Abstract
Although cytokine-induced sickness behavior is now well-established, the mechanisms by which chronic inflammation and depression are linked still remain elusive. Therefore this study aimed to develop a suitable model to identify the neurobiological basis of depressive-like behavior induced by chronic inflammation, independently of sickness behavior. We chose to measure the behavioral consequences of chronic inoculation of mice with Bacillus Calmette-Guerin (BCG), which has been shown to chronically activate both lung and brain indoleamine 2,3-dioxygenase (IDO), a tryptophan-catabolizing enzyme that mediates the occurrence of depressive-like behavior following acute innate immune system activation. BCG inoculation induced an acute episode of sickness (approximately 5 days) that was followed by development of delayed depressive-like behaviors lasting over several weeks. Transient body weight loss, reduction of motor activity and the febrile response to BCG were dissociated temporarily from a sustained increase in the duration of immobility in both forced swim and tail suspension tests, reduced voluntary wheel running and decreased preference for sucrose (a test of anhedonia). Moreover, we show that a distinct pattern of cytokine production and IDO activation parallels the transition from sickness to depression. Protracted depressive-like behavior, but not sickness behavior, was associated with sustained increase in plasma interferon-γ and TNF-α concentrations and peripheral IDO activation. Together, these promising new data establish BCG inoculation of mice as a reliable rodent model of chronic inflammation-induced depressive-like behaviors that recapitulate many clinical observations and provide important clues about the neurobiological basis through which cytokines may have an impact on affective behaviors.
Keywords: Interferon-γ; Tumor necrosis factor-α; Indoleamine 2,3-dioxygenase; Inflammation; Tail suspension test; Forced swim test; Sucrose preference test; Voluntary wheel running; Anhedonia; Depression
1. Introduction
Major depressive disorders are commonly observed in many medical illnesses that share chronic inflammation as a common denominator (Evans et al., 2005; Raison et al., 2006). Inflammation-associated sickness behavior can be a confounding factor since it partially overlaps with several clinical symptoms of depression, including mood and cognitive changes, decreased motor activity, anhedonia (reduced sensitivity to reward), anorexia, alterations in brain monoaminergic neurotransmission and activation of the hypothalamic–pituitary–adrenal axis (reviewed in Castanon et al., 2002; Dantzer et al., 2008). However, longitudinal clinical studies performed on patients undergoing cytokine immunotherapy for the treatment of viral diseases or certain cancers (Capuron et al., 2001; Raison et al., 2005) have revealed that the overlap between cytokine-induced sickness and depression is only partial. Whereas sickness symptoms invariably appear in all patients immediately after the first administration of recombinant cytokines, only one-third of patients who receive cytokine immunotherapy eventually develop delayed major depressive disorders (Capuron et al., 2000, 2001). Furthermore, pre-treatment with the selective serotonin reuptake inhibitor paroxetine prevents the occurrence of symptoms of depression, but not sickness (Capuron et al., 2002a). Together, these data point to a functional dissociation between the cytokine-induced sickness response and depression.
Patients who receive cytokine immunotherapy and develop major depression exhibit a prolonged decrease in circulating concentrations of tryptophan (TRP) that is accompanied by elevated concentrations of kynurenine (KYN) compared to non-depressed patients (Capuron et al., 2003). Since KYN is the major product of TRP degradation (Dale et al., 2000), these results can be explained by an enhanced activation of the TRP-catabolizing enzyme indoleamine 2,3-dioxygenase (IDO) (Wirleitner et al., 2003). This extrahepatic enzyme is activated in monocytes, macrophages and brain microglia in response to proinflammatory cytokines, mainly interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) (Fujigaki et al., 2006; Takikawa et al., 1999). In cancer patients treated by cytokine immunotherapy, the fall in plasma levels of TRP is correlated with the intensity of depressive symptoms (Capuron et al., 2002b), indicating that IDO activation might play a role in cytokine-induced depressive symptoms (Dantzer et al., 2008). By reducing TRP availability, IDO activation may impact brain serotoninergic neurotransmission, as TRP is the limiting factor for the synthesis of serotonin that plays a crucial role in the regulation of mood (Mattson et al., 2004). Concurrently, IDO activation results in an increased production of several neuroactive glutamatergic metabolites (Guillemin et al., 2005), and heightened glutamate receptor activity has been recently evidenced in major depression (Muller and Schwarz, 2007; Wichers et al., 2005).
Most studies aimed at identifying mechanisms of the depressive-like effects of cytokines have been carried out in rodents and are based on acute immune activation by the cytokine inducer lipopolysaccharide (LPS). The results of these studies can therefore be biased by the profound lethargy and motor impairment that occurs in the first few hours following immune stimulation (Deak et al., 2005; Dunn and Swiergiel, 2005; Renault and Aubert, 2006; Yirmiya, 1996). However, there are ways to circumvent this problem. In particular, it is possible to differentiate the initial phase of decreased motor activity induced by LPS with the delayed depressive-like behavior, measured by increased immobility in the forced swim and tail suspension tests, by carrying out these tests of depressive-like behavior 24 h after administration of LPS, when the sickness has dissipated and locomotor activity returns to normal (Frenois et al., 2007). In a similar manner, LPS-induced anhedonia, as assessed by reduced consumption of a sweetened solution, can be observed even after normalization of food intake (Frenois et al., 2007). More importantly, blockade of IDO activation either indirectly with the anti-inflammatory tetracycline derivative minocycline, or directly with the IDO competitive antagonist 1-methyltryptophan, prevents the LPS-induced development of depressive-like behavior, but not sickness behavior (O’Connor et al., in press). Taken together, these results clearly show that it is possible to dissociate sickness from depression in an acute model of immune activation. However, similar findings have not yet been reported in a chronic model of immune activation that would certainly be more relevant to the clinical situation.
We have selected for this purpose the inoculation of mice with Bacillus Calmette-Guerin (BCG), an attenuated form of Mycobacterium bovis that is used in several countries outside the United States as a vaccine for tuberculosis (Brewer, 2000). Intraperitoneal administration of BCG to mice is rapidly followed by long-lasting mycobacterial dissemination in all organs (particularly the lungs) except the brain (Tsenova et al., 1999) and by a drastic increase in circulating IFN-γ (Sander et al., 1995), a key cytokine for the activation of IDO (Takikawa et al., 1999). We first demonstrated that BCG inoculation causes a persistent activation of peripheral and brain IDO in mice for up to 3 weeks, resulting in decreased TRP levels (Moreau et al., 2005). The present series of studies was carried out to assess the short- and long-term behavioral consequences of BCG inoculation in mice. We hypothesized that the initial macrophage activation by BCG should be associated with a transient episode of sickness behavior followed by a longer occurrence of depressive-like behavior temporarily associated to IFN-γ-related IDO activation. Using various measures of sickness and depressive-like behaviors, we present exciting new data that are consistent with this hypothesis.
2. Materials and methods
2.1. Animals and treatments
Male CD1 mice 8- to 10-week old were obtained from Charles River Laboratories. They were housed individually under a normal 12:12 h light:dark cycle (lights on at 08:00). Food and water were available ad libitum and room temperature was controlled (22 ± 1 °C). Mice were handled daily for at least 1 week before the onset of the experiment to minimize stress reactions to manipulation. They were randomly allocated to treatment conditions and tested in counterbalanced order. All animal care and use were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NRC) and approved by the Institutional Animal Care and Use Committee.
Mice were inoculated with an attenuated live strain of BCG (Connaught strain, ImmuCyst Aventis). On the inoculation day, the stock solution was diluted in sterile physiological saline, properly dispersed and administered intraperitoneally (ip) in a volume of 200 μl/mouse. Control mice received the same amount of saline. The dose of BCG (107 CFU/mouse) was selected on the basis of its ability to reliably increase peripheral and central IDO activity (Moreau et al., 2005). All mice were weighed for 4 days before treatment and for 7 consecutive days after. Then, body weight was measured twice a week until the end of the experiments. All experimental subgroups were matched for body weight before treatment. A transient loss of body weight shortly after BCG inoculation together with an enlargement of the spleen at the end of the experiment and a significant increase in lung IDO activity served as positive controls for BCG treatment efficiency.
2.2. Experimental procedure
All behavioral experiments were performed between 9:00 and 12:00 AM, under conditions of dim light and low noise. Behavior was monitored via a video camera and videotaped to be scored later by a trained observer blind to drug treatments, using “The Observer Basic” software (Noldus, The Netherlands). In order to avoid possible interferences between the different measures of depressive-like behavior, only one depressive-like behavioral endpoint was considered in each experiment.
For measurement of body temperature, biotelemetry transmitters (e-mitters) were surgically implanted into the peritoneal cavity of mice. Mice were then individually housed in cages set atop electronic receiving/energizing pads and allowed to recover for 1 week prior to data collection. Data were collected hourly throughout the duration of the study using the “Mini-Mitter VitalView” data acquisition system (The Mini-Mitter Company, Inc., Bend, OR, USA) and the average daily body temperature was calculated for comparison.
2.2.1. Experiment 1
This experiment aimed at measuring the time-course of BCG-induced: (1) sickness behavior, as assessed by locomotor impairment and febrile response, (2) depressive-like behavior, as assessed by increased duration of immobility in the forced swim test (FST) and (3) concomitant peripheral neurobiochemical alterations (plasma cytokine levels and lung IDO activity). The increase in plasma cytokine levels observed in response to peripheral infection results from the activation of both peritoneal and tissue macrophages, including pulmonary macrophages (Dantzer, 2006). This measure provides therefore an accurate indication of the entire systemic peripheral cytokine response to ip BCG inoculation. On the other hand, although IDO is present in most extrahepatic tissues (Wirleitner et al., 2003), lungs have been chosen as preferential peripheral target tissue to control the efficiency of the response to BCG on the basis of their ability to show a huge and sustained increase of IDO in these conditions (Moreau et al., 2005) and as the main site of mycobacteria development (Costello and Izumi, 1971).
2.2.1.1. Motor activity
The motor effects of BCG were assessed as previously described (Frenois et al., 2007) in a polypropylene cage (30 × 11 × 12 cm) divided into two communicating compartments which were separated by a plexiglas wall containing a small opening (2.5 × 3 cm) allowing the mouse to freely move between compartments. Motor activity was evaluated by counting the number of between-compartments crossings and rearings performed over a period of 6 min. The cage was cleaned thoroughly between each session. This test was performed at 4, 8, 24, and 48 h after BCG or saline treatment, each time with a new group of naïve mice.
2.2.1.2. Forced swim test
The forced swim test (FST) was conducted as described by Porsolt (2000). Briefly, each mouse was placed individually in a cylinder (diameter: 16 cm; height: 31 cm) containing 15 cm of water maintained at 25 ± 1 °C. The water was changed and the cylinders were cleaned thoroughly between testing sessions. Mice were tested for 6 min and then returned to their home cage. The duration of immobility, swimming and climbing was evaluated during the last 5 min of the test. For the purpose of the present experiment, mice previously tested for their motor activity were tested in the FST 7, 14, or 21 days post-BCG. Each mouse was tested once only.
2.2.1.3. Experimental protocol
One hour after completion of the FST session, mice (n = 6 mice per group) were sacrificed by decapitation within a few seconds after being picked up from their home cage. Trunk blood was collected on EDTA (10%) in chilled tubes. After centrifugation (4000g, 15 min, 4 °C), aliquots of plasma were frozen until assayed for determination of cytokines levels. Lungs were also immediately collected and kept at −80 °C until IDO assay.
Concurrently, an independent group of mice were sacrificed 24 h after BCG inoculation in the same experimental conditions. Lungs and whole brains were immediately collected and stored at −80 °C until measurement of acute effect of BCG on both peripheral and brain IDO activity.
2.2.1.4. Measurement of plasma levels of cytokines
Plasma cytokines were measured by Luminex™ technology using carboxylated polystyrene beads with a distinct emitting fluorescence pattern and coupled covalently with capture antibodies specific for individual cytokines. A Luminex 100 IS instrument (Biosource, France) was used to process the data. The service was provided by the “Functional Exploration Platform” from the Toulouse Genopole, France (http://ifr31.toulouse.inserm.fr/PFT/ExplPhysioPatho/). The mouse multiplex-5 bead array assay kit (Linco Research Inc., St. Charles, MO, USA) was used to quantify 4 proinflammatory cytokines: interleukin-1β (IL-1β), IL-6, TNF-α, IFN-γ and the anti-inflammatory cytokine IL-10. All analyses were performed according to the manufacturer’s protocol and samples were run in duplicate.
2.2.1.5. Measurement of IDO activity
IDO activity was measured as previously described (Lestage et al., 2002; Moreau et al., 2005). Briefly, tissues were disrupted using a Polytron in ice-cold 20 mM potassium phosphate buffer (pH 7.0) containing KCl 140 mM. After centrifugation of the homogenates (13,000g, 30 min, 4 °C), supernatants were incubated with the reaction mixture (0.4 mM L-TRP, 20 mM ascorbate, 10 μM methylene blue, 100 μg catalase in 50 mM phosphate buffer, pH 6.5) for 3 h at 37 °C. The reaction was blocked by adding 30% trichloroacetic acid (TCA) and then incubated for 30 min at 50 °C to convert N-formylkynurenine to L-kynurenine (L-KYN). After centrifugation (13,000g, 10 min, 4 °C) and ultrafiltration (cut-off of 10,000 Mr), the amount of L-KYN produced from TRP were determined by Reversed Phase High Pressure Liquid Chromatography (RP-HPLC). The reaction product was injected onto a 5 μm C18 RP-HPLC column (Lichrospher, Alltech, Deerfield, IL, USA) at a flow rate of 1.0 ml/min with a mobile phase containing 0.1 M ammonium acetate acetic acid buffer and 5% acetonitrile (pH 4.65). KYN was detected by UV absorbency at 360 nm and concentration was determined by comparison with known L-KYN standards. One unit of activity was defined as 1 nmol KYN/h/mg protein at 37 °C. The amount of protein was determined with a BCA assay (Uptima, France).
2.2.2. Experiment 2
In order to complete results collected over time in the FST, another sample of naïve mice (n = 6–7 mice per group) were tested in two additional tests of depressive-like behavior: either the classic tail suspension test (TST) or in a computerized voluntary wheel running paradigm.
2.2.2.1. Tail suspension test
The TST was carried out as previously described (Steru et al., 1985). Briefly, an adhesive tape was fixed to the mouse tail (distance from the tip of the tail = 2 cm) and hooked to a horizontal ring stand bar placed 30 cm above the floor. The test was conducted for a period of 6 min in a visually isolated area. The apparatus was cleaned thoroughly after each mouse. Mice demonstrated several escape attempts interspersed with immobility periods during which they hung passively and completely motionless. Mouse behavior was first assessed in the TST 1 week before BCG or saline treatment, in order to balance treatment groups for basal immobility level. Then, each mouse was again repeatedly tested 7, 14 and 21 days after treatment. It has been previously shown that exposure to the TST can be repeated without causing any significant habituation (El Yacoubi et al., 2003).
2.2.2.2. Voluntary wheel running
Mice were housed individually in cages equipped with a stainless steel running wheel. An electronic sensor affixed to the outside of each cage detected the passing of a magnet on the running wheel and recorded the number of wheel turns. Data were collected using the “Mini-Mitter VitalView” data acquisition system (The Mini-Mitter Company, Inc., Bend, OR, USA) and the daily wheel turns were calculated for comparison. Mice were allowed to acclimate to the wheel cages for 2–3 weeks prior to treatment, until daily voluntary wheel running stabilized. Mice exhibited >90% of their wheel running activity during the dark phase of the dark:light cycle. Cages and wheels were cleaned and sanitized weekly during the light phase of the dark:light cycle.
2.2.3. Experiment 3
This experiment was designed to assess the effect of BCG on the preference for a palatable solution using a two-bottle paradigm in which mice has the choice between a bottle of water and a bottle containing a sucrose solution. Blunted sucrose intake in this test reflects impaired sensitivity to reward, which allows to model anhedonia, a core symptom of major depression (Monleon et al., 1995).
2.2.3.1. Sucrose preference test
This test has been conducted as previously described (Frenois et al., 2007). During the weeks preceding the experiment onset, all mice were given repeated experience with the test procedure in their home cage to reduce reaction to novelty and ensure stability of the sucrose consumption baseline. For each session, a bottle containing a freshly prepared 2% sucrose solution was juxtaposed to the bottle of water for 12 h, beginning just at the lights offset. In order to avoid any place preference, the relative location (left or right) of the sucrose bottle was changed whenever fluid intake was measured. Mice were not food deprived before or during the test. Fluid consumption was measured by weighing bottles before and after each test session. As sucrose consumption depends on body weight, this variable was expressed as a percent body weight by calculating the ratio between the amount of sucrose that was consumed and the body weight ×100. The habituation period lasted until a stable sucrose intake level was reached, and the mean volume ingested over the 3 last training sessions was used as baseline value for each mouse. Mice were inoculated with BCG or saline 2 days after the last training session. Post-treatment sucrose consumption was then measured in a first set of mice (n = 10 mice per treatment) 24 h after saline or BCG inoculation and for 8 consecutive nights, whereas the remaining mice were left undisturbed. The long-term effect of BCG was assessed by measuring sucrose preference from 21 days post-treatment in this second set of mice (n = 7 mice per treatment).
2.3. Statistical analysis
Data are represented as means ± SEM. All measures were analyzed using one-way (treatment) or two-way (treatment × time) analysis of variance (ANOVA) with repeated measurement on the time factor where appropriate, followed by the Student–Newman–Keuls post hoc test if the treatment × time interaction was significant.
3. Results
3.1. BCG induces transient symptoms of sickness behavior
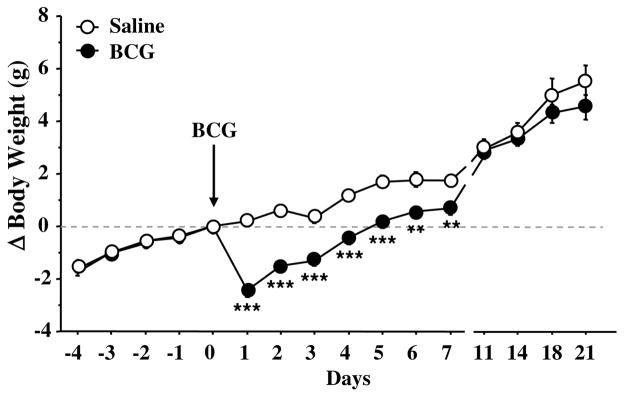
In order to assess sickness behavior induced by BCG, 3 classical sickness symptoms have been assessed, namely body weight loss, locomotor impairment and febrile response (Dantzer, 2006). Fig. 1 represents the time-course of the effect of BCG on body weight. Treatment (F(1,210) = 13.8, p < .01) and time (F(10,210) = 183.3, p < .001) factors, as well as their interaction (F(10,210) = 6.8, p < .001) were significant. Both groups presented the same growth curve before treatment. BCG inoculation induced a marked body weight loss during the first week post-treatment (BCG vs. controls: p < .001 to p < .01) and then body weight progressively recovered. As expected, BCG also induced splenomegaly in all mice, as confirmed visually (data not shown).
Fig. 1.
Temporal evolution of body weight changes relative to treatment day (expressed in grams) measured in saline- and BCG-treated mice. Saline and BCG (107 CFU/mouse) were injected intraperitoneally. Values are means ± SEM of 24 mice/treatment. ***p < .001; **p < .01 for the effect of treatment (BCG vs. saline).
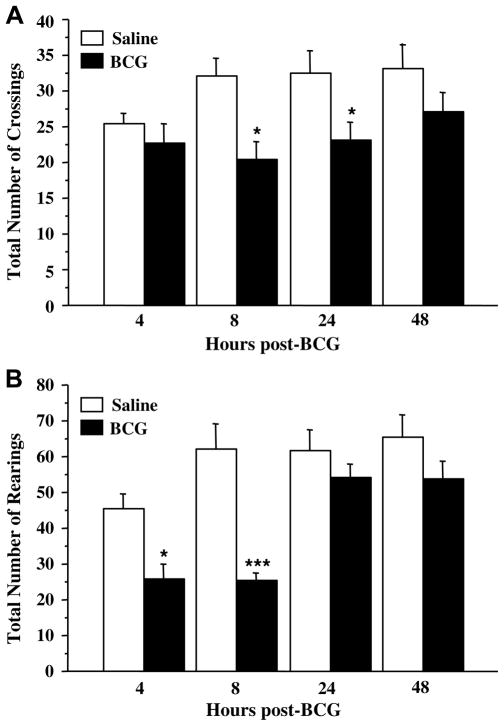
BCG decreased motor activity, as evidenced by a significantly smaller number of crossings (Fig. 2A: F(1,37) = 14.4, p < .001) and rearings (Fig. 2B: treatment: F(1,37) = 26.3, p < .001; time: F(3,37) = 9.4 p < .001; treatment × time: F(3,37) = 3.1, p < .05) in the activity cage. This effect was particularly marked during the first 24 h post-BCG inoculation. Although saline-treated mice were less active at 4 h post-treatment, this effect was not significant (p > .10) and the activity level was back to normal as soon as 8 h post-treatment.
Fig. 2.
Temporal evolution of the effect of BCG on motor activity as assessed by (A) the total number of between-compartment crossings and (B) the total number of rearings performed in the activity cage over the 6 min of test. Saline and BCG (107 CFU/mouse) were injected intraperitoneally. Each bar represents mean ± SEM of 6 mice. ***p < .001; *p < .05 for the effect of treatment (BCG vs. saline).
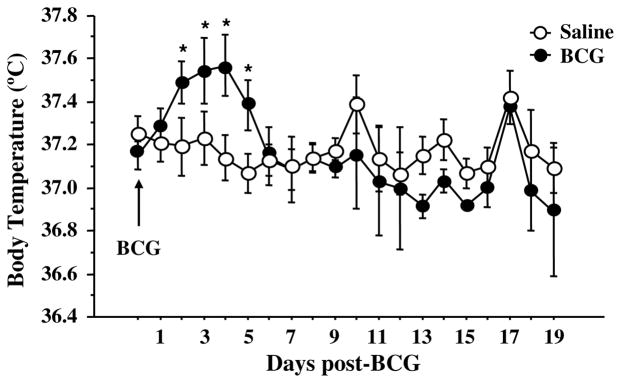
An elevated febrile response represents a third distinctive sign of the acute sickness response following infection. In this study, BCG induced a significant increase in core body temperature 2–5 days post-inoculation (Fig. 3: treatment × time: F(1,95) = 2.0, p < .05). The peak increase in body temperature occurred 3–4 days following inoculation with BCG-treated mice exhibiting an average body temperature about 0.5 °C higher than saline-treated controls.
Fig. 3.
Febrile response to BCG. Mice were injected intraperitoneally with either saline or BCG (107 CFU/mouse) and core body temperature was measured via biotelemetry e-mitters. Average daily body temperature (°C) was measured for 19 days post-BCG. Data represent means ± SEM (n = 6–7 mice/treatment). *p < .05 for the effect of treatment (BCG vs. saline).
3.2. BCG induces sustained depressive-like behavior
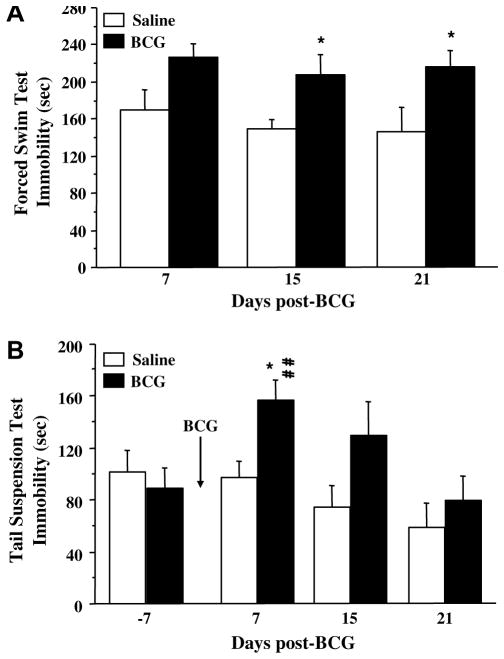
In order to thoroughly characterize depressive-like effects of BCG, several classical tests of depressive-like behavior modeling different core symptoms of major depression have been used. Although BCG-treated mice exhibited normal locomotor activity by the time of the first FST that was performed 1 week after treatment, BCG induced a sustained increase of immobility in the FST (Fig. 4A: F(1,30) = 14.7, p < .001) that could be evidenced up to 3 weeks after treatment. This increase was accompanied by a marked decrease in the time spent swimming (F(1,30) = 8.4, p < .01) and climbing (F(1,30) = 4.0, p = .05) (data not shown).
Fig. 4.
Temporal evolution of the depressive-like behavioral effects of BCG in (A) the forced swim test and (B) the tail suspension test. In both cases, mice were injected intraperitoneally with either saline or BCG (107 CFU/mouse). (A) 7, 15 or 21 days later, mice were placed into the forced swim test for 6 min and the duration of immobility was measured during the last 5 min of the test. Each bar represents mean ± SEM of 6 mice. (B) Concurrently, an independent group of mice were placed into the tail suspension test for 6 min, firstly 7 days before treatment, then again 7, 15 and 21 days after. The duration of immobility was measured during the last 5 min of the test. Bars represent means ± sem of 6 mice/treatment. *p < .05 for the effect of treatment (BCG vs. saline) and ##p < .005 for the difference between pre-and post-treatment (day 7) immobility measured in BCG-treated mice.
BCG also induced a marked increase of immobility in the TST (Fig. 4B: time: F(3,33) = 11.5, p < .001; treatment × time: F(3,33) = 3.9, p < .05). An important and significant increase of immobility was observed after treatment in BCG-treated mice compared to their pre-treatment baseline value (p < .01), whereas no significant difference was observed in control mice between pre- and post-treatment immobility.
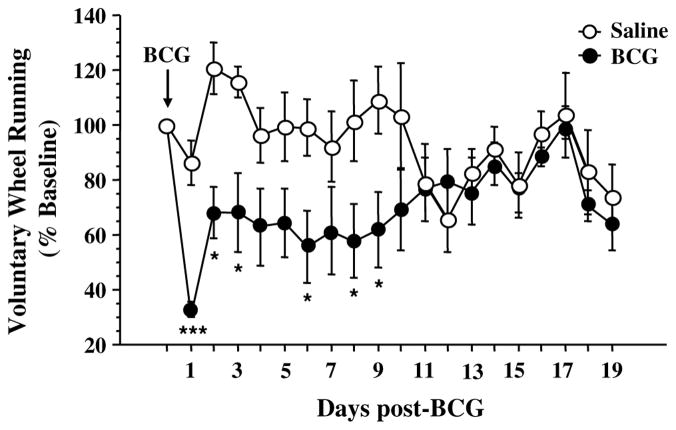
Similarly, BCG induced a chronic reduction in voluntary wheel running activity (Fig. 5: time: F(1,209) = 2.6, p < .001; treatment: F(1,209) = 5.9, p < .05; time × treatment: F(1,209) = 2.6, p < .001). There was a peak reduction in wheel running 1 day post-inoculation, followed by a partial, yet significant, increase in voluntary wheel running that did not return to baseline levels until 10–11 days post-BCG. Importantly, there was a wide intermouse variation in baseline wheel running, but it was not significantly different between saline and BCG-treated groups.
Fig. 5.
Reduced voluntary wheel running following BCG. Mice were allowed access to a freely moving running wheel in their home cage. Following ip injection with either saline or BCG (107 CFU/mouse), daily turns of the running wheel were measured for 19 days post-BCG and expressed as a percent of baseline. Data represent means ± SEM (n = 6–7 mice/group). ***p < .05 day 1 BCG vs. all other days (except day 6 BCG, p < .09); *p < .05 for the effect of treatment (BCG vs. saline).
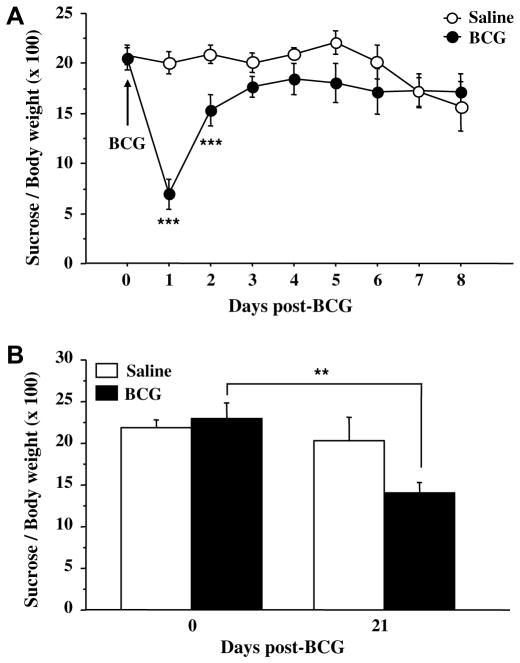
Fig. 6A shows the time-course of sucrose intake expressed as a percent of body weight. At the end of the habituation period, sucrose and water intake did not differ according to the future treatment and a clear preference for sucrose was established (6.79 ± 0.26 g/day vs. 1.35 ± 0.07 g/day for sucrose and water intake, respectively). However, sucrose consumption declined during the first 24 h after treatment in BCG-treated mice (treatment: F(1,126) = 5.0, p < .05; time: F(7,126) = 7.2, p < .001; treatment × time: F(7,126) = 7.8, p < .001) and stayed under baseline levels up to 6 days later. In contrast to sucrose intake, water intake did not significantly change in response to BCG (p > .10; data not shown). A separate ANOVA for each treatment group with repeated measures on the time factor revealed a significant effect of time in BCG-treated mice (F(7,63) = 9.9, p < .001), but also in saline-treated mice (F(7,63) = 4.4, p < .001), indicating that daily exposure to the test might in the end hide the potential long-term effect of BCG. In order to test for this possibility, an independent group of mice exhibiting before treatment a marked sucrose preference (7.01 ± 0.62 g/day vs. 1.24 ± 0.12 g/day for sucrose and water intake, respectively) was submitted to a single post-treatment sucrose preference test carried out 21 days after BCG inoculation. This procedure revealed a significant reduction of sucrose intake in BCG-treated mice (Fig. 6B: time: F(1,24) = 7.9, p < .01; time × treatment: F(1,24) = 4.0, p < .05) compared to controls.
Fig. 6.
Depressive-like behavioral effect of BCG in the sucrose preference test. Mice were injected intraperitoneally with either saline or BCG (107 CFU/mouse) and their relative sucrose intake, expressed as percent bodyweight, was measured (A) overnight during 8 consecutive days post-BCG (n = 10 mice/treatment), or (B) once only, 21 days post-BCG (n = 7 mice/treatment). Values represent means ± sem of 10 mice/group. ***p < .001; **p < .01 for the effect of treatment (BCG vs. saline).
3.3. BCG induces sustained cytokine expression and IDO activation
In order to determine how cytokine expression and IDO activation paralleled the transition from sickness to depression, their respective time-course has been measured in response to BCG. Table 1 shows the time-course of the effect of BCG on levels of cytokines. Plasma levels of IL-1β, a key cytokine in the development of short-term sickness behavior (Dantzer, 2006), were very low from 1 to 3 weeks post-BCG and did not vary according to treatment or time. In contrast, BCG induced a drastic elevation of IFN-γ (F(1,26) = 11.9, p < .01), TNF-α (F(1,26) = 43.8, p < .001) and IL-6 (F(1,28) = 22.9, p < .001) concentrations, the respective time-course of this effect differing however according to the cytokine. Whereas the increase in IFN-γ levels induced by BCG did not vary according to time, BCG-treated mice exhibited over the 3 weeks a progressive elevation of TNF-α concentrations (time: F(2,26) = 6.8, p < .01) compared to controls. IL-6 levels varied according to time (F(2, 28) = 5.7, p < .01), in interaction with the treatment factor (time × treatment: F(2,28) = 5.7, p < .01). Whereas plasma levels of IL-6 were undetectable at all time points in control mice, BCG-induced IL-6 increase was significantly higher at 1 week after treatment than at 2 and 3 weeks later (p < .05). Plasma levels of the anti-inflammatory cytokine IL-10 differed according to treatment (F(1,26) = 7.0, p < .05) in interaction with time (F(2,26) = 4.4, p < .05). A significant increase of IL-10 was observed in BCG-treated mice 3 weeks after treatment (saline vs. BCG: p < .05).
Table 1.
Time-course of plasma cytokine concentrations
| Cytokines (pg/ml) | 1 week |
2 weeks |
3 weeks |
|||
|---|---|---|---|---|---|---|
| Controls | BCG | Controls | BCG | Controls | BCG | |
| IL-1β | 0.63 ± 0.05 | 1.19 ± 0.37 | 0.84 ± 0.16 | 1.04 ± 0.17 | 1.07 ± 0.24 | 1.02 ± 0.22 |
| IFN-γ | 6.75 ± 2.09 | 25.71 ± 5.29** | 17.24 ± 6.77 | 73.89 ± 17.75* | 13.99 ± 2.29 | 51.04 ± 30.19 |
| TNF-α | 3.44 ± 0.67 | 12.88 ± 2.57** | 9.01 ± 2.17 | 18.15 ± 3.56 | 6.96 ± 1.34 | 25.46 ± 2.81*** |
| IL-6 | n.d. | 69.58 ± 16.74** | n.d. | 19.46 ± 9.47* | n.d. | 17.27 ± 8.86* |
| IL-10 | 6.94 ± 3.22 | 10.34 ± 3.14 | 2.56 ± 0.98 | 1.83 ± 1.31 | 0.93 ± 0.50 | 18.32 ± 7.28* |
(Interleukin-1β, IL-1β; interferon-γ, IFN-γ; tumor necrosis factor-α, TNF-α; IL-6 and IL-10) measured 1, 2 or 3 weeks after saline (controls) or BCG (107 CFU/mouse) inoculation. There was no detectable (n.d.) IL-6 in the plasma of control mice. Data represent means (pg/ml) ± sem of 6 mice per group.
p < .01;
p < .05 for the effect of treatment (BCG vs. saline).
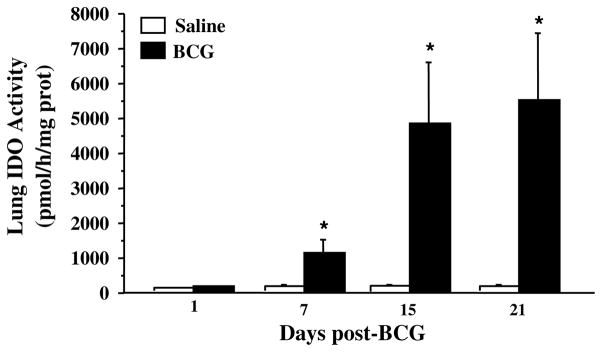
Because activation of IDO at the peripheral and brain levels has been shown to be positively correlated from 1 to 3 weeks post-BCG inoculation (Moreau et al., 2005), we here measured over this period IDO activity in the lungs only to serve as a control of BCG efficiency and to allow assessing correlations with plasma levels of cytokines. On the contrary, both lung and brain IDO activities have been assessed 24 h post-treatment since no data were available on the acute effect of BCG on these measures. By the time of maximal development of sickness symptoms, i.e. 24 h after BCG inoculation, no significant increase of lung IDO activity was observed in BCG-treated mice compared to their saline-treated controls (Fig. 7: p > .10). Similarly, no significant increase of brain IDO activity was observed by that time in response to BCG (saline vs. BCG: 16.56 ± 1.96 vs. 20.49 ± 2.62, p > .10). This treatment induced however a marked and sustained increase in lung IDO activity (Fig. 7: F(1,26) = 13.9, p < .001) from 1 week post-treatment up to 3 weeks (treatment × time: F(2,26) = 3.4, p < .05). These results confirm that BCG induced a chronic activation of peripheral IDO, which was probably associated with concomitant activation of brain IDO, as previously described (Moreau et al., 2005). Lung IDO activity was positively correlated with plasma levels of IFN-γ (r = 0.96; p < .05) and TNF-α (r = 0.96; p = .05) at 2 and 3 weeks post-treatment, respectively, in BCG-treated mice, which corresponds to the peak plasma levels for each cytokine.
Fig. 7.
Time-course of lung IDO activity (expressed as pmol of kynurenine produced by h and by mg protein) in response to BCG (107 CFU/mouse). Each bar represents mean ± sem of 6 mice. *p < .05 for the effect of treatment (BCG vs. saline).
4. Discussion
Although cytokine-induced sickness behavior is no longer a curiosity, the mechanisms by which chronic inflammation and depression are linked still remain elusive (Dantzer et al., 2008; Raison et al., 2006). Attempts to elucidate these mechanisms have been hampered by the lack of a suitable animal model to study the delayed behavioral and neurochemical alterations induced by chronic systemic inflammation. In the present study, the use of a murine model of chronic immune activation by BCG inoculation (Moreau et al., 2005) provides revealing behavioral data consistent with the induction of sustained depressive-like behavior for up to 3 weeks post-inoculation, i.e. well beyond the period during which signs of sickness behavior are present. These findings are consistent with clinical data reporting the delayed occurrence of depressive symptoms in patients submitted to cytokine immunotherapy developing on an early background of sickness symptoms (Capuron et al., 2000, 2001). They also extend our previous findings that acute immune activation by LPS induces depressive-like behavior independently of sickness behavior (Frenois et al., 2007). Furthermore, the present study shows for the first time that development of depressive-like behavioral alterations is associated with sustained IDO activation whereas behavioral signs of sickness occur in the absence of any significant elevation in IDO activity. These results are consistent with clinical data reporting that the delayed development of depressive symptoms observed in patients undergoing cytokine immunotherapy is associated with a drastic decrease in circulating levels of TRP (Capuron et al., 2002b). Moreover, we have recently demonstrated in mice that IDO activation is an essential molecular mediator of depressive-like behavior induced by acute immune stimulation with LPS (O’Connor et al., in press). The temporal association between the occurrence of depressive-like behavior and the increase in IDO activity that is observed in the present study can be interpreted to suggest that IDO activation probably plays the same role in the development of depressive-like behavior during chronic immune activation.
Although measuring depression in rodents could appear limiting (Dunn and Swiergiel, 2005; Dunn et al., 2005), the development of consistent and reliable behavioral tests modeling different core symptoms of major depression rather than the entire depressive syndrome has provided very useful tools to study the pathophysiology of depression (Cryan and Slattery, 2007). In the present study, the results of four different behavioral tests of depression, including the FST, TST, voluntary wheel running and sucrose preference test, converge to indicate that the depression-like condition induced by BCG is probably responsible for the behavioral alterations observed in the present study. The obvious tautological nature of this reasoning is somewhat attenuated by the specificity of the behavioral signs of depression that were observed in BCG-treated mice since they occurred in the absence of any possibly confounding sign of sickness behavior. The FST and TST were initially developed to identify new antidepressant drugs, based on the reduction of the time mice spent immobile in a helplessness or resignated-like state. Of note, it has been suggested that they are probably different in terms of the biological substrates that under-lie the observed behavioral performances (see for review: Cryan and Mombereau, 2007), which strengthens the necessity of using complementary behavioral paradigms to accurately measure depressive-like behavior in mice. In the present experiment, BCG increased the duration of immobility in both tests over several weeks, as previously shown shortly after acute immune stimulation with LPS or IL-1 (Dunn and Swiergiel, 2005; Jain et al., 2001; Renault and Aubert, 2006) and this effect was protracted in the FST compared to the TST.
BCG also induced depressive-like behavior in the voluntary wheel running paradigm. Exercise has been demonstrated to have antidepressant properties in humans (Hunsberger et al., 2007), and numerous animal studies have corroborated an interaction between exercise and depression. When given free access to a running wheel, mice run considerable distances, spending much of their active period on the wheel (De Bono et al., 2006). Interestingly, mice injected with the viral mimetic, polyriboinosinic:polyribocytidylic acid, exhibit a reduction in voluntary wheel running behavior that is temporally dissociated from general locomotor activity in the open field (Katafuchi et al., 2003). Our results (Fig. 5) are in agreement with this group’s findings, as BCG injection resulted in a dramatic acute reduction in wheel running followed by a modest chronic reduction that parallels BCG-induced chronic inflammation and IDO activation. While these earlier results were interpreted to distinguish between physical vs. mental fatigue, reduced voluntary exercise can reasonably be interpreted to represent a symptom of depression (e.g. diminished motivation or engagement in normal daily activities). Another strong support to this interpretation is provided by the fact that BCG also induced depressive-like behavior in the sucrose preference test. Instead of being based on its sensitivity to antidepressant drugs, this animal test of depression directly models a core symptom of depression, namely anhedonia, i.e. the lack of interest in pleasurable activities including consumption of palatable foods (Monleon et al., 1995; Shen et al., 1999). Interestingly, BCG still reduced sucrose intake 3 weeks after inoculation, whereas food and water consumption were normalized by the end of the first week (data not shown). Such a dissociation between relatively short-term reducing effects of cytokines on food intake and more sustained anhedonic effects has already been reported in response to chronic IL-2 treatment (Anisman and Merali, 2003) and in mice chronically infected with an IFN-γ adenovector (Kwant and Sakic, 2004). The delayed establishment of this long-term depressive-like behavior probably reflects the time required for the cytokine-induced neurobiochemical changes underlying depressive-like symptoms to fully develop. Moreover, it is important to note that our experimental paradigm allowed mice free access to food and water before and during the test, which increased the probability that intake of the highly palatable sucrose solution was mostly based on its rewarding properties (De La Garza, 2005; Merali et al., 2003; Shen et al., 1999). All these data point therefore to the ability of BCG to induce chronic depressive-like behavior, independently of its sickness inducing properties.
In general, the depressive-like behavioral effects of BCG were easier to evidence on the long-term when mice were tested only once than when they were submitted to repeated behavioral testing. Although this feature was only directly characterized in the sucrose preference test, the attenuation in the behavioral differences between BCG- and saline-treated mice that occurred over time in the TST and voluntary wheel running indicates that this could be a general phenomenon. Besides the TST which is claimed to be insensitive to repeated testing in mice (El Yacoubi et al., 2003; Liu and Gershenfeld, 2001), this aspect has not been systematically investigated in murine models of depression. Whether this habituation phenomenon is specific to conditions of immune stimulation or to the present experimental protocol cannot be determined from the present set of studies but is certainly worthy of further investigation.
The temporal dissociation between BCG-induced sickness and depressive-like behavior might be dependent on the differential profile of cytokines that is induced over time in response to BCG. Plasma levels of IL-1β, the main proinflammatory cytokine involved in induction of sickness behavior (Dantzer, 2006), were very low from 1 week post-BCG inoculation, i.e. by the time of disappearance of sickness behavior. Similarly, plasma levels of IL-6, another cytokine involved in regulating sickness behavior, were elevated in BCG-treated mice 1 week after treatment, but drastically decreased thereafter. Chronic induction of depressive-like behavior by BCG was accompanied by sustained increase of plasma concentrations of TNF-α and IFN-γ, the two major cytokines that are responsible for IDO activation (Fujigaki et al., 2006; Takikawa et al., 1999). Although not directly tested in the present set of experiments, this last increase in plasma levels of TNF-α and IFN-γ probably explains the prolonged stimulation of lung IDO activity measured over the same period of time, as indicated by the positive correlation between IDO activity and the plasma levels of these cytokines. An inverse relationship has usually been reported between pro- and anti-inflammatory cytokines. However, the time-course of their respective changes are not strictly separated in time, particularly under conditions of chronic inflammation (Jander et al., 1998; Link, 1998). Indeed, the delayed increase in IL-10 that we report in this manuscript, which occurred while pro-inflammatory cytokines remained elevated (Table 1), is part of the normal host immune response to BCG (Ordway et al., in press; Sander et al., 1995). It has been suggested that such a complex balance between pro- and anti-inflammatory cytokines probably corresponds to the necessity of preventing an overexpression of potentially tissue-damaging cytokines, while still eliminating pathogenic agents (Turner et al., 2002).
We have previously shown that chronic BCG-induced lung IDO activation is significantly correlated with a concomitant activation of brain IDO (Moreau et al., 2005). These data suggest therefore that chronic induction of depressive-like behavior by BCG in mice is associated with sustained activation of both peripheral and brain IDO, in contrast with sickness behavior taking place before detection of any significant IDO activation. We have recently shown that global inhibition of IDO prevents LPS-induced depressive-like behavior, but not LPS-induced sickness behavior (O’Connor et al., in press). Taken together, these data suggest that central IDO activation may play a role in mediating chronic inflammation-induced depressive-like behaviors. The direct implication of IDO in this phenomenon is currently under study, but our recent study reporting that prolonged depressive-like behavior observed in aged LPS-treated mice is associated with a more pronounced induction of peripheral and brain IDO compared to young counterparts (Godbout et al., in press) supports this hypothesis.
Cytokine-induced IDO activation results in a decrease in circulating TRP levels, as observed in cancer patients undergoing chronic cytokine immunotherapy (Capuron et al., 2002b) or in subjects suffering from viral infection (Bonaccorso et al., 2002; Launay et al., 1988). In mice, BCG inoculation reduced both peripheral and brain concentrations of TRP, with differences in the amplitude and the time-course of this effect between tissues (Moreau et al., 2005). These changes in the bioavailability of TRP could ultimately impact on the synthesis of brain serotonin and therefore serotoninergic neurotransmission. However, an alternative interpretation is the generation of TRP-neuroactive metabolites as a result of IDO activation, such as kynurenine which by itself is able to induce at least acutely depressive-like behavior (O’Connor et al., in press). Although not addressed in the present set of experiments, the mechanisms by which IDO activation ultimately induces depressive-like behavior in conditions of both acute and chronic immune stimulation remain to be elucidated.
In conclusion, the present study clearly shows that prolonged immune activation by BCG is a suitable animal model to study the pathophysiological basis of the depressive-like behavior induced by chronic inflammation. Moreover, although only correlative and with the limitations inherent to the measurement of depressive-like behavior in rodents, the present findings report a temporal association between the occurrence of depressive-like behavioral changes and the increase in IDO activity. These results are highly suggestive of a role of IDO as a potential molecular mediator of chronic inflammation-associated depression. Based on these findings, the causative role of IDO in depressive-like behavior can be determined by directly measuring the behavioral consequences of IDO inhibition. Future experiments will also assess the respective participation of the different downstream enzymes activated by IDO in inflammation-associated depression. It will be important to determine whether the same mechanisms apply to patients suffering from chronic cytokine-induced impairment of TRP metabolism are also associated with depressive symptoms. The present findings create a new vista to search for pharmacological strategies aimed at treating mood disorders that occur in a wide range of chronic diseases that share systemic inflammation in their pathology.
Acknowledgments
MM was supported by a doctoral fellowship from the FRM (Fondation pour la Recherche Médicale). This study was funded by INRA, CNRS, Région Aquitaine, the French Ministry of Research (ACI “Neurosciences Intégratives et Computationnelles” to N.C.) and National Institutes of Health (NIH) to KWK (MH-51569 and AG-029573) and RD (R01 MH-71349 and MH-079829).
References
- Anisman H, Merali Z. Cytokines, stress and depressive illness: brain–immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Brewer TF. Preventing tuberculosis with Bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31 (Suppl 3):S64–S67. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26:797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002a;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002b;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Castanon N, Leonard BE, Neveu PJ, Yirmiya R. Effects of antidepressants on cytokine production and actions. Brain Behav Immun. 2002;16:569–574. doi: 10.1016/s0889-1591(02)00008-9. [DOI] [PubMed] [Google Scholar]
- Costello R, Izumi T. Measurement of resistance to experimental tuberculosis in albino mice. The immune phase. J Exp Med. 1971;133:362–375. doi: 10.1084/jem.133.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: Recent developments. Curr Opin Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Dale WE, Dang Y, Brown OR. Tryptophan metabolism through the kynurenine pathway in rat brain and liver slices. Free Radic Biol Med. 2000;29:191–198. doi: 10.1016/s0891-5849(00)00341-5. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–133. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–R934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- De La Garza R., 2nd Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81:688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci USA. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O’Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, Connor JO, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301649. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS. Antidepressant actions of the exercise-regulated gene VGF. Nat Med. 2007;13:1476–1482. doi: 10.1038/nm1669. [DOI] [PubMed] [Google Scholar]
- Jain NK, Kulkarni SK, Singh A. Lipopolysaccharide-mediated immobility in mice: reversal by cyclooxygenase enzyme inhibitors. Methods Find Exp Clin Pharmacol. 2001;23:441–444. doi: 10.1358/mf.2001.23.8.662131. [DOI] [PubMed] [Google Scholar]
- Jander S, Pohl J, D’Urso D, Gillen C, Stoll G. Time course and cellular localization of interleukin-10 mRNA and protein expression in autoimmune inflammation of the rat central nervous system. Am J Pathol. 1998;152:975–982. [PMC free article] [PubMed] [Google Scholar]
- Katafuchi T, Kondo T, Yasaka T, Kubo K, Take S, Yoshimura M. Prolonged effects of polyriboinosinic:polyribocytidylic acid on spontaneous running wheel activity and brain interferon-alpha mRNA in rats: a model for immunologically induced fatigue. Neuroscience. 2003;120:837–845. doi: 10.1016/s0306-4522(03)00365-8. [DOI] [PubMed] [Google Scholar]
- Kwant A, Sakic B. Behavioral effects of infection with interferon-gamma adenovector. Behav Brain Res. 2004;151:73–82. doi: 10.1016/j.bbr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Launay JM, Copel L, Callebert J, Corvaia N, Lepage E, Bricaire F, Saal F, Peries J. Decreased whole blood 5-hydroxytryptamine (serotonin) in AIDS patients. J Acquir Immune Defic Syndr. 1988;1:324–325. [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Link H. The cytokine storm in multiple sclerosis. Mult Scler. 1998;4:12–15. doi: 10.1177/135245859800400104. [DOI] [PubMed] [Google Scholar]
- Liu X, Gershenfeld HK. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol Psychiatry. 2001;49:575–581. doi: 10.1016/s0006-3223(00)01028-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Merali Z, Brennan K, Brau P, Anisman H. Dissociating anorexia and anhedonia elicited by interleukin-1beta: antidepressant and gender effects on responding for “free chow” and “earned” sucrose intake. Psychopharmacology. 2003;165:413–418. doi: 10.1007/s00213-002-1273-1. [DOI] [PubMed] [Google Scholar]
- Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology. 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, Castanon N. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192:537–544. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. doi: 10.1038/sj.mp.4002148. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway D, Henao-Tamayo M, Smith E, Shanley C, Harton M, Troudt J, Bai X, Basaraba RJ, Orme IM, Chan ED. Animal model of Mycobacterium abscessus lung infection. J Leukoc Biol. doi: 10.1189/jlb.1007696. in press. [DOI] [PubMed] [Google Scholar]
- Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci. 2000;11:53–58. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]
- Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav. Immun. 2005;19:23–27. doi: 10.1016/j.bbi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault J, Aubert A. Immunity and emotions: lipopolysaccharide increases defensive behaviours and potentiates despair in mice. Brain Behav Immun. 2006;20:517–526. doi: 10.1016/j.bbi.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Sander B, Skansen-Saphir U, Damm O, Hakansson L, Andersson J, Andersson U. Sequential production of Th1 and Th2 cytokines in response to live Bacillus Calmette-Guerin. Immunology. 1995;86:512–518. [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Connor TJ, Nolan Y, Kelly JP, Leonard BE. Differential effect of chronic antidepressant treatments on lipopolysaccharide-induced depressive-like behavioural symptoms in the rat. Life Sci. 1999;65:1773–1786. doi: 10.1016/s0024-3205(99)00430-0. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Takikawa O, Tagawa Y, Iwakura Y, Yoshida R, Truscott RJ. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv Exp Med Biol. 1999;467:553–557. doi: 10.1007/978-1-4615-4709-9_68. [DOI] [PubMed] [Google Scholar]
- Tsenova L, Bergtold A, Freedman VH, Young RA, Kaplan G. Tumor necrosis factor alpha is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc Natl Acad Sci USA. 1999;96:5657–5662. doi: 10.1073/pnas.96.10.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, Cooper AM. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Wirleitner B, Neurauter G, Schrocksnadel K, Frick B, Fuchs D. Interferon-gamma-induced conversion of tryptophan: immunologic and neuropsychiatric aspects. Curr Med Chem. 2003;10:1581–1591. doi: 10.2174/0929867033457179. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]