Abstract
Intensive research efforts have been placed on the development of nanospheres for targeted drug delivery for treating a variety of diseases, including coronary restenosis, cancer, and inflammatory reactions. Although most of these drug-bearing spheres are delivered via intravenous administration, little is known about the effect of sphere physical characteristics on the responses of vascular and blood cells. To find the answer, this work was aimed to investigate the cellular uptake of nano- (100nm) and micro-sized hydrogel spheres (1 µm) made of poly (N-isopropylacrylamide) by vascular cells and phagocytes under various flow conditions in vitro. We found that the cellular uptake of nanospheres depended on incubation times and sphere concentrations as well as on the introduced shear stress levels of the medium. Measurements of the intracellular released fluorescence and confocal fluorescence microscopy revealed that nanospheres were internalized by endothelial cells and smooth muscle cells more than microspheres, whereas microspheres were rapidly taken up by phagocytes, especially at high concentration. Our results strongly suggest that hydrogel nanospheres are more effective as an intra-vascular delivery system compared to microspheres in the term of vascular cellular uptake and biocompatibility.
Keywords: poly(N-isopropylacrylamide), nanospheres, microspheres, hydrogel, phagocytes, vascular cells, restenosis
Introduction
Coronary atherosclerosis is still the leading cause of mortality and morbidity in the United States. Over the last decade, several strategies to treat obstructive atherosclerotic vessels have been developed. Those include percutaneous transluminal coronary angioplasty (PTCA), excisional atherectomy, endoluminal stenting, laser ablation, and systematic administration of various drugs such as anti-thrombosis, anti-inflammation, and anti-proliferation agents. However, the restenosis rate continues to be up to 40% within 6 months after these treatments.1–3 Despite extensive research on the mechanical and pharmacological interventions in human and animal models, no therapy effectively prevents the problem of restenosis. The unsuccessful attempts to control restenosis by these interventions have prompted extensive research efforts to search for novel therapeutic approaches including locally delivered therapeutics using colloidal spheres.
Small colloidal spheres, such as microspheres and nanospheres, have been introduced in the seventies for use as locally drug and gene delivery.4–6 Therapeutic agents of interest can be either encapsulated within the polymeric matrix or adsorbed/conjugated onto the surface of these spheres. Local drug delivery using spheres can minimize this requirement and other problems such as the inability to use pharmacologically active doses due to systemic side effects and inactivity due to rapid metabolism and/or hydrolysis before reaching the target.4,5 To improve the efficacy of drug and gene therapies, it is important to deliver a therapeutic agent into specific tissues and cells at a sustained rate.4 It has been shown that, for intraluminal delivery, the size of a carrier is an important parameter for targeting, internalization, and effectiveness of a therapeutic cargo in drug and gene delivery strategies. For example, research has revealed that vascular cells, especially the endothelium, are only able to take up nanospheres (less than 500 nm).4,7–9
Nanospheres have several advantages as a carrier system for intra-arterial drug delivery compared to microspheres.4,10–12 One of the major advantages of nanospheres is that nanospheres have relatively higher intracellular uptake into the vessel wall compared to microspheres. For instance, nanospheres of about 100 and 200 nm have been found to deposit in the inner regions of the vessel wall, whereas 514 nm nanospheres accumulated primarily at the luminal surface of the aorta.8,12 In addition, various in vitro studies show that only the submicron size spheres are taken up efficiently but not the larger size microspheres in some cell lines.4,13 Furthermore, nanospheres usually cause little or no local inflammation, while microspheres in the size range of 5–10 µm produce prominent inflammatory reactions with subsequent fibrosis.4,10,11,14 Moreover, nanospheres can deliver any therapeutic agents including small molecular weight drugs, proteins, or genes.4–6,15 Finally, spheres with small size such as nanospheres are suitable and easier for the infusion through the pores of a delivery balloon.4
Although nanospheres provide several attractive properties for drug and gene therapy delivery application, they have low drug loading capacity and do not provide a sustained drug release. On the other hand, many studies have shown that microspheres have much better drug capacity and sustained drug release property.7 To better engineer intra-luminal drug delivery systems, it is important to investigate cellular uptake of these nanoparticles and microspheres in interested cell types. For the vascular system, it is also important to study cellular uptake of these particles in vascular cells under blood flow condition. In addition, it has been suggested that injected spheres are quickly removed by the reticuloendothelial system (RES), which includes circulating phagocyte cells.10,16–19 Therefore, for developing spheres with improved therapeutic efficacy, it is important to understand the relationship between sphere sizes, blood flow, and cellular uptake of spheres in vascular and phagocyte cells.
This work was focused on in vitro investigation of cellular uptake of poly-N-isopropylacrylamide (PNIPAm) nano and microspheres in various cell culture systems including mouse peritoneal phagocyte cells (PCs), rat aorta endothelial cells (ECs) and smooth muscle cells (SMCs). PNIPAm nano and microspheres were selected because these spheres could release a therapeutic agent in response to changes in temperature, exhibiting themselves as attractive intravascular drug carriers.20–22 Although various properties of PNIPAm based polymer gels and their biomedical applications including drug delivery have been investigated,20–23 the potential influence of sphere size and flow condition on cellular uptake of these spheres in various cell types has yet to be established. In addition, many experiments have explored cellular uptake of nanospheres in vascular cells such as ECs and SMCs,9,24–26 though none have studied cellular uptake of both PNIPAm nanospheres and microspheres in both vascular and phagocyte cells. Therefore, in our approach, several studies were developed to examine different parameters including, the effect of dose, incubation time, and shear stress on the subsequent cellular internalization of PNIPAm spheres in ECs, SMCs, and PCs in vitro. Intracellular uptake of spheres was assessed using both fluorescent confocal microscopy for visualization and fluorometry for quantitative analysis. Using a parallel flow plate system,27 this project was also aimed to investigate the cellular sphere uptake under static and dynamic flow conditions in vitro.
Materials and Methods
Materials
Cell culture media and supplements for cell culture were purchased from Gibco Laboratories (Lenexa, KS). Tissue culture plates and flasks were purchased from Fisher Scientific (Pittsburgh, PA). Total protein BCA assay kits were obtained from Pierce (Rockford, IL). FITC was purchased from Fluka BioChemika (Buchs, Switzerland). N-Isopropylacrylamide, allylamine, N,N’-methylenebisacrylamide (BIS), potassium persulfate, sodium dodecyl sulfate (SDS), and other chemicals (if not specified) were purchased from Sigma-Aldrich (St. Louis, MO).
Nano- and micro- spheres preparation
The PNIPAm nano-spheres (~100 nm diameter) and micro-spheres (~1 µm diameter) were prepared using free radical precipitation polymerization method.22 In brief, for the preparation of 1.0µm microspheres, a free radical polymerization of 1.9 g of NIPAM and 0.01g of allylamine was carried out in 150ml of aqueous medium containing 0.03 g of N, N’-methylene-bisacrylamide (BIS) as the cross-liking agent and 0.08g of potassium persulfate as the initiator. PNIPAm nano-spheres (~100 nm diameter) were synthesized by the same method as the 1.0 µm microsphere preparation except that 0.2 g of Sodium dodecyl sulphate (SDS) was used as a surfactant to control the sphere size. The reaction was kept at 68–70 °C under nitrogen atmosphere for 4 hours to ensure that all of the monomers reacted. After cooling the solution to room temperature, the unreacted chemicals and SDS was removed via dialyzing (MW cut off 12,000) against excess distilled sterilized deionized water for a week. The concentration of the dialyzed dispersion was calculated from the weight of a 1 ml dialyzed dispersion induced by a drying process. The size distribution of the spheres was analyzed in water by dynamic light scattering using Nanotrac (Microtrac, Inc., Montgomeryville, PA). The instruments optics insure that there is minimum penetration of light into the sample. The Microtrac Flex software (Microtrac, Inc., Montgomeryville, PA) analyzes the signals to calculate the Doppler shifts corresponding to sphere size. The spheres were conjugated with FITC by incubation of PNIPAm spheres and FITC dyes with the ratio of 40 µg FITC/mg spheres overnight at 4 °C as previously published.28 Free FITC was removed from FITC-labeled PNIPAm spheres by dialysis for 7 days.
Mouse peritoneal activated phagocytes
An in vitro culture system using mouse peritoneal phagocyte cells was employed to assess cellular uptake of spheres. Briefly, male Balb/C mice (20–25 gram body weight) were implanted intraperitoneally with sterile disks of polyethylene terephthalate.29 After implantation for 48 hours, the time for maximal phagocyte recruitment, implant-bearing animals were euthanized by CO2 inhalation. Peritoneal lavage was performed with injection of 4 ml of sterile complete DMEM (with 10% fetal bovine serum + 1% penicillin-streptomycin solution). The fluid was carefully recovered with sterile plastic transfer pipettes. The lavage phagocyte solution was diluted in complete DMEM to 3.0 × l06 cells/ml; and 1.0 ml of this cell-containing fluid was placed on the 24-well tissue culture plates overnight for the next day experiments as mentioned later.
Cell culture and seeding of ECs and SMCs for sphere uptake studies
Rat aorta endothelial cells and smooth muscle cells were isolated from Lewis rats (250–300 gram body weight) by collagenase using standard enzyme isolation protocols.30,31 After isolation, cells were subcultured in complete growth media (M199 containing 10% FBS and 1% penicillin-streptomycin solution for endothelial cells and DMEM supplemented with 10% FBS and 1% penicillin-streptomycin solution for SMCs). Cells were grown at 37 °C in a 5% CO2 atmosphere. After reaching to confluence, cells were passaged in a split ratio of 1:3. All experiments were performed with cells between passages 2–9. Aortic endothelial cells have typical endothelial cell characteristics including a "cobbled" morphology at confluence and positive staining for von Willebrand factor.30 On the other hand, SMCs possess three typical cellular markers, alpha-SM actin, desmin and SM myosin.31 To study the cellular uptake of nanospheres, subconfluent cells in the T-75 flasks were detached by trypsin-EDTA, centrifuged, resuspended in growth media. Prior to every experiment, cells were seeded in a 24-well plate at a density of 10,000 cells/well and allowed to grow for 3 days.
Studies of intracellular sphere uptake
Three sets of experiments were carried out in this study. First, to determine the dose-dependent sphere uptake of nanospheres or microspheres, various amounts of FITC-labeled spheres were added to cell-seeded wells to make final concentrations of 0, 100, 200, 400, and 1600 µg/ml. After incubation at 37 °C for 30 minutes, the cells were ready for the analyses. Second, to study the effect of incubation times on nanosphere uptake, the cells were exposed to 200 µg/ml suspension of nanospheres (100 nm) at 37 °C for 15 minutes, 1, 3, or 6 hours. Third, to assess the effects of shear stress on cellular sphere uptake, cells were seeded on the glass slides as previously described.27 These glass slides were then exposed to the parallel flow plate system at various shear stress levels, 1 and 10 dyn/cm2, in the culture media containing spheres for 15 minutes. Static slides incubated with sphere suspension at the same conditions served as controls.
At the end of experiments, the culture media was recovered and then stored at −80 °C for LDH assays to monitor the cell death or injury as described earlier.32,33 The adherent cells were rinsed three times with serum free culture media to remove excess fluorescent spheres. The cells were then lysed by incubating them with 0.5% Triton-X 100 solution at 37 °C for 30 minutes. A portion of the cell lysate from each sample was analyzed for the total cell protein content to reflect the total number of adherent cells using the Pierce BCA protein assay following manufacture’s instruction (Pierce Chemical Company, Rockford, IL). Portions of the cell lysates were also used for the measurement of fluorescence intensity using a fluorometer (FluoroMax-3, Jobin Yvon Inc., Edison, NJ) with a fluorescent detector of excitation 492 nm and emission 518 nm. Based on the standard curves established for both nano- and micro-spheres, the fluorescence intensities in different lysates were used to estimate the amounts of intracellular spheres. The extent of sphere uptake by cells was calculated and then expressed as the amount of spheres (µg) taken up per mg cell protein.
Confocal microscopy studies of sphere uptake
For confocal laser scanning microscopy, cells were placed on the glass slides coated with a 1% gelatin solution as described previously.27 Cells were allowed to grow for 2 days and then incubated with nano or microspheres at a concentration of 400 µg/ml at 37 °C for 30 minutes. The glass slides were rinsed with PBS three times to remove excess, extracellular, and unbound spheres. The glass slides were then examined under Zeiss Confocal Microscope (LSM 510 equipped with Argon-Krypton laser, Zeiss Imaging Inc, Thornwood, NY). Cells were imaged using the FITC filter (Ex 495 nm, Em 520 nm) and Carl Zeiss LSM Aim software. A representative cell was selected, and a series of z-sections (20 of sections total at 1 µm in distance per section) were performed from the surface of the cellular monolayers through inside the cells.
Results
Sphere size and size distribution
The sphere sizes were determined at 22 °C and below low critical solution temperature. Both nanosphere and microspheres used in this study have uniform size distribution. The nanospheres ranged from 61 to 243 nm with the mean diameter of 109 nm, whereas the microspheres ranged from 0.82 to 2.75 µm with the mean diameter of 1.38 µm. The distribution indicated that the PNIPAm spheres had a relatively narrow Gaussian distribution.
Sphere biocompatibility
The cytotoxicity of nano-spheres was evaluated in vitro using LDH assays of the incubated medium. Cells incubated with media containing spheres at low concentration (≤ 800 µg/ml) were viable similarly to those of exposed to media without spheres. However, nanospheres at high concentration (1600 µg/ml) caused some cell death as shown by more cytoplasmic LDH released into the medium (LDH release mU/ml = 101.4 ± 5.7 vs. 94.3 ± 2.3 of controls (e.g. without spheres) for ECs, 130.5 ± 4.9 vs. 101.7 ± 1.4 for SMCs, and 143.1 ± 2.4 vs. 110.8 ± 3.0 for PCs, n =6, P <0.05). This was surprising, as PNIPAm spheres are not noted for cytotoxic effects. It may be possible that upon internalization, the spheres with high concentration could aggregate inside the cells, which may influence cell integrity. Similar observation was obtained for micro-spheres (results not shown).
Effects of sphere concentrations on cellular uptake
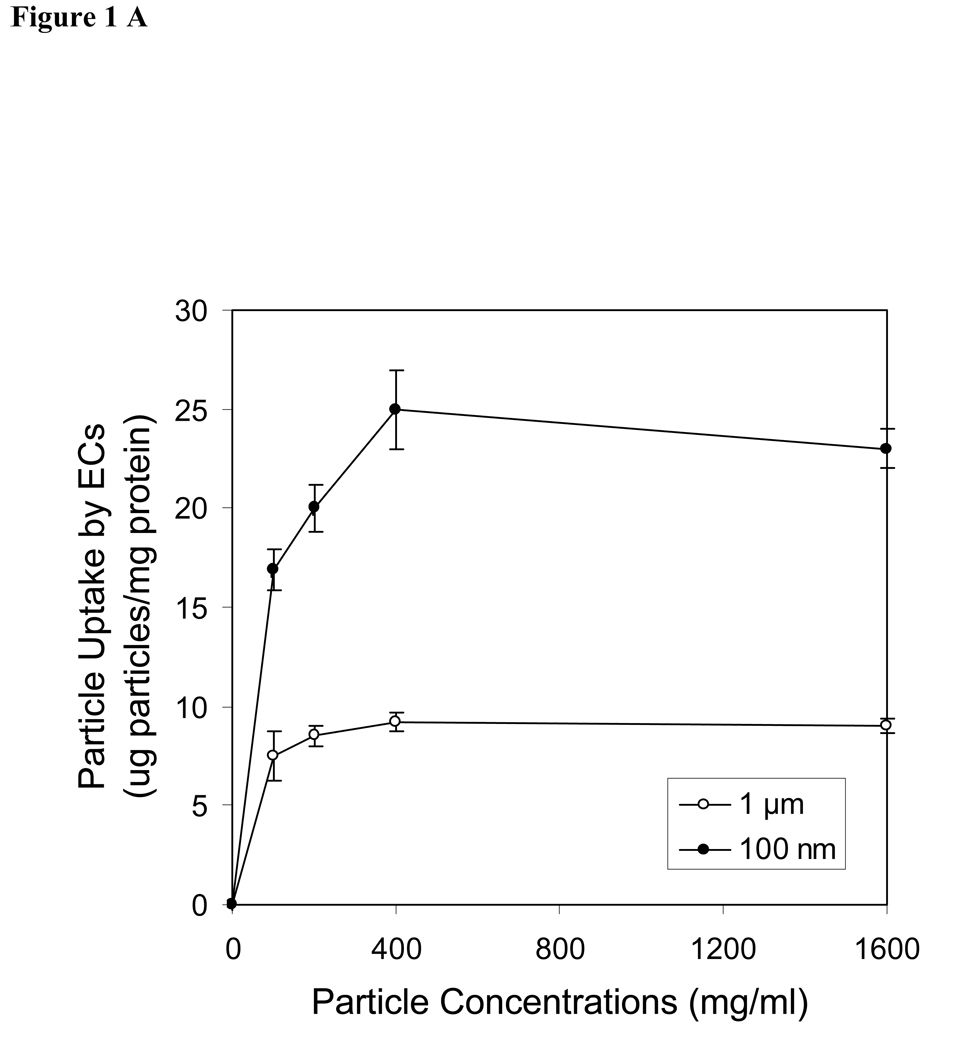
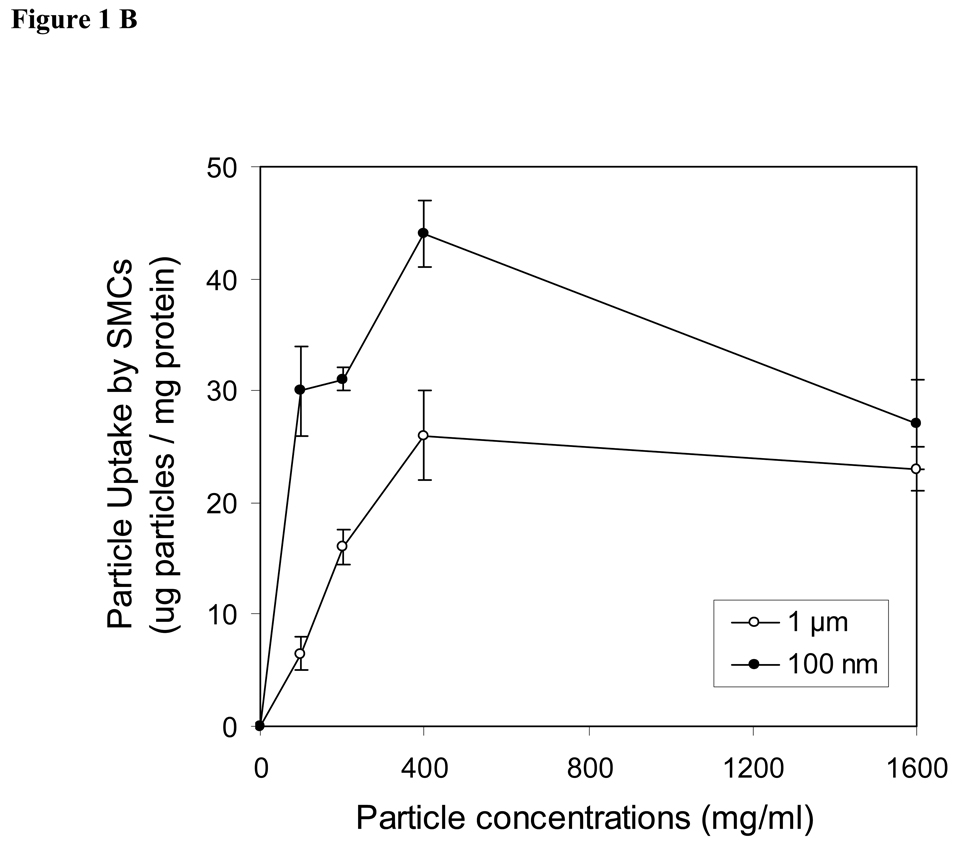
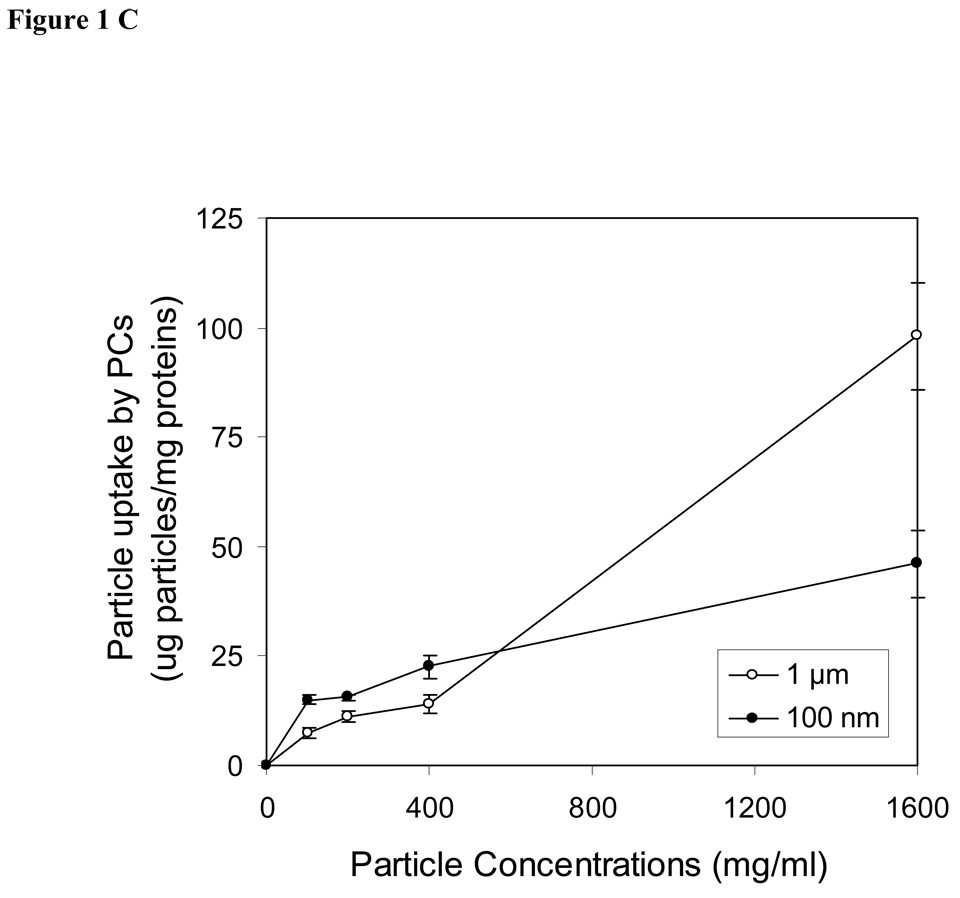
The uptake of spheres by endothelial cells, smooth muscle cells and peritoneal phagocytes were shown as the dose-dependence of spheres in the medium (Fig. 1A–C). In other words, the degree of cellular uptakes of spheres is enhanced with increasing sphere concentrations. The maximal cellular uptake occurred at a concentration of 400 µg/ml for both endothelials cells and smooth muscle cells (Fig. 1A & 1B). Furthermore, we observed that the uptakes of nanospheres was substantially greater than the uptake of microspheres in both endothelial cells and smooth muscle cells. In contract to the observation seen in vascular cells, peritoneal phagocytes devoured larger amounts of both microspheres and nanospheres. The amounts of engulfed spheres in peritoneal phagocytes are concentration dependent. Based on the conditions used in this study, the maximal sphere uptake by peritoneal cells can not be achieved and is >1600 µg/ml (Fig. 1C). Interestingly, we found that microspheres were engulfed by peritoneal phagocytes at high concentration (1600 µg/ml), whereas no significant difference was observed in the cellular uptake of nano and microspheres by phagocytes at lower concentration below 500 µg/ml.
Figure 1.
Effects of sphere concentrations on cellular uptake by (A) aorta endothelial cells (ECs), (B) smooth muscle cells (SMCs) and (C) peritoneal activated phagocytes (PC). Filled circles (●) present the uptake of nanospheres (100 nm) and open circles (○) demonstrate the uptake of microspheres (1 µm). Values represent as mean ± SEM (n = 6).
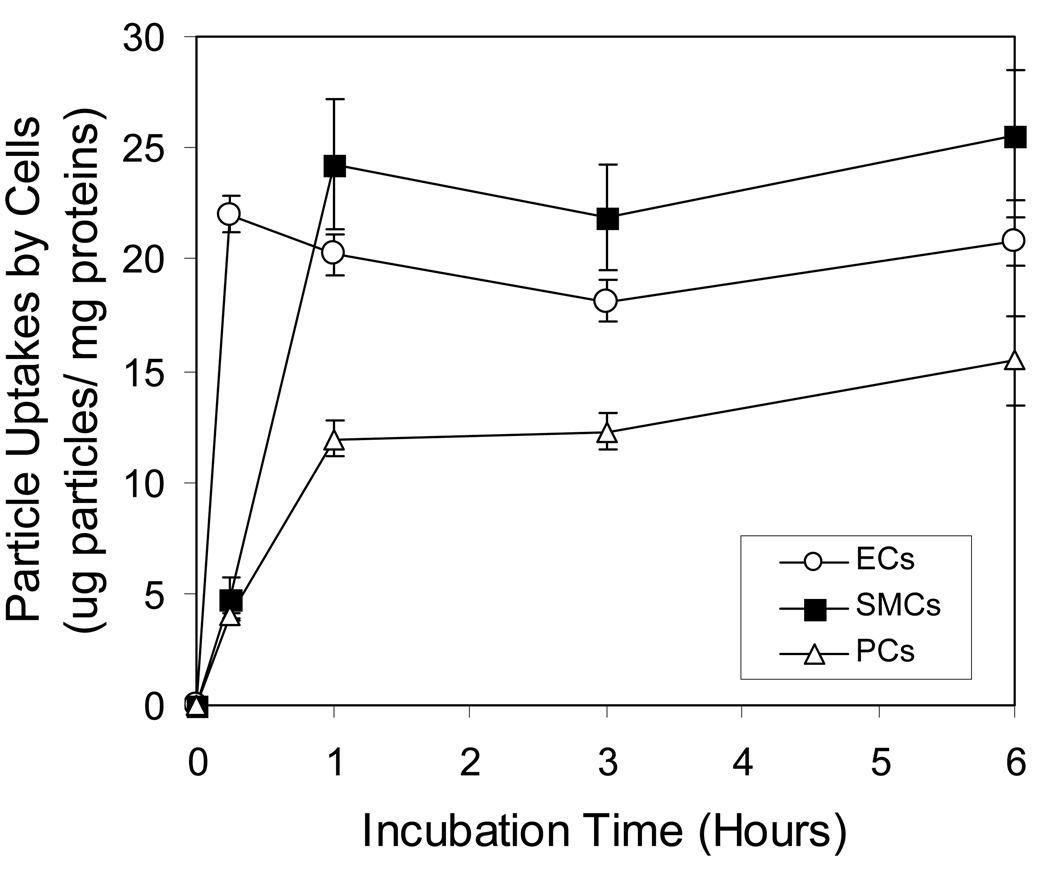
Effects of incubation time on cellular sphere uptake
For subsequent incubation time experiments, incubation conditions were set at concentration of 200 µg/ml spheres because this concentration was in the non-saturated portion for both endothelial cells and smooth muscle cells. Our results have shown that the cellular uptake of nanospheres by phagocytes, endothelial cells and smooth muscle cells was rather fast. The intracellular sphere uptake for endothelial cells achieves a plateau in 15 minutes (Fig 2). Compared with the two other cell types, smooth muscle cells’ sphere uptake were initially slow and then maximized in 60 minutes. The majority of sphere uptake by phagocytes occurs within 60 minutes. Phagocytes were continuously taking up nanospheres for several hours (Fig. 2) contrary to endothelial and smooth muscle cells
Figure 2.
Effects of incubation time on cellular uptake of nanospheres by endothelial cells (ECs), smooth muscle cells (SMCs) and peritoneal phagocytes (PC). Open triangles (Δ) present the uptake in peritoneal phagocytes, whereas open circles (○) and closed squares (■) demonstrate the uptake in endothelials (ECs) and smooth muscle cells (SMCs), respectively. Values represent as mean ± SEM (n = 6).
Confocal images on cellular sphere uptake
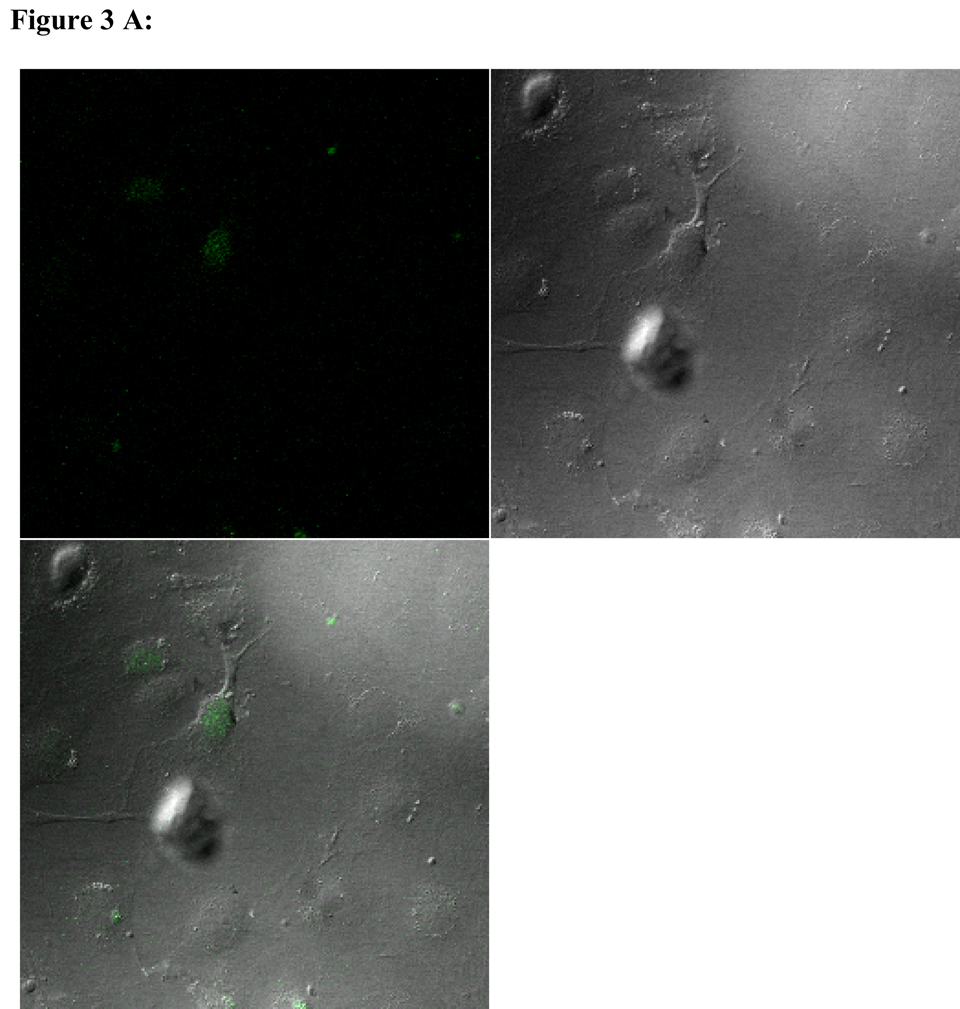
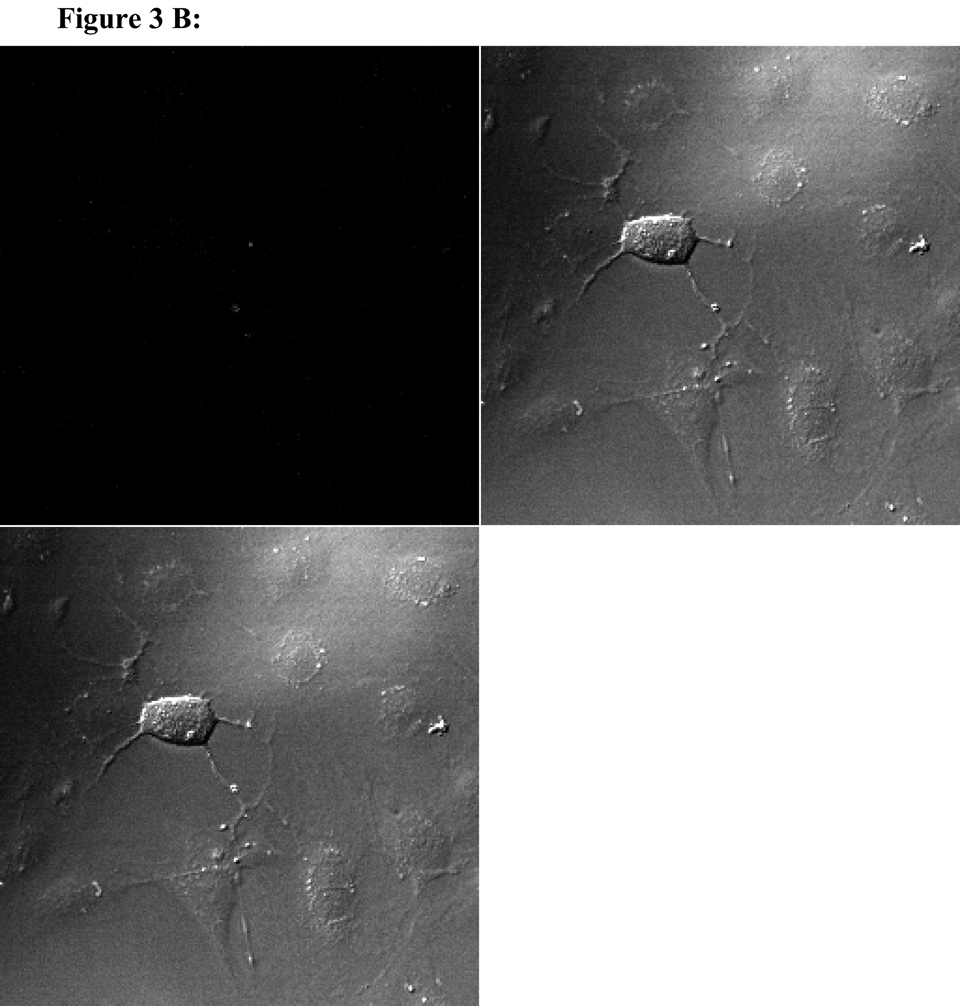
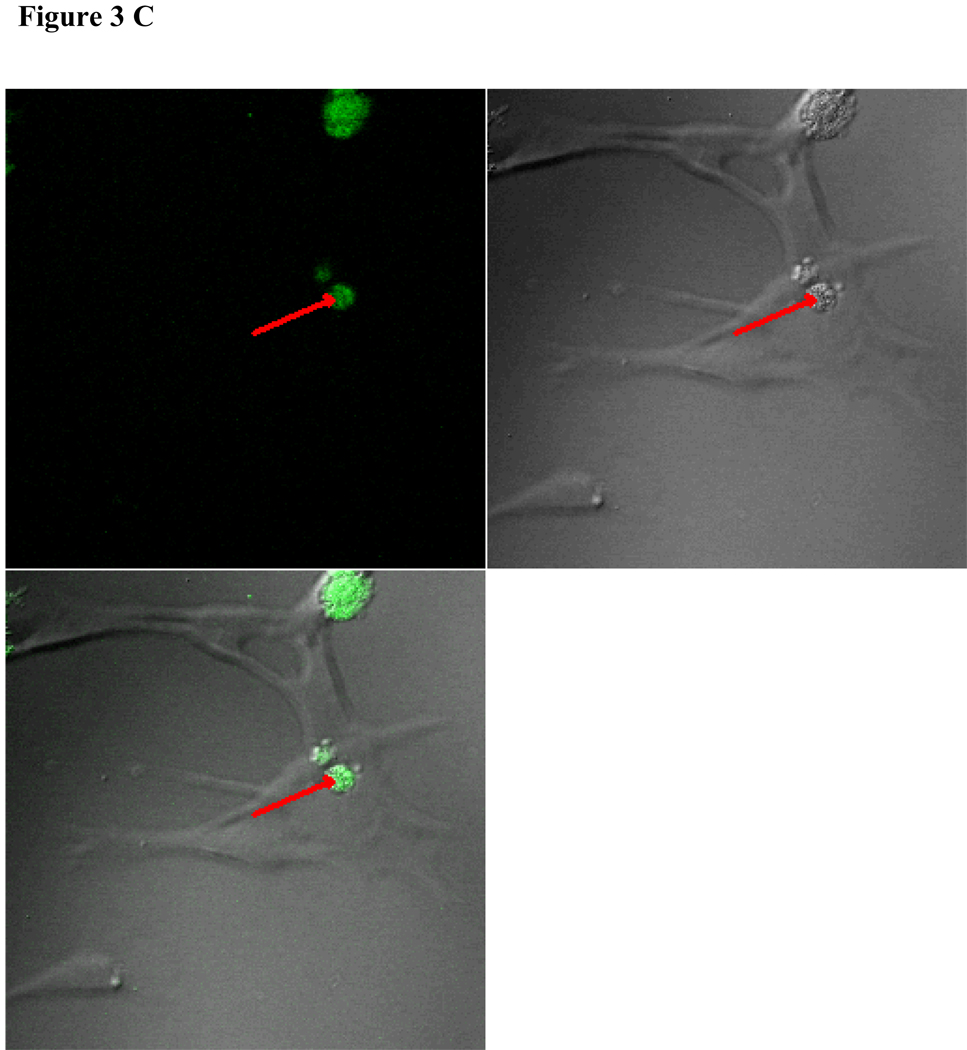
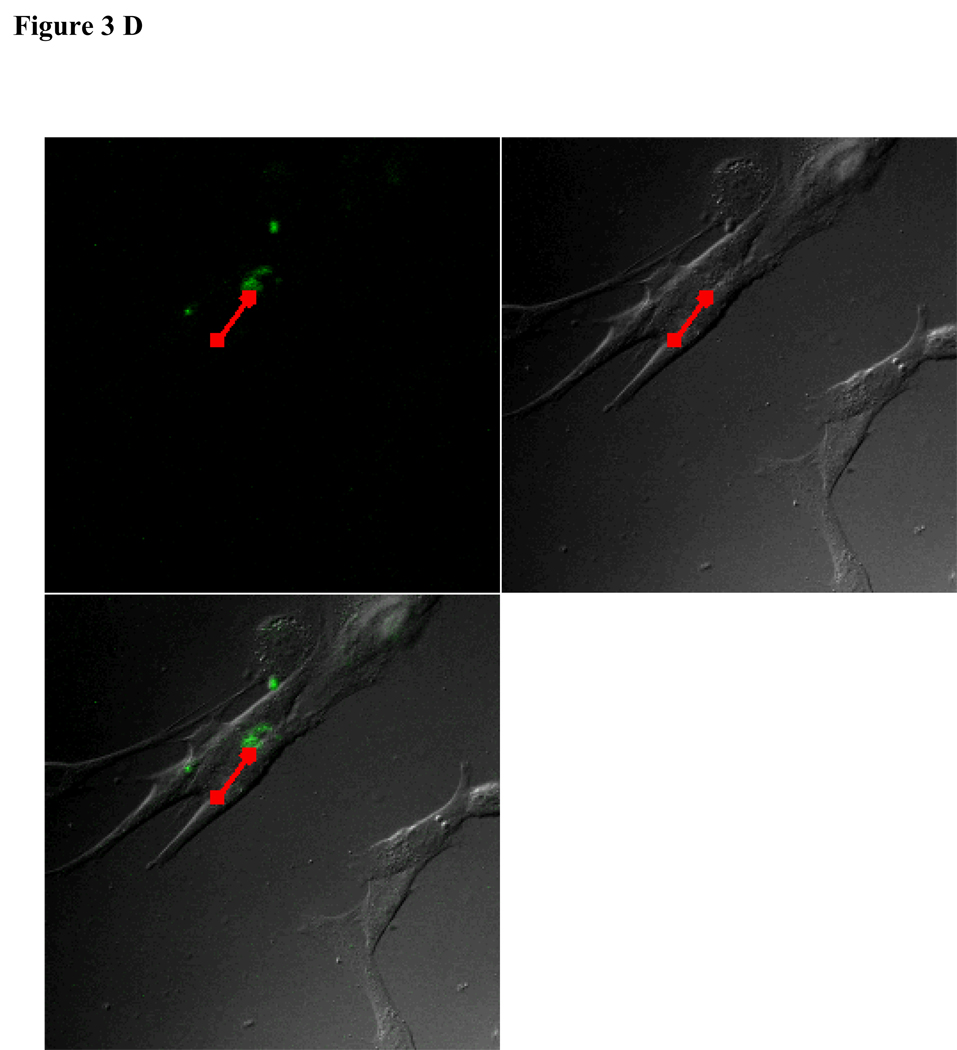
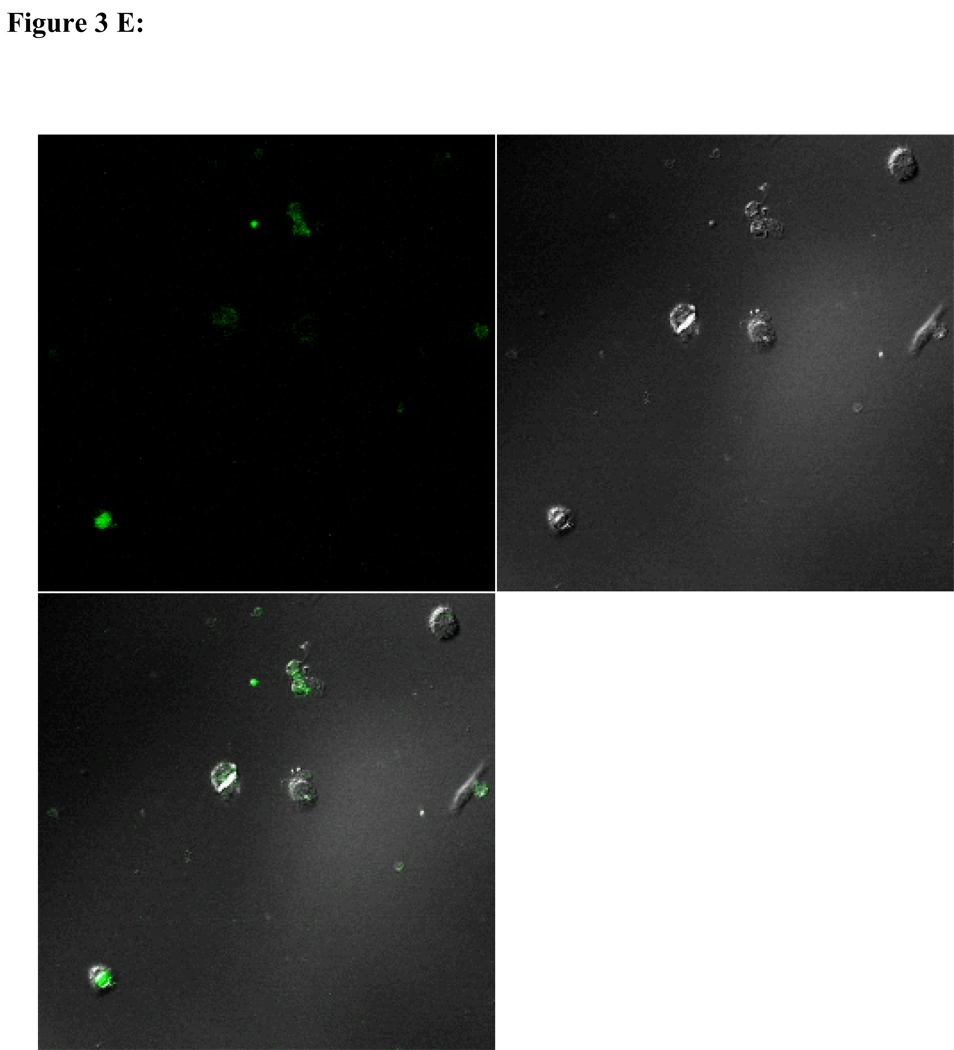
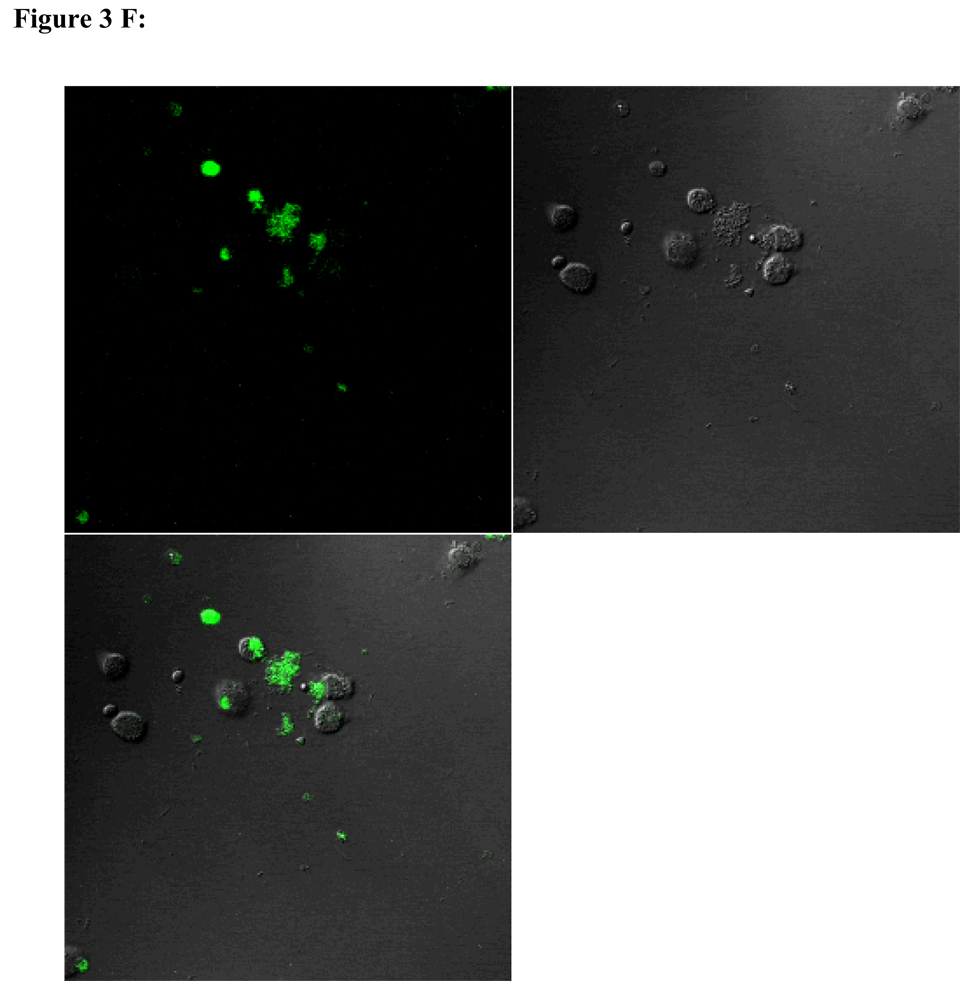
To visualize cellular responses to different spheres, confocal images were taken on cells previously exposed to fluorescence-labeled spheres with serial z-sections performed from the surfaces of the cells through the inside of the cells to investigate whether the spheres were internalized by the cells. Each photograph (Fig. 3A–F) shown is a z-section through the middle of the cells and hence the fluorescence seen reveals significant fluorescent signal in the middle focal plane suggesting that the spheres have been endocytosed. Z-sections at the surface of the cells detecting no fluorescent signals demonstrated that the numbers of surface-bound spheres are insignificant (results not shown). Focal planes were chosen based on their location at the midpoint between the upper and lower surface (section 10) of the cellular monolayers. For the responses of individual cell types, confocal images are in complete agreement with the results of in vitro sphere uptake study (Fig. 1A, 1B, and 1C). Specifically, compared with microspheres (Figure 3B), nanospheres (Figure 3A) are internalized better by endothelial cells. Similarly, nanospheres (Figure 3C) are easier internalized by smooth muscle cells than microspheres (Figure 3D). On the other hand, peritoneal phagocytes have a higher capacity for microspheres (Figure 3F) than nanospheres (Fig. 3E).
Figure 3.
Confocal fluorescence microscopy: cellular uptake of nanospheres (A) and microspheres (B) by endothelial cells, cellular uptake of nanospheres (C) and microspheres (D) by smooth muscle cells, and cellular uptake of nanospheres (E) and microspheres (F) by peritoneal phagocytes. The pictures are taken at the midsection of the cells.
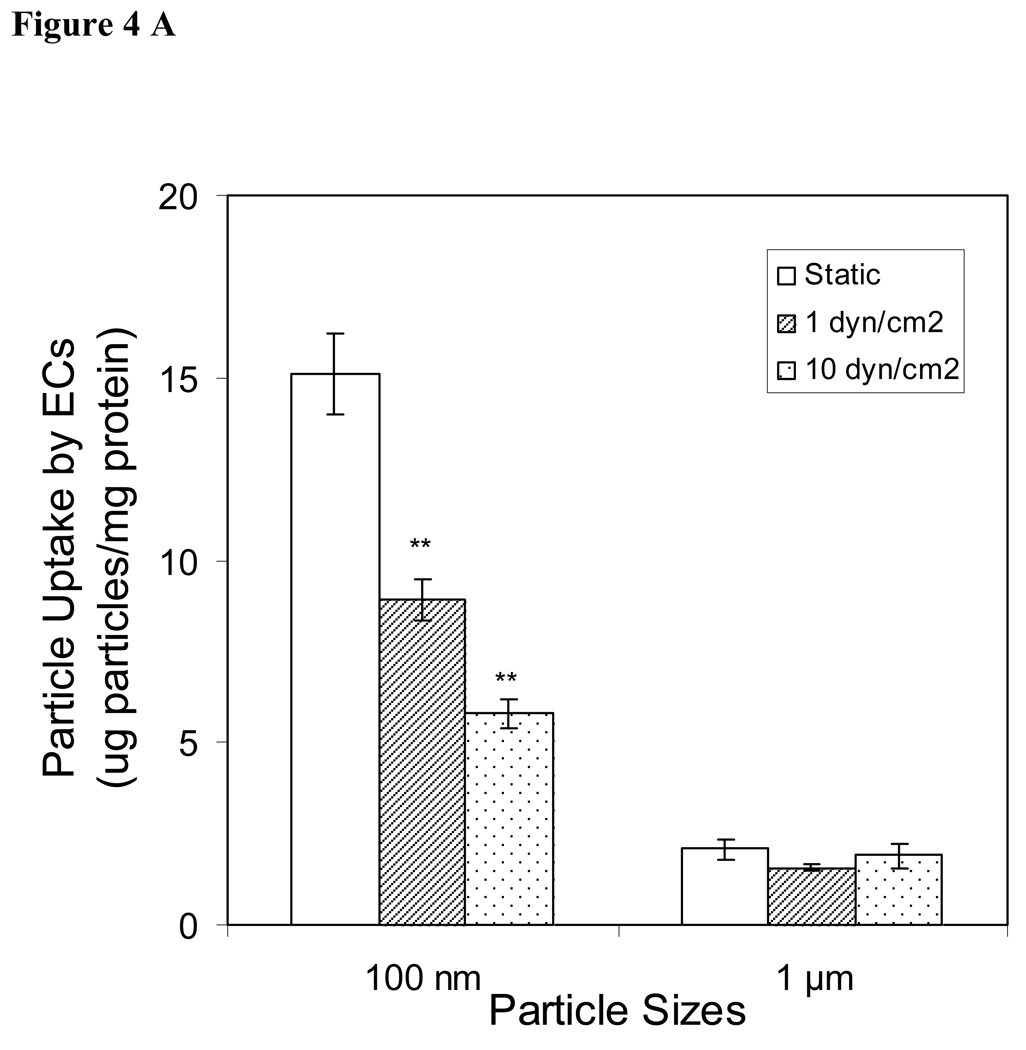
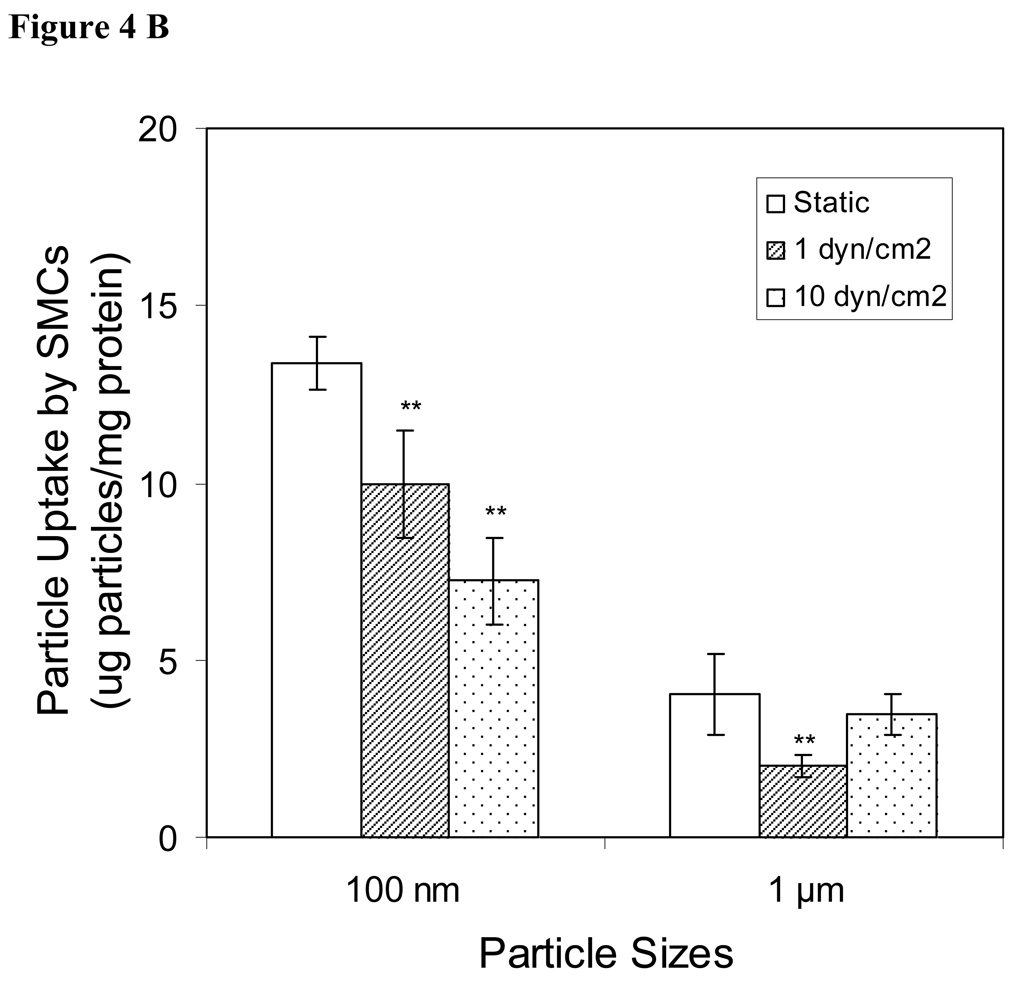
Effects of shear stress on cellular sphere uptake
For achieving optimal intra-vitreous drug delivery, it is important to study the sphere:cell interaction in a fluid dynamic environment. To achieve this goal, endothelial cells and smooth muscle cells were exposed to nanospheres and microspheres under flow conditions designed to mimic the blood circulation in vivo. Overall, we find that the influence of shear stress on cellular sphere uptake is similar among endothelial cells and smooth muscle cells. As shown in Fig. 4A and 4B, cellular uptake of nanospheres was higher in static rather than shear conditions. Shear force has no influence on microsphere uptake by endothelial cells (Fig. 4A), although shear force at 1 dyn/cm2, but not 10 dyn/cm2, reduced microsphere uptake by smooth muscle cells (Figure 4B).
Figure 4.
Cellular uptake of nanospheres and microspheres by (A) endothelial cells (ECs) and (B) smooth muscle cells (SMCs) under static or fluid flow conditions (1 or 10 dyn/cm2). Values represent as mean ± SEM (n = 6). * indicated significant difference compared to control samples (static samples).
Conclusion
Several strategies have recently developed toward local delivery systems such as nano and microspheres designed to achieve local sustained drug release at the wall site of balloon injury and to avoid the potential systemic side effects. Vascular cells such as endothelial cells and smooth muscle cells are important targets for drug or gene therapy because these cell types are involved in several normal and patho-physiological conditions such as angiogenesis and atherosclerosis. Spheres could also be taken up rapidly by the circulating phagocytes for clearance. Characterization of the cellular uptake of spheres such as nano and microspheres in vascular cells and phagocytes presented here provides a clearer understanding of the relationships between several parameters including sphere size, flow rate, and cellular uptake. This information may provide better strategies for engineering targeted drug delivery vehicles.
Sphere size is one of the key parameters affecting cellular uptake and biodistribution of drug- or gene-releasing spheres.4,5,34 We have observed that nanospheres were internalized more efficiently by endothelial cells and smooth muscle cells than microspheres. Conversely, the sphere uptake increases with increasing size for phagocytes. In support of our findings, early reports has shown that nanospheres preferentially accumulate in the arterial wall. Furthermore, uptake of spheres smaller than 350 nm but not spheres larger than 500 nm has been observed in human endothelial and mesothelioma cells;35 whereas uptake of spheres larger than 500 nm has been shown in phagocytic cells.36 The poor uptake of microspheres by endothelial cells in vitro may help explaining in vivo observations that only a negligible number of spheres (larger than 500 nm in size) were detected in the inner parts of the vessel wall.12,37 The endothelial layer might act as a strong barrier to inhibit the penetration of micro-spheres through the connective tissue.
Cellular uptake of PNIPAm nanospheres by vascular cells including endothelial cells and smooth muscle cells was dose-dependent and time-dependence. Recent work by others has observed similar behavior in the cellular uptake of poly(D,L-lactide-co-glycolide) (PLGA) nanospheres by endothelial cells and smooth muscle cells.9,25,38 It has also been found that cellular uptake of PLGA nanospheres by vascular smooth muscle cells were through an energy-dependent endocytosis process involving both nonspecific receptor mediated endocytosis and fluid-phase endocytosis.25 Furthermore, in vivo studies of arterial uptake of nanospheres found that increasing the concentration of biodegradable PLGA nanospheres (70–160 nm) in the infusate applied to dog carotid arteries significantly increased arterial nanosphere uptake levels.8
Interestingly, in contrast to vascular cells, immune cells have higher affinity to microspheres than nanospheres. This finding is in agreement with early observations by Kawaguchi H et al.39 that the rate of oxygen consumption by stimulated phagocytes increased with increasing sphere size of polystyrene spheres. In addition, in vitro work has shown that macrophages belonging to the phagocytic cells can take up microspheres up to 12 µm in diameter.5,17,36 In vivo studies have also observed that delivering of PLLA microspheres (1–5 µm) in the rabbit carotid artery was associated with some degree of inflammatory response and increase in intimal arteriosclerosis tissue.40 On the other hand, nanospheres appeared to be biocompatible and caused little or no local inflammation.37 Taken together, results in both vascular cells and phagocytes suggest that nanospheres might be more effective as an intra-vascular delivery system compared to microspheres in the term of vascular cellular uptake and biocompatibility.
Since drug carriers interact with vascular cells in a fluid dynamic environment in vivo, adhesion and cellular uptake of spheres by vascular cells in the presence of flow or shear model is needed to provide a better understanding of vascular sphere adhesion and uptake in vivo. In one such adhesion study, Blackwell et al.24 found that in some shear regimes the level of fluid shear stress can significantly reduce the adhesion of polystyrene nanospheres to monolayers of the endothelium compared to static cells. For instance, this study demonstrated that increasing shear rate from 150 to 300 s−1 cause no significant effect on the adhesion of nanospheres, whereas shear rates greater than 300 s−1 resulted in significant reduction in adhesion. In addition, previous studies using animal models have noted a low efficiency of nanosphere retention in the arterial wall after restoring the blood flow.7,37 Another study found that multiple short infusions resulted in better arterial wall retention than with a single-prolonged infusion.8 Although effects of shear stress on the adhesion of spheres to vascular cells have been well-investigated,24,41–44 the effects of shear stress on cellular uptake of these particles in vascular cells have not been studied. Using an in vitro flow system, we found that increasing the shear stress significantly decreased the cellular uptake of nanospheres in both endothelial cells and smooth muscle cells. Taken together, results from literature and our studies suggest that vascular cells including endothelial cells and smooth muscle cells exhibit shear-dependent cellular sphere uptake and this behavior also depends on the sphere size. Thus a major challenge of nanosphere administration within an artery is to overcome the potential of arterial blood flow to wash away a significant fraction of infused nanospheres. Conjugating nanospheres with many adhesion ligands to the endothelium might help to overcome this challenge.
In this study, we have shown the relationship between parameters such as size and intracellular uptake of spheres by vascular cells and phagocytes. We have shown that delivery of micron-sized and nanometer-sized spheres at high concentrations leads to increased internalization in vascular cells than phagocytes. Nanospheres at low concentration and long incubation time are also biocompatible with the cells. In addition, cellular uptake of nanospheres are dose, time, and shear dependent in both endothelial cells and smooth muscle cells. A number of important questions remain to be addressed by future research in this area. Those include (i) investigating the mechanisms of the cellular sphere uptake in these various cell types, (ii) defining optimal conditions for nanospheres drug delivery in vivo, and (iii) modifying the surfaces of nanospheres with specific ligands45 for targeted drug or gene delivery. Although the dynamics of cellular uptake and retention in vivo could be different from that observed in vitro, it is important to understand the factors affecting the cellular uptake of spheres and the underlying mechanisms to further explore the drug delivery applications of nanospheres. A greater understanding of sphere cellular uptake, such as presented here, would be useful in exploring degradable nanospheres and microspheres for drug and gene delivery, and thus should aid in disease treatments.
Acknowledgement
Part of this work was supported by NIH grants RO1 GM074021 (LT), Advanced Research Program from Higher Education Coordinating Board (ZH and LT) and an AHA Established Investigator Award (LT).
References
- 1.Chorny M, Fishbein I, Golomb G. Drug delivery systems for the treatment of restenosis. Critical reviews in therapeutic drug carrier systems. 2000;17(3):249–284. [PubMed] [Google Scholar]
- 2.Fishbein I, Chorny M, Rabinovich L, Banai S, Gati I, Golomb G. Nanoparticulate delivery system of a tyrphostin for the treatment of restenosis. Journal of controlled release : official journal of the Controlled Release Society. 2000;65(1–2):221–229. doi: 10.1016/s0168-3659(99)00244-8. [DOI] [PubMed] [Google Scholar]
- 3.Uwatoku T, Shimokawa H, Abe K, Matsumoto Y, Hattori T, Oi K, Matsuda T, Kataoka K, Takeshita A. Application of nanoparticle technology for the prevention of restenosis after balloon injury in rats. Circulation research. 2003;92(7):e62–e69. doi: 10.1161/01.RES.0000069021.56380.E2. [DOI] [PubMed] [Google Scholar]
- 4.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55(3):329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 5.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56(11):1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Vasir JK, Labhasetwar V. Polymeric nanoparticles for gene delivery. Expert opinion on drug delivery. 2006;3(3):325–344. doi: 10.1517/17425247.3.3.325. [DOI] [PubMed] [Google Scholar]
- 7.Labhasetwar V, Song C, Humphrey W, Shebuski R, Levy RJ. Arterial uptake of biodegradable nanoparticles: effect of surface modifications. J Pharm Sci. 1998;87(10):1229–1234. doi: 10.1021/js980021f. [DOI] [PubMed] [Google Scholar]
- 8.Song C, Labhasetwar V, Cui X, Underwood T, Levy RJ. Arterial uptake of biodegradable nanoparticles for intravascular local drug delivery: results with an acute dog model. J Control Release. 1998;54(2):201–211. doi: 10.1016/s0168-3659(98)00016-9. [DOI] [PubMed] [Google Scholar]
- 9.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm. 2002;233(1–2):51–59. doi: 10.1016/s0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- 10.Barratt G. Colloidal drug carriers: achievements and perspectives. Cellular and molecular life sciences : CMLS. 2003;60(1):21–37. doi: 10.1007/s000180300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas SJ, Davis SS, Illum L. Nanoparticles in drug delivery. Crit Rev Ther Drug Carrier Syst. 1987;3(3):233–261. [PubMed] [Google Scholar]
- 12.Westedt U, Barbu-Tudoran L, Schaper AK, Kalinowski M, Kissel T. Deposition of nanoparticles in the arterial vessel by porous balloon catheters: localization by confocal laser scanning microscopy and transmission electron microscopy. AAPS pharmSci. 2002;4(4):E41. doi: 10.1208/ps040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharmaceutical research. 1997;14(11):1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- 14.Speiser PP. Nanoparticles and liposomes: a state of the art. Methods and findings in experimental and clinical pharmacology. 1991;13(5):337–342. [PubMed] [Google Scholar]
- 15.Vasir JK, Labhasetwar V. Targeted drug delivery in cancer therapy. Technology in cancer research & treatment. 2005;4(4):363–374. doi: 10.1177/153303460500400405. [DOI] [PubMed] [Google Scholar]
- 16.Bazile D, Prud'homme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. Journal of pharmaceutical sciences. 1995;84(4):493–498. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- 17.Mèuller RH, Maassen S, Weyhers H, Mehnert W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. Journal of drug targeting. 1996;4(3):161–170. doi: 10.3109/10611869609015973. [DOI] [PubMed] [Google Scholar]
- 18.Vittaz M, Bazile D, Spenlehauer G, Verrecchia T, Veillard M, Puisieux F, Labarre D. Effect of PEO surface density on long-circulating PLA-PEO nanoparticles which are very low complement activators. Biomaterials. 1996;17(16):1575–1581. doi: 10.1016/0142-9612(95)00322-3. [DOI] [PubMed] [Google Scholar]
- 19.Zambaux MF, Bonneaux F, Gref R, Dellacherie E, Vigneron C. MPEO-PLA nanoparticles: effect of MPEO content on some of their surface properties. Journal of biomedical materials research. 1999;44(1):109–115. doi: 10.1002/(sici)1097-4636(199901)44:1<109::aid-jbm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Pelton R. Temperature-sensitive aqueous microgels. Advances in colloid and interface science. 2000;85(1):1–33. doi: 10.1016/s0001-8686(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman AS. Hydrogels for biomedical applications. Advanced drug delivery reviews. 2002;54(1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 22.Ramanan RM, Chellamuthu P, Tang L, Nguyen KT. Development of a temperature-sensitive composite hydrogel for drug delivery applications. Biotechnology progress. 2006;22(1):118–125. doi: 10.1021/bp0501367. [DOI] [PubMed] [Google Scholar]
- 23.Weng H, Zhou J, Tang L, Hu Z. Tissue responses to thermally-responsive hydrogel nanoparticles. Journal of biomaterials science. (Polymer edition) 2004;15(9):1167–1180. doi: 10.1163/1568562041753106. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell JE, Dagia NM, Dickerson JB, Berg EL, Goetz DJ. Ligand coated nanosphere adhesion to E- and P-selectin under static and flow conditions. Ann Biomed Eng. 2001;29(6):523–533. doi: 10.1114/1.1376697. [DOI] [PubMed] [Google Scholar]
- 25.Panyam J, Labhasetwar V. Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res. 2003;20(2):212–220. doi: 10.1023/a:1022219003551. [DOI] [PubMed] [Google Scholar]
- 26.Panyam J, Labhasetwar V. Sustained cytoplasmic delivery of drugs with intracellular receptors using biodegradable nanoparticles. Molecular pharmaceutics. 2004;1(1):77–84. doi: 10.1021/mp034002c. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen KT, Eskin SG, Patterson C, Runge MS, McIntire LV. Shear stress reduces protease activated receptor-1 expression in human endothelial cells. Ann Biomed Eng. 2001;29(2):145–152. doi: 10.1114/1.1349700. [DOI] [PubMed] [Google Scholar]
- 28.Haugland RP. Handbook of fluorescent probes and research products. 9th Edition. 2002. pp. 112–114. [Google Scholar]
- 29.Tang L, Eaton JW. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. The Journal of experimental medicine. 1993;178(6):2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire PG, Orkin RW. Isolation of rat aortic endothelial cells by primary explant techniques and their phenotypic modulation by defined substrata. Laboratory investigation; a journal of technical methods and pathology. 1987;57(1):94–105. [PubMed] [Google Scholar]
- 31.Bochaton-Piallat ML, Gabbiani F, Ropraz P, Gabbiani G. Cultured aortic smooth muscle cells from newborn and adult rats show distinct cytoskeletal features. Differentiation; research in biological diversity. 1992;49(3):175–185. doi: 10.1111/j.1432-0436.1992.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 32.Tang L, Lucas AH, Eaton JW. Inflammatory responses to implanted polymeric biomaterials: role of surface-adsorbed immunoglobulin G. The Journal of laboratory and clinical medicine. 1993;122(3):292–300. [PubMed] [Google Scholar]
- 33.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. Journal of immunological methods. 1988;115(1):61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 34.Panyam J, Labhasetwar V. Targeting intracellular targets. Current drug delivery. 2004;1(3):235–247. doi: 10.2174/1567201043334768. [DOI] [PubMed] [Google Scholar]
- 35.Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99(3):912–922. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]
- 36.Kruth HS, Chang J, Ifrim I, Zhang WY. Characterization of patocytosis: endocytosis into macrophage surface-connected compartments. European journal of cell biology. 1999;78(2):91–99. doi: 10.1016/S0171-9335(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 37.Guzman LA, Labhasetwar V, Song C, Jang Y, Lincoff AM, Levy R, Topol EJ. Local intraluminal infusion of biodegradable polymeric nanoparticles. A novel approach for prolonged drug delivery after balloon angioplasty. Circulation. 1996;94(6):1441–1448. doi: 10.1161/01.cir.94.6.1441. [DOI] [PubMed] [Google Scholar]
- 38.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16(10):1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi H, Koiwai N, Ohtsuka Y, Miyamoto M, Sasakawa S. Phagocytosis of latex particles by leucocytes. I. Dependence of phagocytosis on the size and surface potential of particles. Biomaterials. 1986;7(1):61–66. doi: 10.1016/0142-9612(86)90091-8. [DOI] [PubMed] [Google Scholar]
- 40.Gradus-Pizlo I, Wilensky RL, March KL, Fineberg N, Michaels M, Sandusky GE, Hathaway DR. Local delivery of biodegradable microparticles containing colchicine or a colchicine analogue: effects on restenosis and implications for catheter-based drug delivery. Journal of the American College of Cardiology. 1995;26(6):1549–1557. doi: 10.1016/0735-1097(95)00345-2. [DOI] [PubMed] [Google Scholar]
- 41.Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci U S A. 2003;100(26):15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang KC, Hammer DA. Adhesive dynamics simulations of sialyl-Lewis(x)/E-selectin-mediated rolling in a cell-free system. Biophys J. 2000;79(4):1891–1902. doi: 10.1016/S0006-3495(00)76439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omolola Eniola A, Hammer DA. In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. Biomaterials. 2005;26(34):7136–7144. doi: 10.1016/j.biomaterials.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Eniola AO, Krasik EF, Smith LA, Song G, Hammer DA. I-domain of lymphocyte function-associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow. Biophysical journal. 2005;89(5):3577–3588. doi: 10.1529/biophysj.104.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nature biotechnology. 2005;23(11):1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]