Abstract
PURPOSE
To investigate the feasibility of electrically stimulating the lateral rectus muscle to recover its physiologic abduction ability in cases of complete sixth cranial (abducens) nerve palsy.
METHODS
In the feline lateral rectus muscle model, the effects of a charge-balanced, biphasic, current-controlled stimulus on the movement of the eye were investigated while stimulation frequency, amplitude, and pulse duration was varied. Eye deflection was measured with a force transducer. Denervated conditions were simulated by injection of botulinum toxin A.
RESULTS
Three chemically denervated and 4 control lateral rectus muscles were analyzed. In control lateral rectus muscles, the minimum fusion frequency was approximately 170 Hz, and the maximum evoked abduction was 27°. The minimum fusion frequency was unchanged after 4 weeks of chemical denervation. Stimulation of chemically denervated lateral rectus muscle resulted in 17° of abduction. For both innervated and chemically denervated lateral rectus muscle, frequencies greater than 175 Hz yielded very little increase in abduction. Modulating amplitude produced noticeable movement throughout the tested range (0.2 to 9 mA).
CONCLUSIONS
Results from the feline lateral rectus muscle showed that electrical stimulation is a feasible approach to evoke a contraction from a denervated lateral rectus muscle. The degree of denervation of the feline lateral rectus muscle was indeterminate. Varying the stimulation amplitude allowed greater eye movement. It is very likely that both frequency and amplitude must be modulated for finer control of static eye position.
Congenital or acquired anomalous innervation and palsy of the extraocular muscles may result in severe amblyopia, loss of stereopsis, marked deficits in binocular visual fields, and diplopia.1 The goal of strabismus treatment is to restore fusion and stereopsis and to enlarge the binocular visual field. Existing treatment for anomalous-innervation strabismus is limited to surgical procedures that are only partially successful in achieving these goals.
A complete extraocular muscle palsy results in permanent denervation and muscle atrophy, which limits the outcome from surgical intervention on the affected muscle. Surgical correction is challenging and outcomes are limited, with many patients requiring several surgical procedures to reach alignment in the primary position.
Several techniques have been described to remobilize a denervated striate muscle, including nerve-to-nerve anastomosis, neuromuscular pedicle transfer, and functional electrical stimulation (FES).2–11 The use of FES has long been proposed as a potential treatment for restoring motor function of denervated motor systems.2,4–11 Recent success has been noted in the ability of FES to allow paraplegics to gain the ability to stand.5,6,9 FES may protect muscles from atrophy, promote muscle reinnervation from appropriate native motoneurons, and reconnect a muscle to the original endplate and its nerve fiber without interrupting the natural course of reinnervation and the potential for spontaneous recovery.12–18 The validity of this approach has been demonstrated in humans.
Zealear and colleagues19,20 and Tobey and Sutton21 first proposed that axial muscles such as those in the larynx or the extraocular muscles could be stimulated by using a normally innervated agonist muscle as a “master” to control a paralyzed “slave” muscle. The effectiveness of coordinating pairs of muscles with FES was proven feasible for clinical research.22,23 Zealear and colleagues22–24 and Billante and colleagues25 applied FES to a laryngeal muscle (posterior cricoarytenoid) by using input respiratory signals to help patients breathe. In their work, they timed the stimulated laryngeal muscle to contract during the inspiratory phase of respiration.
The extraocular muscle system is an optimal candidate for Zealear and colleagues’ approach in using paired muscles as master and slave. By coordinating the electrical stimulus between a paralyzed muscle and its antagonist muscle, it may be possible to excite a paralyzed extraocular muscle to improve ocular alignment and binocular motor function. To simplify our study, we chose the lateral rectus muscle because of its exclusive innervation by the sixth cranial nerve. Several authors have conducted motoneuron or nerve stimulations of innervated and chemically denervated lateral rectus muscles in cats and monkeys.26–32
Our ultimate goal is to develop a miniature implantable electronic system that can perform closed-loop electrical stimulation of extraocular muscles to substantially restore ocular motility. A conceptual system sketch is shown in Figure 1. In this study, we present the feasibility of evoking adequate contractions of chemically denervated feline lateral rectus muscles by using electrical stimulation applied directly to the muscle.
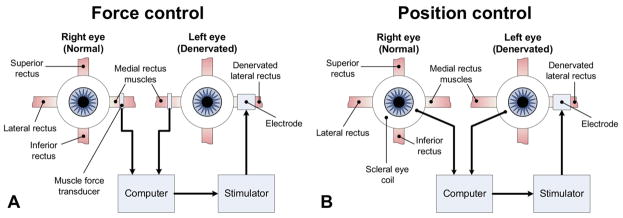
FIG 1.
System sketch of lateral rectus stimulation apparatus to align eye positions. Two types of feedback inputs are under consideration: A, ipsilateral medial-rectus muscle force, and B, contralateral eye position. In both systems, the input is processed by a computer that delivers the stimulus that drives a desired muscle response. The stimulus is adjusted based on the feedback of the input to fine-tune the stimulus.
Methods
We designed a study to deliver FES to the lateral rectus muscle. The overall study consists of several feasibility substudies, including correlation between eye position and extraocular muscle force output, determination of stimulation parameters, and muscle electrical stimulation followed by the design of an implantable device based on proven algorithm and electronics.
The present feasibility experiment is a prospective study in which we used an intermediate animal model to evaluate electrical stimulation of the lateral rectus muscle. The study was divided into 3 steps: (1) determine lateral rectus dimension and properties, (2) evaluate electrode design and muscle properties and correlated muscle response, and (3) eye movement and stimulation parameters. This project fulfilled animal-research-protection regulations of the University of California Los Angeles.
Seven adult cats (mean age, 2 years) were involved in the study. One cat was used to determine the anatomical and physiological characteristics of the lateral rectus muscle, and produced the following results. The muscle was inserted on the sclera at 6.5 mm from the limbus. The width of the muscle near the insertion was 5.5 mm and the resting length was about 35 mm. The areas within the lateral rectus muscle with the strongest response to stimulation were determined with the use of TECA needles and a randomized stimulation protocol, which combined different ranges of amplitude (2 to 8 mA), pulse duration (0.5 to 2.0 ms), and frequency (50 to 250 Hz). Stronger muscle contraction was observed when the electrical stimulus was delivered closer to the neuromuscular junction. Fusion frequency for fused tetanus was found to be between 100 and 200 Hz. The combination of 200 Hz and 1 ms was able to produce a constant contraction (no fatigue) for 1 second. Modulating the amplitude appeared to adequately control lateral rectus muscle contractions. The minimum stimulation duration to first observe eye rotation was 90 ms.
Three cats underwent acute stimulation procedures on the lateral rectus. To determine the ability of the implanted electrodes to stimulate chronically denervated lateral rectus muscle, 5 units (5 units/0.1 mL) of botulinum toxin A (Botox Allergan Inc., Irvine, CA) were injected into one lateral rectus muscle of the remaining 3 cats 4 weeks before the stimulation procedures. The contralateral lateral rectus muscle was used as a control.
Detail descriptions of the experimental procedures, equipment, and protocol are available online as e-Supplement 1 (available at jaapos.org). Surgeries were performed with the cats under general anesthesia. A limbal-conjunctival incision was fashioned temporally. An opening was created between the Tenon’s capsule and the sclera. An extraocular muscle hook was positioned under the lateral rectus muscle. The muscle was cleaned from surrounding connective tissue. A lateral orbitotomy was performed to expose approximately 25 mm of the lateral rectus muscle. The globe and muscle were coated with mineral oil to maintain moisture.
Botulinum toxin type A injection procedures were standardized. The lateral rectus muscle was isolated on a muscle hook. Five units of botulinum toxin were injected into the lateral rectus muscle under direct visualization at approximately 20 mm posterior to the scleral insertion.
For the stimulation experiments, a sleeve-type bipolar electrode (Figure 2) was implanted on the lateral rectus muscle. A diagram of the experimental setup is shown in Figure 3. A force-displacement transducer (Model FT03, Grass Telefactor W., Warwick, RI) was used in series with a spring (0.3 g/cm; Grass Telefactor W.) to measure the eye deflection generated by the stimulated lateral rectus muscle. A computer equipped with a data-acquisition card (PCI-6025E, National Instruments Corp., Austin, TX) was used to automatically conduct both recording (10 kHz sampling rate) and stimulation operations.
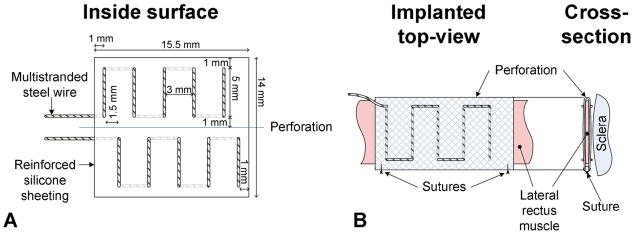
FIG 2.
A, A sleeve-type electrode was constructed from multistranded stainless-steel wire stitched into silicone mesh material. The bipolar electrode positioned one set of stimulating leads on the global surface of the lateral rectus and another set on the orbital surface of the lateral rectus. B, The electrode wraps around the lateral rectus longitudinally and applies stimulus across the width of the muscle. The side facing away from the muscle is insulated from the stimulation in an attempt to localize the stimulating current.
FIG 3.
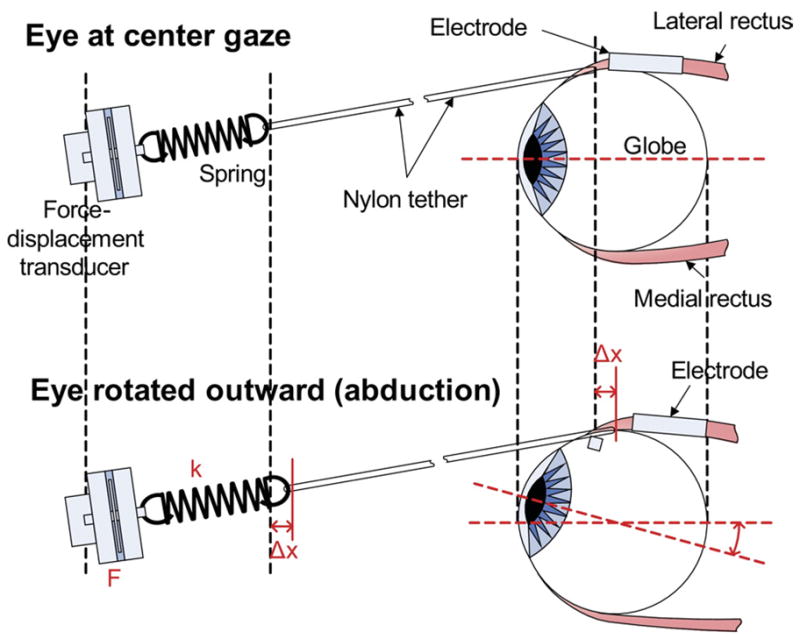
The experimental setup for the feline lateral rectus muscle used a force-displacement transducer in series with a spring (0.3 g/cm) to measure eye deflection generated by the stimulated lateral rectus muscle. Values of linear displacement were obtained using Hooke’s Law. A video camera also captured the movements against a ruler.
A biphasic rectangular pulse was used to minimize fatigue and possible metal diffusion into tissue. The computer automatically delivered the stimulation protocol using software developed in LabVIEW 7.0 (National Instruments Corp.). The stimulation amplitude, stimulation frequency, and cathodic and anodic pulse durations were varied within prespecified ranges and delivered in a random sequence. The LabVIEW program recorded data from the FT and converted the raw voltage readings to arc-length displacement through Hooke’s Law (ie, F = k x with measured force F, spring constant k [0.3 g/cm], and displacement x. The program continued to convert the displacement values to eye-deflection angles by simple geometry using an average cat-eye radius of 20.92 mm on the horizontal plane.33
A video-tracking system consisting of a digital video camera (JVC Everio GZ-MG21U, Yokohama, Japan) was used to record and measure eye movement. The eye movement measured by the force-displacement transducer was compared against the physical displacement seen on the image produced by the video tracking system. Data were analyzed in Matlab 7.0 (The Math Works, Natick, MA). Fusion frequency was verified visually and quantified by the presence of a spike at the stimulated frequency on the Fourier spectrum.
Results
From 4 innervated lateral rectus muscles of 3 cats (Cats 2, 3, and 7), the average eye deflection was 21.8° ± 4.5° (range, 9.6° to 28.4°). Fusion frequency was approximately 170 Hz. Although stimulation frequencies as low as 50 Hz produced visible eye deflections, frequencies <170 Hz did not produce a fused tetanus.
After 4 weeks of chemical denervation, 3 denervated lateral rectus muscles from 3 cats (Cat 4, 5, and 6) produced deflections averaging 17° ± 1.5° (range, 13.1° to 20°). The fusion frequency was similar to the innervated case at approximately 175 Hz.
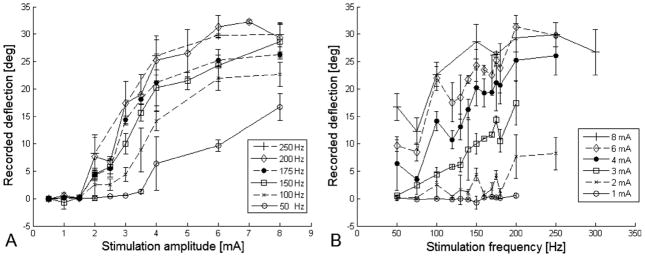
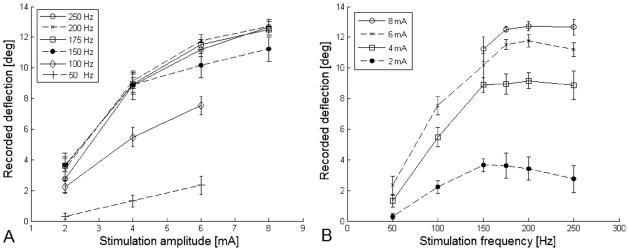
Both stimulation amplitude and frequency elicited a proportional response on ocular rotation. In innervated lateral rectus (Figure 4), frequencies between 100 and 250 Hz responded mostly to changes in amplitude from 1 mA to 3 mA. Amplitude larger than 4 mA had a less but still noticeable effect on ocular rotation. Amplitudes between 2 and 8 mA responded mostly to frequencies of 100 Hz to 175 Hz. There was almost no change in ocular rotation when the frequency was increased above 200 Hz. At 200 Hz, changes in pulse duration from 0.5 ms to 2 ms at amplitudes ranging from 2 mA to 8 mA had no effect on the magnitude of ocular rotations. In the paralyzed muscle, most of the muscle contraction occurred when using amplitudes between 4 mA and 8 mA and frequencies between 100 Hz and 200 Hz (Figure 5). Varying the asymmetry of the biphasic pulse showed no change in ocular rotations for both innervated and denervated lateral rectus.
FIG 4.
Results of stimulation experiments on normal lateral rectus muscle showed that both stimulation amplitude and frequency elicited a proportional response on ocular rotation. (A) Frequencies between 100 Hz and 250 Hz responded mostly to changes in amplitude from 1 mA to 3 mA. (B) Amplitudes between 2 mA and 8 mA responded mostly to frequencies of 100–175 Hz. There was no significant change in ocular rotation when the frequency was increased >200 Hz.
FIG 5.
Results of stimulation experiments on paralyzed lateral rectus muscles showed that most of the muscle contraction occurred when using (A) frequencies between 100 Hz and 200 Hz and (B) amplitudes between 4 mA and 8 mA. The degree of paralysis was not defined.
Discussion
In this study, we demonstrated the feasibility of electrical stimulation to evoke an abduction of the eye from feline innervated lateral rectus muscles and artificially denervated lateral rectus muscles by botulinum toxin type A injection. Stimulation frequency and amplitude were the only parameters that modulated eye deflection. Frequencies less than approximately 170 Hz did not produce a fused tetanus in normal feline lateral rectus muscles. Varying stimulation amplitude was the most effective parameter in eye position using electrical stimulation.
In 1977, Zealear and colleagues17,18 proposed that a signal from a normally innervated muscle could be used to control a stimulation signal that modulates the activity of a contralateral denervated muscle. Preliminary studies were performed in paralyzed dog laryngeal muscles stimulated by an open-loop device. Significantly greater tracking accuracies were observed when closed-loop systems were used.
Microscopic examination of chronically denervated muscles showed atrophy of myofibrils, increased adipocytes, as well as collagen degeneration of the muscle. FES increases muscle–fiber diameter, decreases adipocytes and connective tissue content, and reorganizes myofibril regeneration by promoting selective reinnervation and preventing synkinesis.2,12–16,34
Extraocular muscles differ from other skeletal muscles in the body in many ways, some of which include the presence of multiple innervated muscle-fibers, high mitochondrial content and, in some species, absence of traditional muscle spindles.26,35–37 There is continuous remodeling of the muscle by the addition of myofibers from rapidly activated satellite cells. Compared with other skeletal muscles, extraocular muscles incorporate new myofibrils at a much greater rate. Normal muscle innervation is important in satellite cell number and proliferation. Denervation results in muscle fiber transformation into type-2 white, fast, and anaerobic fibers, which are less resistant to fatigue. Regenerated myofibers express adult fast myosin, which rapidly becomes atrophic and degenerates. FES recovers afferent sensory inputs and improves muscle contraction and resistance by inducing regeneration and transformation of muscle fibers to type 1 red, slow, and aerobic fibers.2,12–16,31,32,34
Slow steady low-force units located primarily in the orbital layer of the extraocular muscle provide primary position. Denervation results in a greater deterioration of the global layer.35 Demer and colleagues38 studied the paralyzed superior oblique muscle. Denervation reduced the whole cross section of the muscle. Although the orbital-layer fiber cross sections were identical in denervated and control muscles, the denervated global-layer fibers had a cross-sectional area 80% lower than the control.
Implantable nerve (epineural), neuromuscular junction (epimysial), or muscle (intramuscular) stimulation systems are ideal for chronic implantation when compared with surface stimulation because of their greater specificity, control, and recruitment. Neural stimulation has the additional advantage of requiring lower current and energy levels. However, the complexity of accessing oculomotor nerves limits its applicability, especially when considering chronic implantation.2 Our study differs from most previous studies of extraocular muscle stimulation because we attempted to directly stimulate the muscle fibers. Our first feline experiment allowed us to design a muscle electrode for acute implantation that effectively transmitted current without damaging the lateral rectus muscle or spreading the stimulation signal to neighboring muscles, such as the orbicularis. Although intramuscular stimulation is ideal for small and deep muscles, it is more difficult to achieve compared with epineural and epimysial stimulation. Greater levels of energy are required to stimulate muscle fibers directly, which may result in the activation of peripheral sensory and pain fibers.
Our study had several limitations. The number of muscles tested was small. Although all experimental procedures were standardized, some variability in the surgical technique and individual response to the botulinum toxin injection could modify the response of the muscle to the stimulation. The effectiveness of denervation was determined by the limitation of abduction and esotropia. No electromyography was used. Previous studies demonstrated an immediate decrease in amplitude and frequency after direct injection of botulinum toxin type A into the feline lateral rectus muscle.32,39 Moreno-Lopez and colleagues38 found that low doses (0.3 ng/kg) of botulinum toxin directly injected to the lateral rectus muscle completely paralyzed the muscle for 15 days, and high doses (3 ng/kg) caused a complete paralysis for 3 months. We limited our study to 4 weeks of chemical denervation using a high-dose (5 units) of botulinum toxin type A to avoid the possibility of muscle recovery. We did not address the degree of paralysis or changes that may result from chronic denervation of extraocular muscles, such as muscle atrophy, which could limit the ability of electrical stimulation to evoke an adequate eye deflection. Our experiments were limited to chemical denervation. It is necessary to create a model in which the lateral rectus muscle is surgically denervated.
Our ultimate goal is a closed-loop system to control the extraocular muscles. Normal muscle activity depends on synchronized muscle contraction and sophisticated sensory feedback. FES must be forceful, controllable, repeatable, and painless. In a closed-loop system, continuous information about contraction, motion, and force should be transmitted to a control unit that modifies the contraction.19–23,40–44 There are many challenges to overcome to achieve such a system, including the difficulty of controlling fast-acting extraocular muscles, the effect of fatigue in constant long-duration stimulation, the power requirements for constant stimulation, and finally addressing the need for integration between motor and sensory systems.
In conclusion, artificial stimulation of the lateral rectus muscle is feasible. Eye position can be modified and controlled by modulating stimulation parameters. Implementation of the protocol-generating algorithm used in this study can greatly reduce surgery and experimentation time. Characterizing the response of the lateral rectus muscle to electrical stimulation was an important first step in our ultimate goal of developing a closed-loop system to improve ocular alignment and enable horizontal pursuit movements using antagonist muscle activity.
Supplementary Material
Acknowledgments
NIH R03 EY015247-01.
This project was funded by NIH Grant 5R03EY15247-3, and the Knights Templar Eye Foundation and Research to Prevent Blindness to Federico G. Velez, MD.
The authors are grateful to John D, Schlag, PhD, Madeline Schlag-Rey, PhD, Dario Ringach, PhD, and Joseph L. Demer, MD, PhD, for their contribution with project design, development, training and implantation; Joel Miller, PhD, for his contribution with the MFT (Miller Force Transducer); Cheryl Billante for her thorough review of this article.
Footnotes
Presented at the 34th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, Washington, DC, April 2–6, 2008.
References
- 1.Adams D. Normal and abnormal visual development. In: Taylor D, Hoyt C, editors. Pediatric ophthalmology and strabismus. Philadelphia, PA: Elsevier Saunders; 2005. pp. 9–22. [Google Scholar]
- 2.Ragnarsson KT. Functional electrical stimulation after spinal cord injury: Current use, therapeutic effects and future directions. Spinal Cord. 2007;46:255–74. doi: 10.1038/sj.sc.3102091. [DOI] [PubMed] [Google Scholar]
- 3.Hockman CH, Gossman MD, Liddell NE, Renehan WE. Restoration of orbicularis oculi function by contralateral orbicularis oculi innervated muscle flap vs neuromuscular pedicle technique. Exp Neurol. 1992;177:307–12. doi: 10.1016/0014-4886(92)90140-l. [DOI] [PubMed] [Google Scholar]
- 4.Kumar VP, Lau HK, Liu J, Pereira BP, Pho RW. Clinical applications of functional electrical stimulation. Ann Acad Med Singapore. 1995;24:428–35. [PubMed] [Google Scholar]
- 5.Zhang D, Zhu K. Modeling biological motor control for human locomotion with functional electrical stimulation. Biol Cybern. 2007;96:79–97. doi: 10.1007/s00422-006-0107-3. [DOI] [PubMed] [Google Scholar]
- 6.Davoodi R, Andrews BJ. Computer simulation of FES standing up in paraplegia: A self-adaptive fuzzy controller with reinforcement learning. IEEE Trans Neural Systems Rehabil. 1998;6:151–61. doi: 10.1109/86.681180. [DOI] [PubMed] [Google Scholar]
- 7.Salmons S, Ashley Z, Sutherland H, Russold MF, Li F, Jarvis JC. Functional electrical stimulation of denervated muscles: Basic issues. Artif Organs. 2005;29:199–202. doi: 10.1111/j.1525-1594.2005.29034.x. [DOI] [PubMed] [Google Scholar]
- 8.Modlin M, Forstner C, Hofer C, Mayr W, Richter W, Carraro U, et al. Electrical stimulation of denervated muscles: First results of a clinical study. Artif Organs. 2005;29:203–6. doi: 10.1111/j.1525-1594.2005.29035.x. [DOI] [PubMed] [Google Scholar]
- 9.Kern H, Hofer C, Strohhofer M, Mayr W, Richter W, Stohr H. Standing up with denervated muscles in humans using functional electrical stimulation. Artif Organs. 1999;23:447–52. doi: 10.1046/j.1525-1594.1999.06376.x. [DOI] [PubMed] [Google Scholar]
- 10.Kern H, Salmons S, Mayr W, Rossini K, Carraro U. Recovery of long-term denervated human muscles induced by electrical stimulation. Muscle Nerve. 2005;31:98–101. doi: 10.1002/mus.20149. [DOI] [PubMed] [Google Scholar]
- 11.Zealear DL, Rainey CL, Jerles ML, Tanabe T, Herzon GD. Technical approach for the reanimation of the chronically denervated larynx by means of functional electrical stimulation. Ann Otol Rhinol Laryngol. 1994;103:705–12. doi: 10.1177/000348949410300908. [DOI] [PubMed] [Google Scholar]
- 12.Boncompagni S, Kern H, Rossini K, Hofer C, Mayr W, et al. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc Natl Acad Sci USA. 2007;104:19339–44. doi: 10.1073/pnas.0709061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern H, Boncompagni S, Rossini K, Mayr W, Fanò G, Zanin ME, et al. Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): A role for myofiber regeneration? J Neuropathol Exp Neurol. 2004;63:919–31. doi: 10.1093/jnen/63.9.919. [DOI] [PubMed] [Google Scholar]
- 14.Kern H, Rossini K, Carraro U, Mayr W, Vogelauer M, Hoellwarth U, et al. Muscle biopsies show that FES of denervated muscles reverses human muscle degeneration from permanent spinal motoneuron lesion. J Rehabil Res Dev. 2005;42:43–53. doi: 10.1682/jrrd.2004.05.0061. [DOI] [PubMed] [Google Scholar]
- 15.Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36:463–9. doi: 10.1038/sj.sc.3100679. [DOI] [PubMed] [Google Scholar]
- 16.Zealear DL, Billante CL, Chongkolwatana C, Herzon GD. The effects of chronic electrical stimulation on laryngeal muscle reinnervation. J Otorhinolaryngol Relat Spec. 2000;62:87–95. doi: 10.1159/000027723. [DOI] [PubMed] [Google Scholar]
- 17.Zealear DL, Billante CR, Chongkolwatana C, Rho YS, Hamdan A-L, Herzon GD. The effects of chronic electrical stimulation on laryngeal muscle physiology and histochemistry. J Otorhinolaryngol Relat Spec. 2000;62:81–6. doi: 10.1159/000027722. [DOI] [PubMed] [Google Scholar]
- 18.Zealear DL, Rodriguez RJ, Kenny T, Billante MJ, Cho Y, Billante CR, et al. Electrical stimulation of a denervated muscle promotes selective reinnervation by native over foreign motoneurons. J Neurophysiol. 2002;87:2195–9. doi: 10.1152/jn.00451.2001. [DOI] [PubMed] [Google Scholar]
- 19.Zealear DL, Dedo HH. Control of paralyzed axial muscles by electrical stimulation. Acta Otolaryngol. 1977;83:514–27. doi: 10.3109/00016487709128880. [DOI] [PubMed] [Google Scholar]
- 20.Zealear DL, Dedo HH. Control of paralyzed axial muscles by electrical stimulation. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1977;84:310. [PubMed] [Google Scholar]
- 21.Tobey DN, Sutton D. Otolaryngology. Vol. 86. 1978. Contralaterally elicited electrical stimulation of paralyzed facial muscles; pp. ORL-812–18. [DOI] [PubMed] [Google Scholar]
- 22.Zealear DL, Rainey CL, Herzon GD, Netterville JL, Ossoff RH. Electrical pacing of the paralyzed human larynx. Ann Otol Rhinol Laryngol. 1996;105:689–93. doi: 10.1177/000348949610500904. [DOI] [PubMed] [Google Scholar]
- 23.Zealear DL, Swelstad MR, Sant’Anna GD, Bannister RA, Billante CR, Rodriguez RJ, et al. Determination of the optimal conditions for laryngeal pacing with the Itrel II implantable stimulator. Otolaryngol Head Neck Surg. 2001;125:183–92. doi: 10.1067/mhn.2001.118246. [DOI] [PubMed] [Google Scholar]
- 24.Zealear DL, Billante CR, Courey MS, Netterville JL, Paniello RC, Sanders I, et al. Reanimation of the paralyzed human larynx with an implantable electrical stimulation device. Laryngoscope. 2003;113:1149–56. doi: 10.1097/00005537-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Billante CR, Zealear DL, Courey MS, Netterville JL. Effect of chronic electrical stimulation of laryngeal muscle on voice. Ann Otol Rhinol Laryngol. 2002;111:328–32. doi: 10.1177/000348940211100408. [DOI] [PubMed] [Google Scholar]
- 26.Shall MS, Goldberg SJ. Extraocular motor units: Type classification and motoneuron stimulation frequency-muscle unit force relationships. Brain Res. 1992;587:291–300. doi: 10.1016/0006-8993(92)91010-c. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg SJ, Shall MS. Lateral rectus whole muscle and motor unit contractile measures with the extraocular muscles intact. J Neurosci Meth. 1997;78:47–50. doi: 10.1016/s0165-0270(97)00141-6. [DOI] [PubMed] [Google Scholar]
- 28.Shall MS, Goldberg SJ. Lateral rectus EMG and contractile responses elicited by cat abducens motoneurons. Muscle Nerve. 1995;18:948–55. doi: 10.1002/mus.880180905. [DOI] [PubMed] [Google Scholar]
- 29.Shall MS, Wilson KE, Goldberg SJ. Extraocular motoneuron stimulation frequency effects on motor unit tension in cat. Acta Anat (Basel) 1996;157:217–25. doi: 10.1159/000147884. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg SJ, Meredith MA, Shall MS. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J Neurosci. 1998;18:10629–39. doi: 10.1523/JNEUROSCI.18-24-10629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitrova DM, Shall MS, Goldberg SJ. Stimulation-evoked eye movements with and without the lateral rectus muscle pulley. J Neurophysiol. 2003;90:3809–15. doi: 10.1152/jn.00622.2003. [DOI] [PubMed] [Google Scholar]
- 32.Dimitrova DM, Shall MS, Goldberg SJ. Short-term effects of botulinum toxin on the lateral rectus muscle of the cat. Exp Brain Res. 2002;147:449–55. doi: 10.1007/s00221-002-1265-8. [DOI] [PubMed] [Google Scholar]
- 33.Gelatt KM. Textbook of veterinary ophthalmology. Philadelphia, PA: Lea & Febiger; 1981. [Google Scholar]
- 34.McLoon LK, Wirtschafter JD. Continuous myonuclear addition to single extraocular myofibers in uninjured adult rabbits. Muscle Nerve. 2002;25:348–58. doi: 10.1002/mus.10056. [DOI] [PubMed] [Google Scholar]
- 35.Scott AB, Collins CC. Division of labor in human extraocular muscle. Arch Ophthalmol. 1973;90:319–22. doi: 10.1001/archopht.1973.01000050321017. [DOI] [PubMed] [Google Scholar]
- 36.Ruff RL. More than meets the eye: Extraocular muscle is very distinct from extremity skeletal muscle. Muscle Nerve. 2002;25:311–3. doi: 10.1002/mus.10063. [DOI] [PubMed] [Google Scholar]
- 37.Spencer RF, Porter JD, Büttner-Ennever JA. Biological organization of the extraocular muscles. Prog Brain Res. 2006:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- 38.Demer JL, Poukens V, Ying H, Shan X, Tian J, Lee DS. Effects of acute trochlear denervation on the primate SO muscle: Differential sparing of orbital layer. Vol. 49. IOVS; 2008. ARVO E-Abstract 000. [Google Scholar]
- 39.Moreno-Lopez B, de la Cruz RR, Pastor AM, Delgado-Garcia JM. Effects of botulinum neurotoxin type A on abducens motoneurons in the cat: Alterations of the discharge pattern. Neuroscience. 1997;81:437–55. doi: 10.1016/s0306-4522(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 40.Zealear DL, Swelstad MR, Sant’anna GD, Bannister RA, Billante CR, Rodriguez RJ, et al. Determination of the optimal condition for laryngeal pacing with Itrel II implantable stimulator. Otolaryngol Head Neck Surg. 2001;125:183–92. doi: 10.1067/mhn.2001.118246. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y-L, Li Y-C, Kuo T-S, Lai J-S. The development of a closed-loop controlled functional electrical stimulation (FES) in gait training. J Med Eng Technol. 2001;25:41–8. doi: 10.1080/03091900110043612. [DOI] [PubMed] [Google Scholar]
- 42.Quintern J, Riener R, Rupprecht S. Comparison of simulation and experiments of different closed-loop strategies for functional electrical stimulation: Experiments in paraplegics. Artif Organs. 1997;21:232–5. doi: 10.1111/j.1525-1594.1997.tb04656.x. [DOI] [PubMed] [Google Scholar]
- 43.Davis R, Houdayer T, Andrews B, Emmons S, Patrick J. Paraplegia: Prolonged closed-loop standing with implanted nucleus FES-22 stimulator and Andrews’ foot-ankle orthosis. Stereotact Funct Neurosurg. 1997;69:281–7. doi: 10.1159/000099889. [DOI] [PubMed] [Google Scholar]
- 44.Jezernik S, Wassink RGV, Keller T. Sliding mode closed-loop control of FES controlling the shank movement. IEEE Trans Biomed Eng. 2004;51:263–72. doi: 10.1109/TBME.2003.820393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.