Abstract
Stress and alcohol abuse are co-related. Acute alcohol is anxiolytic, and stress is cited as a factor in relapse to alcohol use. A primary mediator of the stress response is the neuropeptide corticotrophin-releasing factor (CRF). The CRF family of endogenous ligands includes urocortin 3 (Ucn 3), which binds selectively to the CRF2 receptor and has been implicated in ethanol consumption in dependent and withdrawing rats. The objective of this study was to examine the effect of Ucn 3, delivered centrally to non-dependent mice, on limited-access ethanol consumption. Adult C57BL/6J mice were trained to self-administer 10% ethanol during daily, 2-hr limited access sessions using lickometers to assess drinking patterns for both ethanol and water. Sterile saline or 0.3, 1, or 3 nmol of Ucn 3 was microinjected into the lateral ventricle immediately before the limited-access session in a within-subjects design. There was a significant decrease in ethanol (both ml and g/kg), but not water, intake following Ucn 3 treatment, explained by a change in size of the largest lick run. Food intake at both 2- and 24-hours after injection was statistically unaffected by Ucn 3 administration. These results establish a role for CRF2R in a non-dependent, mouse model of ethanol self-administration.
Keywords: alcohol drinking, mice, microinjections, CRF2, ingestion
Introduction
Studies in rodents and humans have noted that alcohol use is sometimes motivated by relief of stress and anxiety (Pohorecky, 1990, Sillaber and Henniger, 2004). Acute alcohol use, as well as chronic use that results in neuroadaptation and dependence, cause activation and ultimately disruption of the hypothalamic-pituitary-adrenal axis (HPA axis) (Kiefer and Wiedemann, 2004, Richardson et al., 2008). The proper functioning of the HPA axis is important for adequately responding to stressors. A key mediator of the HPA axis response to a stressor is the corticotropin releasing factor (CRF) family of peptides and receptors. Agonist activity at CRF type 1 receptors (CRF1R) is associated with increased anxiety, and antagonism or deletion of CRF1R is associated with reduced anxiety (Koob and Thatcher-Britton, 1985; Smith et al., 1998; Timpl et al., 1998; Koob and Heinrichs, 1999; Coste et al., 2001; Zorrilla et al., 2002). CRF1R are widely distributed in the brain, but most notably in the amygdala, cortex, cerebellum, hippocampus, and medial septum, as well as in the pituitary (Potter et al., 1994; Chalmers et al., 1995; Van Pett et al., 2000). In contrast, CRF type 2 receptors (CRF2R) are found in fewer areas including the amygdala, bed nucleus of the stria terminalis, dorsal raphe nucleus, hypothalamus, and lateral septum (Lovenberg et al., 1995; Van Pett et al., 2000; Li et al., 2002). Agonist activity at these receptors is associated with anxiolytic effects, and it is hypothesized that CRF2Rmay also modulate the stress-coping response (Coste et al., 2001; De Kloet, 2004; Jamieson et al., 2006). In addition, CRF2R agonists decrease feeding in rats (Inoue et al., 2003; Fekete et al., 2007).
Several studies have examined CRF receptors, non-selectively, for their role in alcohol self-administration in both dependent and non-dependent rodents (Funk et al., 2006; Weitemier and Ryabinin, 2006; Finn et al., 2007; Ryabinin et al., 2008). Others have used methods that selectively target one of the CRF receptor subtypes in their examinations of ethanol-related behaviors. However, relatively few studies have focused on the role of CRF2R, for which another naturally occurring family of peptides, the urocortin (Ucn) family, has high affinity. While Ucn and CRF bind to both CRF1 and CRF2 receptors, Ucn2 and Ucn3 virtually selectively bind to CRF2R (Lewis et al., 2001; Reyes et al., 2001). CRF2R has been implicated in ethanol administration (Valdez et al., 2004; Sharpe et al., 2005; Funk and Koob, 2007), but has no known role in other ethanol-associated behaviors such as sedation, conditioned taste aversion or hypothermia (Sharpe et al., 2005). Rats made dependent on ethanol show increased anxiety and operant ethanol self-administration during early stages of withdrawal, an effect that is attenuated by administration of the CRF2R agonist Ucn 3 into the lateral ventricle or central nucleus of the amygdala (Valdez et al., 2004; Funk and Koob, 2007). The role of CRF2R in non- dependent or withdrawing rodents is less clear, with studies reporting no effect of CRF2R agonist activity on ethanol drinking (i.c.v. Ucn 3 administration, Valdez et al., 2004), an increase in ethanol intake (intra-amygdalar Ucn 3 at highest dose tested, Funk and Koob, 2007), or a modest increase in ethanol self-administration in CRF2R null mice (Sharpe et al., 2005).
Previous studies have established that activity at CRF2R is involved in regulation of feeding and ingestive behaviors, and that these receptors are located in areas of the brain that are associated with self-administration of drugs of abuse, including alcohol. It is possible that the discrepancy in the role of CRF2R regulation of ethanol intake in non-dependent, rodent models is due to species differences between mice and rats, developmental compensation in the genetic knockout model, differences associated with operant versus non-operant drinking models, or other potential dissimilarities between the studies. The objective of the study presented herein, was to determine the effect of centrally administered Ucn 3 on ethanol self-administration in mice in a two-bottle limited access ethanol drinking procedure, with regard to both pattern and amount of intake. Based on the increased ethanol consumption seen in CRF2R knockout mice, we hypothesized that Ucn 3 microinjected into the lateral ventricle would decrease ethanol intake. This could occur via a change in number of runs of ethanol drinking or run duration.
Methods
Animals
Male, adult (age 123–129 days at 1st microinjection) C57BL/6J (B6) mice (n=10; bred in house at the VA Medical Center from breeding stock obtained from The Jackson Laboratory) were individually housed in clear acrylic chambers (4″ wide × 6.5″ long × 5″ high) with a metal grid floor. The vivarium was kept on a 12 hour light/dark cycle with lights off from 9 am to 9 pm. Mice had ad libitum access to food (Purina Rodent Chow, 5001) and water throughout the study except as noted. Water and ethanol were available through inverted conical tubes fitted with a stopper and bent metal sipper tube inserted through one of two holes on the front of the home cage. Licking was measured via lickometers attached to both metal sipper tubes, and patterns of licking were monitored and recorded by a personal computer equipped with Med-PC software (Med-Associates, St. Albans, VT).
Drugs
Ethanol solutions (v/v) were prepared by diluting 95% ethanol (Pharmco, Shelbyville, KY) in tap water. Urocortin 3 (Phoenix Pharmaceuticals, Burlingame, CA) was reconstituted in sterile saline (0.3 and 1 nmol Ucn 3 per 1 μl saline or 3 nmol Ucn 3 per 2 μl saline) immediately before each session and kept on ice until used.
Procedure
Limited access sessions were conducted beginning 2 hours into the dark portion of the light cycle. This procedure produces high levels of ethanol intake and ataxia in the B6 mouse strain (Sharpe et al., 2005). Immediately before the limited access session, the water bottle was removed from the home cage, and mice were weighed and then returned to the home cage. The appropriate pre-weighed bottles were then placed on the home cage. To initiate ethanol drinking during the limited access session, initially mice were given access to only a 10% ethanol tube. No water was available during these 2-h periods, but water was freely available for the remaining 22 h of the day. After 8 sessions with only ethanol available, both water and 10% ethanol containing tubes were placed on the cage front during the limited-access session and mice were free to drink from either bottle. After the 2-h session, the bottle or bottles were removed and re-weighed to determine how much fluid the mouse had consumed. A water bottle was then returned to the cage for the remainder of the day. Six days into the 2-bottle choice phase, mice underwent surgery to place a cannula for injection of Ucn 3 into the lateral ventricle. Mice were anesthetized by inhalation of isofluorane and placed in a sterotaxic apparatus (SAS75 Stereotaxic Alignment System, Cartesian Research, Inc, Sandy, OR). Anesthesia was maintained during surgery by isoflurane and oxygen delivered via the stereotaxic nose cone. A 26 gauge, 10-mm stainless steel cannula (Small Parts Inc, Miami Lakes, FL) was placed −1.2 mm M/L and −0.46 mm A/P relative to bregma, and 1.85 mm below the skull. Durelon (carboxylate cement from 3M ESPE, St. Paul, MN) was used to fix the cannula and anchor screws to the skull. All cannula were fitted with a stylet (33 gauge wire, 9 mm long, Small Parts Inc, Miami Lakes, FL) to prevent clogging and entry of materials that could result in infection. Mice were allowed 10 days to recover from surgery before daily limited access sessions were resumed. Two-bottle choice sessions were identical to those before surgery, except that after the mice were weighed, they were lightly restrained by the experimenter and the stylet was removed and replaced each day before being returned to the home cage. This process acclimated the mice to being handled for microinjections. After 18 sessions post-surgery that allowed drinking levels to normalize, the microinjection portion of the study began. At the usual time for the daily limited access session, mice were weighed, lightly restrained, the stylet was removed, and an 11-mm injector (33 gauge stainless steel tubing, Small Parts Inc, Miami Lakes, FL) was inserted into the cannula. Once the injector was inserted, the mouse was allowed to freely move about in the experimenter’s hand while a pump (Harvard Apparatus, Holliston, MA) injected the solution (either vehicle or Ucn 3) over a period of 1 min. The injector was left in place for 1 min after the injection to allow the solution to disperse away from the site of injection. All mice received a 1 μl injection of sterile saline, followed by 1 injection of each dose of Ucn 3 (0.3, 1, and 3 nmol) given in a pseudo-randomized order, followed at the end of the study by a 2 μl sterile saline injection in all mice. Due to solubility concerns, the highest dose of Ucn 3 (3 nmol) was diluted in 2 μl saline while the two lowest doses were given in 1 μl saline. To assure that the volume of the injection did not affect behavior, the saline injections were given at both volumes. Sham injections, where the pump did not contact the syringe and thus no liquid was delivered, were conducted for 3 sessions before the 1st vehicle injection and on all days between subsequent injections. At least 2 sessions were conducted between vehicle or Ucn 3 injections to allow behavior to return to baseline before the next injection. Food consumption was monitored during the limited access session and 24-h intake by weighing the food before and after the limited access session each day. Mice were euthanized and immediately injected (i.c.v.) with 1 μl India ink via an 11-mm injector. Brains were then removed, post fixed, cut, and examined to confirm correct cannula placement.
Statistics
To assess possible changes in behavior over the length of the study or due to volume injected, a paired t-test was used to compare the dependent variables collected after the two vehicle injections. Data were analyzed via linear regression analysis with dose (0, 0.3, 1, and 3) as the predictor variable. Ethanol data were expressed in g/kg for consumption and as the ratio of ml consumed from the ethanol tube to ml consumed from both tubes for preference. For the lick analysis, a “run” of licking was defined as at least 25 licks with no more than 300 seconds between consecutive licks (Gannon et al., 1992). Other lick measures are self-explanatory.
Results and Discussion
During the two days prior to microinjections, when mice were sham injected, the average ethanol intake was 2.85 ± 0.27 g/kg, with a preference ratio for ethanol of 0.87 ± 0.03. There were no significant differences between the two vehicle injections for intake, preference or any other dependent variable. This indicated that microinjection volume (1.0 μl vs. 2.0 μl) had no significant impact and that there was no significant drift in baseline response to vehicle over the term of the study. Thus, vehicle injection data were averaged to create a single vehicle value for subsequent data analysis.
Injection of Ucn 3 dose-dependently decreased the volume of ethanol consumed when expressed in ml (Figure 1; F(1,38)=5.1, P<0.03) or normalized to the weight of the mice (g/kg; Figure 2A; F(1,38)=5.3, P<0.03). Ucn 3 did not significantly affect water intake (Figure 1; P=0.42) or ethanol preference ratio (Table 1; P=0.44). However, Ucn 3 did decrease the size of the largest run of licking for ethanol during the 2-h limited access session (Figure 2B; F(1,38)=6.5, P<0.02). No other variable reflecting pattern of drinking was significantly affected by Ucn 3 (Table 1), nor did the amount of food consumed change significantly with dose of Ucn 3 during the 2 hour session or in the 24 hours following the injection (Table 1).
Figure 1.
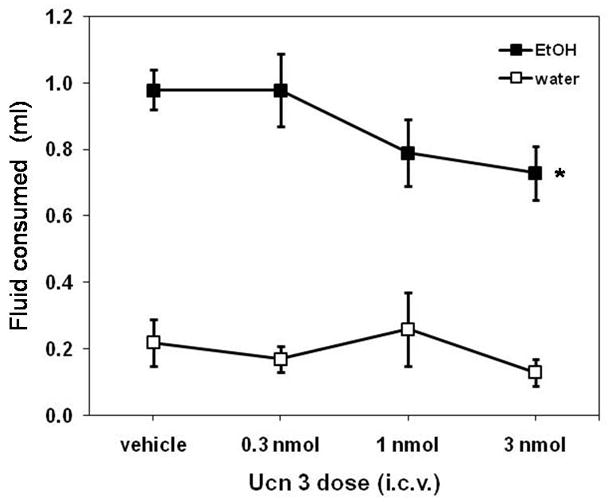
Ucn 3 significantly decreased ethanol intake (* P<0.03), but not water intake (P=0.42), when measured as volume consumed during the 2-hr limited access session. Data are presented as means ± SEM.
Figure 2.
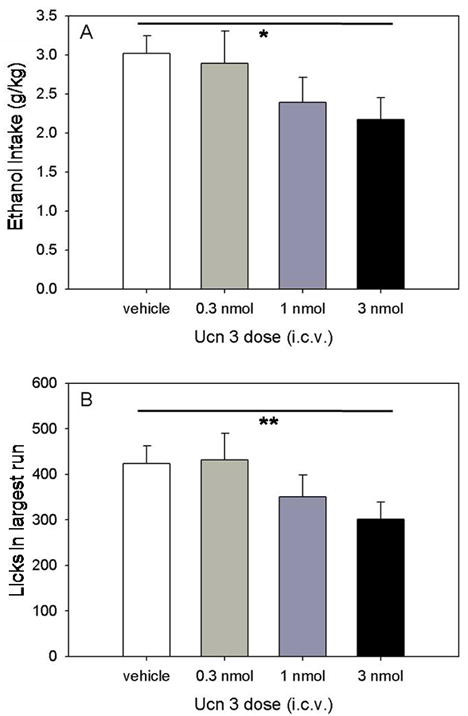
Ucn 3 significantly decreased g/kg ethanol consumption (panel A; * P<0.03) and the size of the largest run of licking for ethanol (panel B; * P<0.02). A run of licking was defined as a minimum of 25 licks with no separation between licks of more than 300 s. Data are presented as means ± SEM.
Table 1.
Ethanol drinking and food consumption data (mean ± SEM) from the 2-hr limited-access session.
| Vehicle | 0.3 nmol | 1 nmol | 3 nmol | |
|---|---|---|---|---|
| Ethanol preference ratio | 0.83±0.04 | 0.84±0.04 | 0.78±0.05 | 0.87±0.03 |
| Latency to 1st run (s) | 3.20±0.72 | 1.57±0.43 | 1.13±0.34 | 1.70±0.68 |
| 1st run size (licks) | 348±32 | 322±50 | 323±56 | 290±41 |
| 1st run time (min) | 6.6±1.3 | 5.6±1.4 | 6.8±1.9 | 5.5±1.1 |
| 1st run rate (licks/min) | 80±9 | 130±55 | 62±10 | 76±18 |
| # runs | 5.65±0.47 | 5.00±0.45 | 5.20±0.47 | 5.67±0.59 |
| 2 hr food intake (g) | 0.63±0.08 | 0.63±0.06 | 0.53±0.06 | 0.53±0.06 |
| 24 hr food intake (g) | 5.11±0.31 | 5.00±0.32 | 5.00±0.38 | 4.86±0.42 |
The findings presented here suggest that the CRF2R is involved in regulation of ethanol intake when ethanol is offered during limited periods of time. Taken with our previous demonstration of an increase in limited access ethanol intake in CRF2R deficient mice (Sharpe et al., 2005), these data suggest that CRF2R activation may decrease ethanol intake. These results also confirm, via pharmacology in non-genetically altered mice, that developmental compensation in the CRF2R null mice was not responsible for our previously published effects (Sharpe et al., 2005). Analysis of the pattern of ethanol consumption using lickometers showed a decrease in the size of the largest run of ethanol drinking, while other parameters were not significantly changed by Ucn 3 administration. Thus, it is not clear from these results if Ucn 3 specifically affects the satiety, initiation or maintenance of ethanol drinking. Subsequent studies using operant self-administration techniques, combined with appetitive vs. consummatory models (e.g., Samson et al., 1998; Samson and Czachowski, 2003), could provide more insight into the role of CRF2R.
Ethanol and food ingestion were both measured in the present study because both processes are regulated by many of the same neurotransmitters, peptides, and neural circuits. Due to the length of the alcohol limited-access session, food intake was measured at 2 and 24 hours post injection. We observed no significant change in food intake in non-food deprived mice in the 2 hours following Ucn 3 injection. This is consistent with previously published reports suggesting that central administration of Ucn 3 decreases food intake in non-food deprived rats after a minimum 2-hour delay (Ohata and Shibasaki, 2004; Fekete et al., 2007). While there was no significant change in food intake during the limited access session, there was a significant decrease in ethanol intake during this same time, suggesting that the effect of CRF2R on ethanol intake may be differentially regulated from that seen with food. In addition to a decrease in food intake following Ucn 3, Ohata and Shibasaki (2004) also observed a significant decrease in locomotor activity in mice injected i.c.v. with Ucn 3. Locomotor activity was not measured in the studies presented here, thus we cannot rule out the possibility that a general decrease in locomotor activity contributed to the decrease in ethanol intake. However, there was no significant difference in the rate of licking in the first ethanol drinking run, or in the 2-h food intake following Ucn 3 injection in our study, suggesting that any effect of Ucn 3 on locomotor behavior that could have affected consumptive behavior was minor.
While there was no significant change in the amount of food consumed during the limited access session, the pattern of food consumption was not monitored. Thus, it is not possible to determine if the pattern of food consumption changed or if the interactions between feeding and drinking changed following Ucn 3 injection. Recently, a drinking-implicit method of meal patterning has been used by Zorrilla and colleagues (2005) that includes both eating and drinking events in determining the definition of a meal, since eating and drinking behaviors are often intertwined. A drinking-inclusive analysis of ingestive behavior for food, ethanol and water, during the 2 hr ethanol limited-access procedure may have yielded more detailed information on the effect of Ucn 3 on both ethanol consumption and feeding. A visual analysis of the cumulative records suggests a possible interaction of feeding and drinking after Ucn 3 injection, specifically a trend towards longer times between drinking runs after Ucn 3 injection (data not shown). This increase in inter-run interval after Ucn 3 could reflect a decrease in feeding between runs, resulting in longer pauses between runs of drinking. Since only drinking (but not eating) behavior was monitored on a constant basis during the limited access session in this study, the effect of Ucn 3 on ethanol drinking pattern is difficult due to the normal interplay between eating and drinking in meals.
These results extend, to non-dependent mice, the previous finding that Ucn 3 decreases ethanol self-administration in dependent and withdrawing rats (Valdez et al., 2004). Dependent, withdrawing mice were not tested in the current study, thus no comparisons between rodent species can be made. In contrast to the current results, Valdez et al. (2004) did not see a significant effect of Ucn 3 in non-dependent and non-withdrawing rats. This may be explained by the considerable differences in the procedures used for the two studies. The design of the Valdez study, with regard specifically to the non-dependent control group, included a break, during which ethanol self-administration was not available and rats were not placed into the operant chambers. Rats were initially trained to self-administer ethanol in daily sessions, followed by 21 days without exposure to ethanol, during which time they consumed a palatable liquid diet containing sucrose. It is possible that use of this procedure with prolonged periods without ethanol or operant chamber exposure may have unintentionally affected the self-administration behavior of these animals. For example, there could have been loss of training to the operant task. In fact, examination of ethanol versus water responding shows that there was little or no preference for ethanol in this control group, following the abstinent period. In addition, ethanol self-administration may have been altered due to the daily exposure to a highly-palatable, sucrose-containing, liquid diet available in the home cage. In the present study, mice self-administered ethanol in continuous, uninterrupted daily sessions prior to all Ucn 3 treatments. An advantage of this procedure (with no breaks in self-administration) is that the consistent treatment produced subjects with very stable day-to-day self-administration and maintained a high ethanol-preference (see Table 1, ethanol preference ratio) despite the Ucn 3-induced decrease in ethanol intake. This stable ethanol-drinking baseline was sensitive to changes in intake, which may not have been possible with a more complicated, variable experimental design. Finally, the differences between the results of the two studies could also be explained by the use of operant self-administration by Valdez et al. (2004), as opposed to the non-operant, home cage self-administration presented here. Due to the significant differences in procedure between the two studies, it is not possible to conclude if there is a differential effect of Ucn 3 on ethanol drinking between mice and rats.
The results of this study establish a role for CRF2R in a non-dependent mouse model of ethanol self-administration. Although much of the focus in the alcohol field has been on CRF and activity at CRF1R, results from this study and others suggest that CRF2R may also regulate ethanol self-administration in both dependent and non-dependent animals. This effect may be mediated by the hypothalamus, which is involved in regulation of ingestive processes, or limbic areas such as the amygdala or bed nucleus of the stria terminalis that are hypothesized to regulate drug reward and use. Future work using microinjections could elucidate the specific region or regions of the brain where CRF2R regulate ethanol intake, and perhaps determine if chronic drug use has an effect on endogenous levels of Ucn 3 or CRF2R.
Acknowledgments
Supported by NIH grants from the National Institute on Alcohol Abuse and Alcoholism AA014747 to ALS, AA013331 to TJP, and AA010760 to TJP, and by the Department of Veterans Affairs.
References
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotrophin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides. 2001;22:733–41. doi: 10.1016/s0196-9781(01)00386-2. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Hormones and the stressed brain. Ann N Y Acad Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szücs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–68. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–8. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–32. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon KS, Smith JC, Henderson R, Hendrick P. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav. 1992;51:515–21. doi: 10.1016/0031-9384(92)90173-y. [DOI] [PubMed] [Google Scholar]
- Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP. Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther. 2003;305:385–93. doi: 10.1124/jpet.102.047712. [DOI] [PubMed] [Google Scholar]
- Jamieson PM, Li C, Kukura C, Vaughan J, Vale W. Urocortin 3 modulates the neuroendocrine stress response and is regulated in rat amygdala and hypothalamus by stress and glucocorticoids. Endocrinology. 2006;147:4578–88. doi: 10.1210/en.2006-0545. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Wiedemann K. Neuroendocrine pathways of addictive behavior. Addict Biol. 2004;9:205–12. doi: 10.1080/13556210412331292532. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Thatcher-Britton K. Stimulant and anxiogenic effects of corticotropin releasing factor. Prog Clin Biol Res. 1985;192:499–506. [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–5. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–42. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- Ohata H, Shibasaki T. Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides. 2004;25:1703–9. doi: 10.1016/j.peptides.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Interaction of ethanol and stress: research with experimental animals—an update. Alcohol Alcohol. 1990;25:263–76. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko PE, Vale W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994;91:8777–81. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–8. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–53. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Yoneyama N, Tanchuck MA, Mark GP, Finn DA. Urocortin 1 microinjection into the mouse lateral septum regulates the acquisition and expression of alcohol consumption. Neuroscience. 2008;151:780–90. doi: 10.1016/j.neuroscience.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–43. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–7. [PubMed] [Google Scholar]
- Sharpe AL, Coste SC, Burkhart-Kasch S, Li N, Stenzel-Poore MP, Phillips TJ. Mice deficient in corticotropin-releasing factor receptor type 2 exhibit normal ethanol-associated behaviors. Alcohol Clin Exp Res. 2005;29:1601–9. doi: 10.1097/01.alc.0000179371.46716.5e. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Henniger MS. Stress and alcohol drinking. Ann Med. 2004;36:596–605. doi: 10.1080/07853890410018862. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–6. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–72. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience. 2006;137:1439–45. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:1450–67. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–99. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]


