Abstract
Dendrimers have emerged as topical microbicides to treat vaginal infections. This study explores the in-vitro, in-vivo antimicrobial activity of PAMAM dendrimers, and the associated mechanism. Interestingly, topical cervical application of 500 µg of generation-4 neutral dendrimer (G4-PAMAM-OH) showed potential to treat the Escherichia coli induced ascending uterine infection in guinea pig model of chorioamnionitis. Amniotic fluid collected from different gestational sacs of infected guinea pigs post treatment showed absence of E. coli growth in the cultures plated with it. The cytokine level [tumor necrosis factor (TNFα) and interleukin (IL-6 and IL-1β)] in placenta of the G4-PAMAM-OH treated animals were comparable to those in healthy animals while these were notably high in infected animals. Since, antibacterial activity of amine-terminated PAMAM dendrimers is known, the activity of hydroxyl and carboxylic acid terminated PAMAM dendrimers was compared with it. Though the G4-PAMAM-NH2 shows superior antibacterial activity, it was found to be cytotoxic to human cervical epithelial cell line above 10µg / mL, while the G4-PAMAM-OH was non cytotoxic upto 1mg / mL concentration. Cell integrity, outer (OM) and inner (IM) membrane permeabilization assays showed that G4-PAMAM-OH dendrimer efficiently changed the OM permeability, while G4-PAMAM-NH2 and G3.5-PAMAM-COOH damaged both OM and IM causing the bacterial lysis. The possible antibacterial mechanism are; G4-PAMAM-NH2 acts as polycation binding to the polyanionic lipopolysaccharide in E. coli, the G4-PAMAM-OH forms hydrogen bonds with the hydrophilic O-antigens in E. coli membrane and the G3.5-PAMAM-COOH acts as a polyanion, chelating the divalent ions in outer cell membrane of E. coli. This is the first study which shows that G4-PAMAM-OH dendrimer acts as an antibacterial agent.
Keywords: PAMAM dendrimer, antimicrobial activity, Gram-negative bacteria, cytotoxicity, cell membrane
1. Introduction
Dendrimers are emerging as the new topical antimicrobial agents (Svenson and Tomalia, 2005). Recent developments show that the polylysine dendrimers are effective against the simplex herpes virus (Bourne et al., 2000; Svenson and Tomalia, 2005). The polylysine (SPL7013) dendrimer is currently undergoing Phase I and Phase II human clinical trials to test its efficacy against genital herpes and HIV, safety, tolerability, retention and duration of activity (Halford, 2005; Mumper et al., 2009). Different approaches have been used to enhance the antimicrobial activities employing dendrimers and the most conventional being carriers for the antimicrobial agents, as encapsulating or complexing agents for antibacterial agents, by competitively inhibiting the binding of bacteria to the host cells (Cloninger, 2002; Hou et al., 2009) and by exerting the microbial activity by themselves. Quinolone drugs encapsulated in PAMAM dendrimers were active and useful for topical delivery of microbicides (Cheng Y, 2007). Triazine-based antibiotics encapsulated in the dendrimer beads provided enhanced inhibition of microbial growth when compared to the non-denrimerized triazine antibiotics (Lebreton, 2003). The silver complexes and nanocomposites with PAMAM dendrimer resulted in increased antibacterial activity towards the S. aureus, P. aeruginosa and E. coli due to the improved contact of microbes with the organized silver domains (Balogh, 2001).
Literature reveals that the dendrimer core, the surface charge and functionality, 3D structure and the size of the dendrimer are key factors that affect the antibacterial activity. The PAMAM (polyamidoamine) dendrimer with amine terminations exhibited antibacterial activity against gram negative E. coli, P. aeruginosa and gram positive S. aureus (Calabretta et al., 2007; Lopez et al., 2009). Glycodendrimers are known as antimicrobial agents (Cloninger, 2002). Ortega et al (Ortega, 2008) reported that the amine and ammonium terminated cationic carbosilane dendrimers were more potent towards the gram positive bacteria than the gram negative bacteria. Amine-terminated dendrimers with poly(propyleneoxide) amine core and methylacrylate and ethylenediamine core were found to demonstrate antibacterial and antifungal activities comparatively higher or equipotent to the antibacterial and antifungal agents used conventionally (Tulu et al., 2009). The quaternary ammonium terminated poly(propyleneimine) (PPI) dendrimer were found to be antibacterial against gram negative and gram positive bacteria (Chen et al., 2000; Chen and Cooper, 2002). The mechanism of antibacterial activity of the polycationic PPI dendrimers was attributed to the displacement of the divalent cations from the outer membrane of the gram negative bacteria causing cell wall disruption and lysis. Though the amine-terminated PPI dendrimers show excellent transfection and antibacterial activity, the major limitation to their use is the cytotoxicity and hence surface modification was sought to reduce cytotoxicity (Dutta et al., 2008; Tziveleka et al., 2007; Yang et al., 2008). Considerable effort has been directed in the past towards the surface group modification of the amine-terminated PAMAM dendrimers for reduce the cytotoxicity (Jevprasesphant et al., 2003; Kim et al., 2008; Kolhatkar et al., 2007; Yang et al., 2008). Size of the dendrimer affects its ability to penetrate the bacterial cell and therefore its antibacterial activity. For example, G3 PAMAM dendrimers were more effective antibacterial agents than G5 PAMAM dendrimers (Lopez et al., 2009). Similarly, lower generation carbosilane dendrimers were found to be more effective than higher generation dendrimers (Ortega, 2008). Typically, dendrimers have been investigated as carriers for drugs and imaging agents, but these recent findings present the perspective of dendrimers as new antimicrobial agents (Svenson and Tomalia, 2005).
Efforts continue to identify the newer and better antimicrobial agents. Antibacterial activity of amine-terminated PAMAM dendrimers is well recognized (Calabretta et al., 2007; Lopez et al., 2009), to the best of our knowledge, the assessment of the antibacterial activity of carboxylic and hydroxyl terminated PAMAM dendrimers has not been previously reported. In the present study, we have evaluated mechanisms by which hydroxyl and carboxylic acid terminated PAMAM dendrimers affect the cell wall of E. coli. Interestingly, we found that the hydroxyl terminated PAMAM dendrimer exhibited antibacterial activity in-vitro. We evaluated the ability of the hydroxyl terminated PAMAM dendrimer to treat the E. coli induced uterine infection in-vivo in pregnant guinea pig. Since, the guinea pig model of chorioamnionitis is well established to demonstrate the injury caused to the fetus resulting from E. coli infections (Patrick et al., 2004), we used this model for evaluating the in-vivo activity of G4-PAMAM-OH.
Intrauterine infection is usually caused by microorganisms ascending from vaginal and affecting the fetus and amniotic fluid leading to chorioamnionitis, cerebral palsy, increased efficiency of HIV seroconversion, miscarriage, and spontaneous preterm birth (Chaim et al., 1997),(Romero, 2003; Ugwumadu, 2007). Chorioamnionitis is known to cause fetal brain injury (Patrick et al., 2004) due to the generation of pro-inflammatory cytokines (Dickinson et al., 2009; Harnett et al., 2007). Antibacterial and antifungal agents are applied to vagina and cervix to treat intrauterine infections in the pregnant women (Chaim et al., 1997; Ugwumadu, 2007). In the present study we show that E. coli infection in pregnant guinea pig can be treated by topical vaginal and cervical application of G4-PAMAM-OH dendrimer. This is the first report using the guinea pig model of chorioamnionitis to induce E. coli infections and show the effective inhibition of bacterial growth by treatment with G4-PAMAM-OH. Our results show that the cytokine levels in placenta of the G4-PAMAM-OH treated animals were comparable to those in healthy animals and significantly less than infected animals. Although PAMAM dendrimers are the most extensively studied dendrimers the antimicrobial activity of unmodified G4-PAMAM-OH and G3.5-PAMAM-COOH has not been reported previously. Though G4-PAMAM-NH2 dendrimer shows strong antibacterial activity it shows high cytotoxicity to human cervical cell line and the antibacterial activity of G4-PAMAM-OH dendrimer is notable since it is non-cytotoxic at higher concentrations. Our findings bring out that G4-PAMAM-OH has a potential as antibacterial agent.
2. Experimental Section
2.1. Materials
The PAMAM dendrimers (generation 4, with end groups OH, NH2 and generation 3.5 COOH 14.93 % w/w in methanol) were purchased from Dendritech. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), 2-Nitrophenyl-β-D- galactopyranoside (ONPG), Osmium tetrozxide N-Phenyl-1-naphthylamine (NPN), glutaraldehyde and hexamethyldisilazane were purchased from Invitrogen. Nutrient broth and nutrient agar were purchased from BD Biosciences. Mouse TNFα, IL-6 and IL-1β ELISA kits were purchased from R&D Systems.
2.2. Preparation of bacteria
Escherichia coli (ATCC 11775) isolated from human urine is the bacterial strain used in this study. Single colony on nutrient agar was used to inoculate 5 mL of nutrient broth at 37 °C overnight. A small volume (100 µL) of this growth was used to inoculate 20 mL of nutrient broth media at 37 °C for 6 h. The bacteria were resuspended at 106 colony forming units (CFU)/mL for the experiments.
2.3. Bacterial growth inhibition assays
The inhibitory concentration (IC50) of dendrimers was determined using the broth microdilution method (Lopez et al., 2009; Wiegand et al., 2008). Briefly, serial dilutions of dendrimers (0.76g/mL to 200 mg/mL) were prepared in PBS and combined 1:1 v/v with bacteria at 106 CFU/mL in a 96 well polypropylene plate. After incubation at 37 °C for 18 h, the absorbance was measured at 650 nm using a microplate reader to assess the cell growth. The positive-control wells contained PBS and nutrient broth medium inoculated with bacteria (5 × 105 CFU/mL), and the negative-control wells contained PBS and nutrient broth medium without bacteria. The IC50 value was determined as the concentration of the dendrimers which inhibits 50% of microbial growth after 18–24 h incubation (Lopez et al., 2009; Wiegand et al., 2008). The % survival of the bacteria was determined on the basis of the positive control which was considered as 100 %.
2.4. Morphology of the bacteria
Scanning Electron Microscopy (SEM) analyses were performed to investigate the morphological changes of the E. coli following 8h treatment with dendrimers G4-PAMAM-OH, G3.5-PAMAM-COOH and G4-PAMAM-NH2 at concentrations arrived at from bacterial growth inhibition assays. E. coli were fixed with 2.5 % glutaraldehyde for 2 h. The samples were washed thrice with deionised water, and postfixed with 1% osmium tetraoxide for 2 h, followed by washing thrice with deionised water. The samples were dehydrated with a series of ethanol solutions (50 %, 70 %, 90 % and 100 %) followed by 10 min in 100 % hexamethyldisilazane (HMDS), and were air dried. The samples were coated with gold and observed under SEM (HITACHI S-2400 Scanning Electron Microscope) at 20 kV.
2.5. Cell Integrity
The bacterial cell membrane integrity was examined by determination of the release of nuclear acids (RNA/DNA) material absorbing at 260 nm (Je and Kim, 2006b; Ibrahim, 1991). E. coli were harvested, washed and resuspended in 0.5% NaCl solution with an absorbance of 0.7 at 420 nm. 150 µL of G4-PAMAM-OH, G3.5-PAMAM-COOH and G4-PAMAM-NH2 solutions at concentrations arrived at from bacterial growth inhibition assays were mixed with 150µL of bacterial suspension in a 96-well plate, control was carried out with 0.5% NaCl alone, and the release over time of nuclear materials was monitored with a UV spectrometer by measuring the absorbance at 260 nm.
2.6. Outer Membrane (OM) Permeabilization Assay
The OM permeabilization of E. coli by dendrimers was evaluated using the hydrophobic NPN (1-N-phenylnaphthylamine) fluorescent probe (Helander and Mattila-Sandholm, 2000; Je and Kim, 2006a). E. coli were harvested, washed, and resuspended in 0.5% NaCl solution with an absorbance of 1.0 at 420 nm. The concentration of dendrimers was the same as described in previous section. 100 µL of E. coli and 50 µL of dendrimers were mixed with 50 µL of 40 µM NPN in black fluorotiter plate, control was carried out with 0.5% NaCl alone. An increase in fluorescence due to partitioning of NPN into the OM was recorded as a function of time until no further increase in intensity was observed. Excitation and emission wavelengths were set at 350 and 420 nm, respectively.
2.7. Inner Membrane (IM) Permeabilization Assay
IM permeabilization was determined by measuring the release of cytoplasmic β-galactosidase activity from E. coli into the culture medium using ONPG as the substrate (Je and Kim, 2006b). E. coli were harvested, washed and resuspended in 0.5% NaCl solution an absorbance of 1.2 at 420 nm, then re-suspended in half volume of 0.5% NaCl solution. The concentration of dendrimers was the same as described in previous section. 100 µL of E. coli and 100 µL of dendrimers were mixed with 10 µL of ONPG (30 mM) in a 96-well plate, control was carried out with 0.5% NaCl alone. The production of 0-nitrophenol over time was determined by monitoring the change in absorbance at 420 nm using a spectrophotometer.
2.8. Cell culture
Human cervical epithelial End1/E6E7 cells were obtained from ATCC, maintained in DMEM/F-12K medium supplemented with 20 % fetal bovine serum (FBS). Mouse microglial BV-2 cells were obtained from Children’s Hospital of Michigan Cell Culture Facility and maintained in DMEM supplemented with 5 % FBS. These cells were cultured at 37 °C with 5 % CO2 and the media was replaced at 2 days interval.
2.9. Evaluation of normal cell cytotoxicity
End1/E6E7 and BV-2 (passage 19) cells were seeded into a 96-well plate at 1.5 × 104 / well, and 5 × 103 /well, respectively. After 24 h, cells were exposed to various concentrations of dendrimers (10 ng/mL to 1 mg/mL) in serum free medium for 24 h. Controls were carried out with medium alone. Cytotoxic effect was determined using MTT assay. The proportion of viable cells in the treated group was compared to that of the control.
2.10. Flow Cytometry Analysis
E. coli (106 CFU) and BV-2 (106) cells were treated with 10 µg/mL of FITC-labeled G4-PAMAM-OH dendrimer. The FITC-labeled G4-PAMAM-OH dendrimer was prepared as previously reported (Kolhe et al., 2006). The E. coli and cells were washed 3 times with PBS, resuspended in 1% formaldehyde, and analyzed using a flow cytometer (FACS caliber, Becton Dickinson) by counting 20,000 and 10,000 events, respectively. The mean fluorescence intensity of cells was calculated using the histogram plot.
2.11. Confocal Laser Scanning Microscopy
Microglial cells (106) were treated for 2 h with 10 µg/mL of G4-PAMAM-OH-FITC. The cells were washed with PBS three times and fixed with 4 % para-formaldehyde for 20 min. Images were captured using a confocal microscope (Zeiss LSM 310) using a magnification of 400×. The excitation and emission wavelengths were 488 and 518 nm respectively, for FITC.
2.12. Evaluation the antimicrobial activity in guinea pig model of chorioamnionitis
All the animal experimental procedures were approved by the institutional animal care and use committee of Wayne State University. Intracervical bacterial inoculation was performed as previously reported (Patrick et al., 2004). Briefly, pregnant Dunkin-Hartley strain guinea pigs (Charles River) at 52 days of gestation were anesthetized with 1.5% isoflurane using the mask. An endoscope was used to visualize the cervix. Guinea pigs were inoculated intra-cervically with 150 CFU E. coli (n=11) to induce infection. Dendrimer G4-PAMAM-OH 500 µg was injected into the cervix 5 min after E. coli inoculation in the treatment group 3 (n=4). The E. coli inoculated guinea pigs without treatment (group 2) were used as positive control (n=4). The guinea pigs without any treatment (group 1) and inoculation were used as negative controls (n=3). Forty eight hours after intervention, guinea pigs were euthanized with pentobarbital sodium (120 mg/kg) and midline laparotomy was performed to expose uterus. Amniotic fluid was collected from each gestational sac and 50 µL was plated on nutrient agar to determine the presence of microbiologic chorioamnionitis.
2.13. Cytokine quantification in placenta
The placental tissue (0.3 g) was homogenized in 1 mL RIPA lysis buffer. The homogenate was kept on ice for 30 min, centrifuged at 10,000 g for 25 min at 4°C and the protein concentration of supernatant was determined. Cytokines; tumor necrosis factor (TNFα), interleukin (IL-6 and IL-1β) concentrations were measured in the total protein fraction using ELISA kits (Ethier-Chiasson, 2008).
2.13. Statistical analysis
Data are presented as mean ± SD. Specific comparisons between control and individual experiment were analyzed by ANOVA test with p-value less than 0.05 considered as statistically significant.
3. Results
3.1. Antimicrobial Assay
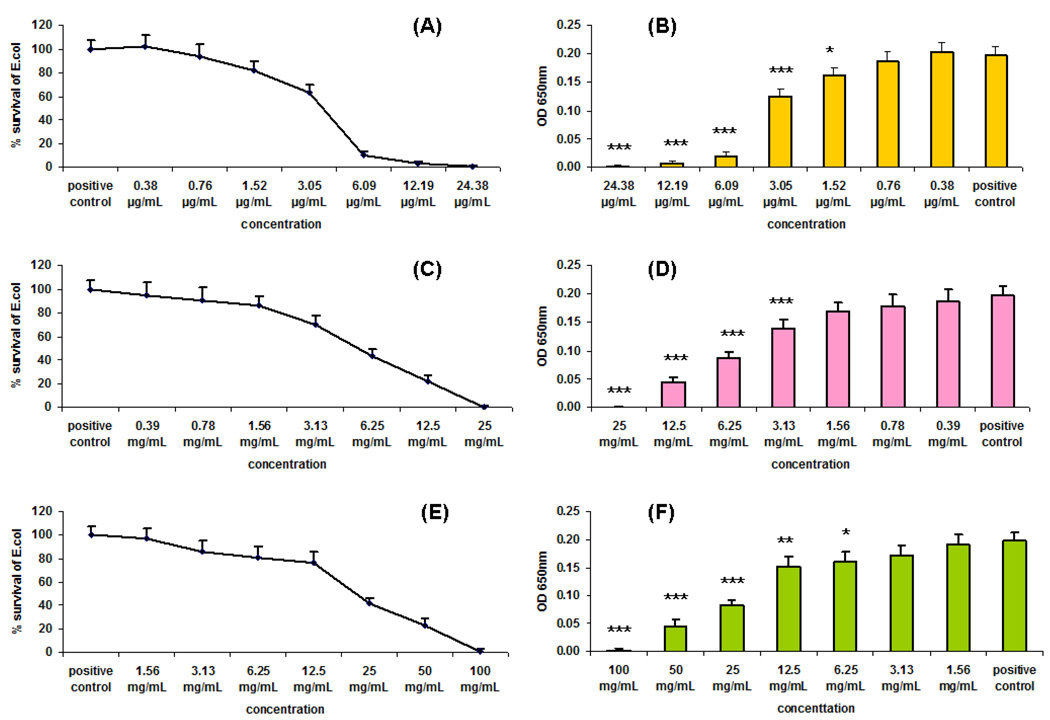
We used the antibacterial assay procedure reported previously (Lopez et al., 2009) to assess the antimicrobial activity of G4-PAMAM-OH and G3.5-PAMAM-COOH dendrimers towards the gram negative bacteria E. coli and compared it with the activity of G4-PAMAM-NH2. We used E. coli in this study since it is known to cause the chorioamnionitis in pregnancy, in an established guinea pig model based on E. coli infection (Patrick et al., 2004). We have used E. coli for in-vitro and in-vivo evaluations to demonstrate the antibacterial activity of PAMAM dendrimers. In the present study we have measured the IC50 values of PAMAM dendrimers using a modified broth microdilution assay in a 96-well plate format. The optical density of the suspension of bacteria in different dendrimer solutions was measured at 650 nm. The IC50 value of the dendrimer was then obtained from the plot of % survival of bacteria vs. the concentrations of the dendrimer and the plot of optical densities vs. the concentrations of the dendrimer. G4-PAMAM-OH, G3.5-PAMAM-COOH and G4-PAMAM-NH2, dendrimers inhibited the growth of E. coli in a concentration-dependent manner as seen from 18 h treatment (Fig. 1). The strong antimicrobial activity of G4-PAMAM-NH2 is consistent with that reported previously (Calabretta et al., 2007). It is interesting to note that G4-PAMAM-OH markedly inhibited the growth of E. coli from 3.13 mg/mL to 25.0 mg/mL concentration. G3.5-PAMAM-COOH also inhibited the growth of E. coli but at relatively higher concentrations 6.25 mg/mL to 100 mg/mL. For our set of conditions we observed the IC50 values for G4-PAMAM-OH, G3.5-PAMAM-COOH and G4-PAMAM-NH2 as 5.4 mg/mL, 22.0 mg/mL and 3.8µg/mL respectively. Since G4-PAMAM-NH2 dendrimer exhibits high cytotoxicity, the G4-PAMAM-OH was considered for in-vivo evaluations in guinea pigs.
Fig. 1.
Bacterial growth inhibition assays. E.coli was treated with the indicated concentration of G4-PAMAM-NH2 (A) and (B), G4-PAMAM-OH (C) and (D), G3.5-PAMAM-COOH (E) and (F) dendrimers for 18 h. The initial concentration used for bacterial seeding was 5×105 CFU/mL. Three samples were in each group. Bacterial growth was measured by turbidity as the optical density at 650 nm using a microplate reader. * P<0.05, ** P<0.01, *** P<0.001 VS Positive control.
For the amine-terminated PAMAM dendrimers, it is proposed that the amino groups form nanoscale holes in supported lipid bilayers of bacterial membrane causing its rupture and cell lysis (Calabretta et al., 2007; Hong et al., 2006; Mecke et al., 2005; Milovic et al., 2005). The quaternary ammonium dendrimers adsorb onto negatively charged bacterial cell surfaces, diffuse through the cell wall, bind to cytoplasmic membrane, disrupt and disintegrate the cytoplasmic membrane, release of electrolytes such as potassium ions and phosphate from the cell and release nucleic materials such as DNA and RNA, all contributing to the death of the bacterial cell (Chen et al., 2000). These reports suggest that dendrimers mediate their antimicrobial activity by disrupting the bacterial outer and inner membrane. The antibiotic ampicillin is known to penetrate the outer membrane of gram negative bacteria and inhibits the bacterial cell wall synthesis. The antibacterial activity of dendrimers is limited to its effect on bacterial membrane permeabilities. However, there has not been any experimental data to support this suggestion and we performed the outer and inner membrane permeabilization assays to gain insights into the bactericidal mechanism of action of the PAMAM dendrimers. This is discuss0ed in the subsequent sections in the manuscript.
3.2. SEM analysis of the E. coli
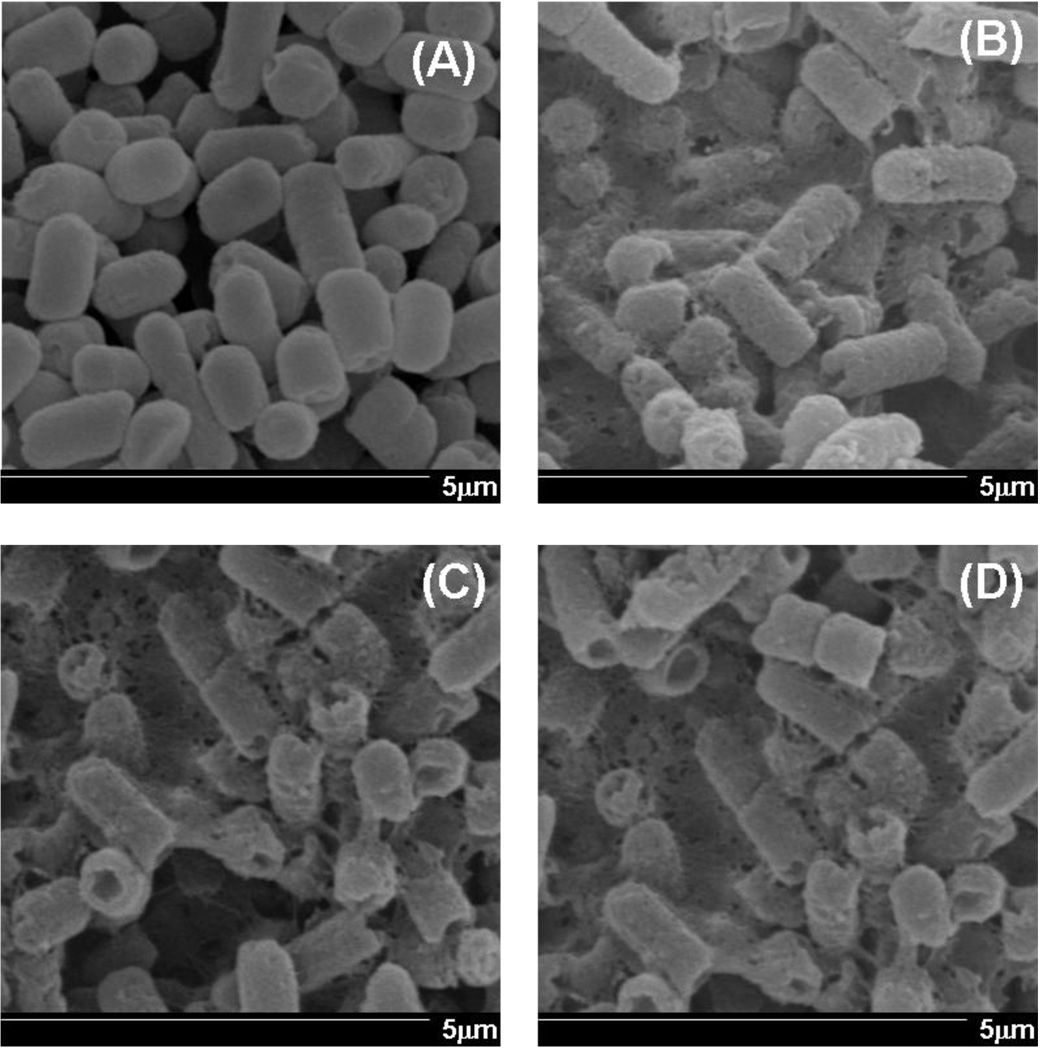
SEM studies were performed to examine if dendrimers damage the cell wall of E. coli. The concentration representing the IC50 values of dendrimers was used. The SEM images collected after 8 h of treatment with dendrimers showed a marked difference in morphology (Fig. 2). The untreated E. coli (Fig. 2A) shows distinct rod shape with the intact cell wall. The treatment of E. coli with G3.5-PAMAM-COOH (Fig. 2B), G4-PAMAM-OH (Fig. 2C) and G4-PAMAM-NH2 (Fig. 2D) shows shrinking, significant disruption of cell walls, and erosion of cell membrane. The major morphological changes observed by SEM support the antibacterial activity obtained in previous section. These studies indicated that major changes in the bacterial cell wall were induced by the dendrimers at the IC50 concentrations, indicative of their antibacterial potential. In order to understand the underlying mechanism for the possible damage to the bacterial cell wall, the cell integrity, outer and inner membrane permeabilization studies were conducted.
Fig. 2.
SEM images of E.coli. (A) untreated E.coli (B) 8h treatment of G3.5-PAMAM-COOH (C) 8h treatment of G4-PAMAM-OH (D) 8h treatment of G4-PAMAM-NH2. Magnification 20000 ×. Scale bars indicate 5 µm. The treatment with dendrimers shows the damage to the bacterial cell wall.
3.3. Cell Integrity
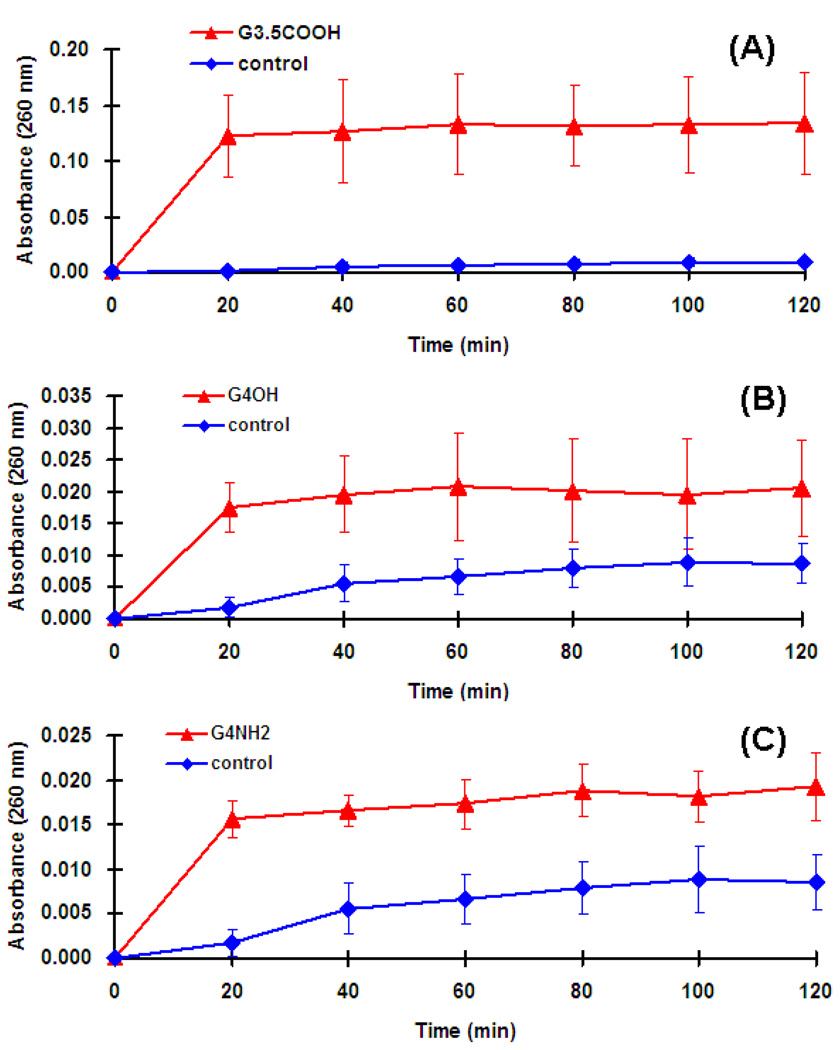
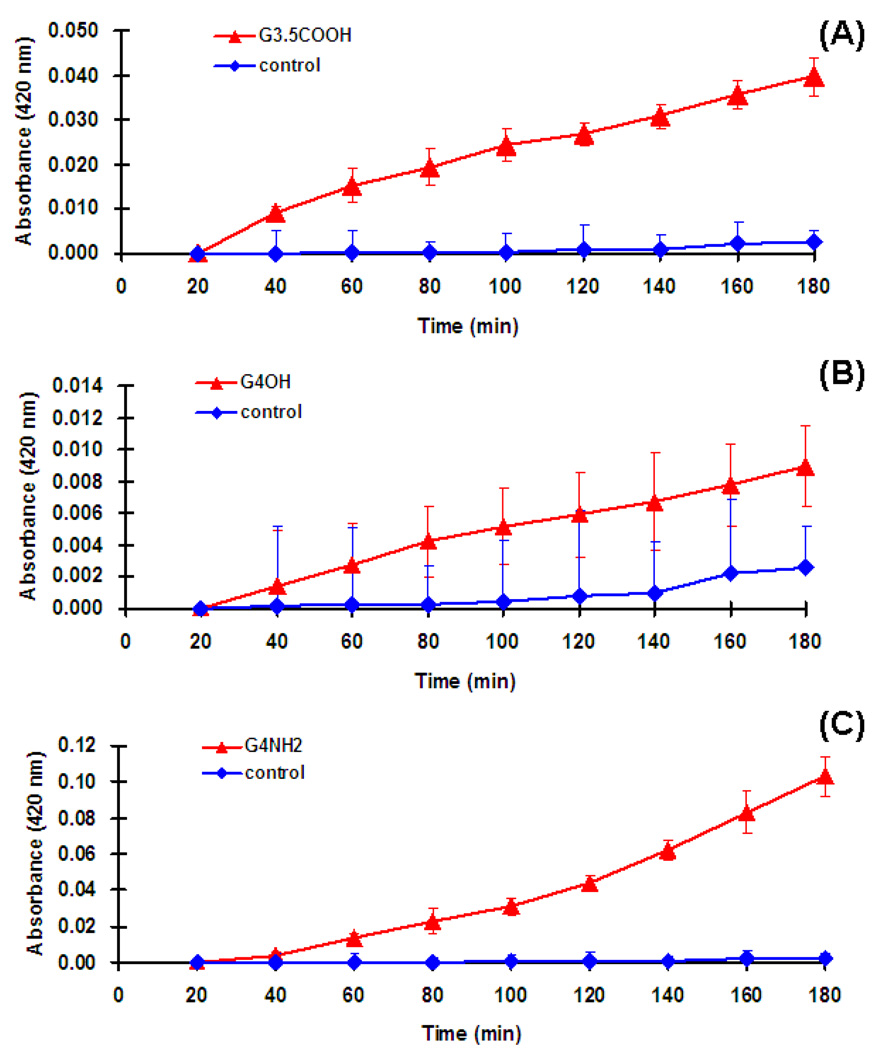
The cytoplasmic bacterial cell membrane undoubtedly is the target for many antimicrobial agents. The release of intracellular components is a good indicator of the compromise in cytoplasmic membrane integrity of gram negative bacteria resulting from the microbicidal activity of the tested compounds. In the present study, E. coli suspensions were treated with G4-PAMAM-OH, G3.5-PAMAM-COOH and G4-PAMAM-NH2 dendrimers at the IC50 concentrations and the absorbance of the suspensions was recorded at 260 nm. The absorbance significantly increased up to 20 min for each dendrimer at the tested concentration and thereafter the absorbance was almost unchanged (Fig. 3). The increase in the absorbance at 260 nm is due to the leaching of the small ions such as potassium and phosphate, but largely it’s attributed to the large nucleotides; DNA and the RNA released from the compromised bacterial cell. Similar observation was reported previously (Chen and Cooper, 2002) for QAC-functionalized dendrimers which exhibited fast killing kinetics till 10 min for E. coli. The increased absorbance is an indicator that at tested concentrations the three dendrimers were capable of altering the cytoplasmic membrane (outer cell membrane and cell wall) integrity for the gram negative bacteria.
Fig. 3.
Release of intracellular components of E. coli suspensions treated with (A) G3.5-PAMAM-COOH, (B) G4-PAMAM-OH and (C) G4-PAMAM-NH2. Four samples were evaluated in each group. The increase in the absorbance is an indicator of the compromised cell integrity resulting in leaching of the nuclear components which are absorbed at 260nm.
3.4. Outer Membrane (OM) permeabilization
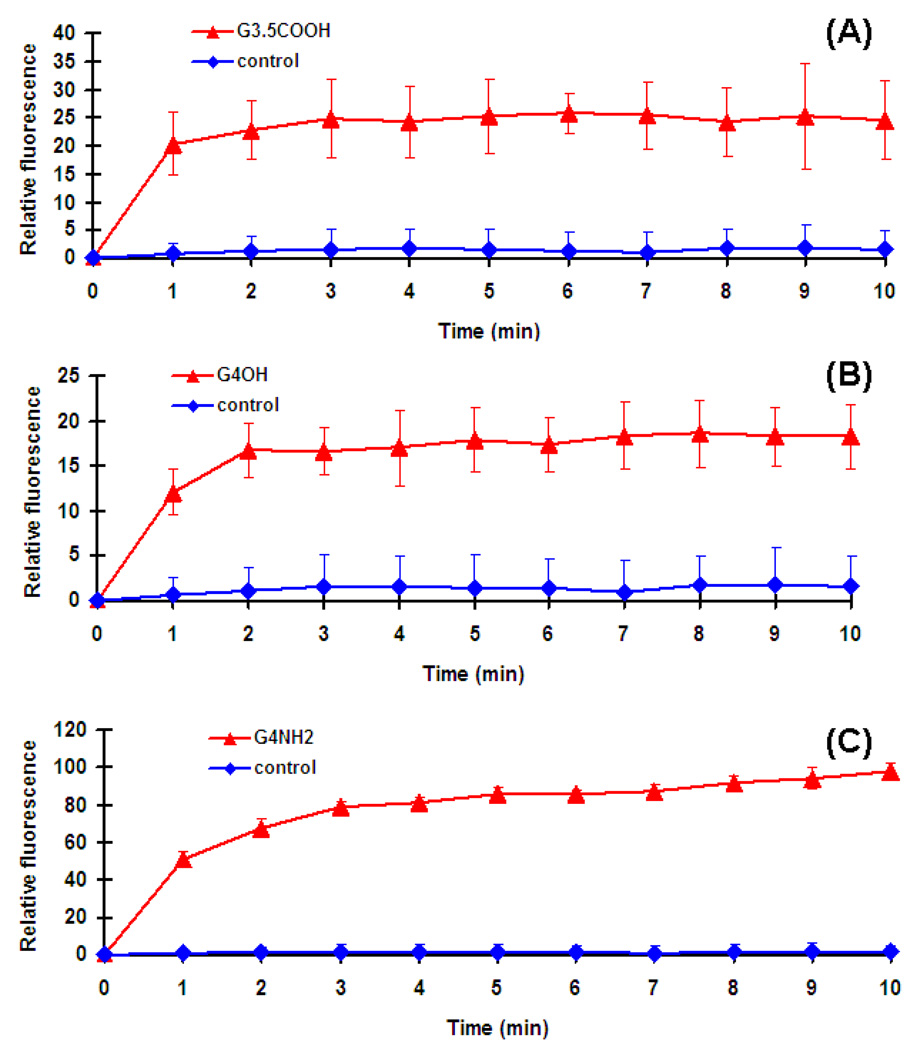
The outer membrane in the gram negative bacteria acts as a strong barrier to foreign materials such as drugs. When the bacterial outer membrane is intact, it is impervious to the hydrophobic probe NPN. However once the outer membrane is compromised the NPN partitions in the perturbed outer membrane. Hence NPN is used to determine the changes induced by the biocides in the outer cell membrane. Typically, the greater the damage induced on the membrane, higher is the fluorescence reading, meaning greater is the NPN partitioning in membrane (Je and Kim, 2006b). The E. coli was treated with solutions of dendrimers as described in previous section. The G4-PAMAM-NH2 dendrimer with amine groups on surface showed the highest fluorescence indicative of the rapid and bulk changes induced in the outer membrane of E. coli (Fig. 4). The divalent ions; calcium and magnesium play a key role in the stabilization of the bacterial membrane and are electrostatically bound to the anionic lipopolysaccharides (Chen and Cooper, 2002). The displacement of these divalent cations from the phospholipid membrane structure results in the structural instability, causing the disruption and eventually leading to cell death. At physiological pH, all of the primary amines and half of the tertiary amines in the dendrimer core of G4-PAMAM-NH2 are protonated (Cakara, 2007; Cakara, 2003). The G4-PAMAM-NH2 dendrimer is a polycation and it possibly replaces the divalent ions binding to the polyanions present in the cell membrane. This could lead to the insoluble polyanion-polycation complex and efflux of the calcium ions both contributing to major structural changes in outer cell membrane resulting in bacterial cell death.
Fig. 4.
Uptake of NPN by E.coli suspensions treated with (A) G3.5-PAMAM-COOH, (B) G4-PAMAM-OH and (C) G4 -PAMAM-NH2. Four samples were in each group.
It is interesting to note that the hydroxyl and carboxylic acid terminated dendrimers also showed an increase in the fluorescent intensity suggesting the NPN partitioning in treated E. coli (Fig. 4). These results clearly indicate that both G4-PAMAM-OH and G3.5-PAMAM-COOH alter the outer membrane structure of the E. coli. The underlying mechanism by which these dendrimers induce changes in the OM needs to be investigated. Several approaches can be considered to explain how the hydroxyl terminated dendrimer might act on the OM of bacteria. The microscopic protonation mechanism showed that at pH 7.4 and 7.5 the tertiary amines in the core of PAMAM dendrimers are protonated (Cakara, 2007; Cakara, 2003). The number of tertiary amines in the core is abundant when compared to the number of surface groups. Hence at pH 7.4, the core tertiary amines of G4-PAMAM-OH dendrimer are protonated and the surface hydroxyls are expected to bear negative charge and behave as anions. Anions are known to be bactericidal (Ellison et al., 1988; Khan M. A. S, 2000). This could be one of the possible mechanisms by which G4-PAMAM-OH dendrimer affects the E. coli cell wall permeability. Further, the outer membrane of gram negative bacteria is extremely hydrophilic (Benz, 1988). The permeability of gram negative bacterial cell is altered by interaction of its O-antigens (outer polysaccharide) with hydroxyl groups and surface bound water (Jucker, 1997; Jucker, 1998). The lipopolysaccharide of E. coli forms with hydroxyls and amines in the chitosan forming a complex which contributes to bactericidal effect of chitosan (Naberezhnykh et al., 2008). The lipopolysaccharide from E. coli forms ionic, electrostatic and hydrogen bonds with chitosan and the hydroxyl groups of chitosan act as proton donors contributing towards these interactions. Further, the antibacterial activity due to hydroxyl groups is well established (Aslama, 2009; Myers, 1928). The literature clearly reveals the ability of the outer membrane of gram negative bacteria to form hydrogen bonds with hydroxyl groups. Our SEM images and OM permeability assay measurements together show the changes brought out in the bacterial cell membrane due to the activity of G4-PAMAM-OH dendrimer. Based on experimental and the inferential evidence, one can interpret that G4-PAMAM-OH dendrimer forms hydrogen bonds with the outer membrane of E. coli, another possible mechanism by which it could brings the permeability changes. The in-depth understanding on the mechanism by which G4-PAMAM-OH works is required and it is beyond the scope of this paper.
At physiological pH, the carboxylic acid terminated dendrimer acts as polyanion and chelates the positive calcium and magnesium ions in the outer membrane altering its structure and permeability. The phosphate group in the phosphatidyl choline (lipid) layers of bacteria has free carboxyl groups and phosphate groups bearing a negative charge which bind to cations. The outer membrane of gram negative bacteria is stabilized by the ionic interaction and binding of the positive ions (calcium and magnesium) to the polar anionic residues on the lipid head groups. This complex is reversible. Since this complex is reversible, the carboxylic groups from dendrimer replace the carboxylic groups from the lipids and bind to positive calcium ions destabilizing the membrane. This could be a possible mechanism by which the G3.5-PAMAM-COOH alters the outer membrane of the E. coli. There are reports which support this assumption. The ethylenediamine tetraacetic acid is antibacterial as it binds to the membrane stabilizing cations and releases of lipopolysaccharides (LPS)(Ellison et al., 1988). Incorporation of palmitic acid residues in lysozyme rendered it bactericidal to E. coli as hydrophobic interactions occur between the two in LPS zone (Ibrahim, 1991). The anionic phosvitin shows antibacterial activity towards E. coli by chelating calcium ions in outer membrane (Khan M. A. S, 2000). Further, anionic peptide MDpep5 acts as antimicrobial by binding to the bacteria through hydrophobic interactions causing disorders in bilayer structure. The membrane disruption is not complete for anionic MDpep5 when compared to the cationic (AMPs) antimicrobial peptide (Tang et al., 2008). Based on the literature, when we review the results of present study, it appears that at physiological pH the carboxylate ions on the G3.5-PAMAM-COOH act as polyanions interacting with the calcium ions in the outer membrane of gram negative bacteria affecting its permeability. As compared to the anionic G3.5-PAMAM-COOH, the cationic G4-PAMAM-NH2 causes more damage to outer membrane structure, which is again consistent with the observations reported by Tang et al (Tang et al., 2008). From our studies we found that PAMAM dendrimers with amine, hydroxyl and carboxyl terminal group, could each show a different path for interacting with the bacterial outer membrane.
3.5. IM permeabilization
Destabilization of the outer membrane in bacteria is essential for establishing contact with the inner membrane (Benz, 1988; Je and Kim, 2006b). The inner layer of bacteria consists of phosphatidyl glycerol and cardiolipin (Je and Kim, 2006a). The cytoplasmic β-galactosidase is released as a consequence of alteration / compromise in inner membrane and it reacts with ONPG to yield ortho-nitrophenol which is absorbed at 420 nm. The concentrations of three dendrimers were the same as IC50 values. There was a lag of 20 min followed by which a slow release of cytoplasmic β-galactosidase was observed for all the dendrimers tested. The release of cytoplasmic β-galactosidase was found to be time dependant (Fig. 5). Amongst the dendrimers evaluated, the G4-PAMAM-NH2 showed a marked increase in OD values indicative its capability to induce major permeability changes in inner membrane. The G3.5-PAMAM-COOH dendrimer treated bacteria showed increase in OD with respect to time, again indicating the changes brought in the structure of inner wall. The OD values were low for G4-PAMAM-OH dendrimer as compared to the other dendrimers but they increased slowly over time as seen from Fig. 5. These results indicate that the G4-PAMAM-OH dendrimer seems to induce profound permeability changes in outer membrane as compared to the inner membrane. While the G3.5-PAMAM-COOH dendrimer appears to release the cell intracellular nucleotides more than the other dendrimers, the cationic G4-PAMAM-NH2 dendrimer appears to disrupt the IM and OM to a significantly higher extent compared to the other dendrimers.
Fig. 5.
Release of cytoplasmic β-galactosidase of E.coli treated with (A) G3.5-PAMAM-COOH, (B) G4-PAMAM-OH and (C) G4-PAMAM-NH2. Four samples were in each group.
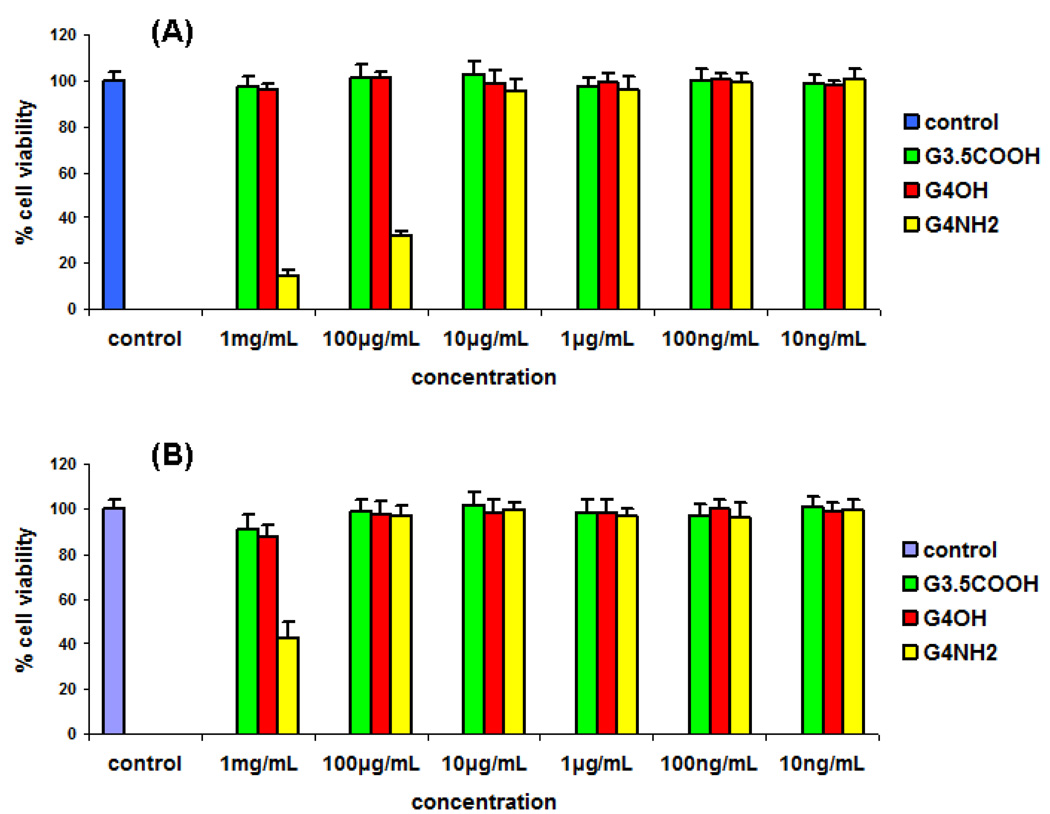
3.6. Cytotoxicity assay
The cytotoxicity of PAMAM dendrimers was evaluated against human cervical epithelial (End1/ E6E7) and immune cells; mouse microglial cells (BV-2). MTT assay showed that G4-PAMAM-OH and G3.5-PAMAM-COOH dendrimers were non cytotoxic to End1/E6E7 cells and BV-2 cells in 24 h treatment at concentrations 10 ng / mL - 1 mg / mL (Fig. 6). The G4-PAMAM-NH2 showed high cytotoxicity above 10 µg / mL concentration to human cervical epithelial End1/E6E7 cells. Also the G4-PAMAM-NH2 exhibited cytotoxicity at 1mg / mL concentration to microglial cells. On the basis of the MTT assay, G4-PAMAM-OH did not exhibit cytotoxicity upto 1mg / mL concentration, while the G4-PAMAM-NH2 was found to be cytotoxic at higher concentrations. E.coli infections in the vagina can lead to preterm birth. The experimental data shows that G4-PAMAM-OH dendrimer is non cytotoxic to the human cervical cell line and also exhibits antibacterial activity towards E. coli, hence it was chosen as antibacterial agent to prevent preterm birth in pregnant guinea pigs. In our in-vivo experiments we applied a total of 500 µg of G4-PAMAM-OH to the cervix of E. coli infected pregnant guinea pigs and at this concentration the dendrimer showed efficacy.
Fig. 6.
Cytotoxicity assay. (A) Human cervical epithelial End1/E6E7 cells and (B) mouse microglial cells were treated with the G4-PAMAM-OH, G3.5-PAMAM-COOH and G4-PAMAM-NH2 dendrimers at concentrations indicated for MIC values. Three samples were in each group. Cell viability was assessed by MTT method. The proportion of viable cells in the treated group was compared to that of negative control.
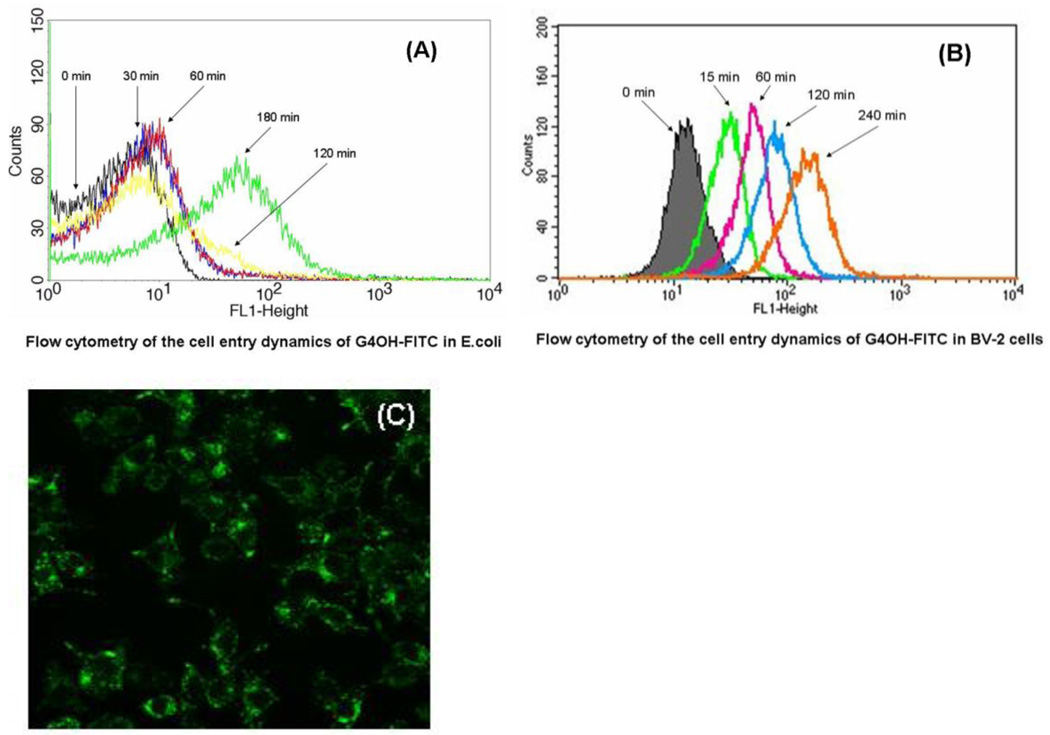
3.7. Cell entry dynamics of dendrimer in E. coli and microglial cells
The size of the dendrimer affects its antibacterial activity and G5-PAMAM-NH2 was less bactericidal than G3-PAMAM-NH2 which could penetrate the bacterial cell because of relatively smaller size (Lopez et al., 2009). In the present study, we have evaluated the generation-4 PAMAM dendrimers and hence the ability of G4-PAMAM-OH to enter the cells was determined using a FITC tagged G4-PAMAM-OH-FITC conjugate. The E. coli did not show significant increase in fluorescence intensity after treatment with G4-PAMAM-OH-FITC for 2 h. However, a significant increase in fluorescence intensity was observed after 3 h (Fig. 7A) confirming a slow entry of G4-PAMAM-OH-FITC conjugate into the E. coli. This demonstrates that G4-PAMAM-OH enters the E. coli effectively while the exact location of G4-PAMAM-OH in the bacteria remains unclear and needs to be further elucidated using other methods. These results support the previous studies where the G4-PAMAM-OH shows changes in the permeability of the bacterial membrane. The microglial cells showed a significant fluorescence intensity increase within 15 min, as evidenced by a two-order of magnitude increase in the fluorescence intensity. This was followed by a moderate increase in fluorescence over the next 4 h (Fig. 7B) indicating that G4-PAMAM-OH-FITC conjugate entered the microglial cells. In case of BV-2 cells the cellular entry was visualized by using confocal microscopy showing the localization of G4-PAMAM-OH-FITC in cytoplasm (Fig. 7C). The uptake of the dendrimer by E. coli was slower than that in microglial cells. The difference in the structure of cell membrane may result in different cell entry dynamics of dendrimer in E. coli and microglial cells.
Fig. 7.
Flow cytometry of the cell entry dynamics of (A) G4-PAMAM-OH-FITC in E. coli and (B) BV-2 microglial cell line. The log of FITC absorption intensity (FL1-H on x-axis) is plotted against the number of cells (counts on y-axis). The maximum uptake of G4-PAMAM-OH-FITC in E. coli occurs at 3h. The rapid cellular uptake of G4-PAMAM-OH-FITC within 15 min in microglial cells is evident. The transport of conjugate into microglial cell increased with increasing time. Confocal microscopy images (400×) showed that G4-PAMAM-OH-FITC appeared to be mainly localized in the cytoplasm of BV-2 cells while the nucleus appeared to be relatively free of the presence of any fluorescence at this time point (C).
3.8. Antimicrobial activity in guinea pig model of chorioamnionitis
The in-vitro studies brought out antibacterial potential of G4-PAMAM-OH dendrimer as seen from the antibacterial assay, OM and IM permeabilization assays and bulk changes in morphology seen from SEM analysis. These interesting results coupled with its non cytotoxicity to human cervical epithelial cells encouraged the evaluation of G4-PAMAM-OH as an antibacterial agent in-vivo using the guinea pig model of chorioamnionitis. Though this model is established for creating infection and assessing injury to the fetus, it has not been previously used to demonstrate the effective treatment. This is the first study to our knowledge, which shows the treatment of the pregnant guinea pig using the model of chorioamnionitis. The ascending E. coli infection causes chorioamnionitis which is associated with development of cerebral palsy, a motor disorder in children due to stimulation of proinflammatory cytokines causing white matter damage and fetal brain injury (Patrick et al., 2004). We optimized the dose of the E. coli inoculation in the guinea pigs (n=17) in the pilot experiments for the strain (ATCC 11775). We observed that 1000 CFU of E. coli effectively induced the infection causing extreme sickness in mother, leading to abortion of dead fetuses within 48 to 72 h. The lower CFU of E. coli were subsequently inoculated to identify the optimum dose which lead to infection and yet the guinea pigs did not abort upto 48 h. Based on this evaluation a dose of 150 CFU of E. coli was found to effectively induce the infection in the pregnant guinea pigs without leading to abortion of fetuses.
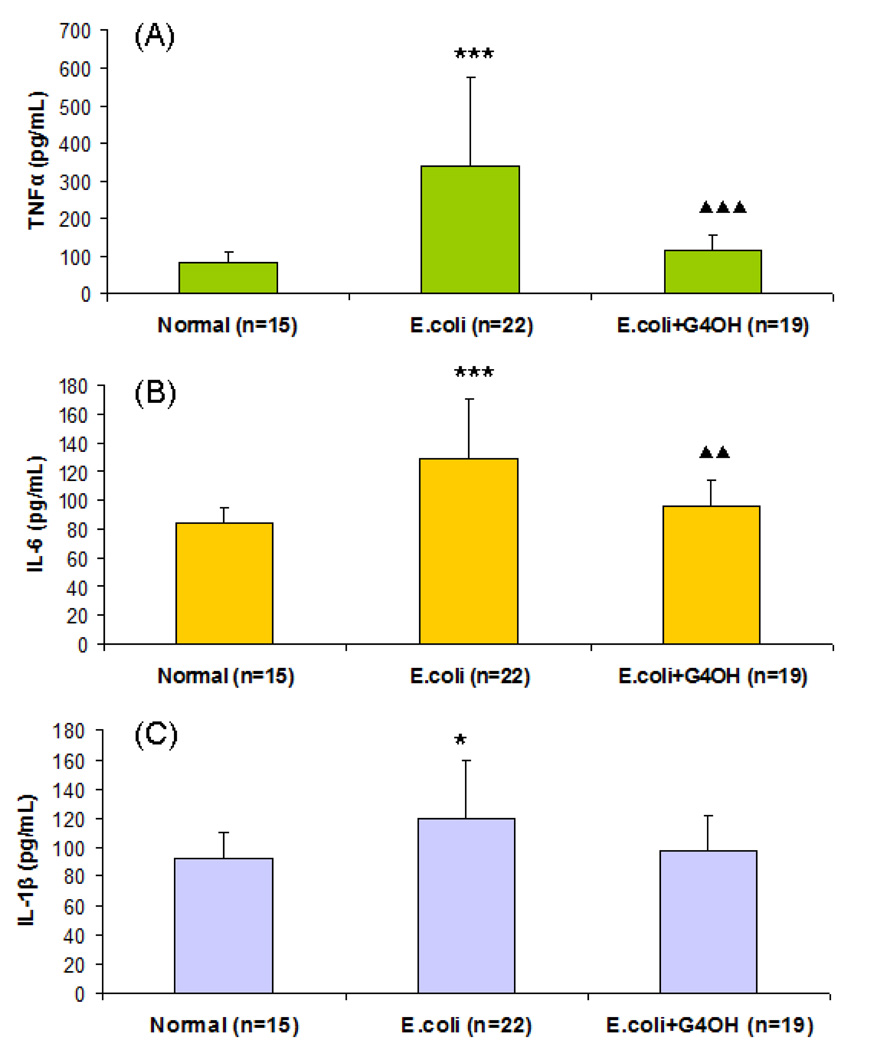
In the present study, chorioamnionitis was induced after intracervical inoculation with E. coli in 8 guinea pigs of which n=4 were considered as positive control (group 2) and n=4 were used for treatment with G4-PAMAM-OH (group 3). None of the amniotic fluid samples plated from the negative control group-1 (n=3) showed evidence of microbiologic chorioamnionitis. Of the pregnant guinea pigs (group 2, n=4) that were inoculated with 150 CFU of E. coli, 57.1% of the amniotic fluid samples for different fetus were positive with bacterial growth (indicative of induced infection) as seen from the culture inoculated with it (see Table 1). Prenatal exposure to maternal infection alters cytokine expression in the placenta (Urakuboa, 2001). Abundance of cytokines in placental tissues is an indicator of activation of inflammatory response in gestational membranes with term and preterm parturition (Keelan et al., 1999). The cytokine IL-6 is known to peak after 48 h of infection (Dickinson et al., 2009) and hence in present study animals were sacrificed after 48 h to determine the cytokine level in positive and treated animals. When we compared the expression of cytokine levels in negative control vs the positive controls, the cytokines, especially TNFα and IL-6, increased significantly in the placenta of positive controls after 48 h of inoculation with E. coli (Fig. 8). These results demonstrated that chorioamnionitis was successfully induced after 48 h of cervical inoculation with 150 CFU of E. coli.
Table 1.
The inhibition of E. coli growth after treatment with G4-PAMAM-OH dendrimer in guinea pig model of chorioamnionitis
| Inoculation with E.coli | Treatment : With G4-OH after E. coli inoculation |
|||||
|---|---|---|---|---|---|---|
| Guinea Pig | Amniotic fluid from different gestational sacs |
Guinea Pig | Amniotic fluid from different gestational sacs |
|||
| Bacterial growth | % | Bacterial growth | % | |||
| Mother 1 | Fetuses (4/5) | 80.0 | Mother 1 | Fetuses (0/5) | 0 | |
| Mother 2 | Fetuses (5/6) | 83.3 | Mother 2 | Fetuses (0/6) | 0 | |
| Mother 3 | Fetuses (1/4) | 25.0 | Mother 3 | Fetuses (0/5) | 0 | |
| Mother 4 | Fetuses (2/5) | 40.0 | Mother 4 | Fetuses (0/3) | 0 | |
| Average | 57.1 | Average | 0 | |||
Fig. 8.
The placental tissue (0.3 g) was homogenized in 1 ml RIPA lysis buffer. The homogenate was kept on ice for 30 min and the protein concentration of supernatant was determined. Cytokines concentrations were measured in the total protein fraction using ELISA. (A) TNFα measurements in normal, E.coli infected and G4-PAMAM-OH treated guinea pigs (B) IL-6 measurements in normal, E.coli infected and G4-PAMAM-OH treated guinea pigs (C) IL-1β measurements in normal, E.coli infected and G4-PAMAM-OH treated guinea pigs * P<0.05, *** P<0.001 Vs. Normal control. ▲▲ P<0.01, ▲▲▲ P<0.001 Vs. E.coli group.
The G4-PAMAM-OH dendrimer was applied topically at a dose of (625 µg/kg) on the cervical endometrium of guinea pigs (group-3, n = 4) in form of aqueous solution in saline after E. coli inoculation. The total amount applied was 500µg dissolved in saline based on the average weight of the guinea pigs (800 g / animal). The amniotic fluid samples for different fetus were collected after 48 h and were plated on the culture plates and evaluated for the bacterial growth. All these samples did not show any bacterial growth (0 %) on the culture plates (Table 1). The study shows that the treatment with G4-PAMAM-OH dendrimer completely eliminated the bacterial growth and prevented bacteria ascension into uterine cavity and amniotic fluid i.e. from 57.1 (positive control) to 0 % (treatment group) bacterial growth. Earlier, the in-vitro data showed antibacterial nature of G4-PAMAM-OH at higher concentration and the in-vivo results show that amniotic fluid samples for different fetus in treatment group-3 were found to be negative. The comparison of cytokine expression in placenta of the treatment group, negative and positive control groups shows that the cytokine levels (TNFα and IL-6) in treatment group are comparable to the negative control while they are overly expressed in positive controls (Fig. 8). These results indicate the potential of G4-PAMAM-OH to effectively kill gram negative bacteria E. coli in cervix of guinea pig and prevent chorioamnionitis. This is a significant finding since the chorioamnionitis is known to cause fetal brain injury (Patrick et al., 2004) which could possibly be averted by treatment with G4-PAMAM-OH as indicated from these findings. It is interesting to note that the G4-PAMAM dendrimers show very negligible transport across the fetal membranes and can therefore be possibly used for selective treatment of mother without crossing into the fetus when administered by intravaginal route (Menjoge et al., 2010).
4. Conclusion
The bactericidal activity of hydroxyl and carboxylic acid terminated PAMAM dendrimer was evaluated against gram negative E. coli and compared with amine terminated PAMAM dendrimers. The antimicrobial assay, SEM analysis, cell integrity, inner and outer membrane permeability assays showed that G4-PAMAM-OH and G3.5-PAMAM-COOH dendrimers affect the cell wall of E. coli, and were antibacterial at the concentrations evaluated. The major finding was the bactericidal effect of G4-PAMAM-OH dendrimer and its ability to treat E. coli infections in-vivo in pregnant guinea pigs. Topical cervical application of 500 µg of G4-PAMAM-OH treated the E. coli infections induced in guinea pig model of chorioamnionitis. The amniotic fluid collected from different fetus in the infected guinea pigs, post treatment showed absence of E. coli growth in the cultures plated with it. The cytokines levels were higher in the positive controls confirming presence of infection after inoculation with E. coli. The cytokine expression (TNFα and IL-6) in the treatment group was comparable to that in negative control showing the efficacy of G4-PAMAM-OH to treat the E. coli infections. The G4-PAMAM-NH2 dendrimer is known to be potent antibacterial agent, however, it was found to be highly cytotoxic to above 10 µg/ mL to human cervical epithelial (End1/E6E7) cells and immune cells (BV-2) while the G4-PAMAM-OH dendrimer was non cytotoxic upto 1mg /mL concentrations to both cell lines. Each dendrimer appears to affect the bacterial cell wall in a different way. The possible mechanisms involve the G4-PAMAM-NH2 acting as polycation binding to the polyanionic lipopolysaccharide, the G4-PAMAM-OH binding via hydrogen bonds to the hydrophilic O-antigens and the G3.5-PAMAM-COOH acting as a polyanion chelating the divalent ions in outer cell membrane. The outer and inner membrane permeabilization assay shows that G4-PAMAM-OH brings major structural changes to the outer membrane whereas G4-PAMAM-NH2 brings major changes to both outer and inner membrane.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS, and the Pediatric Critical Care Scientist Development Program NICHD-K08.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- http://clinicaltrials.gov/ct2/results?term=SPL7013.
- Aslama SNS, Kokubun T, Halla DR. Antibacterial and antifungal activity of cicerfuran and related 2-arylbenzofurans and stilbenes. Microbiol Res. 2009;164:191–195. doi: 10.1016/j.micres.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Balogh LSDR, Tomalia DA, Hagnauer GL, McManus AT. Dendrimer-Silver Complexes and Nanocomposites as Antimicrobial Agents. Nano Lett. 2001;1:18–21. [Google Scholar]
- Benz R. Strcture and fuction of porins from gram negative bacteria. Microbial. 1988;42:359–393. doi: 10.1146/annurev.mi.42.100188.002043. [DOI] [PubMed] [Google Scholar]
- Bourne N, Stanberry LR, Kern ER, Holan G, Matthews B, Bernstein DI. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrobial agents and chemotherapy. 2000;44:2471–2474. doi: 10.1128/aac.44.9.2471-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakara D, Borkovecb Microscopic M, Protonation Mechanism of Branched Polyamines: Poly(amidoamine) versus Poly(propyleneimine) Dendrimers. Croat Chem Acta. 2007;80:421–428. [Google Scholar]
- Cakara DKJ, Borkovec M. Protonation Equilibria of Poly(amidoamine) Dendrimers from Macroscopic Titrations. Macromolecules. 2003;36:4201–4207. [Google Scholar]
- Calabretta MK, Kumar A, McDermott AM, Cai C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules. 2007;8:1807–1811. doi: 10.1021/bm0701088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaim W, Mazor M, Leiberman JR. The relationship between bacterial vaginosis and preterm birth. A review. Archives of gynecology and obstetrics. 1997;259:51–58. doi: 10.1007/BF02505309. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Beck-Tan NC, Dhurjati P, van Dyk TK, LaRossa RA, Cooper SL. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: structure-activity studies. Biomacromolecules. 2000;1:473–480. doi: 10.1021/bm0055495. [DOI] [PubMed] [Google Scholar]
- Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi: 10.1016/s0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- Cheng YQH, Ma M, Xu Z, Xu P, Fang Y, Xu T. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials. An in vitro study Eur J Med Chem. 2007;42:1032–1038. doi: 10.1016/j.ejmech.2006.12.035. [DOI] [PubMed] [Google Scholar]
- Cloninger MJ. Biological applications of dendrimers. Current opinion in chemical biology. 2002;6:742–748. doi: 10.1016/s1367-5931(02)00400-3. [DOI] [PubMed] [Google Scholar]
- Dickinson MA, Harnett EL, Venditti CC, Smith GN. Transient lipopolysaccharide-induced cytokine responses in the maternal serum and amniotic fluid of the guinea pig. American journal of obstetrics and gynecology. 2009;200:534 e531–534 e536. doi: 10.1016/j.ajog.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Dutta T, Garg M, Jain NK. Poly(propyleneimine) dendrimer and dendrosome mediated genetic immunization against hepatitis B. Vaccine. 2008;26:3389–3394. doi: 10.1016/j.vaccine.2008.04.058. [DOI] [PubMed] [Google Scholar]
- Ellison RT, 3rd, Giehl TJ, LaForce FM. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988;56:2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier-Chiasson MFJ-C, Giguere Y, Masse A, Marseille-Tremblay C, Levy E, Lafond J. Modulation of placental protein expression of OLR1: implication in pregnancy-related disorders or pathologies. Reproduction. 2008;136:491–502. doi: 10.1530/REP-08-0082. [DOI] [PubMed] [Google Scholar]
- Halford B. Dendrimers branch out. C&EN. 2005;83:30–36. [Google Scholar]
- Harnett EL, Dickinson MA, Smith GN. Dose-dependent lipopolysaccharide-induced fetal brain injury in the guinea pig. American journal of obstetrics and gynecology. 2007;197:179 e171–179 e177. doi: 10.1016/j.ajog.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Helander IM, Mattila-Sandholm T. Fluorometric assessment of gram-negative bacterial permeabilization. J Appl Microbiol. 2000;88:213–219. doi: 10.1046/j.1365-2672.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- Hong S, Leroueil PR, Janus EK, Peters JL, Kober MM, Islam MT, Orr BG, Baker JR, Jr, Banaszak Holl MM. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- Hou S, Zhou C, Liu Z, Young AW, Shi Z, Ren D, Kallenbach NR. Antimicrobial dendrimer active against Escherichia coli biofilms. Bioorg Med Chem Lett. 2009;19:5478–5481. doi: 10.1016/j.bmcl.2009.07.077. [DOI] [PubMed] [Google Scholar]
- Ibrahim HRKA, Kobayashi K. Antimicrobial Effects of Lysozyme against Gram-Negative Bacteria Due to Covalent Binding of Palmitic Acid. J Agric Food Chem. 1991;39:2077–2082. [Google Scholar]
- Je JY, Kim SK. Antimicrobial action of novel chitin derivative. Biochim Biophys Acta. 2006a;1760:104–109. doi: 10.1016/j.bbagen.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Je JY, Kim SK. Chitosan derivatives killed bacteria by disrupting the outer and inner membrane. J Agric Food Chem. 2006b;54:6629–6633. doi: 10.1021/jf061310p. [DOI] [PubMed] [Google Scholar]
- Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D'Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252:263–266. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- Jucker BAHH, Hug SJ, Zehnder AJB. Adsorption of bacterial surface polysaccharides on mineral oxides is mediated by hydrogen bonds Colloids and Surfaces B: Biointerfaces. 1997;9:331–343. [Google Scholar]
- Jucker BZABD, Harms H. Quantification of Polymer Interactions in Bacterial Adhesion. Environ Sci Technol. 1998;32:2909–2915. [Google Scholar]
- Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. American journal of obstetrics and gynecology. 1999;181:1530–1536. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- Khan MASNS, Ogawa M, Akita E, Azakami H, Kato A. Bactericidal Action of Egg Yolk Phosvitin against Escherichia coli under Thermal Stress. J Agric Food Chem. 2000;48:1503–1506. doi: 10.1021/jf990700r. [DOI] [PubMed] [Google Scholar]
- Kim Y, Klutz AM, Jacobson KA. Systematic investigation of polyamidoamine dendrimers surface-modified with poly(ethylene glycol) for drug delivery applications: synthesis, characterization, and evaluation of cytotoxicity. Bioconjug Chem. 2008;19:1660–1672. doi: 10.1021/bc700483s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhatkar RB, Kitchens KM, Swaan PW, Ghandehari H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug Chem. 2007;18:2054–2060. doi: 10.1021/bc0603889. [DOI] [PubMed] [Google Scholar]
- Kolhe P, Khandare J, Pillai O, Kannan S, Lieh-Lai M, Kannan RM. Preparation, cellular transport, and activity of polyamidoamine-based dendritic nanodevices with a high drug payload. Biomaterials. 2006;27:660–669. doi: 10.1016/j.biomaterials.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Lebreton SNN, Bradley M. Antibacterial single-bead screening. Tetrahedron. 2003;59:10213–10222. [Google Scholar]
- Lopez AI, Reins RY, McDermott AM, Trautner BW, Cai C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol Biosyst. 2009;5:1148–1156. doi: 10.1039/b904746h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecke A, Majoros IJ, Patri AK, Baker JR, Jr, Holl MM, Orr BG. Lipid bilayer disruption by polycationic polymers: the roles of size and chemical functional group. Langmuir. 2005;21:10348–10354. doi: 10.1021/la050629l. [DOI] [PubMed] [Google Scholar]
- Menjoge AR, Navath RS, Asad A, Kannan S, Kim CJ, Romero R, Kannan RM. Transport and biodistribution of dendrimers across human fetal membranes: implications for intravaginal administration of dendrimer-drug conjugates. Biomaterials. 2010;31:5007–5021. doi: 10.1016/j.biomaterials.2010.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovic NM, Wang J, Lewis K, Klibanov AM. Immobilized N-alkylated polyethylenimine avidly kills bacteria by rupturing cell membranes with no resistance developed. Biotechnol Bioeng. 2005;90:715–722. doi: 10.1002/bit.20454. [DOI] [PubMed] [Google Scholar]
- Mumper RJ, Bell MA, Worthen DR, Cone RA, Lewis GR, Paull JR, Moench TR. Formulating a sulfonated antiviral dendrimer in a vaginal microbicidal gel having dual mechanisms of action. Drug development and industrial pharmacy. 2009;35:515–524. doi: 10.1080/03639040802488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RP. The Effect of Hydroxyl Ion Concentration on the Thermal Death Rate of Bacterium Coli. J Bacteriol. 1928;15:341–356. doi: 10.1128/jb.15.5.341-356.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naberezhnykh GA, Gorbach VI, Likhatskaya GN, Davidova VN, Solov'eva TF. Interaction of chitosans and their N-acylated derivatives with lipopolysaccharide of gram-negative bacteria. Biochemistry (Mosc) 2008;73:432–441. doi: 10.1134/s0006297908040081. [DOI] [PubMed] [Google Scholar]
- Ortega PC-PJL, Munoz-Fernandez MA, Soliveri J, Gomez R, Mata F, Jdela Amine and ammonium functionalization of chloromethylsilane-ended dendrimers Antimicrobial activity studies. Org Biomol Chem. 2008;6:3264–3269. doi: 10.1039/b809569h. [DOI] [PubMed] [Google Scholar]
- Patrick LA, Gaudet LM, Farley AE, Rossiter JP, Tomalty LL, Smith GN. Development of a guinea pig model of chorioamnionitis and fetal brain injury. American journal of obstetrics and gynecology. 2004;191:1205–1211. doi: 10.1016/j.ajog.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Espinoza J. Micronutrients and intrauterine infection, preterm birth and the fetal inflammatory response syndrome. The Journal of nutrition. 2003:1668S–1673S. doi: 10.1093/jn/133.5.1668S. [DOI] [PubMed] [Google Scholar]
- Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Advanced drug delivery reviews. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Tang YL, Shi YH, Zhao W, Hao G, Le GW. Insertion mode of a novel anionic antimicrobial peptide MDpep5 (Val-Glu-Ser-Trp-Val) from Chinese traditional edible larvae of housefly and its effect on surface potential of bacterial membrane. J Pharm Biomed Anal. 2008;48:1187–1194. doi: 10.1016/j.jpba.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Tulu M, Aghatabay NM, Senel M, Dizman C, Parali T, Dulger B. Synthesis, characterization and antimicrobial activity of water soluble dendritic macromolecules. Eur J Med Chem. 2009;44:1093–1099. doi: 10.1016/j.ejmech.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Tziveleka LA, Psarra AM, Tsiourvas D, Paleos CM. Synthesis and characterization of guanidinylated poly(propylene imine) dendrimers as gene transfection agents. J Control Release. 2007;117:137–146. doi: 10.1016/j.jconrel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Ugwumadu A. Role of antibiotic therapy for bacterial vaginosis and intermediate flora in pregnancy. Best practice & research. 2007;21:391–402. doi: 10.1016/j.bpobgyn.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Urakuboa AJLF, Liebermana JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophrenia Research. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature protocols. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- Yang H, Lopina ST, DiPersio LP, Schmidt SP. Stealth dendrimers for drug delivery: correlation between PEGylation, cytocompatibility, and drug payload. J Mater Sci Mater Med. 2008;19:1991–1997. doi: 10.1007/s10856-007-3278-0. [DOI] [PubMed] [Google Scholar]