SUMMARY
It is unclear why disease occurs in only a small proportion of persons carrying common risk alleles of disease susceptibility genes. Here we demonstrate that an interaction between a specific virus infection and a mutation in the Crohn’s disease susceptibility gene Atg16L1 induces intestinal pathologies in mice. This virus-plus-susceptibility gene interaction generated abnormalities in granule packaging and unique patterns of gene expression in Paneth cells. Further, the response to injury induced by the toxic substance dextran sodium sulfate was fundamentally altered to include pathologies resembling aspects of Crohn’s disease. These pathologies triggered by virus-plus-susceptibility gene interaction were dependent on TNFα and IFNγ and were prevented by treatment with broad spectrum antibiotics. Thus, we provide a specific example of how a virus-plus-susceptibility gene interaction can, in combination with additional environmental factors and commensal bacteria, determine the phenotype of hosts carrying common risk alleles for inflammatory disease.
INTRODUCTION
Common genetic polymorphisms predispose to complex diseases such as type I diabetes, multiple sclerosis, Crohn’s disease, and ulcerative colitis (The Wellcome Trust Case Control Consortium, 2007; Altshuler et al., 2008). It is not clear why some individuals with a given polymorphism acquire disease while others remain unaffected. The concept that environmental factors including infections trigger disease in individuals with certain genetic backgrounds is broadly recognized. In animal models, autoimmune disease can be influenced by viral infections. For example, glomerulonephritis is exacerbated by LCMV and polyoma virus infections in certain inbred backgrounds (Tonietti et al., 1970), and LCMV infection inhibits development of diabetes in NOD mice or BB rats (Dyrberg et al., 1988; Oldstone, 1988). The genes responsible for these differences in outcome have not been defined, but these studies indicate that virus infection can alter disease in specific genetic backgrounds.
Crohn’s disease is a common type of inflammatory bowel disease involving mucosal ulceration and inflammation occurring in the distal small intestine (ileum) and variable discontinuous regions of the colon. A distinguishing feature of inflammation in Crohn’s disease is involvement of the entire thickness of the bowel wall. This transmural inflammation leads to atrophy of ileal villi, fibrosis, hypertrophy of smooth muscle and autonomic nerve cells in the outer layer of the bowel wall, and an inflammatory response including lymphoid aggregates and granulomas (Day et al, 2003). Polymorphisms in over 30 loci have been associated with increased risk of Crohn’s disease (Barrett et al., 2008). Yet these genetic components individually, or in combination (Weersma et al., 2007), confer limited risk. Environmental factors such as exposure to pathogens are potential cofactors for disease development, but the etiology of Crohn’s disease remains a controversial topic (Packey and Sartor, 2009).
One Crohn’s disease susceptibility allele is in the autophagy gene ATG16L1 (The Wellcome Trust Case Control Consortium, 2007; Rioux et al., 2007; Hampe et al., 2007). The ATG16L1 disease variant is frequent (~50% in European-derived populations) and confers less than a twofold increase in susceptibility. We previously established two mouse lines in which Atg16L1 expression is disrupted by gene trap mutagenesis (Cadwell et al., 2008a). Mutant mice displayed hypomorphic (HM) Atg16L1 protein expression and reduced autophagy (Cadwell et al., 2008a; Ju et al., 2009). Conventionally raised Atg16L1HM mice display striking abnormalities in Paneth cells, epithelial cells at the base of ileal crypts that are important in mucosal immunity (Porter et al., 2002; Ouellette, 2006; Vaishnava et al., 2008). In addition to aberrant packaging and exocytosis of antimicrobial granules, Paneth cells from Atg16L1HM mice display a surprising gain-of-function transcriptional profile in which transcripts associated with lipid metabolism, pro-inflammatory cytokines, and other pathways were enriched. Importantly, we observed similar Paneth cell abnormalities in Crohn’s disease patients homozygous for the risk allele of ATG16L1 but not in control patients (Cadwell et al., 2008a). Therefore, these aspects of intestinal pathology link Crohn’s disease patients carrying the susceptibility allele of ATG16L1 to mice that are hypomorphic for Atg16L1.
Here, we report a striking genetic interaction between Atg16L1 mutation and a specific strain of an enteric virus, murine norovirus (MNV). This interaction determines multiple pathologic abnormalities in the intestine including several similar to those observed in Crohn’s disease patients. Noroviruses are positive-sense encapsidated RNA viruses responsible for the majority of epidemic non-bacterial gastroenteritis in humans (Mead et al., 1999). Multiple strains of MNV are prevalent in mouse facilities around the world (Hsu et al., 2005; Pritchett-Corning et al., 2009; Goto et al., 2009), and some MNV strains persist for months after initial infection (Thackray et al., 2007). We demonstrate that Paneth cell abnormalities in Atg16L1HM mice are triggered by infection with an MNV strain that establishes persistent infection (persistent strain). This virus-plus-susceptibility gene interaction alters the transcriptional signature of Paneth cells and the nature of the inflammatory response in mice treated with the toxic substance dextran sodium sulfate (DSS). The mechanism for this pathologic response driven by the virus-plus-susceptibility gene interaction involved the cytokines TNFα and IFNγ as well as commensal bacteria. Our results provide evidence for a multi-hit model of inflammatory disease defined in a highly specific way by a viral infection only in the presence of a mutation in a disease susceptibility gene. This observation provides a basis for understanding how a common allele can be linked to an infrequent severe disease, and why mice carrying mutations in human disease susceptibility genes do not always spontaneously reproduce human pathology.
RESULTS
Viral infection triggers Paneth cell abnormalities in Atg16L1HM mice
Atg16L1HM mice raised in a conventional barrier facility were re-derived into an enhanced barrier facility (Experimental Procedures) by embryo transfer. Surprisingly, in contrast to observations made in mice from a conventional barrier (Cadwell et al., 2008a), Paneth cells in re-derived Atg16L1HM mice were indistinguishable morphologically from those in littermate wild-type control mice (herein referred to as WT) (Figure 1A) and failed to display aberrant packaging of the granule protein lysozyme (Figure 1B). All subsequent experiments were performed in mice raised in the enhanced barrier facility.
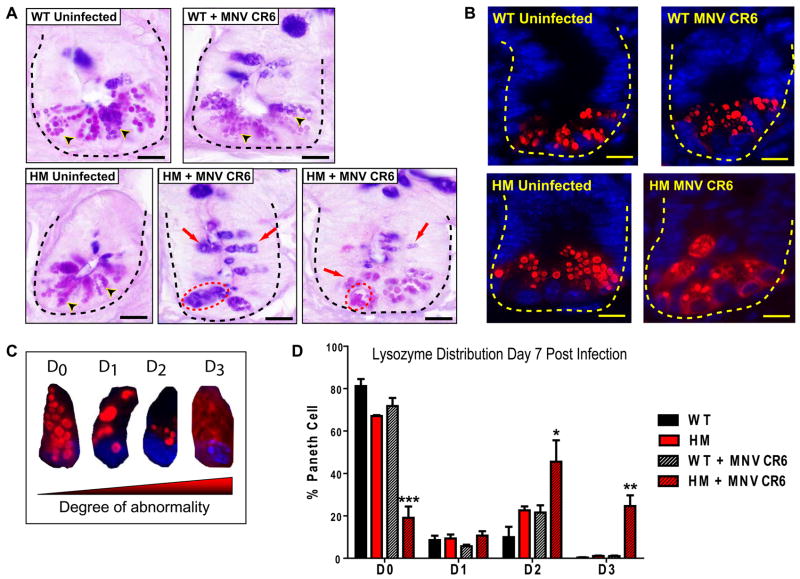
Figure 1. Murine norovirus (MNV) infection triggers Paneth cell abnormalities in Atg16L1 mutant mice.
(A-B) Atg16L1 hypomorph (Atg16L1HM) and WT mice in the MNV-free barrier facility were orally inoculated with 3×107 particle forming units (pfu) of MNV CR6 for 7 days or left untreated (n > 6 mice for each group).
(A) Light microscopy images of ileal sections stained with PAS-alcian blue. Dotted line denotes crypt unit containing several Paneth cells each, and arrowheads indicate typical granules. For the two representative images of Atg16L1HM mice infected with MNV CR6, red dotted circle denotes aggregated granules and red arrows indicate granules with abnormal staining and size. Scale bar represents 10 μm.
(B) Indirect immunofluorescence of ileal sections stained for lysozyme (red) and nuclei (blue). Dotted line denotes crypt unit. Scale bar represents 10 μm.
(C) Four types of lysozyme distribution patterns observed in Paneth cells: normal (D0), disordered (D1), depleted (D2), diffuse (D3).
(D) Percentage of Paneth cells displaying each of the four types of lysozyme distribution patterns from WT and Atg16L1HM mice that were uninfected or inoculated with MNV CR6 (n > 5,700 cells from 3 mice/condition, *p < 0.05, **p < 0.01, ***p < 0.001, mean +/−SEM).
See also Figure S1.
These results suggest that Paneth cell abnormalities require an exogenous factor present in the conventional barrier facility. We previously identified the first murine norovirus (MNV) in mice from this facility (Karst et al., 2003). We therefore considered the possibility that MNV triggers Paneth cell abnormalities in Atg16L1HM mice. To test this, mice were orally inoculated with an MNV strain that establishes persistent infection (MNV CR6) (Thackray et al., 2007). Seven days post-inoculation, Atg16L1HM but not WT mice displayed Paneth cell abnormalities including aberrant granule numbers, size, and distribution (Figure 1A). UV-inactivated virus did not induce these abnormalities (Figure S1A) indicating that productive virus infection was required.
MNV CR6 infection for seven days also induced abnormal lysozyme distribution and abnormal ultrastructural morphology in Paneth cells from Atg16L1HM mice (Figure 1B, S1B, and S1C). We used a previously established scale to blindly identify cells with increasingly abnormal lysozyme staining (Figure 1C) (Cadwell et al., 2008a). Only Atg16L1HM mice that were infected with MNV CR6 displayed Paneth cells with the two most severe abnormalities (Figure 1D). Analysis of Paneth cells by transmission electron microscopy also revealed a substantial proportion of cells that were depleted of secretory granules in MNV CR6-infected Atg16L1HM mice (Figure S1B and S1C). Moreover, many of the Paneth cells from these mice contained distended rough endoplasmic reticulum (Figure S1B and S1C). Other cytoplasmic organelles including mitochondria were not obviously altered in virally-infected Atg16L1HM mice. Thus, Paneth cell morphological and granule packaging abnormalities were induced by the combination of viral infection and mutation in the Crohn’s disease susceptibility gene Atg16L1. Hereinafter we will refer to this as a virus-plus-susceptibility gene interaction.
Properties of MNV associated with Paneth cell abnormalities
Since autophagy is important in innate immunity (Virgin and Levine, 2009), a mutation in Atg16L1 might lead to enhanced viral replication and cytopathicity in Paneth cells. When comparing WT and Atg16L1HM mice, we did not observe significant differences in viral shedding in the stool or viral titers in organs including the distal ileum where Paneth cell abnormalities were observed (Figure S2A). Consistent with a previous report in which MNV was detected in the lamina propria (Mumphrey et al., 2007), we did not detect MNV in Paneth cells by immunohistochemistry, and viral RNA was not detected in Paneth cell RNA procured by laser capture microdissection (data not shown, Experimental Procedures). Thus, direct infection of Paneth cells is not responsible for triggering the abnormalities described above.
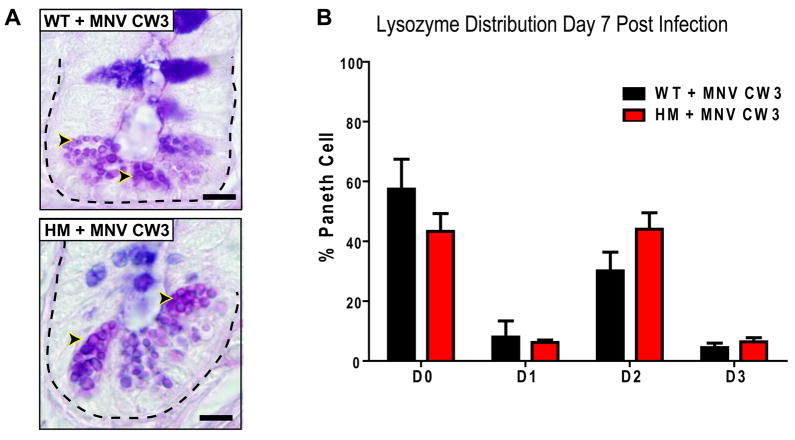
To determine if Paneth cell abnormalities develop after infection with any virus, we investigated MNV CW3 which does not persist in immunocompetent mice despite sharing 95% amino acid sequence identity across the genome with MNV CR6 (Mumphrey et al., 2007; Thackray et al., 2007). As in WT mice, shedding of MNV CW3 in Atg16L1HM mice is initially high but reduced or undetectable at later time points (Figure S2B). MNV CW3 failed to trigger aberrant Paneth cell morphology seven days post infection (Figure 2A). While MNV CW3 induced some changes in lysozyme distribution (increase in D2), the most abnormal distribution pattern (D3) was not detected (Figure 2B). Since the lysozyme staining pattern seen after MNV CW3 infection was similar in both WT and Atg16L1HM mice, these mild changes may constitute a normal response to viral infection. Nevertheless, induction of Paneth cell abnormalities specific to Atg16L1HM mice is virus strain-dependent and associated with viral persistence, a property of MNV CR6 but not MNV CW3.
Figure 2. Properties of MNV associated with Paneth cell abnormalities in Atg16L1 mutant mice.
(A) Light microscopy of ileal sections stained with PAS-alcian blue of WT and Atg16L1HM mice 7 days post infection with 3×107 pfu of MNV CW3 (n = 6 mice/genotype). Dotted line denotes crypt unit and arrowheads indicate Paneth cell granules. Scale bar represents 10 μm.
(B) Number of Paneth cells displaying abnormal lysozyme distribution from WT and Atg16L1HM mice in (A) (n > 6100 cells from 3 mice/condition, mean +/−SEM).
See also Figure S2.
Since MNV CW3 shedding is reduced or undetected seven days post infection, we also evaluated lysozyme distribution at day three post infection when viral shedding is high. The majority of Paneth cells were normal (D0 pattern; Figure S2C) in WT and Atg16L1HM mice infected with either MNV CR6 or CW3. However, Atg16L1HM mice infected with MNV CR6 for 35 days displayed Paneth cell abnormalities similar to mice infected for seven days (data not shown). These results are consistent with a requirement for the continual presence of virus past the first several days of initial infection to induce Paneth cell abnormalities.
Virus-plus-susceptibility gene interaction results in a unique and virus strain-specific transcriptional profile in Paneth cells
We previously reported aberrant gene expression in Paneth cells of conventionally raised Atg16L1HM mice (Cadwell et al., 2008a). To determine if gene expression is influenced by the virus-plus-susceptibility gene interaction, we compared gene expression in Paneth cells from WT and Atg16L1HM mice seven days after infection with MNV CR6 (Figure 3). Data was analyzed from three biological replicate experiments. Among genes whose expression were increased or decreased at least 1.5 fold (p < 0.05) in response to MNV infection, 39 were shared between WT and Atg16L1HM mice (Zones C and F in Venn diagram, Figure 3). Of these, 27 exhibited expression changes of similar magnitude and in the same direction in WT and Atg16L1HM mice (Figure S3).
Figure 3. MNV infection induces a distinct transcriptional response in Paneth cells from Atg16L1 mutant mice.
Factorial design analysis was performed to identify gene sets showing differential gene expression in Paneth cells from WT and Atg16L1HM mice with or without MNV CR6 infection. Venn diagram displays the subsets of genes found to be differentially expressed (p < 0.05 as defined using a linear model and exhibiting a fold-change > 1.5) in the respective comparisons as indicated (circles 1 to 3). The ‘differential-of-differential’ analysis in the factorial design identified genes that respond differently to infection in Atg16L1HM mice compared to WT mice (Zones A, B, C, and D bounded by the red circle). These were found to be enriched for genes associated with intracellular protein traffic, protein targeting and localization, and amino acid metabolism. Also noteworthy is the sizable representation of genes associated with carbohydrate metabolism. The expression profiles for genes in these biological process categories are displayed as Log2-transformed fold-changes in the heatmaps. The ‘differential-of-differential’ analysis also identified genes that were not significantly altered in response to infection when compared within each genotype but exhibited significant differences when comparing responses to infection between genotypes as highlighted in Zone A. Genes with transporter functions or those with ligase activity were significantly enriched in this subset.
See also Figure S3 and S4.
We next identified gene expression changes attributable to virus-plus-susceptibility gene interaction employing a factorial design approach incorporating ‘differential-of-differential’ analysis that examined factor combinations of the two experimental dimensions (infection states and genotypes). 759 genes were altered differentially between the two genotypes as a result of MNV CR6 infection (Zones A-D in Venn diagram, Figure 3). Enrichment analysis of these genes identified a significant over-representation of the biological processes of intracellular protein traffic, protein targeting and localization, and amino acid metabolism (Figure 3). These results are consistent with autophagy deficiency and Paneth cell ER and granule packaging abnormalities observed in virally-infected Atg16L1HM mice (Figure 1, S1A, and S1B). We also identified transcripts that were not significantly altered in response to infection within a genotype, but displayed significant expression differences between genotypes (Zone A in Venn diagram, Figure 3). These transcripts were enriched for those encoding transporters and ligases, particularly those involved in processing ubiquitin.
Strikingly, when comparing the direction of gene expression changes between WT and Atg16L1HM Paneth cells, entire sets of genes were altered in opposite directions (Figure 3). For example, changes in the expression of genes encoding ligases or proteins associated with amino acid metabolism are regulated in different directions in WT versus Atg16L1HM mice after MNV CR6 infection. In some functional groups (intracellular protein traffic and protein targeting and localization), changes in gene expression are reciprocal with gene sets that increase in one genotype decreasing in the other. Therefore, the virus-plus-susceptibility gene interaction changes the fundamental nature, and not just the extent, of transcriptional responses during infection.
To determine if the transcriptional profile of Paneth cells from Atg16L1HM versus WT mice was unique to MNV CR6 infection, we defined the transcriptional profile of Paneth cells in mice infected with MNV CW3 seven days post infection. Infection with MNV CW3 led to changes in 2,226 transcripts in Atg16L1HM not altered in WT mice (Figure S4). Of these, 2,181 were unique to MNV CW3 compared to MNV CR6 infection. Enrichment analyses of the MNV CR6 and CW3 datasets supported the virus strain-specific nature of this effect; there was no overlap in significantly enriched functional groups (Figure S4).
Virus-plus-susceptibility gene interaction generates an aberrant intestinal injury response
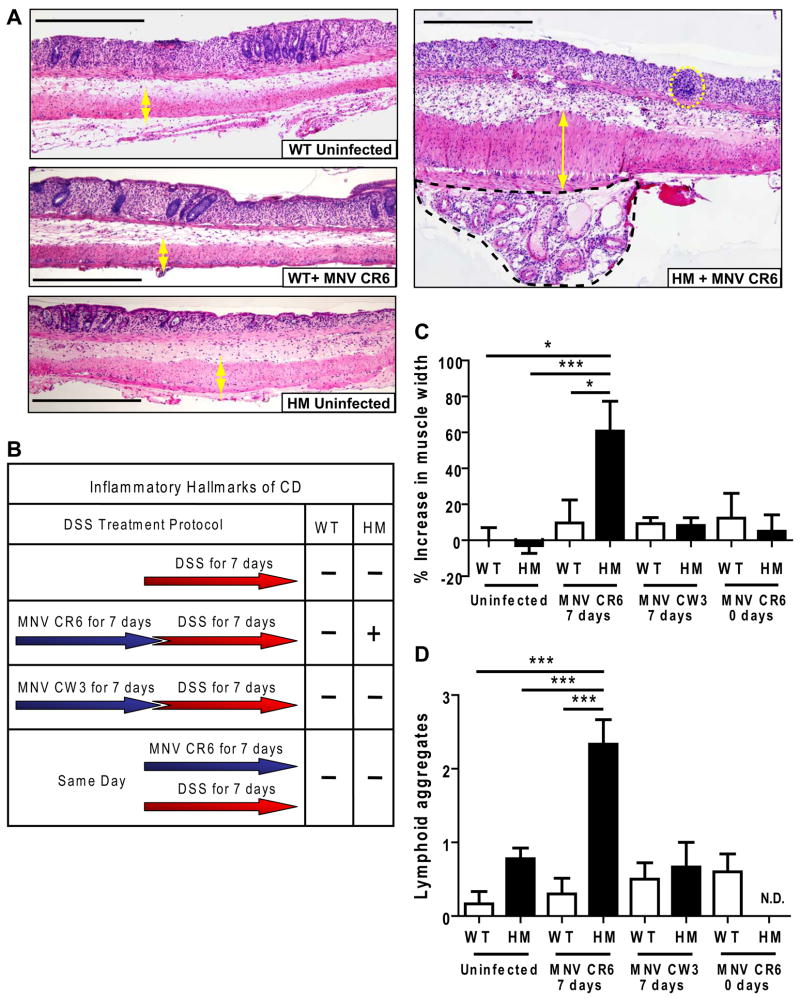
Since changes in Paneth cells depended on a virus-plus-susceptibility gene interaction, intestinal responses to an additional environmental ‘hit’ might also be influenced by this interaction. Administration of dextran sodium sulfate (DSS) induces intestinal injury. We chose a concentration of DSS (2.5%) that causes mild pathology and no lethality seven days post-treatment in immunocompetent mice. Instead, this treatment induces a reproducible programmed response in immunocompetent mice including decreased epithelial cell proliferation, loss of epithelial integrity, and generation of ulcers in specific colonic regions (Pull et al., 2005). As expected, uninfected Atg16L1HM and both uninfected and MNV CR6-infected WT mice fed DSS developed a typical response including focal ulcers in the transverse colon and a single confluent ulcer adjacent to the ano-rectal junction while the ileum was not ulcerated (Figure 4A). Thus, Atg16L1 mutation alone does not lead to altered responses to DSS-induced intestinal injury.
Figure 4. Atg16L1 mutant mice display a virus-dependent aberrant response to DSS in the colon.
(A) WT and Atg16L1HM mice were orally inoculated with MNV CR6 for 7 days or uninfected, and subsequently given 2.5% DSS for an additional 7 days at which point intestines where harvested. Light microscopy images of H+E stained sections of ulcerated regions located immediately adjacent to the ano-rectal junction are shown. Yellow double-headed arrows indicate the muscularis propria thickness. In the MNV CR6-infected Atg16L1HM sample, lymphoid aggregates are indicated by yellow dashed circles, and the black dashed region contains submusocal fibrosis and inflammation. Scale bar represents 500 μm.
(B) Table summarizing the outcome of DSS treatment. All intestines were harvested at the end of DSS treatment for analysis. + and − refer to the presence or absence of inflammatory hallmarks of Crohn’s disease respectively (n ≥ 6 mice/condition).
(C) Quantification of the muscularis propria thickness in the region adjacent to the anorectal junction from DSS treated mice. 7 days and 0 days refer to the amount of time mice were inoculated with the virus prior to DSS treatment. The Increase in muscle thickness was normalized to the average of uninfected WT mice treated with DSS (*p < 0.05, ***p < 0.001, mean +/−SEM).
(D) Number of lymphoid aggregates in the region adjacent to the ano-rectal junction from DSS treated mice (***p < 0.001, mean +/−SEM). N.D. refers to not detected.
See also Figure S5.
Remarkably, Atg16L1HM mice infected with MNV CR6 for a week prior to DSS treatment exhibited aberrant responses to injury in the colon (Figure 4). In ulcers adjacent to the ano-rectal junction, infected Atg16L1HM mice unexpectedly exhibited multiple hallmarks of human Crohn’s disease including increased inflammation in the muscularis and associated mesenteric fat and blood vessels, increases in lymphoid aggregates, development of subserosal fibrosis, hypertrophy of the muscularis propria, and hyperplasia of the proximal epithelium (Figure 4). These aspects of intestinal pathology in our experimental system were similar to the typical intestinal pathology of Crohn’s disease patients (Roberts, 2009). Thus, virus-plus-susceptibility gene interaction resulted in a significantly altered intestinal injury response.
In contrast to MNV CR6, infection of Atg16L1HM mice with the non-persistent strain MNV CW3 did not induce inflammatory hallmarks of Crohn’s disease after DSS treatment (Figure 4C and 4D). Further, Atg16L1HM mice receiving MNV CR6 and DSS concurrently did not develop inflammatory hallmarks of Crohn’s disease and were indistinguishable from similarly treated WT mice (Figure 4C and 4D). In contrast, Atg16L1HM mice infected with MNV CR6 for 28 days prior to DSS treatment developed similar inflammatory pathologies to those seen seven days after infection (data not shown). These data are consistent with a role for viral persistence. Taken together, these results indicate that in the context of DSS treatment, virus strain specificity and timing of infection are important factors in virus-plus-susceptibility gene interaction.
Unexpected ileal pathology dependent on virus-plus-susceptibility gene interaction
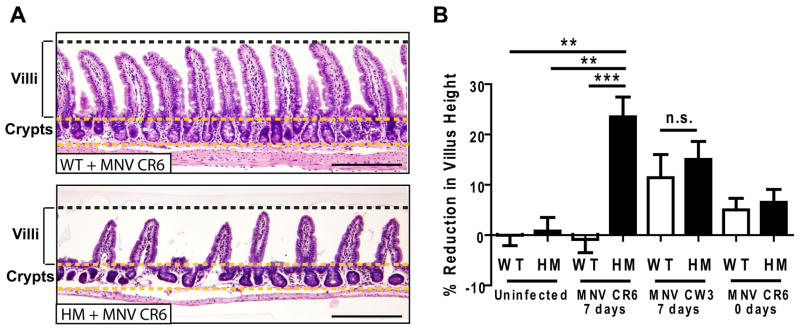
Atg16L1HM mice infected with MNV CR6 prior to DSS administration displayed ileal pathology not previously reported in DSS treated mice. In contrast to WT mice, Atg16L1HM mice receiving DSS exhibited virus-dependent mucosal atrophy manifested as blunting of villi (Figure 5) similar in degree to that observed in Crohn’s disease (Roberts, 2009), celiac disease (Chand and Mihas, 2006), or norovirus-induced gastroenteritis (Agus et al., 1973; Dolin et al., 1975). Villus blunting in response to DSS was inconsistent in Atg16L1HM mice infected with the non-persistent MNV CW3 strain (2/6 mice, Figure 5B). MNV CR6 infection for a week prior to DSS administration was required for induction of villus blunting (Figure 5B).
Figure 5. Virus-infected Atg16L1 mutant mice display mucosal atrophy in the ileum in response to DSS.
(A) Light microscopy of H+E stained ileal sections from the same mice in Figure 4. Scale bar represents 200 μm.
(B) Quantification of average villus height normalized to uninfected WT mice treated with DSS. 7 days and 0 days refer to the amount of time mice were inoculated with the virus prior to DSS treatment (an average of 286 total villi from at least 5 mice/condition was measured, **p < 0.01, ***p < 0.001, mean +/−SEM).
See also Figure S5.
One explanation for aberrant DSS-induced pathologies observed in virus-infected Atg16L1HM mice would be uncontrolled viral replication. However, there were no significant differences in viral replication in the ileum and colon where aberrant pathology is observed prior to or after DSS treatment (Figure S2A and S5). Less MNV CR6 was detected in the mesenteric lymph nodes from Atg16L1HM compared to WT mice receiving DSS, indicating that virus-plus-susceptibility gene effects were not mediated by increased viral replication.
TNFα, IFNγ, and commensal bacteria mediate intestinal injury responses dependent on virus-plus-susceptibility gene interaction
To identify mediators of DSS-induced pathology dependent on virus-plus-susceptibility gene interaction, we examined the role of cytokines. TNFα is a major mediator of inflammation in Crohn’s disease; administration of blocking antibodies that interfere with TNFα signaling is a powerful therapeutic approach (Targan et al., 1997). Furthermore, excess TNFα induces villus blunting as observed in Figure 5 (Piguet et al., 1999). Upon in vitro stimulation, intestinal lymphocytes from Crohn’s disease patients secrete increased IFNγ (interferon-γ) (Fuss et al., 1996; Fais et al., 1991), which can function in concert with TNFα in inflammatory processes (Suk et al., 2001; Lake et al., 1994), and IFNγ may have a role in DSS-induced intestinal injury (Brem-Exner et al., 2008).
MNV CR6-infected, DSS-treated Atg16L1HM mice given TNFα or IFNγ blocking antibodies displayed dramatically reduced muscular hypertrophy, fewer lymphoid aggregates adjacent to the ano-rectal junction, and decreased ileal villus blunting (Figure 6A, 6B, and 6C). Importantly, only the pathologies specific to virally-infected Atg16L1HM mice were altered as blocking antibodies had no effect on the superficial ulceration typical of DSS treatment present in all conditions. Thus, TNFα and IFNγ were each necessary for the DSS-induced pathologies in both ileum and colon that were dependent on virus-plus-susceptibility gene interaction.
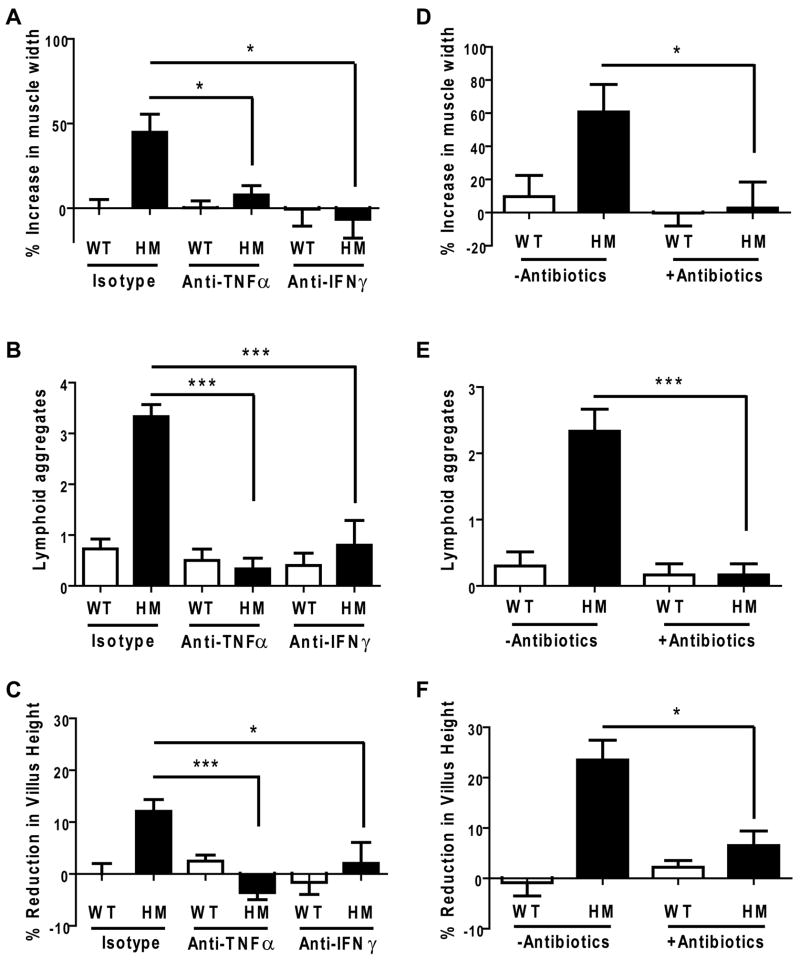
Figure 6. TNFα and IFNγ inhibition or antibiotics treatment ameliorates DSS-induced disease in virus-infected Atg16L1 mutant mice.
(A) Isotype control or blocking antibodies against TNFα and IFNγ were administered to WT and Atg16L1HM mice infected with MNV CR6 for 7 days followed by additional 7 days of DSS treatment at which point intestines where harvested. The muscularis propria thickness in the region adjacent to the ano-rectal junction was quantified and normalized to the average of WT mice receiving isotype control antibodies (n ≥ 6 mice/condition, *p < 0.05, mean +/−SEM).
(B) Number of lymphoid aggregates in the region adjacent to the ano-rectal junction from mice in (A) (*p < 0.001, mean +/−SEM).
(C) Quantification of average villus height from mice in (A) normalized to the average of WT mice receiving isotype control antibodies (an average of 263 total villi from at least 5 mice/condition was measured, *p < 0.05, ***p < 0.001, mean +/−SEM).
(D) WT and Atg16L1HM mice were orally inoculated with MNV CR6 for 7 days and then given 2.5% DSS and broad spectrum antibiotics concurrently for another 7 days at which point intestines where harvested. The muscularis propria thickness was normalized to the average of uninfected WT mice treated with DSS from Figure 4D (n = 6 mice/condition, *p < 0.05, mean +/−SEM).
(E) Number of lymphoid aggregates from mice in (D) (***p < 0.001, mean +/−SEM).
(F) Quantification of average villus height from mice in (C) normalized to the average of uninfected WT mice treated with DSS from Figure 5B (an average of 320 total villi from at least 5 mice/condition was measured, *p < 0.05, mean +/−SEM).
See also Figure S5.
Commensal bacteria are important in inflammatory bowel disease (Sartor, 1997; Kang et al., 2008), and there is precedence for a role of bacteria in disease triggered by viral infection as in secondary infection with Streptococcus pneumoniae after influenza (McCullers, 2006). To determine if the intestinal injury response dependent on virus-plus-susceptibility gene interaction requires commensal bacteria, Atg16L1HM mice were infected with MNV CR6 and treated with both DSS and broad spectrum antibiotics. Antibiotics uptake was similar across groups and did not interfere with the administration of DSS (Extended Experimental Procedures). Administration of antibiotics to virally-infected Atg16L1HM mice prevented abnormal DSS-induced pathologies in both ileum and colon reported above (Figure 6D, 6E, and 6F). Thus, aberrant injury responses dependent on virus-plus-susceptibility gene interaction require commensal bacteria.
DISCUSSION
An important goal is to identify specific genetic, infectious, or environmental factors responsible for a given disease. When such an etiologic smoking gun is detected, the full power of medicine, chemistry, and vaccine development can be brought to bear on the illness. However, if complex diseases are combinatorial in etiology, then an individual gene or pathogen may display only poor association with disease incidence and severity. This model of complex disease is widely recognized but experimental evidence has not been reported.
In this study, we define a complex inflammatory disease in mice that depends on an interaction between a specific enteric virus strain and a single host disease susceptibility gene. Several of the pathologic features observed in this model resemble those in many Crohn’s disease patients. In particular, Paneth cell structural and granule packaging abnormalities observed in virus-infected Atg16L1HM mice are remarkably similar to those in patients homozygous for the risk allele of ATG16L1. Since Crohn’s disease patients homozygous for the non-risk allele of ATG16L1 do not display this pathology, virus-plus-susceptibility gene interaction may contribute to heterogeneity between patients. Importantly, Paneth cells from Atg16L1HM mice infected with virus have a fundamentally different gene expression profile than controls. These results provide a specific example of how a genetic factor and an environmental agent, each innocuous by itself, can have profound effects on the host when combined. These results also provide one explanation for why persons in clinical trials may display widely variable responses to treatment for a single pathologically defined disease.
Mechanisms of pathology induced by virus-host susceptibility gene interaction
Despite the importance of autophagy genes in regulating the intracellular replication of pathogens, Atg16L1 mutant mice did not display increased MNV replication, virus was not detected in Paneth cells, and we found no role for Atg16L1 in control of MNV replication in vitro using primary macrophages from Atg16L1HM mice (data not shown). In this context, it is interesting that Paneth cell abnormalities, the transcriptional response to infection, and DSS-induced pathologies were dependent on infection with MNV CR6, a strain capable of persistent infection. Notably, the two strains of MNV used in this study generate serologically indistinguishable antibody responses (Thackray et al., 2007) despite the dramatic difference in ability to induce disease in Atg16L1 mutant mice. Additionally, aberrant DSS-induced pathologies were dependent on the timing of MNV infection. The relationship between an infectious agent and a complex disease is typically established through serology or the presence of a pathogen in infected tissue. These conventional approaches may dismiss etiologic agents if the disease is dependent on a specific pathogen strain or the timing of the infection. Detection of virus-plus-susceptibility gene interactions in humans may therefore require advances in the capacity to identify viruses present in tissues in an unbiased way, allowing a better understanding of the relationship between the human virome and disease pathogenesis (Virgin et al., 2009). To be revealing, such studies will have to be coupled to high power epidemiological studies and analysis of when patients were exposed to environmental factors.
The aberrant intestinal injury response we described in virally-infected Atg16L1HM mice was dependent on three factors associated with Crohn’s disease pathogenesis in humans – TNFα, IFNγ, and commensal bacteria. This common role of two cytokines and commensal bacteria suggest that the human disease and the observed pathologies in mice share an underlying mechanism, and supports the relevance of the virus-plus-susceptibility gene interaction reported here. Consistent with the efficacy of TNFα and IFNγ blockade in our model, cytokine-mediated injury following viral infection is receiving attention as an attractive target for therapeutic intervention (Marsolais et al., 2009; Brockman et al., 2009). There is also evidence for an intersection between viral infection and commensal bacteria (Brenchley et al., 2006; Xi et al., 2008). The antibiotic responsiveness of the DSS-induced disease in our model reveals an important bacterial component to the virus-plus-susceptibility gene interaction which may be exploited therapeutically.
The gene expression and morphological changes in Paneth cells we report are consistent with an important role of Atg16L1 in the secretory pathway and the response of epithelial cells to infection. These functions of Atg16L1 may be related to the membrane trafficking component of autophagy. Consistent with a cell autonomous role of Atg16L1 in Paneth cells, intestinal epithelium-specific deletion of two other autophagy genes also leads to Paneth cell granule defects (Cadwell et al., 2008a; Cadwell et al., 2008b). Since Atg16L1 and its binding partner Atg5 can limit cytokine production (Saitoh et al., 2008; Jounai et al., 2007; Tal et al., 2009), viral infection may trigger an altered cytokine response in Atg16L1 mutant mice. Although the changes we see are in the epithelium, the complex inflammatory phenotype reported here may also depend on Atg16L1 function in other cell types. For example, the necessity of autophagy genes for T cell function (Pua et al., 2007; Nedjic et al., 2008) may be relevant given the role of T cells in colitis after adoptive transfer into Rag2-deficient mice (Barnes and Powrie, 2009); the role of MNV has not been explored in that model.
It is tempting to speculate that Paneth cell abnormalities contribute to aberrant DSS-induced pathologies. Local production of inflammatory molecules by Paneth cells may drive villus blunting in the ileum. Moreover, virally-infected Atg16L1HM mice can have alterations in commensal bacteria since mice with Paneth cells that have lost α-defensin activation develop intestinal dysbiosis including the emergence of segmented filamentous bacteria (Salzman et al., 2010) that stimulate the development of Th17 lymphoctyes (Ivanov et al., 2009). Paneth cells are not present in the mouse colon where viral infection alters injury responses in Atg16L1 mutant mice. However, the effects of Paneth cells need not be restricted to the ileum since active peptides from their granules can be detected in the distal colonic lumen (Mastroianni and Ouellette, 2009). Since it is unclear how diverse pathologies are related to one another in Crohn’s disease patients, it will be important to address the relationship between Paneth cells, the microbiome, and the virome in the context of the aberrant injury response dependent on the virus-plus-susceptibility gene interaction in our model.
Disease penetrance in mouse models of mucosal immunity
Based on our observations in Atg16L1 mutant mice, we speculate that other traits attributed to specific mutations are also dependent on viral infections. The presence of an infectious agent can lead to different experimental outcomes between laboratories. For example, experimental allergic encephalomyelitis (EAE) correlates with mouse facility-specific pathogen exposure (Goverman et al., 1993). Experimental discrepancies between facilities are particularly germane for models of intestinal disease since MNV is an enteric virus commonly detected in specific pathogen free facilities (Hsu et al., 2005; Pritchett-Corning et al., 2009; Goto et al., 2009).
The effect of viral infection can be remarkably specific. In our model, the MNV CR6 strain altered experimental outcomes only in Atg16L1HM mice. MNV CR6 infection does not alter immune responses in C57/BL6 mice challenged with Friend leukemia virus, influenza, vaccinia, or MCMV (Ammann et al., 2009; Hensley et al., 2009; Doom et al., 2009). In contrast, MNV infection of Mdra1−/− mice enhances immune responses to Helicobacter bilis infection (Lencioni et al., 2008). Thus, the effect that a particular pathogen has on the host cannot be generalized. While we focus on a single virus and gene, multiple interactions may exist between different susceptibility genes and an array of commensals and pathogens. Much remains to be learned about the interactions between susceptibility genes and specific pathogens to understand what may be a general ‘microbe plus susceptibility gene’ contribution to a range of complex diseases.
Understanding the contributions of genetic and environmental factors in complex diseases
Epidemiological studies have not identified a specific infectious cause for inflammatory bowel disease. Reported associations between disease risk and infectious gastroenteritis are correlative (Porter et al., 2008; Garcia Rodriguez et al., 2006; Gradel et al., 2009), but are of interest given our data since human noroviruses related to MNV cause human gastroenteritis (Mead et al., 1999). Since many bacteria and viruses cause gastroenteritis, the infectious trigger of a complex disease need not be a single specific agent. Rather several agents that affect similar immunologic pathways can be involved. Interestingly, the Crohn’s disease associated gene NOD2 may recognize viral RNA in addition to bacterial peptidoglycan (Sabbah et al., 2009; Shapira et al., 2009), raising the possibility that a viral infection can interact with multiple susceptibility genes.
Although Atg16L1 mutant mice do not display all pathology found in Crohn’s disease patients, we have reproduced several disease hallmarks in a mouse with a mutation in a single susceptibility gene. It is commonly assumed that the failure to recreate the entirety of Crohn’s disease in various mouse models is due to inherent differences between humans and mice. However, in the combinatorial view of complex disease supported by our results, reproducing full disease may require combinations of specific alleles of multiple genes with certain environmental agents. It is worth noting that not all Crohn’s disease patients exhibit identical symptoms or pathologies, and the nature of Crohn’s disease varies over time even within one individual. In addition, therapeutic interventions that improve conditions for some do not always alleviate disease in others. Therefore, complex diseases may represent a combinatorial confluence of pathologic responses, each with overlapping but non-identical genetic and environmental causes and therefore therapeutic responses.
This paradigm has profound implications for how we view the relationship between genetic heterogeneity and phenotype in humans. We propose that studies examining associations between disease susceptibility and genetic variation should consider the history and current status of viral infections in the individuals. Similarly, studies examining the correlation between viral infections and disease would benefit from sorting individuals based on genetic background. If we can improve our knowledge in this area, the concept of personalized medicines may become closer to clinical application.
EXPERIMENTAL PROCEDURES
Mice
Atg16L1HM mice were described (Cadwell et al., 2008a). The Atg16L1HM line housed in a conventional barrier facility was re-derived by embryo transfer into the enhanced facility with the following specialized features: all cages, bedding, chow, and water are autoclaved prior to use; access to breeding mice in this facility is restricted to specially trained personnel; and sentinel mice are routinely screened for common mouse pathogens including MNV. Mice were generated by mating heterozygotes for the gene trap mutagenized Atg16L1 locus. Progeny homozygous for the mutation and wild-type littermates aged 7–15 weeks were used in experiments.
Viruses
Generation of concentrated stocks was described (Chachu et al., 2008). 25 μl of concentrated stocks (3×107 pfu) of MNV CR6 and CW3 was orally inoculated into mice. For virus quantification, total RNA was harvested per one stool pellet, 1 cm of intestine, or total mesenteric lymph nodes. qRT-PCR for MNV genome copy numbers was as described (Thackray et al., 2007). For detection of virus in Paneth cells, ~300 crypts from 3 mice/genotype were procured by laser capture microdissection (Extended Experimental Procedures) 7 days post infection with MNV CR6. As a positive control, MNV was detected in whole gut RNA from similarly prepared tissue.
Statistical Analysis
Lysozyme distribution, muscle thickness, lymphoid aggregates, and villus height were analyzed by two-tailed unpaired t tests. Viral genome copies were analyzed with the nonparametric Mann-Whitney test. Analyses except for microarray data used GraphPad Prism (version 5.00).
Supplementary Material
Acknowledgments
This research was supported by grant U54 AI057160 Project 6 and the Broad Foundation (K.C., N.M., and H.W.V.), R01 AI084887 (T.S.S. and H.W.V.), the Lallage Feazel Wall Fellowship DRG-1972-08 from the Damon Runyon Cancer Research Foundation (K.C.), training grant NIH T32-AI007172 (N.M.), the Pew Foundation (K.K.P. and T.S.S.), Washington University Digestive Diseases Research Core Center DK52574, Pfizer biomedical agreement with Washington University, fellowship award from the Crohn’s and Colitis Foundation of America (A.C.Y.N), and grants DK83756, DK086502, and DK043351 (R.J.X.). Washington University holds U.S. patents 7,041,444 B2, 7,264,923, and US 7,455,972 related to growth and detection of MNV. Washington University and H.W.V. receive income based on licenses for MNV technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agus SG, Dolin R, Wyatt RG, Tousimis AJ, Northrup RS. Acute infectious nonbacterial gastroenteritis: intestinal histopathology. Histologic and enzymatic alterations during illness produced by the Norwalk agent in man. Ann Intern Med. 1973;79:18–25. doi: 10.7326/0003-4819-79-1-18. [DOI] [PubMed] [Google Scholar]
- Altshuler D, Daly MJ, Lander ES. Genetic mapping in human disease 4. Science. 2008;322:881–888. doi: 10.1126/science.1156409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann CG, Messer RJ, Varvel K, Debuysscher BL, Lacasse RA, Pinto AK, Hasenkrug KJ. Effects from acute and chronic murine norovirus infections on immune responses and recovery from Friend retrovirus infection. J Virol. 2009 doi: 10.1128/JVI.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Barrett JC, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem-Exner BG, Sattler C, Hutchinson JA, Koehl GE, Kronenberg K, Farkas S, Inoue S, Blank C, Knechtle SJ, Schlitt HJ, Fandrich F, Geissler EK. Macrophages driven to a novel state of activation have anti-inflammatory properties in mice. J Immunol. 2008;180:335–349. doi: 10.4049/jimmunol.180.1.335. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le GS, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114:346–356. doi: 10.1182/blood-2008-12-191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008a;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Komatsu M, Virgin HW, Stappenbeck TS. A common role for Atg16L1, Atg5, and Atg7 in small intestinal Paneth cells and Crohn’s disease. Autophagy. 2008b;5:250–252. doi: 10.4161/auto.5.2.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 2008;4:e1000236. doi: 10.1371/journal.ppat.1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand N, Mihas AA. Celiac disease: current concepts in diagnosis and treatment. J Clin Gastroenterol. 2006;40:3–14. doi: 10.1097/01.mcg.0000190644.01661.2b. [DOI] [PubMed] [Google Scholar]
- Day DW, Morson BC, Williams GT. Morson and Dawson’s gastrointestinal pathology. Malden, Massachusetts: Blackwell Publishing; 2003. [Google Scholar]
- Dolin R, Levy AG, Wyatt RG, Thornhill TS, Gardner JD. Viral gastroenteritis induced by the Hawaii agent. Jejunal histopathology and serologic response. Am J Med. 1975;59:761–768. doi: 10.1016/0002-9343(75)90461-1. [DOI] [PubMed] [Google Scholar]
- Doom CM, Turula HM, Hill AB. Investigation of the impact of the common animal facility contaminant murine norovirus on experimental murine cytomegalovirus infection. Virology. 2009;392:153–161. doi: 10.1016/j.virol.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrberg T, Schwimmbeck PL, Oldstone MB. Inhibition of diabetes in BB rats by virus infection. J Clin Invest. 1988;81:928–931. doi: 10.1172/JCI113405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S, Capobianchi MR, Pallone F, Di MP, Boirivant M, Dianzani F, Torsoli A. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn’s disease. Kinetics of in vitro response to interferon gamma inducers. Gut. 1991;32:403–407. doi: 10.1136/gut.32.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss IJ, Neurath M, Boirivant M, Klein JS, de La MC, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastro. 2006;130:1588–1594. doi: 10.1053/j.gastro.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Goto K, Hayashimoto N, Yasuda M, Ishida T, Kameda S, Takakura A, Itoh T. Molecular detection of murine norovirus from experimentally and spontaneously infected mice. Exp Anim. 2009;58:135–140. doi: 10.1538/expanim.58.135. [DOI] [PubMed] [Google Scholar]
- Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastro. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De LV, Briggs J, Gunther S, Prescott NJ, Onnie CM, Hasler R, Sipos B, Folsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- Hensley SE, Pinto AK, Hickman HD, Kastenmayer RJ, Bennink JR, Virgin HW, Yewdell JW. Murine norovirus infection has no significant effect on adaptive immunity to vaccinia virus or influenza A virus. J Virol. 2009;83:7357–7360. doi: 10.1128/JVI.00623-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Wobus CE, Steffen EK, Riley LK, Livingston RS. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin Diagn Lab Immunol. 2005;12:1145–1151. doi: 10.1128/CDLI.12.10.1145-1151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K, Okuda K. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci U S A. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju JS, Miller SE, Jackson E, Cadwell K, Piwnica-Worms D, Weihl CC. Quantitation of selective autophagic protein aggregate degradation in vitro and in vivo using luciferase reporters. Autophagy. 2009;5 doi: 10.4161/auto.5.4.7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Bloom SM, Norian LA, Geske MJ, Flavell RA, Stappenbeck TS, Allen PM. An antibiotic-responsive mouse model of fulminant ulcerative colitis. PLoS Med. 2008;5:e41. doi: 10.1371/journal.pmed.0050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- Lake FR, Noble PW, Henson PM, Riches DW. Functional switching of macrophage responses to tumor necrosis factor-alpha (TNF alpha) by interferons. Implications for the pleiotropic activities of TNF alpha. J Clin Invest. 1994;93:1661–1669. doi: 10.1172/JCI117148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencioni KC, Seamons A, Treuting PM, Maggio-Price L, Brabb T. Murine norovirus: an intercurrent variable in a mouse model of bacteria-induced inflammatory bowel disease 1. Comp Med. 2008;58:522–533. [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, Kawaoka Y, Rosen H, Oldstone MB. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci U S A. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroianni JR, Ouellette AJ. Alpha-defensins in enteric innate immunity: functional Paneth cell alpha-defensins in mouse colonic lumen. J Biol Chem. 2009;284:27848–27856. doi: 10.1074/jbc.M109.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. Murine Norovirus 1 Infection Is Associated with Histopathological Changes in Immunocompetent Hosts, but Clinical Disease Is Prevented by STAT1-Dependent Interferon Responses. J Virol. 2007;81:3251–3263. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science. 1988;239:500–502. doi: 10.1126/science.3277269. [DOI] [PubMed] [Google Scholar]
- Ouellette AJ. Paneth cell alpha-defensin synthesis and function. Curr Top Microbiol Immunol. 2006;306:1–25. doi: 10.1007/3-540-29916-5_1. [DOI] [PubMed] [Google Scholar]
- Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292–301. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet PF, Vesin C, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis and detachment in mice: induction of caspases and prevention by a caspase inhibitor, ZVAD-fmk. Lab Invest. 1999;79:495–500. [PubMed] [Google Scholar]
- Porter CK, Tribble DR, Aliaga PA, Halvorson HA, Riddle MS. Infectious gastroenteritis and risk of developing inflammatory bowel disease 1. Gastro. 2008;135:781–786. doi: 10.1053/j.gastro.2008.05.081. [DOI] [PubMed] [Google Scholar]
- Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett-Corning KR, Cosentino J, Clifford CB. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim. 2009;43:165–173. doi: 10.1258/la.2008.008009. [DOI] [PubMed] [Google Scholar]
- Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor RB. The influence of normal microbial flora on the development of chronic mucosal inflammation. Res Immunol. 1997;148:567–576. doi: 10.1016/s0923-2494(98)80151-x. [DOI] [PubMed] [Google Scholar]
- Shapira SD, Gat-Viks I, Shum BO, Dricot A, de Grace MM, Wu L, Gupta PB, Hao T, Silver SJ, Root DE, Hill DE, Regev A, Hacohen N. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell. 2009;139:1255–1267. doi: 10.1016/j.cell.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, Shong M, Lee MS. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol. 2001;166:4481–4489. doi: 10.4049/jimmunol.166.7.4481. [DOI] [PubMed] [Google Scholar]
- Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent aplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan SR, Hanauer SB, Van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- Thackray LB, Wobus CE, Chachu KA, Liu B, Alegre ER, Henderson KS, Kelley ST, Virgin HW. Murine Noroviruses Comprising a Single Genogroup Exhibit Biological Diversity despite Limited Sequence Divergence. J Virol. 2007;81:10460–10473. doi: 10.1128/JVI.00783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonietti G, Oldstone MB, Dixon FJ. The effect of induced chronic viral infections on the immunologic diseases of New Zealand mice. J Exp Med. 1970;132:89–109. doi: 10.1084/jem.132.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Levine B. Autophagy genes in immunity. Nat Immunol. 2009;10:461–470. doi: 10.1038/ni.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Weersma RK, Zhernakova A, Nolte IM, Lefebvre C, Rioux JD, Mulder F, van Dullemen HM, Kleibeuker JH, Wijmenga C, Dijkstra G. ATG16L1 and IL23R Are Associated With Inflammatory Bowel Diseases but Not With Celiac Disease in The Netherlands. Am J Gastroenterol. 2007 doi: 10.1111/j.1572-0241.2007.01660.x. [DOI] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.