Abstract
Purpose
Intracerebral microdialysis (ICMD) is an accepted methodology for monitoring changes in neurochemistry from acute brain injury. The goal of this pilot study was to determine the feasibility of using ICMD to examine the neuropharmacokinetics (nPK) of temozolomide (TMZ) in brain interstitium (BI) following oral administration.
Experimental Design
Patients with primary or metastatic brain tumors had a microdialysis catheter placed in peritumoral brain tissue at the time of surgical debulking. CT scan confirmed the catheter location. Patients received a single oral dose of TMZ (150 mg/m2) on the first post-operative day, serial plasma and ICMD samples were collected over 24 hrs, and TMZ concentrations were determined by tandem mass spectrometry.
Results
Nine patients were enrolled. Dialysate and plasma samples were successfully collected from 7 of the 9 patients. The mean TMZ area-under-the-concentration-time-curve (AUC) in plasma and BI were 17.1 and 2.7 μg/ml × hr, with an average BI/plasma AUC ratio of 17.8%. The mean peak TMZ concentration in brain was 0.6 ± 0.3 μg/ml, and the mean time to reach peak level in brain was 2.0 ± 0.8 hrs.
Conclusions
The use of ICMD to measure the nPK of systemically administered chemotherapy is safe and feasible. Concentrations of TMZ in BI obtained by ICMD are consistent with published data obtained in a pre-clinical ICMD model, as well as from clinical studies of cerebrospinal fluid. However, the delayed time required to achieve maximum TMZ concentrations in brain suggests that current chemoradiation regimens may be improved by administering TMZ 2-3 hours before radiation.
Keywords: pharmacokinetics, temozolomide, blood-brain barrier, microdialysis, brain tumor
Introduction
The treatment of malignant brain tumors continues to challenge clinicians and scientists alike. A major obstacle to successful pharmacologic management of central nervous system (CNS) tumors is the presence of the blood-brain barrier (BBB), which prevents most anti-cancer agents from entering the CNS. As a result of the ever-expanding list of new targeted agents, and given the pharmacologic limitations associated with the BBB, there exists a significant need for tools that will allow assessment of a new drug’s CNS biodistribution prior to applying that drug for brain tumor therapy.
Microdialysis is a technique for continuously sampling the concentration of a drug or biomolecule in the extracellular fluid (ECF) of body tissues, without significantly disturbing tissue function. This technique consists of implanting into a body tissue a catheter that contains a semi-permeable membrane at the end. The dialysis membrane acts as an artificial capillary, so that when perfusion fluid is pumped through the microdialysis catheter, diffusion of molecules occurs down their concentration gradients as the ECF equilibrates with the perfusion fluid. The dialysate, or solution that exits the probe, is then collected at regular intervals for analysis. The fraction of drug that is recovered in the dialysate is an indirect measurement of the free drug concentration in the interstitium.
A microdialysis catheter (CMA Microdialysis, Solna, Sweden), with a molecular weight cut-off membrane of 20,000 daltons, has received FDA (510k) clearance for intracerebral use in humans. This catheter is smaller in caliber than that typically used for performing ventriculostomies and monitoring intracranial pressure. With the use of image guidance, the catheter can be safely and accurately placed in brain interstitium (BI). A gold filament at the catheter tip is visible on CT scan, enabling confirmation of correct positioning of the catheter with a non-contrast CT scan of the brain. Collection of interstitial fluid samples can be done while patients are awake and mobile.
Clinically, the technique of ICMD has mainly been applied to the study of head trauma (1-4), subarachnoid hemorrhage (5-7), and epilepsy (8). In these settings, microdialysis catheters are typically used to monitor changes in biochemical markers, such as glucose, lactate, and glutamate, in order to evaluate the effects or detect possible complications of a therapeutic intervention. ICMD is also a suitable method for performing neuropharmacokinetic (nPK) studies (9-11) of potential chemotherapy agents for the treatment of brain tumors, because microdialysis catheters can serially sample free drug concentrations in the peritumoral cerebral cortex or within the brain tumor itself (12, 13).
Temozolomide (TMZ) is an orally bioavailable alkylating agent that is converted to its active metabolite 5-(3-methyl triazen-1-yl)imidazole-4-carbozamide (MTIC) in a spontaneous process that does not require hepatic activation. Maximum plasma TMZ concentrations occur within 30-90 mins following an oral dose (14), and drug levels in the cerebrospinal fluid (CSF) have been reported to be approximately 20% of those measured in the systemic circulation (15). In addition to its activity as a single agent, TMZ is a potent radiosensitizer and a key component of chemoradiation therapy for patients with newly-diagnosed glioblastoma (16).
In this pilot feasibility study, we applied ICMD for determining the nPK of TMZ. Although the primary endpoint of this trial was safety and feasibility, a major secondary objective was to assess the difference in both the magnitude and time course of drug levels measured in BI versus systemic circulation.
Materials and Methods
Study Subjects
In order to be eligible to participate in this pilot feasibility study, patients had to be at least 18 years of age and have either a primary or metastatic brain tumor for which TMZ would be an appropriate treatment post-operatively. Patients had to be in need of a surgical debulking or a stereotactic brain biopsy for the purpose of diagnosis or differentiating between tumor progression versus treatment-induced effects following radiation therapy and/or chemotherapy. Other conditions required for study participation included: a) Karnofsky performance status (KPS) ≥ 60%; b) recovery from toxicity of any prior therapy; c) adequate bone marrow function (absolute neutrophil count ≥ 1500 cells/mm3, platelet count ≥ 100,000 cells/mm3); d) adequate hepatic function (total bilirubin ≤ 2.0 mg/dL, serum levels of aspartate aminotransferase ≤ 4 × the institutional upper limit of normal); e) adequate renal function (serum creatinine ≤ 1.5 mg/dL); and f) a Mini Mental Status Exam score ≥ 15. Patients were excluded from participating in this study if they a) were receiving chemotherapy, radiation therapy or were enrolled in another clinical trial; b) were allergic to TMZ; c) were pregnant or breast-feeding; d) had a serious medical or psychiatric illness that could, in the investigator’s opinion, potentially interfere with the completion of treatment according to the protocol. All participating patients gave written informed consent. The clinical protocol and the informed consent document were approved by the City of Hope Institutional Review Board.
Study Design
Eligible brain tumor patients were asked to participate in this non-therapeutic study at the time plans were being made for them to undergo a debulking craniotomy or stereotactic brain biopsy. If the frozen section indicated the presence of viable tumor, the study neurosurgeon inserted a CMA 70 Microdialysis Brain Catheter (membrane length 10 mm; shaft length 100 mm; ref. no. P000050, CMA, Solna, Sweden) into residual tumor or peritumoral BI, within 5 mm of the resection cavity. After a post-operative non-contrast CT scan of the brain confirmed proper placement of the intracerebral catheter, the inlet tubing of the catheter was connected to a portable syringe pump (CMA 107 Microdialysis Pump, ref. no. P000127, CMA, Solna Sweden), which perfused the catheter with artificial CSF (Perfusion Fluid CNS, ref. no. P000151, CMA, Solna, Sweden) at a rate of 1μl/min.
At least 24 hrs after the surgery, when patients were alert and tolerating oral intake, they were given a single dose of TMZ (150 mg/m2) by mouth. To determine the nPK profile of TMZ, dialysate samples were collected continuously during the next 24 hrs. The microvial at the end of the catheter’s outlet tubing was changed to a new one every 30 mins during the first 2 hrs, and then every 60 mins for the remainder of the 24 hr collection period. In order to stabilize TMZ, 6 μL of acetic acid (HOAc) were added to each microvial prior to use. Blood samples for defining the plasma concentration-time profiles of TMZ were obtained prior to the dose of TMZ, then 30 mins, 60 mins, 90 mins, 2, 3, 4, 8, and 24 hrs after the dose of TMZ was taken by the patient. The samples of blood were collected in tubes containing 12 μL of hydrochloric acid (HCl). After all of the dialysate samples were collected, the microdialysis catheter was removed at the bedside, and patients were discharged home when medically ready.
Analytical Method for Determining TMZ Concentrations in Plasma and Dialysate
HPLC grade acetonitrile (ACN) and methanol (MeOH) were purchased from Fisher Scientific (Fair Lawn, NJ, USA). ACS grade formic acid (FA), hydrochloric acid (HCl) and glacial acetic acid (HOAc) were purchased from J.T.Baker (Phillipsburg, NJ, USA). Water was purified using the Millipore Milli-Q system (Milford, MA, USA). Caffeine (trimethyl-13C3, 99%) was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA) and used as an internal standard (IS). TMZ was kindly provided by Schering-Plough (Kenilworth, NJ, USA).
Concentrations of TMZ were determined in plasma and dialysate. For plasma analysis, 50 μl of 2.5M HCl was added per ml of plasma. Standard solutions containing TMZ over the range of 0.1-10 μg/ml were prepared in blank plasma with HCl. High and low controls were prepared at 7μg/ml and 0.2μg/ml respectively. The IS was diluted in MeOH to 2 μg/ml. A 20 μl aliquot of plasma sample, standard, or control was added to 80 μl MeOH with IS and vortex mixed for 10 s then centrifuged at 14K rpm for 5 mins at 4°C. A 20 μl aliquot of the supernatant was diluted with 300 μl 0.5% HOAc. The diluted supernatant was transferred to an assay vial, and 20 μl was injected per assay. For dialysate analysis, 2 μl of 6% HOAc was added per 10 μl fluid. In order to measure concentrations of TMZ over the range of 5-1500 ng/ml, working standard solutions were prepared over the range of 0.5 -150 ng/ml in 0.5% HOAc. High and low control solutions were prepared at 120 ng/ml and 1 ng/ml, respectively. The IS was prepared at a concentration of 500 ng/ml in MeOH / 0.5% HOAc (50:50). A 12 μl aliquot of blank perfusion fluid (or sample) with HOAc was mixed with 10 μl IS solution and 100 μl working standard or control solution (or 0.5% HOAc for sample) and transferred to the assay vial. 20 μl was injected per assay.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis was performed using an Agilent Technologies LC 1100 series system (Palo Alto, CA,USA) interfaced with a Micromass Quattro Ultima Triple Quadrupole Mass Spectrometer (Micromass, Inc., Milford, MA, USA). HPLC separation was achieved using a Prodigy 5 micron 250 × 2.0 mm analytical column (Phenomenex, Torrance, CA USA). The auto-injector temperature was maintained at 5°C and the column temperature at 25°C. An isocratic mobile phase of 13.8% ACN, 0.1% FA in water was used to elute the analytes from the column at a flow rate of 0.2 ml/min. Total run time was 12 mins. The electrospray ionization source of the mass spectrometer was operated in positive ion mode with a cone gas flow of 190 L/hr and a desolvation gas flow of 550 L/hr. The capillary voltage was set to 1 kV. The cone voltage was optimized at 10 V for both TMZ and IS. The collision cell energy was 10 eV for TMZ and 21 eV for IS. The source temperature was 125°C and the desolvation temperature was 350°C. A solvent delay program was used from 0 to 3 mins and from 10 to 12 mins to minimize the mobile phase flow to the source. MassLynx version 4.1 software was used for data acquisition and processing.
Positive electrospray ionization of TMZ and IS produced abundant protonated molecular ions (MH+). The fragmentation of these compounds was induced under collision induced dissociation conditions and acidic mobile phase. The precursor→product ion combinations at m/z 194.89→137.75 for TMZ and 197.98→139.82 for IS were used in multiple reaction monitoring (MRM) mode to determinate these compounds. The use of MRM provided sufficient specificity and sensitivity. LC-MS/MS experimental conditions, such as collision energy and collision cell pressure, were optimized from continuous flow injection sample introduction of standard solutions. Under optimized assay conditions, the retention time was 4.8 mins for TMZ and 8.3 mins for IS.
In Vitro Determination of the Fractional Recovery of TMZ by the CMA 70 Microdialysis Catheter
In preparation for the clinical microdialysis study, an in vitro assessment was done to estimate the recovery of TMZ at a given flow rate and length of catheter. Slower perfusion rates produce a higher fractional recovery (i.e. a concentration of drug in the dialysate that is close to the true interstitial drug concentration), but a longer sample collection interval is needed. The in vitro fractional recovery of TMZ is used as a correction factor for the in vivo results later.
A CMA 70 Microdialysis Brain Catheter was placed into a reservoir containing a 1 μg/ml solution of TMZ in artificial CSF. A CMA 107 Microdialysis Pump perfused the catheter with artificial CSF. Serial dialysate samples were collected at different flow rates (0.5 – 5 μl/min).
Pharmacokinetic Analysis
Pharmacokinetic parameters were determined using the measured plasma and microdialysis TMZ concentration versus time data for each individual using non-compartmental methods. The collection times assigned to the concentration for the vials of dialysate from BI were determined by subtracting half the measurement time plus 5 mins (allowing travel time through the tubing into the vial) from the time of ingestion of TMZ. The maximum concentration (Cmax) and time of maximum concentration (Tmax) were determined directly from the measured data. The area-under-the concentration-time curve (AUC) was determined using the rule of linear trapezoids extrapolated to infinity using the elimination rate constant (Kel) derived from the last 4 measured concentrations. Half-lives (t1/2) were calculated from the elimination rate constant derived from the last 4 measured concentrations.
The primary objective of the study was to determine the feasibility of using the microdialysis technique to assess the distribution of an anticancer drug in the brain. The objective measures of feasibility included incidence rates of clinically symptomatic intracerebral hemorrhage, CNS infection, and catheter malfunction. All toxicities were recorded and summarized by incidence rates, severity, duration, and attribution. The secondary objective of this study was to determine the systemic and intracerebral pharmacokinetic profile of TMZ using a microdialysis catheter.
Results
Feasibility/safety/tolerability
Nine patients were enrolled in this pilot feasibility study from June 2006-August 2007. Table 1 summarizes the patients’ characteristics. The majority of patients had high-grade gliomas. All patients underwent debulking craniotomies. Dexamethasone was administered to every patient peri-operatively.
Table 1.
Patient Characteristics
| Patient | Age | Gender | KPS | Diagnosis |
|---|---|---|---|---|
| 1 | 48 | Female | 100 | Glioblastoma |
| 2 | 71 | Male | 80 | Non-small cell lung cancer |
| 3 | 56 | Male | 90 | Non-small cell lung cancer |
| 4 | 57 | Male | 100 | Glioblastoma |
| 5 | 20 | Male | 80 | Anaplastic Oligodendroglioma |
| 6 | 75 | Female | 60 | Non-small cell lung cancer |
| 7 | 54 | Male | 90 | Glioblastoma |
| 8 | 72 | Male | 90 | Glioblastoma |
| 9 | 56 | Male | 80 | Anaplastic Astrocytoma |
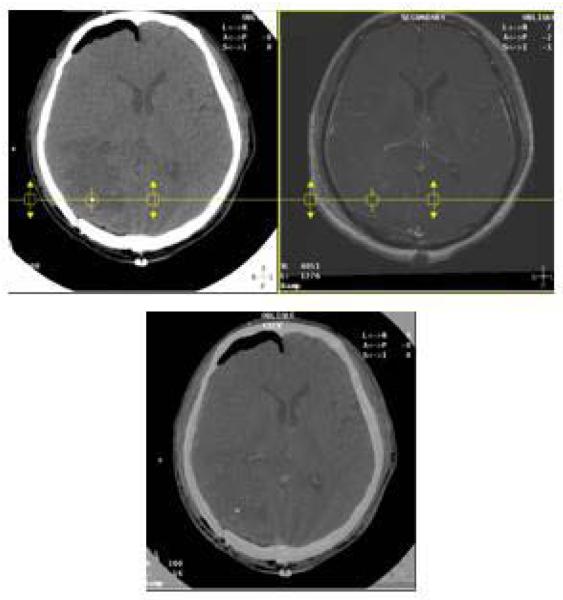
All microdialysis catheters were placed in peri-tumoral BI (non-enhancing brain tissue), as was determined by fusing images from post-operative non-contrast CT scans (where catheter tip is visible) and brain magnetic resonance imaging (MRI), Figure 1.
Figure 1.
Fused images of a non-contrast CT scan and a T1 post-contrast MRI of the brain of a 70 year old man with non-small cell lung cancer are displayed side by side. The blended image is shown at the bottom. Images were auto-registered with mutual information matching algorithm using Varian Eclipse ™ version 8.0 treatment planning system. The patient underwent a craniotomy for gross total resection of a right occipital lobe metastasis. The gold filament at the tip of the microdialysis catheter is visible as bright signal on the CT scan shown inside the circle. Fused corresponding MRI images show that the catheter is placed in non-enhancing brain.
Dialysate samples were obtained from 7 of the 9 patients. One patient declined TMZ after catheter placement. Catheter occlusion occurred in 1 patient, resulting in no collection of dialysate samples for that patient.
All patients tolerated placement of the microdialysis catheter and collection of the dialysate samples well; however, 1 patient subsequently developed several grade 3 and 4 neurologic adverse events whose etiologies remain unclear. This 20 year old man underwent resection of a recurrent right frontal lobe high-grade glioma. He did well in the immediate post-operative period. Administration of TMZ, collection of dialysate samples, and removal of the microdialysis catheter proceeded uneventfully. He was discharged home on post-operative day 3, ambulating and without any neurologic complaints. The next day he began experiencing back pain, nausea and became obtunded. A CT scan of his brain done at an outside hospital showed no hemorrhage or significant edema. He was treated with a single dose of ceftriaxone for presumed meningitis and was transferred to our institution. A lumbar puncture revealed an elevated opening pressure of 55 mmHg. CSF total protein was elevated at 180 mg/dL (nl 15-44); CSF glucose was <10 mg/dL (nl 50-70). The white blood cell count (WBC) in the CSF was 18 /μL, with a differential of 3% segmented neutrophils (segs), 6% lymphocytes (lymphs), and 69% monocytes (monos). The CSF red blood cell count was 10,000 /μL. Gram stains were negative for bacteria and no fungal elements were seen on direct exam. All bacterial, viral and fungal cultures from the CSF were negative. His peripheral complete blood count was within normal range except for an elevated WBC of 14,200, with a differential of 91% segs, 6% lymphs, and 3% monos. The patient was on a tapering dose of dexamethasone, which may explain his elevated WBC.
Mild subarachnoid hemorrhage in the left occipital, left frontal, and right parietal regions was seen on brain MRI. Diffusion-weighted images showed increased signal in the right frontal lobe deep to the post-operative changes, consistent with acute ischemia. An MR angiogram showed no vasospasm or arterial narrowing in the intracranial circulation. MRI of his thoracic and lumbar spine was negative for spinal cord compression or leptomeningeal enhancement. The patient was treated with broad-spectrum antibiotics and acyclovir. Because of persistently elevated intracranial pressure, a lumbar drain was placed for diversion of CSF. When the patient became more alert, it was discovered that he had cortical blindness, cranial neuropathies (left 6th and right 12th nerve palsies), hearing loss, an expressive aphasia, and bilateral lower extremity paralysis. All of his neurologic deficits subsequently improved, but did not resolve completely.
Neuropharmacokinetics of Temozolomide
From the in vitro recovery experiments, it was determined that the highest fractional recovery of TMZ was 87±5.5% and was obtained using a flow rate of 1 μl/min. Therefore, measured TMZ concentrations in the BI were corrected for the fractional recovery determined in vitro. TMZ is a prodrug that spontaneously converts to its active metabolite, MTIC, at physiologic pH. Attempts to recover MTIC by microdialysis were unsuccessful due to instability of the drug in the perfusion solution and possibly due to drug binding to the catheter.
The TMZ plasma and BI pharmacokinetic data for each subject are summarized in Table 2. Peak TMZ concentrations in plasma and brain ECF were: 5.5 ± 3.2 and 0.6 ± 0.3 μg/ml, respectively. Mean Tmax in plasma and brain were 1.8 ± 1.2 and 2.0 ± 0.8 hrs, respectively. TMZ AUCs in plasma and brain ECF were 17.1 ± 6.8 and 2.7 ± 1.0 μg/mLxhr. The ECF/plasma AUC ratio was 17.8 ± 13.3%.
Table 2.
Summary of temozolomide pharmacokinetics in plasma and brain interstitium.
| Plasma TMZ | Brain Interstitial TMZ | Brain/Plasma Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # |
Cmax (ug/ml) |
Tmax (hr) |
Kel (hr-1) |
t1/2 (hr) | AUC 0-inf (ug/mlxhr) |
Cmax (ug/ml) |
Tmax (hr) | Kel (hr-1) |
t1/2 (hr) |
AUC 0-inf (ug/mlxhr) |
Cmax (%) |
AUC 0-inf (%) |
| 1 | 5.2 | 1.0 | 0.32 | 2.2 | 20.9 | 0.9 | 1.2 | 0.33 | 2.1 | 3.4 | 17.0 | 16.4 |
| 2 | 11.8 | 0.5 | 0.47 | 1.5 | 18.9 | 0.2 | 1.7 | 0.11 | 6.3 | 2.9 | 1.9 | 15.5 |
| 3 | 6.4 | 1.0 | 0.41 | 1.7 | 20.6 | 0.4 | 2.4 | 0.21 | 3.3 | 1.7 | 5.8 | 8.1 |
| 4 | 6.8 | 1.5 | 0.43 | 1.6 | 21.0 | 0.6 | 1.2 | 0.21 | 3.3 | 2.2 | 8.5 | 10.6 |
| 5 | 6.5 | 3.0 | 0.41 | 1.7 | 27.0 | 0.4 | 2.4 | 0.34 | 2.0 | 1.5 | 6.4 | 5.7 |
| 7 | 1.4 | 4.0 | 0.14 | 5.0 | 11.4 | 0.3 | 3.4 | 0.39 | 1.8 | 2.6 | 24.6 | 23.3 |
| 8 | 2.5 | 1.5 | 0.46 | 1.5 | 7.8 | --- | --- | --- | --- | --- | --- | --- |
| 9 | 3.3 | 1.5 | 0.42 | 1.7 | 9.5 | 1.1 | 1.4 | 0.4 | 1.7 | 4.3 | 33.2 | 44.9 |
| Avg = | 5.5 | 1.8 | 0.38 | 2.1 | 17.1 | 0.6 | 2.0 | 0.28 | 2.9 | 2.7 | 13.9 | 17.8 |
| SD = | 3.2 | 1.2 | 0.11 | 1.2 | 6.8 | 0.3 | 0.8 | 0.11 | 1.6 | 1.0 | 11.5 | 13.3 |
| %CV = | 59.2 | 66.6 | 28.2 | 56.1 | 39.5 | 56.6 | 42.7 | 38.3 | 55.4 | 36.3 | 82.4 | 74.8 |
AUC 0-inf, area-under-the-concentration-time-curve extrapolated to infinity; Avg, average; %CV, percent coefficient of variation.
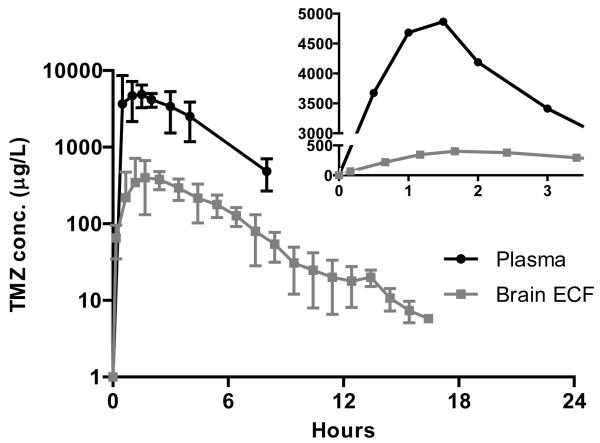
The TMZ concentration versus time data demonstrate that TMZ levels in BI rise more gradually over time and stay elevated slightly longer than those in plasma (Figure 2). This is particularly evident in the inset, which is a plot of the average TMZ concentrations in the BI and plasma during the first 4 hours using a linear rather than a log scale. The linear scaling makes it easier to appreciate the difference in the rate of rise and decline in TMZ levels in plasma and BI between 2-4 hours. Furthermore, the rate of rise in TMZ levels in the BI is more rapid than the rate of fall.
Figure 2.
Concentrations of temozolomide in plasma (triangles) and brain interstitium (squares) obtained by intracerebral microdialysis. The inset panel is an expanded view of the initial 3.5 hr interval following the dose. Symbols represent the means and the bars are the standard deviations.
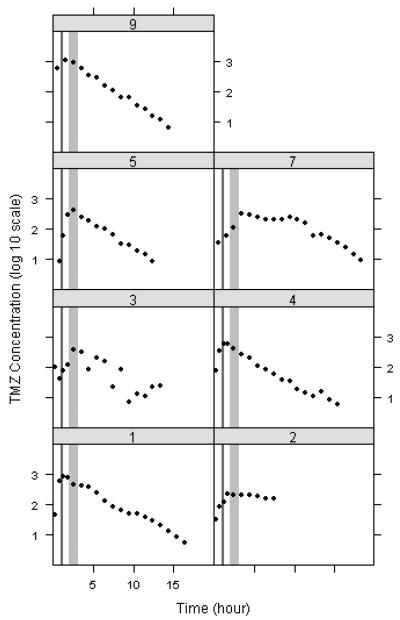
Trellis plots (TMZ concentration vs time) for collected dialysate samples demonstrate that Cmax values occur later than 1 hr (range 1.2 -3.4 hrs) in every subject (Figure 3). The mean Tmax value of 2 hrs is significantly different from the fixed value of 1 hr with a p-value of 0.02 using a two-sided single sample t-test. The light grey bars highlight the portion of the concentration time curve from 2-3 hrs which represents a high TMZ concentration period in every subject.
Figure 3.
Trellis plot of log10 temozolomide concentrations in the brain interstitium as a function of time for each subject (black circles). The dark grey bar represents the 1 hr time point and the light grey bar represents the 2-3 hr time span.
The TMZ concentrations in BI at 1 hr and at Cmax for each individual are compared in Table 3. The Cmax levels are as high or higher than the levels at 1 hr in every patient, with a median fold difference of 1.8 (range 1.0- 7.0).
Table 3.
Comparison of brain temozolomide levels at 1 hour with levels at Cmax.
| Patient # | C 1 hour (μg/ml) |
Cmax (μg/ml) |
Fold difference |
|---|---|---|---|
| 1 | 0.9 | 0.9 | 1 |
| 2 | 0.1 | 0.2 | 1.8 |
| 3 | 0.1 | 0.4 | 4.8 |
| 4 | 0.6 | 0.6 | 1 |
| 5 | 0.1 | 0.4 | 7.0 |
| 7 | 0.1 | 0.3 | 5.3 |
| 8 | --- | --- | --- |
| 9 | 1.1 | 1.1 | 1 |
Discussion
The results of this study show that ICMD is both a safe and feasible technique for measuring concentrations of chemotherapy in the brain. ICMD is a promising research tool that can be incorporated into phase I studies of potential new anti-cancer therapies for the treatment of brain tumors. As described above, 1 study patient did develop multiple severe adverse events approximately 48 hrs after the microdialysis catheter was removed. The cause of his significant neurologic deficits remains unclear. Possible explanations include bacterial or chemical meningitis, or diffuse microvasospasm precipitated by subarachnoid hemorrhage; nonetheless, it is unlikely that the microdialysis catheter was related to either of these potential etiologies. If the patient’s neurologic sequelae were due to infection from the catheter, one would have expected to see enhancement on the MRI along the tract of the catheter. Similarly, if the catheter was the cause of his subarachnoid hemorrhage, one would have expected to see a focus of blood in the region of the catheter tip on the MRI. Neither of these findings was present on any of his serial brain MRIs. Furthermore, ICMD catheters have been safely placed in hundreds of patients with acute brain injuries. There are no reported cases of catheter-associated infections. In the largest published study (17) of ICMD catheter use in neuro-intensive care patients, 4 out of 97 patients (3%) were found to have a small (≤ 1 mL), collection of blood around the catheter on follow-up CT scans. None of these bleeds were clinically significant.
Although an initial goal of the study was to insert microdialysis catheters into residual tumor tissue, we found this task to be more complex for several reasons. First, intraoperative visualization of residual invasive gliomas at the resection margin can be difficult with current surgical techniques. Second, brain shift during and after surgery can significantly diminish the accuracy of the intra-operative navigation system and result in post-operative displacement of the catheter tip. Finally, the length and flexibility of the catheters can limit their precise placement into deeper targets. To more accurately study drug nPK, future ICMD studies could be performed in patients undergoing stereotactic biopsies to improve the precision of catheter placement into tumor tissue.
Although it is desirable to determine drug concentrations in tumor, it is arguably just as important to know whether a drug is penetrating into non-enhancing peritumoral brain tissue, where the advancing edge of the tumor lies, in order to assess whether the drug is reaching areas of growth that “hide behind” intact BBB. Placement of more than 1 microdialysis catheter at the same time is well-tolerated (12, 18-20). While in this pilot feasibility study only 1 catheter was placed per patient, in order to perform more comprehensive nPK studies of potential drugs for the treatment of brain tumors, the design of future protocols could include simultaneous placement of catheters in tumor, peritumoral tissue and/or normal brain.
It is possible that the in vivo recovery of TMZ may be lower than predicted from our in vitro recovery experiments, resulting in an underestimation of the true concentration of TMZ in the brain. Use of in vitro recovery to calibrate the catheter has its limits because it cannot take into account conditions in individual patients that may affect in vivo recovery, such as tortuosity of the tissue and blood flow (21). In vivo calibration using the retrodialysis method is ideally preferable. With retrodialysis, a small amount of the drug is first perfused through the catheter, and the loss of drug is used to determine its relative recovery. After a washout period, the drug is then administered systemically and microdialysis is performed. TMZ is only available in an oral formulation, which is unsterile, making it infeasible to perform retrodialysis for catheter calibration in this study.
The concentrations of TMZ in human brain tissue determined here are similar to reported CNS levels of TMZ in rats using the technique of microdialysis (22). Zhou and colleagues measured TMZ concentrations in the brains of rats via ICMD to gather data for development of a predictive pharmacokinetic model of drug concentrations in tumor. The model derived from their pre-clinical data predicted a mean TMZ brain to plasma AUC ratio in humans of approximately 20%, which corresponds to our findings in patients. Therefore, our data confirm that the physiologically-based predictive model proposed by Zhou and colleagues may indeed be very useful for developing clinical dosing regimens, as well as providing guidance for the appropriateness of testing new agents for the treatment of brain tumors.
More recently, Rosso and colleagues reported their results using a PET imaging-based approach for the prediction of TMZ concentrations in CNS tissues of patients with brain tumors (23). By combining PET imaging data following a single dose of [methyl-11C]-TMZ with individual plasma pharmacokinetic data, the authors were able to develop a mathematical model to predict TMZ concentrations in normal brain and brain tumor over time and non-invasively. Following doses of 75-200 mg/m2, predicted peak TMZ concentrations ranged from 1.8 to 3.7 μg/ml in normal brain, which are 3-6 fold higher than the levels measured using ICMD in the current study. This large difference is primarily due to the difference in the physiologic compartment being sampled. The PET-imaging approach is measuring TMZ both in the vasculature and interstitial spaces, whereas ICMD samples the interstitium only. Indeed, the peak concentrations in the brain reported by Rosso and colleagues are more similar to the plasma concentrations measured in the current study. Therefore, although the ideal approach to studying nPK in patients would be minimally invasive, such as the one proposed by Rosso and colleagues, ICMD still provides important additional information that can be used to further refine the model and allow discrimination between drug concentrations in the blood from those in the tissue microenvironment.
One criticism of the technique of ICMD is that it may produce falsely elevated drug levels due to disruption of the BBB that inevitably occurs to some degree with insertion of the catheter into BI. When performing ICMD studies in patients with high-grade gliomas and brain metastases, where the BBB is already disrupted (as evidenced by the presence of contrast enhancement on brain MRI), this theoretical concern is less of a consideration. Moreover, the TMZ concentrations in brain that we determined with ICMD are similar to levels of TMZ measured in the CSF via lumbar puncture (15), indicating that it is unlikely that ICMD significantly overestimated the concentration of TMZ in BI.
Ultimately, determining absolute drug concentrations in the brain may not be as clinically important as being able to detect changes in intracerebral concentrations of a particular drug when given in combination with another chemotherapy agent or having the ability to know when the peak concentration of a drug occurs in the brain. Taking TMZ as an example, it can act as a radiosensitizer (24-27) and is given concurrently with focal brain RT as part of standard treatment for newly-diagnosed glioblastoma (16). When used as a radiosensitizer, low-dose daily TMZ is typically taken 1 hr prior to RT based on data from phase I studies of TMZ (28,29), which determined that the peak plasma concentration of TMZ occurs 1 hr after oral administration. Our nPK data obtained using the technique of ICMD, show that peak levels of TMZ in BI occur between 1.2 and 3.4 hrs after an oral dose, and the 2- 3 hr period represents a time of high TMZ concentration. Furthermore, TMZ concentrations measured in the BI at 1 hour were as much as 7-fold lower than the maximum levels achieved between 2-3 hrs. These findings suggest that the current recommendation for the schedule of chemoradiation for glioma may be sub-optimal. While the data presented here are hypothesis-generating in nature, and the true optimal schedule for chemoradiation will have to be determined by prospective investigation, the current study demonstrates the utility of ICMD for the assessment of nPK and may lead to further refinement of therapeutic regimens for glioma.
Statement of Translational Relevance.
Microdialysis is a technique for continuously sampling the concentration of a drug or biomolecule in a body tissue without significantly disturbing the function of the tissue. The fraction of drug that is recovered in the dialysate is an indirect measurement of the free drug concentration in the interstitium. We applied intracerebral microdialysis to study the neuropharmacokinetics of temozolomide. Our findings have potential implications for the timing of temozolomide administration relative to daily brain radiation treatment and demonstrate the value of intracerebral microdialysis as a research tool for use in the early development of appropriate new drugs to be tested in brain tumor clinical trials.
Acknowledgments
We would like to thank Ms. Brenda Williams, the research study nurse, Mr. Bixin Xi and Ms. Susan Markel in the Analytical Pharmacology Core Facility for their assistance with the TMZ assays, Ms. Jan Walthers for her assistance with preparing the manuscript, and Ms. Leslie Smith-Powell for her help with the collecting, processing, and tracking of clinical specimens.
Grant support This research was supported in part by a General Clinical Research Center grant awarded to the City of Hope (M01 RR00043), an NIH training grant (K12 CA01727; J. Portnow), and a Cancer Center Support Grant (P30 CA33572).
References
- 1.Goodman JC, Valadka AB, Gopinath SP, Uzura M, Robertson CS. Extracellular lactate and glucose alterations in the brain after head injury measured by microdialysis. Crit Care Med. 1999;27:1965–73. doi: 10.1097/00003246-199909000-00041. [DOI] [PubMed] [Google Scholar]
- 2.Vespa PM, McArthur D, O’Phelan K, et al. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J Cereb Blood Flow Metab. 2003;23:865–77. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- 3.Vespa P, Bergsneider M, Hattori N, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab. 2005;25:763–74. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vespa P, Boonyaputthikul R, McArthur DL, et al. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;34:850–6. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- 5.Staub F, Graf R, Gabel P, Köchling M, Klug N, Heiss WD. Multiple interstitial substances measured by microdialysis in patients with subarachnoid hemorrhage. Neurosurgery. 2000;47:1106–15. doi: 10.1097/00006123-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Sarrafzadeh AS, Sakowitz OW, Kiening KL, Benndorf G, Lanksch WR, Unterberg AW. Bedside microdialysis: a tool to monitor cerebral metabolism in subarachnoid hemorrhage patients? Crit Care Med. 2002;30:1062–70. doi: 10.1097/00003246-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Kett-White R, Hutchinson PJ, Al-Rawi PG, Gupta AK, Pickard JD, Kirkpatrick PJ. Adverse cerebral events detected after subarachnoid hemorrhage using brain oxygen and microdialysis probes. Neurosurgery. 2002;50:1213–21. doi: 10.1097/00006123-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 8.During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–10. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin RK, Hochberg FH, Fox E, et al. Review of microdialysis in brain tumors, from concept to application: first annual Carolyn Frye-Halloran Symposium. Neuro Oncol. 2004;6:65–74. doi: 10.1215/S1152851703000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lange EC, de Boer AG, Breimer DD. Methodological issues in microdialysis sampling for pharmacokinetic studies. Adv Drug Deliv Rev. 2000;45:125–48. doi: 10.1016/s0169-409x(00)00107-1. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Gallo JM. In vivo microdialysis for PK and PD studies of anticancer drugs. AAPS J. 2005;7:E659–67. doi: 10.1208/aapsj070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergenheim AT, Capala J, Roslin M, Henriksson R. Distribution of BPA and metabolic assessment in glioblastoma patients during BNCT treatment: a microdialysis study. J Neurooncol. 2005;71:287–93. doi: 10.1007/s11060-004-1724-0. [DOI] [PubMed] [Google Scholar]
- 13.Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG. Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol. 2009;91:51–8. doi: 10.1007/s11060-008-9678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brada M, Judson I, Beale P, et al. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br J Cancer. 1999;81:1022–30. doi: 10.1038/sj.bjc.6690802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–36. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 16.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 17.Poca MA, Sahuquillo J, Vilalta A, de los Rios J, Robles A, Exposito L. Percutaneous implantation of cerebral microdialysis catheters by twist-drill craniostomy in neurocritical patients: description of the technique and results of a feasibility study in 97 patients. J Neurotrauma. 2006;23:1510–7. doi: 10.1089/neu.2006.23.1510. [DOI] [PubMed] [Google Scholar]
- 18.Roslin M, Henriksson R, Bergstrom P, Ungerstedt U, Bergenheim AT. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol. 2003;61:151–60. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]
- 19.Bergenheim AT, Roslin M, Ungerstedt U, Waldenstrom A, Henriksson R, Ronquist G. Metabolic manipulation of glioblastoma in vivo by retrograde microdialysis of L-2, 4 diaminobutyric acid (DAB) J Neurooncol. 2006;80:285–93. doi: 10.1007/s11060-006-9186-1. [DOI] [PubMed] [Google Scholar]
- 20.Tabatabaei P, Bergstrom P, Henriksson R, Bergenheim AT. Glucose metabolites, glutamate and glycerol in malignant glioma tumours during radiotherapy. J Neurooncol. 2008;90:35–9. doi: 10.1007/s11060-008-9625-2. [DOI] [PubMed] [Google Scholar]
- 21.Chu J, Gallo JM. Application of microdialysis to characterize drug disposition in tumors. Adv Drug Deliv Rev. 2000;45:243–53. doi: 10.1016/s0169-409x(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Q, Guo P, Kruh GD, Vicini P, Wang X, Gallo JM. Predicting human tumor drug concentrations from a preclinical pharmacokinetic model of temozolomide brain disposition. Clin Cancer Res. 2007;13:4271–9. doi: 10.1158/1078-0432.CCR-07-0658. [DOI] [PubMed] [Google Scholar]
- 23.Rosso L, Brock CS, Gallo JM, et al. A new model for prediction of drug distribution in tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients. Cancer Res. 2009;69:120–7. doi: 10.1158/0008-5472.CAN-08-2356. [DOI] [PubMed] [Google Scholar]
- 24.Wedge SR, Porteous JK, Glaser MG, Marcus K, Newlands ES. In vitro evaluation of temozolomide combined with X-irradiation. Anticancer Drugs. 1997;8:92–7. doi: 10.1097/00001813-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Kil WJ, Cerna D, Burgan WE, et al. In vitro and in vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res. 2008;14:931–8. doi: 10.1158/1078-0432.CCR-07-1856. [DOI] [PubMed] [Google Scholar]
- 26.van Rijn J, Heimans JJ, van den Berg J, van der Valk P, Slotman BJ. Survival of human glioma cells treated with various combination of temozolomide and X-rays. Int J Radiat Oncol Biol Phys. 2000;47:779–84. doi: 10.1016/s0360-3016(99)00539-8. [DOI] [PubMed] [Google Scholar]
- 27.van Nifterik KA, van den Berg J, Stalpers LJ, et al. Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys. 2007;69:1246–53. doi: 10.1016/j.ijrobp.2007.07.2366. [DOI] [PubMed] [Google Scholar]
- 28.Dhodapkar M, Rubin J, Reid JM, et al. Phase I trial of temozolomide (NSC 362856) in patients with advanced cancer. Clin Cancer Res. 1997;3:1093–100. [PubMed] [Google Scholar]
- 29.Reid JM, Stevens DC, Rubin J, Ames MM. Pharmacokinetics of 3-methyl-(triazen-1-yl) imidazole-4-carboximide following administration of temozolomide to patients with advanced cancer. Clin Cancer Res. 1997;3:2393–8. [PubMed] [Google Scholar]