Abstract
Purpose
Pirenzepine is suggested to be a relatively selective muscarinic (M1) antagonist and is currently under investigation for the treatment of myopia. Atropine, a nonselective M-type antagonist, is used in the treatment of myopia, but has undesired ocular and systemic side effects. An M1-specific antagonist may decrease side effects and remain effective at reducing the progression of myopia. In the current study, the effects of pirenzepine on pupil diameter, resting refraction, and accommodation were studied in rhesus monkeys.
Methods
The time course and extent of mydriasis from subconjunctival injection of 2% pirenzepine were determined in five normal rhesus monkeys, and the effects on static and dynamic accommodation were determined in four rhesus monkeys with permanent indwelling electrodes in the Edinger-Westphal (EW) nucleus of the midbrain. Subconjunctival injections of 0.0002% to 0.2% pirenzepine in log unit dilutions were tested in three monkeys to determine the effects on static EW-stimulated accommodation. At 40 to 50 minutes after pirenzepine injection, accommodation was stimulated pharmacologically in both eyes, and the response was measured for 30 minutes.
Results
After 2% pirenzepine injection, pupil size increased 2.02 ± 0.41 mm, there was a hyperopic shift in resting refraction of 1.07 ± 0.23 D, and nearly complete cycloplegia occurred. Maximum EW-stimulated accommodation was significantly decreased 20 to 40 minutes after 0.02% or greater pirenzepine. Carbachol-stimulated accommodation was significantly decreased after 0.2% or greater pirenzepine.
Conclusions
Subconjunctival injections of 0.02% or greater pirenzepine result in a significant decrease in accommodation and are probably acting through nonselective muscarinic antagonism. Subconjunctival injections of 0.002% or less pirenzepine do not decrease EW-stimulated accommodation.
Pirenzepine is thought to be a relatively selective cholinergic type I muscarinic antagonist1,2 and has been undergoing human U.S. Food and Drug Administration (FDA) clinical trials for the treatment of myopia (Siatkowski R, et al. IOVS 2003;45:ARVO E-Abstract 4778). It has been shown to prevent the development of form-deprivation myopia in chicks,3 tree shrews,4 and rhesus monkeys.5 In chicks, pirenzepine prevents form-deprivation myopia through a nonaccommodative mechanism,6 by preventing axial elongation through transient modulation of scleral glycosaminoglycan synthesis.7
Pirenzepine has a history of clinical use in the treatment of peptic ulcers as an inhibitor of gastric secretion and motility. It has been reported to cause ocular side effects, including accommodative difficulties.8 For the purposes of peptic ulcer treatment, pirenzepine is taken in 50-mg doses, twice daily by mouth. The concentration of pirenzepine used for the treatment of myopia in FDA clinical trials is substantially lower, but it is applied directly to the eyes in a gel formulation (Siatkowski R, et al. IOVS 2003;45:ARVO E-Abstract 4778). Therefore, some parasympathetic antagonistic ocular side effects may occur after administration of pirenzepine. In human FDA clinical trials, “blurred vision at near” was listed as one of three most common adverse events accounting for the 11% withdrawal rate (Siatkowski R, et al. IOVS 2003;45:ARVO E-Abstract 4778). A study in children with myopia showed that subjectively measured accommodative amplitude decreased 2 to 3 D compared with the placebo group after 1% and 2% pirenzepine gel instillation.9 Measurements were taken 60 minutes after instillation, and reduced amplitudes were present approximately 12 hours after instillation. The effect continued through month 9 of the 1-year study.
Atropine, a nonselective muscarinic antagonist that has also been used for the treatment of myopia, causes ocular and systemic side effects due to its nonselective parasympathetic antagonism. Ocular side effects include mydriasis, leading to increased aberrations and photophobia,10 and paralysis of the ciliary muscle, leading to an inability to focus on near objects and the need for bifocal correction during treatment.11 It is believed that many of these side effects would be eliminated by using an M1-selective cholinergic antagonist such as pirenzepine at appropriate concentrations.
Accommodation, the ability to change the focus of the eyes from distance to near, occurs after parasympathetic stimulation of the ciliary muscle. The human ciliary body and iris contain mainly M3-type receptors.12 Therefore, at concentrations for which pirenzepine is selective for M1 receptors, it is believed to have little effect on the ciliary muscle. In vitro, a continuous perfusion of 1.8 μM pirenzepine (0.07% solution) has been shown to inhibit contraction of the circular and longitudinal portions of the isolated rhesus monkey ciliary muscle stimulated by the muscarinic agonists carbachol, aceclidine, and oxotremorine.13 In vivo in rhesus monkeys, accommodation and pupil constriction elicited by pilocarpine are antagonized by continuous intracameral perfusion of 1.4 μM pirenzepine (0.059% solution),14 and responses elicited by aceclidine are antagonized by 7 μM pirenzepine (0.3% solution).16 At these relatively high concentrations, it is likely that pirenzepine acts through nonselective antagonism of the ciliary muscle through M3 receptors.13,14,15
Pirenzepine has been shown to reduce the progression of myopia in animal studies. In tree shrews, 7500 μg pirenzepine (i.e., 75 μL of 10% solution) injected subconjunctivally each day resulted in a significant reduction in form-deprivation myopia.4 In rhesus monkeys, a 5% pirenzepine solution delivered as 2 drops topically per day (approximately 5000 μg) was as effective as atropine at reducing the progression of form-deprivation myopia.5 In studies in chicks, intravitreal and subconjunctival injections of 0.0035 to 7500 μg have been used,17–19 and an ED50 for reducing myopia was achieved with subconjunctival injection of 5000 μg pirenzepine.18
It is of interest to determine what effects pirenzepine has on objectively measured accommodation at concentrations similar to those shown to be effective at reducing the progression of myopia in animals. Although there have been several studies on the use of pirenzepine for the treatment of myopia, there have been no published studies specifically exploring the effects of pirenzepine on objectively measured accommodation in humans or monkeys. For this reason, in the present study, the effects on accommodation of 0.2 mL of subconjunctivally injected 2% pirenzepine (i.e., 4000 μg), as well as lower concentrations, were studied. Although it was expected that 0.2 mL of 2% would result in nonspecific muscarinic effects, 4000 μg pirenzepine is comparable to, and indeed in many cases lower than, the amount shown to be effective at reducing the progression of myopia in animals. Because of the expected non-specificity of 2% pirenzepine, and because of the profound effects on accommodation (see results), after the 2% concentration was tested, additional testing of log unit dilutions, guided by studies in other tissues2 was undertaken to determine the pirenzepine concentrations at which accommodation would not be affected.
This study was conducted in rhesus monkeys because they have high accommodative amplitudes20–22 and an accommodative mechanism23 and anatomy24 similar to that of humans. Accommodation in rhesus monkeys can be stimulated, rigorously controlled, and studied with objective methods to understand the effects of drugs on the ciliary muscle.23,25–29 In this study, the effects of pirenzepine—in concentrations similar to and lower than those shown to be effective at reducing the progression of myopia in animal models—on pupil size, resting refraction, and pharmacologically and centrally stimulated accommodative amplitude and dynamics were examined in rhesus monkeys.
Materials and Methods
Experiments were performed on nine rhesus monkeys (Macaca mulatta). All experiments conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were conducted under an institutionally approved animal protocol. Initially, pupil size and resting refraction experiments were performed on five normal rhesus monkeys after subconjunctival injection of 2% pirenzepine. Effects of subconjunctival injection of 2% pirenzepine on accommodative amplitude and dynamics were studied in four rhesus monkeys with permanent indwelling electrodes in the Edinger-Westphal (EW) nucleus of the midbrain. Subsequently, static accommodation experiments were performed using subconjunctival injections of log unit dilutions from 0.2% to 0.0002%.
Pirenzepine Preparation and Administration
Pirenzepine dihydrochloride powder, at least 98% pure in assay (Sigma-Aldrich, St. Louis, MO), was mixed into 1-mL aliquots of sterile saline, to formulate 0.0002% to 2% solutions. Each solution was prepared within 1 hour before use on each occasion. Nasal and temporal bulbar subconjunctival injections, approximately 2 mm posterior of the limbus, each of 0.1 mL, were given with a 27-gauge needle. This resulted in subconjunctival delivery of 0.4 to 4000 μg pirenzepine.
Pupil Size and Resting Refraction after 2% Pirenzepine
Monkeys were anesthetized with 10 mg/kg intramuscular ketamine and 0.5 mg/kg intramuscular acepromazine. Anesthesia was supplemented with 6.25 mg/kg ketamine approximately every 30 minutes throughout the experiment, as needed. Each monkey was placed prone with the head facing forward in a head holder. The eyelid was held open with a lid speculum, and a custom-made PMMA contact lens (MetroOptics, Austin, TX) was placed on the cornea to maintain optical quality and corneal hydration.
The time course and extent of mydriasis were determined by videographic pupillography after subconjunctival injections of 2% pirenzepine. The same protocol was tested in one eye each of five normal rhesus monkeys, and one monkey served as the control in which 0.2 mL saline was injected subconjunctivally instead of pirenzepine. Pupil images were captured with an infrared-sensitive charge-coupled device (CCD) video camera (Cohu, USA, San Diego, CA) at 0.3 m, with a coaxial infrared light source. All experimental sessions took place in a dim room with a constant ambient illuminance of 7 lux (spot photometer, model LS-100; Minolta Camera Co., Ltd., Osaka, Japan). Ten baseline pupil images were captured over a period of 2 minutes and saved to a computer. The contact lens was removed, the pirenzepine or saline was injected subconjunctivally (as described above), and the contact lens was replaced. Three pupil images were then captured approximately 15 seconds apart to the computer every 3 minutes for 70 minutes. Calibrated images were analyzed off-line with image-analysis software (Optimas; Media Cybernetics, Silver Springs, MD). The measured pupil diameters, 10 for baseline and 3 for each subsequent period, were averaged for each 3-minute period.
Baseline resting refraction was measured with a Hartinger coincidence refractometer (Carl Zeiss Meditec, Jena, Germany).30 Refraction measurements were made through the contact lens, three times before pirenzepine or saline injection and three times within 15 seconds every 3 minutes after injection for 70 minutes. The three measurements were averaged for each period.
Accommodative Amplitude and Response Dynamics after 2% Pirenzepine
Accommodative amplitude and response dynamics were studied in four rhesus monkeys, ages 12 (monkey 4), 4 (monkey 111), and 5 (monkeys 38 and 70) years. The monkeys had undergone bilateral, complete iridectomies31 and had stimulating electrodes surgically implanted in the EW nucleus of the midbrain.23,26 The electrode was implanted into the EW nucleus to stimulate accommodation in both eyes simultaneously, although different stimulus current amplitudes are necessary to elicit the same response in each eye due to possible midline decentration of the electrode.28 In one monkey (monkey 4) the stimulating electrode elicited an accommodative response in only one eye. Therefore, centrally stimulated accommodation measurements on contralateral control eyes were performed in only three of the four monkeys. The monkeys are used repeatedly in multiple experimental protocols,28,32 and the iridectomies,31 the justification for them,33 and the absence of an effect on centrally stimulated accommodation34 have been described previously. Accommodation was stimulated and measured before and after subconjunctival injection of pirenzepine in one eye. As a control, the protocol was repeated on one monkey (monkey 38) with subconjunctival injection of saline instead of pirenzepine.
For the accommodation experiments, monkeys were initially anesthetized with 10 mg/kg intramuscular ketamine and 0.5 mg/kg intramuscular acepromazine. Surgical-depth anesthesia was maintained for the duration of the experiment with intravenous propofol (an initial bolus of 0.15 mg/kg, followed by constant perfusion at 0.5 mg/kg per minute). Contact lenses were placed on the corneas, and sutures were tied beneath the lateral and medial rectus muscles to reduce eye movements during accommodation.23
For the 2% pirenzepine experiments, static EW-stimulated accommodative responses were measured before and 35 minutes after injection of pirenzepine or saline in one eye. First, baseline refraction was measured three times with a Hartinger coincidence refractometer. An accommodative stimulus response function was then generated. Accommodation was stimulated using 2-second stimulus trains, from 0 μA up to a current amplitude sufficient to produce the maximum accommodative response available to that eye. The accommodative response to 10 increasing stimulus current amplitudes was measured. For each stimulus amplitude, the accommodative response was stimulated and measured three times in succession and averaged. An accommodative stimulus response function was generated in both eyes of each monkey (except monkey 4) before and 35 minutes after injection of 2% pirenzepine or saline.
Infrared Photorefraction and Dynamic Accommodation Measurements
Infrared photorefraction was used to measure accommodation dynamically, and the dynamics of accommodation and disaccommodation were studied to determine the relationships of peak velocity and amplitude (main sequence). The instrumentation, calibration, and analysis of infrared photorefraction to measure accommodative dynamics in monkeys have been described previously.28
Before the injection of 2% pirenzepine or saline, a sequence of dynamic accommodative responses to increasing stimulus currents was recorded with infrared photorefraction. Responses to eight increasing stimulus amplitudes were measured spanning the full accommodative range available to each eye. Five, 4-second stimulus trains were delivered for each stimulus amplitude. The last three accommodative responses for each stimulus amplitude were analyzed off-line, frame by frame, and the three responses were averaged. The first two of the five responses were recorded but not analyzed or considered further.
After the baseline recordings, the contact lens was removed from the eye, 2% pirenzepine or saline was injected subconjunctivally (as described earlier), and the contact lens was replaced. Dynamic accommodation measurements were performed every 2 minutes for 30 minutes for a single stimulus amplitude previously determined to give the maximum response. Five responses to 4-second stimulus trains were recorded with photorefraction every 2 minutes, and the last three responses were analyzed.
The effects of 2% pirenzepine on dynamic accommodation were established in terms of peak velocities of accommodation and disaccommodation. Amplitude of accommodation, peak velocity of accommodation, and peak velocity of disaccommodation were determined for each stimulus amplitude of the pre-pirenzepine sequence and for the single maximum amplitude stimuli after injection of pirenzepine, as described previously.28 Maximum accommodative amplitude was determined for each stimulus amplitude as the difference between the baseline refraction and the accommodated refraction.
Carbachol-Stimulated Accommodation
To understand the pharmacological basis of the cycloplegic effect, approximately 40 minutes after injection of 2% pirenzepine into one eye, accommodation was stimulated with carbachol iontophoresis in both eyes. The contact lenses were removed, and 40% carbachol in agar gel was applied iontophoretically for 8 seconds to the nasal and temporal corneal margins.28 The eyes were immediately irrigated with saline, and the contact lenses were replaced on the eyes. Refraction was measured three times in each eye with a Hartinger coincidence refractometer, every 1 minute for the first 14 minutes and then every 2 minutes, for a total duration of 30 minutes.
Pirenzepine Dilution Experiments
Four additional pirenzepine dilution experiments were performed on three of the four centrally stimulated monkeys (monkeys 4, 111, and 38) at least 1 week apart, using log unit dilutions of 0.0002% to 0.2% pirenzepine. Each concentration (0.2%, 0.02%, 0.002%, and 0.0002%) was tested on each monkey. Anesthesia and drug instillation were the same as described earlier. For the dilution experiments, only the effects on static accommodative responses were recorded.
A static stimulus response function was generated in both eyes of each monkey (except monkey 4) using the same current amplitudes described earlier, and the response was measured with a Hartinger coincidence refractometer. A total of 0.2 mL pirenzepine at the various concentrations was injected subconjunctivally, as described earlier. Accommodative responses to a maximum stimulus current amplitude were measured every 2 minutes for 40 minutes in the pirenzepine-injected eye. After 40 minutes, a Hartinger-measured stimulus response function was repeated on both eyes. Carbachol iontophoresis was performed on both eyes, 50 minutes after pirenzepine injection. The response was measured for 30 minutes.
Results
Pupil and Baseline Refraction Measurements after 2% Pirenzepine
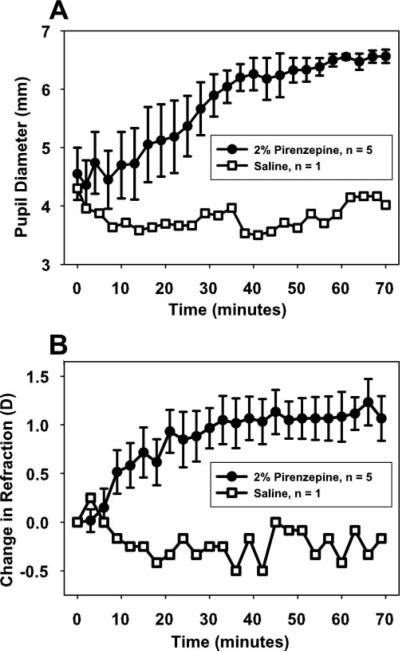
Mean pre-pirenzepine pupil diameter in the five normal monkeys was 4.55 ± 0.45 mm (Fig. 1A). Seventy minutes after pirenzepine injection, pupil diameter was 6.57 ± 0.12 mm, a significant increase (2.01 ± 0.41 mm; t-test: t =−4.94, df = 4, P < 0.05). In the control monkey, in which saline was injected rather than pirenzepine, pupil diameter between baseline and 70 minutes after saline injection was not significantly different.
Figure 1.
Subconjunctival injection of 2% pirenzepine caused (A) a gradual increase in pupil diameter and (B) a systematic hyperopic shift in resting refraction during 70 minutes in the eyes of five normal monkeys. Error bars, SEM. Subconjunctival injection of saline in one monkey did not cause an increase in pupil diameter or a hyperopic shift in resting refraction.
All monkeys had hyperopic pre-pirenzepine resting refractions as measured through the contact lens. Resting refraction in the five normal monkeys was +6.90 ± 0.65 D before pirenzepine and +7.97 ± 0.63 D 70 minutes after pirenzepine injection (Fig. 1B), a significant hyperopic shift (+1.07 ± 0.23 D; t-test: t =−4.66, df = 4, P < 0.05). The resting refraction of the saline control monkey was not different before and 70 minutes after saline injection.
Static Accommodation Measures after 2% Pirenzepine
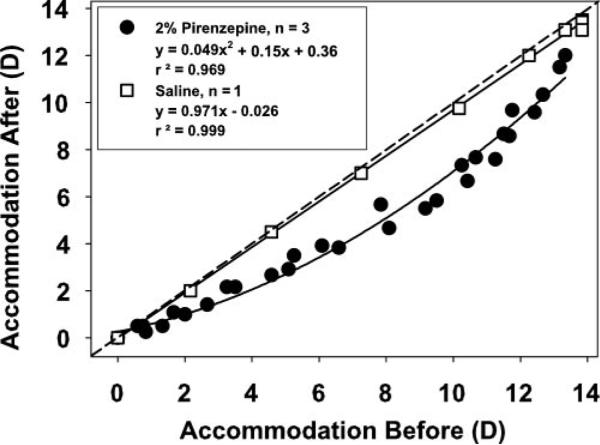
In the four monkeys in which accommodation testing was performed, mean maximum accommodative amplitude in the eyes receiving 2% pirenzepine was 12.45 ± 0.93 D before pirenzepine and 0.63 ± 0.40 D 40 minutes after pirenzepine, a significant decrease (11.82 ± 1.17 D; t-test: t = 10.11, P < 0.005, df = 3; Table 1). Maximum accommodative amplitude of the untreated control eyes decreased from 11.92 ± 0.77 to 9.78 ± 1.25 D, a significant decrease (2.14 ± 0.48 D; t-test: t = 4.42, P < 0.05, df = 2). For each stimulus current amplitude, accommodative amplitude in the untreated control eye decreased 40 minutes after pirenzepine was injected into the experimental eye (Fig. 2). The accommodative amplitude of the noninjected eyes before and after pirenzepine injection in the contralateral experimental eyes is different from the 1:1 line, and is significantly fit with a second-order function (r2 = 0.969), verified by normality test of the residuals.
Table 1.
Centrally and Pharmacologically Stimulated Static Accommodative Amplitude
| Accommodative Amplitude before Pirenzepine |
Accommodative Amplitude after Pirenzepine |
|||||
|---|---|---|---|---|---|---|
| Central Stimulation |
Central Stimulation |
Carbachol Stimulation |
||||
| Monkey | Experimental Eye | Control Eye | Experimental Eye | Control Eye | Experimental Eye | Control Eye |
| 111 | 14.08 | 11.75 | 0.00 | 9.67 | 1.60 | 18.71 |
| 38 | 13.33 | 13.33 | 0.00 | 12.00 | 0.00 | 15.46 |
| 70 | 12.58 | 10.67 | 1.67 | 7.67 | 3.02 | 11.56 |
| 4 | 9.80 | NA | 0.84 | NA | 2.25 | 14.38 |
| Average ± SEM | 12.45 ± 0.93 | 11.92 ± 0.77 | 0.63 ± 0.40 | 9.78 ± 1.25 | 1.70 ± 0.64 | 15.03 ± 1.48 |
Centrally stimulated amplitude was measured with a Hartinger coincidence refractometer in both eyes before and 35 minutes after subconjunctival injection of 2% pirenzepine into one eye. Carbachol was applied iontophoretically 40 minutes after pirenzepine injection. Data are expressed in diopters.
Figure 2.
In the nontreated control eyes of the monkeys that received subconjunctival injections of 2% pirenzepine, static Hartinger-measured, EW-stimulated accommodative responses at 35 minutes after injections were reduced relative to the amplitudes recorded before pirenzepine injection. The data are best fit with a second-order function. In the nontreated eye of the control monkey that received a subconjunctival saline injection rather than pirenzepine, there was no change in the Hartinger-measured, EW-stimulated accommodative responses before and 35 minutes after the saline injection.
In the saline-injected control monkey, maximum accommodative amplitude did not decrease significantly in either the saline-injected eye or the untreated contralateral eye. Accommodative amplitude in the injected and control eyes was 13.88 and 12.50 D before saline injection and 12.50 and 13.50 D 40 minutes after saline injection, respectively. Orthogonal regression analysis shows that the slope of accommodative amplitude of the untreated control eye before and after saline injection in the experimental saline-injected eye was not significantly different from 1 (95% confidence interval: 0.939–1.025).
Dynamic Accommodation Measures after 2% Pirenzepine
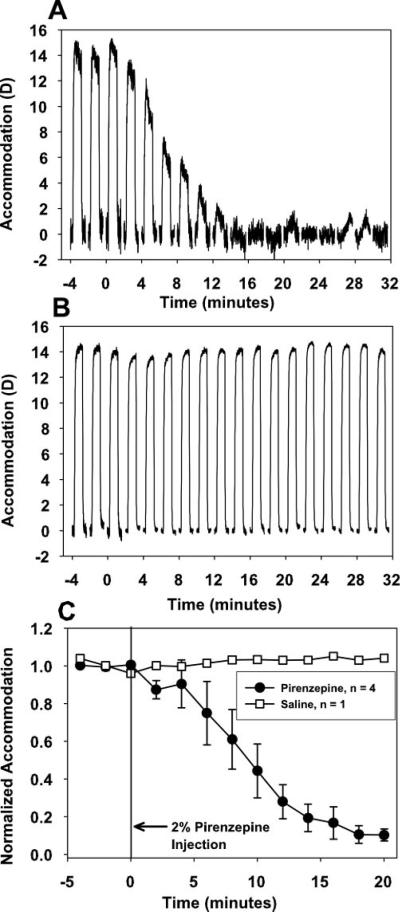
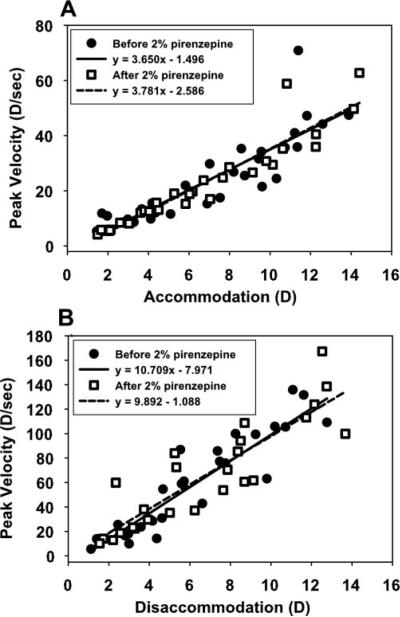
Dynamic accommodation measurements made with infrared photorefraction in the pirenzepine-injected eye of each monkey showed that for a single, maximum stimulus current amplitude, response amplitude decreased every 2 minutes for 30 minutes after 2% pirenzepine injection but did not change after injection of saline (Figs. 3A, 3B: monkey 38). Each response shown is an average of the last three of five, 4-second accommodative responses, delivered just before and every 2 minutes for 30 minutes after pirenzepine or saline injection. In all monkeys, amplitude dcreased to near noise levels by 20 minutes (Fig. 3C). The main sequence of accommodative amplitude versus peak velocity determined before 2% pirenzepine injection for stimulus current amplitudes spanning the full accommodative range available for each eye was compared with the amplitude versus peak-velocity relationship as the response amplitude gradually decreased over 30 minutes after 2% pirenzepine injection (Fig. 4). Linear regression analyses of the main sequence before and after 2% pirenzepine injection were not significantly different for accommodation (P = 0.78) or disaccommodation (P = 0.575).
Figure 3.
Representative dynamic photorefraction-measured accommodative responses before and after subconjunctival injection of (A) 2% pirenzepine and (B) saline in monkey 38. Each trace is the average of three responses recorded from 4-second stimuli, delivered every 2 minutes for 30 minutes after pirenzepine or saline injection. The time scale on the graph is in minutes, but the individual accommodative responses, lasting only 4 seconds each, are scaled so the individual responses can be seen. (C) After 2% pirenzepine injection, mean normalized photorefraction-measured accommodative response amplitude in response to a single, maximum stimulus current amplitude delivered every 2 minutes decreased to approximately 10% of the pre-pirenzepine amplitude. Accommodative amplitude in response to a maximum stimulus current amplitude did not change over 20 minutes after subconjunctival injection of saline in the control monkey. Error bars, SEM.
Figure 4.
The relationship between accommodative response amplitude and peak velocity for the eyes receiving pirenzepine was similar before and after (recorded over 30 minutes after pirenzepine injection) injection of 2% pirenzepine for (A) accommodation (P = 0.783) and (B) disaccommodation (P = 0.575).
Pirenzepine Dilution Experiments
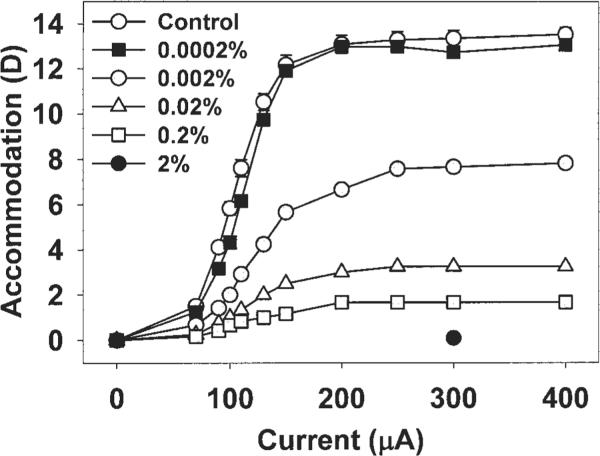
Pirenzepine concentrations of 0.002% or greater caused a decrease in the maximum centrally stimulated accommodative amplitude (Fig. 5, monkey 111). For each concentration of pirenzepine, a further increase in stimulus current amplitude over the maximum stimulus amplitude (200 μA) did not result in an increase in accommodative amplitude.
Figure 5.
The Hartinger-measured static EW-stimulated response functions for the treated eye of one monkey (monkey 111) recorded on different occasions at 40 minutes after injection of various concentrations of pirenzepine is shown. Accommodative amplitude decreased after injection of 0.002% pirenzepine or greater. Error bars on the control data, SEM.
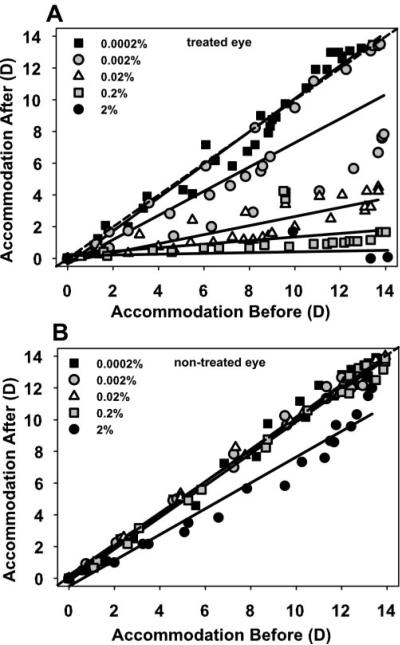
In all monkeys, pirenzepine concentrations of 0.02% or greater resulted in a significant decrease in maximum centrally stimulated static accommodation 20 to 40 minutes after injection (Fig. 6), with a maximum decrease of 11.6 ± 0.96 D after 2% (P < 0.05), 9.9 ± 1.7 D after 0.2% (P < 0.05), and 3.2 ± 1.1 D after 0.02% (P < 0.05). Orthogonal regression analysis shows that the stimulus response function over the range of current amplitudes in the three monkeys decreased significantly in the treated eye at 40 minutes after injection of pirenzepine in concentrations of 0.02% or greater (Fig. 7A). Centrally stimulated accommodation was significantly reduced in the contralateral control eyes at 40 minutes after injection of 2% pirenzepine in the treated eye, but not at any of the lower concentrations (Fig. 7B).
Figure 6.
(A) After subconjunctival injection of 0.0002% to 2% pirenzepine, accommodation to a maximum current stimulus amplitude was measured every 2 minutes up to 40 minutes. Error bars, SEM. (B) Centrally stimulated accommodation to a maximum stimulus current amplitude was significantly decreased 20 to 40 minutes after subconjunctival injection of 0.02% pirenzepine or greater (paired t-test, *P < 0.05).
Figure 7.
Accommodation was significantly reduced at 40 minutes after pirenzepine injection compared with before pirenzepine injection (A) in the treated eye after injection of 0.02% pirenzepine or greater and (B) in the control eye after 2% pirenzepine injection in the treated eye (orthogonal regression analysis, 95% confidence interval).
Post-pirenzepine Carbachol Stimulation
Carbachol-stimulated accommodation decreased significantly at 70 (for 2%) and 80 (for all other concentrations) minutes after injection of pirenzepine in concentrations of 0.2% or more compared with the control eyes (t-test: P < 0.05, Fig. 8A). Accommodation of 1.70 ± 0.64 D was achieved after 2% pirenzepine injection, 4.22 ± 1.54 D after 0.2% pirenzepine injection, and 15.03 ± 1.48 D in the nontreated control eyes (Fig. 8B). In the control monkey, the saline-injected eye achieved 16.25 D and the nontreated control eye achieved 15.50 D of accommodation (Fig. 8C).
Figure 8.
(A) Injection of 0.2% pirenzepine or greater resulted in a significant decrease in carbachol iontophoresis-stimulated accommodation 70 to 80 minutes after pirenzepine injection. (B) Carbachol caused a strong accommodative response in the uninjected control eyes over 30 minutes, but little accommodation in the 2% pirenzepine-injected eyes. Error bars, SEM. (C) In the monkey that received a subconjunctival injection of saline, carbachol iontophoresis produced an accommodative response that was not different in the injected and uninjected eyes during 30 minutes.
Discussion
The goal of this study was to determine the effects of subconjunctival injection of 2% pirenzepine on pupil diameter, resting refraction, and dynamic accommodation and the effects of lower concentrations of subconjunctivally injected pirenzepine on centrally and pharmacologically stimulated static accommodation in rhesus monkeys. An increase in pupil diameter, a hyperopic shift in resting refraction and nearly complete cycloplegia resulted within 20 to 30 minutes after subconjunctival injection of 2% pirenzepine. Pirenzepine concentrations of 0.02% or greater resulted in a significant decrease in accommodation.
A 2% pirenzepine solution was initially used in the study, the same concentration that has been delivered as a topical gel formulation in the FDA human clinical trials of myopia treatment (Siatkowski R, et al. IOVS 2003;45:ARVO E-Abstract 4778; Tan DT, et al. IOVS 2003;44:ARVO E-Abstract 801). The 0.2 mL of 2% pirenzepine injected subconjunctivally in the present study in monkeys (i.e., 4000 μg per eye) is similar to the amount of pirenzepine delivered to the eyes and a similar delivery method as has been used and shown to be effective at reducing the progression of myopia in previous animal studies. In tree shrews, 7500 μg of pirenzepine was injected subconjunctivally each day.4 In rhesus monkeys, a 5% pirenzepine solution was delivered as 2 drops topically per day,5 and in chicks, up to 7500 μg was injected intravitreally and subconjunctivally.17–19 The results from the present study in monkeys show that subconjunctival injection of 0.2 mL of 2% solution, a dose and delivery method previously shown to be effective for reducing the progression of myopia in animals, causes complete cycloplegia in monkeys. There have been very few M1 receptors localized to the ciliary muscle in many species, including chick (Yin GC, et al. IOVS 2003;44:ARVO E-Abstract 4339), bovine,35 dog,36 rhesus monkey,13 and human.16,37 Therefore, it is likely that pirenzepine works through a nonselective mechanism in concentrations of 0.02% to 2%, when delivered subconjunctivally.
The pupil diameter and resting refraction measurements were performed with the monkeys under ketamine and acepromazine anesthesia. The approximately 1 D hyperopic shift in resting refraction may result from cycloplegic block of tonic accommodation.38 If such a hyperopic shift in resting refraction were to occur in conscious human subjects after pirenzepine treatment, and were to persist throughout the period of pirenzepine treatment, progression of myopia should be assessed only using pre- and post-pirenzepine cycloplegic refraction or A-scan measurements of axial length to avoid the potential confound that may be introduced by a hyperopic shift.
Here in monkeys, after injecting 2% pirenzepine subconjunctivally in the experimental eye, the maximum accommodative response was reduced in the uninjected control eye, indicating some systemic crossover into the untreated eye. A previous study in chicks reported that systemic absorption of pirenzepine occurred after both intravitreal and subconjunctival injections of 200 μg.18 It was shown that systemic levels were two times greater for subconjunctival delivery compared with intravitreal delivery. With topical administration of 2% pirenzepine solution in humans, systemic levels were “barely” detectable in adults (Sheddon A, et al. IOVS 1998;39:ARVO Abstract 1273), although some systemic parasympathetic antagonistic effects were reported in children (Tan DT, et al. IOVS 2003;44:ARVO E-Abstract 801).
In this study, after injection of 2% pirenzepine, carbachol stimulated accommodation was decreased to approximately 10% of the amplitude achieved in the control eyes and also significantly decreased after injection of 0.2% pirenzepine. Carbachol is a direct-acting cholinergic agonist39 that stimulates muscarinic receptors nonselectively and is capable of eliciting a maximum accommodative response in rhesus monkeys.20,28 Atropine, a nonselective muscarinic antagonist, inhibits carbachol-induced stimulation of the ciliary muscle.13,40 In the current study, pirenzepine acted in a competitive manner with carbachol, and subconjunctival injection of 2% pirenzepine almost completely blocked carbachol-stimulated accommodation. This suggests that the injected 2% pirenzepine causes a cycloplegic effect through nonselective blockage of muscarinic receptors in rhesus monkeys in the same way that atropine does. A study of form deprivation myopia in tree shrews included a control experiment to test the effects of pirenzepine on pupil size and accommodation.4 Pirenzepine (10%) was injected subconjunctivally in one eye each of three tree shrews, and carbachol was applied iontophoretically 90 minutes after pirenzepine injection. Although a significant increase in pupil size was found after pirenzepine, carbachol-stimulated accommodation was not significantly different in pirenzepine-treated versus control eyes of each animal 10 to 60 minutes after carbachol iontophoresis. We are aware of no other accommodation studies performed in tree shrews, and limited information is available about the accommodative amplitude, mechanism, and structure of the ciliary muscle in the tree shrew. It is possible that the differing cycloplegic effects of pirenzepine in tree shrews and rhesus monkeys may be due to species differences in anterior segment anatomy and receptor types. In humans and rhesus monkeys, pupil constriction and accommodation are both inhibited by the same receptor type, M3 receptors, but in the tree shrew, pirenzepine had an effect on only pupil size and not accommodation.
In human FDA trials, pirenzepine is titrated gradually from 0.5% to 2% in a gel formulation, applied topically twice daily.9 In the present study in monkeys, pirenzepine was injected subconjunctivally on one occasion in an attempt to deliver a controlled, known amount of the drug. Topical administration is notoriously variable in terms of how much drug remains on the cornea or penetrates the eye. The gel formulation is used in the human clinical trials to prolong the duration of exposure to the drug. This in turn is expected to increase the amount of drug entering the eye. Subconjunctival injection of 2% pirenzepine would allow pirenzepine to be absorbed at higher concentrations than would be possible with topical delivery of a gel formulation. The lower concentrations absorbed with topical administration of a 2% gel formulation may maintain M1-specificity. In the most recent human study of the treatment of myopia with pirenzepine, 11% of subjects were reported to have withdrawn, some due to blurred vision at near (Siatkowski R, et al. IOVS 2003;45:ARVO E-Abstract 4778). Only minor effects on accommodation were reported, although the authors did not report objectively measured accommodation. In another recent report of the tolerability of pirenzepine in children, the dose, administered morning and evening, was titrated from 0.5% for 1 week to 1% for 1 week, and finally to 2% for the remainder of 1 year.8 One child inadvertently received the 2% concentration as the first dose, and withdrew from the study due to accommodative insufficiency.9 The remaining subjects were reported to have a 2 to 3 D decrease in subjectively measured accommodative amplitude.8 It is possible that titrating the pirenzepine concentration may lessen the cycloplegic effect of the pirenzepine. However, if mild cycloplegia occurred in these myopic children, it is possible that they would simply remove their spectacles to read with their myopic refractive error, and therefore mild cycloplegia in myopes with high accommodative amplitudes is unlikely to represent a debilitating side effect.
A limitation of the present study is that only the short-term effects of pirenzepine were tested. This study was an initial attempt to understand with more certainty what effects pirenzepine has on accommodation using concentrations and delivery methods similar to those previously shown to be effective at reducing the progression of myopia in animals. This does not necessarily relate to how accommodation may be affected in humans who undergo long-term topical pirenzepine therapy. Therefore, these results from subconjunctival injection in rhesus monkeys should not be extrapolated to attempt to predict side effects of pirenzepine on accommodation in human clinical trials. The use of rhesus monkeys with central stimulation of the Edinger-Westphal nucleus allows controlled, repeatable testing of the effects of pharmacological agents on accommodation.29
The methodology presented here has been used to test the effects of various concentrations of pirenzepine. This may allow determination of a concentration of the drug which maintains M1-specificity. It will be of clinical importance to undertake objective accommodation testing in children or young adults using the gel formulation so the possible side effects of pirenzepine on accommodation in human patients can be know with certainty.
Acknowledgments
The authors thank Abhiram S. Vilupuru and Siddharth Poonja for technical assistance and Ying-Sheng Hu for statistical support.
Supported in part by National Eye Institute Grants 1 R01 EY014651 (AG), P30 EY07751 to the University of Houston, College of Optometry, and 5 T32 EY07024 to the University of Texas Health Science Center at Houston (AG, LAO), and a research grant from Beta Sigma Kappa.
Footnotes
Disclosure: L.A. Ostrin, None; L.J. Frishman, None; A. Glasser, None
References
- 1.Collison DJ, Coleman RA, James RS, et al. Characterization of muscarinic receptors in human lens cells by pharmacologic and molecular techniques. Invest Ophthalmol Vis Sci. 2000;41:2633–2641. [PubMed] [Google Scholar]
- 2.Caulfield M, Birdsall N. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 3.Leech EM, Cottriall CL, McBrien NA. Pirenzepine prevents form deprivation myopia in a dose dependent manner. Ophthalmic Physiol Opt. 1995;15:351–356. [PubMed] [Google Scholar]
- 4.Cottriall CL, McBrien NA. The M1 muscarinic antagonist pirenzepine reduces myopia and eye enlargement in the tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1368–1379. [PubMed] [Google Scholar]
- 5.Tigges M, Iuvone P, Fernandes A, et al. Effects of muscarinic cholinergic receptor antagonists on postnatal eye growth of rhesus monkeys. Optom Vis Sci. 1999;76:397–407. doi: 10.1097/00006324-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 6.McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–215. [PubMed] [Google Scholar]
- 7.Truong H, Cottriall CL, Gentle A, McBrien NA. Pirenzepine affects scleral metabolic changes in myopia through a non-toxic mechanism. Exp Eye Res. 2002;74:103–111. doi: 10.1006/exer.2001.1107. [DOI] [PubMed] [Google Scholar]
- 8.Stacher G, Havlik E, Bergmann H, et al. Effects of oral pirenzepine on gastric emptying and antral motor activity in healthy man. Scand J Gastroenterol Suppl. 1982;72:153–158. [PubMed] [Google Scholar]
- 9.Bartlett JD, Niemann K, Houde B, et al. A tolerability study of pirenzepine ophthalmic gel in myopic children. J Ocul Pharmacol Ther. 2003;19:271–279. doi: 10.1089/108076803321908392. [DOI] [PubMed] [Google Scholar]
- 10.Chiang MF, Kouzis A, Pointer RW, Repka MX. Treatment of childhood myopia with atropine eye drops and bifocal spectacles. Binocul Vis Strabismus Q. 2001;16:209–215. [PubMed] [Google Scholar]
- 11.Syniuta LA, Isenberg SJ. Atropine and bifocals can slow the progression of myopia in children. Binocul Vis Strabismus Q. 2001;16:203–208. [PubMed] [Google Scholar]
- 12.Woldemussie E, Feldmann BJ, Chen J. Characterization of muscarinic receptors in cultured human iris sphincter and ciliary smooth muscle cells. Exp Eye Res. 1993;56:385–392. doi: 10.1006/exer.1993.1052. [DOI] [PubMed] [Google Scholar]
- 13.Poyer JF, Gabelt BT, Kaufman PL. The effect of muscarinic agonists and selective receptor subtype antagonists on the contractile response of the isolated rhesus monkey ciliary muscle. Exp Eye Res. 1994;59:729–736. doi: 10.1006/exer.1994.1159. [DOI] [PubMed] [Google Scholar]
- 14.Gabelt BT, Kaufman PL. Inhibition of outflow facility and accommodative and miotic responses to pilocarpine in rhesus monkeys by muscarinic receptor subtype antagonists. J Pharmacol Exp Ther. 1992;263:1133–1139. [PubMed] [Google Scholar]
- 15.Gabelt BT, Kaufman PL. Inhibition of aceclidine-stimulated outflow facility, accommodation and miosis in rhesus monkeys by muscarinic receptor subtype antagonists. Exp Eye Res. 1994;58:623–30. doi: 10.1006/exer.1994.1057. [DOI] [PubMed] [Google Scholar]
- 16.Pang I-H, Matsumoto S, Tamm E, DeSantis L. Characterization of muscarinic receptor involvement in human ciliary muscle cell function. J Ocul Pharmacol. 1994;10:125–136. doi: 10.1089/jop.1994.10.125. [DOI] [PubMed] [Google Scholar]
- 17.Rickers M, Schaeffel F. Dose-dependent effects of intravitreal pirenzepine on deprivation myopia and lens-induced refractive errors in chickens (letter) Exp Eye Res. 1995;61:509–516. doi: 10.1016/s0014-4835(05)80147-2. [DOI] [PubMed] [Google Scholar]
- 18.Cottriall CL, McBrien NA, Annies R, Leech EM. Prevention of form-deprivation myopia with pirenzepine: a study of drug delivery and distribution. Ophthalmic Physiol Opt. 1999;19:327–335. [PubMed] [Google Scholar]
- 19.Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia (letter) Exp Eye Res. 1991;52:755–758. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 20.Koretz JF, Bertasso AM, Neider MW, et al. Slit-lamp studies of the rhesus monkey eye. II Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res. 1987;45:317–326. doi: 10.1016/s0014-4835(87)80153-7. [DOI] [PubMed] [Google Scholar]
- 21.Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- 22.Tornqvist G. Effect of topical carbachol on the pupil and refraction in young and presbyopic monkeys. Invest Ophthalmol Vis Sci. 1966;5:186–195. [Google Scholar]
- 23.Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- 24.Koretz JF, Neider MW, Kaufman PL, et al. Slit-lamp studies of the rhesus monkey eye. I. Survey of the anterior segment. Exp Eye Res. 1987;44:307–318. doi: 10.1016/s0014-4835(87)80014-3. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman PL. Anticholinesterase-induced cholinergic subsensitivity in primate accommodative mechanism. Am J Ophthalmol. 1978;85:622–631. doi: 10.1016/s0002-9394(14)77094-1. [DOI] [PubMed] [Google Scholar]
- 26.Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- 27.Croft MA, Kaufman PL, Erickson-Lamy K, Polansky JR. Accommodation and ciliary muscle muscarinic receptors after echothiophate. Invest Ophthalmol Vis Sci. 1991;32:3288–3297. [PubMed] [Google Scholar]
- 28.Vilupuru AS, Glasser A. Dynamic accommodation in rhesus monkeys. Vision Res. 2002;42:125–141. doi: 10.1016/s0042-6989(01)00260-7. [DOI] [PubMed] [Google Scholar]
- 29.Ostrin LA, Glasser A. The effects of phenylephrine on pupil diameter and accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:215–221. doi: 10.1167/iovs.03-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fincham EF. The coincidence optometer. Proc Phys Soc (London) 1937;49:456–468. [Google Scholar]
- 31.Kaufman PL, Lütjen-Drecoll E. Total iridectomy in the primate in vivo: surgical technique and postoperative anatomy. Invest Ophthalmol Vis Sci. 1975;14:766–771. [PubMed] [Google Scholar]
- 32.Vilupuru AS, Glasser A. Dynamic accommodative changes in Rhesus monkey eyes assessed with A-scan ultrasound biometry. Optom Vis Sci. 2003;80:383–394. doi: 10.1097/00006324-200305000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Bito LZ, Kaufman PL, DeRousseau CJ, Koretz J. Presbyopia: an animal model and experimental approaches for the study of the mechanism of accommodation and ocular aging. Eye. 1987;1:222–230. doi: 10.1038/eye.1987.41. [DOI] [PubMed] [Google Scholar]
- 34.Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of rhesus monkeys. Invest Ophthalmol Vis Sci. 1990;31:2185–2190. [PubMed] [Google Scholar]
- 35.Masuda H, Goto M, Tamaoki S, et al. M3-type muscarinic receptors predominantly mediate neurogenic quick contraction of bovine ciliary muscle. Gen Pharmocol. 1998;30:579–584. doi: 10.1016/s0306-3623(97)00312-1. [DOI] [PubMed] [Google Scholar]
- 36.Choppin A, Eglen R. Pharmacological characterization of muscarinic receptors in dog isolated ciliary and urinary bladder smooth muscle. Br J Pharmacol. 2001;132:835–842. doi: 10.1038/sj.bjp.0703901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa H, DeSantis L, Patil PN. Selectivity of muscarinic agonists including (+/−)-aceclidine and antimuscarinics on the human intraocular muscles. J Ocul Pharmacol Ther. 1998;14:363–373. doi: 10.1089/jop.1998.14.363. [DOI] [PubMed] [Google Scholar]
- 38.Crawford K, Gabelt BT, Kaufman PL, Bito LZ. Effects of various anesthetic and autonomic drugs on refraction in monkeys. Curr Eye Res. 1990;9:525–532. doi: 10.3109/02713689008999592. [DOI] [PubMed] [Google Scholar]
- 39.Bartlett JD, Jaanus SD, Fiscella RG, Sharir M. Ocular hypotensive drugs. In: Bartlett JD, Jaanus SD, editors. Clinical Ocular Pharmacology. 4th ed. Butterworth-Heinemann; Woburn, MA: 2001. chap. 10. [Google Scholar]
- 40.Poyer JF, Kaufman PL, Flugel C. Age does not affect contractile responses of the isolated rhesus monkey ciliary muscle to muscarinic agonists. Curr Eye Res. 1993;12:413–422. doi: 10.3109/02713689309024623. [DOI] [PubMed] [Google Scholar]