Abstract
Background
The relationship between vitamin D metabolites and subclinical vascular disease is controversial. Because low serum levels of 25-hydroxyvitamin D [25(OH)D] have been associated with many cardiovascular disease (CVD) risk factors, we hypothesized that serum 25(OH)D levels would be inversely associated with inflammation as measured by C-reactive protein (CRP) and with subclinical vascular disease as measured by carotid intimal medial thickness (cIMT) and coronary artery calcification (CAC).
Methods
We measured 25(OH)D levels in 650 Amish participants. CAC was measured by computed tomography, and cIMT by ultrasound. The associations of 25(OH)D levels with natural log(CAC+1), cIMT, and natural log(CRP) levels were estimated following adjustment for age, sex, family structure, and season of examination. Additional analyses were carried out adjusting for body mass index (BMI) and other CVD risk factors.
Results
25(OH)D deficiency (<20 ng/ml) and insufficiency (21-30 ng/ml) were common among the Amish (38.2% and 47.7%, respectively). 25(OH)D levels were associated with season, age, BMI, and parathyroid hormone levels. In neither the minimally or fully adjusted analyses were significant correlations observed between 25(OH)D levels and CAC, cIMT, or CRP (R2 < 0.01 for all).
Conclusion
Contrary to our hypothesis, this study failed to detect a cross-sectional association between serum 25(OH)D levels and CAC, cIMT, or CRP. Either there is no causal relationship between 25(OH)D and CVD risk, or, if there is, it may be mediated through mechanisms other than subclinical vascular disease severity.
Introduction
Vitamin D has long been known to be vital to bone health.1 More recently, vitamin D has been shown to play a role in the risk of malignancy2, immune function3, and cardiovascular health.4 The major source of vitamin D is endogenous production via the action of the sun's ultraviolet b light on 7-dehydrocholesterol precursors in the skin, converting them to vitamin D3. Vitamin D3 undergoes 25-hydroxylation in the liver, to form 25-hydroxyvitamin D3 [25(OH)D], the metabolite that reflects stores of vitamin D. The active metabolite, 1,25(OH)2D, (also called calcitriol), is formed after 1-hydroxylation in the kidneys,5 but its serum level does not correlate well with vitamin D deficiency. The optimal level of 25(OH)D has been suggested to be ≥30 ng/ml (75 nmol/L),6 a level associated with maximal suppression of intact parathyroid hormone (iPTH) and reduced fracture rates.7 Using 28 ng/ml as a cut-off, it is estimated that approximately 41% of men and 53% of women in the United States have insufficient levels of 25(OH)D.8
Previous studies have suggested that lower 25(OH)D levels are associated with increased cardiovascular disease (CVD) risk.9,10,11,12,13 Epidemiologically, such an effect is also supported by associations observed between 25(OH)D deficiency and many CVD risk factors, including hypertension, diabetes mellitus, obesity, and elevated serum triglyceride levels.14 In the National Health And Nutrition Examination Survey (NHANES), the multivariable-adjusted odds of the metabolic syndrome decreased progressively across increasing quintiles of 25(OH)D concentrations (p<0.001 for the trend)15 suggesting that higher vitamin D stores may protect against insulin resistance.16
The putative effects of vitamin D and its metabolites on CVD risk could potentially be explained by their anti-inflammatory actions.17 The active vitamin D metabolite, 1,25(OH)2D, has immunoregulatory properties3 and the vitamin D receptor is found on inflammatory cells.18 Treatment with calcitriol, [1,25(OH)2D], has been used as an immunosuppressive agent in preventing cardiac transplant allograft rejection.19 Furthermore, supplementation with calcitriol and vitamin D has also been shown to reduce production of inflammatory markers and cytokines, including C-reactive protein (CRP).20,21
Despite evidence linking low vitamin D levels to CVD risk, there remains wide debate about the role of serum vitamin D metabolites, including 25(OH)D, on CVD risk since some studies have also reported associations between increased levels of vitamin D metabolites and CVD risk.22 Important insights could be attained through assessment of the relationship between 25(OH)D and subclinical vascular disease markers such as coronary artery calcium (CAC) or carotid intimal medial thickness (cIMT), which predict risk of CVD events. In one study of 173 patients at moderately high risk for coronary heart disease, levels of the active vitamin D metabolite, 1,25-dihydroxyvitamin D [1,25(OH)2D] were inversely correlated with the extent of CAC.23 Low 25(OH)D levels have also been associated with increased cIMT in a diabetic population.24 These findings have not been confirmed in other lower-risk populations.
The Old Order Amish (OOA) of Lancaster, Pennsylvania is a closed founder population with homogeneity of lifestyle and environmental exposures. Thus confounding influences such as socioeconomic status, smoking, physical activity, manner of dress, diet, and medication usage are minimized. In the OOA population, we studied the relationship between serum 25(OH)D levels with measures of subclinical vascular disease and markers of inflammation. We hypothesized that increasing levels of serum 25(OH)D may protect against subclinical vascular disease, as measured by CAC and cIMT. Furthermore, we hypothesized that increasing levels of 25(OH)D would also be inversely associated with inflammatory markers such as CRP, and that the postulated anti-inflammatory properties of vitamin D may be one mechanism for potential CVD risk reduction.
Methods
Participants
The OOA population, an Anabaptist sect, originated in Western Europe and immigrated to the United States in the early 1700's to escape religious persecution.25 Approximately 200 founder couples who settled in Lancaster County, Pennsylvania gave rise to the estimated >25,000 OOA living there today.26 Lancaster, PA is located at a latitude of 40.06 degrees North, at a longitude of 76.3 degrees West, and at an elevation of 183 meters. The OOA are a rural population where the men are predominately farmers and the women are homemakers, and cigarette smoking and alcohol consumption are minimal.27
The Amish Family Calcification Study (AFCS) was initiated in 2002 to identify the genetic and environmental determinants of calcification of the coronary arteries and bone. A total of 808 participants were recruited into this study between 2002 and 2006. CAC was measured in all AFCS participants, and serum 25(OH)D levels were measured in a subset of those with available CAC data (n=650) who were additionally enrolled in the Amish Family Osteoporosis Study (AFOS). Given the willingness of the OOA to participate in several overlapping research studies, a subset also had measurement of cIMT and CRP (n=219 and n=496, respectively). Informed consent was obtained from all participants, and the study protocols were approved by the Institutional Review Boards of the University of Maryland and all collaborating institutions.
Study variables
All study participants underwent assessment of potential CVD risk factors through a medical history interview and questionnaire (including assessment of past medical history, medication usage, and smoking status), and a detailed physical examination at the Amish Research Clinic in Strasburg, PA as previously reported.28 Blood pressure was measured twice, and the mean of both measurements was used for analyses. Body mass index (BMI) was calculated as kg/m2.
Blood samples were obtained after an overnight fast for serum 25(OH)D, iPTH, total cholesterol, HDL-cholesterol, triglycerides, and high-sensitivity CRP. Lipid profiles and CRP were assayed by Quest Diagnostics (Maryland and Pennsylvania). LDL cholesterol was calculated using the Friedewald equation.29 Circulating concentrations of serum iPTH and 25(OH)D were measured in aliquots of serum samples stored at -80°C until testing. A radioimmunoassay procedure was used to measure 25(OH)D (DiaSorin, Stillwater, MN) and an immunoradiometric assay was used to measure iPTH (Nichols Institute Diagnostics, San Juan Capistrano, CA). Both assays were run in duplicate at The Johns Hopkins Bayview Medical Center General Clinical Research Center (Baltimore, MD) according to the manufacturer's instructions, and the mean of the duplicate values was used.
Hypertension was defined as a systolic blood pressure ≥140 mm Hg, and/or a diastolic blood pressure ≥ 90 mmHg, and/or use of prescription blood pressure lowering medications. Diabetes was defined by self-report and/or use of hypoglycemic medications. Current smoking included primarily cigars in this population.
A prior history of CVD was considered to be present if participants answered yes to any one or more of the following questions: “Have you been told by a physician that the arteries of your heart may be blocked?”, “Have you ever had a coronary angiogram that suggested a blockage?”, “Have you ever been told by a medical doctor that you had a heart attack?”, “Have your ever had coronary artery bypass surgery, coronary angioplasty, balloon procedure or stent put in because arteries of the heart were blocked?”, “Have you ever been told by a physician that you had a stroke, cerebral hemorrhage, or brain attack?”, or “Have you ever had surgery on a carotid artery in the neck because the artery was blocked?”
EBCT-scanning of the coronary arteries was performed on an Imatron C-150 scanner (Imatron, South San Francisco, CA) located in Timonium, Maryland. Coronary arteries were imaged with rapid acquisition of approximately 30-40 contiguous images of 3 mm slice thickness (with a 26-cm field of view) during end-diastole using ECG-triggering during a single 30-35 second breath hold. CAC was quantified using the previously described Agatston scoring method.30 The total calcium score was calculated by summing CAC scores from the left main, left anterior descending, left circumflex and right coronary arteries.
Participants in the cIMT substudy underwent high resolution B-mode ultrasound to image the right and left common carotid arteries (CCA). IMT was measured between lumen intima and media-adventitia interfaces of the far wall of the CCA (the 1 cm segment proximal to the bifurcation) using an automated edge detection system. The mean cIMT of this 1 cm segment was measured on two separate images of the left and the right CCA at the peak of the R wave on a simultaneous ECG tracing. The mean of these four measurements was used as the cIMT.
Interscan and interreader reproducibility for CAC and cIMT measurements in the OOA have previously been reported by our group.31
Statistical analyses
Because serum 25(OH)D levels vary depending on season32, 25(OH)D levels were adjusted for season of lab draw in all analyses by dividing the calendar year into four seasons and computing the residual of each subject's 25(OH)D level from the mean of that season using a regression analysis. We first tested for correlations between seasonally-adjusted serum 25(OH)D and various clinical characteristics after stratifying seasonally-adjusted serum 25(OH)D measurements into quartiles (see Table I for quartile cut-offs). Because triglycerides and CRP values did not follow a normal distribution, these variables were transformed by their natural logarithms prior to analysis. CAC was also natural logarithm transformed after first adding one to its value.
Table I. Unadjusted clinical characteristics of the Old Order Amish participants by quartiles of seasonally-adjusted serum 25(OH)D levels.
Mean/median ± standard error or % distribution, as appropriate
| Q1 6.7-18.1 ng/ml (N=164) |
Q2 18.2-21.9 ng/ml (N=163) |
Q3 22.0-26.1 ng/ml (N=163) |
Q4 26.2-49.1 ng/ml (N=164) |
p-value for trend* | |
|---|---|---|---|---|---|
| Mean 25(OH)D (ng/ml) | 14.6 ± 0.3 | 20.4 ± 0.2 | 24.1 ± 0.2 | 30.3 ± 0.4 | N/A |
| Mean age, in years | 58.0 ± 1.0 | 53.7 ± 1.1 | 52.8 ± 0.9 | 53.5 ± 1.1 | 0.003 |
| Male sex, % | 39.0 | 44.8 | 45.4 | 44.5 | 0.38 |
| Diabetes mellitus, % | 5.5 | 4.3 | 11.0 | 7.9 | 0.12 |
| Hypertension, % | 16.5 | 12.9 | 16.1 | 13.4 | 0.89 |
| Systolic blood pressure (mmHg) | 120±1 | 120±1 | 121±1 | 115±1 | 0.16 |
| Diastolic blood pressure (mmHg) | 72±1 | 72±1 | 73±1 | 70±1 | 0.17 |
| Smoking, % | 7.3 | 8.6 | 9.2 | 7.3 | 0.70 |
| History of CVD, % | 10.5 | 9.9 | 9.3 | 7.4 | 0.75 |
| Mean BMI, kg/m2 | 29.2 ± 0.5 | 27.9 ±0.4 | 28.5 ± 0.4 | 27.2 ± 0.4 | 0.009 |
| Mean iPTH | 66.4 ± 1.6 | 55.8 ± 1.6 | 55.3 ±1.7 | 51.0 ± 1.3 | <0.0001 |
| Median total cholesterol mg/dl | 208 ± 3 | 212 ± 3 | 213 ± 3 | 212 ± 3 | 0.41 |
| Median HDL-c mg/dl | 57 ± 1 | 55 ± 1 | 55 ± 1 | 59 ± 1 | 0.04 |
| Median LDL-c mg/dl | 133 ± 3 | 135 ± 3 | 136 ± 3 | 133 ± 3 | 0.51 |
| Median triglycerides mg-dl† | 80 ± 5 | 69 ± 5 | 76 ± 5 | 70 ± 4 | 0.10 |
| Use of cholesterol meds, % | 5.5 | 2.5 | 3.1 | 4.9 | 0.85 |
| Framingham Risk Score | 0.086±0.007 | 0.066±0.005 | 0.068±0.005 | 0.058±0.004 | 0.06 |
p-value for all variables (except age and sex) is adjusted for age and sex. P-value for age is adjusted for sex, and p-value for sex is adjusted for age.
p-value based on log-transformed values
25(OH)D= 25-hydroxyvitamin D, CVD=cardiovascular disease, BMI=body mass index, iPTH=intact parathyroid hormone, HDL-c=high density lipoprotein cholesterol, LDL-c=low density lipoprotein cholesterol
The relation of seasonally-adjusted 25(OH)D levels with CRP levels and the subclinical vascular disease measures (cIMT and CAC) was assessed first by comparing mean levels of these variables across quartiles of seasonally-adjusted 25(OH)D, and then by correlating seasonally-adjusted 25(OH)D levels with these measures using linear regression. Logistic regression was also used to determine the risk for the presence of CAC>0 by seasonally-adjusted 25(OH)D levels. These regression analyses were performed first with adjustment for age and sex only, and then again following additional adjustment for a panel of additional CVD risk factors chosen a priori that included BMI, current smoking, hypertension, diabetes, total cholesterol, HDL-cholesterol, use of lipid-lowering medications, and history of prior CVD. Analyses were performed using a variance component approach as implemented in the SOLAR software in order to account for the relatedness among study subjects.33 A supplementary analysis was also performed excluding those participants with prior CVD (n=588 of 654 included). All statistical tests were 2-sided, and p<0.05 was considered statistically significant.
Prior to carrying out these analyses, we estimated the minimum correlations (r) detectable in our sample (at α = 0.05) between 25(OH)D levels and CRP, CAC, and cIMT. These analyses indicated that we would have 80% power to detect correlations of 0.13 for CRP (n = 496 participants), 0.11 for CAC (n = 650 subjects), and 0.19 for cIMT (n = 219 participants).
Results
Vitamin D deficiency was common among the OOA with 38.2% having vitamin D deficiency (levels <20 ng/ml) and 47.7% having suboptimal levels (21-29.9 ng/ml). Mean level of 25(OH)D for the overall study population was 22.3 ± 6.8 ng/ml. Serum 25(OH)D levels varied by season with lower mean levels of 20.2 ± 6.8 ng/ml for winter (October through March) and higher mean levels of 23.8 ± 6.4 ng/ml for summer (April through September) (p<0.0001). Thus all subsequent analyses were seasonally-adjusted using all 4 seasons.
Clinical characteristics of study participants by quartiles of seasonally-adjusted 25(OH)D levels are summarized in Table I. Subjects with lower levels of 25(OH)D were older, had a higher BMI, and a higher iPTH (p<0.01 for all for trend across quartiles). Age and sex adjusted results showed a borderline association of 25(OH)D levels with HDL-cholesterol (Table I). In contrast to published survey data from NHANES,14 25(OH)D levels did not differ significantly between men and women in the Amish population; nor were there any substantive gender-differences in study recruitment by season.
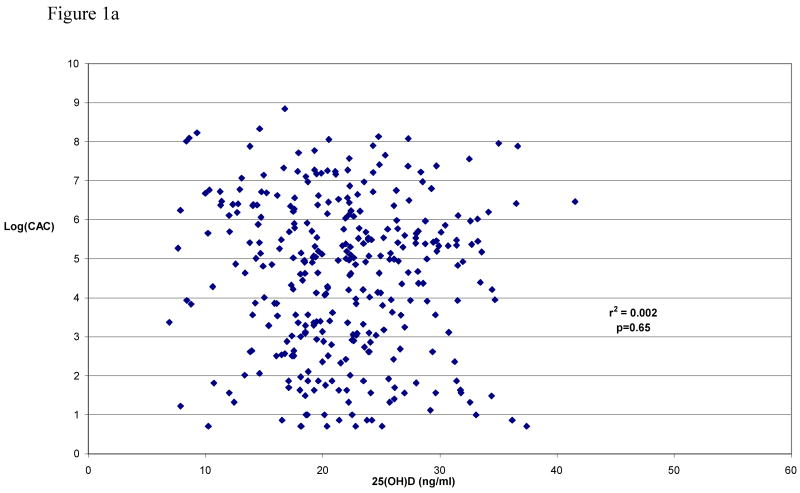
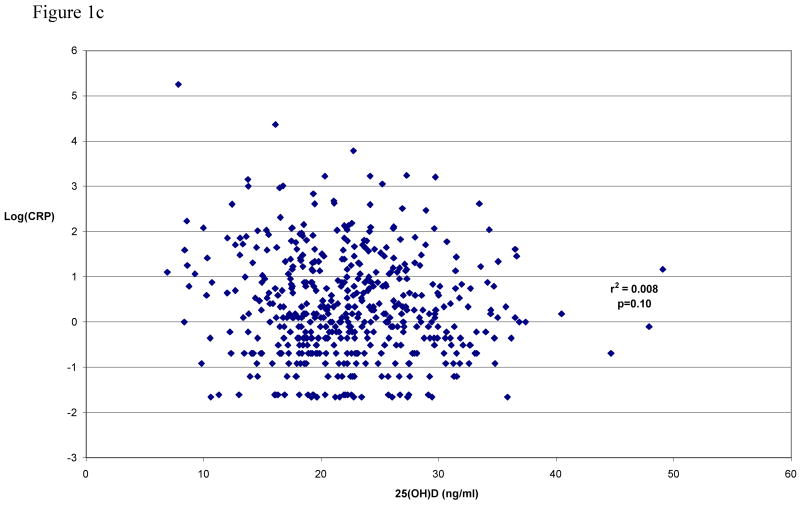
Table II shows the unadjusted median (and inter-quartile range) of CAC, cIMT, and CRP values according to quartile of seasonally-adjusted 25(OH)D levels. The correlations of seasonally-adjusted 25(OH)D levels with each unadjusted variable are shown pictorially in Figure 1. Significance testing, carried out by regressing 25(OH)D levels against each measure with adjustment for age and sex, still revealed no evidence for association. This result was virtually unchanged with additional adjustment for other CVD risk factors (e.g., BMI, current smoking, hypertension, self-reported diabetes status, total cholesterol, HDL-cholesterol, use of cholesterol-lowering medications, and history of prior CVD).
Table II. Median (and interquartile range) of CAC, cIMT, and CRP according to quartile of seasonally-adjusted 25(OH)D levels.
| quartile of seasonally-adjusted 25(OH)D | ||||||
|---|---|---|---|---|---|---|
| n | Q1 | Q2 | Q3 | Q4 | p-value for trend* | |
| CAC score (Agatston units) † | 650 | 6 (0, 224) |
0 (0, 59) |
0 (0, 101) |
0 (0, 147) |
0.80 |
| cIMT (mm) | 219 | 0.62 (0.51, 0.70) |
0.67 (0.51, 0.74) |
0.65 (0.56, 0.72) |
0.60 (0.52, 0.73) |
0.52 |
| CRP (mg/dl)† | 496 | 1.60 (0.60, 4.10) |
1.10 (0.60, 2.40) |
1.35 (0.70, 2.90) |
1.15 (0.60, 2.30) |
0.22 |
adjusted for age and sex
p-value based on log-transformed values
Results were still not statistically significant in alternative linear regression models excluding those with CVD at baseline [p=0.78 for log(CAC+1), p=0.73 for cIMT, p=0.95 for log(CRP)]. Seasonally-adjusted 25(OH)D levels were also not associated with the presence of CAC>0 (yes or no) in fully-adjusted multivariable logistic regression (p=0.55). Because 25(OH)D levels may not confer a linear risk across quartiles, levels of 25(OH)D in the lowest quartile (<18.1 ng/ml) was also additionally compared to levels ≥18.1 (combined quartiles 2-4), but there was still no statistically significant association of the lowest quartile of 25(OH)D with CAC, cIMT, or CRP levels in multivariate models. Finally, we tested formally for a parabolic relation between 25(OH)D and outcome by including in the model a polynominal term for 25(OH)D to allow for a nonlinear and non-monotonic effect on CAC, cIMT, and CRP. In none of these 3 analyses was the effect of the polynomial term statistically significant.
Discussion
We found that vitamin D deficiency and insufficiency were very common among the OOA with 86% having suboptimal levels <30 ng/ml. The high prevalence of vitamin D deficiency in the OOA might be related to the practice of wearing clothing that covers most of the skin. Serum 25(OH)D levels vary with geography, seasonality, latitude, and altitude presumably as a result of sunlight exposure.4 As expected, we observed significantly lower 25(OH)D levels in the Amish during the winter season.
Low 25(OH)D levels have been associated with the CVD risk factors of hypertension,34 obesity,35 glucose intolerance36, and the metabolic syndrome15 and has been implicated in the pathogenesis of stroke10 and congestive heart failure.37 Yet animal models suggest that animals fed a vitamin-D and cholesterol rich diet have accelerated atherosclerosis.38,39 In humans, however, the associations of both 25(OH)D and 1,25(OH)2D levels with CVD events is widely debated in the literature with small case-control studies suggesting both increased22 and decreased risk.9-10 More recently though several prospective cohort studies have found an association of low vitamin D levels with myocardial infarction12 and CVD death.13 A recent analysis from the Framingham Offspring Study found that 25(OH)D deficiency (serum levels <15 ng/ml) was associated with increased risk of incident CVD events (adjusted HR of 1.62, 95% CI 1.11-2.36) compared to those with levels ≥ 15ng/ml.11 These discordant findings between studies may be due to confounding influences such as socioeconomic status, smoking, diet, and physical activity level that were not recognized or adequately taken into account.
However, in generally healthy post-menopausal women participants of the Women's Health Initiative (WHI), vitamin D supplementation (200 IU BID) did not reduce CVD risk over 7 year average follow-up40; although it is widely agreed that the supplementation dose was inadequate for the normal adult requirement of at least 800 IU daily of vitamin D.41 Furthermore 25(OH)D levels were not measured in that trial; thus, whether vitamin D supplementation at higher doses can reduce CVD risk among those with documented vitamin D deficiency is unknown. A more recent meta-analysis of 18 randomized clinical trials, including WHI, did show that participants randomized to vitamin D supplementation experienced fewer deaths compared to those randomized to placebo.42
The association of serum levels of vitamin D metabolites with subclinical disease is also controversial. In a group of 173 patients at moderately high risk for coronary heart disease who underwent EBCT scanning of their coronary arteries, Watson et al found serum 1,25(OH)2 D levels were inversely correlated with the extent of coronary calcification.23 Their findings might be explained by the differentiating effect on cells and the anti-inflammatory effect of 1,25(OH)2D found by others.17-21 However, another study by Arad et al found no correlation between serum 1,25(OH)2D levels and coronary calcification in 50 patients undergoing coronary angiography.43
We did not find a statistically significant relationship between 25(OH)D and CAC despite a larger sample size. However, we studied the association of 25(OH)D (a better marker of vitamin D stores)1 with subclinical atherosclerosis, while Watson et al23 studied 1,25(OH)2D (the active metabolite), so our studies are not directly comparable. While a prior study found that low 25(OHD levels were associated with cIMT among diabetic individuals,24 we did not find an association of 25(OH)D levels and cIMT.
It is possible that the increased risk that vitamin D deficiency confers on CVD may not be mediated through subclinical disease markers such as CAC or cIMT. If the relationship between low 25(OH)D and CVD risk is causal, other potential mechanisms might include incident hypertension34, incident diabetes44, left ventricular hypertrophy45, and/or regulation of the renin/angiotension pathway46 which may not be reflected in the subclinical markers measured, at least cross-sectionally.
Preliminary studies suggest that vitamin D supplementation may decrease serum markers of inflammation. Timms et al found that sensitive CRP correlated inversely with 25(OH)D (R= -0.22, p=0.03),47 and two small clinical trials found that treatment with activated vitamin D (calcitriol) lowered serum CRP levels.47,48 We found a very weak inverse association of serum 25(OH)D levels with CRP, but this association was no longer present after further adjusting for BMI in our multivariable models.
Study Limitations
The OOA have very similar lifestyle and environmental exposures and likely get similar degrees of sunlight exposure because of the confined geographic area in which they live. Thus confounding influences that might falsely create a relationship between 25(OD)D and CVD or mask such a relationship would be expected to be minimized. However, our results may not be generalizable to other racial/ethnic groups or may not be generalizable to others living in different geographical latitudes and altitudes with differing sunlight exposure. There is a relationship in the OOA between traditional CVD risk factors and subclinical disease suggesting that similar mechanisms leading to atherosclerosis exist in the OOA.28 The OOA, being a predominantly farming community, likely spend more time outdoors than the majority of Americans. However despite this, vitamin D insufficiency was very common among OOA participants.
Our study is also limited by its cross-sectional design, and residual confounding and reverse causation are potential concerns, as in any cross-sectional study. Also, a one-time 25(OH)D measurement may not reflect lifetime vitamin D status. Vitamin D stores vary over seasons and time within an individual, and subclinical disease develops over years; however all study variables were measured only once in our population. We did not adjust for serum creatinine in our multivariable analyses, and renal failure is a risk factor for both vitamin D deficiency and CVD. However, only 1% of the AFCS participants had creatinine levels >1.3 and no individuals in the study were on chronic kidney dialysis. The sample size in our study was relatively small, so it remains a possibility that a real association between 25(OH)D and subclinical vascular disease or CRP exists and that our study was underpowered to detect it; however, such an association would likely be small in magnitude. Of note, our sample sizes were similar to previous studies23,47 that did find statistically significant associations.
Finally, 1,25(OH)2D was not measured in our population. Although 25(OH)D is the best marker of vitamin D stores and the best screen for vitamin D deficiency,1 1,25(OH)2D is the active metabolite and 25(OH)D levels might not reflect circulating 1,25(OH)2D levels which are under tight regulation by iPTH. In addition, we have no method to assess local 1,25(OH)2D production in the vasculature, which may be more critical than circulating levels.
Conclusion
Contrary to our hypothesis that higher levels of serum 25(OH)D would confer protection against subclinical vascular disease and inflammation, this study failed to show significant association between 25(OH)D levels and the presence or severity of CAC, nor was there a significant independent association of 25(OH)D with cIMT and CRP, suggesting that circulating 25(OH)D levels do not explain variation in markers of subclinical vascular disease or inflammation measured at the same time in the OOA.
Figure 1.
Figure 1a Seasonally-adjusted serum 25(OH)D levels vs. log transformed coronary artery calcification (n=321, excluding subjects with CAC=0)
Figure 1b Seasonally-adjusted serum 25(OH)D levels vs. carotid IMT (n=219)
Figure 1c Seasonally-adjusted serum 25(OH)D levels vs. log transformed CRP (n=496)
Acknowledgments
We gratefully acknowledge our Amish liaisons and field workers and the extraordinary cooperation and support of the Amish community without which these studies would not have been possible. This work was supported by research grants R01 HL088119, R01 AR046838, U01 HL72515 and University of Maryland General Clinical Research Center (GCRC), Grant M01 RR 16500, Johns Hopkins University GCRC, Grant M01 RR 000052, GCRC, National Center for Research Resources, Maryland Clinical Nutrition and Research Unit, Grant P30 DK072488, NIH; Baltimore Veterans Administration Geriatric Research and Education Clinical Center. Dr. Michos is funded by a Clinician Scientist Award at Johns Hopkins School of Medicine and a Career Development Award through American College of Cardiology Foundation in conjunction with Pfizer.
Footnotes
Conflicts of Interest: EDM received a one-time minimal honorarium from Abbott pharmaceuticals. The other authors have no conflict of interest related to this manuscript.
References
- 1.Streeten EA, Levine MA. Vitamin D metabolism or action. In: Emery AE, Rimoin D, editors. Principles and Practice of Medical Genetics. 4th. Churchill Livingston; 2002. pp. 2566–2623. [Google Scholar]
- 2.Spina CS, Tangpricha V, Uskokovic M, et al. Vitamin D and cancer. Anticancer Res. 2006;26(4A):2515–24. [PubMed] [Google Scholar]
- 3.Mathieu C, Adorini L. The coming of age of 1,25 dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends in Molecular Medicine. 2002;8:174–8. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 4.Zitterman A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. British Journal of Nutrition. 2005;94:483–92. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 5.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D(3)-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–21. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 6.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from NHANES III. Ethn Dis. 2005;15S5:97–101. [PubMed] [Google Scholar]
- 9.Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–63. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 10.Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, Warburton EA. Reduced Vitamin D in Acute Stroke. Stroke. 2006;37:243–45. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin D and 1,25 dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 14.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care. 2005;28:1228–30. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 16.Baynes KC, Boucher BJ, Feskens EJ, Kromhout D. Vitamin D, glucose intolerance, and insulinemia in elderly men. Diabetologia. 1997;40:344–7. doi: 10.1007/s001250050685. [DOI] [PubMed] [Google Scholar]
- 17.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25 dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–55. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25 dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–93. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 19.Briffa NK, Keogh AM, Sambrook PN, Eisman JA. Reduction of immunosupressant therapy requirement in heart transplantation by calcitriol. Transplantation. 2003;75:2133–4. doi: 10.1097/01.TP.0000065179.06731.99. [DOI] [PubMed] [Google Scholar]
- 20.Lange U, Jung O, Teichmann J, Neeck G. Relationship between disease activity and serum levels of vitamin D metabolites and parathyroid hormone in ankylosing spondylitis. Osteoporos Int. 2001;12:1031–5. doi: 10.1007/s001980170013. [DOI] [PubMed] [Google Scholar]
- 21.Mahon BD, Gordon SA, Cruz J. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. Journal of Neuroimmunology. 2003;134:128–32. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 22.Rajasree S, Rajpal K, Kartha CC, Sarma PS, Kutty VR, Iyer CS, Girija G. Serum 25-hydroxyvitamin D3 levels are elevated in South Indian patients with ischemic heart disease. European Journal of Epidemiology. 2001;17:567–71. doi: 10.1023/a:1014559600042. [DOI] [PubMed] [Google Scholar]
- 23.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–60. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 24.Targher G, Bertolini L, Padovani R, Zenari L, Scala L, Cigolini M, Arcaro G. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol. 2006;65:593–7. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 25.McKusick VA. Medical Genetics Studies of the Amish. Baltimore, MD: The Johns Hopkins University Press; 1978. [Google Scholar]
- 26.Church Directory of the Lancaster County Amish. Gordonsville, PA: Peqaea Publishers; 1996. [Google Scholar]
- 27.Hsueh WC, Mitchell BD, Schneider JL, et al. QTL influencing blood pressure maps to the region of PPH1 on chromosome 2q31-34 in the Old Order Amish. Circulation. 2000;101:2810–16. doi: 10.1161/01.cir.101.24.2810. [DOI] [PubMed] [Google Scholar]
- 28.Post W, Bielak LF, Ryan KA, Cheng YC, Shen H, Rumberger JA, Sheedy PF, 2nd, Shuldiner AR, Peyser PA, Mitchell BD. Determinants of coronary artery and aortic calcification in the Old Order Amish. Circulation. 2007;115:717–24. doi: 10.1161/CIRCULATIONAHA.106.637512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 31.Rampersaud E, Bielak LF, Parsa A, Shen H, Post W, Ryan KA, Donnelly P, Rumberger JA, Sheedy PF, II, Peyser PA, Shuldiner AR, Mitchell BD. The association of coronary artery calcification and carotid artery intima-media thickness with distinct, traditional coronary artery disease risk factors in asymptomatic adults. Am J Epidemiol. 2008 Sep 19; doi: 10.1093/aje/kwn211. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–7. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 33.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and the risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 35.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–61. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 36.Hypponen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care. 2006;29:2244–6. doi: 10.2337/dc06-0946. [DOI] [PubMed] [Google Scholar]
- 37.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 38.Kunitomo M, Kinoshita K, Bando Y. Experimental atherosclerosis in rats fed a vitamin D, cholesterol-rich diet. J Pharmacobiodyn. 1981;4:718–23. doi: 10.1248/bpb1978.4.718. [DOI] [PubMed] [Google Scholar]
- 39.Ito M, Cho BH, Kummerow FA. Effects of a dietary magnesium deficiency and excess vitamin D3 on swine coronary arteries. J Am Coll Nut. 1990;9:155–63. doi: 10.1080/07315724.1990.10720365. [DOI] [PubMed] [Google Scholar]
- 40.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, Heckbert SR, Johnson KC, Manson JE, Sidney S, Trevisan M, Women's Health Initiative Investigator Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115:827–8. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 41.Michos ED, Blumenthal RS. Vitamin D supplementation and cardiovascular disease risk. Circulation. 2007;115:827–8. doi: 10.1161/CIRCULATIONAHA.106.686238. [DOI] [PubMed] [Google Scholar]
- 42.Autier P, Gandini S. Vitamin D Supplementation and Total Mortality: A Meta-analysis of Randomized Controlled Trials. Arch Intern Med. 2007;167(16):1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 43.Arad Y, Spadaro LA, Roth M, Scordo J, Goodman K, Sherman S, Lerner G, Newstein D, Guerci AD. Serum concentration of calcium, 1,25 vitamin D and parathyroid hormone are not correlated with coronary calcifications. An electron beam computed tomography study. Coron Artery Dis. 1998;9:513–8. doi: 10.1097/00019501-199809080-00007. [DOI] [PubMed] [Google Scholar]
- 44.Knekt P, Laaksonen M, Mattila C, Härkänen T, Marniemi J, Heliövaara M, Rissanen H, Montonen J, Reunanen A. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology. 2008;19:666–71. doi: 10.1097/EDE.0b013e318176b8ad. [DOI] [PubMed] [Google Scholar]
- 45.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288(1):E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 46.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D, and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJ Med. 2002;95:787–96. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 48.Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R. Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab. 2003;88:4623–32. doi: 10.1210/jc.2003-030358. [DOI] [PubMed] [Google Scholar]