Abstract
Sleep disorders are observed in Parkinson’s disease, Dementia with Lewy Bodies and Alzheimer’s disease, however the underlying mechanisms are unclear.
Reduced hypocretin (orexin) levels are reported in Parkinson’s disease and sleep disorders including narcolepsy, however levels in Dementia with Lewy Bodies and Alzheimer’s disease and their relationship to sleep disturbances in these disorders remain undetermined.
We examined hypocretin levels in Dementia with Lewy Bodies and Alzheimer’s disease cases and correlated these with sleep habits and clinical characteristics. Whilst limited hypocretin alterations were observed in Alzheimer’s disease, we demonstrate reduced neocortical hypocretin-immunoreactivity in Dementia with Lewy Bodies patients correlating with hypersomnolence and alpha-synuclein levels. These results suggest the involvement of hypocretin in sleep disorders in Dementia with Lewy Bodies.
Keywords: Sleep, Parkinson’s disease, hypersomnolence, leg movement
INTRODUCTION
Sleep disorders are reported in Parkinson’s disease, Dementia with Lewy Bodies and Alzheimer’ Disease [1,2]. Whilst the pathophysiology of sleep disorders in these diseases remains unclear, it has been linked with reduced hypocretin levels in other sleep disorders such as narcolepsy [3,4]. Hypocretins 1 and 2, (orexins A and B) are hypothalamic neuropeptides, initially identified and investigated as regulators of food intake but which have more recently been shown to stimulate wakefulness [5,6].
Whilst recent studies in patients diagnosed with Parkinson’s disease have reported a 60% reduction in hypothalamic hypocretin neurons [7] and Lewy bodies in hypocretin neurons of patients with advanced Parkinson’s disease [8], neuropathological hypocretin levels in Dementia with Lewy Bodies or Alzheimer’s disease remain unexamined.
This study aimed to examine neocortical hypocretin levels in Dementia with Lewy Bodies or Alzheimer’s disease patients and to correlate these with patient-reported sleep habits and clinical characteristics.
METHODS
Case Selection and Neuropathological Evaluation
Autopsy material, from a total of 43 cases (Table 1), was obtained from patients who received neurological and psychometric testing at the Alzheimer Disease Research Center, San Diego in the 12 months before death. A sleep evaluation was obtained including sleep difficulty, frequency and leg movements during sleep.
Table 1.
Summary of demographics
| Dementia with Lewy Bodies (n=21) | Alzheimer’s Disease (n=19) | Controls (n=3) | |
|---|---|---|---|
| Age, years | 79.5 (7.7) | 82.5 (9.0) | 85.7 (4.6) |
| Education, years | 15.4 (2.9) | 14.2 (3.8) | 15.3 (3.1) |
| Disease duration, yr | 9.0 (4.4) | 10.4 (4.5) | N/A |
| MMSE (0–30) | 10.7 (8.6) | 9.6 (10.3) | 28.5 (0.7) |
| DRS (0–144) | 67.4 (34.3) | 52.5 (44.4) | 135.7 (4.5) |
MMSE=Mini-mental state examination; DRS= Dementia Rating Scale
At autopsy, brains were divided sagittally, and left temporal cortex samples were fixed in 4% paraformaldehyde and sectioned at 40 μm for immunohistochemical analysis. Frozen samples from the right hemisphere were used for immunoblot analysis. The temporal cortex was selected as previous studies have shown pathology and accumulation of alpha-synuclein in this region in Dementia with Lewy Bodies patients [9,10].
For neuropathological diagnosis, paraffin sections from neocortical, limbic and subcortical regions were stained with heamatoxylin and eosin or thioflavine-S [11,12] and Braak stage was assessed [13]. Based on published clinical and pathological findings [14], cases were subdivided into: non-demented age-matched controls (n=3), Alzheimer’s disease cases (n=19), and Dementia with Lewy Bodies cases (n=21). All Alzheimer’s disease cases met the Consortium to Establish a Registry for Alzheimer’s disease and National Institute of Aging criteria for diagnosis and displayed neuritic plaques and tangle formation in the neocortex and limbic system [15]. The diagnosis of Dementia with Lewy Bodies was based on clinical presentation of dementia and pathological findings of Lewy Bodies in the locus coeruleus, substantia nigra, or nucleus basalis of Meynert, as well as in cortical regions. Lewy Bodies were detected using an alpha-synuclein antibody as recommended by the Consortium on Dementia with Lewy Bodies criteria [15]. In addition to Lewy Bodies the majority of these cases displayed sufficient plaques and tangles to be classified as Braak stages III-IV, they had abundant plaques in the neocortex and limbic system but fewer tangles compared to Alzheimer’s disease cases.
Immunohistochemistry
As previously described [16], vibratome sections were washed in Tris buffered saline (TBS, pH 7.4) and incubated at 4°C overnight with anti-hypocretin 1 (1:500, Millipore, CA). Sections were incubated in secondary antibody (1:75, Vector, CA), followed by Avidin D-horseradish peroxidase (Vector, CA) and reacted with 0.2 mg/ml diaminobenzidine in 50 mM Tris (pH 7.4) with 0.001% H2O2. Sections were imaged with a digital Olympus microscope and analysis of hypocretin immunoreactivity was performed using Image-Pro Plus (Media Cybernetics, MD). For each case three sections (10 images per section) were analyzed to estimate the average number of immunolabeled cells per unit area (mm2) and the intensity of the immunostaining above background levels (corrected optical density).
In control experiments designed to test the specificity of the hypocretin antibody pre-incubating the antibody with 20-fold excess of purified hypocretin protein eliminated immunoreactivity in sections from the hypothalamus of a control case (data not shown).
Immunoblot Analysis
Brains were homogenized in lysis buffer (1% Triton X-100, 10% glycerol, 50mM HEPES, pH7.4, 140mM NaCl, 1mM EDTA, 1mM Na3VO4, 20mM β-glycerophosphate, and proteinase inhibitor cocktails) and separated into cytosolic and membrane fractions by centrifugation (100,000rpm, 60 mins). For immunoblot, 20μg of protein was resolved by SDS-PAGE on 4–12% Bis-Tris gels (Invitrogen, CA) and transferred onto Immobilon membranes (Millipore, CA). Membranes were blocked with phosphate-buffered saline (PBS) with 0.2% Tween-20 containing 3% skim milk, followed by incubation with polyclonal antibodies against alpha-synuclein (Millipore, CA) or hypocretin (Millipore,CA). Membranes were incubated with secondary antibodies (1:5000, American Qualex, CA), visualized with enhanced chemiluminescence (PerkinElmer, MA) and analyzed with the VersaDoc gel imaging system (BioRad, CA). Beta-Actin (Sigma, CA) was used as a loading control.
Statistical Analysis
Unless otherwise noted, all data are presented as mean ± standard deviation. ANOVA with post-hoc analyses were performed on biochemical markers. Given the degree of variability in the group sizes, non-parametric correlations were performed between biochemical markers and sleep questions for Dementia with Lewy Bodies and Alzheimer’s disease subjects. Simple linear regression was performed on biochemical markers and sleep characteristics. Bonferroni’s multiple comparison tests were conducted to account for multiple comparisons.
RESULTS
Immunoblot analysis of cytosolic and membrane fractions from neocortical brain homogenates of Dementia with Lewy Bodies, Alzheimer’s disease and controls was conducted to examine hypocretin levels. The anti-hypocretin antibody recognized a major band at approximately 4kDa representing mature hypocretin and an additional band at 8kDa likely representing pro-hypocretin. Levels of mature hypocretin were significantly reduced in the cytosolic fraction of Dementia with Lewy Bodies cases compared to controls (Fig. 1a). A similar reduction was observed in the membrane fraction. No differences in cytosolic or membrane hypocretin levels were detected between controls and Alzheimer’s disease cases.
Figure 1. Hypocretin and alpha-synuclein levels in Dementia with Lewy Bodies and Alzheimer’s disease.
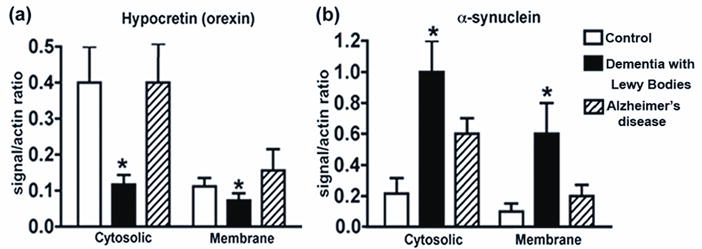
Analysis of hypocretin (a) and alpha-synuclein (b) levels in the cytosolic and membrane fractions of brain homogenates from Dementia with Lewy Bodies patients, Alzheimer’s disease patients and controls.
* p<0.05, one-way ANOVA and post-hoc Fisher
Abnormally aggregated accumulation of oligomeric alpha-synuclein, a natively soluble and unfolded protein, is a key characteristic of Lewy bodies. Immunoblot analysis of alpha-synuclein levels in Dementia with Lewy Bodies, Alzheimer’s disease and controls demonstrated significantly higher levels in Dementia with Lewy Bodies versus Alzheimer’s disease patients and controls in both the cytosolic and membrane portions (Fig. 1b).
Consistent with the immunoblot analysis, immunohistochemical analysis of neocortical hypocretin levels in Dementia with Lewy Bodies, Alzheimer’s disease and controls demonstrated reduced levels of neuronal (Fig. 2c (arrowhead)) and fiber (Fig. 2d) hypocretin compared to controls (Fig. 2a, b, g). No differences in patterns of neuronal or fiber hypocretin immunoreactivity were detected between control and Alzheimer’s disease cases (Fig. 2e (arrowheads), f (arrow), g).
Figure 2. Hypocretin levels in Dementia with Lewy Bodies and Alzheimer’s disease.
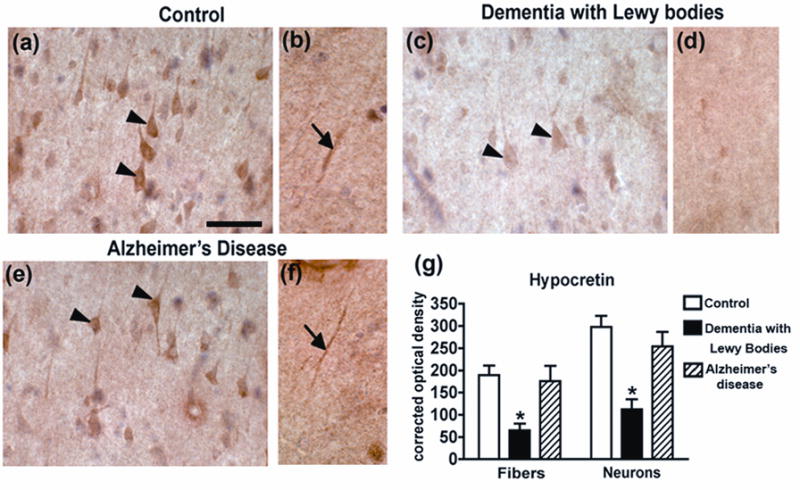
Immunohistochemical analysis of hypocretin levels in neocortical neurons (a, arrowheads) and of a representative hypocretin-immunopositive neocortical fiber (b, arrow) from a control patient. Immunohistochemical analysis of hypocretin levels in neocortical neurons (c, arrowheads) and of a representative hypocretin-immunonegative neocortical fiber (d) Dementia with Lewy Bodies patient. Immunohistochemical analysis of hypocretin levels in neocortical neurons (e, arrowheads) and of a representative hypocretin-immunopositive neocortical fiber (f, arrow) from an Alzheimer’s disease patient. Scale bar = 50μM
* p<0.05, one-way ANOVA and post-hoc Fisher
Correlational analysis of clinical measures reported by Dementia with Lewy Bodies patients and levels of hypocretin or alpha-synuclein demonstrated a significant correlation between cytosolic hypocretin levels and hypersomnolence and alpha-synuclein levels in the membrane fraction. Also in these cases, levels of alpha-synuclein in the membrane fraction correlated with the presence and amount of leg movement. In Alzheimer’s disease cases hypocretin levels in the membrane fraction correlated with the amount of leg movement and sleep difficulties.
DISCUSSION
This study sought to investigate the levels of hypocretin, a hypothalamic neuropeptide associated with wakefulness, in Dementia with Lewy Bodies and Alzheimer’s disease patients and its relationship to sleep abnormalities in these disorders. We show reduced neocortical hypocretin immunoreactivity in Dementia with Lewy Bodies compared to Alzheimer’s disease patients and controls. Hypocretin levels in Dementia with Lewy Bodies patients correlated with hypersomnolence and alpha-synuclein accumulation. These results suggests that altered hypocretin levels may play a role in sleep abnormalities reported in Dementia with Lewy Bodies patients, as has been proposed for Parkinson’s Disease [7,17].
Recent studies have examined cerebrospinal fluid levels of hypocretin in Parkinson’s disease and Dementia with Lewy Bodies, however results have been inconclusive, with some studies reporting reductions [8,17,18] and others finding no alterations [19,20] in either Parkinson’s disease or Dementia with Lewy Bodies. These inconsistencies may be related to the reported need for a 50% reduction in hypocretin neurons to identify cerebrospinal fluid alterations [17].
Our results are consistent with studies showing reduced cerebrospinal fluid levels of hypocretin in Parkinson’s disease cases [8,17,18] and similar findings in experimental animal models [21]. We show a reduction of neocortical hypocretin-immunoreactive fibers and neurons in Dementia with Lewy Bodies cases compared to Alzheimer’s disease and control. As most hypocretin is produced in the lateral hypothalamus [5,22], these hypocretin-immunoreactive neurons in the neocortex may represent uptake from projecting fibers. Alternatively, the presence of hypocretin in these neurons may indicate it is being produced by them, this is consistent with a study reporting the presence of pre-pro hypocretin mRNA in pyramidal neurons in the hippocampus under basal conditions and during epilepsy [23] indicating that hypocretin may be made in cell populations other than the lateral hypothalamus.
Mechanisms underlying neocortical hypocretin reduction in Dementia with Lewy Bodies are unclear; however we report a correlation between hypocretin levels and alpha-synuclein aggregation in these patients, consistent with previous studies in Parkinson’s disease which have shown that alpha-synuclein accumulates in hypocretin-containing neurons in the hypothalamus [7]. Alpha-synuclein has been shown to accumulate in axons and interfere with axonal transport [24], therefore one possibility is that accumulation of alpha-synuclein in the neocortex may interfere with hypocretin transport, however further investigation of the relationship between alpha-synuclein aggregation and hypocretin levels is needed to clarify this.
CONCLUSION
Neocortical hypocretin levels are reduced in Dementia with Lewy Bodies patients and correlate with hypersomnolence in these cases suggesting a role for hypocretin in the sleep disturbances observed in Dementia with Lewy Bodies.
Table 2.
Clinical and Neuropathological Correlations in Dementia with Lewy Bodies
| Leg movement | Amount of leg movement | Hypersomnolence | Sleep difficulties | Hypocretin (Cytosolic) | Hypocretin (Membrane) | Alpha-synuclein (Cytosolic) | Alpha-synuclein (Membrane) | |
|---|---|---|---|---|---|---|---|---|
| Leg movements | 1.000 | 0.966(**) | 0.444 | −0.423 | 0.000 | 0.317 | 0.195 | 0.586(*) |
| Amount of leg movements | 0.966**) | 1.000 | 0.499(*) | −0.373 | −0.031 | 0.256 | 0.300 | 0.656(**) |
| Hypersomnolence | 0.444 | 0.499(*) | 1.000 | −0.404 | 0.443 (*) | 0.220 | −0.050 | 0.165 |
| Sleep difficulties | −0.423 | −0.373 | −0.404 | 1.000 | −0.066 | −0.225 | −0.048 | −0.165 |
| Hypocretin (Cytosolic) | 0.000 | −0.031 | 0.443 (*) | −0.066 | 1.000 | −0.090 | −0.242 | −0.447(*) |
| Hypocretin (Membrane) | 0.317 | 0.256 | 0.220 | −0.225 | −0.090 | 1.000 | −0.092 | 0.074 |
| Alpha-synuclein (Cytosolic) | 0.195 | 0.300 | −0.050 | −0.048 | −0.242 | −0.092 | 1.000 | 0.639(**) |
| Alpha-synuclein (Membrane) | 0.586(*) | 0.656(**) | 0.165 | −0.165 | −0.447(*) | 0.074 | 0.639(**) | 1.000 |
Results are presented as just Spearman’s rank correlation coefficients or Spearman’s rank correlation coefficient (significance indicator)
indicates significance at the 0.01 level (2-tailed) and
indicates significance at the 0.05 level (2-tailed).
Significant results are presented in boldtype.
Table 3.
Clinical and Neuropathological Correlations in Alzheimer’s disease
| Leg movement | Amount of leg movement | Hypersomnolence | Sleep difficulties | Hypocretin (Cytosolic) | Hypocretin (Membrane) | Alpha-synuclein (Cytosolic) | Alpha-synuclein (Membrane) | |
|---|---|---|---|---|---|---|---|---|
| Leg movements | 1.000 | 0.964(**) | −0.030 | 0.489(*) | 0.396 | 0.424 | −0.123 | 0.232 |
| Amount of leg movements | 0.964(**) | 1.000 | 0.055 | 0.514(*) | 0.355 | 0.512(*) | −0.137 | 0.250 |
| Hypersomnolence | −0.030 | 0.055 | 1.000 | 0.161 | 0.190 | 0.158 | −0.136 | 0.219 |
| Sleep difficulties | 0.489(*) | 0.514(*) | 0.161 | 1.000 | 0.485 | 0.515(*) | −0.408 | −0.019 |
| Hypocretin (Cytosolic) | 0.396 | 0.355 | 0.190 | 0.485 | 1.000 | 0.252 | −0.571(*) | −0317 |
| Hypocretin (Membrane) | 0.424 | 0.512(*) | 0.158 | 0.515(*) | 0.252 | 1.000 | −0.309 | −0.057 |
| Alpha-synuclein (Cytosolic) | −0.123 | −0.137 | −0.136 | −0.408 | −0.571(*) | −0.309 | 1.000 | 0.587(*) |
| Alpha-synuclein (Membrane) | 0.232 | 0.250 | 0.219 | −0.019 | −0.317 | −0.057 | 0.587(*) | 1.000 |
Results are presented as just Spearman’s rank correlation coefficients or Spearman’s rank correlation coefficient (significance indicator)
indicates significance at the 0.01 level (2-tailed) and
indicates significance at the 0.05 level (2-tailed).
Significant results are presented in bold type.
Acknowledgments
This work was funded by NIH grants AG 022074, AG 5131, AG 18440 and NS 044233
References
- 1.Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chokroverty S. Sleep and neurodegenerative diseases. Semin Neurol. 2009;29:446–467. doi: 10.1055/s-0029-1237124. [DOI] [PubMed] [Google Scholar]
- 3.Nightingale S, Orgill JC, Ebrahim IO, de Lacy SF, Agrawal S, Williams AJ. The association between narcolepsy and REM behavior disorder (RBD) Sleep Med. 2005;6:253–258. doi: 10.1016/j.sleep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Schenck CH, Garcia-Rill E, Segall M, Noreen H, Mahowald MW. HLA class II genes associated with REM sleep behavior disorder. Ann Neurol. 1996;39:261–263. doi: 10.1002/ana.410390216. [DOI] [PubMed] [Google Scholar]
- 5.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroeger D, de Lecea L. The hypocretins and their role in narcolepsy. CNS Neurol Disord Drug Targets. 2009;8:271–280. doi: 10.2174/187152709788921645. [DOI] [PubMed] [Google Scholar]
- 7.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drouot X, Moutereau S, Nguyen JP, Lefaucheur JP, Creange A, Remy P, et al. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology. 2003;61:540–543. doi: 10.1212/01.wnl.0000078194.53210.48. [DOI] [PubMed] [Google Scholar]
- 9.Hansen L. The Lewy body variant of Alzheimer disease. JNeural Transm. 1997;51:111–121. doi: 10.1007/978-3-7091-6846-2_7. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247(Suppl2):II3–10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 11.Hansen L, Daniel S, Wilcock G, Lowe S. Neocortical synaptophysin in Lewy body disease: relationship to Alzheimer’s disease and dementia. JNeurolNeurosurgPsych. 1998 doi: 10.1136/jnnp.64.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen L, Masliah E, Quijada-Fawcett S, Rexin D. Entorhinal neurofibrillary tangles in Alzheimer disease with Lewy bodies. NeurosciLett. 1991;129:269–272. doi: 10.1016/0304-3940(91)90478-c. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 14.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- 15.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 16.Masliah E, Alford M, Adame A, Rockenstein E, Galasko D, Salmon D, et al. Abeta1–42 promotes cholinergic sprouting in patients with AD and Lewy body variant of AD. Neurology. 2003;61:206–211. doi: 10.1212/01.wnl.0000073987.79060.4b. [DOI] [PubMed] [Google Scholar]
- 17.Fronczek R, Overeem S, Lee SY, Hegeman IM, van Pelt J, van Duinen SG, et al. Hypocretin (orexin) loss in Parkinson’s disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 18.Asai H, Hirano M, Furiya Y, Udaka F, Morikawa M, Kanbayashi T, et al. Cerebrospinal fluid-orexin levels and sleep attacks in four patients with Parkinson’s disease. Clin Neurol Neurosurg. 2009;111:341–344. doi: 10.1016/j.clineuro.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Baumann C, Ferini-Strambi L, Waldvogel D, Werth E, Bassetti CL. Parkinsonism with excessive daytime sleepiness--a narcolepsy-like disorder? J Neurol. 2005;252:139–145. doi: 10.1007/s00415-005-0614-5. [DOI] [PubMed] [Google Scholar]
- 20.Compta Y, Santamaria J, Ratti L, Tolosa E, Iranzo A, Munoz E, et al. Cerebrospinal hypocretin, daytime sleepiness and sleep architecture in Parkinson’s disease dementia. Brain. 2009;132:3308–3317. doi: 10.1093/brain/awp263. [DOI] [PubMed] [Google Scholar]
- 21.Gerashchenko D, Murillo-Rodriguez E, Lin L, Xu M, Hallett L, Nishino S, et al. Relationship between CSF hypocretin levels and hypocretin neuronal loss. Exp Neurol. 2003;184:1010–1016. doi: 10.1016/S0014-4886(03)00388-1. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 23.Morales A, Bonnet C, Bourgoin N, Touvier T, Nadam J, Laglaine A, et al. Unexpected expression of orexin-B in basal conditions and increased levels in the adult rat hippocampus during pilocarpine-induced epileptogenesis. Brain Res. 2006;1109:164–175. doi: 10.1016/j.brainres.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 24.Roy S. The Paradoxical Cell Biology of alpha-Synucle. Results Probl Cell Differ. 2009;48:159–172. doi: 10.1007/400_2009_23. [DOI] [PubMed] [Google Scholar]


