Abstract
Liquid-ordered (Lo) and liquid-disordered (Ld) phase coexistence has been suggested to partition the plasma membrane of biological cells into lateral compartments, allowing for enrichment or depletion of functionally relevant molecules. This dynamic partitioning might be involved in fine-tuning cellular signaling fidelity through coupling to the plasma membrane protein and lipid composition. In earlier work, giant plasma membrane vesicles, obtained by chemically induced blebbing from cultured cells, were observed to reversibly phase segregate at temperatures significantly below 37 °C.
In this contribution, we compare the temperature dependence of fluid phase segregation in HeLa and Rat basophilic leukemia (RBL) cells. We find an essentially monotonic temperature dependence of the number of phase separated vesicles in both cell types. We also observe a strikingly broad distribution of phase transition temperatures in both cell types. The binding of peripheral proteins, such as cholera toxin subunit B (CTB), as well as annexin V, is observed to modulate phase transition temperatures, indicating that peripheral protein binding may be a regulator for lateral heterogeneity in vivo. The partitioning of numerous signal protein anchors and full length proteins is investigated. We find Lo phase partitioning for several proteins assumed in the literature to be membrane raft associated, but observe deviations from this expectation for other proteins, including caveolin-1.
Keywords: Liquid-ordered, liquid-disordered, bleb, giant plasma membrane vesicle, phase transition, phase partitioning
Introduction
The membrane raft hypothesis assumes lateral lipid membrane heterogeneity to critically influence cellular functions involving signaling, sorting, and trafficking [1, 2]. This hypothesis evolved from findings from biochemical assays based on the detergent resistance of membrane components at low temperature (4 °C). However, it is becoming increasingly understood and recognized that detergents [3, 4], as well as temperature [5, 6], crucially influence membrane phase behavior (related to lateral heterogeneity). These findings have challenged the field of research on biologically relevant membrane heterogeneity [7, 8].
Lateral segregation of plasma membrane components is often described within the context of Lo and Ld type membrane phases [9, 10]. The physicochemical basis of Lo/Ld phase coexistence has been investigated extensively in research based on membranes self-assembled from defined mixtures of synthetic or purified lipids [11–15]. It is increasingly being appreciated that non-ideal mixtures of lipids (i.e. mixtures where lipids show non-random intermolecular interactions) at temperatures even above any mixing/demixing transition temperatures can show heterogeneity (dynamic compositional fluctuations) below optical resolution [16, 17]. Importantly, closer to a critical mixing/demixing temperature of Lo/Ld phase coexistence, both model membranes [18] and cell-derived membranes [5] can show microscopically visible composition fluctuations, demanding their occurrence at higher temperatures with sub-microscopic length scales [5, 18].
While membranes obtained from synthetic lipids are helpful in understanding fundamental aspects of membrane biophysics, only limited inference can be made regarding any functional aspects of biological membranes. Undoubtedly, protein/protein interactions are important contributors to plasma membrane heterogeneity [19, 20], and specific protein/protein and protein/lipid interactions may define various classes of membrane microdomains. The incorporation of complex membrane signaling machineries into self-assembled membranes, however, remains challenging. In order to circumvent protein purification and reconstitution into model membranes, giant plasma membrane vesicles (GPMVs) were recently introduced as plasma membrane models for studying cellular membrane phase behavior [6]. GPMVs consist of plasma membranes unsupported by cortical actin, and are obtained by chemical induction of membrane blebbing [6]. GPMVs were observed to phase segregate in a temperature dependent manner [6] into two coexisting fluid membrane phases with Lo- and Ld-like character, based on the partitioning of a set of Lo/Ld discriminating membrane fluorophores [21]. Such vesicles have earlier been demonstrated to preserve the lipid composition of the plasma membrane[22], as well as to contain numerous functionally relevant proteins [6, 23]. More recently, the protein content of GPMVs has been analyzed by proteomics techniques. It was found that 93% of GPMV membrane protein content is plasma membrane proteins, with the remainder consisting of intracellular membrane proteins [24]. This further indicates that membrane compositions of GPMVs are similar to cellular plasma membranes. A previous study has furthermore shown that a fluorescent version of the PIP2 binding pleckstrin homology (PH) domain is found to be membrane associated in GPMVs [23]. This observation emphasizes that GPMVs contain a sufficient amount of PIP2 to allow membrane anchoring of PIP2 binding proteins.
GPMVs have already enabled the re-investigation of Lo phase partitioning of several membrane associated proteins [6, 23] and the characterization of the partitioning of lipid dyes among Lo-like and Ld-like phases.
The present contribution is organized as follows. We first investigate the temperature dependence of the phase behavior of GPMVs obtained from both HeLa and Rat basophilic leukemia (RBL-2H3) cells. We also investigate the effects of peripherally binding proteins on membrane heterogeneity and observe that peripheral protein binding can significantly shift the phase behavior. We proceed with an investigation of the phase partitioning preference of several signaling proteins, their membrane anchors, and mutants of these anchors. We find significant Lo phase partitioning of Lck anchors, as well as of the transmembrane protein hemagglutinin, consistent with predictions from the membrane raft hypothesis. Conversely, we observe preferential Ld phase partitioning of the proteins caveolin, H-ras, and Fyn.
Materials and Methods
Cells
HeLa cells were obtained from G. Kao (University of Pennsylvania). Rat basophilic leukemia (RBL-2H3) cells, a mast cell model, were obtained from the Baird lab (Cornell University). RBL cells represent an extensively studied cell line to investigate lipid domain involvement in receptor signaling [25, 26].
Blebbing procedures
GPMVs were obtained as previously described [6], with modifications mentioned below. Briefly, all cell lines were grown as monolayer cultures in a 5% CO2 atmosphere at 37 °C. RBL cells were cultured in minimum essential medium containing 10% fetal bovine serum (FBS). HeLa cells were grown in Dulbecco’s modified Eagle medium with 10% FBS. Approximately 3–4 days after passage, cells grown to 80% confluency were washed with GPMV (“blebbing”) buffer composed of 2 mM CaCl2, 10 mM Hepes, and 0.15 M NaCl at pH 7.4, to which formaldehyde (HCHO) and dithiothreitol (DTT) at final concentrations of 25 mM and 2 mM, respectively, were added prior to washing. After three consecutive washing steps, the cell monolayer was covered with a thin layer of GPMV buffer (2 mL per culture flask with an area of 25 cm2 for non-transfected cells), and 0.7 mL per well of a six-well plate (area ~ 7 cm2) in case of transfected cells, and shaken at 60 RPM and 37 °C for 1 h (RBL cells) and 2.5 h (HeLa cells), respectively. Following incubation, GPMVs that had separated from the cell monolayer were gently decanted into a 15 mL conical tube and stored at 4 °C to settle. After 30 minutes, 20% of the sample was transferred from the bottom of the conical tube into a 1 mL Eppendorf tube, of which 50 µL aliquots were stained at room temperature and imaged as described below. The GPMV yield was ≈ 200–500 vesicles per 5µL sample used for microscopy. An alternative protocol substituting 2 mM N-ethylmaleimide for HCHO and DTT was used to confirm that chemical agents did not affect the results obtained [6].
Commercial fluorescent proteins and lipids
AlexaFluor488-Cholera Toxin Subunit B (CTB, Invitrogen, Carlsbad, CA) was added in amounts of 0.5 µL from a stock solution (0.2 mg/mL in phosphate buffered saline (PBS)) per 50 µL GPMV dispersion. Cholera Toxin binds the ganglioside GM1, a lipid that has been observed to target Lo membrane phases of GPMVs [6].
AlexaFluor555-Annexin V Conjugate (A35108, Invitrogen) was added to GPMVs after blebbing. Annexin-bound phosphatidylserine (PS) has previously been shown to be Ld preferring [6]. 0.50 µL of the fluorophore stock solution (100 assays per 500 µL) was used per 40 µL GPMV dispersion.
1,2- dipalmitoyl-sn-glycero-3-phospho-ethanol-amine-x-Texas red (TR, Invitrogen) was added from a stock solution (200 µg/mL dye/methanol). 0.50 µL of this solution was used per 40–50 µL GPMV aliquot. In a small number of experiments, instead of a methanol solution, we used a previously developed bovine serum albumin (BSA) shuttling approach[27] to label GPMVs, and we did not find measurable differences in the temperature dependent phase behavior comparing these two approaches.
In the present contribution we use TR to identify Ld phases. This probe choice is based on the fact that in model membrane research, TR has been shown to preferentially partition into Ld phases, as opposed to Lo phases [28, 29]. Furthermore, the Texas red emission spectrum leads to reduced overlap with green dye emission spectra compared to rhodamine dyes [30] that we have previously used as Ld markers [6]. GPMVs, however, represent a significantly more complex lipid environment compared to ternary model membrane mixtures, and fluorophore membrane phase partitioning preference depends not only on phase state, but also on particular thermodynamic properties of co-existing phases [31, 32]. We therefore compared the partitioning of TR in HeLa and RBL cell blebs to the partitioning of molecules that previously were observed to display unique Ld phase preference in RBL cell blebs (such as the GFP-labeled acyl chain anchor palmitoyl-myristoyl (PM-GFP) [6], as well as molecules with Lo phase preference, such as GPI anchors [23] and CTB [6]. This comparison (data not shown) confirmed exclusive Ld phase partioning of TR in HeLa and RBL cell blebs. We furthermore note that through diffusion measurements a related fluorophore (rhodamine-stearoyl-oleyol-phosphatidylethanolamine) recently was shown to be Ld phase preferring in fibroblast blebs [33], Proteins. FYN-eGFP, CD59-eGFP, the H-ras anchor H-ras-eGFP (Clontech) consisting of the 20 amino acid farnesylation signal of c-Ha-ras, the glycosyl phosphatidyl inositol anchored eGFP construct, GPI-eGFP, and a hemagglutinin eGFP fusion protein, HA-eGFP, were obtained from A. Kenworthy (Vanderbilt University). Hras-eGFP was obtained from M. Philips (New York University). N- and C-terminally eGFP tagged caveolin-1 (Cav-eGFP and eGFP-Cav, respectively) were from A. Helenius (ETH Zurich). Lck and Fyn eGFP anchor constructs (in a pCMV5 vector) were obtained from L.G. Berthiaume (University of Alberta). These constructs consisted of the first ten N-terminal amino acids of the wild type protein plus a seven-amino acid linker corresponding to amino acids TKLTEER [34] (see Table 2 and Table 3).
Table 2.
Lck protein anchor variants and phase partitioning preferences. Assignment of phase partitioning followed the same principles as explained in the materials and methods section.
| Anchor Constructs |
Amino acid sequence | Charge | Phase preference |
Ld | Lo | NP |
|---|---|---|---|---|---|---|
| Lck wild type |
(M) GCGCSSHPEDTKLTEER | −2 | Ld and NP | 35% | 30% | 35% |
| Lck neutral | (M) GCGCSSHPQNTKLTEER | 0 | NP | 21% | 12% | 67% |
| Lck all neutral |
(M) GCGCSSHPQNTQLTQQQ | 0 | NP | 29% | 29% | 42% |
| Lck(−2) | (M) GCGCSSHPEDTQLTQQQ | −2 | Ld | 50% | 23% | 27% |
| Lck(+2) | (M) GCGCSSHPKKTKLTEER | +2 | Ld | 81% | 3% | 16% |
| LckG4V | (M) GCVCSSHPEDTKLTEER | −2 | Ld | 67% | 11% | 22% |
| LckG4VH8N | (M) GCVCSSNPEDTKLTEER | −2 | Ld and NP | 57% | 0% | 43% |
| LckG4VH8R | (M) GCVCSSRPEDTKLTEER | −1 | Ld and NP | 41% | 9% | 50% |
| LckH8N | (M) GCGCSSNPEDTKLTEER | −2 | Lo | 14% | 54% | 32% |
| LckH8R | (M) GCGCSSRPEDTKLTEER | −1 | Lo and NP | 8% | 50% | 42% |
| Lck CGGC | (M) GCGGCSSHPEDTKLTEER | −2 | Cytosolic | - | - | - |
Table 3.
Fyn protein anchor variants and phase partitioning preferences. Assignment of phase partitioning followed the same principles as explained in the materials and methods section.
| Anchor Constructs |
Amino acid sequence | Charge | Phase preference |
|---|---|---|---|
| Fyn wild type | (M) GCVQCKDKEATKLTEER | 0 | >90 % Ld |
| Fyn neutral linker | (M) GCVQCKDKEATQLTQQQ | 0 | > 90 % Ld |
| Fyn neutral | (M) GCVQCQNQQATKLTEER | 0 | > 90 % Ld |
| Fyn(−2) | (M) GCVQCKDEEATKLTEER | −2 | > 90 % Ld |
| Fyn(−1) | (M) GCVQCKDKEATQLTEER | −1 | > 90 % Ld |
| Fyn(+4) | (M) GCVQCKKKKATKLTEER | +4 | > 90 % Ld |
| Fyn(−4) | (M) GCVQCEDEEATKLTEER | −4 | Cytosolic |
| Fyn all neutral | (M) GCVQCQNQQATKQLTQQQ | 0 | Cytosolic |
| LckFyn | (M) GCVCKDKEATKLTEER | 0 | Cytosolic |
| LckFynV4G | (M) GCGCKDKEATKLTEER | 0 | Cytosolic |
Mutagenesis
Fyn and Lck anchor constructs in pCMV5 vectors were observed to show lower expression levels compared to similar constructs expressed by means of a pEGFP vector. To obtain higher expression levels, Fyn anchor sequence was cloned into the pEGFP (Clontech) vector. The resulting plasmid was used as a template for the construction of the Fyn and Lck mutants. Mutagenesis was performed by means of a standard protocol using the QuikChange Site Directed Mutagenesis kit (Stratagene) for primers of length smaller than 45 bases. For mutations needing longer primers, a modified protocol including separate primer extension reactions for forward and reverse primers was performed [35]. All constructs described in the present work contained the eGFP A206K mutation that reduces the tendency of GFP to dimerize at high concentrations [36]. Comparison of the same anchor constructs with and without the A206K mutations did not reveal measurable partitioning differences (data not shown).
HeLa cells were transiently transfected with fusion protein plasmids using Lipofectamine (Invitrogen), according to the supplier’s instructions. RBL cells were transfected by a modified procedure including incubation for 1 h with 0.1 µL phorbol 12,13-dibutyrate (Sigma Aldrich) as described [37].
Imaging
GPMVs were imaged immediately after preparation. Room temperature measurements were conducted on standard microscope slides with coverslips. 5µL of sample was enclosed within a border of vacuum grease totaling approximately 2.5 cm2 in area. Fluorescence imaging at additional temperatures was performed by encasing 5 µL of sample between two circular cover slips in a vacuum grease “cage” (ring) enclosing an area of roughly 2.0 cm2. The two coverslips were then sealed with clear nail polish (Maybelline: New York Express Finish Advanced Wear #10). A stainless steel disk was then super-glued to the back of the sample for convenient mounting on the microscope stage via a suspended magnetic rod. Room temperature samples were imaged through a water immersion objective (60×, 1.2 NA, Olympus). Temperature-controlled samples were submerged via magnetic rod into a small water bath (the size of a small Petri dish) that was mounted onto the microscope objective (60×, 0.9NA, Olympus). Both the water bath and the objective were temperature regulated by means of tubing that was thermostated via a circulating, temperature-controlled water bath (temperature fluctuations were below ± 0.2 °C). Temperatures were measured near the sample with a thermocouple (type K, Fisher Scientific). Alternatively to the method described above, temperature was regulated using a peltier device regulated by a commercial temperature controller (temperature fluctuations below ± 0.1 °C. Model: PTC 5 K-CH, Wavelength Electronics, Bozeman, MT). GPMVs were imaged using a confocal laser scanning imaging system (IX81, Fluoview 1000, Olympus, Bethlehem, PA). Fluorophores were excited at wavelengths of λ = 488 nm and λ = 543 nm.
The fraction of phase-separated GPMVs was determined at each temperature by counting the number of phase-separated GPMVs versus homogenous GPMVs in single fields of view (FoV). Numerous FoVs were observed for each temperature measurement and averaged. Typically, 50 vesicles were imaged and counted to determine both the temperature dependence of phase-coexistence and phase partitioning preference of proteins. For quantitative analysis of fluorescence intensity ratios, a smaller subset of high quality images (e.g. those with low background intensity, large vesicles, low noise levels) were selected. Protein partitioning was always determined at room temperature (22 ± 1 C°). For each protein, partitioning data were obtained from at least three independent GPMV preparations from different cell culture flasks.
Quantitative image analysis to determine protein partitioning
Fluorescence intensities in brighter and darker regions of phase-separated, randomly chosen GPMVs were determined by averaging the fluorescence intensities in a square-shaped region of interest (ROI) on four randomly chosen positions in each brighter and each darker membrane phase. Vesicles which showed no detectable phase coexistence at optical resolution were excluded from analysis. Background levels, obtained from averaging fluorescence intensities measured using the software ImageJ (NIH, Bethesda, MD) in eight ROIs near the GPMV circumference were subtracted. For every given vesicle, all ROIs had the same size of ≈ 1 µm2. The phase state was assigned by the partitioning of TR. The degree of apparent Lo phase partitioning was defined as the base-10 logarithm of the Lo-phase versus Ld-phase fluorescence intensity ratio. We assigned a protein to be Lo-phase partitioning if this value exceeded 0.15, and Ld-phase partitioning for a value below that of −0.15. These boundaries correspond to a fluorescence intensity ratio of ≈1.4. This fluorescence intensity ratio refers roughly to the minimum intensity difference that could be detected by visually inspecting the protein channel of our images. Non-preferential protein partitioning was defined as a value in between and including these two boundaries. We emphasize that values defined as such do not necessarily directly correspond to thermodynamic partition coefficients, due to reasons inherent in the fluorescence approach used here [21]. Quantitative data were summarized by means of histograms [38], of which we show three representative examples in Figure 2. Protein anchors were assigned a partitioning preference (Ld, Lo, or NP) if more than 1/3 of the counted vesicles showed the respective partitioning preference as quantitatively defined above.
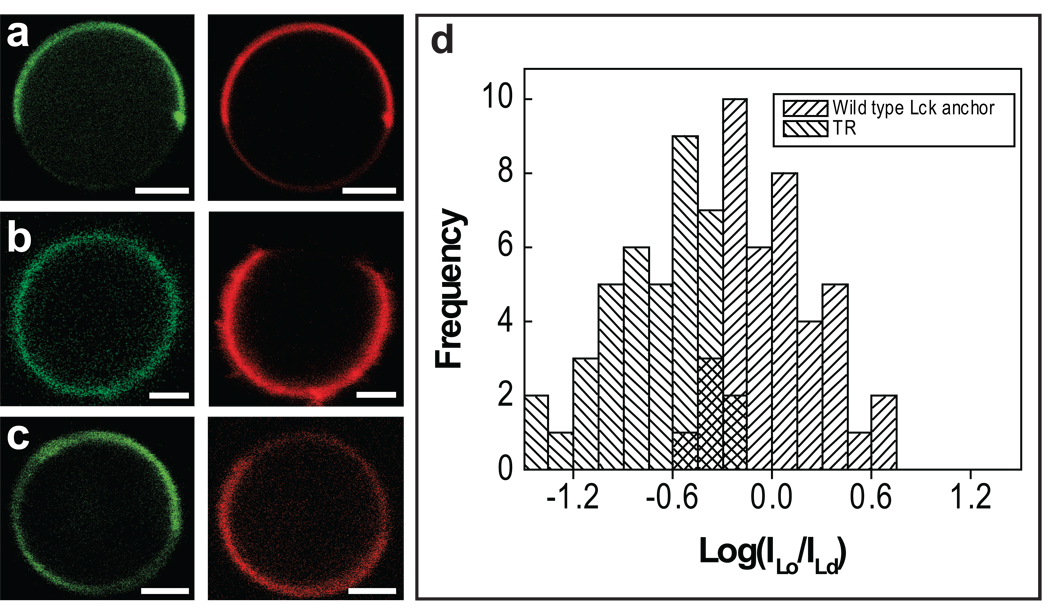
Figure 2.
Variable partitioning of wild type Lck anchor eGFP construct. (a)–(c): Representative images of vesicles displaying preferential Ld phase partitioning (a), non-preferential partitioning (b), and preferentially ordered phase partitioning (c) wt Lck anchors (green channel, left) in HeLa cell giant plasma membrane vesicles labeled with the lipid dye TR. Scale bars: 2 µm. (d) Histogram of fluorescence intensity ratios of Lo phase versus Ld phase fluorescence, comparting protein and lipid dye. Fluorescence intensity ratios are shown as base-10 logarithmic values. The broad histogram reflects the variable partitioning behavior shown in the fluorescence images.
Results
I) Temperature dependence of fluid phase coexistence
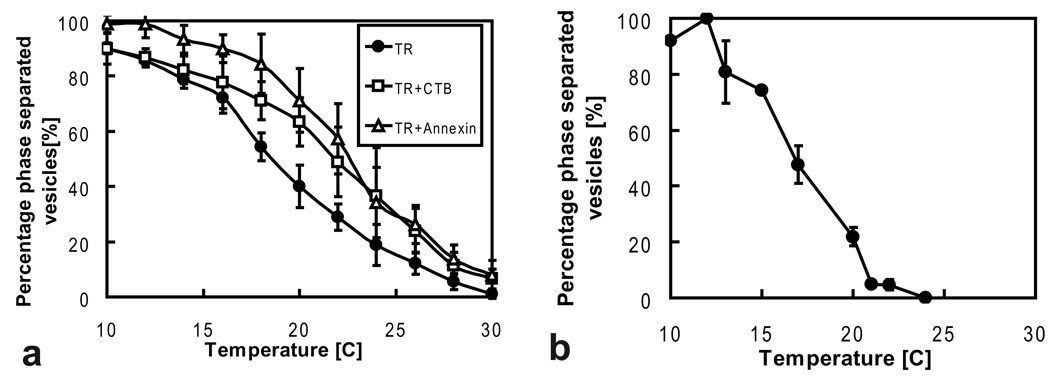
Giant plasma membrane vesicles from HeLa and RBL cells were prepared from cells grown in adhesion culture and induced to bleb as described in the materials and methods section. We first investigated the effect of temperature on the fraction of phase separated vesicles. Figure 1 indicates that GPMVs labeled with TR of HeLa (Figure 1a) and RBL cells (Figure 1b) phase segregate in a temperature-dependent manner. Similar temperature dependence of the fraction of phase separated vesicles is observed both for HeLa and RBL cells. Single GPMVs displayed a sharp phase transition (i.e. with a temperature spread smaller than our measurement uncertainty), and heating / cooling cycles indicate absence of measurable hysteresis effects under the conditions of our experiments (temperature accuracy ± 0.2 °C, scanning speed ≈ 1 °C/min). Also deduced from Figure 1 is a large range of phase transition temperatures. We observe an essentially monotonic temperature dependence (consistent with results presented in Ref. [5]) of the fraction of phase separated vesicles. GPMVs phase separated over a temperature range of roughly 10 – 30 °C. Because the phase behavior of HeLa and RBL cell GPMVs was sensitively dependent on cellular growth conditions (confluency, time after last medium exchange), care had to be taken to perform all blebbing experiments under standardized conditions (see materials and methods section).
Figure 1.
Temperature dependence of phase coexistence in HeLa and RBL cell bleb membranes. (a) HeLa cells were labeled either with the lipid fluorophore TR, or with both TR and either CTB or annexin V. In all cases, the fraction of phase-separated membrane vesicles is observed to depend essentially monotonically on temperature, with a broad spread of transition temperatures. Both the addition of CTB and Annexin V raise transition temperatures relative to those GPMVs that were labeled with TR only. The effect of Annexin V on phase behavior appears to be slightly larger at low temperatures compared to CTB, although the error bars overlap. (b) RBL cell blebs were labeled with TR. Similar temperature-dependent phase behavior is found compared to HeLa cell blebs labeled with TR.
In order to examine whether the large range of mixing-demixing transition temperatures was due to the fact the cells shed GPMVs at varying times after initiation of the blebbing process, we harvested GPMVs from cell culture monolayers in half-hour intervals. Factors which would contribute to such a time-dependence include potential time-dependent plasma membrane compositional changes. After each time interval, the GPMV-containing blebbing buffer was collected and replaced by fresh blebbing buffer. GPMVs were examined immediately for their temperature dependent mixing-demixing properties. No dependence (beyond sample-to-sample variations, see Figure 1) of blebbing time on phase behavior could be identified with this experiment (data not shown).
We furthermore observed GPMV phase behavior to be influenced by peripheral protein binding. In particular, cholera toxin subunit B (CTB), which binds to the ganglioside GM1, and annexin V, which binds to scrambled (i.e. leaflet randomized) phosphatidylserine, led to an increase in the fraction of phase separated vesicles, compared to GPMVs that were labeled with TR only. This influence is observed when comparing vesicles labeled with TR in combination with either annexin V or CTB to GPMVs labeled with TR only (Figure 1a). This finding confirms earlier results in model membranes, where cross-linking of GM1 by CTB raised phase transition temperatures[39].
The effects of peripherally binding proteins on membrane phase behavior were similar in HeLa cell and in RBL cell GPMVs (not shown).
II) Protein partitioning in GPMVs
In previous contributions, the phase partitioning of several membrane proteins was examined in RBL cell GPMVs [6, 23]. Here we describe the partitioning of additional proteins, including transmembrane proteins, as well as outer and inner leaflet acyl chain anchored proteins, and short peptide chains with eGFP anchors in HeLa cells.
II.1) Partitioning of wild type protein anchors
In order to focus on the contribution of the membrane anchor (rather than protein/protein interactions) of signaling proteins such as Src-like protein tyrosine kinases [34], we investigated short N-terminal sequences of signaling proteins containing myristoylation and palmitoylation sites that were C-terminally labeled with eGFP. It has already been observed that anchors with a single palmitoylation site and a single myristoyl chain (PM-eGFP and Lyn-eGFP) show preference for Ld phases [6]. Below we will test the hypothesis that anchors with an additional palmitoylation site increase the Lo phase partitioning preference in phase separated GPMVs. We furthermore examine to what extent mutations in the amino acid sequence of protein anchors affect partitioning.
Previous reports based on fluorescence quenching and detergent resistance assays applied to ternary lipid mixtures of DPPC, DOPC, and cholesterol had reported Lo phase and detergent resistant membrane (DRM) partitioning preference of membrane anchors similar to those of the Src-like protein tyrosine kinases Lck and Fyn [40]. We therefore examined the partitioning of their anchor sequences in GPMVs. Both of these membrane anchors contain one myristoyl and typically two palmitoyl groups that are post-translationally attached, resulting in plasma membrane association [34].
Protein partitioning was quantified by measuring the fluorescence intensity ratios in Lo versus Ld phases, as described in the methods section. Table 1 summarizes the phase partitioning of the Lck wild type (wt) anchor, the sequence of which is shown in Table 2. As shown in the fluorescence images (Figure 2a–c) and the histogram comparing fluorescence intensity ratio distributions of wt Lck and TR (Figure 2d), this protein anchor displayed variable partitioning ranging from Ld (Figure 2a) over non-preferential (Figure 2b) to Lo preference (Figure 2c). All images in this manuscript show fluorescence from the protein chimera in the left panel, and TR fluorescence in the right panel. Variable phase partitioning was a common observation that likely indicates compositional differences among GPMVs obtained from the same cell culture flask, a hypothesis which is in line with the dispersity of measured phase transition temperatures (Figures 1). Our observations suggest that cells can effectively regulate the phase preference of signaling molecules by carefully tuning plasma membrane compositions.
Table 1.
Membrane phase partitioning of signal protein anchors and of several full length proteins. Partitioning was analyzed as described in the materials and methods section.
| Anchor Construct/Protein |
Phase preference | Ld | Lo | NP |
|---|---|---|---|---|
| Lck anchor | NP | 35% | 30% | 35% |
| Fyn anchor | Ld | >90% | ||
| H-ras anchor | Ld | >90% | ||
| GPI anchor | Lo | >90% | ||
| H-ras (full length) | Ld | >90% | ||
| CD-59 | Lo | >90% | ||
| Caveolin-1 | Ld | >90% | ||
| Hemagglutinin | Lo | 26% | 58% | 16% |
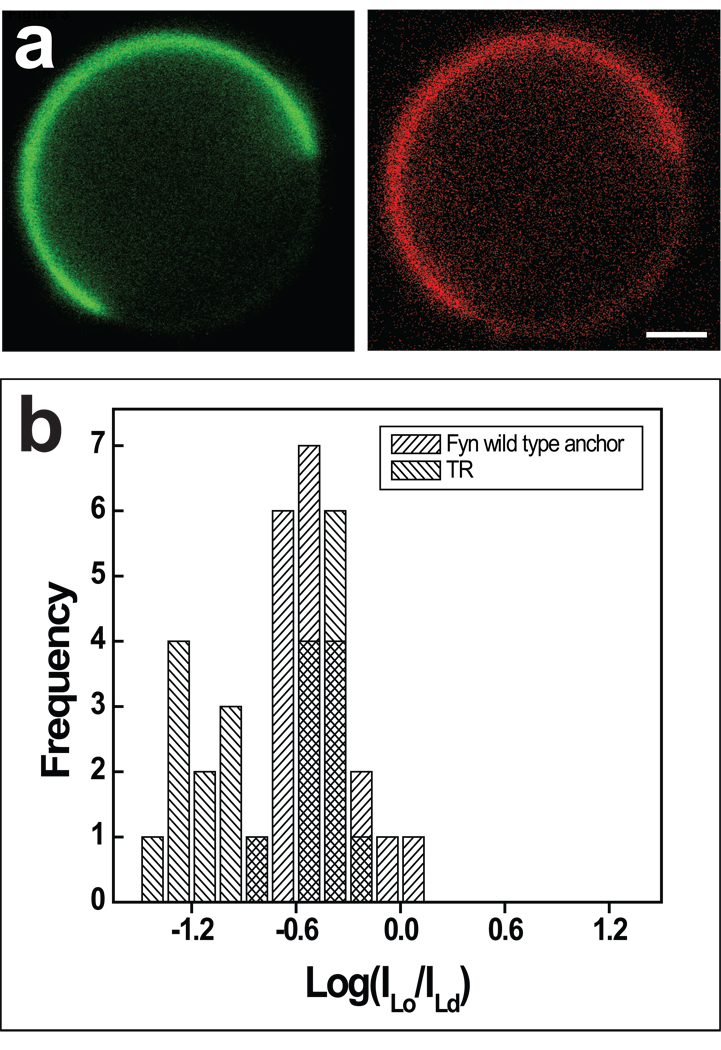
The wt Fyn anchor (see Table 2 for the sequence) was predominantly Ld phase partitioning. Typical fluorescence images of wt Fyn are shown in Figure 3a, and the associated fluorescence intensity histogram is shown in Figure 3b. This difference in phase partitioning preference of Lck and Fyn wt anchors might contribute to the reported differences in their trafficking and signaling behavior [41], although protein/protein interactions have been shown to be critically important as well [42].
Figure 3.
Partitioning of wt Fyn anchor eGFP construct. (a) Representative fluorescence images comparing protein (green, left) and lipid dye (red, right) fluorescence in a phase separated HeLa cell GPMV. Scale bars, 2 µm. (b) Fluorescence intensity ratio distribution for protein and lipid dye demonstrates primarily disordered phase partitioning of the wt Fyn protein anchor. Scale bars, 2 µm.
In addition to Src-like kinase anchors, we investigated an eGFP labeled H-ras anchor construct. Ras GTPases are proteins that act as plasma membrane localized molecular switches that regulate several signal transduction pathways [43]. The acyl anchor of H-ras consists of two palmitoyl chains and one farnesyl chain. The truncated anchor sequence of H-ras was found to associate with cholesterol sensitive microdomains [43] and to co-localize with GDP-loaded, but not with GTP-loaded (i.e. activated), H-ras.
In HeLa cell GPMVs, we observed strong disordered phase targeting of the H-ras anchor (Table 1) and the fluorescence intensity ratio histogram (see supplementary information, SI) indicates unique disordered phase preference. This observation departs from the hypothesis that H-ras-eGFP (also called tH-ras) targets cholesterol-enriched membrane raft domains with liquid-ordered nature. The eGFP labeled version of the full length H-ras protein also was not associated with Lo phases (Table 1 & SI).
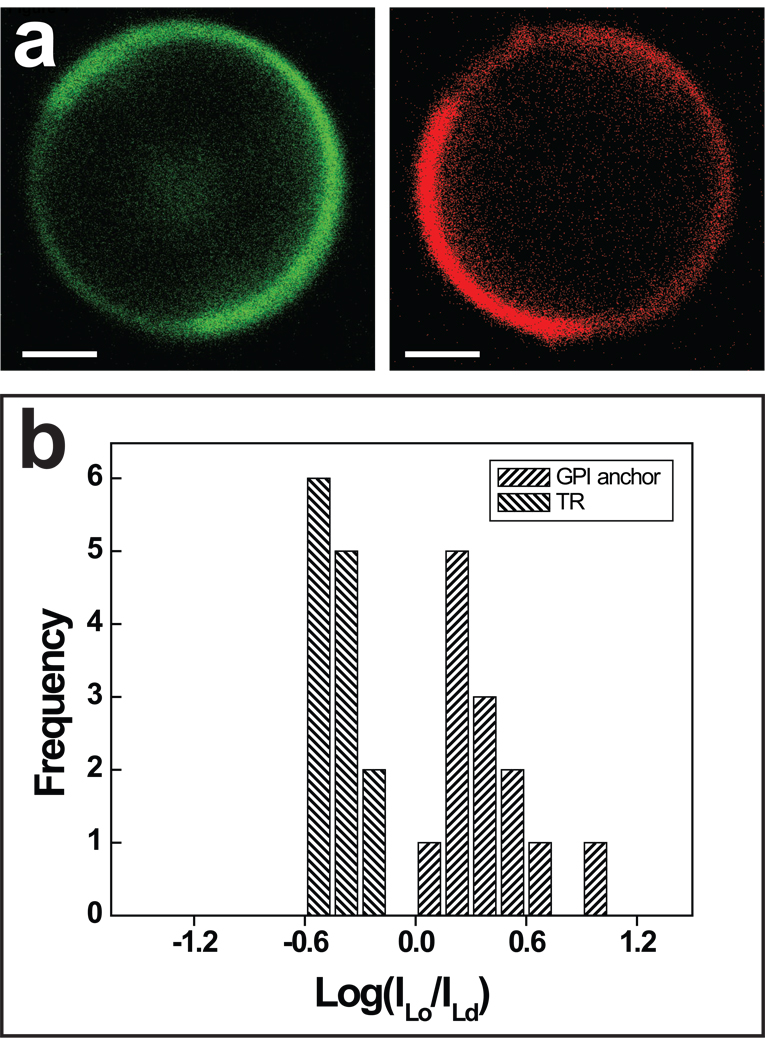
In addition to inner leaflet bound signal protein anchors, we studied outer leaflet associated (GPI-anchored) constructs. As the fluorescence images in Figure 4a and the histogram of fluorescence intensity ratio distribution (Figure 4b) shows, the GPI-anchored eGFP construct was observed to partition oppositely to Ld phase markers in HeLa cell GPMVs. This Lo phase preference of the GPI anchor in HeLa cells is in accordance with the phase partitioning of the GPI-anchored protein Thy1 investigated in RBL cell GPMVs [6] and the partitioning of GFP tagged GPI anchors in RBL cell GPMVs [23]. Having now shown examples of fluorescence images and histograms of strong ordered phase (GPI anchor), disordered phase (wt Fyn), and non-preferential / mixed partitioning (wt Lck) molecules, we confine ourselves to summarizing our data in Table 1–Table 3 and refer the reader to the SI for fluorescence intensity ratio histograms of additional membrane proteins to be discussed in the following.
Figure 4.
Partitioning of GPI anchor eGFP construct. (a) Representative fluorescence images comparing protein (green, left) and lipid dye (red, right) fluorescence in a phase separated HeLa cell GPMV. Scale bars, 2 µm. (b) Fluorescence intensity ratio distribution for protein and lipid dye demonstrates primarily ordered phase partitioning of the GPI anchor.
II.2) Partitioning of full length proteins
In addition to GPI anchors, we examined the GPI-anchored protein CD59. This molecule is a small, globular, highly glycosylated, outer leaflet associated membrane protein found in almost all tissues and expressed in all circulating cells. The most investigated role of CD59 is in complement regulation, but a variety of additional functions have been proposed [44]. Among these, cross-linking of CD59 by monoclonal antibodies was observed to initiate tyrosine kinase activation and associated downstream signaling in T-cells [44]. CD59 is classified as a raft associating protein [45]. The eGFP labeled form of this outer leaflet anchored protein exhibits Lo phase partitioning preference, consistent with the partitioning of GPI anchors (Table 1 & SI).
We also investigated the phase partitioning of the trans-membrane raft protein hemagglutinin (HA, from the influenza virus) in eGFP labeled form. Previous reports have suggested that membrane raft domains are selectively incorporated into the influenza virus envelope [46], suggesting that HA itself might be raft and liquid-ordered phase preferring[45]. HA-eGFP shows variable phase partitioning in HeLa cell GPMVs, including Ld phase partitioning, non-preferential partitioning, and a majority of Lo phase partitioning GPMVs (Table 1 & SI). This variable partitioning again underscores the hypothesis of compositional differences among different vesicles and suggests an influence of membrane lipid composition on lateral targeting of membrane proteins.
Finally, we tested eGFP labeled versions of caveolin-1. Caveolin-1 is a major constituent of caveolae, which are invaginations of the plasma membrane enriched in cholesterol and sphingomyelins [47]. Caveolins are multiply palmitoylated at the C-terminal domain and contain both a putatively membrane-inserting hair-pin and a scaffolding domain that improves membrane binding through basic and bulky hydrophobic residues [47]. Because of the specific caveolae lipid composition, one might expect caveolin to be ordered phase preferring. Caveolin has therefore been described to stabilize ordered membrane domains[48] but has also been proposed to remain excluded from liquid-ordered domains [49].
We observed that caveolin-1 partitions out of Lo-like phases in HeLa cell GPMVs (Table 1 & SI). This observation likely indicates that the concept of caveolae as a liquid-ordered phase contained in a coexisting Ld membrane phase is oversimplified. It seems to be in accordance, however, with studies that have described newly synthesized caveolin in the Golgi apparatus not to be associated with detergent resistant membranes [50] and with a model membrane study that described the caveolin scaffolding domain of caveolin-1 to be Ld phase preferring[51].
II.3) Partitioning of Lck anchor mutants
In order to characterize molecular determinants that govern the partitioning of Src-like protein tyrosine kinase membrane anchors between fluid domains in GPMVs, we investigated the partitioning of GFP-fused truncated versions of these proteins. It is known that the plasma membrane targeting signals of these proteins consist of a myristoyl group and a double palmitate group [34], rather than a polybasic domain. We therefore hypothesized that the plasma membrane targeting of these proteins is not significantly affected by any potential reduction in phosphatidylserine asymmetry that may occur during blebbing [6].
We examined anchor sequences of human Lck and compared the partitioning behavior to numerous mutants. As described above, the wt anchor displayed variable partitioning in phase separated GPMVs. We note that this observation contrasts with the strong Ld phase partitioning behavior of PM-GFP, a peptide sequence membrane anchored via a myristoyl/palmitoyl anchor [6].
We first examined to what extent the net charge of the anchor and the distribution of charged residues affect phase partitioning. The iso-steric mutation E10QD11N results in zero net charge and leads to increased non-preferential phase partitioning (Table 2 & SI), whereas exchanging all charged residues in the anchor sequence by polar neutral ones (Lck-all neutral, Table 2 & SI), leads to phase partitioning more similar to the wild type (wt) anchor. Eliminating charged residues near the C-terminus of the anchor sequences only, while keeping constant the net charge of the construct (Lck -2, Table 2 & SI), also leads to similar partitioning compared to the wt anchor. The mutation E10KD11K (Lck +2) results in a positive net charge and causes increased Ld phase partitioning. We conclude that altering the net charge and charge distribution particularly close to the acyl chain anchor residues can affect phase partitioning. We also emphasize the fact that changes in anchor sequences affect the partitioning of our constructs, despite the presence of a large GFP label that might be expected to influence the observed partitioning behavior [52].
We next examined how amino acids separating the two cysteines of the anchor sequence affect partitioning. Based on the partitioning behavior of the Lck wild type and Fyn wild type anchors, we hypothesized that both the spacing of the cysteines, as well as the residue size of the spacer amino acids, might affect partitioning. Interestingly, exchanging a single amino acid (G4V) that separates the two cysteines of the palmitate anchor changes the partitioning preference to Ld. We found that the double mutation G4VH8N, resulting in a sequence found in mouse Lck, switched the human wt Lck anchor to an Ld phase preferring / non-preferential construct, with no observable Lo phase partitioning. Because of the notable partitioning differences between human and mouse Lck anchors, it would be interesting to examine if these Lck anchors lead to functional differences in signaling pathways.
In order to further characterize the observed difference in partitioning behavior comparing human and mouse Lck anchors, we investigated to what extent mutation in position 8 affects partitioning. Comparing G4VH8N to G4VH8R does not reveal a significant change in partitioning. However, the single site mutations H8N and H8R both lead to increased Lo phase partitioning (Table 2 & SI). This observation allows for the hypothesis that variable protonation states of the histidine residue at position 8 in human Lck might be a way to modulate phase partitioning.
Strikingly, the sequence (M)GCGGC results in loss of membrane association, indicated by absence of detectable membrane fluorescence and a diffuse fluorescence signal from the GPMV interior in the green channel (not shown). This observation could either indicate that the configuration CGGC leads to orientations of palmitates unsuitable for efficient membrane insertion, or that the sequence CGGC interferes with N-myristoylation of the anchor by N-myristoyltransferase (NMT). Because of the reduction in membrane binding affinity, impaired myristoylation would also lead in loss of palmitoylation, as palmitoyl acyltransferases are typically membrane associated [53]. A consensus sequence for NMT substrates is (M)-G-X-X-X-S/T, although there are several exceptions, including the Fyn anchor (M)-G-C-V-Q-C-K [54]. More generally, NMTs show a preference for serines at position 6 and basic residues at positions 7 and 8 of the peptide substrate [54]. We note that palmitoylation by palmitoyl acyltransferases (PATs) might also be sequence specific, although no consensus sequence has been identified [55]. Further interpretation of our results will therefore likely require evaluation of palmitoylation degrees of our anchor constructs; such an analysis was beyond the scope of the present study.
To summarize our findings, it is evident that mutations affecting amino acids both in the immediate vicinity of the palmitate anchoring cysteines, as well as amino acids further separated from the palmitate groups, can have effects on membrane domain partitioning.
II.4) Partitioning of Fyn anchor mutants
Fyn anchor sequences, summarized in Table 3 (see SI for fluorescence intensity ratio histograms), ubiquitously showed Ld phase partitioning, independent of net charge and charge distribution, as shown for the wt protein in Figure 3. An exception was a construct with significant negative charge (−4), which was observed not to be membrane associated. This observation can likely be explained by electrostatic interactions reminiscent of the myristoyl/electrostatic-switch [54] of the peripherally membrane associating protein MARCKS. After phosphorylation increases its negative net charge, this protein unbinds from the inner plasma membrane leaflet. Loss of membrane association for the all neutral Fyn construct displayed in Table 3 is likely a consequence of loss in myristoylation since, as mentioned above, NMTs prefer substrates with basic residues in positions 7 and 8 [54]. More difficult to explain is the loss of membrane association for Fyn palmitate spacer sequences (“LckFyn” and “LckFynV4G”) shortened by one amino acid to resemble the mouse or human Lck, respectively (Table 3). As these palmitate spacers generally show sufficient membrane association for the constructs shown in Table 2, we speculate that the “LckFyn” and “LckFynV4G” of Table 3 suffer from a myristoylation defect that results from a loss of NMT recognition of the Fyn myristoylation sequence.
To summarize, GPMVs provide an alternative system for the investigation of plasma membrane partitioning. The observations presented above indicate that the amino acid sequence in the N-terminal anchor region of Src-like protein tyrosine kinases sensitively influences both membrane targeting and membrane domain partitioning. Our present observations do not yet clarify whether Ld versus Lo partitioning is determined primarily through modulated membrane interaction of the anchor peptide sequence through variations in net charge, charge distribution, or steric considerations, or whether such changes primarily affect the degree of palmitoylation, thereby leading to a secondary effect contributing to domain partitioning. This question can be answered by measurements of palmitoylation status, which was beyond the scope of the present contribution. We also note that the partitioning of Lck and Fyn constructs was comparable in HeLa and in RBL cells (not shown).
Discussion
Our measurements of GPMV phase behavior temperature dependence suggest that plasma membranes are not macroscopically (i.e. on a scale resolvable by the optical microscope) phase separated at physiological temperatures, consistent with results shown in Refs [5,6]. There are two scenarios that have been suggested to explain the existence of membrane rafts in the framework of equilibrium thermodynamics.
One of these is the microemulsion model [56] that has been theoretically examined by Frolov et al. [57]. This model assumes that membrane rafts consist of domains with a sharp phase boundary (the so-called “strong segregation limit”), where the line energy associated with the steep change in membrane properties orthogonally to this boundary (i.e. the line tension [14, 15, 58]) could be reduced by line-actants [56]. These line-actants would lead to an “entropic trapping” of small domains [57], below the critical temperature of phase coexistence, Tc. A critical emulsification temperature exists in this case (below Tc) that is determined by the balance between energetic line tension penalty and a term stemming from the increase in entropy through emulsification[57].
A second model considers that plasma membrane compositions are poised to be near a critical point of phase coexistence[59, 60]. In that case, macroscopic phase coexistence disappears at the critical temperature. Above Tc, compositional fluctuations (transient clusters) within a non-ideal mixture [61] lead to spatial regions that are enriched in components with preferential interactions [6]. Such correlated concentration fluctuations [62] could be pivotal in dynamically modulating the encounter probability of plasma membrane associated signaling molecules [10], thereby influencing the fidelity of membrane associated signaling pathways [6]. Importantly, critical composition fluctuations have been recently observed and thoroughly characterized in GPMVs of RBL cells [5].
Our observation that annexin V binding influences lateral membrane heterogeneity confirms earlier suggestions for members of the annexin family of membrane binding proteins to function as regulators of cellular membrane heterogeneity [63, 64]. It is known that the calcium-mediated binding of the Annexin V monomer involves several membrane lipid binding sites [65], which leads to lipid cross-linking that is likely to affect phase behavior [39]. Annexin AII is also known to cluster phosphatidylserines [66] and PIP2 [67] and has furthermore been suggested to be involved in domain regulation [66]. Annexins may therefore contribute to linking calcium signaling and functionally relevant dynamic lateral membrane organization [64]. Effects on phase transition temperatures by cross-linking lipids through protein binding have previously been demonstrated in self-assembled mixed model membranes [39, 68].
In addition to these effects of peripherally binding proteins on membrane heterogeneity, we have recently demonstrated that the cholesterol content of GPMVs sensitively affects the temperature-dependent phase behavior [69].
Findings in GPMVs may not always reflect the behavior of proteins in native cells. The limitations of GPMVs as a model membrane system have previously been discussed [6]. In particular, it has been found that phosphatidylserine, a negatively charged lipid normally concentrated on the inner plasma membrane leaflet, is likely to flip during bleb formation. To what extent flipping occurs, and if the observed degree of PS externalization influences protein partitioning in GPMVs, will be an important aspect of future studies.
Conclusions
We have investigated the temperature-dependent phase behavior of giant plasma membrane vesicles. The phase behavior of these model membranes suggests that plasma membranes do not show a tendency to macroscopically phase separate at physiological temperature in the thermodynamic limit. We have shown that peripheral protein binding affects transition temperatures in GPMVs.
We observed that the proteins CD-59 and hemagglutinin, which are believed to be raft associated, showed noticeable Lo phase partitioning. Furthermore, Lck anchors showed increased Lo phase preference compared to Fyn anchors, and we found membrane phase partitioning preference differences in human and mouse Lck anchors. Surprisingly, the protein caveolin, in GFP labeled form, was not found to be associated with Lo phases.
Supplementary Material
Acknowledgments
This work was funded by NIH grant R21AI073409.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons K, Ikonen E. Functional Rafts in Cell Membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 2.Pike LJ. The challenge of lipid rafts. Journal of Lipid Research. 2009;50:S323–S328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heerklotz H. Triton Promotes Domain Formation in Lipid Raft Mixtures. Biophysical Journal. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends in Biochemical Sciences. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Veatch SL, Cicuta P, Sengupta P, Honerkamp-Smith A, Holowka D, Baird B. Critical fluctuations in plasma membrane vesicles. Acs Chemical Biology. 2008;3:287–293. doi: 10.1021/cb800012x. [DOI] [PubMed] [Google Scholar]
- 6.Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka D, Baird B, Webb WW. Large scale fluid/fluid phase separation of proteins and lipds in giant plasma membrane vesicles. PNAS. 2007;104:3165. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edidin M. The State of Lipid Rafts: From Model Membranes to Cells. Annual Review of Biophysics and Biomolecular Structure. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- 8.Munro S. Lipid Rafts: Elusive or Illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 9.Holowka D, Gosse JA, Hammond ATH, Han X, Sengupta P, Smith NL, Wagenknecht-Wiesner A, Wu M, Young RM, Baird B. Lipid segregation and IgE receptor signaling: A decade of progress. Biochimica et Biophysica Acta. 2005;1746:252–259. doi: 10.1016/j.bbamcr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nature Reviews Molecular Cell Biology. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. Biochimica et Biophysica Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Feigenson GW. Phase behavior of lipid mixtures. Nature Chemical Biology. 2006;2:560–563. doi: 10.1038/nchembio1106-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacia K, Schwille P, Kurzchalia T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. PNAS. 2005;102:3272–3277. doi: 10.1073/pnas.0408215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 15.Tian A, Johnson C, Wang W, Baumgart T. Line Tension at Fluid Membrane Domain Boundaries Measured by Micropipette Aspiration. Physical Review Letters. 2007;98:2081021–2081024. doi: 10.1103/PhysRevLett.98.208102. [DOI] [PubMed] [Google Scholar]
- 16.Feigenson GW, Buboltz JT. Ternary Phase Diagram of Dipalmitoyl-PC/Dilauroyl-PC/Cholesterol: Nanoscopic Domain Formation Driven by Cholesterol. Biophysical Journal. 2001;80:2775–2788. doi: 10.1016/S0006-3495(01)76245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korlach J, Baumgart T, Webb WW, Feigenson GW. Detection of motional heterogeneities in lipid bilayer membranes by dual probe fluorescence correlation spectroscopy. Biochimica Et Biophysica Acta-Biomembranes. 2005;1668:158–163. doi: 10.1016/j.bbamem.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Honerkamp-Smith AR, Cicuta P, Collins MD, Veatch SL, den Nijs M, Schick M, Keller SL. Line tensions, correlation lengths, and critical exponents in lipid membranes near critical points. Biophysical Journal. 2008;95:236–246. doi: 10.1529/biophysj.107.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willig KI, Rizzoli SO, Westphal V, Jahn R, Hell SW. STED microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis. Nature. 2006;440:935–939. doi: 10.1038/nature04592. [DOI] [PubMed] [Google Scholar]
- 21.Baumgart T, Hunt G, Farkas E, Webb WW, Feigenson GW. Fluorescence probe partitioning between Lo / Ld phases in lipid membranes. Biochimica et Biophysica Acta Biomembranes. 2007;1768:2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridriksson EK, Shipkova PA, Sheets E, Holowka D, Baird BA, McLafferty FM. Quantitative Analysis of Phospholipids in Functionally Important Membrane Domains from RBL-2H3 Mast Cells Using Tandem High-Resolution Mass Spectroscopy. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 23.Sengupta P, Hammond A, Holowka D, Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochimica Et Biophysica Acta-Biomembranes. 2008;1778:20–32. doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer B, Davidson M, Orwar O. Proteomic Analysis of Plasma Membrane Vesicles. Angewandte Chemie-International Edition. 2009;48:1656–1659. doi: 10.1002/anie.200803898. [DOI] [PubMed] [Google Scholar]
- 25.Field KA, Holowka D, Baird B. Fc-Epsilon-Ri-Mediated Recruitment of P53/56(Lyn) to Detergent-Resistant Membrane Domains Accompanies Cellular Signaling. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheets ED, Holowka D, Baird B. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of Fc epsilon RI and their association with detergent-resistant membranes. Journal of Cell Biology. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee S, Soe TT, Maxfield FR. Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails. Journal of Cell Biology. 1999;144:1271–1284. doi: 10.1083/jcb.144.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumgart T, Hunt G, Farkas ER, Webb WW, Feigenson GW. Fluorescence probe partitioning between L-o/L-d phases in lipid membranes. Biochimica Et Biophysica Acta-Biomembranes. 2007;1768:2182–2194. doi: 10.1016/j.bbamem.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veatch SL, Keller SL. Separation of Liquid Phases in Giant Vesicles of Ternary Mixtures of Phospholipids and Cholesterol. Biophysical Journal. 2003;85:3074–3083. doi: 10.1016/S0006-3495(03)74726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brismar H, Trepte O, Ulfhake B. Spectra and Fluorescence Lifetimes of Lissamine Rhodamine, Tetramethylrhodamine Isothiocyanate, Texas Red, and Cyanine-3.18 Fluorophores - Influences of Some Environmental-Factors Recorded with a Confocal Laser-Scanning Microscope. J Histochem Cytochem. 1995;43:699–707. doi: 10.1177/43.7.7608524. [DOI] [PubMed] [Google Scholar]
- 31.Bagatolli LA. To see or not to see: Lateral organization of biological membranes and fluorescence microscopy. Biochimica Et Biophysica Acta-Biomembranes. 2006;1758:1541–1556. doi: 10.1016/j.bbamem.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Okonogi TM, McConnell HM. Contrast inversion in the epifluorescence of cholesterol-phospholipid monolayers. Biophysical Journal. 2004;86:880–890. doi: 10.1016/S0006-3495(04)74163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levental I, Byfield FJ, Chowdhury P, Gai F, Baumgart T, Janmey PA. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochemical Journal. 2009;424:163–167. doi: 10.1042/BJ20091283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCabe JB, Berthiaume LG. Functional roles for fatty acylated amino-terminal domains in subcellular localization. Molecular Biology of the Cell. 1999;10:3771–3786. doi: 10.1091/mbc.10.11.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang WY, Malcolm BA. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange (TM) site-directed mutagenesis. Biotechniques. 1999;26:680–682. doi: 10.2144/99264st03. [DOI] [PubMed] [Google Scholar]
- 36.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 37.Larson DR, Gosse JA, Holowka DA, Baird BA, Webb WW. Temporally resolved interactions between antigen-stimulated IgE receptors and Lyn kinase on living cells. Journal of Cell Biology. 2005;171:527–536. doi: 10.1083/jcb.200503110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gudheti MV, Mlodzianoski M, Hess ST. Imaging and shape analysis of GUVs as model plasma membranes: Effect of Trans DOPC on membrane properties. Biophysical Journal. 2007;93:2011–2023. doi: 10.1529/biophysj.106.103374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammond AT, Heberle FA, Baumgart T, Holowka D, Baird B, Feigenson GW. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang TY, Leventis R, Silvius JR. Partitioning of lipidated peptide sequences into liquid-ordered lipid domains in model and biological membranes. Biochemistry. 2001;40:13031–13040. doi: 10.1021/bi0112311. [DOI] [PubMed] [Google Scholar]
- 41.Filipp D, Julius M. Lipid rafts: resolution of the 'fyn problem"? Molecular Immunology. 2004;41:645–656. doi: 10.1016/j.molimm.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Filipp D, Moemeni B, Ferzoco A, Kathirkamathamby K, Zhang J, Ballek O, Davidson D, Veillette A, Julius M. Lck-dependent Fyn activation requires C terminus-dependent targeting of kinase-active Lck to lipid rafts. Journal of Biological Chemistry. 2008;283:26409–26422. doi: 10.1074/jbc.M710372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hancock JF, Parton RG. Ras plasma membrane signalling platforms. Biochemical Journal. 2005;389:1–11. doi: 10.1042/BJ20050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimberley FC, Sivasankar B, Morgan BP. Alternative roles for CD59. Molecular Immunology. 2007;44:73–81. doi: 10.1016/j.molimm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. Journal of Cell Biology. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. Journal of Biological Chemistry. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 47.Parton RG, Simons K. The multiple faces of caveolae. Nature Reviews Molecular Cell Biology. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 48.Brown DA, London E. Structure of detergent-resistant membrane domains: Does phase separation occur in biological membranes? Biochemical and Biophysical Research Communications. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- 49.Almeida PFF, Pokorny A, Hinderliter A. Thermodynamics of membrane domains. Biochimica Et Biophysica Acta-Biomembranes. 2005;1720:1–13. doi: 10.1016/j.bbamem.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the golgi complex and between the cell surface and lipid bodies. Molecular Biology of the Cell. 2005;16:2091–2105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horton MR, Radler J, Gast AP. Phase behavior and the partitioning of caveolin-1 scaffolding domain peptides in model lipid bilayers. Journal of Colloid and Interface Science. 2006;304:67–76. doi: 10.1016/j.jcis.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 52.Gorfe AA, Hanzal-Bayer M, Abankwa D, Hancock JF, McCammon JA. Structure and dynamics of the full-length lipid-modified H-ras protein in a 1,2-dimyristoylglycero-3-phosphocholine bilayer. Journal of Medicinal Chemistry. 2007;50:674–684. doi: 10.1021/jm061053f. [DOI] [PubMed] [Google Scholar]
- 53.Planey SL, Zacharias DA. Palmitoyl acyltransferases, their substrates, and novel assays to connect them (Review) Molecular Membrane Biology. 2009;26:14–31. doi: 10.1080/09687680802646703. [DOI] [PubMed] [Google Scholar]
- 54.Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochimica Et Biophysica Acta-Molecular Cell Research. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 55.Bijlmakers MJ. Protein acylation and localization in T cell signaling (Review) Molecular Membrane Biology. 2009;26:93–103. doi: 10.1080/09687680802650481. [DOI] [PubMed] [Google Scholar]
- 56.Simons K, Vaz WLC. Model systems, lipid rafts, and cell membranes. Annual Review of Biophysics and Biomolecular Structure. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 57.Frolov VAJ, Chizmadzhev YA, Cohen FS, Zimmerberg J. "Entropic traps"in the kinetics of phase separation in multicomponent membranes stabilize nanodomains. Biophysical Journal. 2006;91:189–205. doi: 10.1529/biophysj.105.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esposito C, Tian A, Melamed S, Johnson C, Tee SY, Baumgart T. Flicker spectroscopy of thermal lipid bilayer domain boundary fluctuations. Biophysical Journal. 2007;93:3169–3181. doi: 10.1529/biophysj.107.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dietrich C, Bagatolli LA, Volovyk ZN, Thompson NL, Levi M, Jacobson K, Gratton E. Lipid Rafts Reconstituted in Model Membranes. Biophysical Journal. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tserkovnyak Y, Nelson DR. Conditions for extreme sensitivity of protein diffusion in membranes to cell environments. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15002–15007. doi: 10.1073/pnas.0606992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang JY, Feigenson GW. Monte-Carlo Simulation of Lipid Mixtures - Finding Phase-Separation. Biophysical Journal. 1993;65:1788–1794. doi: 10.1016/S0006-3495(93)81234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirkwood JG, Goldberg RJ. Light Scattering Arising from Composition Fluctuations in Multi-Component Systems. Journal of Chemical Physics. 1950;18:54–57. [Google Scholar]
- 63.Draeger A, Wray S, Babiychuk EB. Domain architecture of the smooth-muscle plasma membrane: regulation by annexins. Biochemical Journalf. 2005;387:309–314. doi: 10.1042/BJ20041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerke V, Creutz CE, Moss SE. Annexins: Linking Ca2+ signalling to membrane dynamics. Nature Reviews Molecular Cell Biology. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 65.Richter RP, Him JLK, Tessier B, Tessier C, Brisson AR. On the kinetics of adsorption and two-dimensional self-assembly of annexin A5 on supported lipid bilayers. Biophysical Journal. 2005;89:3372–3385. doi: 10.1529/biophysj.105.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menke M, Gerke V, Steinem C. Phosphatidylserine membrane domain clustering induced by annexin A2/S100A10 heterotetramer. Biochemistry. 2005;44:15296–15303. doi: 10.1021/bi051585i. [DOI] [PubMed] [Google Scholar]
- 67.Gokhale NA, Abraham A, Digman MA, Gratton E, Cho W. Phosphoinositide specificity of and mechanism of lipid domain formation by annexin A2-p11 heterotetramer. Journal of Biological Chemistry. 2005;280:42831–42840. doi: 10.1074/jbc.M508129200. [DOI] [PubMed] [Google Scholar]
- 68.Liu AP, Fletcher DA. Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophysical Journal. 2006;91:4064–4070. doi: 10.1529/biophysj.106.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levental I, Choudhourie P, Gai F, Baumgart T, Janmey P. Cholesterol, but not sphingomyelin, determines phase behavior in cell-derived giant plasma membrane vesicles, submitted. 2008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.