PREFACE
The vast majority of Alzheimer’s disease (AD) cases are late-onset and their development is likely influenced by both genetic and environmental risk factors. A strong genetic risk factor for late-onset AD is the presence of the ε4 allele of the apolipoprotein E (APOE) gene, which encodes a protein with crucial roles in cholesterol metabolism. Mounting evidence demonstrates that apoE4 contributes to AD pathogenesis by modulating the metabolism and aggregation of amyloid-β peptide and by directly regulating brain lipid metabolism and synaptic functions through apoE receptors. Emerging knowledge on the contribution of apoE to the pathophysiology of AD presents new opportunities for AD therapy.
INTRODUCTION
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly. Extracellular amyloid plaques and intracellular neurofibrillary tangles are defining lesions in AD1,2. Mounting genetic and biochemical data support the hypothesis that amyloid-β(Aβ) accumulation and aggregation in the brain is an early and central event in the pathogenesis of AD2,3. Aβ is derived from sequential proteolytic processing of the amyloid precursor protein (APP) by β- and γ-secretases. Mutations associated with early-onset familial AD (FAD) are dominantly inherited and are found in the APP gene itself or in the genes of presenilin 1 (PSEN1) and PSEN2, whose products, together with nicastrin, APH-1 and PEN-2, are essential components of a multi-protein complex that is responsible for γ-secretase activity4. A common feature of most FAD mutations is that they increase the generation of Aβ peptides, or increase the proportion of the longer Aβ42 form, which has a higher tendency to aggregate and is more toxic than the shorter Aβ403. Because γ-secretase cleavage of an increasingly recognized panel of substrates is important for synaptic function and neuronal survival, a loss-of-function hypothesis for PSEN mutations in AD pathogenesis has also been proposed5.
FAD genetics and mouse models have shed light on early-onset AD pathogenesis, but the vast majority of AD cases occur late in life. The ε4 allele of the apolipoprotein E (APOE) gene is a major risk for late-onset AD (LOAD). This risk allele, discovered in 1993 by Strittmatter, Roses and colleagues6,7, has been validated in numerous genetic association studies (see AlzGene website at http://www.alzforum.org/res/com/gen/alzgene/default.asp). Although a gene on chromosome 19 had previously been implicated in LOAD risk, the observation that apoE binds to Aβ in the cerebrospinal fluid (CSF) prompted the testing of APOE as a positional candidate6. ApoE is a major apolipoprotein and a cholesterol carrier in the brain8. In humans, the APOE gene exists as three different polymorphic alleles (ε2, ε3 and ε4), which engender six different genotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4). ε3 is the most (77%) and ε2 the least (8%) common allele8. The ε4 allele frequency is about 15% in general populations, but is ~40% in AD patients. Individuals with one ε4 allele are 3- to 4-fold more likely to develop AD than those without ε4 alleles7,9. This odds ratio (OR) is much greater than those for other AD-risk alleles, which are typically <1.59. The maximal effects of the ε4 allele on AD risk is between 60 and 70 years of age10, and the prevalence of the ε4 allele in AD patients is >50%7. Interestingly, although rare, the ε2 allele is associated with protection against LOAD compared to the ε3 allele7. The ε4 allele is also a risk factor for atherosclerosis8, HIV disease progression11 and additional neurological disorders including cerebral amyloid angiopathy (CAA) and CAA-associated cerebral hemorrhages12, tauopathies and Lewy body disease13, Parkinson’s disease14 and multiple sclerosis15. These observations suggest that the ε4 allele may be associated with accelerated neurodegeneration in the development and progression of several neurodegenerative diseases.
The three apoE isoforms (apoE2, apoE3 and apoE4) differ from each other by a single amino acid8. In most, if not all, AD putative pathogenic pathways, apoE4 either diminishes protection or augments toxicity when compared to apoE2 and apoE3. In the brain, apoE/lipoprotein particles are produced primarily by astrocytes and deliver cholesterol and other essential lipids to neurons via members of the low-density lipoprotein receptor (LDLR) family16,17. Although the mechanisms underlying the pathogenic nature of apoE4 in AD are still not completely understood, several pathways have been identified in vitro and in vivo. In this Review, I describe Aβ-dependent and Aβ-independent pathways by which apoE isoforms and apoE receptors contribute to AD pathogenesis, with particular attention to the distinct functions of apoE receptors, and the recent advances and challenges in targeting apoE and apoE receptors for AD therapy.
Biochemical properties of apoE and apoE/lipoproteins
ApoE is an ~34-kDa protein that transports cholesterol and other lipids in the plasma and the central nervous system (CNS) by binding to cell surface apoE receptors8. Its highest concentration is in the liver and brain. In the periphery, apoE transports liver-synthesized very low-density lipoprotein (VLDL), a subclass of high-density lipoprotein (HDL) and intestinally synthesized chylomicrons. In the brain, apoE is predominantly synthesized by astrocytes and to some extent by microglia18,19. ApoE/lipoprotein particles from human CSF are the size and density of plasma HDL, are spherical and contain a core lipid: esterified cholesterol20,21. Interestingly, nascent apoE/lipoprotein particles secreted by cultured astrocytes are primarily discoid and contain little lipid21. It is possible that astrocyte-secreted apoE/lipoprotein particles acquire a core of esterified cholesterol before reaching the CSF. Alternatively, native apoE/lipoprotein particles produced by astrocytes in the brain may differ from those secreted by cultured astrocytes. The stability of apoE in the brain requires its association with lipids, as evidenced by the marked reduction in brain apoE levels when the murine Abca1 gene, whose product lipidates apoE, is deleted22,23.
Human apoE contains 299 amino acid residues and has independently folded N-terminal and C-terminal domains, which are joined together by a flexible hinge region24,25 (FIG. 1). The apoE region (residues 136-150) that interacts with apoE receptors is in the N-terminal domain, whereas the major lipid-binding region (residues 244-272) is within the C-terminal domain. The three isoforms, apoE2, apoE3 and apoE4, differ at positions 112 and 1588,24,26. These single amino acid differences among the three apoE isoforms alter the protein structure and influence lipid association and receptor binding24. For example, apoE3 and apoE4 bind to LDLR with affinities that are ~50-fold greater than the affinity of apoE2. As a result, apoE2 less efficiently transports lipids, and its presence is associated with type III hyperlipoproteinemia. ApoE4 preferentially binds to large lipoprotein particles and is also associated with a modestly increased risk of cardiovascular disease. The preference of apoE4 to larger lipoprotein particles is attributable to the presence of Arg112, which affects the conformation of the side chain of Arg61 resulting in ‘domain interaction’ between this Arg61 in the N-terminal domain and Glu255 in the C-terminal domain.
Figure 1. Schematic representation of human apoE.

The 299-amino acid human apoE contains two independently folded domains: an N-terminal domain that includes the receptor-binding region and a C-terminal domain that contains the major lipid-binding region. The residues that distinguish the apoE isoforms (112 and 158) are marked. ApoE2 has cysteines at both positions, apoE4 has arginines at both positions, and apoE3 has Cys at position 112 and Arg at position 158. Domain interaction between Arg61 and Glu255 in apoE4 is also indicated.
In all animal species except human, apoE does not have genetic variants and contains threonine at the position equivalent to Arg61 in human apoE4. Therefore, with respect to domain interaction, apoE in other species behaves functionally like apoE3, regardless of whether it has arginine at position 11224. By introducing an Arg61 residue in murine apoE, murine apoE was converted to a human apoE4-like structure with domain interaction27. This conformation of human apoE4 and mouse Arg61 apoE lowers apoE levels in mouse brains, suggesting that domain interaction directly impacts apoE stability. ApoE isoforms also differ in their conformation and stability, with apoE4 assuming an unstable ‘molten globule’ state24. The unique properties of the domain interaction and the molten globule state underlie the pathogenic role of apoE4 in AD24,25.
ApoE receptors, members of the LDLR family
ApoE binds primarily to a group of receptors known as the LDLR family16,17. The prototype of this family is LDLR, which is the main receptor for cholesterol homeostasis28,29. Loss-of-function mutations in the LDLR gene cause high plasma LDL levels, which lead to familial hypercholesterolemia and atherosclerosis. LDLR binds both apoB-containing LDL and apoE-containing particles of different densities via electrostatic interactions between the basic amino acid residues on apoB/apoE and the acidic residues on the receptor. In 1988, a 4,544-amino acid protein containing the signature complement-type repeats and epidermal growth factor (EGF) precursor homology domains in LDLR was cloned30 (FIG. 2). This protein, named LDLR-related protein (LRP, now commonly known as LRP1 to distinguish it from other LRPs), is highly expressed in the liver and brain, and binds >30 ligands, including apoE, α2-macroglobulin (α2M), tissue-type plasminogen activator (tPA) and APP. LRP1 is synthesized as a single glycosylated protein (~600 kDa) and then cleaved by furin in the trans-Golgi network (TGN) to generate a 515-kDa extracellular subunit and an 85-kDa transmembrane subunit, which remain non-covalently associated with one another31. LRP1 is subjected to additional proteolysis that resembles APP/Notch processing (see BOX 1). Like LDLR, LRP1 also undergoes constitutive receptor-mediated endocytosis to transport ligands from the cell surface to intracellular compartments; however, LRP1 has a faster endocytosis rate than LDLR32. LRP1 is essential for early embryonic development as conventional deletion of the Lrp1 gene in mice results in lethality33. Brain-specific knockout of the Lrp1 gene demonstrates that LRP1 plays important roles in synaptic transmission and motor function in the CNS34. The 100-amino acid cytoplasmic tail of LRP1 binds to a variety of adaptor proteins that function in receptor trafficking and signalling16.
Figure 2. ApoE receptors, members of the LDLR family.
Structural organization of the LDLR family members. All receptors are type I receptors, each containing a single membrane-spanning domain and a relatively short cytoplasmic tail. The extracellular regions of these receptors contain three characteristic modules: ligand-binding repeats (also called complement-type repeats), EGF repeats and YWTD-containing β-propeller domains. The furin cleavage sites in LRP1 and LRP1B are indicated with purple arrow heads. The four clusters of ligand-binding repeats in LRP1 are labelled (I-IV). Highlighted in bright green are the two extra sequences in LRP1B encoded by two extra exons (compared to LRP1): a ligand-binding repeat in the fourth ligand-binding domain and a 33-amino acid insert in the cytoplasmic tail. LRP5/6 and sorLA/LR11 are distant members of the family with atypical structural arrangements. Several other LDLR family members, including LRP3, LRP9 and LRP12, whose functions are poorly defined, are not depicted here.
Box 1. LRP1 undergoes APP/Notch-like proteolytic processing and shedding.
In addition to furin cleavage in the trans-Golgi network (TGN) during its biogenesis, mature LRP1 is also subjected to several sequential proteolytic events that resemble APP/Notch processing (see FIG). First, LRP1 undergoes ectodomain shedding, which is mediated by metalloproteases (MMPs), from a region close to the membrane, releasing a soluble form of LRP1 (sLRP1) that is capable of binding ligands (ligand-binding domains shown in pink)146,147. LRP1 shedding occurs under physiological conditions, as evidenced by abundant sLRP1 in human plasma146,148. Second, LRP1 associates with BACE1 in lipid rafts and is a substrate for BACE1149. Third, LRP1 can be cleaved at an intramembrane site by the γ-secretase150, releasing a LRP1 intracellular domain (LICD), which is capable of regulating gene transcription151,152. The major enzyme(s) responsible for LRP1 shedding and the extent, if any, of altered LRP1 processing in AD brains remain unknown. Furthermore, how these processing events and their cleavage products affect LRP1 cellular function is not clear. Other LDLR family members, such as apoER2153, also undergo similar proteolytic processing, but their mechanisms and significance are also poorly understood.

Over ten receptors have now been identified as members of the LDLR family; they all contain characteristic ligand-binding repeats, EGF-like repeats and β-propeller-like structures with YWTD motifs (FIG. 2). These receptors bind a diverse array of ligands, including apoE, to mediate their transport and/or signalling (Supplementary Table). In addition to LRP1, this family also includes two additional ~600-kDa large receptors, megalin/LRP2 and LRP1B. Megalin is expressed abundantly on the apical surface of epithelial cells of proximal tubules in the kidney and functions in ligand resorption35. LRP1B is a putative tumour suppressor that is highly homologous to LRP1 (59% identity at the amino acid level) and binds overlapping ligands with LRP136. However, because of its slow rate of endocytosis36, LRP1B antagonizes LRP1 function in ligand uptake37,38. The very low-density lipoprotein receptor (VLDLR) and apoE receptor 2 (apoER2)/LRP8 are structurally similar to LDLR, but instead of functioning as typical endocytic receptors, they primarily serve as reelin signalling receptors39. Multiple EGF-like domain 7 (MEGF7)/LRP4 structurally resembles a portion of LRP1 and functions in limb patterning40 and in neuromuscular synapse formation by serving as a receptor for Agrin41. Sortilin-related receptor with A-type repeats (sorLA)/LR11/SORL1, LRP5 and LRP6 are structurally more diverse than the core members of the LDLR family described above. Whereas sorLA functions primarily in intracellular protein trafficking, LRP5 and LRP6 are co-receptors for canonical Wnt/β-catenin signalling42. Several of these LDLR family members play important roles in neuronal development and synaptic plasticity17,43. All members of the LDLR family bind apoE and receptor-associated protein (RAP), a molecular chaperone that promotes receptor folding and maturation in the secretory pathway44. RAP also universally antagonizes ligand-binding to LDLR family members44,45.
LDLR and LRP1 are the main apoE/lipoprotein metabolic receptors in the brain
Deletion of the Ldlr gene in mice increases apoE levels in brain parenchyma and CSF46, suggesting impaired metabolism of apoE. Similarly, conditional deletion of the Lrp1 gene in mouse forebrain neurons increases apoE levels47 and overexpression of a functional LRP1 minireceptor in mouse brain decreases brain apoE levels48. Although both LDLR and LRP1 play roles in brain apoE/lipoprotein metabolism, there are important differences between them. First, whereas LRP1 is highly expressed in neurons and to a lesser degree in glia, LDLR is more prominently expressed in glia than neurons49,50. Second, deletion of the Lrp1 gene in mouse forebrain neurons reduces brain cholesterol levels47, whereas cholesterol levels in Ldlr knockout mice are unchanged46. Third, apoE/lipoprotein particles secreted by astrocytes have higher affinity for LDLR than LRP146, whereas recombinant apoE51, apoE-enriched lipoprotein particles52 and CSF-isolated HDL particles53 bind more avidly to LRP1. The receptor-binding specificity of apoE is probably influenced by its conformation and lipidation state. For example, native apoE-containing β-VLDL is a ligand for LDLR but not LRP1. However, apoE enrichment, with either recombinant apoE or apoE extracted from native β-VLDL, converts these particles into high-affinity ligands for LRP152. Therefore, the inability of apoE on native β-VLDL to bind LRP1 is not due to any permanent modification of apoE but rather to lack of an apoE conformation that is generated at higher concentrations of apoE. It is possible that apoE/lipoprotein particles secreted by astrocytes recruit additional apoE molecules, perhaps bound to heparan sulfate proteoglycan (HSPG), before being transported to the CSF or binding to LRP1 at the neuronal cell surface.
ApoE and apoE receptors in neuronal signalling
In addition to transporting ligands to the cells, apoE receptors also mediate cellular signalling by binding to a variety of extracellular ligands and intracellular adaptor proteins17. The best-characterized signalling pathway is triggered by reelin and mediated by apoER2 and VLDLR17. Reelin signalling is crucial for neuronal migration39, dendritic spine development54 and synaptic plasticity55,56. Although biochemical evidence suggests that apoE interferes with reelin binding to apoE receptors57, the in vivo significance of a relationship between reelin and apoE is not clear. In primary neurons, apoE isoforms differentially affect several signalling cascades through apoE receptors, including increased phosphorylation of disabled 1 (Dab1), activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway and inhibition of the c-Jun N-terminal kinase 1/2 (JNK1/2) pathway58. ApoE4, but not apoE3, significantly increases resting calcium, calcium response to N-methyl-D-aspartate (NMDA) and neurotoxicity in a manner that depends on the function of LRP159. ApoE3/lipoprotein affords greater protection from apoptosis than apoE4/lipoprotein via LRP1-mediated signalling that involves activation of protein kinase Cδ (PKCδ) and inactivation of glycogen synthase kinase-3β (GSK3β)60. The isoform-specific signalling function of apoE and the potential in vivo relevance to AD pathogenesis require further investigation.
ApoE and apoE receptors in APP trafficking and processing to Aβ
Aβ accumulation, oligomerization and deposition in brain are central events in the pathogenesis of AD. The Aβ level in the brain is the net balance of Aβ production and clearance. Accordingly, Aβ accumulation in AD brains could reflect overproduction, inefficient clearance or both. Mounting evidence demonstrates that apoE and apoE receptors play important roles in both processes.
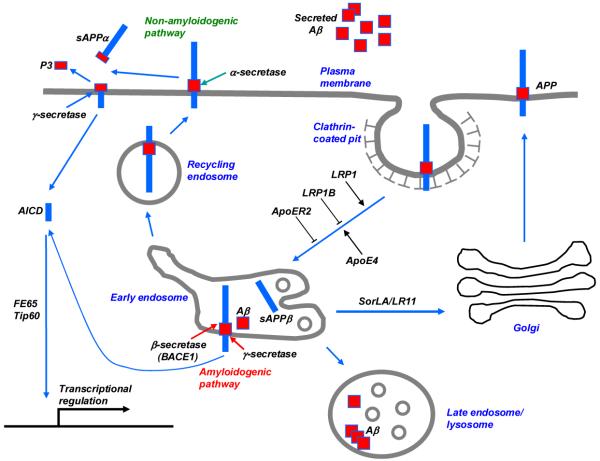
Several apoE receptors interact with APP and modulate its trafficking and processing to Aβ. Kounnas et al.61 first demonstrated that LRP1 binds soluble APP that contains the Kunitz-type protease inhibitor (KPI) domains (APP751 and APP770) extracellularly, and mediates its cellular catabolism. Further studies demonstrated that LRP1 also interacts with the neuronal isoform of APP lacking the KPI domain (APP695). Two different phosphotyrosine binding (PTB) domains within the adaptor protein FE65 bind to the NPxY motifs within APP and LRP1, thus bridging an interaction between these two membrane proteins intracellularly62,63. The extracellular and intracellular interactions between APP and LRP1 were confirmed in living cells by fluorescence resonance energy transfer (FRET) measurements64. The consequence of APP and LRP1 interaction is accelerated APP endocytic trafficking and processing to Aβ65, due to the fast rate of LRP1 endocytosis compared with that of APP66 (FIG. 3). Because the β-secretase BACE1 is abundantly present and active in acidic endosomes67, increased distribution of APP in endosomes leads to increased amyloidogenic processing and Aβproduction. The role of LRP1 in facilitating APP processing to Aβ is also supported by evidence that overexpression of a functional LRP1 minireceptor in neurons in the PDAPP amyloid mouse model causes an age-dependent increase in soluble brain Aβ68. Because LRP1 also has a role in Aβ clearance (see below), no significant changes in either total Aβ or plaque burden were detected. ApoE isoforms also differentially regulate APP processing to Aβ by a mechanism that depends on LRP1 function. In a neuronal cell line overexpressing APP, apoE4 has been shown to increase Aβ production to a greater extent than apoE369. This difference between the effects of apoE4 and apoE3 was abolished by incubating cells with the receptor antagonist RAP or by reducing LRP1 expression with small interfering RNA.
Figure 3. APP processing pathways regulated by LDLR family members and apoE.
Newly synthesized APP traffics through the secretory pathway to the plasma membrane, where it is primarily cleaved by the α-secretase in the non-amyloidogenic pathway, producing a minimally toxic peptide P3. When APP is internalized through the clathrin-mediated pathway, it is delivered to endosomes, where it is cleaved first by the β-secretase (BACE1), and then by the γ-secretase to generate highly toxic Aβ. Although most Aβ peptides are secreted to extracellular space, some Aβ peptides may aggregate in the late endosomes/lysosomes contributing to intraneuronal Aβ accumulation. The fast endocytosis of LRP1 enhances APP endocytosis and processing to Aβ, whereas the slow endocytosis of LRP1B and apoER2 contributes to retaining APP at the cell surface and promotes non-amyloidogenic processing. SorLA/LR11 likely shuttles APP to the Golgi compartments and reduces its processing by β-secretase in the early endosomes, thus decreasing Aβ production. ApoE4 promotes APP amyloidogenic processing in a manner that depends on LRP1 function. APP’s intracellular domain (AICD), a product of both non-amyloidgenic and amyloidgenic processing, interacts with FE65 and Tip60, and regulates gene transcription in the nucleus.
Three other apoE receptors also interact with APP and regulate its trafficking and processing to Aβ (FIG. 3). LRP1B, due to its slow rate of endocytosis, contributes to retaining APP at the cell surface and reduces its processing to Aβ38. ApoER2 either decreases or increases Aβ production depending on the experimental conditions. In the presence of F-spondin, which bridges the extracellular interaction between APP and apoER2, the slow endocytosis rate of apoER2 inhibits APP endocytic trafficking and reduces Aβ production70. However, in the absence of common ligands, apoER2 increases the distribution of APP into lipid rafts and APP processing to Aβ. A third apoE receptor that modulates APP trafficking and processing to Aβ is sorLA. Expression of sorLA is significantly reduced in AD brains71 and overexpression of sorLA in neurons shifts APP distribution to the Golgi compartment and decreases its processing to Aβ72. Importantly, a deletion of the Sorla gene in mice increases concentration of Aβ in the brain72. More direct evidence linking sorLA to AD comes from a recent study demonstrating that inherited variants in the SORL1 gene are associated with LOAD73. Whether apoE isoforms play roles in sorLA regulation of APP trafficking and processing is not known.
ApoE and apoE receptors in Aβ clearance and aggregation
It is widely believed that impaired Aβ clearance is a major pathogenic event for LOAD. Aβ has a relatively short half-life in the brain. Using in vivo microdialysis and γ-secretase inhibitor, it has been shown that Aβ has a half-life of ~2 h and ~4 h in young and aged mice, respectively74. In human brains, Aβ clearance rate is 8.3% per hour75 indicating that Aβ is actively and efficiently cleared from the brain.
There are two major pathways by which Aβ is cleared from the brain: receptor-mediated clearance by cells in brain parenchyma (microglia, astrocytes, neurons), along the interstitial fluid (ISF) drainage pathway or through the blood-brain barrier (BBB); and through endopeptidase-mediated proteolytic degradation (FIG. 4). Receptor-mediated clearance of Aβ in the brain is likely to be mediated by the apoE receptors LRP1, LDLR and VLDLR, which are widely expressed in neurons, astrocytes and microglia of brain parenchyma, as well as in endothelial cells, astrocytes and smooth muscle cells at the BBB and cerebral arteries. ApoE as well as LRP1 and several other LRP1 ligands (e.g., α2M and lactoferrin) are present in amyloid plaques49. These receptors can bind Aβ directly76 or indirectly via Aβ chaperones. ApoE is the best-characterized Aβ chaperone. ApoE immunoreactivity is found in amyloid plaques6,49, suggesting that apoE interacts with Aβ directly in AD brains. The region of apoE that is responsible for Aβ binding is in the C-terminal domain, overlapping with the lipid-binding region77,78; this suggests that the lipophilic Aβ peptide associates with apoE in a process that is analogous to lipid binding. Indeed, Aβ binding to apoE compromises its lipid-binding function78. Furthermore, Aβ peptides modulate the binding of apoE isoforms differently to apoE receptors79,80. These results demonstrate that Aβ peptides can interfere with the normal function of apoE in brain lipid metabolism and thus contribute to AD pathogenesis. ApoE3/lipoprotein binds to Aβ with higher affinity than apoE4/lipoprotein81. Accordingly, apoE3 clears through cell surface apoE receptors more efficiently than apoE4. Indeed, several studies using different amyloid mouse models expressing either human apoE3 or apoE4 demonstrate that apoE3 mice develop fewer amyloid plaques than apoE4 mice82-84. Postmortem studies have demonstrated increased amyloid plaque load in the brains of ε4 allele carriers of both sporadic85 and genetic AD cases86, and this notion is now confirmed by PET imaging studies from ‘cognitively normal’ controls87. Interestingly, a recent study shows that Aβ binding to apoE4 redirects its clearance from LRP1 to VLDLR, which internalizes Aβ-apoE4 complexes at the BBB more slowly than LRP188. In contrast, Aβ-apoE2 and Aβ-apoE3 complexes are cleared at the BBB via both VLDLR and LRP1 at a substantially faster rate than Aβ-apoE4 complexes. The important function of apoE receptors in brain Aβ clearance was demonstrated in the PDGF-APPSw,Ind amyloid mouse model with decreased apoE receptor expression due to a deletion of their chaperone RAP89. Recombinant RAP and LRP1 antibody also reduce Aβ efflux from mouse brain90.
Figure 4. Major Aβ clearance pathways in the brain: role of apoE isoforms.
Aβ accumulation in the brain parenchyma leads to formation of Aβ oligomers and amyloid plaques, which are toxic to neurons, whereas its accumulation in the perivascular region leads to the formation of CAA, which disrupts vessel function and is associated with cerebral haemorrhage. Aβ has a relatively short half-life in the brain, ~4 h in older mice and ~6 h in humans. Major Aβ clearance pathways include receptor-mediated clearance by cells in brain parenchyma (by neurons and glia), along the interstitial fluid (ISF) drainage pathway, through the blood-brain barrier (BBB) and proteolytic degradation by endopeptidases. The comparative effects of apoE3 and apoE4 are indicated. In all major cellular Aβ clearance pathways, LRP1 is involved and is likely to clear Aβ either directly or when Aβ binds to its chaperones that are also LRP1 ligands (e.g. apoE, α2M). LDLR function in Aβ clearance is likely to involve Aβ-apoE complexes. VLDLR also has a role in the clearance of Aβ-apoE complexes at the BBB.
The receptor-mediated clearance is, in principle, an efficient way of reducing brain Aβ because most Aβ that is internalized by apoE receptors is delivered to lysosomes for degradation or transcytosed into the plasma (FIG. 4). However, it is possible that receptor-mediated clearance of Aβ into neurons can lead to intraneuronal Aβ, which is toxic91. A portion of Aβ that is internalized by neurons, particularly oligomeric Aβ42, accumulates in multivesicular bodies (MVBs)/late endosomes and lysosomes, and contributes to lysosomal dysfunction and neuronal toxicity. By contrast, receptor-mediated internalization of Aβ by astrocytes92 and microglia93 is likely to represent a more functional pathway to clear and eventually degrade Aβ.
ApoE4 is also associated with increased risk for CAA, which is characterized by Aβ deposits along the blood vessel walls in the CNS and is associated with cerebral hemorrhage94. Interestingly, the Aβ40/42 ratio in cerebral vascular Aβ deposits exceeds that of senile plaques95. Therefore, an increased Aβ40/42 ratio can shift Aβ deposition from brain parenchyma to the vascular wall, perhaps by increasing the solubility of Aβ to diffuse to the vasculature. ApoE4 alters the Aβ40/42 ratio and promotes the formation of CAA in the Tg2576 amyloid mouse model96. CAA is associated with eventual degeneration of smooth muscle cells, which express high levels of LRP1. It is possible that smooth muscle cells contribute significantly to receptor-mediated Aβ clearance in normal brains. However, when Aβ accumulates under pathological conditions such as AD, it can exceed the capacity of this clearance pathway, resulting in local Aβ aggregation and toxicity to smooth muscle cells. Further studies are needed to test this hypothesis.
Aβ can also be removed from the brain by proteolytic degradation. Several Aβ degrading enzymes play important roles in Aβ clearance97,98. Most of these enzymes are produced by neurons or glia, but some are also expressed in the cerebral vasculature. Neprilysin and insulin-degrading enzyme (IDE) are the two most extensively studied Aβ proteases that are expressed both in neurons and vascular cells. Reduced expression of these two enzymes in AD has been associated with the ε4 allele. Other Aβ-degrading enzymes include plasmin, endothelin-converting enzymes (ECE-1 and ECE-2), matrix metalloproteinases (MMP)2, MMP3 and MMP9, and angiotensin-converting enzyme (ACE). The specific roles for each of these enzymes in AD pathogenesis are still unclear. Interestingly, apoE promotes Aβ degradation by neprilysin and IDE, with apoE3 functioning more efficiently than apoE499. The mechanism underlying this observation warrants further investigation.
ApoE in Aβ-induced neurotoxicity
Aβ assemblies, particularly Aβ oligomers, are highly toxic to neurons. Synthetic Aβ42 oligomers inhibit neuronal viability of cultured neurons 10-fold more than Aβ42 fibrils and 40-fold more than unaggregated peptides100. ApoE4 augments Aβ42 oligomer-induced neurotoxicity to a larger extent than apoE3101. Aβ dimers from human AD brains102 and Aβ dodecamer, also known as Aβ*56, from Tg2576 mouse brains103 inhibit long-term potentiation (LTP) and memory in animal models, but it is not known if these toxicities are further regulated by apoE. Recently, apoE4 and Aβ aggregates, induced by inhibiting Aβ-degrading neprilysin, were found to act synergistically to induce neurodegeneration in mouse brain104. Because neurodegeneration was not reported in several amyloid mouse models expressing human apoE4, it is not clear why Aβ accumulation induced by inhibiting neprilysin caused neurodegeneration, whereas Aβ accumulation induced by over-production from mutant APP did not. Studies of the potential roles of soluble APPs and APP intracellular domain (AICD)105, which are more abundant in APP-overexpressing mice, might explain this difference.
ApoE fragmentation, tau phosphorylation and mitochondrial dysfunction
Hyperphosphorylated microtubule-associated protein tau is toxic to neurons and is the major component of neurofibrillary tangles. Tau is essential for Aβ- and excitotoxin-induced neuronal dysfunction; reducing endogenous tau blocks Aβ-induced cognitive impairments in the PDGF-APPSw,Ind amyloid model mice106. Transgenic over-expression of apoE4 in neurons, but not in astrocytes, increases tau phosphorylation in mice107,108, suggesting a neuron-specific effect of apoE4 on tau phosphorylation. The pathophysiological significance of this pathway is not clear, as apoE is generally produced by astrocytes and microglia, not neurons. However, apoE expression in neurons has been reported after injury109. Although apoE is not expressed in hippocampal neurons under normal conditions, neuronal injury induced by kainic acid upregulates apoE expression in neurons110. Therefore, it is possible that in stressed AD brains, abnormal apoE expression in neurons facilitates tau hyperphosphorylation.
Another question that remains to be solved is how apoE and tau, which are normally separated by the plasma or organelle membrane, come in physical contact. One hypothesis is that the C-terminal-truncated fragments of apoE, detected in both human AD brains and in a neuronal apoE4 transgenic mouse model, enter the cytosol and interact directly with tau108. Alternatively, apoE isoforms might differentially regulate apoE receptor-mediated signalling cascades that in turn modify the function of tau kinases and phosphatases.
The receptor- and lipid-binding regions of apoE4 fragments act together to cause mitochondrial dysfunction and neurotoxicity111. Using a cultured neuronal cell line, it was shown that the receptor-binding region of apoE4 C-terminal truncated fragments is required for its escape from the secretory pathway and the lipid-binding region mediates interaction with mitochondria leading to mitochondrial dysfunction. The in vivo relevance of this apoE4-mediated toxic pathway requires further investigation112.
ApoE and apoE receptors in brain cholesterol and lipid transport
The primary function of apoE in the brain is to transport cholesterol, mainly from astrocytes to neurons. Cholesterol is an essential component of membranes and myelin sheaths and is crucial for synaptic integrity and neuronal function113. The reduced synthesis and greater need for cholesterol by neurons in adult brains require active cholesterol transport to support synaptic functions and repair. Cholesterol associated with apoE/lipoprotein particles secreted by astrocytes is essential for the formation of mature synapses in vitro through a mechanism that requires functional apoE receptors114 (FIG. 5). Interestingly, an association between cholesterol metabolism and the risk of AD has been proposed115. In humans, early studies indicated that the use of statins, which inhibit cholesterol synthesis, is associated with a significant decrease in AD prevalence; however, several recent retrospective studies do not support such a conclusion115. Because statins also exert other pleiotrophic effects including regulation of gene expression and protein prenylation and isoprenylation, the specific effects of statins on AD pathology in different model systems are likely to be influenced by various biological and pathological processes such as the efficiency of blood flow to the brain and the presence of other disease states including hypertension, diabetes and hypercholesterolaemia. At the cellular level, the effect of cholesterol on the amyloidogenic processing of APP to Aβ remains controversial116,117. Cholesterol and statins clearly modulate APP processing in vitro and in vivo118,119. Much of the effects of cholesterol on APP processing relates to the important role of cholesterol in lipid rafts, where APP processing by β- and γ-secretase cleavage is favoured119,120.
Figure 5. Synapse formation and repair depend on cholesterol transport from astrocytes to neurons via the apoE/apoE receptor pathway.

ApoE, secreted by astrocytes, assembles cholesterol and other lipids into lipoprotein particles. The ATP-binding cassette transporter subfamily A member 1 (ABCA1) at the plasma membrane transports and loads lipids to apoE. Liver X receptors (LXRs) increase the expression of both ABCA1 and apoE. ApoE/lipoprotein particles may undergo modifications such as recruiting oligodendrocyte-specific lipids and additional apoE molecules prior to binding to neuronal apoE receptors LRP1/LDLR or being transported to the cerebrospinal fluid (CSF). Cholesterol and other lipids transported to neurons play important roles in synaptic formation and repair (see main text for details). The inset shows the main components of apoE/lipoprotein particles: cholesterol, cholesterol esters and phospholipids.
A reduction of brain cholesterol levels is observed in AD brains121,122. Interestingly, apoE4 knock-in mice have decreased brain cholesterol concentrations even though the peripheral cholesterol levels are increased123. This finding confirms that brain apoE/lipoprotein metabolism is distinct from that in plasma. Although the exact functional differences among the three apoE isoforms in brain cholesterol metabolism are still not clear, there is some evidence that apoE4 might be less efficient than apoE3 in transporting brain cholesterol50. ApoE4 is also less efficient than apoE3 in promoting cholesterol efflux from both neurons and astrocytes124. Using cultured astrocytes from apoE-TR mice, it was shown that apoE3 has the ability to generate similarly sized lipid particles with less number of apoE molecules than apoE4, suggesting that apoE3-expressing astrocytes can supply more cholesterol to neurons than apoE4-expressing astrocytes125. Despite these interesting observations, the roles of apoE isoforms in brain cholesterol metabolism require further investigation. The structural differences among apoE isoforms that determine their lipid and receptor binding specificities in the brain environment may account for their differences in modulating brain cholesterol metabolism. It is important to note that there are likely to be LRP1-mediated cholesterol transport mechanisms in the brain that are independent of apoE because LRP1-deficiency in the brain, but not apoE-deficiency, leads to decreased brain cholesterol levels. Other LRP1 ligands such as lipoprotein lipase might also play a role. Alternatively, LRP1 may serve as a cholesterol sensor that influences cholesterol synthesis and/or intracellular transport.
In addition to cholesterol, apoE also mediates the transport of other brain lipids, some of which are not produced in astrocytes. For example, sulfatide, an oligodendrocyte-synthesized lipid that is crucial for neuronal spine and myelin sheath integrity, is actively transported by an apoE- and LRP1-dependent mechanism126. It is possible that apoE/lipoprotein particles are modified by myelin-associated lipids prior to being transported into neurons. Interestingly, sulfatide is a potential biomarker for AD diagnosis as its levels are decreased in AD brains126.
ApoE and apoE receptors in synaptic plasticity, repair and dendritic spine integrity
Synaptic failure is an early pathological feature of AD127. ApoE/apoE receptor-mediated lipid redistribution and signalling play important roles in synaptic integrity and plasticity. Increasing evidence demonstrates that apoE isoforms differentially regulate synaptic plasticity and repair. Human apoE4-targeted replacement (apoE4-TR) mice display synaptic deficits in the absence of neuropathology128. LTP in the hippocampus of apoE4-TR mice is also significantly impaired compared with apoE3-TR mice and WT mice129.
ApoE expression is up-regulated in rat brains after ischemia130 and in injured neurons upon kainic acid treatment110. The primary function of injury-induced apoE is probably to redistribute lipids and strengthen apoE-mediated signalling for neuronal and synaptic repairs. Using β-VLDL enriched with apoE isoforms or apoE-containing HDL isolated from CSF, it was shown that apoE3 increases neurite outgrowth, whereas apoE4 either decreases outgrowth or has no effect53,131,132. Similarly, apoE3-containing lipoprotein particles are more effective than apoE4 particles in protecting CNS neurons from apoptosis60. Collectively, these studies indicate that apoE4 is less effective than apoE3 in maintaining and repairing synapses and neurons, which may explain why under certain neuronal injury conditions, such as severe head trauma and stroke, individuals with apoE4 tend to have poorer outcome. It is possible that LOAD is a result of cumulative injury from Aβ and other neuronal stresses, such as oxidation and inflammation, and that apoE4 is less effective in maintaining and repairing injured synapses and neurons.
ApoE isoforms also differentially regulate dendritic spines during aging. ApoE3 and WT mice have a higher density of dendritic spines than apoE4 and apoE-KO mice at 1-2 years of age but not at 3 weeks of age133. This age-dependent difference suggests that the effects of apoE isoforms on neuronal integrity might relate to the increased risk of dementia in aged individuals with apoE4. In human brains, apoE4 dose correlates inversely with dendritic spine density in both AD and aged normal controls133. Interestingly, apoE2 overexpression ameliorates dendritic spine loss in Tg2576 amyloid model mice, suggesting that apoE isoforms functionally interact with Aβ to regulate neuronal integrity134. Moreover, a recent in vitro study showed that rosiglitazone, a peroxisome proliferator-activated receptor-gamma (PPAR-gamma) agonist, increases dendritic spine density and rescues spine loss caused by apoE4 in primary neurons135. Whether the different roles of apoE isoforms in dendritic spine density relate to apoE/apoE receptor-mediated lipid metabolism, signalling and/or synaptic plasticity and repair is not currently clear.
ApoE and apoE receptors as therapeutic targets for AD
Most AD therapeutic approaches have been designed to reduce Aβ production or aggregation, or promote its clearance. Because apoE and apoE receptors play critical roles in both Aβ-dependent and Aβ-independent AD pathogenic pathways, it is logical to also consider strategies to regulate their expression and functions (TABLE 1). As apoE4 is a strong risk factor for AD compared with apoE3, one attractive approach is to convert an apoE4 to an apoE3-like molecule. The unique domain interaction in apoE4 but not apoE3 seems to be responsible for most of apoE4-associated neuropathology and provides a target for agents to disrupt this interaction. Indeed, several molecules that can disrupt apoE4 domain interaction have been identified. Among these, GIND-25, a disulfonate, and GIND-105, a monosulfoalkyl, decrease Aβ production induced by apoE4 to a similar level to that induced by apoE3112. Whether or not these compounds can diminish apoE4 toxic functions in animals or humans in vivo is currently unknown.
Table 1. Strategies of apoE/apoE receptor-based AD therapy.
| Strategy | Method | Benefit |
|---|---|---|
| Convert apoE4 to apoE3 | Disrupt apoE4 ‘domain interaction’ with pharmacological approach |
Increase apoE3-related function, decrease apoE4-related function |
| Increase apoE level | LXR agonists, pharmacological approach |
Increase Aβ clearance, lipid homeostasis, synaptic function |
| Pseudo apoE | ApoE-mimetic peptides | Decrease neurotoxicity, inflammation |
| Block apoE4/Aβ interaction | Aβ12-28P, pharmacological approach |
Decrease Aβ aggregation |
| Block apoE fragmentation | Pharmacological approach | Decrease tau phosphorylation, mitochondria toxicity |
| Increase apoE lipidation | Increase ABCA1 expression with LXR agonists, other pharmacological approach |
Increase apoE half-life, decrease amyloid deposition |
| Increase LRP1/LDLR level | Pharmacological approach | Increase Aβ clearance, cholesterol transport, synaptic plasticity |
| Increase apoER2/VLDLR level | Pharmacological approach | Increase apoE signalling, synaptic plasticity |
Another potential strategy is to regulate apoE expression levels in the brain. However, this strategy obligates careful consideration as to whether apoE4 effects in AD brains are caused by loss-of-protection, gain-of-toxicity or both. It has been shown that the promoter polymorphisms of the APOE gene may lead to changes in apoE expression levels by altering transcription of the APOE gene136. Therefore, regulating APOE promoter function might be one strategy to alter apoE expression. Compounds that alter brain apoE expression might also be identified through screening. Using an apoE4-specific immunoassay, a recent study demonstrated that apoE4 has a faster turnover and lower steady-state concentration in apoE4-TR mice than in apoE3-TR mice137, suggesting that low levels of total apoE4 might directly contribute to AD pathogenesis, perhaps by reducing apoE function in lipid metabolism, synaptic repair and Aβ clearance. Therefore, increasing the expression of apoE in all apoE genotypes might prevent or slow the progression of AD. However, increasing the expression of apoE, particularly apoE4, may also generate deleterious effects such as slowing Aβ clearance through the BBB88 and augmenting Aβ neurotoxicity101. The specific effects of altering apoE expression on AD pathogenesis will likely depend on apoE isoforms, stage of the disease and the presence of other pathophysiological conditions.
Liver X receptors (LXRs) are oxysterol receptors that serve as transcription factors to regulate cholesterol homeostasis. In brain, LXRs up-regulate apoE and ABCA1, thereby promoting cholesterol efflux in neurons and glia138. In Tg2576 amyloid mouse model, LXR agonists facilitate the clearance of Aβ42 and reverse the contextual memory deficit in these mice139. Thus, LXR agonists may represent an important therapeutic approach to AD. Another related approach is to identify apoE peptides that perform apoE-related functions and cross the BBB140. In this regard, apoE-mimetic peptides suppress glial inflammation and protect neurons from injury141,142. Because these apoE peptides do not contain lipid-binding domains, their exact mechanism of function is currently unknown. Nonetheless, their promising effects make them strong candidates for AD therapy.
Because apoE is essential for amyloid deposition in mice143, probably by promoting Aβ fibrillization, disrupting apoE and Aβ interaction could be another therapeutic approach. An Aβ12-28P synthetic peptide, which is homologous to the apoE binding site on the full-length Aβ BBB-permeable and nontoxic, reduced the total brain Aβ levels, Aβ plaques, CAA burden and memory performance in two amyloid mouse models144. This suggests that inhibiting apoE and Aβ interaction in vivo might decrease total brain Aβ.
ApoE receptors can also be potential targets for AD therapy. LRP1 and LDLR play critical roles in brain apoE/lipoprotein metabolism and in Aβ clearance. Because LRP1 level is decreased in AD brains145, it will be interesting to test whether restoring LRP1 expression in AD brains augments Aβ clearance and reduces AD pathology. ApoER2 and VLDLR are both implicated in reelin/apoE signalling, which is important for synaptic repair and plasticity. Therefore, these apoE signalling receptors might be novel targets for compounds that up-regulate their expression and/or function. One caveat, however, is that up-regulating apoE receptor expression/function might increase the risk of other diseases such as cancer.
Other apoE/apoE receptor-related strategies include reducing apoE fragmentation, which might block interactions between apoE fragments and tangles or mitochondria, preserving brain apoE levels by facilitating apoE recycling efficiency, and blocking the interaction between APP and certain apoE receptors (such as LRP1) that promotes APP processing. Strategic combinations of modulating Aβ and apoE pathways are highly promising avenues of investigations for combating AD.
Perspectives
ApoE4 is a strong risk factor for LOAD. An emerging body of data has identified multiple pathways that could explain the pathogenic nature of apoE4. These include Aβ production, Aβ clearance, Aβ fibrillization, tangle formation, cholesterol homeostasis, synaptic plasticity and repair, and neuronal toxicity (FIG. 6). These multiple pathways regulated by apoE isoforms and apoE receptors present opportunities to identify the pathways that are most relevant to AD pathogenesis and novel targets with tractable efficacy for AD therapy. Greater understanding of the differential expression and function of apoE isoforms has been facilitated by novel animal models including human apoE isoform-TR mice and humanized mouse apoE expressed in the apoE-KO background. Inducible expression of apoE isoforms in aged animals should further clarify the roles of apoE isoforms in aging brains. Furthermore, the relationship between brain apoE-mediated cholesterol/lipid metabolism and apoE-mediated Aβ/tau pathology should be studied in vivo. Finally, the functional relationship between apoE isoforms and multiple apoE receptors requires more in-depth investigation. Because apoE4 affects a much greater AD population than early-onset AD, therapies based on apoE/apoE receptors as targets might benefit more AD patients and offer hope to those reaching old age.
Figure 6. Roles of apoE isoforms in the healthy brain and AD pathogenesis.

Summary of apoE functions in normal brain function and the pathogenic processes of AD. Differential regulations by apoE3 and apoE4 are indicated.
Supplementary Material
Acknowledgements
The author wishes to thank P. Tarr, D. Owyoung and members of the Bu laboratory for critical reading and comments on this Review. I apologize to those whose work is not cited in this review due to either space limitations or the specific focus of this review. Work in the author’s laboratory is supported by the National Institutes of Health (grants R01AG027924 and R01AG031784) and a Zenith Fellows Award from the Alzheimer’s Association.
GLOSSARY
- Polymorphic alleles
Natural variants in a gene that occur with fairly high frequency (>1%) in the general population.
- Cerebral amyloid angiopathy (CAA)
A neurological condition in which amyloid protein is deposited onto the walls of the arteries of the brain. CAA increases the risk of bleeding into the brain causing haemorrhagic stroke.
- Molten globule
A stable, partially folded protein state found in mildly denaturing conditions such as low pH, mild denaturant or high temperature. It is also used to refer to certain protein folding intermediates.
- PDAPP amyloid mouse model
Mouse model of AD in which a human APP transgene bearing the amyloidogenic V717F mutation is overexpressed under the control of the human platelet derived growth factor β (PDGF-β) chain gene promoter.
- Endopeptidases
A group of enzymes that catalyze the hydrolysis of peptide bonds in the interior of a polypeptide chain or protein molecule.
- PDGF-APPSw,Ind amyloid mouse model
Mouse model of AD in which a human APP transgene bearing the amyloidogenic V717F and K670N/M671L mutations is overexpressed under the control of the human platelet derived growth factor β (PDGF-β) chain gene promoter.
- Tg2576 amyloid mouse model
Mouse model of AD in which a human APP transgene bearing the amyloidogenic K670N/M671L mutations is overexpressed under the control of the hamster prion protein gene promoter.
- Protein prenylation and isoprenylation
Covalent attachment of hydrophobic prenyl or isoprenyl groups to a protein. Play roles in protein attachment to cell membranes and protein-protein interactions.
- ApoE4-TR mice
Mouse model of human apoE4 in which human APOE-ε4 gene is inserted into the mouse Apoe gene locus. Expression of human apoE4 in these mice temporally and spatially resembles that of mouse endogenous apoE.
Footnotes
Competing interests statement
The author declares no competing financial interest.
REFERENCES
- 1.Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J. Clin. Invest. 2002;110:1375–1381. doi: 10.1172/JCI16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Kelleher RJ., III The presenilin hypothesis of Alzheimer’s disease: Evidence for a loss-of-function pathogenic mechanism. Proc. Natl. Acad. Sci. USA. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strittmatter WJ, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. Together with reference 7, these papers were the first to report a genetic association between the ε4 allele of the APOE gene and late-onset AD.
- 7.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 8.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. This is a classic review on the biochemical properties and cholesterol-transport function of apoE.
- 9.Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. This paper provides a comprehensive review on the current state of AD genetics.
- 10.Blacker D, et al. ApoE-4 and age at onset of Alzheimer’s disease: the NIMH genetics initiative. Neurology. 1997;48:139–147. doi: 10.1212/wnl.48.1.139. [DOI] [PubMed] [Google Scholar]
- 11.Burt TD, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc. Natl. Acad. Sci. USA. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg SM, Rebeck GW, Vonsattel JP, Gomez-Isla T, Hyman BT. Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann. Neurol. 1995;38:254–259. doi: 10.1002/ana.410380219. [DOI] [PubMed] [Google Scholar]
- 13.Josephs KA, Tsuboi Y, Cookson N, Watt H, Dickson DW. Apolipoprotein E epsilon 4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch. Neurol. 2004;61:1579–1584. doi: 10.1001/archneur.61.10.1579. [DOI] [PubMed] [Google Scholar]
- 14.Martinez M, et al. Apolipoprotein E4 is probably responsible for the chromosome 19 linkage peak for Parkinson’s disease. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2005;136B:72–74. doi: 10.1002/ajmg.b.30196. [DOI] [PubMed] [Google Scholar]
- 15.Masterman T, Hillert J. The telltale scan: APOE epsilon4 in multiple sclerosis. Lancet Neurol. 2004;3:331. doi: 10.1016/S1474-4422(04)00763-X. [DOI] [PubMed] [Google Scholar]
- 16.Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu. Rev. Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 17.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 18.Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 19.Uchihara T, et al. ApoE immunoreactivity and microglial cells in Alzheimer’s disease brain. Neurosci. Lett. 1995;195:5–8. doi: 10.1016/0304-3940(95)11763-m. [DOI] [PubMed] [Google Scholar]
- 20.Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. J. Biol. Chem. 1987;262:14352–14360. [PubMed] [Google Scholar]
- 21.LaDu MJ, et al. Nascent astrocyte particles differe from lipoproteins in CSF. J. Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch-Reinshagen V, et al. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 23.Wahrle SE, et al. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 24.Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem. Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. This is a comprehensive review on the biophysical and structural properties of apoE isoforms.
- 25.Zhong N, Weisgraber KH. Understanding the association of apolipoprotein E4 with Alzheimer’s disease: Clues from its structure. J. Biol. Chem. 2008 Oct 22; doi: 10.1074/jbc.R800009200. doi:10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisgraber KH, Rall SC, Jr., Mahley RW. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J. Biol. Chem. 1981;256:9077–9083. [PubMed] [Google Scholar]
- 27.Ramaswamy G, Xu Q, Huang Y, Weisgraber KH. Effect of domain interaction on apolipoprotein E levels in mouse brain. J. Neurosci. 2005;25:10658–10663. doi: 10.1523/JNEUROSCI.1922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein JL, Brown MS, Anderson RGW, Russell DW, Schneider WJ. Receptor-mediated endocytosis: Concepts emerging from the LDL receptor system. Ann. Rev. Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- 29.Jeon H, Blacklow SC. Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 2005;74:535–562. doi: 10.1146/annurev.biochem.74.082803.133354. [DOI] [PubMed] [Google Scholar]
- 30.Herz J, et al. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. This paper reports the initial cloning and structural properties of LRP1, a major apoE receptor in the brain.
- 31.Herz J, Kowal RC, Goldstein JL, Brown MS. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990;9:1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Lu W, Marzolo MP, Bu G. Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J. Biol. Chem. 2001;276:18000–18006. doi: 10.1074/jbc.M101589200. [DOI] [PubMed] [Google Scholar]
- 33.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 34.May P, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol. Cell. Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willnow TE, Nykjaer A, Herz J. Lipoprotein receptors: new roles for ancient proteins. Nat. Cell. Biol. 1999;1:E157–162. doi: 10.1038/14109. [DOI] [PubMed] [Google Scholar]
- 36.Liu CX, Li Y, Obermoeller-McCormick LM, Schwartz AL, Bu G. The putative tumor suppressor LRP1B, a novel member of the low density lipoprotein (LDL) receptor family, exhibits both overlapping and distinct properties with the LDL receptor-related protein. J. Biol. Chem. 2001;276:28889–28896. doi: 10.1074/jbc.M102727200. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, et al. Low density lipoprotein (LDL) receptor-related protein 1B impairs urokinase receptor regeneration on the cell surface and inhibits cell migration. J. Biol. Chem. 2002;277:42366–42371. doi: 10.1074/jbc.M207705200. [DOI] [PubMed] [Google Scholar]
- 38.Cam JA, et al. The low density lipoprotein receptor-related protein 1B retains beta-amyloid precursor protein at the cell surface and reduces amyloid-beta peptide production. J. Biol. Chem. 2004;279:29639–29646. doi: 10.1074/jbc.M313893200. [DOI] [PubMed] [Google Scholar]
- 39.Trommsdorff M, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. This paper describes the role of apoER2 and VLDL receptor function in reelin signalling, which is critical for neuronal migration during development.
- 40.Johnson EB, Hammer RE, Herz J. Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum. Mol. Genet. 2005;14:3523–3538. doi: 10.1093/hmg/ddi381. [DOI] [PubMed] [Google Scholar]
- 41.Kim N, et al. Lrp4 Is a Receptor for Agrin and Forms a Complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 43.May P, Herz J. LDL receptor-related proteins in neurodevelopment. Traffic. 2003;4:291–301. doi: 10.1034/j.1600-0854.2003.00086_4_5.x. [DOI] [PubMed] [Google Scholar]
- 44.Bu G. Receptor-associated protein: a specialized chaperone and antagonist for members of the LDL receptor gene family. Curr. Opin. Lipidol. 1998;9:149–155. doi: 10.1097/00041433-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Bu G, Marzolo MP. Role of rap in the biogenesis of lipoprotein receptors. Trends Cardiovasc. Med. 2000;10:148–155. doi: 10.1016/s1050-1738(00)00045-1. [DOI] [PubMed] [Google Scholar]
- 46.Fryer JD, et al. The low density lipoprotein receptor regulates the level of central nervous system human and murine apolipoprotein E but does not modify amyloid plaque pathology in PDAPP mice. J. Biol. Chem. 2005;280:25754–25759. doi: 10.1074/jbc.M502143200. [DOI] [PubMed] [Google Scholar]
- 47.Liu Q, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. This paper reports that APP and apoE are functionally linked in brain cholesterol metabolism via the apoE receptor LRP1.
- 48.Zerbinatti CV, et al. Apolipoprotein E and low density lipoprotein receptor-related protein facilitate intraneuronal Abeta42 accumulation in amyloid model mice. J. Biol. Chem. 2006;281:36180–36186. doi: 10.1074/jbc.M604436200. [DOI] [PubMed] [Google Scholar]
- 49.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. This paper describes the finding that both apoE and its receptor LRP1 are present in amyloid plaques.
- 50.Rapp A, Gmeiner B, Huttinger M. Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie. 2006;88:473–483. doi: 10.1016/j.biochi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Narita M, et al. Cellular catabolism of lipid poor apolipoprotein E via cell surface LDL receptor-related protein. J. Biochem. 2002;132:743–749. doi: 10.1093/oxfordjournals.jbchem.a003282. [DOI] [PubMed] [Google Scholar]
- 52.Kowal RC, et al. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J. Biol. Chem. 1990;265:10771–10779. [PubMed] [Google Scholar]
- 53.Fagan AM, Bu GJ, Sun YL, Daugherty A, Holtzman DM. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J. Biol. Chem. 1996;271:30121–30125. doi: 10.1074/jbc.271.47.30121. [DOI] [PubMed] [Google Scholar]
- 54.Niu S, Yabut O, D’Arcangelo G. The reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 2008;28:10339–10348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beffert U, et al. Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission. J. Neurosci. 2004;24:1897–1906. doi: 10.1523/JNEUROSCI.4084-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beffert U, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 57.D’Arcangelo G, et al. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 58.Hoe HS, Harris DC, Rebeck GW. Multiple pathways of apolipoprotein E signaling in primary neurons. J. Neurochem. 2005;93:145–155. doi: 10.1111/j.1471-4159.2004.03007.x. [DOI] [PubMed] [Google Scholar]
- 59.Qiu Z, Crutcher KA, Hyman BT, Rebeck GW. ApoE isoforms affect neuronal N-methyl-D-aspartate calcium responses and toxicity via receptor-mediated processes. Neuroscience. 2003;122:291–303. doi: 10.1016/j.neuroscience.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi H, Campenot RB, Vance DE, Vance JE. Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J. Neurosci. 2007;27:1933–1941. doi: 10.1523/JNEUROSCI.5471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kounnas MZ, et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. This was the first report describing an interaction between APP and LRP1. Subsequent studies show that LRP1 regulates APP trafficking and processing.
- 62.Trommsdorff R, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J. Biol. Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- 63.Pietrzik CU, et al. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J. Neurosci. 2004;24:4259–4265. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kinoshita A, et al. Demonstration by fluorescence resonance energy transfer of two sites of interaction between the low-density lipoprotein receptor-related protein and the amyloid precursor protein: role of the intracellular adapter protein Fe65. J. Neurosci. 2001;21:8354–8361. doi: 10.1523/JNEUROSCI.21-21-08354.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ulery PG, et al. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. J. Biol. Chem. 2000;275:7410–7415. doi: 10.1074/jbc.275.10.7410. [DOI] [PubMed] [Google Scholar]
- 66.Cam JA, Zerbinatti CV, Li Y, Bu G. Rapid endocytosis of the low density lipoprotein receptor-related protein modulates cell surface distribution and processing of the beta-amyloid precursor protein. J. Biol. Chem. 2005;280:15464–15470. doi: 10.1074/jbc.M500613200. [DOI] [PubMed] [Google Scholar]
- 67.Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE1. Mol. Neurodegener. 2007;2:22. doi: 10.1186/1750-1326-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zerbinatti CV, et al. Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein. Proc. Natl. Acad. Sci. USA. 2004;101:1075–1080. doi: 10.1073/pnas.0305803101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye S, et al. Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target. Proc. Natl. Acad. Sci. USA. 2005;102:18700–18705. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoe HS, et al. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol. Cell Biol. 2005;25:9259–9268. doi: 10.1128/MCB.25.21.9259-9268.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scherzer CR, et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch. Neurol. 2004;61:1200–1205. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- 72.Andersen OM, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cirrito JR, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J. Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. This paper describes an in vivo microdialysis technique to assess Aβ concentration in brain interstitial fluid. It also shows that Aβ half-life in mouse brain is 2-4 hours.
- 75.Bateman RJ, et al. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat. Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deane R, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. This paper reports an important function of LRP1 in brain Aβ efflux through the BBB. It also demonstrates that Aβ binds directly to LRP1.
- 77.Strittmatter WJ, et al. Binding of human apolipoprotein E to synthetic amyloid b peptide: Isoform-specific effects and implications for late-onset Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamamizu-Kato S, et al. Interaction with amyloid beta peptide compromises the lipid binding function of apolipoprotein E. Biochemistry. 2008;47:5225–5234. doi: 10.1021/bi702097s. [DOI] [PubMed] [Google Scholar]
- 79.Beffert U, et al. Beta-amyloid peptides increase the binding and internalization of apolipoprotein E to hippocampal neurons. J. Neurochem. 1998;70:1458–1466. doi: 10.1046/j.1471-4159.1998.70041458.x. [DOI] [PubMed] [Google Scholar]
- 80.Hone E, et al. Alzheimer’s disease amyloid-beta peptide modulates apolipoprotein E isoform specific receptor binding. J. Alzheimers Dis. 2005;7:303–314. doi: 10.3233/jad-2005-7406. [DOI] [PubMed] [Google Scholar]
- 81.LaDu MJ, et al. Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 1994;269:23403–23406. This paper shows that apoE3/lipoprotein binds to Aβ with higher affinity than apoE4/lipoprotein.
- 82.Holtzman DM, et al. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer’s disease. J. Clin. Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holtzman DM, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. This paper describes the differential in vivo effects of human apoE isoforms in amyloid deposition in mouse models.
- 84.DeMattos RB, et al. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- 85.Schmechel DE, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bogdanovic N, Corder E, Lannfelt L, Winblad B. APOE polymorphism and clinical duration determine regional neuropathology in Swedish APP(670, 671) mutation carriers: implications for late-onset Alzheimer’s disease. J Cell Mol Med. 2002;6:199–214. doi: 10.1111/j.1582-4934.2002.tb00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Small GW, et al. Influence of cognitive status, age, and APOE-4 genetic risk on brain FDDNP positron-emission tomography imaging in persons without dementia. Arch Gen Psychiatry. 2009;66:81–87. doi: 10.1001/archgenpsychiatry.2008.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deane R, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Uden E, et al. Increased extracellular amyloid deposition and neurodegeneration in human amyloid precursor protein transgenic mice deficient in receptor-associated protein. J. Neurosci. 2002;22:9298–9304. doi: 10.1523/JNEUROSCI.22-21-09298.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shibata M, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 92.Koistinaho M, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-[beta] peptides. Nat. Med. 2004;10:719–726. doi: 10.1038/nm1058. This paper shows that apoE secreted by astrocytes plays a critical role in degrading Aβ associated with amyloid; this process depends on the function of apoE receptors.
- 93.Khoury JE, Luster AD. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends in Pharmacol. Sci. 2008;29:626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 94.McCarron MO, Nicoll JAR. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol. 2004;3:484–492. doi: 10.1016/S1474-4422(04)00825-7. [DOI] [PubMed] [Google Scholar]
- 95.Roher AE, et al. Beta-amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: Implications for the pathology of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90:10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fryer JD, et al. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J. Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leissring MA. The ABCs of abeta-cleaving proteases. J. Biol. Chem. 2008;283:29645–29649. doi: 10.1074/jbc.R800022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 99.Jiang Q, et al. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dahlgren KN, et al. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J. Biol. Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 101.Manelli AM, Bulfinch LC, Sullivan PM, LaDu MJ. Abeta42 neurotoxicity in primary co-cultures: effect of apoE isoform and Abeta conformation. Neurobiol. Aging. 2007;28:1139–1147. doi: 10.1016/j.neurobiolaging.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. Together with reference 103, these papers demonstrate that Aβ oligomers isolated from the brain are highly neurotoxic.
- 103.Lesne S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 104.Belinson H, Lev D, Masliah E, Michaelson DM. Activation of the amyloid cascade in apolipoprotein E4 transgenic mice induces lysosomal activation and neurodegeneration resulting in marked cognitive deficits. J. Neurosci. 2008;28:4690–4701. doi: 10.1523/JNEUROSCI.5633-07.2008. This paper shows that apoE4 and Aβ synergistically activate neurotoxic pathways that lead to neurodegeneration and cognitive deficits in mice.
- 105.Muller T, Meyer HE, Egensperger R, Marcus K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-Relevance for Alzheimer’s disease. Prog. Neurobiol. 2008;85:393–406. doi: 10.1016/j.pneurobio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 107.Tesseur I, et al. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am. J. Pathol. 2000;156:951–964. doi: 10.1016/S0002-9440(10)64963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brecht WJ, et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J. Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004.. This paper demonstrates that neuronal expression of apoE4 leads to increased tau phosphorylation.
- 109.Aoki K, et al. Increased expression of neuronal apolipoprotein E in human brain with cerebral infarction. Stroke. 2003;34:875–880. doi: 10.1161/01.STR.0000064320.73388.C6. [DOI] [PubMed] [Google Scholar]
- 110.Xu Q, et al. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J. Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang S, et al. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc. Natl. Acad. Sci. USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol. Life Sci. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mauch DH, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. This paper shows that astrocyte-secreted cholesterol is essential for synaptogenesis.
- 115.Shobab LA, Hsiung G-YR, Feldman HH. Cholesterol in Alzheimer’s disease. Lancet Neurol. 2005;4:841–852. doi: 10.1016/S1474-4422(05)70248-9. [DOI] [PubMed] [Google Scholar]
- 116.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat. Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 117.Kaether C, Haass C. A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation. J. Cell Biol. 2004;167:809–812. doi: 10.1083/jcb.200410090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reid PC, Urano Y, Kodama T, Hamakubo T. Alzheimer’s disease: cholesterol, membrane rafts, isoprenoids and statins. J. Cell Mol. Med. 2007;11:383–392. doi: 10.1111/j.1582-4934.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marzolo MP, Bu G. Lipoprotein receptors and cholesterol in APP trafficking and proteolytic processing, implications for Alzheimer’s disease. Semin Cell Dev Biol. 2008 Oct 17; doi: 10.1016/j.semcdb.2008.10.005. doi:10.1016/j.semcdb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ledesma MD, Abad-Rodriguez J, Galvan C, Biondi E, Navarro P, Delacourte A, Dingwall C, Dotti CG. Raft disorganization leads to reduced plasmin activity in Alzheimer’s disease brains. EMBO Rep. 2003;4:1190–1196. doi: 10.1038/sj.embor.7400021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ledesma MD, Dotti CG. Amyloid excess in Alzheimer’s disease: What is cholesterol to be blamed for? FEBS Lett. 2006;580:5525–5532. doi: 10.1016/j.febslet.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 123.Hamanaka H, et al. Altered cholesterol metabolism in human apolipoprotein E4 knock-in mice. Hum. Mol. Genet. 2000;9:353–361. doi: 10.1093/hmg/9.3.353. [DOI] [PubMed] [Google Scholar]
- 124.Michikawa M, Fan QW, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J. Neurochem. 2000;74:1008–1016. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- 125.Gong JS, et al. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem. 2002;277:29919–29926. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- 126.Han X. Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stage of Alzheimer’s disease: a tale of shotgun lipidomics. J. Neurochem. 2007;103:171–179. doi: 10.1111/j.1471-4159.2007.04708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 128.Wang C, et al. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol. Dis. 2005;18:390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 129.Trommer BL, et al. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004;15:2655–2658. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- 130.Hayashi T, et al. Different expression of low density lipoprotein receptor and ApoE between young adult and old rat brains after ischemia. Neurol. Res. 2006;28:822–825. doi: 10.1179/016164105X40002. [DOI] [PubMed] [Google Scholar]
- 131.Holtzman DM, et al. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl. Acad. Sci. USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nathan BP, et al. Differential effects of apolipoprotein E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. Together with reference 131, these papers describe the differential effects of apoE isoforms in promoting neurite outgrowth.
- 133.Ji Y, et al. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 134.Lanz TA, Carter DB, Merchant KM. Dendritic spine loss in the hippocampus of young PDAPP and Tg2576 mice and its prevention by the ApoE2 genotype. Neurobiol. Dis. 2003;13:246–253. doi: 10.1016/s0969-9961(03)00079-2. [DOI] [PubMed] [Google Scholar]
- 135.Brodbeck J, et al. Rosiglitazone increases dendritic spine density and rescues spine loss caused by apolipoprotein E4 in primary cortical neurons. Proc. Natl. Acad. Sci. USA. 2008;105:1343–1346. doi: 10.1073/pnas.0709906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laws SM, Hone E, Gandy S, Martins RN. Expanding the association between the APOE gene and the risk of Alzheimer’s disease: possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J Neurochem. 2003;84:1215–1236. doi: 10.1046/j.1471-4159.2003.01615.x. [DOI] [PubMed] [Google Scholar]
- 137.Riddell DR, et al. Impact of apolipoprotein E (apoE) polymorphism on brain apoE levels. J. Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J. Neurochem. 2007;102:1727–1737. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- 139.Riddell DR, et al. The LXR agonist TO901317 selectively lowers hippocampal Abeta42 and improves memory in the Tg2576 mouse model of Alzheimer’s disease. Mol Cell Neurosci. 2007;34:621–628. doi: 10.1016/j.mcn.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 140.Laskowitz DT, Vitek MP. Apolipoprotein E and neurological disease: therapeutic potential and pharmacogenomic interactions. Pharmacogenomics. 2007;8:959–969. doi: 10.2217/14622416.8.8.959. [DOI] [PubMed] [Google Scholar]
- 141.Aono M, et al. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116:437–445. doi: 10.1016/s0306-4522(02)00709-1. [DOI] [PubMed] [Google Scholar]
- 142.Laskowitz DT, Fillit H, Yeung N, Toku K, Vitek MP. Apolipoprotein E-derived peptides reduce CNS inflammation: implications for therapy of neurological disease. Acta. Neurol. Scand. Suppl. 2006;185:15–20. doi: 10.1111/j.1600-0404.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 143.Bales KR, et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat. Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 144.Sadowski MJ, et al. Blocking the apolipoprotein E/amyloid-beta interaction as a potential therapeutic approach for Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2006;103:18787–18792. doi: 10.1073/pnas.0604011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kang DE, et al. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J. Clin. Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grimsley PG, Quinn KA, Owensby DA. Soluble low-density lipoprotein receptor-related protein. Trends Cardiovasc. Med. 1998;8:363–368. doi: 10.1016/s1050-1738(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 147.Quinn KA, Pye VJ, Dai YP, Chesterman CN, Owensby DA. Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP) Exp. Cell Res. 1999;251:433–441. doi: 10.1006/excr.1999.4590. [DOI] [PubMed] [Google Scholar]
- 148.Sagare A, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat. Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.von Arnim CA, et al. The low density lipoprotein receptor-related protein (LRP) is a novel beta-secretase (BACE1) substrate. J. Biol. Chem. 2005;280:17777–17785. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- 150.May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J. Biol. Chem. 2003;278:37386–37392. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- 151.Kinoshita A, Shah T, Tangredi MM, Strickland DK, Hyman BT. The intracellular domain of the low density lipoprotein receptor-related protein modulates transactivation mediated by amyloid precursor protein and Fe65. J. Biol. Chem. 2003;278:41182–41188. doi: 10.1074/jbc.M306403200. [DOI] [PubMed] [Google Scholar]
- 152.Zurhove K, Nakajima C, Herz J, Bock HH, May P. Gamma-secretase limits the inflammatory response through the processing of LRP1. Sci Signal. 2008;1:ra15. doi: 10.1126/scisignal.1164263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoe HS, Rebeck GW. Regulated proteolysis of APP and ApoE receptors. Mol. Neurobiol. 2008;37:64–72. doi: 10.1007/s12035-008-8017-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.