disease and Huntington’s chorea. Neuroscience. 1988 May;25(2):419–38. doi: 10.1016/0306-4522(88)90249-7. [DOI] [PubMed] [Google Scholar]
- 35.Rinne UK, Riekkinen P, Sonninen V, Laaksonen H. Brain acetylcholinesterase in Parkinson’s disease. Acta Neurol Scand. 1973;49(2):215–26. doi: 10.1111/j.1600-0404.1973.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd KG, Mohler H, Heitz P, Bartholini G. Distribution of choline acetyltransferase and glutamate decarboxylase within the substantia nigra and in other brain regions from control and Parkinsonian patients. J Neurochem. 1975 Dec;25(6):789–95. doi: 10.1111/j.1471-4159.1975.tb04409.x. [DOI] [PubMed] [Google Scholar]
- 37.Reisine TD, Fields JZ, Yamamura HI. Neurotransmitter receptor alterations in Parkinson’s disease. Life Sci. 1977 Aug 1;21(3):335–43. doi: 10.1016/0024-3205(77)90514-8. [DOI] [PubMed] [Google Scholar]
- 38.Marsden CD, Parkes JD. Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet. 1977 Feb 12;1(8007):345–9. doi: 10.1016/s0140-6736(77)91146-1. [DOI] [PubMed] [Google Scholar]
- 39.Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol. 2005 Oct;252(Suppl 4):IV37–IV42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 40.Wolters EC, Francot C, Bergmans P, Winogrodzka A, Booij J, Berendse HW, et al. Preclinical (premotor)Parkinson’s disease. J Neurol. 2000 Apr;247(Suppl 2):II103–II109. [PubMed] [Google Scholar]
- 41.Tomer R, Aharon-Peretz J, Fisher T, Shamay-Tsoory SG. Differential pattern of cognitive decline in Parkinson’s patients with left- vs. right-sided onset of symptoms. Mov Disord. 2001;16(Suppl 1):S25. [Google Scholar]
- 42.Wang EQ, Metman LV, Bakay RAE, Arzbaecher J, Bernard B, Corcos DM. Hemisphere-specific effects of subthalamic nucleus deep brain stimulation on speaking rate and articulatory accuracy of syllable repetitions in Parkinson’s disease. Journal of Medical Speech-Language Pathology. 2006 Dec;14(4):323–33. [PMC free article] [PubMed] [Google Scholar]


