Abstract
Background
Diagnosis of post-breast cancer lymphoedema is difficult because of inconsistent measurement approaches, measurement reliability and validity, and lymphoedema definition and criterion.
Aims
To examine lymphoedema occurrence using a body mass index (BMI)-adjusted limb volume change (LVC) as a potentially sensitive alternative criterion for assessment and diagnosis of lymphoedema. Secondary aims were to examine the risk of lymphoedema occurrence in relation to post-operative swelling and limb dominance and the cancer-affected side.
Methods
The volume calculated from circumferences of 193 breast cancer survivors was used to analyse lymphoedema assessment. A change ≥5% in affected-arm volume over percent change in BMI in comparison to pre-operative baseline was considered indicative of lymphoedema.
Results
For all participants, 63% met the 5% BMI-adjusted LVC criterion. Dominant limb and cancer-affected side were significantly related to lymphoedema occurrence only in those whose BMI ≥30 (p=0.02), while post-operative swelling significantly increased the lymphoedema risk irrespective of BMI (p=0.01).
Conclusions
The proposed 5% BMI-adjusted LVC criterion provides a more sensitive estimation of post-breast cancer lymphoedema occurrence.
Keywords: Breast cancer, Arm dominance, Post-operative swelling, Diagnosis of lymphoedema, Body mass index (BMI)
Over 200,000 American women and over one million women around the world are newly affected by breast cancer each year (American Cancer Society, 2007; Office for National Statistics, 2007). The two million breast cancer survivors living in the US and ten million worldwide are at lifetime risk of developing lymphoedema (Ferlay et al, 2004; American Cancer Society, 2006;), a chronic condition involving accumulation of protein-rich fluid that affects physical, functional and psychosocial health and well-being (Hull, 1998; Beaulac et al, 2002;
Second only to breast cancer recurrence, lymphoedema is the most feared sequela of breast cancer treatment (Disa and Petrek, 2001).
Geller et al, 2003; Voogd et al, 2003; Radina and Armer, 2004). Second only to breast cancer recurrence, lymphoedema is the most feared sequela of breast cancer treatment (Disa and Petrek, 2001).
The percentage of breast cancer survivors who develop lymphoedema is not precisely known, although it is conservatively estimated that as many as half of survivors may experience lymphoedema during their lifetime (Armer and Stewart, 2005; Armer, 2008, in press). The discrepancy between the reported percentages of 3% to 62.5% (Passik and McDonald, 1998; Petrek and Heelan, 1998; Sener et al, 2001) in the literature stems from difficulties in measurement, diagnosis, and follow up of lymphoedema (Meek, 1998; Passik and McDonald, 1998; Petrek and Heelan, 1998; Rockson, 1998; Armer, 2005; Armer and Stewart, 2005). Common quantitative criteria for lymphoedema include: two or more centimetres difference in limb girth between the affected and non-affected limb; a 200ml limb volume difference; or a 10% limb volume change (LVC) (Petlund, 1991; Armer and Stewart, 2005).
The reported incidence fluctuates greatly among groups of individuals at risk of developing lymphoedema (Armer et al, 2004; Armer and Fu, 2005). Although a number of factors have been implicated as being associated with increased risk of lymphoedema, including axillary dissection, radiation therapy, post-operative infection, age, and weight gain (Meek, 1998; Petrek and Heelan, 1998; Coen et al, 2003; Deutsch and Flickinger, 2003; Geller et al, 2003; Voogd et al, 2003; Ozaslan and Kuru, 2004), the diagnostic criteria themselves require further refinement in order to clarify actual occurrence of lymphoedema (Armer and Stewart, 2005). One of the dilemmas of the current forementioned anthropometric criteria for lymphoedema is that they are not calibrated to account for selected individual changes that commonly occur over the course of breast cancer treatment, such as fluid retention and changes in body mass index (BMI) (Armer et al, 2008 in press).
In the same way that it has been identified that increased BMI is associated with a higher risk of breast cancer and poorer outcome (Feigelson et al, 2004), including breast cancer recurrence (Chlebowski et al, 2002), second primary cancers, and higher morbidity and mortality (Johansson et al, 2002; Whiteman, et al, 2005), studies have identified a correlation between both BMI and BMI change and the development of lymphoedema after breast cancer treatment (Petrek et al, 2001; Soran et al, 2006). Unfortunately, the 2cm, 200ml, and even 10% LVC criteria do not take into account the changes experienced in the body that result in weight gain during or following treatment. The aim of this study was to develop and refine a BMI-adjusted criterion for lymphoedema occurrence (Armer et al, 2008 in press) that would consider the commonly-experienced fluctuations in weight during and following breast cancer treatment. In addition, the secondary aims were to examine the risk of lymphoedema occurrence in relation to post-operative swelling, and limb dominance and the cancer-affected side.
Methods
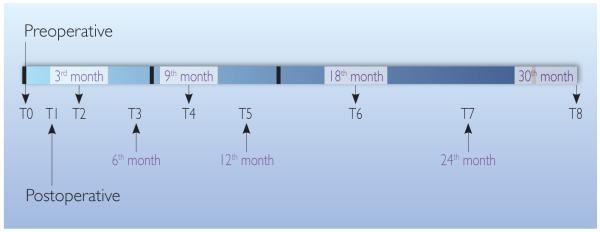
In this National Institute of Health (NIH)-funded prospective repeated-measures study, a convenience sample of 202 women with breast cancer were recruited to participate in the 30-month study starting from a pre-operative visit (visit T0 after breast cancer diagnosis and before surgery). The selection criteria were first breast cancer diagnosis, enrolled prior to surgery, English-speaking, and capable of informed consent. Approval for research with human subjects was received through the University of Missouri Health Sciences Institutional Review Board (IRB) office prior to conducting the study. Participants were seen post-operatively every three months for 12 months, and then every six months for 18 months to a total of 30 months (Figure 1). Of all participants, 193 (95.5%) were unilateral breast cancer survivors. From this group, there were 105 (54.4%) participants whose cancer-affected side was their dominant limb (11 [10.5%] participants were left-handed, 94 [89.5%] participants were right-handed); whereas, there were 88 (45.6%) participants whose cancer-affected side was not their dominant limb. From the same group of 193 participants, there were 37 (19.2%) participants who experienced swelling to the extent of the proposed 5% BMI-adjusted LVC criterion at the post-operative visit (visit T1).
Figure 1.
Timeline for data collection (pre-operative to 30 months following surgery).
Arm circumferences were measured every 4cm using non-stretch tape measures (Callaway et al, 1988; Armer, 2005). Limb volume (LV) was calculated using a summation of cylinder volumes (v). A derived cylinder formula is as follows:
Please note that a cylinder’s base area was inferred from an average of two circular areas associated with two consecutive circumference measurements (c1 and c2) starting from the wrist to the underarm. A cylinder’s height is 4cm.
This research proposes a 5% BMI-adjusted LVC criterion; a participant meets this criterion when there is an LV increase of at least 5% greater than BMI change (with respect to the participant’s pre-operative baseline BMI and LV in the cancer-affected side) during at least one visit after the post-operative visit. The BMI formula (Centers for Disease Control and Prevention, 2008) was defined as:
The 5% BMI-adjusted LVC criterion is a potentially more sensitive measure of lymphoedema occurrence because:
-
▶▶
Increased BMI is associated with higher risk of lymphoedema occurrence following breast cancer
-
▶▶
Current standards rarely consider simultaneous contralateral LVC
-
▶▶
Study participants’ BMI ranged from 17.2 to 54.4 (average 30.5).
BMI categories used in this study follow the guideline of the Centers for Disease Control and Prevention (2008) (Table I). The same table also shows the percentages of women in this study per BMI category, compared to women aged 18 years or older who answered the Behavioral Risk Factor Surveillance System (BRFSS) survey in year 2006 (Centers for Disease Control and Prevention, 2006). The BRFSS 2006 data showed that women in the state of Missouri (MO) had higher percentages for overweight and obese categories than the national percentages. These statistics are consistent with the higher percentages of the same BMI categories among the participants in this study, although in this study, the percentage of the obese category (44%) is considerably higher than the obese category of MO (28.9%).
Table 1. Adult women BMI weight status.
| BMI | Weight status | % of participants |
BRFSS 2006 | |
|---|---|---|---|---|
| % of MO women* |
% of US women* |
|||
| Below 18.5 | Underweight | 1.6% | 37.3% | 40.3% |
| 18.5–24.9 | Normal | 22.8% | ||
| 25.0–29.9 | Overweight | 31.6% | 30.1% | 28.7% |
| 30.0 and above | Obese | 44.0% | 28.9% | 24.0% |
3.7% of MO women and 7% of US women did not respond
Analysis
Occurrence of lymphoedema was calculated from percent change in cancer-affected limb volume at each of eight post-operative time points (starting from one to four weeks to 30 months post-surgery) (T1 or T8), compared to pre-operative LV. Percent change in BMI during the same time periods were then calculated. A change of 5% or greater in affected-arm volume over percent change in BMI with respect to the participant’s pre-operative baseline BMI and LV in the cancer-affected side was considered to be indicative of lymphoedema. Two sets of statistical analyses were conducted between the cancer-affected dominant and non-dominant limbs, and those with and without post-operative (one to four weeks after surgery) swelling. Unpaired (two-sample or independent-sample) t-tests were used to determine statistical significance (Brink and Wood, 1998; Peat and Barton, 2005). Relative risk was calculated to estimate the magnitude of the difference.
Participants were grouped according to their BMI weight status (Centers for Disease Control and Prevention, 2008), as shown in Table I. To find whether there was an increased risk of developing lymphoedema on the dominant limb side that may be used more often, the first analysis compared risks of developing lymphoedema from three months to 30 months post-surgery (visits T2 to T8) between the group of participants whose cancer affected their dominant limb side and the group of participants whose cancer affected their non-dominant side.
To find whether there was an increased risk of developing lymphoedema that may be associated with the swelling caused by breast cancer surgery, the second analysis compared risks of developing lymphoedema during the same time period as the first analysis (visits T2 to T8) between the group of participants who met or exceeded the 5% BMI-adjusted LVC criterion at the post-operative visit (visit T1), and the group that did not meet this criterion at visit T1.
Results
All unilateral cancer-affected limb participants (n=193), 63% (n=121) met the 5% BMI-adjusted LVC criterion at some point following (excluding) the post-operative visit (mean time to criterion=nine months, standard deviation=seven months).
Cancer-affected dominant and non-dominant limbs
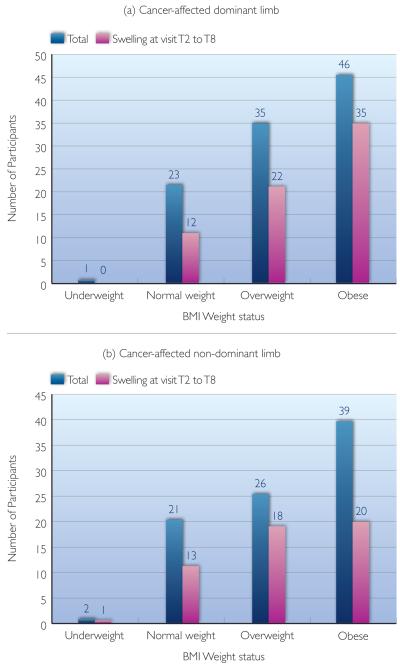
To answer the question of whether there was an increased risk of developing lymphoedema when a participant’s cancer-affected limb was her dominant side, t-test and relative risk analyses were used to compare between two groups of participants: cancer-affected dominant limb group; and cancer-affected non-dominant limb group. Overall, the relative risk between these groups was 1.1, and there was not a significant difference (65.7% compared to 59.1%; t=0.95; p=0.35) (Table 2) (Figure 2).
Table 2. Relative lymphoedema risk analysis between cancer-affected dominant and non-dominant sides.
| Dominance, cancer-affected side, and lymphoedema |
BMI status | Total | ||||
|---|---|---|---|---|---|---|
| Under- weight |
Normal weight |
Over- weight |
Obese | |||
| Cancer-affected dominant limb |
Total number of participants |
1 | 23 | 35 | 46 | 105 |
| Swelling at visits T2 and T8 |
0 of 1 (0%) |
12 of 23 (52.2%) |
22 of 35 (62.9%) |
35 of 46 (76.1%) |
69 of 105
(65.7%) |
|
| Cancer- affected non- dominant limb |
Total number of participants |
2 | 21 | 26 | 39 | 88 |
| Swelling at visits T2 and T8 |
1 of 2 (50%) |
13 of 21 (61.9%) |
18 of 26 (69.2%) |
20 of 39 (51.3%) |
52 of 88
(59.1%) |
|
Figure 2.
Number of participants whose (a) cancer affected their dominant limb or (b) cancer affected their non-dominant limb, categorised by body mass index status.
Tests of statistical significance were also conducted for lymphoedema occurrence and non-occurrence in the three BMI categories—normal weight, overweight, and obese, with the reported relative risks of 0.84, 0.91, and 1.48, reported t values of 0.64, 0.51, and 2.44, and reported p values of 0.53, 0.61, and 0.02, respectively. Even though in the larger group analysis, limb dominance and cancer-affected side were not significantly associated with the risk of developing lymphoedema, participants with BMI 30 and above had a significantly higher risk of developing lymphoedema if their cancer treatment was on the dominant side (relative risk [rr]=1.48, 48% higher risk). Please note that the underweight group was not tested due to its small sample size.
With and without post-operative swelling
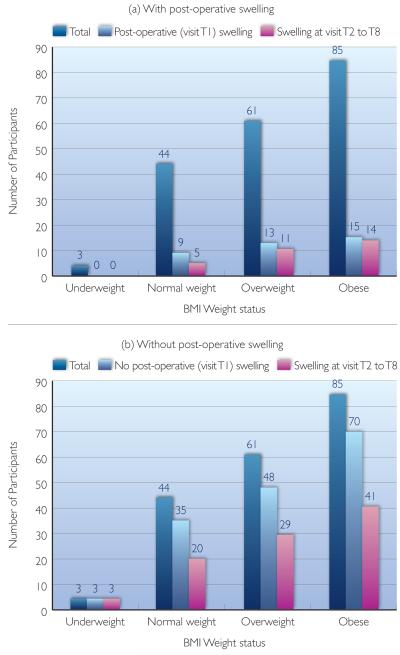
A relative risk analysis was calculated to compare the risk of developing lymphoedema at a later visit (visits T2 to T8) between the groups of participants with and without post-operative (visit T1) swelling. Overall, the relative risk between these two groups was 1.4, and there is a significant difference between the groups (81.1% compare to 58.3%; t=2.6; p=0.01) (Table 3) (Figure 3). Those with post-operative swelling had a 1.4 greater risk of developing lymphoedema at some later point compared to those without post-operative swelling.
Table 3. Relative lymphoedema risk between participants with and without post-operative swelling.
| With and without post- operative swelling |
BMI status | Total | ||||
|---|---|---|---|---|---|---|
| Under- weight |
Normal weight |
Over- weight |
Obese | |||
| Total number of participants | 3 | 44 | 61 | 85 | 193 | |
| Post-operative swelling |
Swelling at post- operative visit |
0 of 3 (0%) |
9 of 44 (20.5%) |
13 of 61 (21.3%) |
15 of 85 (17.6%) |
37 |
| Swelling at visits T2 to T8 |
0 of 01 (N/A%) |
5 of 9 (55.6%) |
11 of 13 (84.6%) |
14 of 15 (93.3%) |
30 of 37
(81.1%) |
|
| No post- operative swelling |
No swelling at post- operative visit |
3 of 3 (100%) |
35 of 44 (79.5%) |
48 of 61 (78.7%) |
70 of 85 (82.4%) |
156 |
| Swelling at visits T2 to T8 |
1 of 3 (33.3%) |
20 of 35 (57.1%) |
29 of 48 (60.4%) |
41 of 70 (58.6% |
91 of 156
(58.3%) |
|
Figure 3.
Categorised by their body mass index status, number of participants who: (a) experienced post-operative swelling; or (b) did not experience post-operative swelling.
Tests of statistical significance were also conducted for each of the three BMI categories — normal weight, overweight, and obese, with the reported relative risk values of 0.97, 1.4, and 1.6, reported t values of 0.08, 1.64, and 2.63, and reported p values of 0.93, 0.11, and 0.01 respectively. In addition to the larger group analysis in which post-operative swelling was significantly associated with the risk of developing lymphoedema, a sub-category analysis revealed participants with BMI above 25 had a higher relative risk of developing lymphoedema than the normal weight group (rr=1.4 and 1.6, 40% and 60% higher risk for overweight and obese, respectively). Please note that the underweight group was not tested due to its small sample size.
Further analyses showed the significance of having the pre-operative (before surgery) (T0) measurement, when the data from the three-month (following surgery) visit were substituted for the T0 pre-operative data. Without the pre-operative measurement, 49 participants who met the 5% BMI-adjusted LVC criterion at visit T2 would not have been recognised.
Discussion
For all participants, 63% met the 5% BMI-adjusted LVC criterion at some point following (excluding) the post-operative visit. Limb dominance and cancer-affected side were not significantly associated with the development of post-surgery lymphoedema (rr=1.1) in the group as a whole. In the subgroup analysis, those with BMI classified as obese showed a 48% greater lymphoedema risk in women whose cancer occurred on their dominant side. Further, post-operative swelling significantly increased the risk of later developing lymphoedema (rr=1.4) across the group as a whole. This means a person who developed post-operative swelling was 40% more likely to develop lymphoedema at some later time (before 30 months) after surgery. In the subgroup analysis, this relative risk of developing lymphoedema was even higher in the overweight and obese BMI groups than for normal weight women (40% and 60% greater risk).
Also of importance, approximately 40% of those who met the 5% BMI-adjusted LVC criterion in this study would have been overlooked if the pre-operative measurements had not been available. Further, since post-operative swelling is associated with higher risk of developing lymphoedema, having the pre-operative baseline is an essential reference for detection of post-operative swelling. This finding supports the need for pre-operative assessment in the clinical setting.
A strength of the study is the 30-month follow-up with the pre-operative baseline measurements. This follow-up exceeds that of most studies (Deutsch and Flickinger, 2003; Rovere et al, 2003; Voogd et al, 2003; Armer and Stewart, 2005). However, more answers regarding long-term occurrence of lymphoedema will be discovered with seven-year follow-up with this cohort. In early findings from the 36–84 months follow-up, new cases of lymphoedema have been identified. The preliminary findings are consistent with the isolated studies which report late-occurring cases of lymphoedema (Petrek and Heeland, 1998; Petrek et al, 2001).
Conclusions
Using the 5% BMI-adjusted LVC approach to assessment of lymphoedema occurrence provides the opportunity for a more sensitive estimation of post-breast cancer lymphoedema occurrence. Also important is the capability to compare pre-operative LV measurements to post-operative volume. Based on this preliminary analysis, lymphoedema is a risk for approximately two-thirds of breast cancer survivors in the 30 months after surgery. These data suggest increased risk of lymphoedema in survivors with BMI classified as obese whose dominant limb was treated for cancer. Overall, breast cancer survivors with post-operative swelling have a significantly higher risk of developing lymphoedema than those who do not have post-operative swelling. It is the group with higher BMI (overweight or obese) who has the greatest risk of developing lymphoedema. Breast cancer survivors with higher BMI appear to have a cumulative risk of developing lymphoedema if the cancer is on the dominant side or if they experience post-operative swelling. This finding is also consistent with work by Ridner (2005) and Park et al (2008). The survivors who are overweight or obese will benefit from education on maintaining optimal BMI and lymphoedema risk reduction practices, as well as careful monitoring for limb and symptom changes. Further vigilance is required for overweight and obese survivors who have cancer treatment to the dominant side or experience post-operative swelling.
Further research to examine the constellation of risk factors that contribute to the development of lymphoedema in breast cancer survivors must include consideration of:
-
▶▶
Pre-diagnosis BMI
-
▶▶
BMI increase in survivorship
-
▶▶
Occurrence of post-operative swelling
-
▶▶
Cancer treatment to the dominant side.
Increased understanding of the cumulative impact of these and other known risk factors will enable researchers and clinicians to design and implement more targeted risk-reduction interventions.
Key points.
-
▶▶
The unique contribution of this research is a proposed 5% BMI-adjusted limb volume change (LVC) approach, which considers a change of 5% or greater in breast cancer affected-arm volume over percent change in BMI compared to pre-operative baseline to be indicative of lymphoedema.
-
▶▶
Dominant limb and cancer-affected side significantly increased the risk (48% greater risk, p=0.02) of developing lymphoedema only in those whose BMI ≥30.
-
▶▶
Breast cancer survivors who developed post-operative swelling had significantly higher risk (40% greater risk, p=0.01) of developing lymphoedema at some later time (before 30 months) after surgery, than those who did not develop post-operative swelling.
-
▶▶
Pre-operative limb measurement was an essential reference for detection of post-operative swelling. It is not recommended that researchers and clinicians substitute the measurement at the three-month (after surgery) visit, due to occurrences of limb swelling during the post-operative visit (one to four weeks after surgery) and lymphoedema at the three-month (after surgery) visit.
Acknowledgements
The data for this project were supported by Grant Number 1 RO1 NR05342-01 (Armer, PI) from the National Institute for Nursing Research, National Institutes of Health, MU PRIME fund # PRM-01-007, University of Missouri, and Ellis Fischel Cancer Center research gift funds and the first author received funding support by the Nuclear Science and Engineering Institute for Graduate Research Assistantship. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health. The authors thank Tetsuya Kobayashi for preliminary computational implementation, Dr Richard Madsen and Isabella Zaniletti for the original database design and data cleaning, Orawan Nukaew and Nathan Armer for editing assistance, the research nurses and research assistants for data collection and entry, and the breast cancer survivors who participated in the study. The authors would like to thank the reviewers for their comments that helped improve the manuscript.
Footnotes
Declaration of interest: None.
References
- American Cancer Society (ACS) Cancer Facts and Figures 2006. American Cancer Society; Atlanta: 2007. [Google Scholar]
- American Cancer Society (ACS) Lymphedema Understanding and Managing Lymphedema after Cancer Treatment. American Cancer Society; Atlanta: 2006. [Google Scholar]
- Armer JM. Lymphedema occurrence following breast cancer treatment: The first 30 months. In: Lui N, editor. Lymphology; Progress in Lymphology-XXI. Proceedings of the 21st Congress of Lymphology; Shanghai, China. 26–29 September 2007; 2008. in press. [Google Scholar]
- Armer J. The problem of post-breast cancer lymphedema: Impact and measurement issues. Cancer Investigations. 2005;1:76–83. [PubMed] [Google Scholar]
- Armer J, Fu MR, Wainstock JM, Zagar E, Jacobs LK. Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology. 2004;37:73–91. [PubMed] [Google Scholar]
- Armer JM, Fu M. Age differences in post-breast cancer lymphedema signs and symptoms. Cancer Nurs. 2005;28(3):200–7. doi: 10.1097/00002820-200505000-00007. [DOI] [PubMed] [Google Scholar]
- Armer JM, Mahamaneerat WK, Kobayashi T, Nukaew O, Stewart BR, Shyu C-R. Occurrence of lymphedema following breast cancer treatment using BMI-adjusted volume change. In: Lui N, editor. Lymphology; Progress in Lymphology-XXI. Proceedings of the 21st Congress of Lymphology; Shanghai, China. 26–29 September 2007; 2008. in press. [Google Scholar]
- Armer JM, Stewart BR. A comparison of four diagnostic criteria for lymphedema in a post-breast cancer population. Lymphatic Res Biol. 2005;3:208–17. doi: 10.1089/lrb.2005.3.208. [DOI] [PubMed] [Google Scholar]
- Beaulac SM, McNair LA, Scott TE, LaMorte WW, Kavanah MT. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. 2002;137(11):1253–7. doi: 10.1001/archsurg.137.11.1253. [DOI] [PubMed] [Google Scholar]
- Brink PJ, Wood MJ. Advanced Design in Nursing Research. 2nd edn. Sage Publications; Thousand Oaks, California, USA: 1998. [Google Scholar]
- Callaway C, Chumlea W, Bouchard C, et al. Circumferences. In: Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, Illinois, USA: 1998. pp. 39–54. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) [accessed 19/11/2008];BMI — Body Mass Index: BMI for Adults. 2008 www.cdc.gov/nccdphp/dnpa/bmi/
- Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System (BRFSS) Survey Data. US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, Georgia, USA: 2006. [Google Scholar]
- Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20(4):1128–43. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- Coen JJ, Taghian AG, Kachnic LA, Assaad SI, Powell SN. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiation Oncol Biol Phys. 2003;55:1209–15. doi: 10.1016/s0360-3016(02)04273-6. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Flickinger JC. Arm edema after lumpectomy and breast irradiation. Am J Clin Oncol. 2003;26:229–31. doi: 10.1097/01.COC.0000018177.75673.06. [DOI] [PubMed] [Google Scholar]
- Disa JJ, Petrek JA. Rehabilitation after treatment for cancer of the breast. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th edn. Lippincott Williams & Wilkins; Philadelphia, USA: 2001. pp. 1717–26. [Google Scholar]
- Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE. Weight gain, body mass index, hormone replacement therapy, and postmenopausal breast cancer in a large prospective study. Cancer Epidemiol Biomarkers Prevention. 2004;13:220–4. doi: 10.1158/1055-9965.epi-03-0301. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC Cancer Base No. 5, Version 2.0. IARCPress; Lyon, France: 2004. [Google Scholar]
- Geller BM, Vacek PM, O’Brien P, Secker-Walker RH. Factors associated with arm swelling after breast cancer surgery. J Women’s Health. 2003;12:921–30. doi: 10.1089/154099903770948159. [DOI] [PubMed] [Google Scholar]
- Hull MM. Functional and psychosocial aspects of lymphedema in women treated for breast cancer. Innovations in Breast Cancer Care. 1998;3:97–100. [Google Scholar]
- Johansson K, Ohlsson K, Ingvar C, Albertsson M, Ekdahl C. Factors associated with the development of arm lymphedema following breast cancer treatment: A match pair case-control study. Lymphology. 2002;35(2):59–71. [PubMed] [Google Scholar]
- Meek AG. Breast radiotherapy and lymphedema. Cancer Supplement. 1998;83:2788–97. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2788::aid-cncr27>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics . Cancer Statistics Registrations: Registrations of Cancer Diagnosed in 2004, England. National Statistics; London, UK: 2007. Series MB1 no.35. [Google Scholar]
- Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004;187:69–72. doi: 10.1016/j.amjsurg.2002.12.003. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee WH, Chung HS. Incidence and risk factors of breast cancer lymphoedema. J Clinical Nurs. 2008;17(11):1450–9. doi: 10.1111/j.1365-2702.2007.02187.x. [DOI] [PubMed] [Google Scholar]
- Passik S, McDonald M. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. American Cancer Society Lymphedema Workshop: Cancer Supplement. 1998;83:2817–20. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Peat J, Barton B. Medical Statistics: A Guide to Data Analysis and Critical Appraisal. 1st edn. Blackwell Publishing; Massachusetts, USA: 2005. [Google Scholar]
- Petlund CF. Volumetry of limbs. In: Olszewski WL, editor. Lymph Stasis: Pathophysiology, Diagnosis and Treatment. CRC Press; Boston, Massachusetts, USA: 1991. pp. 443–52. [Google Scholar]
- Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83(S12B):2776–81. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–77. doi: 10.1002/1097-0142(20010915)92:6<1368::aid-cncr1459>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Radina ME, Armer JM. Surviving breast cancer and living with lymphedema: Resiliency among women in the context of their families. J Family Nurs. 2004;10:485–505. [Google Scholar]
- Ridner S. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13:904–11. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- Rockson SG. Diagnosis and management of lymphedema. Cancer Supplement. 1998;83:2882–5. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2882::aid-cncr45>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rovere Q, Ahmad G, Singh I, et al. An audit of the incidence of arm lymphoedema after prophylactic level I/II axillary dissection without division of the pectoralis minor muscle. Ann R Coll Surg Engl. 2003;85(3):15–61. doi: 10.1308/003588403321661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener SF, Winchester DJ, Martz CH, et al. Lymphedema after sentinel lymphadenectomy for breast carcinoma. Cancer. 2001;92(4):748–52. doi: 10.1002/1097-0142(20010815)92:4<748::aid-cncr1378>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Soran A, D’Angelo G, Begovic M, et al. Breast cancer-related lymphedema--what are the significant predictors and how they affect the severity of lymphedema? Breast J. 2006;12(6):536–43. doi: 10.1111/j.1524-4741.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- Voogd AC, Ververs JM, Vingerhoets AJ, Roumen RM, Coebergh JW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg. 2003;90:76–81. doi: 10.1002/bjs.4010. [DOI] [PubMed] [Google Scholar]
- Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prevention. 2005;14:2009–14. doi: 10.1158/1055-9965.EPI-05-0106. [DOI] [PubMed] [Google Scholar]