Abstract
We sought to examine the effects of microtubule targeting agents (MTAs) on neural cells to better understand the problem of neurotoxicity, their principal side effect, and to possibly develop a model of clinical toxicity. Studies showed that microtubule-depolymerizing agents (MDAs) not only disassembled microtubules in neural HCN2 cells but also led to rapid disappearance of tubulin and that this was specific for MDAs. Tubulin levels fell to 20% as early as 8 hours after adding vincristine and to 1–30% (mean, 9.8 ± 7.6%; median of 7%) after 100 nM VCR for 24 hours. This disappearance was reversible. An increase in both glu-terminated and acetylated tubulin, markers of stable tubulin, preceded re-accumulation of soluble tubulin, suggesting a priority for stabilizing tubulin first as microtubules, prior to replenishing the soluble pool. Similar results were demonstrated with other MDAs. Furthermore, microtubule reassembly did not arise from a central focus but instead appeared to involve dispersed nucleation, as evidenced by the appearance of small stable microtubule stubs throughout the cytoplasm. In contrast, experiments with four non-neural “normal” cell lines and four cancer cell lines resulted in microtubule de-stabilization but only modest tubulin degradation. Evidence for proteasome-mediated degradation was obtained by demonstrating that adding a proteasome inhibitor prior to vincristine prevented tubulin disappearance. In summary, MDAs lead to rapid disappearance of tubulin in neural but not other normal cells or cancer cells. These results underscore the fine control that occurs in neural cells and may further our understanding of neurotoxicity following MDAs.
Keywords: Microtubule targeting agents, neurotoxicity, tubulin, microtubules, microtubule destabilizing agents, vincristine, dolastatin, proteasome, proteasome degradation, MG132, neural cells, HCN2
INTRODUCTION
Microtubules (MTs) are filamentous polymers that along with actin and intermediate filaments (vimentin, lamin, and keratin) comprise the cytoskeleton. MTs are composed of α/β tubulin heterodimers that form linear protofilaments and then arrange in parallel to create cylindrical “tubules”. The continuous equilibrium of MT assembly (growth) and disassembly (shortening) make the MTs dynamic structures that maintain cell shape, polarity and motility, provide a scaffold for cellular trafficking of proteins and organelles, and play an integral role in mitosis. Because MTs and their dynamics are required for mitotic spindle formation and chromosome separation during mitosis, they are an important target for a chemically diverse group of anticancer drugs that induce mitotic arrest and cell death in vitro.
Microtubule targeting agents (MTAs) have been used clinically since the 1960’s as single agents or in combinatorial regimens for the effective treatment of leukemia, lymphoma and various solid tumors. MTAs inhibit cell proliferation by disrupting the dynamics of MT polymerization (1, 2). These mitotic inhibitors include the vinca alkaloids (vincristine, vinblastine, vinorelbine, vinflunine) that destabilize or depolymerize MTs and the taxanes (paclitaxel, docetaxel) and epothilones (ixabepilone) that polymerize MTs thus causing mitotic arrest. While it is often stated that MTAs kill proliferating cancer cells by effecting mitotic arrest that in turn leads to apoptosis (3–6), such a mechanism is unlikely to be important in patients whose tumors divide on average every 30 to 60 days (7, 8). Evidence that such agents can bring about cell death without affecting mitotic arrest includes the primary toxicity of these agents, i.e. the neurotoxicity that inflicts damage on somatic nerve cells that rarely divide (9).
While neurotoxicity is the principal toxic effect observed clinically with MTAs how and why this happens is not well understood (10), although it is thought to be due at least in part to the inhibition of axonal MTs that are necessary for axonal transport in neurons (11). Other than the obvious explanation that neural cells have large amounts of tubulin, studies addressing this phenomenon have been hampered by the lack of suitable in vitro or animal models.
The starting point for this work was a motivation to further understand the effects of MTAs on neural cells to: (1) comprehend what role if any they might have in the phenomenon of chemo-brain (chemotherapy associated cognitive dysfunction presumably due to penetration of the blood brain barrier by anticancer agents); (2) attempt to better understand the problem of neurotoxicity, often a treatment limiting side effect of this class of drugs and (3) possibly develop a model of some relevance to neurotoxicity. In the conduct of these studies we noticed that the microtubule destabilizing agents (MDA) not only led to the dissolution of MTs when administered to neural cells but also to the rapid disappearance of tubulin - an observation we had never made in hundreds of similar experiments with cancer cell lines. Prior studies have described the tight regulation of tubulin in neural cells (12, 13). This regulation is not surprising given that: a) tubulin makes up more than 20% of the soluble protein in brain, and b) the precise polarity of MT can differ in different regions of a single neuron and this polarity is essential for proper intracellular transport and hence for synaptic function. We sought to investigate further our observation of tubulin degradation in neural cells and compare the results with those obtained in cancer cells treated under similar conditions. We describe herein the results of these experiments.
METHODS
Cell Culture and Reagents
HCN2 and HCN1A neural cells and CRL 2127 (skin fibroblasts) were obtained from ATCC (Manassas, VA) and were grown in DMEM supplemented with 10% FBS, 2 mM glutamine, 100 units/ml penicillin and 100 ug/ml streptomycin. MCF 10A (breast epithelial) were grown in DMEM/F12 supplemented with 5% horse serum, 10 ng/ml EGF, 500 ng/ml hydrocortisone, 10 μg/ml insulin and penicillin and streptomycin as described above. HUVEC cells were obtained from Lonza (Walkersville, MD) and grown in endothelial cell growth media (EGM BulletKit, Lonza). Cancer cell lines utilized include MCF-7 (breast), A549 (lung), SY5Y (neuroblastoma and obtained from ATCC), and 1A9 (ovarian, a A2780 subclone) (14) were grown in RPMI and were supplemented as described above. All cells obtained from cell repositories were grown in culture for less than 6 months.
The following chemicals were used in this study: vincristine (VCR), cycloheximide (CHX), doxorubicin (DOX), paclitaxel (PTX), 5-fluorouracil (5-FU), 3-isobutyl-1-methylxanthine (IBMX), 12-O-tetradecanoylphorbol-13-acetate (TPA), nerve growth factor (NGF) and bafilomycin A (Baf) (Sigma, St. Louis, MO), ixabepilone (Bristol Myers Squibb), dolastatin and MG132 (Calbiochem, San Diego, CA) and ammonium chloride (NH4Cl) (Fisher Scientific, Pittsburgh, PA). Reagents were reconstituted and stored according to the manufacturer’s instructions.
MTT Assays
Cells were plated in 96 well plates and treated with increasing concentrations of vincristine. Vincristine IC50 values for each cell line described in Table 1 were determined using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) as per manufacturer’s protocol. Plates were read on a BioRad Imager. (BioRad, Hercules, CA)
Table 1.
Vincristine Cytotoxicity
| Cell Line | IC50 |
|---|---|
| A549 (Lung) | 40nM |
| MCF-7 (Breast) | 5nM |
| 1A9 (Ovarian) | 4nM |
| SY5Y (Neuroblastoma) | 2nM |
| HCN2 | 10μM |
Immunoblots
Cells grown to 70–80% confluency in 12 well plates, were harvested after drug treatment in 100 μl/well of protein lysis buffer containing 1 mM MgCl2, 2 mM EGTA, 0.5% NP40 and 20 mM Tris HCl, pH 6.8 supplemented with protease inhibitors (protease inhibitor cocktail tablets, Roche, Indianapolis, IN) plus 200 units/ml aprotinin (Sigma Aldrich, St. Louis, MO) and 10 μM trichostatin A (Cayman Chemical, Ann Arbor, MI). Protein concentrations were determined using the BioRad Protein Assay Reagent (Hercules, CA) and 20 μg protein was loaded per well onto 10% or 4–15% gradient SDS/PAGE gels. After transfer onto nitrocellulose, blots were blocked in 0.5% milk and each blot was probed with an anti-actin antibody (C4 monoclonal, Chemicon) as an internal control. Other primary antibodies used were anti-p53 (Ab-6 monoclonal Do1, Oncogene Science), anti-acetylated tubulin (clone 6–11B-1, Sigma) and antiα-tubulin (DM1A monoclonal, Sigma). Infrared species-specific secondary antibodies (LiCor, Lincoln, NE) were used and protein expression was quantitated using the Odyssey Infrared Imager (Licor, Lincoln, NE).
Real Time RT PCR
HCN2 cells were treated with 100 nM VCR for 0h, 8h, 24h, or 24h VCR followed by 24h in drug-free medium. RNA was isolated from HCN2 cells at these time points using the Qiagen RNeasy method (Qiagen, Valencia, CA). One microgram of RNA was reverse transcribed. Real Time PCR was performed on cDNA template using Taqman Master UPL kit (Roche, Indianapolis, IN) and the following primers:
α-Tub 5′: CCTTCGCCTCCTAATCCCTA
α-Tub 3′: AGCAGGCATTGCCAATCT
28s 5′: AAACTCGGTGGAGGTCCGT
28s 3′: CTTACCAAAAGTGGCCCACTA.
Tubulin levels were normalized to 28s RNA and compared to untreated HCN2 control RNA.
Immunofluorescence and confocal microscopy
Cells plated on glass coverslips in 24 well dishes were incubated either with or without vincristine for 24 hours, rinsed in PBS twice, and either fixed immediately in 100% methanol at −20°C for 10 minutes (15) or incubated in media without drug for washout [periods of 20 minutes, 1, 4, 8, or 24 hours] prior to fixation. Following fixation, all coverslips were rinsed in PBS and incubated for 45 minutes in 20% goat serum in PBS, followed by incubation in either mouse monoclonal anti α-tubulin (DM1A) or mouse anti acetylated α-tubulin (Sigma, St. Louis, MO), and rabbit anti pericentrin (ABCAM, Cambridge, MA). Following rinses in PBS after each primary antibody incubation, the coverlsips were incubated in either fluorescein (FITC)-conjugated goat anti mouse IgG or rhodamine (RHOD)-conjugated donkey anti rabbit secondary IgG, (both from Jackson Immuno Research Labs, Inc., West Grove, PA). Dapi (Sigman, St. Louis, MO) was used to counterstain nuclei. Antibody control cells were either incubated without any primary or secondary antibodies, or with only nonspecific primary IgG, or with only secondary antibodies. Stained cells were visualized on a Zeiss Axiovert 100 M microscope equipped with a Plan-NeoFluar 100×/1.3 oil immersion objective and confocal images were generated using a Zeiss LSM510 META. Images in Results are shown as 3-dimensional maximal projections reconstructed from Z-stacks.
RESULTS
In an attempt to better understand how MTAs affect neural cells we began exploratory studies using a neural cell line (HCN2) established from normal neural cells derived from the cerebral cortical tissue of a patient with Rasmussen’s encephalitis (16). While we recognize the limitations of such in vitro studies, we felt that given the lack of suitable models, these cells at a minimum presented us a starting point.
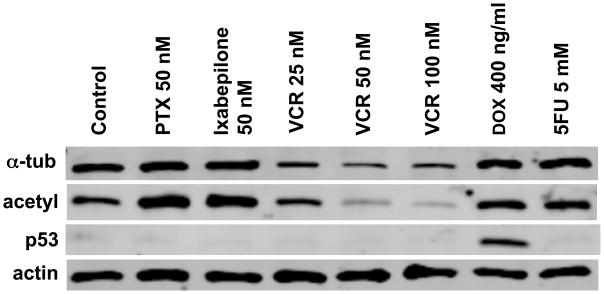
Figure 1 demonstrates results from one of the initial experiments performed to assess the response of the neural cell line, HCN2, to several chemotherapeutic agents. An increase in p53 levels was seen in response to the DNA damaging agent doxorubicin (Dox 400 ng/ml) when HCN2 cells were treated for 24 hours, an observation that was expected in these cells with wild type p53. Consistent with our experience in other models, treatment with paclitaxel (PTX 50 nM) or ixabepilone (50 nM) for 24 hours stabilized MTs as shown by increased levels of the acetylated form of alpha tubulin (acetyl tubulin) a marker of stable MTs (similar results were observed with an antibody that detects the de-tyrosinated form of alpha tubulin, not shown). We would note here that the relative increases observed in these cells that under normal circumstances have a very stable MT network is not as pronounced as seen with cancer cell lines. As expected, overnight treatment of HCN2 cells with VCR destabilized MTs and lowered acetyl tubulin levels (and de-tyrosinated tubulin not shown). Surprisingly, however, overnight treatment with vincristine (25, 50, or 100 nM) also resulted in a marked reduction of tubulin levels as measured with several alpha tubulin antibodies. The DNA damaging agent, 5 Fluorouracil (5FU 5mM) had no effect on tubulin levels or stability.
Figure 1. Tubulin expression in HCN2 cells following drug treatment.
HCN2 cells were treated for 24h with paclitaxel (PTX, 50 nM), ixabepilone (50 nM), vincristine (VCR 25 nM, 50 nM and 100 nM), doxorubicin (DOX 400 ng/ml) and 5-fluorourocil (5-FU 5 mM). Immunoblots were hybridized with anti-tubulin (total α-tubulin and acetylated α-tubulin) and anti-p53 antibodies. Actin was used as a loading control.
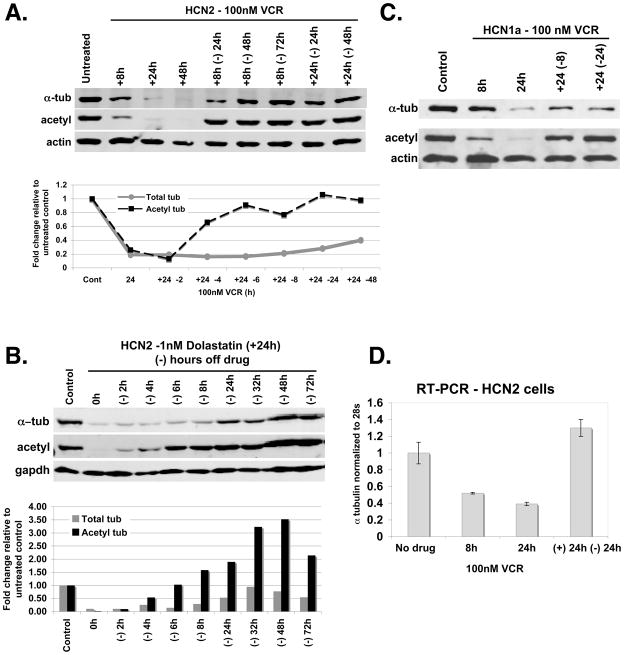
While we anticipated that treatment with vincristine would destabilize MTs, the fall in total tubulin levels was unexpected and not previously noted in our work with numerous cancer cell lines. Figure 2A shows a representative immunoblot of HCN2 cells treated with 100 nM vincristine (IC50 ≈ 11μM) from 8 to 48 hours. Tubulin levels fall to 30% of their starting levels as early as eight hours after the addition of vincristine to the culture medium, a decrease that was detectable after 4 hours in other experiments. A further decrease in tubulin levels occurs by 24 hours and tubulin is undetectable by 48 hours. As the figure shows, the vincristine induced tubulin degradation is reversible. An increase in acetylated tubulin, a marker of stable MTs, preceded the increase in total tubulin, suggesting a priority for incorporating any remaining tubulin as well as newly synthesized tubulin into stable MTs, followed subsequently by a replenishment of the soluble tubulin pool. This is seen when after 8 or 24 hours of drug treatment, vincristine is removed from the medium. In fact, the results of a separate experiment tabulated in the graph in Fig. 2A shows that acetyl tubulin levels increase as early as 4 hours after the removal of VCR from the culture media. We would emphasize at this point that the marked reductions in tubulin levels observed in these experiments were detected with four different antibodies, making it unlikely these results could be explained by the loss or masking of an epitope rather than an actual reduction in tubulin levels. While tubulin levels varied slightly, in 18 successive experiments, total tubulin levels were reduced to an average of averaged 9.8% ± 7.6% that of the starting level (median of 7%) after treating HCN2 cells with 100 nM vincristine for 24 hours.
Figure 2. Tubulin levels fall dramatically in neural cells after treatment with MDAs (VCR and dolastatin), but recover in the absence of drug.
2A: Immunoblot of tubulin levels in HCN2 cells treated with 100 nM VCR (+8h, +24h, and+ 48h) and when drug was removed for 24 to 72 hrs (−24 from the culture media following 8h of VCR (+8h −24h, +8h, −24h, +8h −72h) and 24h (+24h −24h, +48h −48h) of VCR treatment. A graph of tubulin levels tabulated from a separate experiment shows the reappearance of acetyl tubulin, a marker of stable microtubules, as early as 4h following withdrawal of drug after 24h of VCR treatment. Tubulin levels were normalized to actin and compared to untreated HCN2 cells. 2B: Immunoblot of HCN2 probed with tubulin and actin antibodies after treatment with 1 nM dolastatin for 24h followed by removal of drug for the time points indicated. Results are tabulated. 2C: Immunoblot showing tubulin expression in HCN1a cells treated with 100 nM VCR for 8h and 24h, and following drug removal for 8h (+24 −8) and 24h (+24 −24). 2D: Tubulin RNA expression in untreated HCN2 cells compared to cells treated with 100 nM VCR for 8h and 24h and following drug removal after 24h VCR treatment (+24h −24h). Experiments were done in triplicate to obtain error bars.
That the effect seen with vincristine was not drug specific but rather a consequence of MT destabilization was confirmed by demonstrating similar results with the MT destabilizing agent dolastatin. To ensure that the tubulin recovery seen when vincristine was withdrawn from the culture media was not due to a wash out effect of vincristine, dolastatin, a drug that has sustained intracellular retention due to its high affinity for tubulin was chosen and these results are shown in Figures 2B. HCN2 cells were treated for 24h with 1 nM dolastatin (IC50 ≈ 0.5 nM) and after drug removal the effect on total and acetyl tubulin was assessed at various time points. A representative immunoblot shown in Figure 2B shows the nearly total disappearance of total tubulin after 24 hours of dolastatin treatment (0 hours off drug). Similar to the results observed after treatment with vincristine, tubulin loss is reversible and again the increase in acetylated tubulin occurs sooner than the increase in total tubulin, consistent with a preferential incorporation of newly synthesized tubulin into stabilized MTs. However, the results tabulated in Figure 2B demonstrate an increase that far exceeds the starting level, an observation that became clear when the cells were examined by immunofluorescence (see below). Although dolastatin uptake is considered irreversible, the time course of the recovery was similar to that following vincristine treatment and is consistent with the synthesis of new tubulin, which in the absence of free dolastatin is then preferentially incorporated into MTs. We also observed similar results with combretastatin, another depolymerizing agent that binds not the vinca but the colchicine site of tubulin – establishing not drug binding but MT depolymerization as the essential event in tubulin degradation (data not shown). In addition, we observed the destabilization of MTs and the disappearance of total tubulin with VCR treatment in HCN1a cells, a second neural cell line (16) (Fig. 2C). Like in the HCN2 cells, acetylated tubulin and total alpha tubulin levels were reconstituted after drug removal in the HCN1 cells. Finally, we examined tubulin gene expression in the HCN2 cells in the presence and absence of VCR. The graph in Fig 2D shows tubulin RNA levels normalized to the control gene 28S. RNA levels only decreased to 52% at 8h and 39% by 24h with vincristine treatment indicating factors other than reduced transcription were responsible for the marked decrease in tubulin protein levels to less than 10% of control (Figure 2A). As expected, mRNA levels then increase when drug is removed, likely as a result of reduced tubulin protein levels and the absence of a repressive feedback effect on transcription. (17)
Although the HCN2 cells were isolated from human cerebral cortex, to confirm that they had maintained their neural properties in culture we differentiated them as previously described (16) and observed neurite extensions within 4 hours of drug exposure (data not shown). Supplementary Figures 1A–1B show phase microscopy of neurite production and increased syaptophysin levels respectively in HCN2 cells after differentiation for 48h. However, total tubulin levels were still markedly decreased after 8h and 24h treatment with 100nM VCR (Supplementary Figure 1C).
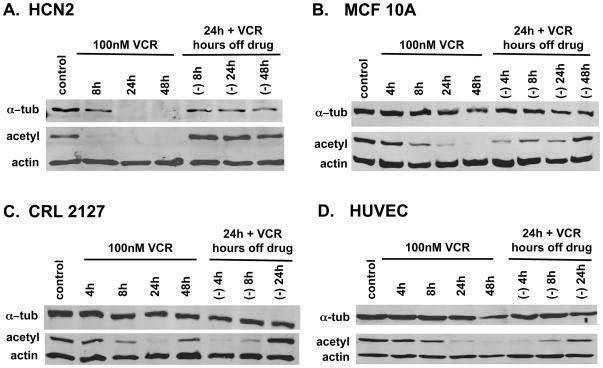
To establish that the extent of tubulin degradation seen in HCN2 cells exceeded that in other “normal” cells, we compared the results in HCN2 cells with additional “normal” cell lines as shown in Figure 3. Relative to HCN2 cells (3A), when 100 nM vincristine was added to MCF10A (breast), CRL2127 (fibroblast), and HUVEC (umbilical endothelium) cells (3B–3D), only minimal α-tubulin degradation occurred despite clear evidence of MT destabilization as demonstrated by the marked decrease in acetylated tubulin. MT destabilization is reversible in the absence of VCR in the non-neural normal cell lines although recovery occurs at a later time point (24h to 48h) than in the neural cells (8h).
Figure 3. Compared to HCN2 cells a decrease in total α-tubulin levels in “normal” cells is less pronounced despite MT destabilization.
Immunoblots of tubulin protein levels in 3A: HCN2 cells and 3 normal epithelial cell lines 3B: MCF10A, breast 3C: CRL 2127, fibroblast and 3D: HUVEC, endothelial cells after treatment with 100 nM VCR.
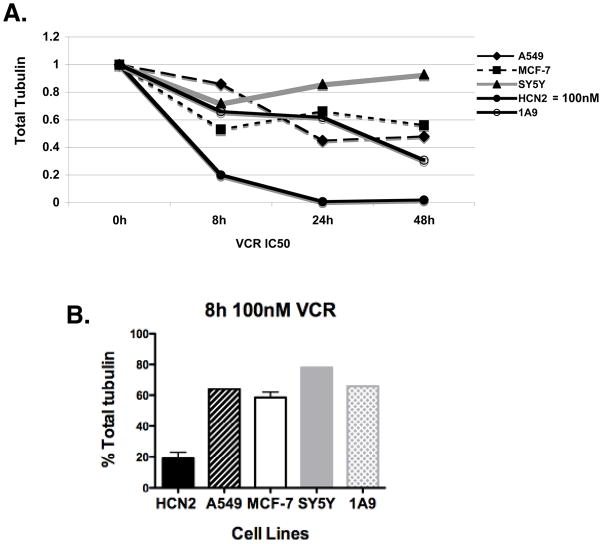
We considered the effect on the HCN2 cells unique having never observed such results when working with numerous cancer cell lines in the laboratory. However, to exclude the possibility that we had missed such an effect we performed similar experiments with cancer cell lines representing four different tissues of origin: A549 non-small cell lung cancer cells, A2780 (1A9) ovarian carcinoma cells (14), MCF7 breast cancer cells and SY5Y neuroblastoma cells. As shown in Supplementary Table 1 in a 4-day cytotoxicity experiment the IC50 for the HCN2 neural cells was found to be 11 μM – far in excess of the 100 nM maximum used in these experiments – while that of the cancer cell lines was 1.6 nM to 40 nM. When the stability of tubulin was examined using vincristine concentrations equal to the IC50 values, large differences were noted between the extent of tubulin disappearance in the cancer cell lines, compared to the HCN2 cells (Figure 4A). Only at higher concentrations that exceeded the IC50 was degradation observed in the cancer cell lines, but at these concentrations this was accompanied by cell death and the associated protein degradation. A comparison at 100 nM vincristine was only possible at 8 hours before the onset of widespread cell death, and as shown in the graph, demonstrate preferential degradation in the HCN2 neural cells (Figure 4B).
Figure 4. VCR treatment decreases tubulin levels in cancer cell lines but not to the levels seen in neural cells.
4A: Cancer cells (A549, MCF-7, SY5Y and 1A9) were treated with IC50 concentrations of VCR (Supplementary Table 1) for 8h, 24h and 48h compared to 100 nM VCR treatment in HCN2 cells. Total α-tubulin levels in cancer cells compared to HCN2 cells are shown. 4B: Total α-tubulin levels in cancer cells are plotted and compared to HCN2 cells when treated for 8h with 100 nM VCR.
Because in HCN2 cells tubulin degradation occurred in the absence of cytotoxicity and was reversible we assumed that new protein synthesis must have been occurring. Support for this assertion is found in Figure 5. As seen in Figure 5A even after 48 h inhibition of protein synthesis with CHX (10 μg/ml) very little turnover of tubulin had occurred. This underscores active tubulin degradation as the process behind the fall in tubulin levels following exposure to a MDA. In addition, following vincristine withdrawal in the presence of the protein synthesis inhibitor cycloheximide, total tubulin levels do not recover, but there occurs a recovery of “stable” tubulin levels as seen by the increase in acetylated tubulin shown in Figures 6B demonstrating stabilization of the remaining tubulin.
Figure 5. Decreased tubulin levels in HCN2 cells after vincristine treatment is not due to increased protein degradation of soluble tubulin when protein synthesis is inhibited but is partially mediated via the proteosome.
5A: HCN2 cells were treated with 100 nM VCR, 10μg/ml cycloheximide (CHX) or in combination (100 nM VCR + 10 μg/ml CHX) for 8h, 24h, and 48h. 5B: Immunoblot of HCN2 cells treated with 100 nM VCR, 10 μg/ml CHX, or in combination for 24 h after which the VCR was withdrawn (−4h, −8h, −24h, −48, −72h) but 10μg/ml CHX was maintained in the media. Blots were hybridized withα-tubulin, acetyl-α-tubulin and actin antibodies. Results are tabulated. 5C: Immunoblot of HCN2 cells were pretreated with lysosome inhibitors – Baf 10 nM (bafilomycin) and 10 mM NH4Cl, for 24h followed by the addition of 100 nM VCR for 24h in the presence of the inhibitor compared to 48h of lysosome inhibitors alone, untreated HCN2 cells and HCN2 cells treated for 24h with 100nM VCR. 5D: Immunoblot of HCN2 cells treated with either 100 nM VCR alone, MG 132 (50 μM) alone or in combination (VCR + MG132) for 8h or 24h. A 2h pretreatment with MG132 preceded the drug treatment in combination (VCR + MG132). Results are tabulated.
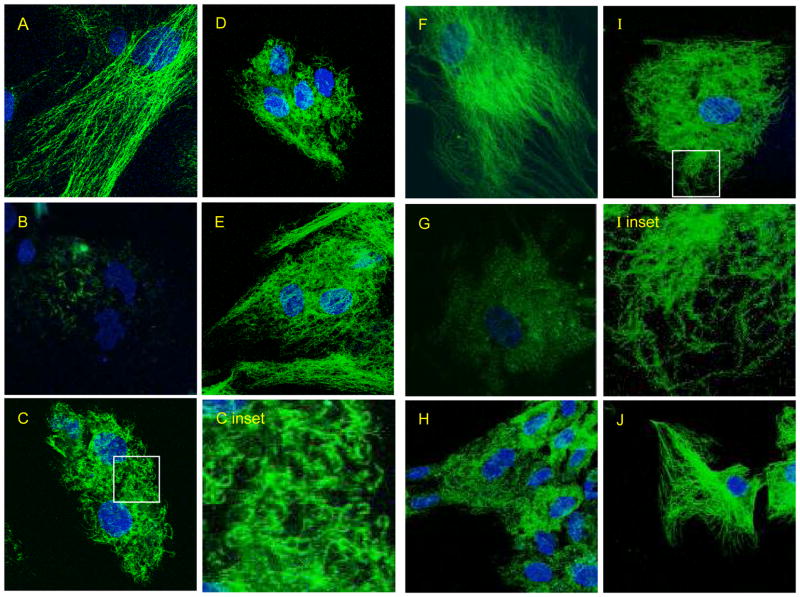
Figure 6. Confocal microscopy of acetylated tubulin (6A–6E) and total alpha tubulin (6F–6J) levels in HCN2 cells treated for 24 h with 100 nM VCR and following drug removal.
6A and 6F: Untreated control 6B and 6G: 24h VCR 6C and 6H: 24h VCR followed by 4h washout of VCR 6D and 6I: 8h washout 6E and 6J: 24h washout.
Degradation via the lysosomal or proteasomal pathways seemed logical starting points as possible mechanisms of active tubulin degradation. To that end we sought to determine the extent to which inhibition of these pathways would impact the disappearance of tubulin. We investigated the lysosomal pathway by pre-treating the HCN2 cells with the lysosome inhibitors bafilomycin (10 nM) and ammonium chloride (10 mM) for 24h followed by 100 nM VCR for 24h in the presence of the inhibitors. The results in Figure 5C show that the inhibitors did not prevent the VCR induced α-tubulin degradation. We concluded that degradation via the lysosome was not a major contributor to the rapid disappearance of α-tubulin.
However, pre-treatment of cells with the proteasome inhibitor MG 132 (50 μM) for 2h prior to the addition of 100 nM VCR, as shown in Figure 5D, prevented the disappearance of tubulin at 8 hours (MG 132 + VCR) and to a lesser extent after 24 hours when compared to 8h or 24h VCR alone. While this is consistent with proteasome-mediated degradation of tubulin, we cannot conclude that proteasome degradation is the only mechanism for the MG132 effect, since as we have shown previously, and confirm here, inhibition of the proteasome stabilizes MTs (18). This is shown clearly by the increase in stabilized (acetylated) tubulin seen with the addition of the proteasome inhibitor alone. But this also shows that VCR alone works in the presence of MG132. Because of this one cannot exclude the possibility that to some extent, the stabilization that occurs as a result of proteasome inhibition is in part mediated by the stabilization effect preventing tubulin degradation. That the protection seen after 24 hours is not as marked as that seen at eight hours in part reflects cellular toxicity. Nonetheless the loss of acetylated tubulin when cells were treated with VCR + MG132 indicates that the MTs still depolymerized but the tubulin was not degraded, consistent with MG132 inhibition of the proteosome.
The immunoblots indicated that during the initial recovery of HCN2 cells from MDA exposure, most tubulin was incorporated into stable MTs, but we often saw what appeared to be an excessive amount of compensation, resulting in acetylated tubulin levels higher than drug-free controls. Thus we sought to investigate this further by performing immunofluorescence experiments on HCN2 cells using acetylated tubulin (Fig 6A–E) and total α-tubulin antibodies (Fig. 6F–7J) in the presence or absence of 100 nM VCR. In Fig. 6A and 6F, untreated HCN2 cells display long MTs that extend throughout the cell and disappear after a 24h treatment with VCR (panel 6B and 6G). Four hours after drug is removed short irregular MTs appear throughout the cell (panel 6C, 6C inset and 6H). In the untreated control cells the MTs are not organized in a radial array with a defined centrosomal focus. Following drug exposure, most tubulin staining is lost, though a few short Ac tubulin (+) stubs are seen (6B). During recovery from drug, multiple short, Ac tubulin (+) MTs form through out the cell. MT formation does not arise from the centrosome since pericentrin staining showed two centrioles in the majority of cells (Supplemental Figure 2). Eight hours after drug removal (panel 6D, 6I and 6I inset), MTs are found near the periphery of the cell and by 24h (panel 6E and 6J)) these short MTs are replaced by a MT network that can be considered more normal in appearance. Thus for a time of recovery after drug exposure, only short MTs comprise the MT network.
DISCUSSION
First introduced into clinical oncology in the 1960’s, microtubule-targeting agents (MTAs) are essential components in the therapy of many cancers including lymphoma as well as breast, ovarian, lung and head and neck cancers (19). In cancer cells the focus has often been on their ability to interfere with mitosis, a thesis developed with rapidly proliferating in vitro models that has never been proven in patients(20). However, because somatic neurons do not divide, a different explanation must be considered to explain the most common side effect, neurotoxicity (21). The latter has largely centered on the likelihood that microtubule (MT) trafficking -especially crucial in neurons - is adversely impacted. Clinical support for this thesis includes the fact that neuropathy usually begins in the hands and feet, consistent with a greater impact on longer neurons that are more likely to be affected by interference with trafficking (10). In the present study we show that compared to cancer cells, neurons respond differently to MT disruption by rapidly degrading tubulin. While we speculate on the importance this might have in terms of neurotoxicity, the results more directly highlight the delicate balance that exists in neurons with regards to handling tubulin.
Our observations are consistent with previous reports documenting precise regulation of tubulin metabolism in neural cells (13). In this regard we would note that consistent with the importance of MTs in neural cells, the results demonstrate the priority given to assembling stable MTs as recovery occurred, indicated by the increased acetylated tubulin staining, followed later by an increase in the soluble tubulin. However, while previous reports highlighted centrosomal nucleation and release (22), we found that MT reassembly appeared to involve a mechanism of dispersed nucleation, as small stable MT stubs appeared throughout the cytoplasm, not from a central focus. It should be noted that the MT arrays in the control HCN2 cells do not radiate from a clear center. Indeed, staining for pericentrin demonstrates that the MT arrays are not organized around the centrosomes. Therefore the apparently dispersed nucleation observed in drug washout is not surprising. Non-centrosomal MT arrays have been reported in a number of differentiated mammalian cell types (23). A number of different mechanisms have been reported for generation of these non-centrosomal MTs, including nucleation and release from the centrosome as well as dispersed nucleation from membrane sites or MT oligomers. It has also been suggested that spontaneous dispersed nucleation can occur in cells in which the tubulin has all been depolymerized, resulting in a high concentration of tubulin dimers. This is clearly not the case here, since the depolymerized tubulin is degraded, resulting in a low level of total cellular tubulin. Hence recovery from drug exposure starts with a low, not a high concentration of dimeric tubulin.
Cellular control of expression level of tubulin has been studied for many years, but most research has been directed at transcriptional and especially translational control of synthesis, in particular autoregulation of tubulin mRNA stability by the level of unpolymerized dimers. Much less is known about regulation of the degradation of this very stable protein, whose half-life can exceed the doubling time of the cells in which it occurs (control of tubulin expression is reviewed in (24) ). Some reports have addressed the degradation pathways of tubulin, and documented that degradation can be enhanced by drugs, including traditional anti-microtubule agents in normal cells (25), isothiocyanates in some cancer cell lines (26, 27), and by PPAR gamma inhibitors (28, 29). Our study is the first report documenting this effect in neural cells, demonstrating that traditional microtubule depolyerizing agents that are in clinical use can produce significant and rapid loss of tubulin.
While we cannot say with certainty that these results have clinical relevance, there is reason to suspect that they may. The concentrations used in the experiments with the HCN2 cells – 100 nM – are concentrations that can be achieved in patients receiving microtubule-targeting agents (MTAs) (30, 31). And while these concentrations are maintained transiently, and clinically one would expect a recovery of tubulin levels and MT stabilization as we observed in our in vitro models, it is not unreasonable to assume that recurrent events of MT depolymerization and tubulin degradation might take its toll and lead to a worsening of neurotoxicity over time (32). It is also not unreasonable to think that this might explain the often noted observation of transient neurotoxicity where symptoms occur after drug administration, but last only a few days and are followed by recovery (33). Previous reports have demonstrated the neurotoxic effect of epothilone B on dorsal root ganglia and sciatic nerves in Wistar and Fischer rats citing both neurophysical and neuropathologic changes. In addition, increased polymerized tubulin levels were observed in sciatic nerve lysates after treatment with epothilone B as compared to untreated control animals (34). This suggests that examination of the effects of MDAs in an animal model may be possible. Future studies will be directed towards the in vivo effects of VCR in murine models to look for tubulin degradation following drug treatment and the in vitro effects of VCR exposure in differentiated human neural stem cells to support the model of neurotoxicity proposed herein.
In summary, we report rapid degradation of tubulin in normal neural cells following the administration of a MDA. Tubulin levels were markedly reduced by 24 hours following drug treatment and by 48 hours had resulted in the near-complete disappearance of tubulin. Tubulin degradation was also rapidly reversible when the drug was withdrawn. This rapid degradation and recovery appears to be a property that is much more developed in normal neural cells. The latter is not unexpected given that, in neural cells, this protein is not only abundant but also crucial in neural trafficking.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892.
References
- 1.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Jordan MA, Thrower D, Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991;51:2212–22. [PubMed] [Google Scholar]
- 3.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–86. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 4.Kelling J, Sullivan K, Wilson L, Jordan MA. Suppression of centromere dynamics by Taxol in living osteosarcoma cells. Cancer Res. 2003;63:2794–801. [PubMed] [Google Scholar]
- 5.Okouneva T, Hill BT, Wilson L, Jordan MA. The effects of vinflunine, vinorelbine, and vinblastine on centromere dynamics. Mol Cancer Ther. 2003;2:427–36. [PubMed] [Google Scholar]
- 6.Pasquier E, Kavallaris M. Microtubules: a dynamic target in cancer therapy. IUBMB Life. 2008;60:165–70. doi: 10.1002/iub.25. [DOI] [PubMed] [Google Scholar]
- 7.Spratt JS, Meyer JS, Spratt JA. Rates of growth of human neoplasms: Part II. J Surg Oncol. 1996;61:68–83. doi: 10.1002/1096-9098(199601)61:1<68::aid-jso2930610102>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Arai T, Kuroishi T, Saito Y, Kurita Y, Naruke T, Kaneko M. Tumor doubling time and prognosis in lung cancer patients: evaluation from chest films and clinical follow-up study. Japanese Lung Cancer Screening Research Group. Jpn J Clin Oncol. 1994;24:199–204. [PubMed] [Google Scholar]
- 9.Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci. 2007;8:368–78. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- 10.Canta A, Chiorazzi A, Cavaletti G. Tubulin: a target for antineoplastic drugs into the cancer cells but also in the peripheral nervous system. Curr Med Chem. 2009;16:1315–24. doi: 10.2174/092986709787846488. [DOI] [PubMed] [Google Scholar]
- 11.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 12.Laferriere NB, MacRae TH, Brown DL. Tubulin synthesis and assembly in differentiating neurons. Biochem Cell Biol. 1997;75:103–17. [PubMed] [Google Scholar]
- 13.Baas PW. Microtubules and axonal growth. Curr Opin Cell Biol. 1997;9:29–36. doi: 10.1016/s0955-0674(97)80148-2. [DOI] [PubMed] [Google Scholar]
- 14.Giannakakou P, Sackett DL, Kang YK, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–25. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 15.Poruchynsky MS, Giannakakou P, Ward Y, et al. Accompanying protein alterations in malignant cells with a microtubule-polymerizing drug-resistance phenotype and a primary resistance mechanism. Biochem Pharmacol. 2001;62:1469–80. doi: 10.1016/s0006-2952(01)00804-8. [DOI] [PubMed] [Google Scholar]
- 16.Ronnett GV, Hester LD, Nye JS, Snyder SH. Human cerebral cortical cell lines from patients with unilateral megalencephaly and Rasmussen’s encephalitis. Neuroscience. 1994;63:1081–99. doi: 10.1016/0306-4522(94)90574-6. [DOI] [PubMed] [Google Scholar]
- 17.Schedl T, Burland TG, Gull K, Dove WF. Cell cycle regulation of tubulin RNA level, tubulin protein synthesis, and assembly of microtubules in Physarum. J Cell Biol. 1984;99:155–65. doi: 10.1083/jcb.99.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poruchynsky MS, Sackett DL, Robey RW, Ward Y, Annunziata C, Fojo T. Proteasome inhibitors increase tubulin polymerization and stabilization in tissue culture cells: a possible mechanism contributing to peripheral neuropathy and cellular toxicity following proteasome inhibition. Cell Cycle. 2008;7:940–9. doi: 10.4161/cc.7.7.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan MA, Kamath K. How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets. 2007;7:730–42. doi: 10.2174/156800907783220417. [DOI] [PubMed] [Google Scholar]
- 20.Esteve MA, Carre M, Braguer D. Microtubules in apoptosis induction: are they necessary? Curr Cancer Drug Targets. 2007;7:713–29. doi: 10.2174/156800907783220480. [DOI] [PubMed] [Google Scholar]
- 21.McLeod JG, Penny R. Vincristine neuropathy: an electrophysiological and histological study. J Neurol Neurosurg Psychiatry. 1969;32:297–304. doi: 10.1136/jnnp.32.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu W, Centonze VE, Ahmad FJ, Baas PW. Microtubule nucleation and release from the neuronal centrosome. J Cell Biol. 1993;122:349–59. doi: 10.1083/jcb.122.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartolini F, Gundersen GG. Generation of noncentrosomal microtubule arrays. J Cell Sci. 2006;119:4155–63. doi: 10.1242/jcs.03227. [DOI] [PubMed] [Google Scholar]
- 24.Lundin VF, Leroux MR, Stirling PC. Quality control of cytoskeletal proteins and human disease. Trends Biochem Sci. 2010;284:17039–51. doi: 10.1016/j.tibs.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Caron JM, Jones AL, Kirschner MW. Autoregulation of tubulin synthesis in hepatocytes and fibroblasts. J Cell Biol. 1985;101:1763–72. doi: 10.1083/jcb.101.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mi L, Gan N, Cheema A, et al. Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes. J Biol Chem. 2009;284:17039–51. doi: 10.1074/jbc.M901789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin P, Kawamura T, He M, Vanaja DK, Young CY. Phenethyl isothiocyanate induces cell cycle arrest and reduction of alpha- and beta-tubulin isotypes in human prostate cancer cells. Cell Biol Int. 2009;33:57–64. doi: 10.1016/j.cellbi.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaefer KL. PPAR-gamma inhibitors as novel tubulin-targeting agents. Expert Opin Investig Drugs. 2007;16:923–6. doi: 10.1517/13543784.16.7.923. [DOI] [PubMed] [Google Scholar]
- 29.Harris G, Schaefer KL. The microtubule-targeting agent T0070907 induces proteasomal degradation of tubulin. Biochem Biophys Res Commun. 2009;388:345–9. doi: 10.1016/j.bbrc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Kellie SJ, Barbaric D, Koopmans P, Earl J, Carr DJ, de Graaf SS. Cerebrospinal fluid concentrations of vincristine after bolus intravenous dosing: a surrogate marker of brain penetration. Cancer. 2002;94:1815–20. doi: 10.1002/cncr.10397. [DOI] [PubMed] [Google Scholar]
- 31.de Graaf SS, Bloemhof H, Vendrig DE, Uges DR. Vincristine disposition in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 1995;24:235–40. doi: 10.1002/mpo.2950240405. [DOI] [PubMed] [Google Scholar]
- 32.Carbone PP, Bono V, Frei E, 3rd, Brindley CO. Clinical studies with vincristine. Blood. 1963;21:640–7. [PubMed] [Google Scholar]
- 33.Postma TJ, Benard BA, Huijgens PC, Ossenkoppele GJ, Heimans JJ. Long-term effects of vincristine on the peripheral nervous system. J Neurooncol. 1993;15:23–7. doi: 10.1007/BF01050259. [DOI] [PubMed] [Google Scholar]
- 34.Chiorazzi A, Nicolini G, Canta A, et al. Experimental epothilone B neurotoxicity: results of in vitro and in vivo studies. Neurobiol Dis. 2009;35:270–7. doi: 10.1016/j.nbd.2009.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.