Abstract
The repair of open wounds depends on granulation tissue formation and contraction, which is primarily mediated by myofibroblasts. A subset of myofibroblasts originates from bone-marrow-derived monocytes which differentiate into fibroblast-like cells called fibrocytes. Serum amyloid P (SAP) inhibits differentiation of monocytes into fibrocytes. Thus, we hypothesized that the addition of exogenous SAP would hinder the normal wound healing process. Excisional murine dorsal wounds were either injected with SAP (intradermal group) or the mice were treated with systemic SAP (intraperitoneal group) and compared with animals treated with vehicle. Grossly, SAP-treated wounds closed slower than respective controls in both groups. Histologically, the contraction rate was slower in SAP-treated wounds in both groups and the reepithelialization rate was slower in the intraperitoneal group. Furthermore, significantly less myofibroblasts expressing α-smooth muscle actin were noted in the intraperitoneal group wounds compared with controls. These data suggest that SAP delays normal murine dermal wound healing, probably due to increased inhibition of fibrocyte differentiation, and ultimately a decreased wound myofibroblast population. SAP may provide a potential therapeutic target to prevent or limit excessive fibrosis associated with keloid or hypertrophic scar formation. Furthermore, SAP removal from wound fluid could potentially accelerate the healing of chronic, nonhealing wounds.
The repair of open wounds depends on reepithelialization, granulation tissue formation, and contraction. Myofibroblasts are believed to play a key role in wound contraction by exerting tension on the surrounding extracellular matrix (ECM) and secreting ECM proteins such as collagen to stabilize the contraction.1 Originally believed to originate solely from local tissue fibroblasts, it has been shown that a subset of myofibroblasts originate from circulating bone-marrow-derived cells called fibrocytes.2 Fibrocytes differentiate from a CD14+ peripheral blood monocyte precursor population and express markers of both hematopoietic cells (CD45, MHC class II, CD34) and stromal cells (collagen I and III and fibronectin).3–6 These CD34+ spindle-shaped cells have been found in fibrotic lesions that form around schistosome eggs in mice infected with Schistosoma japonicum.4 Fibrocytes are also implicated in fibrosis associated with autoimmune diseases.7–9 Circulating fibrocytes contribute to tissue repair in airway remodeling,10 bleomycin-induced pulmonary fibrosis,11,12 ischemic cardiomyopathy,13 and dermal wound healing. 2,3,8,14–16 Furthermore, mature fibrocytes exposed to TGF-β in vitro are able to further develop into myofibroblasts, which play a fundamental role in wound contraction and healing as well as in systemic fibrotic conditions.5,17,18 Using a chimeric mouse model, Fathke et al. demonstrated that 15–20% of spindle-shaped dermal fibroblasts in healing wounds were bone-marrow-derived, and that these cells were able to contract a collagen matrix and express both collagen types I and III, whereas local dermal fibroblasts expressed only collagen type I.16 Mori et al. confirmed that a subset of the myofibroblast population in wounded skin of mice had in fact differentiated from bone-marrow-derived fibrocytes that were recruited to the injury site from the circulation.2
We previously found that the plasma protein that prevents the rapid appearance of fibrocytes from peripheral blood monocytes in culture is serum amyloid P (SAP).6 SAP is a member of the pentraxin family of proteins that include C-reactive protein (CRP). It is secreted by the liver and circulates as stable pentamers.19–21 While SAP was able to inhibit fibrocyte differentiation, the highly related CRP did not.6 Furthermore, SAP-depleted serum had poor ability to inhibit fibrocyte differentiation as compared with normal serum.6 These observations suggested that SAP inhibits fibrocyte, and ultimately myofibroblast, differentiation. While the role of fibrocytes in wound healing has been established, whether exogenous SAP can modulate wound healing has not been studied. As fibrocytes contribute significantly to the myofibroblast population in healing wounds, we proposed that inhibiting fibrocyte differentiation by adding SAP to wounds would ultimately decrease the myofibroblast population and hinder the normal wound healing process.
MATERIALS AND METHODS
Production of murine SAP
Murine SAP was prepared from murine serum (Gemini BioProducts, Woodland, CA) using calcium-dependent binding to phosphoethanolamine-conjugated agarose, as described previously.6,12,13,22,23 The purified mouse SAP was diluted with 20mM sodium phosphate buffer, pH 7.4, and concentrated with a 15mL centrifugal filter device (UFV2BGC40, Millipore, Bedford, MA). This procedure was repeated to obtain 500 μg/mL SAP in 20mM sodium phosphate buffer, pH 7.4. Endotoxin levels were tested using the Limulus amebocyte lysate assay (E-Toxate, Sigma-Aldrich, St. Louis, MO) and were also tested using HEK293 cells transfected with the lipopolysaccharide (LPS) receptors CD14 and TLR4 (HEK-Blue LPS detection kit, InvivoGen, San Diego, CA). Endotoxin levels were always below detectable levels. Analysis of the SAP by Coomassieand silver-stained polyacrylamide gels (BioRad, Hercules, CA) revealed only one band at 27kDa under reducing conditions, and a single 135 kDa band under nonreducing conditions (data not shown). The identity of the mouse SAP was confirmed by Western blotting using sheep anti-mouse SAP polyclonal antibodies (R&D Systems, Minneapolis, MN), as described previously.6,13
Animals
Following approval by the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine, 8–10-weeks old C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were housed in a temperature-controlled animal facility with a 12-hour light/dark cycle. The mice were allowed food and water ad libitum and were allowed to acclimate to their environment for at least 2 weeks before wounding.
Wounding
Mice were anesthetized using inhaled isoflurane (3%, 1L/minute flow rate, Butler Animal Health Supply, Dublin, OH) delivered via a nose cone. The hair on the dorsal surface was shaved with an electric clipper which was then cleansed with an alcohol swab and sterilized with betadine. The mice were administered a subcutaneous injection of buprenorphine (0.1 mg/kg) and an intramuscular injection of penicillin (106 U/kg). Using a sterile, 8-mm punch biopsy tool, two circular, full-thickness punch wounds were created on the dorsal surface of each mouse. The wounds were photographed using a digital camera (6.0 Megapixels, Sony USA Corp., New York, NY).
Intradermal group
Five mice were used per group. Following wound creation, the wounds of mice in the control group were administered intradermal injections of 250 μL of 20mM sodium phosphate per wound using a 1-mL syringe with a 25-G needle. The fluid was injected into the skin bordering the wounds and into the wound beds. The wounds of mice in the treatment group were administered intradermal injections of 10 μg murine SAP in 250 μL of 20mM sodium phosphate. The wounds of mice in both the control and treatment groups were injected daily until 24 hours before harvest. Mice were harvested at postwounding day 7 (n=5 per group).
Intraperitoneal group
Ten mice were randomized to receive daily intraperitoneal injections of 50 μg of murine SAP in 1mL of 20mM sodium phosphate or vehicle (1mL of 20mM sodium phosphate). After creation of the dorsal back punch wounds, the mice were rotated into a supine position. The hair on the abdomen was shaved using an electric clipper, and the area was cleansed with an alcohol swab and sterilized with betadine. The abdominal wall skin was then elevated using sterile forceps and an intraperitoneal injection of SAP (50 ug in 1mL of 20mM sodium phosphate) or vehicle (1mL 20mM sodium phosphate) was administered. This amount of SAP will approximately double the serum SAP level in C57BL/6 mice.24,25 Daily intraperitoneal injections were administered for 6 days postwounding, and the wounds were harvested on postwounding day 7.
Dressings
Following wounding and injection (intradermal or intraperitoneal), an occlusive (Tegaderm) dressing (3M, St. Paul, MN) was used to stabilize the wounds and keep them as sterile as possible. We have previously noted that, due to the laxity of murine dorsal skin, the wound edges were often inadverdently reapproximated by the natural movements of the mice, causing some wounds to contract and heal quicker than others. Because the rate of wound contraction was an important part of our study, the dressings were a means to maintain consistency of the healing process. A rectangular occlusive dressing with tabs was created by trimming the ends of a 4×4.75 in. Tegaderm dressing (Figure 1). The mouse was then placed in the center of the dressing (dorsal surface contacting the dressing), and the tabs were pulled together over the ventral surface and adhered together. Care was taken to keep the dorsal skin (and wounds) flat so that skin folds did not decrease the wound area in a vertical fashion. The dressing was then secured using the trimmed piece of the Tegaderm dressing by applying this (with the nonstick part) onto the waist of the ventral surface of the animal (above the hips) and adhering the ends together on the dorsal surface (Figure 1). This prevents the dressing from slipping below the waist and onto the animal’s hind legs (which can inhibit mobility or allow the animal to kick off the dressing). The dressings were changed daily in this fashion following reinjection and wound photographs.
Figure 1.
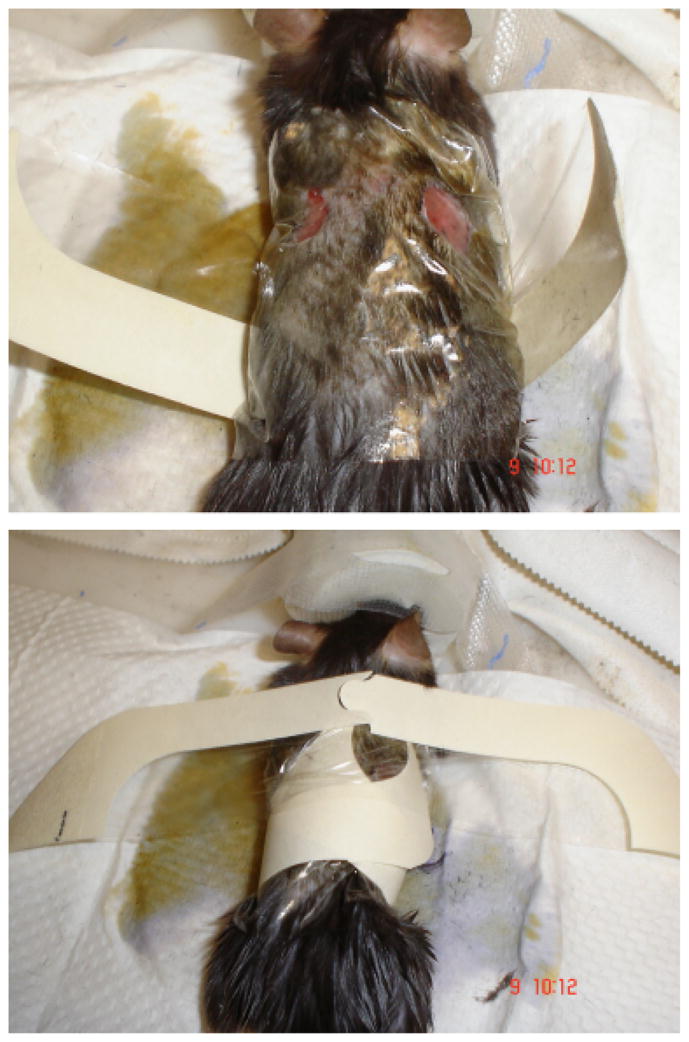
Method of applying simple adherent dressing to keep open wounds splinted and clean while still allowing mice to retain their mobility.
Harvesting
The mice were euthanized by cervical dislocation while under deep isoflurane anesthesia. Following euthanasia, the right-sided wound of every animal was excised with a 2–3mm circumferential margin of normal skin. Dissection was continued down to the muscle layer and excised with a small piece of muscle, so that the wound depth was adequately maintained. The excised tissue was placed into a cassette, fixed in 1% formaldehyde for 24 hours, serially dehydrated in alcohol, and embedded in paraffin blocks. The left-sided wound of every animal was excised with a minimal margin of normal skin, and snap frozen in liquid nitrogen.
Immunohistochemistry
Serial 4-μm thick paraffin sections were cut and placed onto Superfrost Plus slides (Fischer Scientific, Pittsburgh, PA). The slides were dried overnight at room temperature, then placed onto a slide warmer for 30 minutes before staining. After the slides had cooled to room temperature, the tissue sections were deparaffinized in xylene then rehydrated through a graded series of alcohols and distilled water. After eliminating nonspecific staining by sequential incubation of sections in BEAT Blocking Solutions (Histomouse-Plus kit, Zymed Laboratories Inc., San Francisco, CA), sections were covered with 50 μL of an anti α-smooth muscle actin (SMA) antibody (Polyclonal, NeoMarkers, Lab Vision Corporation, Fremont, CA) diluted in phosphate-buffered saline solution (PBS) containing 4% bovine serum albumin (BSA) and incubated overnight at 4 °C in a moist chamber. Following washes in PBS, sections were processed further using a biotinylated, affinity-purified secondary antibody and the enzyme conjugate, both included in the Histomouse-Plus kit (Zymed Laboratories) according to the manufacturer’s instructions. Control sections were stained with a nonspecific rabbit IgG antibody (BD Biosciences, Franklin Lakes, NJ) diluted in PBS. For each tissue section, stained α-SMA + cells were counted in three fields per section. Positive cells surrounding blood vessels were not included in the quantification. For immunohistochemistry with anti- SAP antibody, the sections were dewaxed with xylene, rehydrated through 100%, 95%, and 70% ethanol and then incubated for 10 minutes each in water and then PBS. Nonspecific binding was blocked by incubation in 4% BSA (Fraction V, globulin-free, Sigma-Aldrich) in PBS for 60 minutes at room temperature. Slides were then incubated with 5 μg/mL primary polyclonal sheep anti-murine SAP antibodies (R&D Systems) in PBS containing 4% BSA for 60 minutes at room temperature. Controls included anti-SAP polyclonal antibodies pre-incubated with 100 μg/mL murine SAP for 30 minutes at 37 °C. Slides were then washed in six changes of PBS over 30 minutes and incubated for 30 minutes with 2.5 μg/mL biotinylated donkey F(ab′)2 anti-sheep IgG (Jackson ImmunoResearch, West Grove, PA) in PBS containing 4% BSA. After washing with six changes of PBS over 30 minutes, the biotinylated antibodies were detected with a 1/200 dilution of ExtrAvidin alkaline phosphatase (Sigma) in PBS containing 4% BSA. Staining was developed with Vector Red Alkaline Phosphatase Kit (Vector Laboratories, Burlingame, CA) for 10 minutes. Sections were then counterstained for 20 seconds with 0.02% methylene blue (Fisher Scientific, Kalamazoo, MI) diluted with water, and were then rinsed in water. Slides were dehydrated through 70%, 95%, and 100% ethanol, cleared with xylene, and mounted with Permount (VWR).
Wound analysis
Wound area decrease
The right-side wound of each animal was photographed daily using a digital camera. Each photograph included a ruler placed below the wound. Using Optimas 6.0 software (Media Cybernetics Inc., Silver Spring, MD), wound area was calculated in mm2. Wound area (as a percentage of the original wound area) was calculated for each animal, then the values of the five mice in each group (treatment or control) were averaged. T-tests were then used to compare differences in wound area decrease at each postwounding day between the treatment and control groups.
Histophotometric analysis
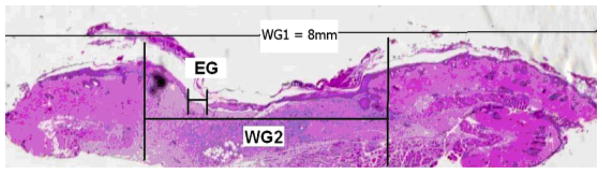
Wound sections underwent routine histological processing and staining with hematoxylin and eosin. Under light microscopy, the sections were photographed using a slide image- scanning software (Super CoolScan 5000 ED, Nikon Inc., Melville, NY). Each image was obtained at a standard magnification. The images were then analyzed with Optimas 6.0 image analysis software, and epithelial gap (EG), defined as the distance between the advancing edges of keratinocyte migration, and wound gap 2 (WG2), defined as the distance between the edge of unwounded skin where hair follicles are present, were calculated. (WG1 = 8 mm, the diameter of the original wound created from an 8mm punch biopsy tool [Figure 2]).
Figure 2.
This wound section demonstrates how histophotometric analysis was performed. EG, epithelial gap; WG2, wound gap 2 (distance between unwounded dermis containing hair follicles). WG1=8mm, the original wound diameter.
The percentage of contraction and percentage of reepithelialization were calculated as follows:
The % contraction and % reepithelialization of the wounds by day 7 were compared among the treatment and control groups using the Student’s t test.
Quantification of SAP staining
Wound sections that had undergone immunohistochemistry with anti-SAP antibody were photographed and analyzed with Image J software (Rasband, W.S., Image J, US National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997–2007) using standard algorithms to define area densitometry.
RESULTS
Both grossly and histologically, the SAP-treated wounds had a decreased rate of wound closure. This was apparent in histological photographs (Figure 3) and wound area calculations from daily wound photographs. In the intradermal group, the percent of the original wound area of the SAP-treated wounds was significantly higher than the control (vehicle only) wounds at 6 days postwounding, (Figure 4A), and a similar difference was noted in the intraperitoneal group at 7 days postwounding (Figure 4B). It is important to note that the intradermally and intraperitoneally treated wounds healed differently; the wounds of the former group tended to close faster, possibly due to the local effect of the injections causing an enhanced inflammatory response that promoted wound closure. Comparisons were only made among the control and SAP-treated wounds that were treated in exactly the same manner, and not between the different treatment groups.
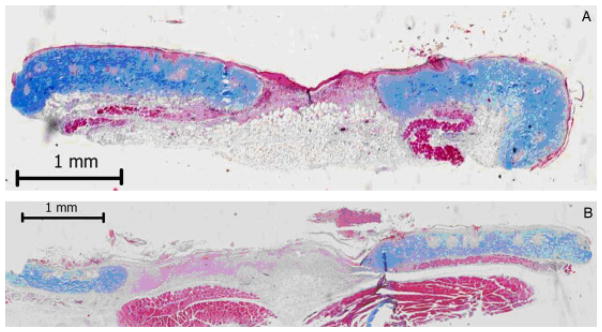
Figure 3.
Representative wound sections (7 days post-wounding) following Masson’s trichrome staining of a control wound (A) and an intraperitoneal serum amyloid P (SAP)-treated wound (B). Note the wider wound gap and the incomplete reepithelialization of the SAP-treated wound.
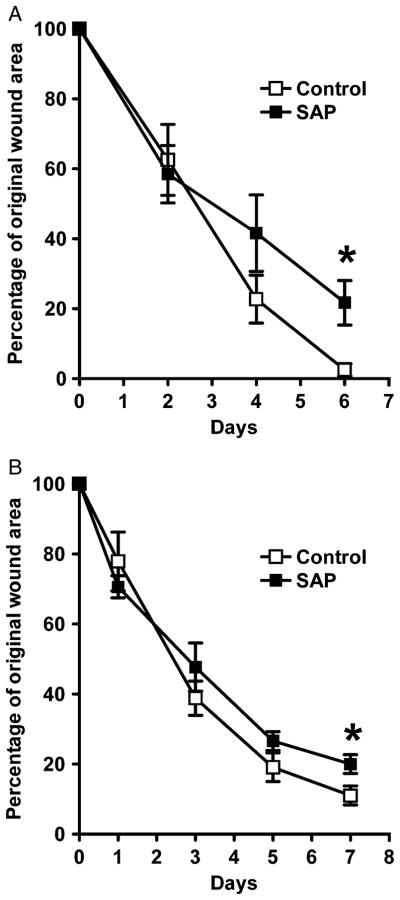
Figure 4.
Serum amyloid P (SAP) treatment delays wound area closure. In both the intradermal group (A) and in the intraperitoneal group (B) at 7 days post-wounding, the percentage of the original wound area was significantly less in the control wounds than in the SAP-treated wounds (*p < 0.05, n=5 animals per group, bars represent standard error of the mean). Group A: SAP=21.7 ± 14.2% vs. Control = 2.5 ± 4.0% (p = 0.02), and group B: SAP = 20.0 ± 5.9% vs. Control = 11.0 ± 6.0% (p = 0.04).
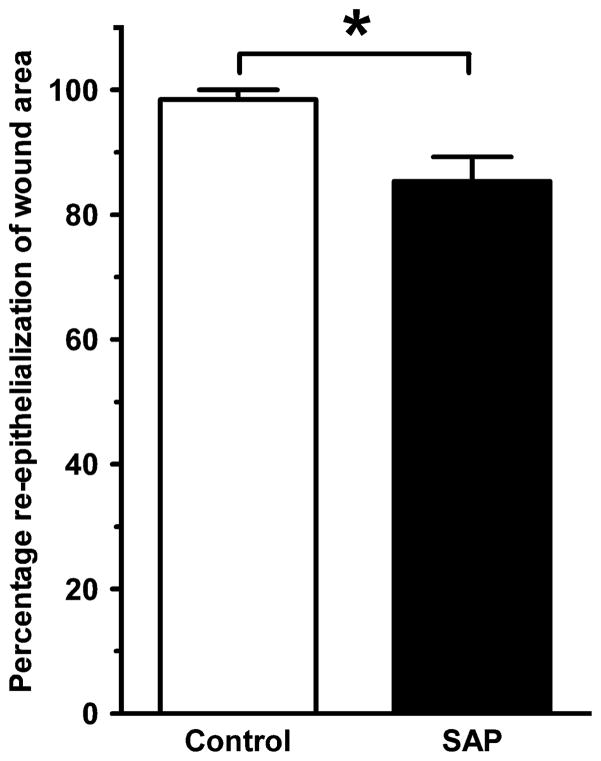
Although the process of excision and fixation appeared to cause wound edges to pull apart somewhat, the extent of contraction was less for the SAP-treated wounds in both the intradermal group and the intraperitoneal group compared with corresponding controls at 7 days postwounding (Figure 5). In the intraperitoneal group, the extent of reepithelialization was also less in the SAP-treated mice than the control mice (Figure 6).
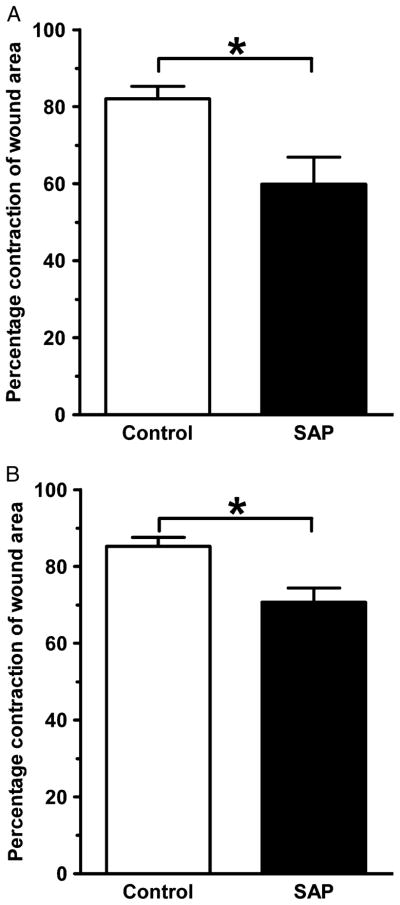
Figure 5.
Wound contracture is delayed by serum amyloid P (SAP) treatment. In both the intradermal group (A) and in the intraperitoneal group (B) at 7 days post-wounding, the control wounds exhibited significantly more contraction than the SAPtreated wounds (*p < 0.05, n = 5 animals per group, bars represent standard error of the mean). Group A: SAP = 60 ± 16% vs. Control = 82 ± 7% (p = 0.02), and group B: SAP = 71 ± 8% vs. Control = 85 ± 5% (p = 0.02).
Figure 6.
Reepithelialization is delayed by serum amyloid P (SAP) treatment in the intraperitoneal group. By 7 days postwounding, control wounds were almost completely reepithelialized, whereas the SAP-treated wounds had significantly less reepithelialization (SAP: 85.3 ± 8.9% vs. Control: 98.5 ± 3.9%, *p < 0.05, n = 5 animals per group, bars represent standard error of the mean).
There was no significant difference between the rate of reepithelialization of the SAP-treated and control wounds in the intradermal group, which may have been a consequence of repeated intradermal injections deterring reepithelialization even in the control wounds (data not shown).
Wound sections were stained with α-SMA in order to quantify the number of myofibroblasts in the healing wounds. There were no significant differences among the SAP-treated and control wounds in the intradermal group at 7 days postwounding; however, the control wounds in the intraperitoneal group did contain significantly more α-SMA-expressing cells than the SAP-treated wounds at 7 days postwounding (Figure 7).
Figure 7.
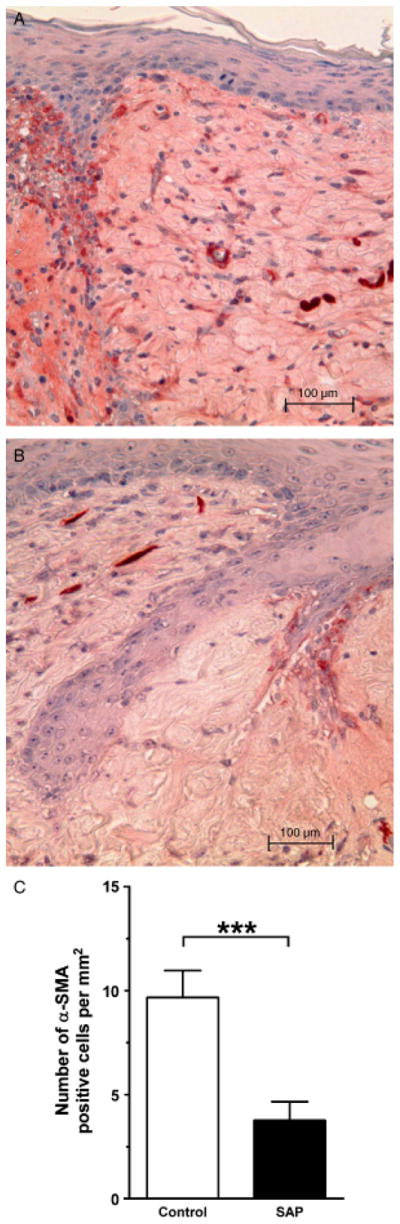
Serum amyloid P (SAP) decreases the number of α-smooth muscle actin (SMA)-expressing cells. (A) α-SMA+cells (stained in red) at the wound margin in a representative control wound, (B) and an intraperitoneal SAP-treated wound at 7 days post-wounding and (C) number of α-SMA positive cells per mm2 in wounds in the intraperitoneal group at 7 days postwounding. The SAP-treated wounds had significantly fewer myofibroblasts than the control wounds (Control: 16.1 ± 8.3 vs. SAP: 6.3 ± 5.8, p < 0.001, *p < 0.001, n = 5 animals per group bars represent standard error of the mean).
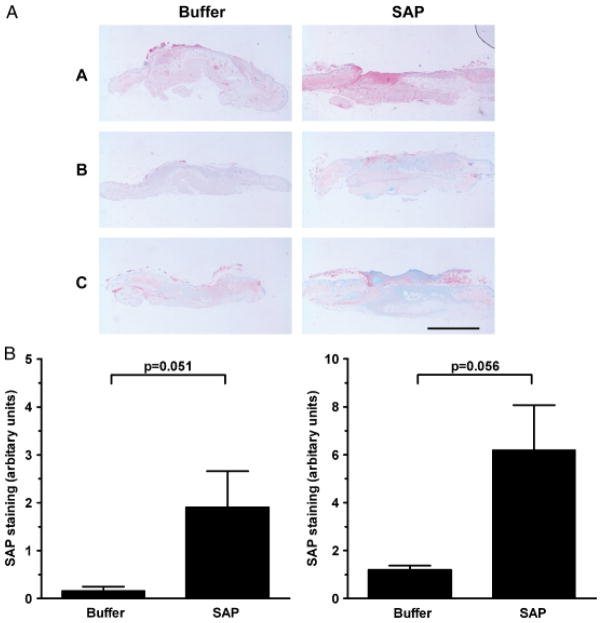
Finally, to examine the distribution of SAP within the wounds, immunohistochemistry with anti-SAP antibody was performed. We observed increased staining of SAP throughout the SAP-treated wounds compared with controls (Figure 8).
Figure 8.
(A) Detection of serum amyloid P (SAP) in SAP-treated wounds 1–3. Photographs of wound sections stained with anti-SAP antibody. Note increased SAP expression (pink stain) in SAP-treated wounds compared with corresponding control wounds. 1: Intradermally treated wounds harvested at post-wounding day 4, 2: Intradermally treated wounds harvested at post-wounding day 7 and 3: Intraperitoneally treated wounds harvested at post-wounding day 7. Bar = 2mm. (B) Significantly increased % SAP staining area was noted in both the intradermally treated wounds (left) and intraperitoneally treated wounds (right) compared with corresponding control wounds.
DISCUSSION
We have demonstrated that addition of exogenous SAP, both locally and systemically, impairs dermal wound healing. Following both local and systemic injections of SAP, murine wounds closed slower after the 6th postwounding day when compared with control wounds. Mori et al.2 showed the presence of fibrocytes in healing wounds at 4 and 7 days postwounding, and demonstrated that a substantially higher portion of fibrocytes had begun to differentiate into α-SMA-expressing myofibroblasts at 7 days postwounding. In our study, the addition of exogenous SAP may have inhibited fibrocyte differentiation, thus decreasing the total myofibroblast population in healing wounds and slowing down the process of wound healing (especially wound contraction). Additionally, we have shown that SAP-treated wounds have fewer myofibroblasts (expressing α-SMA) at 7 days postwounding.
In summary, SAP is a serum protein that inhibits fibrocyte differentiation and subsequently, dermal wound healing. In wounds that heal in a normal fashion, additional SAP appears to impair the rate of healing. In wounds that heal with excessive fibrosis, our preliminary findings suggest that additional SAP may limit the process of abnormal scarring. SAP may provide a potential therapeutic target for wound healing in two ways: depleting SAP from healing wounds to accelerate the normal dermal healing process, and adding it to prevent or limit excessive scarring in wounds that are prone to heal in such a fashion.
Acknowledgments
This work was supported with NIH grants R-01-HL083029, K-08 GM069912, and USDA 6250-51000-046-01.
References
- 1.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 2.Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–25. [PubMed] [Google Scholar]
- 5.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 6.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;17:5537–46. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. PNAS. 1997;94:6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesney J, Bucala R. Peripheral blood fibrocytes: novel fibroblast-like cells that present antigen and mediate tissue repair. Biochem Soc Trans. 1997;25:520–4. doi: 10.1042/bst0250520. [DOI] [PubMed] [Google Scholar]
- 9.Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep. 2000;2:501–5. doi: 10.1007/s11926-000-0027-5. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 11.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilling D, Roife D, Wang M, Ronkainen SD, Crawford JR, Travis EL, Gomer RH. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid p. J Immunol. 2007;179:4035–44. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone Marrow-derived fibroblast precursors mediate Ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–92. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 16.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–22. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol—Cell Physiol. 1999;277:C1–19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 18.Kissin E, Korn JH. Apoptosis and myofibroblasts in the pathogenesis of systemic sclerosis. Curr Rheumatol Rep. 2002;4:129–35. doi: 10.1007/s11926-002-0008-y. [DOI] [PubMed] [Google Scholar]
- 19.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–8. doi: 10.1016/0167-5699(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 20.Gewurz H, Zhang XH, Lint TF. Structure and function of the pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson WL, Hohenester E, Pepys MB. Human serum amyloid P component is a single uncomplexed pentamer in whole serum. Mol Med. 2000;6:482–93. [PMC free article] [PubMed] [Google Scholar]
- 22.Pepys MB. Isolation of serum amyloid P-component (protein SAP) in the mouse. Immunology. 1979;37:637–41. [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson SR, Tennent GA, Sethi D, Gower PE, Ballardie FW, Amatayakul-Chantler S, Pepys MB. Serum amyloid P component in chronic renal failure and dialysis. Clin Chim Acta. 1991;200:191–9. doi: 10.1016/0009-8981(91)90090-y. [DOI] [PubMed] [Google Scholar]
- 24.Pepys MB, Baltz M, Gomer K, Davies AJ, Doenhoff M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature. 1979;278:259–61. doi: 10.1038/278259a0. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen RF, Beisel K, Zeleznik NJ, Le PT. Acute-phase reactants of mice. II. Strain dependence of serum amyloid Pcomponent (SAP) levels and response to inflammation. J Immunol. 1983;130:885–9. [PubMed] [Google Scholar]