Abstract
Background
Peripheral neuropathies affect many people worldwide and are caused by or associated with a wide range of conditions, both genetic and acquired. Current therapies are directed at symptomatic control because no effective regenerative treatment exists. Primary challenge is that mechanisms that lead to distal axonal degeneration, a common feature of all peripheral neuropathies, are largely unknown.
Objective/Methods
To address the role and specific characteristics of dorsal root ganglia (DRG) derived sensory neuron culture system as a useful model in evaluating the pathogenic mechanisms of peripheral neuropathies and examination and validation of potential therapeutic compounds. A thorough review of the recent literature was completed and select examples of the use of DRG neurons in different peripheral neuropathy models were chosen to highlight the utility of these cultures.
Conclusion
Many useful models of different peripheral neuropathies have been developed using DRG neuronal culture and potential therapeutic targets have been examined, but so far none of the potential therapeutic compounds have succeeded in clinical trials. In recent years, focus has changed to evaluation of axon degeneration as the primary outcome measure advocating a drug development strategy starting with phenotypic drug screening, followed by validation in primary complex co-cultures and animal models.
Keywords: Dorsal root ganglion, sensory neuron, neuroprotection, phenotypic screen
I. Introduction
Neuronal cell cultures have been widely and successfully exploited as a powerful tool to answer relevant questions of neurobiology and neurodevelopment; neurite/axon formation and elongation, synapse formation and synaptic properties, neurotrophic factor trafficking and signalling, neurotransmitter release, electrical signalling, and intracellular protein trafficking have all been studied using primary neuronal cultures from various animals. Similarly, primary neuronal cultures represent a tightly controlled system to study the basics of cellular and molecular responses to external or internal disturbances to the nervous system homeostasis and serve as a powerful in vitro model of pathology.
In this review, we will address the role of dorsal root ganglia (DRG) derived sensory neuron culture system as an excellent model in unveiling the pathogenic mechanisms of peripheral nervous system (PNS) diseases to find potential novel targets for neuroprotection and to test and validate new therapeutic compounds. The PNS by definition includes sensory, motor and autonomic nerves that lie outside brain and spinal cord. The afferent branch, i.e. sensory nerves that originate from DRG neurons, is unique in that instead of a typical axon-dendrite structure of other neurons, has unipolar neurons with a single axon stem bifurcating into a peripheral branch that goes to the distal tissues and a central branch that goes to spinal cord. The efferent arms of the PNS include motor neurons and autonomic neurons that originate in the spinal cord or autonomic ganglia and send branches to the skeletal muscle and internal organs, respectively. All components of the PNS can be affected by diseases of the PNS, commonly known as peripheral neuropathies; but often the sensory neurons are affected early and more severely. This is often evident in the constellation of symptoms that the patients experience; sensory symptoms such as pain, paresthesias, loss of perception and gait imbalance often dominate patients' complaints and occur either in isolation or before weakness and autonomic symptoms.
Peripheral neuropathies can be caused by or associated with a wide range of conditions, both genetic and acquired. Common causes consist of diabetes, infections such as leprosy and human immunodeficiency virus (HIV), toxic including chemotherapeutic agents and inflammatory-autoimmune conditions. Apart from autoimmune demyelinating or vascular neuropathies, no effective treatment exist that deal with the underlying axonal degeneration. Current therapies primarily focus on symptom control. Part of the challenge is that mechanisms that lead to distal axonal degeneration, a common feature of all peripheral neuropathies, are largely unknown. Primary DRG neuronal cultures, especially in association with glial cells of the PNS, Schwann cells, represent an ideal system to study cellular and molecular pathways that lead to neurodegeneration in order to gain new insights for drug discovery. Furthermore, the cultures offer a valuable tool for validation of molecular targets identified in high throughput drug screens for neuroprotection and axonal regeneration.
II. Primary culture of dorsal root ganglia sensory neurons: cellular properties and morphological assays
Neurons derived from DRG can be easily grown in culture both from embryonic [1], postnatal [2] and adult animals [3]. Furthermore, when cultured with Schwann cells, they can be myelinated in culture [4]. Once carefully dissected, DRG can be either dissociated by trypsinization or seeded onto culture dishes as explants (Figure 1). Each model offers unique advantages and disadvantages; the scientific question and experimental design often dictate the type of culture. Use of embryos allows a high content of cells with a higher efficiency in isolating neurons in comparison to post-natal animals. They have been shown to be an adequate and predictive model for studying diabetic, HIV and chemotherapy induced neuropathies [5-8]. Both neuronal death, a relatively infrequent event in peripheral neuropathies, and axonal degeneration can be studied in these cultures. Furthermore, relatively high yield and hardiness of cultures represent a good model for drug screening of potential neuroprotective agents. Embryonic DRG sensory neurons are also particularly suitable for compartmentalised cultures. Prototypic compartmentalized culture system was initially developed by Dr. Robert Campenot and is known as Campenot chambers [9]. This sophisticated cell culture system where cell bodies and axons grow in physically separated environments that can be differentially manipulated, allows local study of mechanisms of axonal degeneration independently from the neuronal soma (Figure 2). In addition to their traditional use in developmental neurobiology, embryonic DRG neurons in Campenot chambers have been used successfully to study mechanisms of axonal degeneration in disease models [10-12]. Despite these advantages, embryonic DRG neurons do have potential pitfalls; chief being their dependence on neurotrophins for survival for the first week in culture. This may hinder evaluation of mechanisms of neurodegeneration. Some researchers, however, have successfully “aged” embryonic DRG neurons in vitro by culturing them more than a week and demonstrated that they exhibit properties similar to adult DRG neurons (e.g. response to hyperglycaemia, see below) [13].
Figure 1.
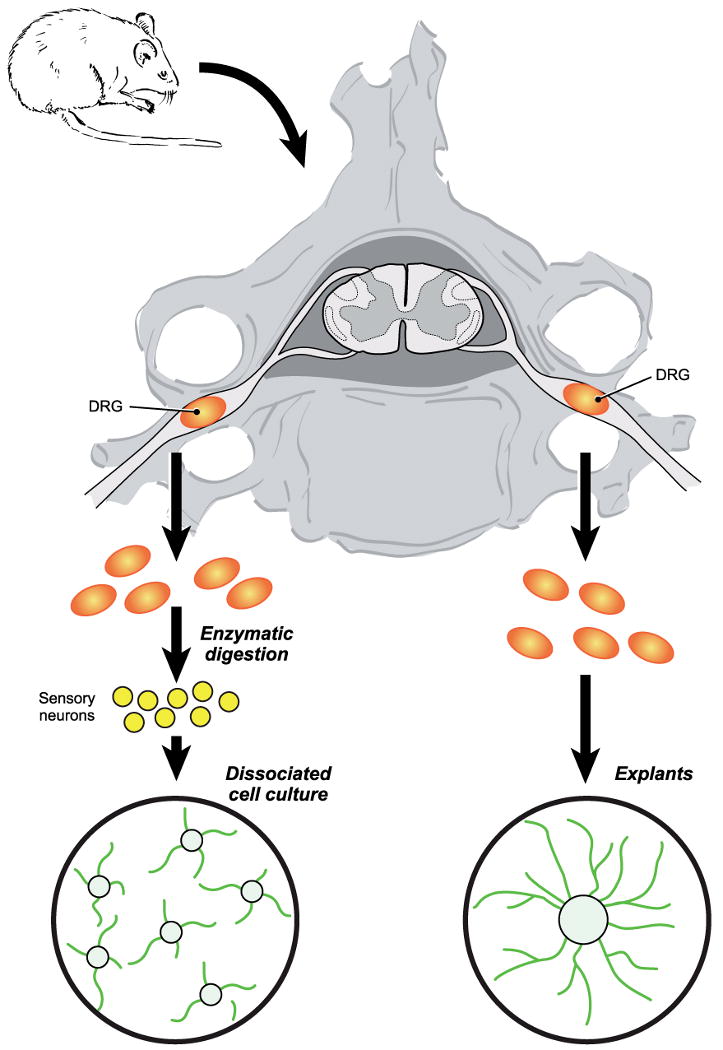
Schematic view of preparation of dissociated and explant DRG sensory neuronal cultures.
Figure 2.
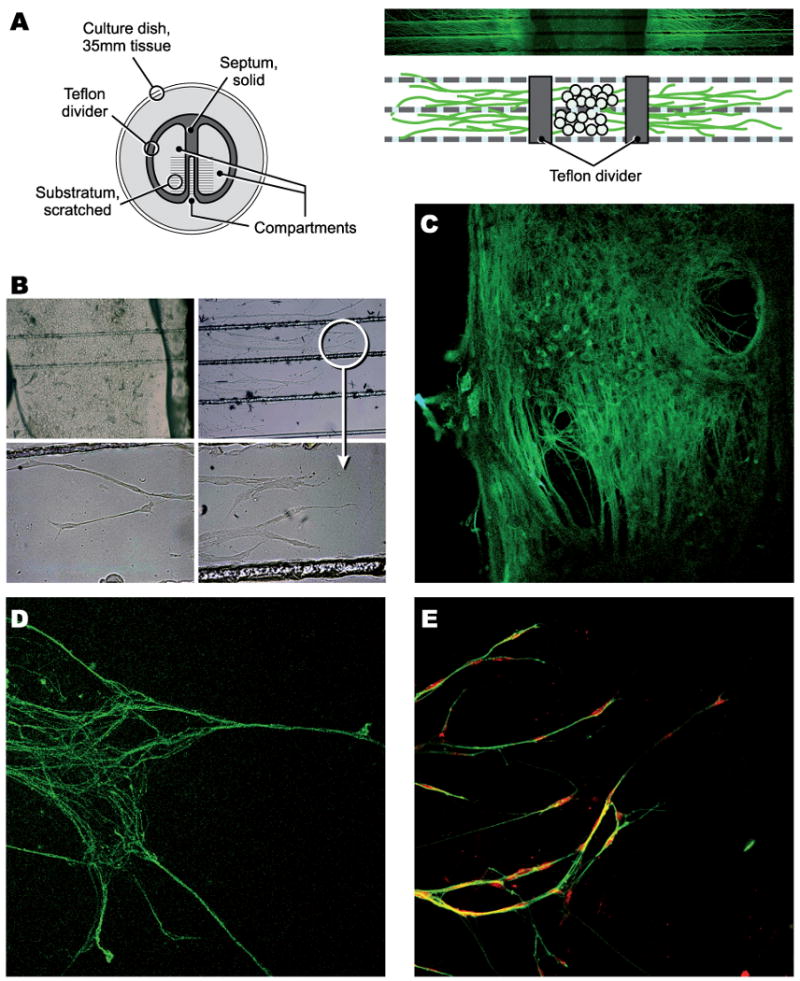
Compartmented sensory neuronal cultures. A: schematic and ß-III tubulin immuno-stained cultures; B: phase contrast images of middle and side chambers (arrow head: growth cone); C-D: TuJ1 immuno-staining of neurons and distal axons; E: Schwann cells (red colored) ensheating axons in side chambers.
In contrast, dissociated postnatal and adult DRG sensory neurons offer the possibility to study mature, completely developed neurons that may resemble the in vivo characteristics of DRG neurons. They have been successfully used to model HIV and diabetic neuropathies [14-16]. One advantage of adult neurons is that they are not dependent on neurotrophins or serum for their survival so they can be cultured in defined media.
Explant cultures have also been used to model peripheral neuropathies and study disease mechanisms. One distinct advantage of DRG explant cultures is that they keep the original architecture of DRG with preserved relationship among neurons, Schwann cells and resident macrophages that are lost in dissociated cultures; this might be relevant in studies on the role of neuronal and glia cell interaction in axonal degeneration and regeneration. The DRG neuron-Schwann cell interactions can also be reconstituted by use of co-culture methods to study axon-neuron/glia interactions in both normal state and in disease conditions. For an example, see below section on hereditary neuropathies.
III. DRG sensory neuronal culture as a model of HIV associated neuropathies
HIV associated sensory neuropathies are the most common neurological complications in HIV infection, affecting one-third of patients with AIDS [17]. They include distal symmetric polyneuropathy (DSP), secondary to HIV infection, and antiretroviral toxic neuropathy (ATN) associated with the use of antiretroviral agents, particularly nucleoside reverse transcriptase inhibitors. They are mainly sensory, characterized by painful dysaesthesias in a length-dependent stocking-and-glove distribution and are clinically indistinguishable from each other. The pathological changes associated with DSP are distal axonal degeneration of sensory fibers in the absence of any significant death of DRG sensory neurons [18]. The pathophysiology of DSP is not completely understood, but most commonly accepted pathogenic mechanism involves neuronal damage secondary to immune activation. Others and our group have used DRG sensory neuron-Schwann cell co-cultures to probe the relationship between the immune mediators and axonal degeneration in vitro. In the DRG neuron-Schwann cell co-culture, HIV envelope protein gp120 binds to chemokine receptor CXCR4 on Schwann cells and induce secretion of RANTES (Regulated upon Activation, Normal T-cell Expressed and Secreted), which in turn binds to CCR5 chemokine receptors on neurons. This results in a TNF-alpha (tumor necrosis factor-alpha)-mediated activation of the caspase pathway and axonal degeneration in vitro [19].
In this in vitro model of HIV associated sensory neuropathy, axonal degeneration is partially blocked by a specific caspase-3 inhibitor, but it is not clear if this effect is a direct action on the mechanism underlying axonal degeneration or it is an indirect effect due to activation of apoptotic pathways in the neuronal cell body. One of the hallmarks of many peripheral neuropathies is distal axonal degeneration without significant cell death at the neuronal level. Most studies directed at neuronal toxicity or degeneration are focused on events that are occurring at the cell body level, but local axonal mechanisms may play a bigger role in mediating distal axonal degeneration and they may be distinct from the intracellular events at the cell body [20].
Compartmentalized culture systems, used successfully in developmental biology, offer a very valuable tool to answer this question. In addition to the ability to manipulate axon and neuronal cell bodies independently of each other one can ask the role peri-neuronal and peri-axonal Schwann cells play in pathogenesis of different peripheral neuropathies. Using Campenot chambers, Melli and colleagues extended the previous work by Keswani et.al., and demonstrated that gp120 has direct axonal toxicity mediated by both CCR5 and CXCR4 chemokine receptors on axons [10]. Furthermore, they showed that there was a difference between peri-neuronal and peri-axonal Schwann cells; peri-neuronal Schwann cells were key players in inducing the gp120 neurotoxicity but the peri-axonal Schwann cells were neuroprotective and presence of Schwann cells in the axonal chamber partially ameliorated the gp120 induced axonal degeneration. This “neuroprotective” role for peri-axonal Schwann cells may be a common theme for other injury models in the peripheral nervous system [21].
Although, these in vitro studies shed some light onto potential mechanisms of HIV-induced axonal degeneration, many unanswered questions remain. For example, how does binding of gp120 onto the chemokine receptors lead to the activation of the caspase pathway and distal axonal degeneration? Which intracellular signalling pathways are involved? Do these pathways share common key elements with other causes of peripheral neuropathies? One potential answer comes from a study by Bodner and colleagues [22]. In this study CEP-1347, an inhibitor of the mixed lineage kinases, prevented the neuronal apoptosis induced by gp120 as well as the neuronal death caused by a nucleoside reverse transcriptase inhibitor (NRTI), didanosine. Both gp120 and didanosine activated the c-Jun N-terminal kinase pathway and caused death of DRG neurons. The investigators, however, did not examine axonal degeneration and it is unclear whether the observation regarding the sensory neuronal death in vitro could be generalized to the in vivo situation, with very limited death in the DRG.
In parallel to these studies, in vitro DRG neuron-Schwann cell cultures have been used to elucidate the mechanism of antiretroviral toxic neuropathy [5]. Nucleoside reverse transcriptase inhibitors such as didanosine cause direct mitochondrial toxicity in neurons; resultant energy failure leads to axonal degeneration and, at high doses, non-apoptotic cell death. This in vitro system was used to demonstrate neuroprotective effects of FK-506 and suggested a potential therapeutic avenue with non-immunosuppressive analogues of FK-506 [5]. Further studies are needed to validate this observation in animal models.
IV. DRG sensory neuronal culture as a model of chemotherapy induced neuropathies
Peripheral neuropathy, manifested by neuropathic pain and axonal degeneration, is one of the major sources of disability in patients following anti-neoplastic therapy [23, 24]. The risk of developing peripheral neuropathy often depends on the cumulative dose of the chemotherapeutic agent and, therefore, increases with longer duration of therapy [25, 26]. The symptoms can be disabling enough that either the dose of chemotherapy is reduced or completely discontinued altogether, limiting the choice of effective anti-cancer therapies. Chemotherapy induced neuropathy is usually characterized by length-dependent axonal degeneration with primary involvement of distal sensory or sensorimotor fibers [27, 28]. Sensory neurons are particularly vulnerable to toxic agents because DRG lie outside of the blood-brain barrier and are supplied with fenestrated capillaries that allow free passage of circulating substances [24]. Moreover, longer peripheral nerves, such as sensory fibers carrying touch, temperature and pain sensation from the feet, are particularly susceptible to any interference with energy metabolism, mitochondrial function, or axonal transport. However, the underlying molecular and cellular mechanisms of neurotoxicity remain unclear for majority of chemotherapeutic compounds and so far no effective regenerative or protective treatment has been found.
DRG sensory-Schwann cell co-cultures offer a unique opportunity to investigate mechanisms of chemotherapy-induced neuropathies. For example, Windebank and colleagues have shown that cisplatin-induced neurotoxicity involves activation of apoptotic pathways, bax redistribution and cytochrome c release in DRG neurons [29]. Furthermore, in vitro models of chemotherapy-induced neuropathies can be used to evaluate the efficacy of potential therapeutic compounds and their mechanisms of action. We have shown that recombinant human erythropoietin prevents axonal degeneration induced by paclitaxel in sensory neurons, and that this effect is associated with downregulation of detyrosinated tubulin [6]. This neuroprotective effect in vitro was further validated in an animal model of paclitaxel-induced neuropathy, suggesting that recombinant erythropoietin could be a potential drug to prevent chemotherapy-induced neuropathies.
Same approach was also used in a study evaluating the efficacy of another potential neuroprotective compound, alpha-lipoic acid [7]. In primary DRG neurons, both cisplatin and paclitaxel induce early mitochondrial dysfunction with loss of membrane potential and induction of autophagic vacuoles. Alpha-lipoic acid prevents axonal degeneration induced by both of these chemotherapeutic drugs. This is mediated by rescue of mitochondrial changes and induction of expression of frataxin, an essential mitochondrial protein with anti-oxidant and chaperone properties. This in vitro study using DRG neuronal cultures demonstrate the importance of early mitochondrial changes in axonal degeneration. Interestingly, in animal models of paclitaxel-induced neuropathy [30, 31] others have found ultrastructural alterations of mitochondria in C-fibers and myelinated axons of sciatic nerves, similar to the pathological changes in our in vitro model. Nevertheless, the neuroprotection observed with alpha-lipoic acid in vitro will need to be validated in the animal models of chemotherapy-induced neuropathies. If confirmed, these findings suggest that alpha-lipoic acid may be an effective neuroprotective therapy for chemotherapy-induced peripheral neuropathies.
V. DRG sensory neuronal culture as a model of diabetic neuropathy
Diabetic neuropathy affects about 60% of diabetic patients [32], and according to some recent studies, sub-clinical impairment of peripheral nerves can occur in all patients. Diabetic neuropathy includes several distinct syndromes, among them symmetric mainly sensory polyneuropathy often accompanied by autonomic neuropathy is the most frequent form. It is the most common late complication of type I diabetes mellitus; close to 100% of patients eventually develop it [33]. Since diabetes is a complex, chronic and multifactorial disease, it is challenging to develop adequate animal models. In vitro, cell culture models offer the unique possibility to study pathogenic mechanisms of disease and biological functions of single molecules in a tightly controlled system. The use of DRG sensory neurons has several advantages: 1) DRG sensory neurons represent the main cellular target of diabetic neurotoxicity; 2) it is possible to enrich the neuronal population with NGF-dependent neurons which are often the most vulnerable ones clinically [34] and in animal models of Type I diabetes [35]; 3) possibility of co-culturing DRG neurons with Schwann cells that are clearly involved in diabetic neuropathy based on electrophysiology and ultrastructural pathology. Furthermore, the close relationship between the sensory axon and the Schwann cell that is established during development and maintained throughout the adult life is likely to play an important role in the pathogenesis of diabetic neuropathy. Axon-glia interactions have been shown to play an important role in the pathogenesis of several neurodegenerative disorders like amyotrophic lateral sclerosis and HIV dementia. Exposure of sensory neurons and Schwann cells to hyperglycaemic conditions in vitro induces morphological and functional abnormalities characteristic of neurotoxicity, such as impairment of cell viability, neuritic outgrowth, mitochondrial activity and electrophysiological abnormalities.
In the past twenty years many laboratories have used in vitro culture system to elucidate mechanisms of neuronal dysfunction in diabetes (reviewed in [36]). To overcome some of the critiques on whether short-term exposure to high glucose can be an adequate model for long-term diabetic complications or reflect the complexity and multifactorial nature of the disease, researchers utilized a variety of culture methods. These include use of dissociated DRG sensory neurons and DRG explants from diabetic animals like streptozotocin treated mice and rats [37, 38], or from spontaneous diabetic BioBred/Worchester rats (BB/W). Furthermore, others have shown that non-diabetic DRG sensory neurons treated with serum from BB/W rats [39] or type II diabetic patients [40] can be used to evaluate the neurotoxicity of diabetes. For example, DRG sensory neurons isolated from diabetic mice show an impaired attachment to extracellular matrix [41]; excessive glycation of type IV collagen and laminin affect attachment, survival and axonal elongation in sensory neurons [42]. Other examples of mechanistic studies include demonstration of impairment of axonal transport of NGF [43], abnormalities in calcium homeostasis [44] [45], mitochondrial dysfunction [46, 47] and increased expression of transient receptor potential cation channel, subfamily V, member 1(TRPV1) in DRG small sensory neurons from diabetic rats [48]. TRPV1 is activated by noxious heat, decreased pH and capsaicin; its increased activity in diabetes may explain hyperalgesia and pain frequently experienced by diabetic patients with neuropathy.
Multiple other in vitro DRG neuronal culture studies have shown that high levels of glucose promote neuronal reactive oxygen species production, leading to loss of mitochondrial membrane potential, depletion of ATP, and death of DRG neurons [49-51]. These observations have led to the use of embryonic rat DRG neuronal cultures to develop a medium-throughput in vitro assay to screen for potential therapeutic compounds for their ability prevent oxidative stress in neurons exposed to hyperglycaemia [52]. The role of glucose induced apoptotic cell death in adult neurons has been questioned on the basis of their independency from neurotrophic support and c-Jun N-terminal kinase (JNK) activation for survival [53]; glucose induced apoptosis would only be a phenomenon observed in embryonic sensory neurons in vitro and not relevant for diabetic neuropathy [54]. However, this issue needs further study because the role of JNK pathways in diabetes is not completely understood. Previous studies have shown that multiple iso-forms are activated in sensory neurons exposed to glucose and that it may have neuroprotective function [55] or glucose-induced neuronal injury stimuli may act through apoptotic pathways independent of JNK.
The use of DRG neuronal cultures to study the effects of hyperglycaemia is not without disagreements in the field. Many researchers have noted that in vitro culture of embryonic DRG neurons require relatively high levels of glucose to induce oxidative stress and apoptosis. In fact, it has been difficult to observe neurotoxicity of hyperglycaemia in embryonic DRG neurons, either as dissociated cultures or as explants [13, 56-58]. However, others have argued that inclusion of IGF-1 and insulin in the culture media allows DRG neurons to utilize supra-normal levels of glucose in vitro without evidence of cellular stress and that in order to observe damaging effects of hyperglycaemia, insulin and IGF-1 needs to be removed from the culture media or levels be drastically reduced. This is in contrast to adult DRG neurons, where levels of hyperglycaemia observed in diabetic patients is neurotoxic to adult DRG neurons in vitro; however, this effect is restricted to the axonal compartment and is not associated with induction of cell death [58].
Another important issue in the diabetic neuropathy field is whether apoptosis observed in vitro has any relevance to neuronal death in vivo. This is a major limitation of the in vitro models of type 1 diabetes. In patients and in long-term models of type 1 diabetes, there is very little neuronal death [59] and the small amount of neuronal death observed is greatly out of proportion to distal axonal loss [60]. Future studies on the mechanisms of neurotoxicity of hyperglycaemia needs to take these observations into consideration and perhaps focus on distal axonal loss as the primary pathogenic mechanism of neuropathic symptoms in patients with diabetic neuropathy.
One advantage of the in vitro models of diabetic neuropathy is that, the in vitro culture system allows one to evaluate the relative contribution of each cell type in the PNS to the pathogenesis of diabetic neuropathy. Hyperglycaemia has been shown to affect Schwann cell proliferation and migration [56], crucial events during axonal regeneration. In fact, axonal regeneration is impaired in diabetes [61]. This observation, perhaps, explains why recovery from nerve traumas is worse in diabetic patients.
VI. Myelinated DRG sensory neuronal culture as a model of hereditary demyelinating neuropathies and inflammatory immune mediated neuropathies
Myelinated DRG sensory neuron-Schwann cell co-cultures have been successfully used as in vitro models of hereditary peripheral neuropathies like Charcot Marie Tooth disease type 1A (CMT1A). When DRG sensory neurons and Schwann cells from CMT1A transgenic rat are grown in culture and induced to myelinate, they exhibit a dysmyelinating phenotype [62-65]. Many axonal segments remain unmyelinated and myelinated segments reveal uncompacted myelin and Schwann cell cytoplasmic abnormalities. Furthermore, the axons that fail to myelinate properly show evidence of degeneration, mimicking the in vivo disease where prolonged demyelination leads to distal axonal degeneration. This model is a valuable tool to investigate the molecular mechanisms of axon-glia interaction in myelination and axon maintenance. Furthermore, the model can be used to test potential neuroprotective drugs for CMT1A.
The relevance of this model extends to immune mediated demyelinating diseases such as chronic inflammatory demyelinating polyneuropathy (CIDP) as cultures can be treated with sera from patients with CIDP or animal models of CIDP. In an in vitro model of Guillain Barre Syndrome (GBS), an acute demyelinating inflammatory polyneuropathy, nitric oxide induced axonal injury and secondary demyelination without injury to Schwann cells directly suggesting that in GBS, the immune attack may be directed at the axon as well [66].
VII. DRG sensory neuronal cultures as tools for drug discovery
As stated above, DRG sensory neuronal cultures, alone or in combination with Schwann cells, have been used to study molecular mechanisms of a variety of diseases affecting the peripheral nervous system, genetic or acquired. These studies have yielded important information about pathogenesis of the disease under study and were useful in identifying new potential therapeutic targets. Furthermore, many of these studies were directed at understanding common mechanisms of axon-glia interaction, axon maintenance, demyelination and axonal degeneration [20].
Although primary DRG neuronal cultures have been used to screen a small library of potential therapeutic drugs in an in vitro diabetic neuropathy model [52] and in an assay to identify drugs that upregulate Nociceptin/orphanin FQ (N/OFQ), an opioid-related peptide [67], they are not suitable for a high-throughput drug screening strategy. Availability of the primary cultures and limited numbers severely restrict the throughput of an assay. An immortalized DRG sensory neuronal line, ideally human, would be more appropriate as long as it maintains characteristics of a DRG sensory neuronal line. A human DRG sensory neuronal line had been developed with typical features of a neuron [68], but is no longer available for use by researchers.
Recently in our lab we immortalized sensory neurons from embryonic rat DRG and further characterized a specific line, 50B11 [69]. This immortalized DRG sensory neuronal line has the receptor characteristics of a nociceptive neuron expressing TRPV1 and receptors for NGF and GDNF. Furthermore, the cells are easy to grow and differentiate, making them suitable for high-throughput drug screening applications. In limited validation assays, the 50B11 neuronal cells exhibited axonal degeneration and other features of neurotoxicity when exposed to toxic drugs that cause neuropathy in patients such as didanosine or paclitaxel. Although, the 50B11 cell line is suitable for high throughput applications, the results would still need to be validated in primary DRG neuronal cultures, preferably in assays that utilize DRG neuron-Schwann cell co-cultures, before moving to animal models.
Despite many years of research on peripheral neuropathies, all of our clinical trials for preventative or regenerative therapies for axonal peripheral neuropathies have failed (excluding immune mediated neuropathies). Our current therapies are limited to symptom control and even at that, most of the available drugs offer limited symptom relief, often at the high cost of side effects. The causes of this failure in developing effective therapies for peripheral neuropathies are multiple, but perhaps its time to rethink our drug screening strategies and utilize appropriate cells and assays that include phenotypic screens to evaluate axonal degeneration as the read-out. Recent advances in automated image analysis systems have made it feasible to do axon length measurements in 96-well plate format neuronal cultures [70, 71]. We propose that a phenotypic screen that starts with cells that are suitable for high-throughput assays, like 50B11 cell line, and goes through validation cycles in primary DRG neuronal cultures and then appropriate animal models, is more likely to yield drug candidates that may be more successful in clinical trials.
Expert Opinion.
Peripheral neuropathies are the most common neurodegenerative diseases affecting almost 20 million people in the USA alone; more common than the combined prevalence of other neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease or multiple sclerosis. Like other neurodegenerative diseases, incidence is higher in an aging population and like other neurodegenerative diseases we do not have an effective preventive or regenerative therapy apart from the relatively uncommon inflammatory autoimmune neuropathies. In the absence of any therapy that can prevent axonal degeneration or allow efficient regeneration of degenerated axons, current therapies are directed at symptomatic control. Since, even symptomatic therapies are only partially effective, there is a significant unmet need to develop preventive and/or regenerative therapies for peripheral neuropathies.
Development of effective therapies can only be accomplished by advances in development of in vitro assays and animal models that reflect the underlying mechanisms of axonal degeneration. Dorsal root ganglion (DRG) neuronal cultures, in association with Schwann cell cultures, offer a unique opportunity to model the various peripheral neuropathies in vitro to elucidate underlying molecular mechanisms of axonal degeneration. Furthermore, the in vitro models utilizing DRG neuronal cultures allow screening of potential therapeutic drugs. However, use of primary DRG neurons for a high-throughput drug screening approach is cumbersome and not practical because of limited availability of cells. Here we review the current literature on the use of primary DRG neuronal cultures in drug discovery and propose a phenotypic screen that starts with cells that are suitable for high-throughput assays, like DRG neuronal cell lines, and goes through validation cycles in primary DRG neuronal cultures and then appropriate animal models. This approach is more likely to yield drug candidates that may be more successful in clinical trials.
Table 1. Selection of representative applications of DRG cultures for the study of peripheral neuropathies.
NRTI: Nucleoside reverse transcriptase inhibitors; RANTES: regulated upon activation normal T-cell expressed and secreted; rc: receptor; JNK: c-Jun N-terminal kinase; STZ: streptozotocin, SC: Schwann cells, ROS: reactive oxygen species; NO: nitric oxide
| Cell culture model | Major findings | References | |
|---|---|---|---|
| HIV-related neuropathies | |||
| Anti-retroviral toxic neuropathy (ATN) | Rat E15 dissociated DRG neurons | NRTIs cause direct mitochondrial damage leading to axonal damage and non-apoptotic cell death. | Keswani et al., 2003[5] |
| Distal symmetric polyneuropathy (DSP) | Rat E15 dissociated DRG neurons | Gp120 induces RANTES in Schwann cells through CXCR4. RANTES induce TNF-α in neurons through CCR5 causing neuronal apoptosis. | Keswani et al, 2003[19] |
| ATN and DSP | Rat Post-natal dissociated DRG neurons | NRTIs and gp120 cause neuronal apoptosis through JNK pathway and inhibitors of the mixed lineage kinases prevents it. | Bodner et al., 2004[22] |
| DSP | Rat E15 dissociated DRG neurons in Campenot chambers | Gp120 causes neuronal apoptosis mediated by Schwann cells when applied to cell bodies and causes local axonal damage through caspases activation. | Melli et al., 2006[10] |
| Diabetic neuropathies | |||
| Diabetic neuropathy | Adult STZ treated mice dissociated DRG neurons | Improvement of neuronal survival and neurite growth from diabetic mice when exposed to insulin or high glucose | Sotelo et al., 1991[37] |
| Diabetic neuropathy | Rat E15 dissociated DRG neurons | High glucose induces ROS production, mitochondrial damage and apoptosis. | Russell et al., 2002[49] |
| Diabetic neuropathy | Adult STZ treated rat dissociated DRG neurons | Loss of insulin-dependent neurotrophic support contributes to mitochondrial membrane depolarization induced by diabetes. | Huang et al., 2003 [46] |
| Diabetic neuropathy | Rat E15 dissociated DRG neurons + rat Schwann cells/myelination | Myelinated SC/DRG cocultures provide a physiologically relevant model for studying demyelination observed in diabetic nerves in vivo. | Yu et al, 2008 [13] |
| Diabetic neuropathy | Rat sciatic nerve derived SC and adult mice DRG explants and dissociated cells | High glucose impairs proliferation and migration of Schwann cells, higher glucose impairs neurite elongation, no substantial apoptosis has been detected. | Gumy et al., 2008[56] |
| Diabetic neuropathy | Normal or 3-5 month STZ rats dissociated DRG neurons | Diabetic DRG neurons express low MnSOD and high ROS in axons, associated with impaired axonal outgrowth and aberrant dystrophic structures. | Zherebitskaya et al, 2009[58] |
| Chemoterapy-associated neuropathies | |||
| Paclitaxel associated neuropathy | Rat E15 dissociated DRG neurons | rhEPO prevents axonal degeneration in sensory neurons and is associated with downregulation of detyrosinated tubulin, further confirmed in the animal model. | Melli et al., 2006[6] |
| Paclitaxel and cisplatin associated neuropathies | Rat E15 dissociated DRG neurons | Alpha-lipoic acid protects sensory neurons through its anti-oxidant and mitochondrial regulatory functions, and induces the expression of frataxin. | Melli et al., 2008[7] |
| Demyelinating neuropathies | |||
| CMT1A | Transgenic PMP22 rat E15 dissociated neurons/myelination | Reproduction in vitro of dysmyelinated internodes and focal myelin swellings, pathological hallmarks of CMT1A. | Nobbio et al., 2001[63] |
| CMTIA | Transgenic PMP22 rat E15 dissociated neurons/myelination | Molecular and morphological signs of axonal damage following persistent demyelination. | Nobbio et al., 2006[65] |
| Inflammatory neuropathies | Rat E15 dissociated DRG neurons/myelination | NO causes demyelination secondary to axonal injury, no damage to Schwann cells viability. | Lehmann et al., 2007[66] |
Acknowledgments
This work was supported by the NIH, Adelson Medical Research Foundation, Neuropathy Association and Foundation for Peripheral Neuropathy.
References
- 1.Wood PM, Bunge RP. Myelination of cultured dorsal root ganglion neurons by oligodendrocytes obtained from adult rats. J Neurol Sci. 1986;74(2-3):153–69. doi: 10.1016/0022-510x(86)90101-2. [DOI] [PubMed] [Google Scholar]
- 2.Horie H, Kim SU. Improved survival and differentiation of newborn and adult mouse neurons in F12 defined medium by fibronectin. Brain Res. 1984;294(1):178–81. doi: 10.1016/0006-8993(84)91327-1. [DOI] [PubMed] [Google Scholar]
- 3.Scott BS. Adult mouse dorsal root ganglia neurons in cell culture. J Neurobiol. 1977;8(5):417–27. doi: 10.1002/neu.480080503. [DOI] [PubMed] [Google Scholar]
- 4.Eldridge CF, Bunge MB, Bunge RP, et al. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105(2):1023–34. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keswani SC, Chander B, Hasan C, et al. FK506 is neuroprotective in a model of antiretroviral toxic neuropathy. Ann Neurol. 2003;53(1):57–64. doi: 10.1002/ana.10401. [DOI] [PubMed] [Google Scholar]
- 6.Melli G, Jack C, Lambrinos GL, et al. Erythropoietin protects sensory axons against paclitaxel-induced distal degeneration. Neurobiol Dis. 2006;24(3):525–30. doi: 10.1016/j.nbd.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Melli G, Taiana M, Camozzi F, et al. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008;214(2):276–84. doi: 10.1016/j.expneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Vincent AM, Russell JW, Sullivan KA, et al. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol. 2007;208(2):216–27. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977;74(10):4516–9. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melli G, Keswani SC, Fischer A, et al. Spatially distinct and functionally independent mechanisms of axonal degeneration in a model of HIV-associated sensory neuropathy. Brain. 2006;129(Pt 5):1330–8. doi: 10.1093/brain/awl058. [DOI] [PubMed] [Google Scholar]
- 11.Silva A, Wang Q, Wang M, et al. Evidence for direct axonal toxicity in vincristine neuropathy. J Peripher Nerv Syst. 2006;11(3):211–6. doi: 10.1111/j.1529-8027.2006.0090.x. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Mehta NR, Conant K, et al. Axonal protective effects of the myelin-associated glycoprotein. J Neurosci. 2009;29(3):630–7. doi: 10.1523/JNEUROSCI.5204-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu C, Rouen S, Dobrowsky RT. Hyperglycemia and downregulation of caveolin-1 enhance neuregulin-induced demyelination. Glia. 2008;56(8):877–87. doi: 10.1002/glia.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh SB, Tran PB, Gillard SE, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21(14):5027–35. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Obrosova IG, Abatan O, et al. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. Am J Physiol Endocrinol Metab. 2005;288(1):E29–36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- 16.Price SA, Gardiner NJ, Duran-Jimenez B, et al. Thioredoxin interacting protein is increased in sensory neurons in experimental diabetes. Brain Res. 2006;1116(1):206–14. doi: 10.1016/j.brainres.2006.07.109. [DOI] [PubMed] [Google Scholar]
- 17.Estanislao L, Carter K, McArthur J, et al. A randomized controlled trial of 5% lidocaine gel for HIV-associated distal symmetric polyneuropathy. J Acquir Immune Defic Syndr. 2004;37(5):1584–6. doi: 10.1097/00126334-200412150-00010. [DOI] [PubMed] [Google Scholar]
- 18.Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6(1):21–7. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 19.Keswani SC, Polley M, Pardo CA, et al. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54(3):287–96. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 20.Hoke A. Neuroprotection in the peripheral nervous system: rationale for more effective therapies. Arch Neurol. 2006;63(12):1681–5. doi: 10.1001/archneur.63.12.1681. [DOI] [PubMed] [Google Scholar]
- 21.Keswani SC, Buldanlioglu U, Fischer A, et al. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56(6):815–26. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- 22.Bodner A, Toth PT, Miller RJ. Activation of c-Jun N-terminal kinase mediates gp120IIIB- and nucleoside analogue-induced sensory neuron toxicity. Exp Neurol. 2004;188(2):246–53. doi: 10.1016/j.expneurol.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 24.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 25.Albers J, Chaudhry V, Cavaletti G, et al. Interventions for preventing neuropathy caused by cisplatin and related compounds. Cochrane Database Syst Rev. 2007;(1):CD005228. doi: 10.1002/14651858.CD005228.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Lipton RB, Apfel SC, Dutcher JP, et al. Taxol produces a predominantly sensory neuropathy. Neurology. 1989;39(3):368–73. doi: 10.1212/wnl.39.3.368. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhry V, Rowinsky EK, Sartorius SE, et al. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol. 1994;35(3):304–11. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]
- 28.Peltier AC, Russell JW. Recent advances in drug-induced neuropathies. Curr Opin Neurol. 2002;15(5):633–8. doi: 10.1097/00019052-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 29.McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis. 2002;9(2):220–33. doi: 10.1006/nbdi.2001.0468. [DOI] [PubMed] [Google Scholar]
- 30.Flatters SJ, Xiao WH, Bennett GJ. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci Lett. 2006;397(3):219–23. doi: 10.1016/j.neulet.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin HW, Flatters SJ, Xiao WH, et al. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol. 2008;210(1):229–37. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leinninger GM, Edwards JL, Lipshaw MJ, et al. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat Clin Pract Neurol. 2006;2(11):620–8. doi: 10.1038/ncpneuro0320. [DOI] [PubMed] [Google Scholar]
- 33.Vinik AI, Holland MT, Le Beau JM, et al. Diabetic neuropathies. Diabetes Care. 1992;15(12):1926–75. doi: 10.2337/diacare.15.12.1926. [DOI] [PubMed] [Google Scholar]
- 34.Sumner CJ, Sheth S, Griffin JW, et al. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60(1):108–11. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- 35.Sima AA, Kamiya H. Diabetic neuropathy differs in type 1 and type 2 diabetes. Ann N Y Acad Sci. 2006;1084:235–49. doi: 10.1196/annals.1372.004. [DOI] [PubMed] [Google Scholar]
- 36.Sango K, Saito H, Takano M, et al. Cultured adult animal neurons and schwann cells give us new insights into diabetic neuropathy. Curr Diabetes Rev. 2006;2(2):169–83. doi: 10.2174/157339906776818613. [DOI] [PubMed] [Google Scholar]
- 37.Sotelo JR, Horie H, Ito S, et al. An in vitro model to study diabetic neuropathy. Neurosci Lett. 1991;129(1):91–4. doi: 10.1016/0304-3940(91)90727-b. [DOI] [PubMed] [Google Scholar]
- 38.Sango K, Horie H, Saito H, et al. Diabetes is not a potent inducer of neuronal cell death in mouse sensory ganglia, but it enhances neurite regeneration in vitro. Life Sci. 2002;71(20):2351–68. doi: 10.1016/s0024-3205(02)02040-4. [DOI] [PubMed] [Google Scholar]
- 39.Ristic H, Srinivasan S, Hall KE, et al. Serum from diabetic BB/W rats enhances calcium currents in primary sensory neurons. J Neurophysiol. 1998;80(3):1236–44. doi: 10.1152/jn.1998.80.3.1236. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan S, Stevens MJ, Sheng H, et al. Serum from patients with type 2 diabetes with neuropathy induces complement-independent, calcium-dependent apoptosis in cultured neuronal cells. J Clin Invest. 1998;102(7):1454–62. doi: 10.1172/JCI2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sango K, Horie H, Okamura A, et al. Diabetes impairs DRG neuronal attachment to extracellular matrix proteins in vitro. Brain Res Bull. 1995;37(5):533–7. doi: 10.1016/0361-9230(95)00057-l. [DOI] [PubMed] [Google Scholar]
- 42.Luo ZJ, King RH, Lewin J, et al. Effects of nonenzymatic glycosylation of extracellular matrix components on cell survival and sensory neurite extension in cell culture. J Neurol. 2002;249(4):424–31. doi: 10.1007/s004150200033. [DOI] [PubMed] [Google Scholar]
- 43.Sango K, Verdes JM, Hikawa N, et al. Nerve growth factor (NGF) restores depletions of calcitonin gene-related peptide and substance P in sensory neurons from diabetic mice in vitro. J Neurol Sci. 1994;126(1):1–5. doi: 10.1016/0022-510x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 44.Meldolesi J. Rapidly exchanging Ca2+ stores in neurons: molecular, structural and functional properties. Prog Neurobiol. 2001;65(3):309–38. doi: 10.1016/s0301-0082(01)00004-1. [DOI] [PubMed] [Google Scholar]
- 45.Verkhratsky A, Fernyhough P. Mitochondrial malfunction and Ca2+ dyshomeostasis drive neuronal pathology in diabetes. Cell Calcium. 2008;44(1):112–22. doi: 10.1016/j.ceca.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Huang TJ, Price SA, Chilton L, et al. Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes. 2003;52(8):2129–36. doi: 10.2337/diabetes.52.8.2129. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan S, Stevens M, Wiley JW. Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49(11):1932–8. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 48.Hong S, Wiley JW. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J Biol Chem. 2005;280(1):618–27. doi: 10.1074/jbc.M408500200. [DOI] [PubMed] [Google Scholar]
- 49.Russell JW, Golovoy D, Vincent AM, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. Faseb J. 2002;16(13):1738–48. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 50.Vincent AM, McLean LL, Backus C, et al. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. Faseb J. 2005;19(6):638–40. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 51.Vincent AM, Stevens MJ, Backus C, et al. Cell culture modeling to test therapies against hyperglycemia-mediated oxidative stress and injury. Antioxid Redox Signal. 2005;7(11-12):1494–506. doi: 10.1089/ars.2005.7.1494. [DOI] [PubMed] [Google Scholar]
- 52.Vincent AM, Feldman EL. Can drug screening lead to candidate therapies for testing in diabetic neuropathy? Antioxid Redox Signal. 2008;10(2):387–93. doi: 10.1089/ars.2007.1815. [DOI] [PubMed] [Google Scholar]
- 53.Walsh GS, Orike N, Kaplan DR, et al. The invulnerability of adult neurons: a critical role for p73. J Neurosci. 2004;24(43):9638–47. doi: 10.1523/JNEUROSCI.1299-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomlinson DR, Gardiner NJ. Diabetic neuropathies: components of etiology. J Peripher Nerv Syst. 2008;13(2):112–21. doi: 10.1111/j.1529-8027.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 55.Price SA, Hounsom L, Purves-Tyson TD, et al. Activation of JNK in sensory neurons protects against sensory neuron cell death in diabetes and on exposure to glucose/oxidative stress in vitro. Ann N Y Acad Sci. 2003;1010:95–9. doi: 10.1196/annals.1299.015. [DOI] [PubMed] [Google Scholar]
- 56.Gumy LF, Bampton ET, Tolkovsky AM. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci. 2008;37(2):298–311. doi: 10.1016/j.mcn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Purves T, Middlemas A, Agthong S, et al. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15(13):2508–14. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- 58.Zherebitskaya E, Akude E, Smith DR, et al. Development of selective axonopathy in adult sensory neurons isolated from diabetic rats: role of glucose-induced oxidative stress. Diabetes. 2009;58(6):1356–64. doi: 10.2337/db09-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng C, Zochodne DW. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes. 2003;52(9):2363–71. doi: 10.2337/diabetes.52.9.2363. [DOI] [PubMed] [Google Scholar]
- 60.Kamiya H, Zhang W, Sima AA. Degeneration of the Golgi and neuronal loss in dorsal root ganglia in diabetic BioBreeding/Worcester rats. Diabetologia. 2006;49(11):2763–74. doi: 10.1007/s00125-006-0379-0. [DOI] [PubMed] [Google Scholar]
- 61.Kennedy JM, Zochodne DW. Impaired peripheral nerve regeneration in diabetes mellitus. J Peripher Nerv Syst. 2005;10(2):144–57. doi: 10.1111/j.1085-9489.2005.0010205.x. [DOI] [PubMed] [Google Scholar]
- 62.Sereda M, Griffiths I, Puhlhofer A, et al. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron. 1996;16(5):1049–60. doi: 10.1016/s0896-6273(00)80128-2. [DOI] [PubMed] [Google Scholar]
- 63.Nobbio L, Mancardi G, Grandis M, et al. PMP22 transgenic dorsal root ganglia cultures show myelin abnormalities similar to those of human CMT1A. Ann Neurol. 2001;50(1):47–55. doi: 10.1002/ana.1034. [DOI] [PubMed] [Google Scholar]
- 64.Nobbio L, Vigo T, Abbruzzese M, et al. Impairment of PMP22 transgenic Schwann cells differentiation in culture: implications for Charcot-Marie-Tooth type 1A disease. Neurobiol Dis. 2004;16(1):263–73. doi: 10.1016/j.nbd.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Nobbio L, Gherardi G, Vigo T, et al. Axonal damage and demyelination in long-term dorsal root ganglia cultures from a rat model of Charcot-Marie-Tooth type 1A disease. Eur J Neurosci. 2006;23(6):1445–52. doi: 10.1111/j.1460-9568.2006.04666.x. [DOI] [PubMed] [Google Scholar]
- 66.Lehmann HC, Kohne A, Meyer zu Horste G, et al. Role of nitric oxide as mediator of nerve injury in inflammatory neuropathies. J Neuropathol Exp Neurol. 2007;66(4):305–12. doi: 10.1097/nen.0b013e3180408daa. [DOI] [PubMed] [Google Scholar]
- 67.Acosta C, Davies A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J Neurosci Res. 2008;86(5):1077–86. doi: 10.1002/jnr.21565. [DOI] [PubMed] [Google Scholar]
- 68.Raymon HK, Thode S, Zhou J, et al. Immortalized human dorsal root ganglion cells differentiate into neurons with nociceptive properties. J Neurosci. 1999;19(13):5420–8. doi: 10.1523/JNEUROSCI.19-13-05420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen W, Mi R, Haughey N, et al. Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line. J Peripher Nerv Syst. 2007;12(2):121–30. doi: 10.1111/j.1529-8027.2007.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Durr O, Duval F, Nichols A, et al. Robust hit identification by quality assurance and multivariate data analysis of a high-content, cell-based assay. J Biomol Screen. 2007;12(8):1042–9. doi: 10.1177/1087057107309036. [DOI] [PubMed] [Google Scholar]
- 71.Narro ML, Yang F, Kraft R, et al. NeuronMetrics: software for semi-automated processing of cultured neuron images. Brain Res. 2007;1138:57–75. doi: 10.1016/j.brainres.2006.10.094. [DOI] [PMC free article] [PubMed] [Google Scholar]


