Abstract
Curcumin is a phenolic natural product isolated from the rhizome of Curcuma longa (tumeric). It was previously described that curcumin had a potent anti-inflammatory effect and inhibited the proliferation of a variety of tumor cells. In the present study, we investigated the inhibitory effects of curcumin on the response of normal murine splenic B cells. Curcumin inhibited the proliferative response of purified splenic B cells from BALB/c mice stimulated with the Toll-like receptor ligands LPS and CpG oligodeoxynucleotides. LPS-induced IgM secretion was also inhibited by curcumin. The proliferative response induced by either the T-independent type 2 stimuli anti-delta-dextran or anti-IgM antibodies was relatively resistant to the effect of curcumin. We investigated the intracellular signaling events involved in the inhibitory effects of curcumin on murine B cells. Curcumin did not inhibit the increase in calcium levels induced by anti-IgM antibody. Western blotting analysis showed that curcumin inhibited TLR ligands and anti-IgM-induced phosphorylation of ERK, IκB and p38. Curcumin also decreased the nuclear levels of NFκB. Our results suggested that curcumin is an important inhibitor of signaling pathways activated upon B cell stimulation by TLR ligands. These data indicate that curcumin could be a potent pharmacological inhibitor of B cell activation.
Keywords: Curcuma longa (tumeric), Immunoglobulin, Signal transduction, Toll like receptors, T-independent antigen
Introduction
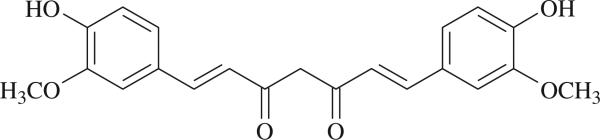
Curcumin (diferuloylmethane) (Fig. 1) is the major compound derived from the rhizome of the plant Curcuma longa. It has anti-inflammatory, anti-microbial, antiviral, anti-fungal, antioxidant and wound healing activities (Aggarwal and Shishodia 2006). Curcumin was shown to modulate the response of macrophages, natural killer cells, T lymphocytes and dendritic cells (Jagetia and Aggarwall 2007). Also, it inhibits EBV-induced immortalization of B cells and the proliferation of several tumor B cells (Jagetia and Aggarwall 2007), while stimulating the proliferation of intestinal B cells (Churchill et al. 2000). In vitro studies showed that low doses of curcumin enhanced IgM production by total rat spleen lymphocytes while high doses have an opposite effect (Kuramoto et al. 1996). This study did not investigate, however, the issue of whether the decrease in antibody response would be due to direct effects of curcumin on B cells.
Fig. 1.
Formula of curcumin.
Several studies have demonstrated that phytochemicals like polyphenols and sesquiterpene lactones inhibit NF-κB activation induced by several stimuli including LPS and TNF-α (Surh 2003). Curcumin was shown to inhibit NFκB activation induced by LPS, PMA, TNF-α and hydrogen peroxide. This effect was mediated through the inhibition of IKKβ-induced phosphorylation of IκBα, which decreased IκB ubiquitinylation and degradation (Singh and Aggarwal 1995).
Human and murine B cells express Toll-like receptors (TLR) and activation through these molecules induces proliferation and immunoglobulin secretion by naïve B cells in vitro (Fillatreau and Manz 2006). The stimulation of B cells by TLRs induces the translocation of NFκB to the nucleus (Fillatreau and Manz 2006). Polyclonal in vitro B cell response can also be stimulated by ligation to surface immunoglobulin like soluble anti-immunoglobulin (Ig) and polysaccharide-conjugated anti-immunoglobulin antibodies (anti-delta-dextran) (Mond et al. 1995).
Despite the extensively characterized anti-inflammatory effect of curcumin and its reported effect on T cells and macrophages, no study have dealt with the characterization of the effect of curcumin on purified normal B cells yet. In the present study we investigated the inhibitory effect of curcumin on B cell response induced by TLR ligands, anti-IgM antibody or soluble dextran-conjugated anti-Ig antibodies. We described that curcumin have a major inhibitory effect on TLR ligands-mediated B cell activation and characterized the signaling pathways inhibited by curcumin.
Material and methods
Materials
Lipopolysaccharide (LPS) W (extracted from E. coli 0111:B4), Goat anti-mouse IgM and curcumin were obtained from Sigma Chem. Co. (St. Louis, MO, USA). The curcumin was diluted in DMSO. Control cultures without curcumin and with DMSO were done in parallel. Curcumin was endotoxin free, as determined by NMR and mass spectroscopy. Pam3Cys-Ser-(Lys)4 (Pam3Cys) was obtained from EMC Microcollections (Tübingen, Germany) and oligodeoxynucleotides with CpG sequences (CpG) were synthesized by Integrated DNA Technologies (Coralville, IA, USA). The dextran-conjugated anti-IgD antibody (anti-delta-dextran) was prepared as described by Brunswick et al. (1988) and was provided by Dr. J. J. Mond (Biosynexus, Gathersburg, MD, USA).
Animals
Male and female BALB/c mice (6–8 weeks of age) were obtained from the animal facility of the Instituto de Microbiologia, Universidade Federal do Rio de Janeiro, Brazil. The animals were bred and housed according to institutional policies for animal care and usage.
B cell purification and culture
The purification of splenic B cells was performed by T cell depletion with anti-CD3, anti-CD4 and anti-CD8 antibodies followed by treatment with low-tox rabbit complement (Cedarlane, Ontario, Canada) (Brunswick et al. 1988). This procedure was followed by fractionation using discontinuous Percoll gradients (Brunswick et al. 1988). High-density (resting) B cells were collected at the 65–70% Percoll interface. Flow cytometry analyses of the purified B cells showed that over 90% of the cells were B220+. Cultures were performed at 37 °C in 7% CO2 atmosphere using RPMI 1640 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal calf serum, l-glutamine (2 mM), 2-ME (50 μM), nonessential aminoacids (100 μM), sodium pyruvate (1 mM) and gentamicin (50 μg/ml).
Proliferation was determined by the measurement of specific incorporation of tritiated thymidine analyzed by liquid scintillation spectroscopy as previously described (Brunswick et al. 1988).
Culture supernatant immunoglobulin levels were determined by capture ELISA as previously described (Snapper and Paul 1987).
Immunoblots
Purified B cells (107 cells/ml) were treated with curcumim. The samples were then stimulated with LPS, CpG oligodeoxynucleotides or Pam3Cys for 30 min. Some cultures were alternatively treated with anti-IgM for 5 min. Incubations were carried out at 37 °C. The cells were lysed, proteins were separated by SDS-PAGE and immunoblots were performed as describe (Benschop et al. 2001). Primary antibodies were obtained from Cell Signaling Technologies (Danvers, MA, USA). Secondary peroxidase-labeled antibodies were purchased from Jackson Immunoresearch (PA, USA). The ratio of total/phosphorylated protein was determined using the ScionImage software.
Eletrophoretic mobility shift assay
Purified B cells (107) were treated with curcumin (10 μM) for 30 min. This treatment was followed by stimulation with LPS 10 μg/ml for 16 h at 37 °C. The nuclear cell extracts were obtained as described previously and a gel shift assay was performed as detailed by Pereira et al. (2005).
Measurement of intracellular calcium
Purified B cells (5 × 106/ml) were loaded for 45 min at 37 °C with 1 μM Indo-1 acetoxymethyl (Indo-AM; Molecular Probes, Eugene, OR, USA). The cells were then stained with anti-B220-FITC antibody and analyzed (1 × 106 cells/ml in RPMI 2% FCS) by flow cytometry (LSR, BD Biosciense, Mountain View, CA, USA). After baseline was established, cells were stimulated with rabbit anti-mouse IgM and treated with curcumin 10 μM. FlowJo software (Tree Star, San Carlos, CA, USA) was used for analysis. The mean intracellular calcium was determined by measurement of the fluorescence ratio of Indo-AM (390 nm/490 nm emission).
Statistical analysis
The results are reported as means±SD. Statistical analysis was performed using the Student t-test for independent samples, with the level of significance set at p≤0.05.
Results and discussion
Effect of curcumin on proliferation and Ig secretion by activated B cells
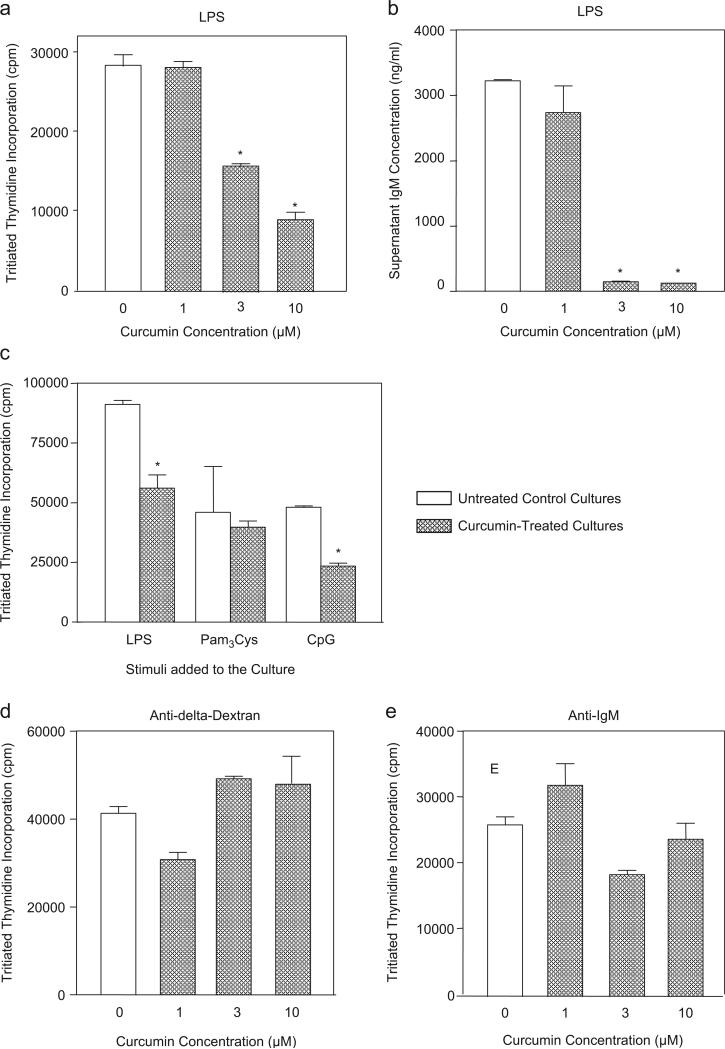
Our main goal in this study was to characterize the effect of curcumin on in vitro-activated normal B cells. We tested whether curcumin would have a direct effect upon normal splenic murine B cells. Curcumin inhibited both proliferation and IgM secretion stimulated by the TLR4 ligand LPS (Figs. 2a and b). The mean IC50 value of curcumin on LPS-stimulated proliferation was 8.31 μM (±2.36). The proliferative response induced by the TLR9 ligand CpG was also inhibited by curcumin, while the response stimulated by the TLR2 ligand Pam3Cys was not modified by this substance (Fig. 2c). Curcumin did not inhibit B cell response stimulated by direct binding to surface Ig (cultures treated with either anti-delta-dextran or soluble anti-IgM) (Figs. 2d and e). The inhibitory effect on LPS-induced response was not due to an unspecific toxic effect, since curcumin did not induce an increase in the percentage of propidium iodide positive cells in LPS-stimulated cultures (data not shown). Also, curcumin did not have an overall inhibitory effect on B cell response since it did not alter in vitro LPS-induced CD86 expression (data not shown).
Fig. 2. Curcumin inhibits B cell proliferation and IgM production induced by TLR ligands but not by ligands to surface Ig.
(a) LPS-induced proliferation. Purified B cells from BALB/c mice (1 × 106/ml) were stimulated with LPS (10 μg/ml). The cultures were set in triplicate in a final volume of 200 μl and were treated simultaneously with the indicated doses of curcumin. No curcumin was added at control cultures. Proliferation was measured in cultures carried out for 48 h by the addition of tritiated thymidine (0.5 μCi per well) during the final 24 h of culture. Data are expressed as mean counts per minute (cpm) levels of triplicate cultures. Control untreated cultures showed <1200 cpm. (b) LPS-induced immunoglobulin secretion. Cultures of purified B cells (2.5 × 105/ml) were set in triplicate in a final volume of 200 μl and were stimulated with LPS (10 μg/ml). Cultures were treated simultaneously with the indicated doses of curcumin. Pooled supernatants from seven days cultures were obtained and IgM levels were determined by ELISA. Control cultures had undetectable levels of IgM (<10 ng/ml). (c) TLR ligands-induced proliferation. Cultures were set as described in (a) and were stimulated with LPS (10 μg/ml), Pam3Cys (1 μg/ml) or CpG (1 μg/ml). Some cultures were also treated with curcumin (10 μM). No curcumin was added to control cultures. Proliferation was measured as described in (a). Control untreated cultures showed <1130 cpm. (d) Anti-delta-dextran-induced proliferation. B cells were stimulated with anti-delta-dextran (10 ng/ml) and curcumin was added at the indicated doses. Cultures were set and analyzed as described in (a). Control untreated cultures showed <1240 cpm. (e) Anti-IgM-induced proliferation. B cells were stimulated with anti-mouse IgM (5 μg/ml) and curcumin was added at the indicated doses. Cultures were set and analyzed as described in (a). Control untreated cultures showed <1210 cpm. Data in all figures are representative of three independent experiments. *statistically significant data (p ≤ 0.05).
The finding that curcumin inhibited LPS-induced response but not the one induced to binding to surface Ig could be explained by the fact that these activators engage distinct signaling transduction pathways. Previous studies have shown, for instance, that the binding of anti-Ig-dextran to surface Ig would induce a potent and sustained increase in intracellular calcium levels (Yamada et al. 1993), while LPS would not modify intracellular calcium levels on B cells (Bijsterbosch et al. 1985).
Inhibition of B cell signal transduction by curcumin
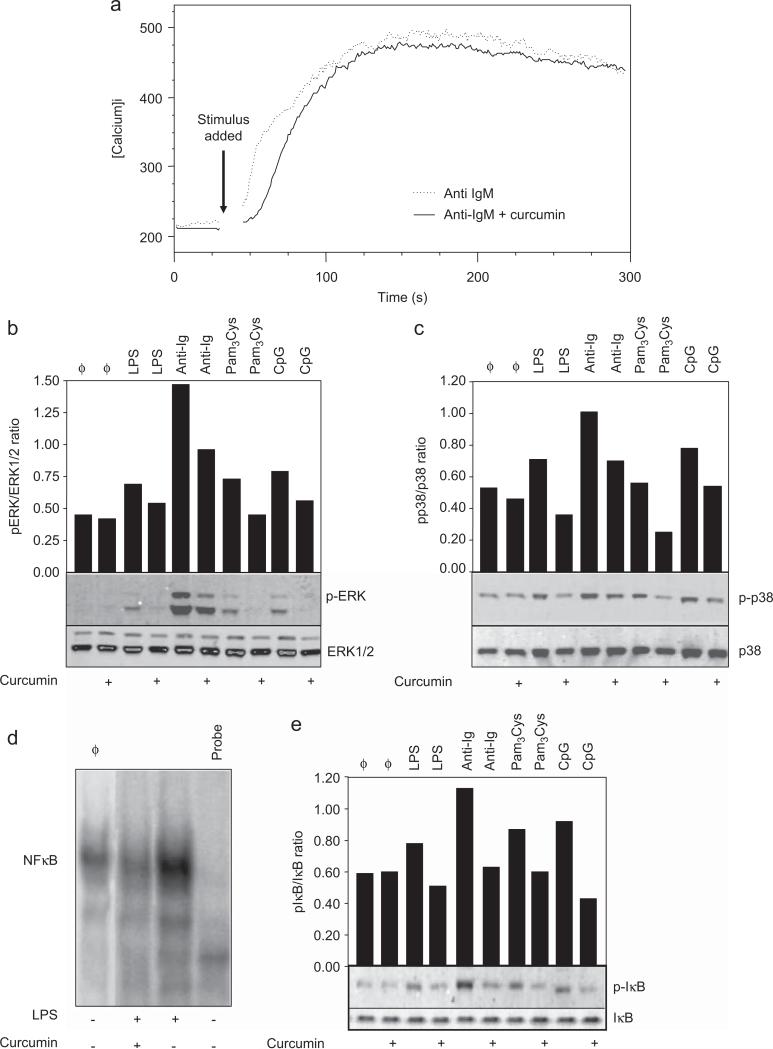
Intracellular calcium rise is induced after BCR-mediated activation and is a downstream signaling event that depends on B cell on Syk-mediated activation (Engelke et al. 2007). We observed that the increase in intracellular calcium levels induced by anti-IgM antibodies was not modified by treatment with curcumin (Fig. 3a). This finding suggests that the above cited initial signaling event is not modified by curcumin.
Fig. 3. Effect of curcumin in B cell signaling transduction.
(a) Intracelular calcium levels. Purified splenic murine B cells were loaded with Indo-AM and then stained with anti-B220-FITC antibody. Baseline calcium levels were determined by flow cytometry and the cells were then stimulated with anti-IgM (10 μg/ml) and treated with curcumin (10 μM). The mean calcium levels were determined by measurement of the fluorescence ratio of Indo-1-AM (390 nm/490 nm emission) and analyzed by the FlowJo software. Data are representative of three independent experiments. (b and c) MAPK phosphorylation. Purified B cells were stimulated with LPS (10 μg/ml), CpG oligodeoxynucleotides (1 μg/ml), Pam3Cys (1 μg/ml) or anti-IgM antibody (10 μg/ml). Some cultures were simultaneously treated with curcumin (10 μM). Cells were then lysed after either a 5 min (anti-IgM-treated cells) or a 30 min (TLR ligands-treated cells) treatment and whole-cell lysates were loaded onto SDS-PAGE gels. Blot were run and probed with the following antibodies: (b) anti-phospho ERK antibody; (c) anti-phospho-p38 antibody. The same blots were then stripped and reprobed with antibodies to nonphosphorylated proteins to determine absolute protein levels. Bar graphics shows the ratio of phosphorylated and total proteins. Data are representative of five independent experiments. (d) NFκB translocation to the nucleus. Purified B cells were treated with curcumin (10 μM) and stimulated with LPS (10 μg/ml) overnight. Nuclear extracts were prepared and tested for NFκB binding activity by EMSA using a double-stranded NF-κB consensus oligonucleotide. A double-stranded mutated oligonucleotide was used to examine the specificity of NFκB binding. The specific NF-κB bands are indicated. (e) IκB phosphorylation. B cells were stimulated with LPS (10 μg/ml), CpG (1 μg/ml), Pam3Cys (1 μg/ml) or anti-IgM antibody (10 μg/ml). Curcumin was added at 10 μM where indicated. Cells were then lysed after either 5 min (anti-IgM-treated cells) or 30 min (TLR ligands-treated cells) treatment and whole-cell lysates were loaded onto SDS-PAGE gels. Blot were run and probed with anti-phospho-IκB antibody. The same blots were then stripped and reprobed with anti-IκB antibody to determine absolute protein levels. Bar graphics shows the ratio of phosphorylated and total proteins. Data shown are representative of three independent experiments.
Phosphorylation of mitogen activated protein kinases (MAPK) is one of the events associated to B cell activation by either TLR or Ig crosslink. We tested whether MAPKs would be a target for curcumin on activated B cells. TLR ligands induced only a low level of phosphorylation of both ERK and p38 and curcumin decreased this response; anti-IgM-induced phosphorylation of ERK and p-38 was also inhibited by curcumin (Figs. 3b and c).
We examined whether curcumin would impair NFκB translocation to the nucleus in TLR ligand-activated B cells. As shown in Fig. 3d, treatment with curcumin reduced nuclear NFκB levels in LPS-activated B cells. Since phosphorylation of IκB is an important event for induction of NFκB migration to the nucleus, we evaluated whether the impaired NFκB migration was due to a block on IκB phosphorylation. We observed a decrease in IκB phosphorylation on cells stimulated with LPS, other TLR ligands, and anti-IgM in the presence of curcumin (Fig. 3e). This suggests that the inhibitory effect of curcumin on NFκB migration to the nucleus is secondary to the inhibition of signaling events that induces IκB phosphorylation.
We used in this study several TLR ligants as B cell activators: bacterial LPS (a TLR4 ligand), CpG (a TLR9 ligand) and Pam3Cys (a TLR2 ligand) (Fillatreau and Manz 2006; Kawai and Akira 2007). Despite a similar curcumin-induced inhibition on both MAPK and IκB phosphorylation regardless of the TLR ligand tested, we observed that the proliferative response induced by Pam3Cys was resistant to the effect of curcumin. A recent study has shown that TLR9 and TLR4 ligands differ in their dependence on c-Rel for signaling (Treml et al. 2007). This finding suggest that there are differences in TLR-induced signal transduction and differences not yet characterized between both TLR2 and TLR9 signaling could explain the resistance to curcumin effect we observed in the response induced by Pam3Cys.
It was suggested that the signaling through TLR mediate the activation of autoimmune B cells present in the normal B cell repertoire (Toubi and Shoenfeld 2004). Also, in animals overexpressing the molecule BAFF, TLR signaling can induce a lupus-like disease (Groom et al. 2007). Based on the effect of curcumin on the B cell response induced by LPS, it would be possible to suggest that curcumin would be a suitable modulator of activation of autoimmune B cells in vivo. In fact, it was shown that treatment of mice subjected to experimental induced encephalomyelitis with curcumin decreased symptoms and disease severity (Natarajan and Bright 2002). Our data on the specific effect of curcumin on some polyclonal TLR-induced response of B cells, therefore, opens the possibility for its use in the treatment of autoimmune diseases where B cells are abnormally activated.
Acknowledgements
Research supported by CNPq, FINEP, PRONEX – MCT, and FAPERJ. K.K.F. Chagas, was the recipient of a CNPq fellowship; D.D. Decoté-Ricardo and J.D.B. Rocha were recipients of CAPES/MEC fellowships. Both K.K.F. Chagas and D. Decoté Ricardo contributed equally in the development of the study. The authors are indebted with the technical support of Sidney Gomes da Costa.
References
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. doi: 10.1016/s1074-7613(01)00087-5. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch MK, Meade CJ, Turner GA, Klaus GG. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985;41:999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Brunswick M, Finkelman FD, Highet PF, Inman JK, Dintzis HM, Mond JJ. Picogram quantities of anti-Ig antibodies coupled to dextran induce B cell proliferation. J. Immunol. 1988;140:3364–3372. [PubMed] [Google Scholar]
- Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J. Surg. Res. 2000;89:169–175. doi: 10.1006/jsre.2000.5826. [DOI] [PubMed] [Google Scholar]
- Engelke M, Engles N, Dittmann K, Stork B, Wienands J. Ca(2+) signaling in antigen receptor-activated B lymphocytes. Immunol. Rev. 2007;218:235–246. doi: 10.1111/j.1600-065X.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Manz RA. Tolls for B cells. Eur. J. Immunol. 2006;36:798–801. doi: 10.1002/eji.200636040. [DOI] [PubMed] [Google Scholar]
- Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupus like disease independent of T cells. J. Exp. Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagetia GC, Aggarwall BB. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kuramoto Y, Yamada K, Tsuruta O, Sugano M. Effect of natural food colorings on immunoglobulin production in vitro by rat spleen lymphocytes. Biosci. Biotechnol. Biochem. 1996;60:1712–1713. doi: 10.1271/bbb.60.1712. [DOI] [PubMed] [Google Scholar]
- Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr. Opin. Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Bright JJ. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through Janus Kinase-STAT pathway in T lymphocytes. J. Immunol. 2002;168:6506–6513. doi: 10.4049/jimmunol.168.12.6506. [DOI] [PubMed] [Google Scholar]
- Pereira RM, Calegari-Silva TC, Hernandez MO, Saliba AM, Redner P, Pessolani MC, Sarno E, Sampaio EM, Lopes UG. Mycobacterium leprae induces NF-kappaB-dependent transcription repression in human Schwann cells. Biochem. Biophys. Res. Commun. 2005;335:20–26. doi: 10.1016/j.bbrc.2005.07.061. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is supressed by curcumin (diferuloylmethane). J. Biol. Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Paul WE. B cell stimulatory factor 1 (Interleukin 4) prepares resting murine B cells to secrete IgG1 upon subsequent stimulation with bacterial lypopolysaccharide. J. Immunol. 1987;139:10–17. [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Toubi E, Shoenfeld Y. Toll-like receptors and their role in the development of autoimmune diseases. Autoimmunity. 2004;37:183–188. doi: 10.1080/08916930410001704944. [DOI] [PubMed] [Google Scholar]
- Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, Cancro MP, Khan WN. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J. Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- Yamada H, June CH, Finkelman F, Brunswick M, Ring MS, Lees A, Mond JJ. Persistent calcium elevation correlates with induction of surface immunoglobulin-mediated B cell DNA synthesis. J. Exp. Med. 1993;177:1613–1621. doi: 10.1084/jem.177.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]