Abstract
In several epidemiological studies, moderate ethanol consumption has been associated with reduced risks of cognitive decline or Alzheimer’s dementia. Of potential relevance is that brain cultures preconditioned with moderate ethanol concentrations are resistant to neurotoxic Alzheimer’s amyloid-β (Aβ) peptides. Using rat cerebellar mixed cultures we investigated whether certain membrane receptors were early ‘sensors’ in moderate ethanol preconditioning (MEP). In a 6-day MEP protocol (30 mM ethanol), neuroprotection from Aβ25–35 was undiminished by antagonism during the first 3 days of either adenosine A1 or Gαi/o protein-coupled receptors. However, similar cotreatment with memantine or DL-2-amino-5-phosphono-pentanoic acid (AP-5), antagonists of NMDA receptors (NMDAR), abolished neuroprotection, indicating key early involvement of this ionotropic glutamate receptor. Also in these cultures, directly activating NMDAR using subexcitotoxic NMDA preconditioning prevented Aβ neurotoxicity. By day 2 of MEP, we observed increased levels of NMDAR subunits NR1, NR2B, and NR2C that persisted through day 6. Interestingly, memantine co-exposure blocked elevations in the obligatory NR1 subunit. Furthermore, 2 days of MEP significantly increased two indicators of synaptic NMDAR localization, NR2B phospho-Tyr1472, and post-synaptic density 95 scaffolding protein. The results indicate that ethanol preconditioning-dependent neuroprotection is associated with early increases in NR subunits concomitant with enhancement of synaptic localization and activity of NMDAR.
Keywords: alcohol, neurotoxicity, tolerance
Preconditioning refers to stress-dependent adaptive responses that generate a tolerant cellular state, such that preconditioned tissues or organs are resistant to a normally lethal insult. Neuroprotective stress examples include brief ischemia, hypoxia, seizures, and an array of chemicals that induce cellular tolerance, possibly via a ‘sensor-transducer-effector’ mechanism (Dirnagl et al. 2003). Our laboratories reported that subchronic preconditioning of rat brain cultures with concentrations of ethanol (20–30 mM) considered moderate to high-moderate in terms of social consumption blood levels (Eckardt et al. 1998; Gunzerath et al. 2004) – termed moderate ethanol preconditioning (MEP) – afforded neuroprotection from the dementia-associated neurotoxic peptides, HIV-1 gp120, or amyloid-β (Aβ) (Collins et al. 2000; Belmadani et al. 2001, 2004).
This study’s objective was to elucidate possible ‘sensor’ roles of certain cell surface receptors that were identified in neuroprotective ischemic preconditioning, in MEP-mediated neuroprotection in vitro against Aβ, the peptide(s) linked to neurodegeneration in Alzheimer’s disease (AD) brain. Although MEP neuroprotection was demonstrated earlier against pathophysiological Aβ1–42 (Belmadani et al. 2004), these experiments used the pathologically active but nonphysiological Aβ25–35 peptide, which has a neurotoxic mechanism apparently identical to Aβ1–42 (Frozza et al. 2009) as well as being the critical sequence within Aβ1–42 responsible for impairment of long-term potentiation (Lee et al. 2009). Here our findings with MEP implicate activation of glutamatergic NMDA receptors (NMDAR) – but rule against adenosine A1 or other Gαi/o protein-coupled receptors – in attaining a neuroprotected state. The ethanol preconditioning appears to modulate NMDAR by increasing subunit expression while enhancing synaptic receptor localization and connectivity, ultimately triggering pathways favoring neuroprotection against toxic peptides that are implicated in synaptic loss, for example, in AD. As such, the findings are consistent with the neuroprotective mechanism of ischemic preconditioning of brain cultures (Lin et al. 2008), in which there is an important role for activation of NMDAR.
We posit that this neuroprotective phenomenon of ethanol may yield insight into epidemiological results often indicating that moderate alcohol consumers have a lower risk of dementia, including AD, compared with abstainers (Zuccala et al. 2001; Mukamal et al. 2003; Peters et al. 2008). A prevalent neurodegenerative disorder of aging, AD has been linked to neurotoxic Aβ peptides (particularly Aβ1–42) that accumulate and form soluble oligomers and amyloid plaques in brain. Soluble oligomeric Aβ levels in AD brain, more so than amyloid plaque burden, correlate with cognitive deficits among AD patients (Naslund et al. 2000). Aside from acetylcholinesterase inhibitors, memantine, which antagonizes the extrasynaptic NMDA subclass of glutamate receptors (NMDAR) to suppress excitotoxic receptor activation (Leveille et al. 2008), is the only FDA-approved drug that has shown promise in delaying AD-related symptoms. Given the increasing prevalence of AD, investigations into mechanisms that prevent or delay the disease and lead to new therapeutics are of the utmost importance.
Materials and methods
Chemicals and antibodies
Hoechst 33342, propidium iodide (PI), DL-2-amino-5-phosphonopentanoic acid (AP-5), 8-Cyclopentyl-1,3-dipropylxanthine, pertussis toxin, memantine, NMDA, deoxyribonuclease I, Tweenr 20, Triton-X, protease and phosphatase inhibitor cocktails, and penicillin/streptomycin were obtained from Sigma Co. (St Louis, MO, USA). Neurobasal-A media, B27, trypsin, and fetal bovine serum (FBS) were obtained from Gibco (Carlsbad, CA, USA). American Peptide (Sunnyvale, CA, USA) provided Aβ25–35 and Aβ35–25. Polyacrylamide gels were obtained from GenScript (Piscataway, NJ, USA). Western blot buffers and enhanced chemiluminescence detection solutions were obtained from Pierce Chemical Co. (Rockford, IL, USA). The antibodies obtained from Upstate Co. (Temecula, CA, USA) were anti NR1-CT (1 : 1000) and anti-NR2B (1 : 1000); anti-NR2C (1 : 1000), post-synaptic density 95 (PSD-95; 1 : 1000), NR2B-pY1472 (1 : 1000), NeuN (1 : 1000), and glial fibrillary acidic protein (GFAP) (1 : 1000) were obtained from Chemicon International (Temecula, CA, USA); and actin (1 : 1000) and horseradish peroxidase-conjugated anti-rabbit IgG (1 : 1000–1 : 20000) were obtained from Cell Signaling (Danvers, MA, USA).
Cerebellar primary cultures
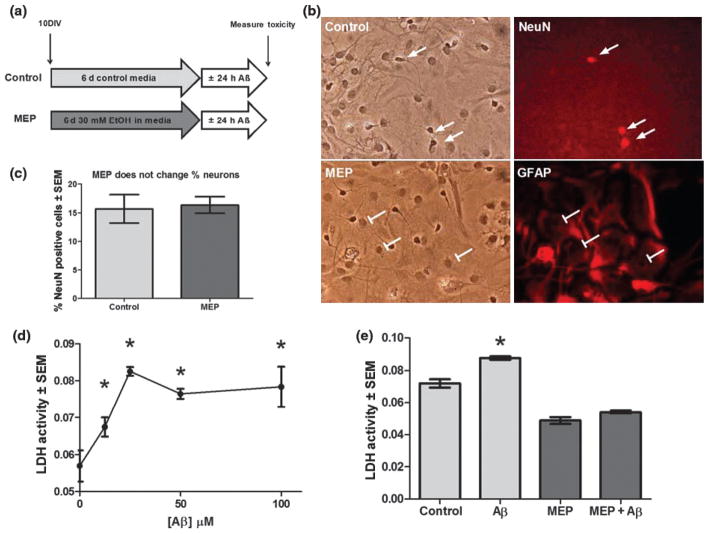
Rats were taken care of according to principles in the Guidelines for the Use of Animals in Neuroscience Research by Society for Neuroscience, and procedures were approved by LUMC IACUC. Rat pups (7-day-old S/D, Harlan, Indianapolis IN, USA) were cold-anesthetized on ice for 2 min and decapitated. Brains were placed in cold Gey’s balanced salt solution with 4.5 mg/mL glucose. After removal of meninges, individual cerebella were divided into approximately six pieces and placed in 37°C calcium-/magnesium-free Hank’s balanced salt solution containing 0.25% trypsin and 0.5 mg/mL DNAse. After 10 min, pieces were transferred to minimal essential medium containing 10% FBS and 5% horse serum. Cells were triturated with two fire-polished pipettes of decreasing size and passing through a 70 μm cell strainer. After determining viable cell density by trypan blue exclusion, cells were diluted to 800 000 cells/mL in Neurobasal-A supplemented with 2% B27, 10% heat-inactivated FBS, 500 μM L-glutamine, and 1% penicillin/streptomycin, and plated on poly-L-lysine-coated 12-well plates at 1 mL/well or 20 mL/10 cm dish. Half of the same growth media less serum was changed every 3 days until 10 days in vitro (DIV), when 100% was changed. This allowed glia to proliferate to confluence and produced neurons with robust processes and intricate neural networks (Fig. 1b).
Fig. 1.
Dose-related Aβ neurotoxicity and neuroprotection by moderate ethanol preconditioning (MEP). (a) MEP protocol: cerebellar cultures (10 DIV) were treated with 30 mM EtOH or control media for 6 days, rinsed, and exposed for 24 h to 25 μM Aβ25–35 or vehicle. (b) Control (top row) and MEP-treated (bottom row) cerebellar cultures (16 DIV) were fixed and analyzed by immunocytochemistry for neural cell type markers [neuronal (NeuN+) and glial (GFAP+) cell types] and co-stained with Hoechst 33342, identifying all nuclei. (c) MEP did not alter neuronal composition of cerebellar cultures. Cerebellar cultures were treated as in b and the percent of total (Hoechst 33342-positive) cells that were positive for NeuN were quantified in MEP and control-treated cultures (n = 3 at least 100 cells counted per well). (d) Doserelated neurotoxicity of cerebellar cultures (16 DIV) to Aβ25–35 as measured by LDH release; *p < 0.05 versus no Aβ25–35 (n = 6). (e) Aβ neurotoxicity in cerebellar cultures was prevented by MEP. *p < 0.05 versus control; 1-way ANOVA with Scheffe post hoc test (n = 6).
Ethanol preconditioning and Aβ exposure
Culture media was changed to media containing 30 mM ethanol or media alone at 10 DIV. Culture plates containing ethanol were placed in covered Tupperware containers containing small open dishes of 0.5% ethanol/water to minimize evaporative ethanol loss from experimental wells. The containers were allowed to equilibrate with incubator atmosphere for 15 min and closed. Culture media were changed with fresh ethanol at day 3. At day 6, ethanol media and control media were completely removed and media with/without Aβ 25–35 or reverse peptide was added. For drug treatments, a parallel set of plates was prepared, differing only by the presence of added drug.
Lactate dehydrogenase assay
Media were collected from cultures and spun at 5000 g for 5 min. The manufacturer’s microplate protocol was used to determine toxicity. Lactate dehydrogenase (LDH) activity correlated linearly (R2 = 0.9992) with the number of live cerebellar cells lysed over the activity ranges in these experiments. Neurotoxicity was reported as LDH activity/100 μL culture media.
Dual fluorescence staining
Cerebellar cell cultures were incubated 15 min at 37°C with 5 μM Hoechst 33342 which labeled nuclei of all cells and 5 μg/mL PI which stained dead/dying neurons. The extent of cytotoxicity defined as percent of dying neurons was obtained by dividing the number of PI-positive neurons by the total number of Hoechst-stained nuclei 100×. Approximately 500 nuclei were counted per well and six wells per experimental group. Images were subsequently analyzed with NIH ImageJ 1.34s (NIH, Bethesda, MD, USA).
Western blot analyses
Following ethanol and drug treatments, cerebellar samples grown on 10 cm dishes were rinsed once with ice-cold Tris-buffered saline, snap-frozen in acetone/dry ice, and stored at −80°C until analysis. Extracts of cells (5–20 μg protein) in the presence of protease and phosphatase inhibitor cocktails were run on 4–12% polyacrylamide gels, transferred to polyvinylidene difluoride membrane blocked with 1–3% bovine serum albumin, and exposed to appropriate primary antibodies, diluted based on manufacturer’s recommendations. Horseradish peroxidase-conjugated secondary antibodies were diluted in Tris-buffered saline wash buffer containing 0.1% Tween 20, incubated for 3 h, and developed using enhanced chemiluminescence detection. X-ray films were scanned and analyzed with NIH ImageJ 1.34s. To establish equal loading and normalize intensities of NMDAR subunits, blots were subsequently stripped and probed for β-actin, which was unaffected by the ethanol preconditioning.
Immunocytochemistry
Following ethanol or control treatment, cultures were washed 2× with pre-warmed phosphate-buffered saline (PBS), fixed for 15 min at 37°C with 4% paraformaldehyde, and stored in PBS containing 0.05% NaN3 for later viewing. Monolayers were permeabilized with PBS containing 0.3% Triton X-100 and blocked with 5% serum of secondary antibody host for 3 h at 27°C. Primary antibodies were incubated overnight at 4°C in blocking buffer, washed, and incubated with corresponding biotinylated secondary antibody. Streptavidin-conjugated Cy2 or Cy3 (1 : 200) was then applied for 2 h at 27°C, washed, and stored in PBS containing 0.05% NaN3. Primary delete experiments showed specificity of cell-type antibodies. Cultures were digitized on an inverted epiluminescent fluorescent microscope equipped with a Nikon digital camera (Nikon Inc., Melville, NY, USA) for image acquisition. Images were subsequently analyzed with NIH ImageJ 1.34s.
Results
Aβ causes dose-related neurotoxicity in cerebellar mixed cultures that is inhibited by ethanol preconditioning
Our laboratories previously reported that the MEP protocol in Fig. 1a blocks Aβ-induced neurotoxicity in hippocampal– cortical slice cultures (Belmadani et al. 2004). Although the cerebellum is relatively resistant to amyloid peptides in vivo (Kim et al. 2003), cerebellar granule neurons are vulnerable to Aβ in vitro (Allen et al. 1999). Our mixed cerebellar cultures contained 15–20% NeuN-positive neuronal cell types and a mixture of non-neuronal, primarily GFAP-positive cell types (Fig. 1b). MEP treatment affected neither the neuronal viability (Fig. 1c) nor the total cell number of these cultures (not shown). At 16 DIV, the cultures displayed dose-related Aβ25–35 neurotoxicity (Fig. 1d, 25 μM for 24 h); the toxicity which was because of Aβ25–35 was significantly counteracted in ethanol-preconditioned cultures (Fig. 1e) as determined by released LDH. As expected, the reverse peptide Aβ35–25 (25 μM) was not neurotoxic (results not shown). Notably, reduced LDH release in MEP cultures compared with control suggested cytoprotection by MEP from toxicity associated with general medium changes. Similar indications of such protection were observed during other MEP experiments, as well as by other investigators who utilized moderate, subchronic ethanol exposures (Thomas and Morrisett 2000).
Acquisition of ethanol preconditioning-induced neuroprotection is NMDAR-dependent
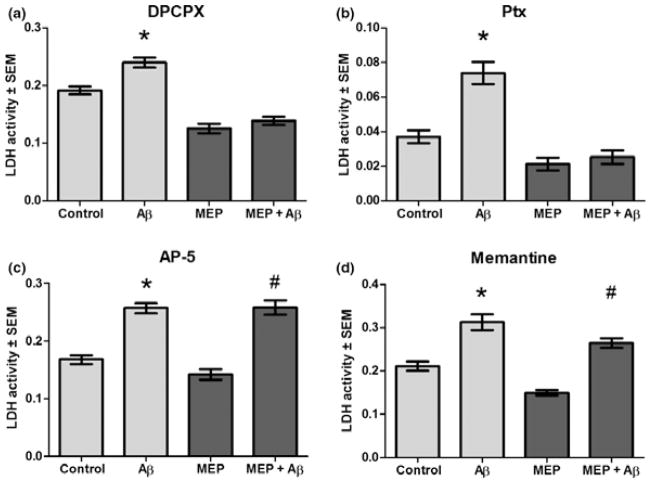
To determine if adenosine/Gαi/o protein-coupled receptors or NMDAR. implicated in other forms of brain preconditioning neuroprotection, were involved in MEP neuroprotection, we examined whether antagonism of these receptors reduced or prevented Aβ neurotoxicity. Figure 2 shows that when present in culture media for the first 3 days of preconditioning, neither a specific adenosine A1 receptor antagonist [dipropylxanthine (DPCPX), Fig. 2a] nor the Gαi/o protein-coupled receptor inhibitor, pertussis toxin (Ptx, Fig. 2b) significantly counteracted neuroprotection from Aβ because of 6 days of MEP. However, AP-5, a specific high affinity NMDAR antagonist, completely abolished the neuroprotective effects of MEP (Fig. 2c). Furthermore, memantine, a moderate affinity open channel NMDAR antagonist, blocked most of the MEP neuroprotection (Fig. 2d). These results are entirely consistent with a relatively early ‘sensor’ role for NMDAR in the MEP mechanism.
Fig. 2.
MEP-induced neuroprotection against Aβ: NMDAR antagonists abolished protection but antagonism of adenosine A1 receptors or Gi/o protein-coupled receptors had no effect. MEP of cerebellar cultures (as in Fig. 1) was performed with the respective receptor antagonists present for the first 3 days in a 6-day protocol, followed by 25 μM Aβ25–35 or vehicle for 24 h and media LDH assays. (a) dipropylxanthine (DPCPX) (100 nM), adenosine A1 receptor antagonist, did not prevent MEP neuroprotection. (b) Pertussis toxin (Ptx, 50 ng/mL), Gi/o protein-coupled receptor antagonist, also did not alter MEP neuroprotection. (c) AP-5 (45 μM), NMDAR antagonist, completely abolished MEP neuroprotection. (d) Memantine (50 μM), NMDAR antagonist, significantly inhibited MEP neuroprotection. *p < 0.001 versus control; #p < 0.001 versus MEP; 1-way ANOVA with Scheffe post hoc test (n = 6).
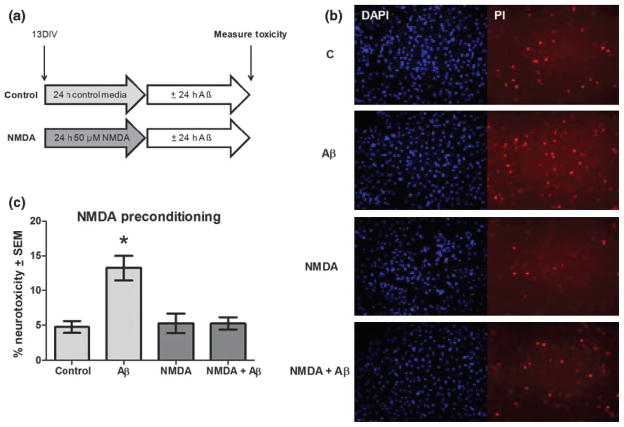
Preconditioning with NMDA neuroprotects from Aβ as determined by dual fluorescence staining
If NMDAR activation is essential in MEP neuroprotection, direct stimulation of this receptor via NMDA preconditioning should elicit similar protection from Aβ insult in our cultures. As shown in Fig. 3a, cultures at 13 DIV were preconditioned for 24 h with 50 μM NMDA, previously determined to be subneurotoxic in this dose range with cerebellar cultures (R. Mitchell, unpublished data), and then exposed for 24 h to 12.5 μM Aβ25–35 or media only. The 13 DIV time point which corresponds to halfway through the MEP exposure was chosen because of our preliminary findings that both subclasses (NR1 and NR2) of NMDAR subunits were expressed at that time. In Fig. 3b are representative live-cell photomicrographs of dual fluorescence staining with Hoechst 33342 and PI, a combination shown to correlate well in cerebellar cultures with other cytotoxic measures (Bachis et al. 2003). Hoechst 33342 labels nuclei of all cells and PI stains dying/dead neurons in the control (C) and NMDA-preconditioned cultures. PI-stained cell densities between control and NMDA preconditioned groups did not differ, verifying that NMDA treatment was not neurotoxic. However, cultures treated with Aβ demonstrated increased density of PI-labeled degenerating neurons compared with C or NMDA, whereas the NMDA-preconditioned cultures treated with Aβ (NMDA + Aβ) appeared similar to C or NMDA alone. Quantification of stained dead cells/total cells, represented as % neurotoxicity in Fig. 3c, showed that Aβ-induced neurodegeneration was completely prevented by NMDA preconditioning. Thus, NMDAR activation by NMDA ligand binding, or by extension, ethanol preconditioning-augmented NMDAR activity, promoted a neuroprotected state in these cerebellar cell cultures, rendering them resistant to Aβ insult.
Fig. 3.
NMDA preconditioning protects against Aβ neurotoxicity as determined by dual fluorescence staining with Hoescht 33342 and PI. (a) Cerebellar cultures (13 DIV) were treated for 24 h with NMDA (50 μM) or control media followed by 24 h in 25 μM Aβ25–35. (b) Representative images of neurotoxicity assayed by live cell dual fluorescence staining. (c) Quantification of Aβ25–35 neurotoxicity (% Hoescht stained nuclei that are PI-positive) in NMDA-preconditioned or control cultures. *p < 0.001 versus control; 1-way ANOVA with Scheffe post hoc test (n = 6).
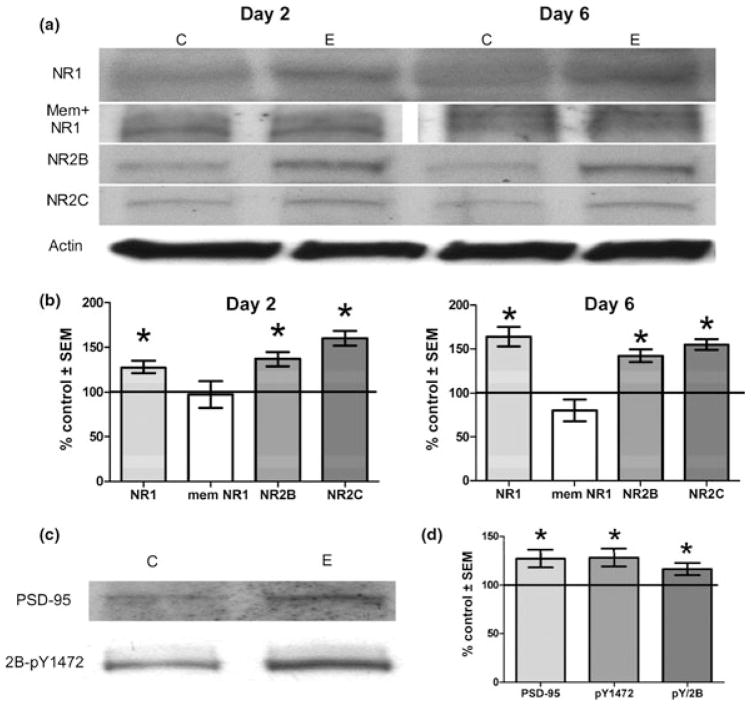
NMDAR subunits are significantly elevated by ethanol preconditioning and memantine abrogates potentiation of obligatory NR1 subunits
As the data implied increased receptor activity because of moderate ethanol exposure, we then examined whether changes in levels of NMDAR subunits could potentially underlie receptor activity. Indeed, the data in Fig. 4 demonstrate that NR1, the obligatory subunit for NMDAR function, and NR2B and NR2C, which contain glutamate binding domain(s), were each significantly elevated by day 2 of 30 mM ethanol exposure and remained elevated after 6 days of preconditioning (Fig. 4a and b). Not shown for simplicity was that the NMDAR subunits were also elevated at day 4 in ethanol preconditioning. It was also apparent that co-treatment with memantine antagonist which counteracted MEP neuroprotection from Aβ and restored the neurotoxic effects of peptides (Fig. 2d), completely prevented the 2 day elevations in the obligatory NR1 subunit (Fig. 4a and b).
Fig. 4.
Increased protein levels of NMDAR subunits by day 2 of MEP persist through day 6 and correlate with indicators of synaptically localized complexes; memantine co-treatment blocks day 2 up-regulation of obligatory NR1 subunit. Cerebellar cultures (10 DIV) were treated with 30 mM ethanol or control media for 2 or 6 days, protein extracted and subjected to immunoblot analyses. (a) Representative immunoblots of NMDAR subunits NR1, NR1 with 45 μM memantine, NR2B, and NR2C after ethanol (E) or control (C) media. (b) Quantification of immunoblot analyses normalized to actin and the results expressed as % control. (c) Representative immunoblots of NMDAR subunit NR2B phosphorylated tyrosine 1472 (2B-pY1472) and synaptic scaffolding protein PSD-95. (d) Immunoblot quantification of PSD-95, 2B-pY1472 and the ratio of 2B-pY1472 to total NR2B expressed as % control. *p < 0.05 versus control by student’s t-test (n = 3–6).
Increases in NR2B phosphotyrosine-1472 and PSD-95 after 2 days of ethanol preconditioning indicate increased synaptically localized NMDAR
Questioning whether MEP-induced NMDAR activation was primarily synaptic, we examined ethanol’s effects on levels of tyrosine 1472-phosphorylated NR2B (NR2B-pY1472) and PSD-95 relatively early in the 6-day preconditioning protocol. The activity and synaptic localization of NMDAR are believed to require phosphorylation of subunits, including tyrosine 1472 of NR2B by Src family tyrosine kinases (Yu et al. 1997; Goebel-Goody et al. 2009). Also, PSD-95 is a cytoskeletal protein involved in the dynamic regulation of synaptic NMDAR complex formation which links the receptors to downstream signaling moieties (Delint-Ramirez et al. 2008; Kurisaki et al. 2008). As shown in Fig. 4c and d, after 2 days of moderate ethanol exposure, the levels of both NR2B-pY1472 (2B-pY1472) and PSD-95 were significantly elevated. Furthermore, although ethanol preconditioning potentiated NR2B protein levels at this time (Fig. 4a and b), tyrosine kinase activity acting on NR2B – most likely of the src family – was evidently increased as well, as demonstrated by the significantly increased ratio of NR2B-pY1472/total NR2B (pY/2B in Fig. 4d) in ethanol-treated cultures. The results point to NMDAR subunit up-regulation in association with enhanced synaptic NMDAR localization as relatively early events of the ethanol preconditioning mechanism.
Discussion
One overall conclusion derived from these experiments is that neuroprotection engendered by preconditioning rat cerebellar cultures with moderate ethanol concentrations involves NMDAR activation as a likely neuroprotective sensor. Recapitulating, pharmacological inhibition of NMDAR early during MEP disallowed later neuroprotection. In contrast, antagonism of adenosine A1 receptors or of Gαi/o protein-coupled receptors generally was without effect. The data agree with evidence for glutamatergic NMDAR involvement in brain preconditioning that originated soon after the discovery of ischemic neuronal tolerance (Kato et al. 1992). More recent reports confirm a neuroprotective role for NMDAR in ischemic preconditioning (Grabb and Choi 1999; Raval et al. 2003).
The fact that preconditioning with subneurotoxic NMDA ligand itself affords neuroprotection from Aβ in our cerebellar cultures lends additional support to the possibility that NMDAR activation mediates moderate ethanol-initiated prosurvival signaling, and agrees with NMDA preconditioning/neuroprotection results in other primary cultures (Soriano et al. 2006; Smith et al. 2008). NMDAR excitability appeared critical as well in preconditioning with glutamate or prolonged elevations in electrical activity, as NMDAR antagonists blocked the resultant neuroprotection against subsequent ischemia (Lin et al. 2008; Tauskela et al. 2008). However, despite this correspondence, we acknowledge that protective pathway of NMDA and mechanism of neuroprotection by ethanol may well differ in other ways.
Ethanol is known to acutely inhibit and chronically potentiate NMDAR currents, although exact mechanisms remain a matter of active investigation (Nagy 2008). We find that persistent increases in subunits NR1, NR2B, and NR2C occur by the first 2 days of moderate ethanol exposure. Such increases in receptor subunits could be a principal reason for increased NMDAR activity leading to neuroprotection. This possibility is borne out by memantine’s blockade of ethanol-dependent increases in NR1, the receptor’s obligatory subunit, thus contributing to a reduction in NMDAR activity and the annulment of MEP neuroprotection. The above results coincide with hippocampal culture expression data (Maler et al. 2005), in which memantine, with negligible effect alone on NMDAR subunits, suppressed the up-regulation of NR1 by 5 days of 50 mM ethanol. Of in vivo relevance to our in vitro findings is that rats chronically fed moderate ethanol-containing diets demonstrated improved memory performance which was dependent on NR1 (Kalev-Zylinska and During 2007). RNA interference knockdown of hippocampal NR1 abolished ethanol’s facilitatory effects in the model, while NR1 over-expression mimicked ethanol’s effects. Although NR2 subunits are known ethanol targets (Woodward et al. 2006), levels of NR1 subunits invariably exert critical effects on NMDAR function.
A further critical aspect of NMDAR dynamics related to MEP is our evidence for early MEP-dependent receptor increases that reflect synaptic as opposed to extrasynaptic NMDAR – specifically, levels of NR2B phospho-tyrosine 1472, which are associated with decreased endocytosis and synaptic enrichment of NMDAR (Yu et al. 1997; Goebel-Goody et al. 2009), and of PSD-95, which implicate increased synaptic receptor localization (Delint-Ramirez et al. 2008; Kurisaki et al. 2008). These findings are in harmony with data showing that increases in synaptic relative to extrasynaptic NMDAR signaling are associated with (or signal) downstream pro-survival events (Soriano et al. 2006; Soriano and Hardingham 2007). Furthermore, the significantly augmented ratio of NR2B-pY1772/total NR2B at an early MEP timepoint of 2 days indicates potentially increased activity of Src-family kinase(s) (Goebel-Goody et al. 2009). This additional appealing aspect of MEP-dependent NMDAR modulation that requires further investigation is consistent with our findings that MEP increases cerebellar levels of a critical non-receptor kinase linked to Src activation, focal adhesion kinase (Sivaswamy et al. 2008).
A recent report showed that Aβ1–42-containing monomeric and oligomeric peptides bind to post-synaptic NMDAR-containing synapses, inhibited NMDAR, and decreased NMDAR immunoreactive spines and surface expression of NR2B-containing NMDAR (Dewachter et al. 2009). The authors also found decreased concentrations of NR2B and PSD-95 in a transgenic mouse model of AD. Others have reported that the suppressive effects of Aβ on NMDAR surface expression correlated with decreased NR2B-pY1472 (Snyder et al. 2005). Our results indicate that ethanol preconditioning may combat neurodegenerative processes induced by Aβ and other neurotoxic proteins (e.g., gp120) by preserving NMDAR-associated synaptic density and connectivity. Speculatively, MEP-mediated neuroprotection could entail the ability of synaptically localized NMDAR to enhance intrinsic antioxidant defenses – specifically, peroxiredoxin and thiol redoxin pathways (Papadia et al. 2008).
Although growing evidence suggests a physiological function of amyloid peptide fragments pertaining to regulation of synaptic transmission and homeostasis (Pearson and Peers 2006), aberrant Aβ peptide overproduction is considered central to AD pathophysiology and possibly age-related mild cognitive decline. The facts that synaptic loss correlates well with cognitive decline in vivo (Terry et al. 1991), and that Aβ peptides experimentally induce alterations in synapse composition and density (Lacor et al. 2007) underscore such losses. AD is reaching epidemic proportions among aging populations (Wang and Ding 2008); however, effective pharmaceutical approaches to slow AD progression are lacking. Investigations into the molecular mechanisms of ethanol preconditioning may help to clarify roles of NMDAR in AD etiology and offer new strategies for halting synaptic degeneration while preserving synaptic connectivity.
Acknowledgments
This project was made possible by funds received from NIH RO1 AA013568 (MAC), Loyola Potts Fund (MAC), Loyola Alcohol Research Program funded by NIH T32 AA013527 (RMM), and the Illinois Department of Public Health Alzheimer’s Disease Research Fund (RMM).
Abbreviations used
- AD
Alzheimer’s disease
- Aβ
amyloid-β
- AP-5
DL-2-amino-5-phosphono-pentanoic acid
- DIV
days in vitro
- FBS
fetal bovine serum
- GFAP
glial fibrillary acidic protein
- LDH
lactose dehydrogenase
- MEP
moderate ethanol preconditioning
- NMDAR
NMDA receptors
- PBS
phosphate-buffered saline
- PI
propidium iodide
- PSD-95
post-synaptic density 95
References
- Allen JW, Eldadah BA, Faden AI. Beta/amyloid-induced apoptosis of cerebellar granule cells and cortical neurons: exacerbation by selective inhibition of group I metabotropic glutamate receptors. Neuropharmacology. 1999;38:1243–1252. doi: 10.1016/s0028-3908(99)00044-1. [DOI] [PubMed] [Google Scholar]
- Bachis A, Major EO, Mocchetti I. Brain-derived neurotrophic factor inhibits human immunodeficiency virus-1/gp120-mediated cerebellar granule cell death by preventing gp120 internalization. J Neurosci. 2003;23:5715–5722. doi: 10.1523/JNEUROSCI.23-13-05715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A, Zou JY, Schipma MJ, Neafsey EJ, Collins MA. Ethanol pre-exposure suppresses HIV-1 glycoprotein 120-induced neuronal degeneration by abrogating endogenous glutamate/Ca2+-mediated neurotoxicity. Neuroscience. 2001;104:769–781. doi: 10.1016/s0306-4522(01)00139-7. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Kumar S, Schipma M, Collins MA, Neafsey EJ. Inhibition of amyloid-beta-induced neurotoxicity and apoptosis by moderate ethanol preconditioning. Neuroreport. 2004;15:2093–2096. doi: 10.1097/00001756-200409150-00019. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Zou JY. HIV-1 gp120 neurotoxicity in brain cultures is prevented by moderate ethanol pretreatment. Neuroreport. 2000;11:1219–1222. doi: 10.1097/00001756-200004270-00015. [DOI] [PubMed] [Google Scholar]
- Delint-Ramirez I, Salcedo-Tello P, Bermudez-Rattoni F. Spatial memory formation induces recruitment of NMDA receptor and PSD-95 to synaptic lipid rafts. J Neurochem. 2008;106:1658–1668. doi: 10.1111/j.1471-4159.2008.05523.x. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Filipkowski RK, Priller C, et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol Aging. 2009;30:241–256. doi: 10.1016/j.neurobiolaging.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Frozza RL, Horn AP, Hoppe JB, Simao F, Gerhardt D, Comiran RA, Salbego CG. A comparative study of betaamyloid peptides Abeta1-42 and Abeta25-35 toxicity in organotypic hippocampal slice cultures. Neurochem Res. 2009;34:295–303. doi: 10.1007/s11064-008-9776-8. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J Neurosci. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzerath L, Faden V, Zakhari S, Warren K. National Institute on Alcohol Abuse and Alcoholism report on moderate drinking. Alcohol Clin Exp Res. 2004;28:829–847. doi: 10.1097/01.alc.0000128382.79375.b6. [DOI] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, During MJ. Paradoxical facilitatory effect of low-dose alcohol consumption on memory mediated by NMDA receptors. J Neurosci. 2007;27:10456–10467. doi: 10.1523/JNEUROSCI.2789-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Liu Y, Araki T, Kogure K. MK-801, but not anisomycin, inhibits the induction of tolerance to ischemia in the gerbil hippocampus. Neurosci Lett. 1992;139:118–121. doi: 10.1016/0304-3940(92)90871-4. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Chae SC, Lee DK, Chromy B, Lee SC, Park YC, Klein WL, Krafft GA, Hong ST. Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J. 2003;17:118–120. doi: 10.1096/fj.01-0987fje. [DOI] [PubMed] [Google Scholar]
- Kurisaki A, Inoue I, Kurisaki K, Yamakawa N, Tsuchida K, Sugino H. Activin induces long-lasting N-methyl-D-aspartate receptor activation via scaffolding PDZ protein activin receptor interacting protein 1. Neuroscience. 2008;151:1225–1235. doi: 10.1016/j.neuroscience.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomerinduced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Kuo YM, Huang CC, Hsu KS. Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol Aging. 2009;30:377–387. doi: 10.1016/j.neurobiolaging.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Leveille F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, Buisson A. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. FASEB J. 2008;22:4258–4271. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- Lin CH, Chen PS, Gean PW. Glutamate preconditioning prevents neuronal death induced by combined oxygen-glucose deprivation in cultured cortical neurons. Eur J Pharmacol. 2008;589:85–93. doi: 10.1016/j.ejphar.2008.05.047. [DOI] [PubMed] [Google Scholar]
- Maler JM, Esselmann H, Wiltfang J, Kunz N, Lewczuk P, Reulbach U, Bleich S, Ruther E, Kornhuber J. Memantine inhibits ethanol-induced NMDA receptor up-regulation in rat hippocampal neurons. Brain Res. 2005;1052:156–162. doi: 10.1016/j.brainres.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289:1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- Nagy J. Alcohol related changes in regulation of NMDA receptor functions. Curr Neuropharmacol. 2008;6:39–54. doi: 10.2174/157015908783769662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson HA, Peers C. Physiological roles for amyloid beta peptides. J Physiol (Lond) 2006;575:5–10. doi: 10.1113/jphysiol.2006.111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Perez-Pinzon MA. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaswamy S, Neafsey EJ, Collins MA. PKC and focal adhesion kinase (FAK): possible transducer roles in ethanol preconditioning-induced neuroprotection from HIV-1 gp120. J Neurochem. 2008;104:32. [Google Scholar]
- Smith AJ, Stone TW, Smith RA. Preconditioning with NMDA protects against toxicity of 3-nitropropionic acid or glutamate in cultured cerebellar granule neurons. Neurosci Lett. 2008;440:294–298. doi: 10.1016/j.neulet.2008.05.066. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Soriano FX, Hardingham GE. Compartmentalized NMDA receptor signalling to survival and death. J Physiol (Lond) 2007;584:381–387. doi: 10.1113/jphysiol.2007.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Hofmann F, Hardingham NR, Bading H, Hardingham GE. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J Neurosci. 2006;26:4509–4518. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauskela JS, Fang H, Hewitt M, Brunette E, Ahuja T, Thivierge JP, Comas T, Mealing GA. Elevated synaptic activity preconditions neurons against an in vitro model of ischemia. J Biol Chem. 2008;283:34667–34676. doi: 10.1074/jbc.M805624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thomas MP, Morrisett RA. Dynamics of NMDARmediated neurotoxicity during chronic ethanol exposure and withdrawal. Neuropharmacology. 2000;39:218–226. doi: 10.1016/s0028-3908(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wang XP, Ding HL. Alzheimer’s disease: epidemiology, genetics, and beyond. Neurosci Bull. 2008;24:105–109. doi: 10.1007/s12264-008-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Ron D, Winder D, Roberto M. From blue states to up states: a regional view of NMDA–ethanol interactions. Alcohol Clin Exp Res. 2006;30:359–367. doi: 10.1111/j.1530-0277.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil GJ, II, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Onder G, Pedone C, Cesari M, Landi F, Bernabei R, Cocchi A. Dose-related impact of alcohol consumption on cognitive function in advanced age: results of a multicenter survey. Alcohol Clin Exp Res. 2001;25:1743–1748. [PubMed] [Google Scholar]