Abstract
Injury to muscle tissue plays a central role in various cardiovascular pathologies. Overexpression of the small heat shock protein Hsp27 protects muscle cells against thermal, oxidative and ischemic stress. However, underlying mechanisms of this protection have not been resolved. A distinctive feature of muscle cells is the stress-induced association of Hsp27 with the sarcomere. The association of Hsp27 with the cytoskeleton, in both muscle and non-muscle cells, is thought to represent interaction with Z-line components or filamentous actin. Here, we examined the association of Hsp27 with myofibrils in adult zebrafish myocardium subjected to hyperthermia and mechanical stretching. Consistent with previously published results, Hsp27 in resting length myofibrils localized to narrowly defined regions, or bands, which colocalized with Z-line markers. However, analysis of stretched myofibrils revealed that the association of Hsp27 with myofibrils was independent of desmin, alpha-actinin, myosin, and filamentous actin. Instead, Hsp27 maintained a consistent relationship with a marker for the titin A/I border over various sarcomeric lengths. Finally, extraction of actin filaments revealed that Hsp27 binds to a component of the remaining sarcomere. Together, these novel data support a mechanism of Hsp27 function where interactions with the titin filament system protect myofibrils from stress-induced degradation.
Keywords: Hsp27, Titin, Myocardium
Introduction
Heat shock proteins (Hsps) are a large family of proteins, many of which are highly conserved, stress-inducible and confer resistance to various forms of cellular stress and injury. Most Hsps can act as chaperones, assisting the folding of nascent proteins, or preventing irreversible aggregation of proteins following loss of structural integrity. Small heat shock proteins (sHsps) are a distinct subclass of Hsps ranging from 12 to 43 kD in mass [1]. All sHsps are characterized by a C-terminal crystallin domain necessary for dimerization, but sHsps also show considerable diversity in sequence, expression pattern and function [1]. Eleven sHsps have been identified in humans and mice [2], while some species of plants have been shown to express up to nineteen small heat shock protein family members [3].
Hsp27, also known as HspB1, is a widely distributed member of the sHsp family. Hsp27 has been shown to play roles in the preservation of cytoskeletal architecture [4], resistance to oxidative [5,6], thermal and ischemic stress [7,8], protection against heavy metal toxicity [9,10], regulation of cellular redox state [11], and inhibition of cellular apoptotic pathways [12,13]. In addition to stress-inducible expression, Hsp27 is constitutively expressed in many cell types [14–16]. High constitutive expression of Hsp27 is detected in muscle tissues, and thus, its function in striated muscle tissue has received considerable attention. Altered Hsp27 expression has been reported in patients diagnosed with primary desminopathies [17] and following prolonged eccentric exercise in skeletal muscles [18,19]. In cardiomyocytes, Hsp27 overexpression has been correlated with resistance to anthracycline toxicity [20], hypoxia [21], and ischemia/reperfusion injury [7,8,22].
Available data support several mechanisms for Hsp27 cytoprotection of muscle cells. Hsp27 acts as a general chaperone in vitro [23], and overexpression of Hsp27 enhances refolding of fluorescent reporter proteins in the nuclear compartment of fibroblasts [24]. Hsp27 also associates with nuclear speckles in cells subjected to various mechanisms of injury [25–27] and enhances the recovery of RNA splicing activity after experimental heat shock [28]. In addition, Hsp27 has been reported to alter the activity of critical elements of the apoptotic signaling pathways including caspases [29,30], cytochrome c [29,31,32] and Akt [33]. However, Hsp27 does not appear to enter the nucleus of differentiated striated muscle cells, and although the expression of Hsp27 inhibits apoptotic signaling in both non-muscle cells and undifferentiated muscle stem cells, there is little data to suggest these mechanisms are significant for the protection of mature muscle cells. Instead, numerous studies have indicated that Hsp27 interacts with and protects specific structural proteins in muscle cells subjected to a variety of injury mechanisms. For example, in differentiated striated muscle cells, Hsp27 translocates from a cytosolic localization to a detergent-insoluble fraction and to the apparent Z- and M-lines of sarcomeres in response to a variety of conditions including heat shock [34,35], dilated cardiomyopathy [36] prolonged eccentric exercise [37,38] and ischemia/reperfusion injury [39,40]. Hsp27 has also been shown to protect desmin and troponins I and T from proteolytic degradation in tissues subjected to ischemia/reperfusion injury [22,41]. Finally, Hsp27 also co-localizes with actin filament arrays in injured non-muscle [42,43] and muscle cells [25,44], and overexpression of Hsp27 enhances the resistance of actin filament arrays in these and other cell types to stress-induced disassembly [5,45–47].
The available data regarding distribution patterns of Hsp27 has led to wide acceptance of the view that Hsp27 interacts directly with filamentous actin or components of the actinomyosin contractile system [4,6,48–50]. However, several recent studies have examined the behavior of the Hsp27 homologue, alpha B-crystallin, within mammalian muscle cells [51,52]. In these studies, apparent Z-line localization patterns seen in the resting length myofibrils were shown to be Z-line independent. Instead, alpha B-crystallin was shown to bind to the N2B domain of the giant myofibrillar protein, titin, in vitro and was localized to the putative N2B region in myofibrils subjected to ischemia and varying degrees of mechanical stretch. This interaction alters the mechanical characteristics of titin [53] and may be important for maintenance of titin and sarcomere stability following stress.
Analysis of Hsp27 localization patterns in stretched myocytes has not been previously conducted. In previous studies, we have demonstrated that heat shock induces recruitment of Hsp27 to myofibrils in embryonic zebrafish in a manner that closely mimics the distribution patterns seen in ischemia/reperfusion injury of mammalian muscle tissues [54]. In the present study, we have used this model system to examine the distribution of Hsp27 in resting length and stretched cardiac myocytes of the adult zebrafish under control conditions and after heat shock. Our results confirm that Hsp27 is recruited to zebrafish cardiac myofibrils after heat shock, as it is in stressed mammalian [55] and zebrafish [54] skeletal muscle cells. We also found that stretching alone was insufficient to recruit Hsp27 to myofibrils, and that heat shock-induced recruitment of Hsp27 to the myofibrils is independent of actin filaments. Additionally, Hsp27 did not colocalize with other major sarcomeric components alpha-actinin, desmin or myosin in this model system. Instead, qualitative and quantitative comparison of Hsp27 localization patterns and a marker for titin filaments indicate that Hsp27 is linked to the titin filament system in cardiomyocytes after heat shock. We propose that the interaction of Hsp27 with titin or a titin-associated protein plays a central role in its protection of muscle tissues.
Materials and methods
Zebrafish husbandry and stress treatment
Zebrafish (Danio rerio) were reared and maintained as previously described [56]. Adult zebrafish were heat-shocked in a beaker with 500 mL of fish system water that was gradually raised from 28 °C to 39 °C in a water bath (Isotemp 202, Fisher Scientific, Pittsburgh, PA). Air was continuously bubbled into the water during the treatment. The temperature of system water in the inner beaker was monitored and a 30 min heat shock begun once the temperature reached 39 °C. After treatment, zebrafish were euthanized by rapid immersion in ice water, and hearts excised for further protocols. All protocols for the use of animals in this study were approved by the Washington State University Animal Care and Use Committee and adhered to National Institute of Health standards established by the Guidelines for the Care and Use of Experimental Animals.
Myofibril stretch protocols
Excised hearts were placed in relaxing solution (100 mM KCl,1 mM MgCl2, 10 mM imidazole, 2 mM EGTA, 20 mM 2,3-butanedione monoxime, 5 mM ATP, 1:500 dilution of Protease inhibitor cocktail (Sigma Aldrich P8340)) and dissected into smaller pieces two to three millimeters in diameter. Tissue fragments were incubated in relaxing solution for 5 additional minutes at room temperature. To stretch myofibrils, we adapted a squash technique previously used to examine mitotic chromosomes [57]. Briefly, relaxed tissue was crushed between poly-(tetrafluoroethylene)-coated plastic and a chrome-gelatin subbed coverslip for 30 s using a fingertip. Tissues which adhered to the coverslip were then immediately placed in 4% paraformaldehyde for 30 min at 4 °C followed by processing for immunofluorescence localization of specific proteins. Myofibrils stretched to varying degrees were found randomly distributed throughout such preparations. For the purposes of this study, sarcomeres with lengths similar to those seen in sectioned intact heart tissues shown in Fig. 1 (≤2 μm) were considered “resting length”. Where noted, tissues were placed in a lysis buffer (relaxing solution containing 0.1% Triton X-100) for 1 h at 4 °C prior to stretch. For these experiments, control tissues were placed in relaxing solution without detergent for 1 h at 4 °C. A total of 41 hearts were prepared by this stretch protocol for examination under 7 different experimental conditions.
Fig. 1.
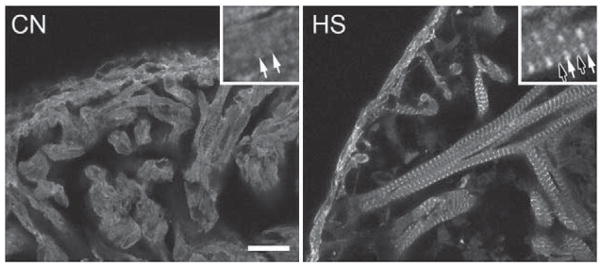
Hsp27 immunolocalization in adult zebrafish myocardium. CN shows the largely diffuse distribution of Hsp27 in the absence of heat shock. The inset shows faintly striated immunostaining of myofibrils with Hsp27 antiserum. HS shows immunolocalization of Hsp27 in myocardium following heat shock. The inset shows bright staining centered on presumptive Z-lines (solid arrows) as well as faint staining of M-bands (open arrows). Scale bar is 20 μm.
F-actin extraction
Extraction of F-actin from tissues was performed as described [52]. Briefly, pellets were incubated in 1 mL of extraction (1 M NaSCN in PBS) or control (PBS) solution and vigorously vortexed. After a 15 min incubation at room temperature, samples were centrifuged at 10,000 g for 5 min, and pellets suspended in fresh extraction solution. This procedure was repeated a total of three times for each sample preparation. Final pellets were washed in PBS and suspended in SDS-PAGE sample buffer (10% v/v glycerol, 62.5 mM Tris–HCl, pH 6.8, 2% w/v SDS, 0.01 mg/mL Bromophenol Blue) for Western blot analysis or processed for cryosectioning and immunofluorescence localization of Hsp27. In total, 14 hearts were utilized for Western blot analysis and 4 hearts were used for immunofluorescence localization.
SDS polyacrylamide gel electrophoresis and Western blotting
For collection of detergent-insoluble protein, whole hearts were homogenized in a Triton X-100 based lysis solution (20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% Triton X-100, pH 7.3) followed by incubation on ice for 20 min. Pellets were collected by centrifuging samples at 10,000 g for 8 min. Supernatants were discarded and pellets solubilized in SDS-PAGE sample buffer. Protein concentrations were measured using a Bio-Rad Dc Protein Assay Kit. 30 μg/lane of protein (approximately 0.5 hearts/sample) was separated on 4% acrylamide stacking and 12% separating gels, transferred to nitrocellulose membranes (Nitrobind, 0.22 μm, GE Water & Process Technologies) and membranes were subsequently stained with Ponceau S to confirm equal loading of lanes (data not shown). Detection of Hsp27 was performed using an anti-Hsp27 serum described previously [14], or an anti-sarcomeric actin antibody (Sigma Aldrich, clone 5C5), and horseradish peroxidase labeled secondary antibodies. Blots were developed using chemiluminescent detection reagents (Amersham Biosciences Inc.) and images obtained with an Autochemi™ System (UVP Bioimaging Systems, LLC Upland, CA). The integrated optical density (IOD) of bands was measured using NIH ImageJ software (rsbweb.nih.gov/ij/). IOD values were expressed as a normalized percent integrated optical density, where the value obtained for each band is compared to the total IOD for all bands on an individual blot and experimental values were normalized to values obtained from control samples [58]. Each experiment was performed four times.
Fluorescent immunolocalization and analysis
Tissues shown in Figs. 1 and Fig. 5B were fixed for 30 min in 4% paraformaldehyde following appropriate experimental treatment, rinsed in PBS and frozen in 100% OCT by immersion in liquid nitrogen. 15 μm thick sections were cut using a Reichert–Jung cryotome (Cryocut model 1800) and mounted on chrome-gelatin subbed coverslips. Cryosections were fixed for an additional 5 min on ice. Tissues shown in Figs. 2, 4B, and 6 were adhered to coverslips as stated in the myofibril stretch protocols section. All samples were permeabilized for 30 min in lysis buffer on ice. Samples were incubated with primary antibodies (1:300 in PBS containing 0.1% Tween 20 (PBS-T) and 10% donor calf serum (DCS)) overnight at 4 °C in a humid chamber. Primary antibodies used were polyclonal anti-Hsp27 serum, anti-desmin (Millipore, MAB3430), anti-alpha-actinin (Sigma Aldrich, clone EA-53), anti-titin, clone T11 (Sigma Aldrich), and anti-MHC F59 (Santa Cruz). The T11 antibody is a specific marker for the A/I boundary of titin filaments [59].
Fig. 5.
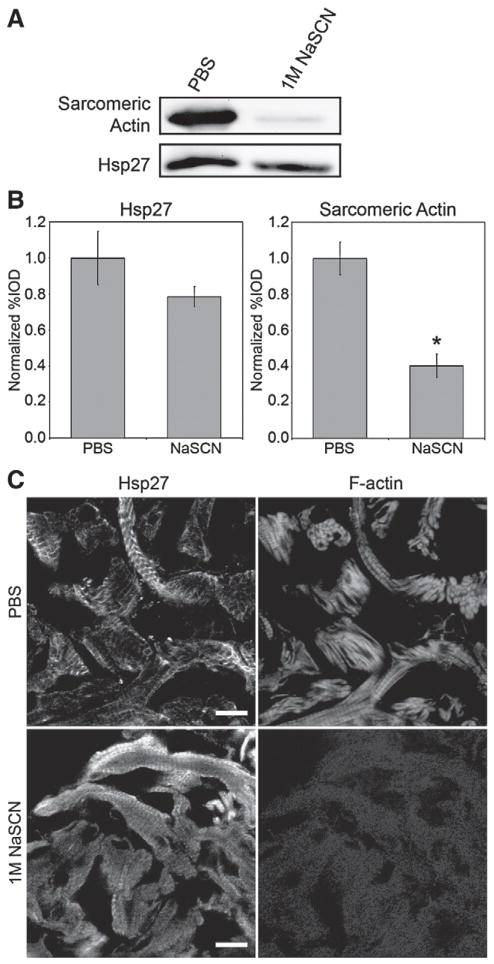
Effect of selective removal of F-actin from detergent-insoluble pellet on Hsp27. (A) Western blot of Hsp27 and α-sarcomeric actin in the detergent-insoluble fraction of heat-shocked myocardium following incubations with PBS or 1 M NaSCN. (B) Quantitative analysis of Hsp27 (left) and sarcomeric actin (right) immunoblots following incubations with PBS or 1 M NaSCN in 4 independent experiments. (*) denotes statistically significant difference when compared to PBS treated samples. Error bars are the SEM. (C) Localization of Hsp27 (left) and F-actin (right) in detergent-insoluble fractions of heat-shocked adult zebrafish myocardium following treatment with PBS or 1 M NaSCN. Hsp27 (lower left) remains in a striated pattern associated with myofibrils after extraction of F-actin. Scale bars are 10 μm.
Fig. 2.
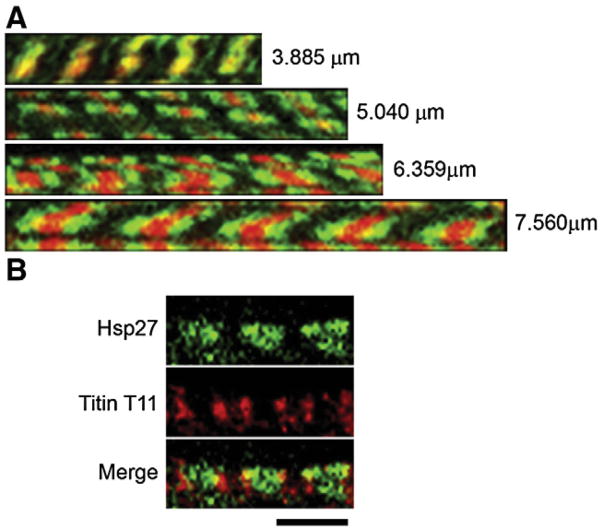
Dual localization of Hsp27 and F-actin or titin in heat-shocked cardiomyocytes. (A) Resting length (top) and stretched myofibrils labeled for Hsp27 (green) and F-actin (red). Stretched myofibrils display a doublet of Hsp27 centered on each Z-line. Values represent average sarcomere length for the corresponding myofibril. Images are representative of squash preparations from 7 hearts. (B) Titin T11 immunolocalization (red) is a doublet centered on each Z-line. Hsp27 (green) is Z-line proximal in relation to the T11 binding epitope in this stretched myofibril. The average length of sarcomeres in this preparation was 4.4 μm. The image is representative of results obtained from preparations of three hearts. Scale bars are 5 μm.
Fig. 4.
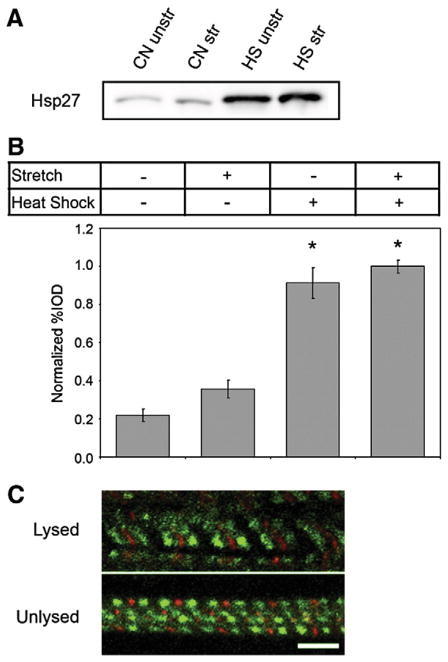
Recruitment of Hsp27 to myofibrils is dependent on heat shock but not stretch. (A) Western blot analysis of detergent-insoluble Hsp27. CN samples were obtained from animals maintained at 28 °C and HS samples were obtained from heat-shocked animals. Insoluble proteins were obtained from hearts without stretch (unstr) or after stretching (str) and probed for Hsp27. Stretching did not alter the amount of detergent-insoluble Hsp27 in these samples. (B) Analysis of Triton X-100 insoluble Hsp27 collected from hearts with or without stretch and heat shock. Data was collected from a total of four independent experiments. (*) denotes a statistically significant difference when compared to hearts after no heat shock and no stretch. Error bars are the SEM. (C) The association of Hsp27 with myofibrils occurs prior to stretch. Hsp27 (green) was localized in heat-shocked, stretched myocytes either without (top) or with (bottom) a prior lysis in detergent containing buffer to remove unbound Hsp27. Lysis of myofibrils prior to stretch and fixation did not alter Hsp27 recruitment patterns. Scale bars are 5 μm.
Fig. 6.
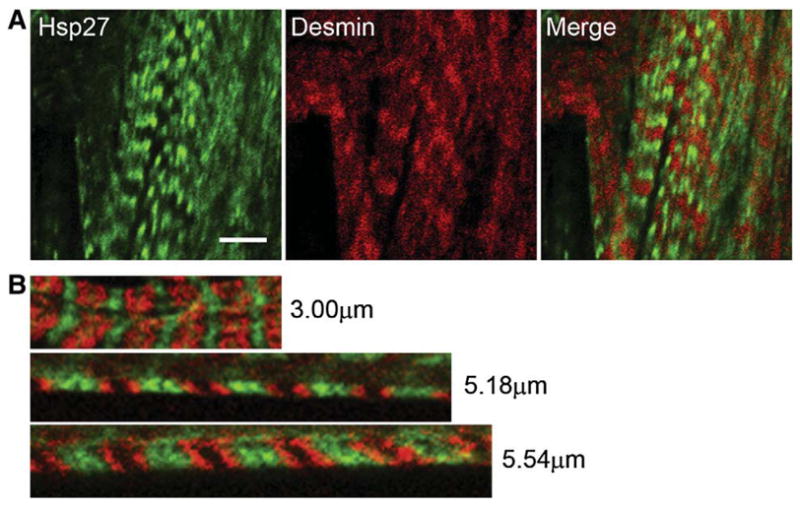
Hsp27 does not colocalize with either myosin or desmin in heat-shocked cardiac muscle. Immunolocalization of Hsp27 (green) and desmin (red, top) or myosin (red, bottom) in heat-shocked zebrafish myocardium subjected to stretch. Desmin and myosin in stretched sarcomeres are found in bands that do not colocalize with Hsp27 (green). The image is representative of results obtained from preparations of 3 hearts. Scale bar is 5 μm.
Fluorochrome labeled secondary antibodies were incubated with samples at a concentration of 1:500 in PBS-T with 10% DCS for 2 h at room temperature. F-actin was detected using rhodamine-conjugated phalloidin (Molecular Probes). Coverslips were mounted on slides using Mowiol containing an antifade agent (DABCO, Sigma Chemical Co). Images were acquired using a Zeiss LSM 510M confocal microscope (Carl Zeiss Inc., Thornwood, NY) with a 63×1.4 NA oil immersion objective or a 20×1.2 NA glycerol immersion objective. Post processing of images was conducted using Adobe Photoshop CS2 to enhance contrast. Images contained within the same figure were processed together and identically. A total of 57 hearts were used for 9 immunolocalization protocols.
Quantification and statistical analyses
Fluorescently labeled areas of myofibrils (band) widths and sarcomere lengths were measured using ImageJ version 1.37 (Wayne Rasband, National Institute of Health, Bethesda, MD). Distance measurements were exported to Microsoft Excel, where scatter plots were created for each data set. Linear regression of band width data was performed using Microsoft Excel software. All other statistical comparisons were performed using Minitab version 15. Fitting data to a general linear model was performed to determine statistical significance of linear regression slope comparisons. Analysis of Western blot densitometry was performed using a one-way ANOVA and Tukey’s W procedure for data in Fig. 4, and Student’s t-tests for data shown in Fig. 5. An alpha of 0.05 was used as a measure of statistical significance in all calculations.
Results
Zebrafish Hsp27 associates with myofibrils in cardiomyocytes after heat shock
In zebrafish heart tissues fixed under control conditions, the distribution of Hsp27 in cardiomyocytes is largely diffuse (Fig. 1, CN), although a faint striated pattern (inset, solid arrows), can be seen, suggesting that a small fraction of Hsp27 may associate with sarcomeres under control conditions. In contrast, cardiomyocytes obtained from hearts of heat-shocked animal display a dramatic and nearly complete association of Hsp27 with myofibrils (Fig. 1, HS). Regularly spaced, narrow, brightly stained regions (inset, closed arrows) alternate with much more faintly stained areas (inset, open arrows). We have demonstrated similar localization patterns for an EGFP-zfHsp27 fusion protein expressed in zebrafish embryonic skeletal muscle under control conditions and after heat shock [54]. In addition, we have recently demonstrated the specificity of the Hsp27 antiserum used here [14]. Finally, this Hsp27 immunolocalization pattern has also been demonstrated by others examining stressed mammalian cardiac and skeletal muscle cells [34,35,37,38]. Therefore, we conclude that this pattern represents specific localization of zebrafish Hsp27 protein. This pattern is consistent with strong recruitment of Hsp27 to the area of the sarcomere near the Z-line (see Fig. 4B), with some, but lesser, association with M-band structures.
Hsp27 associates with the titin filament system independently of actin filaments
To determine if Hsp27 associates with F-actin, co-localized F-actin and Hsp27 in ventricular myocytes were stretched to varying lengths. Fig. 2 provides representative images showing that Hsp27 (green) and F-actin (red) colocalize in resting length myofibrils (Fig. 2A, top). In stretched myofibrils, F-actin remains as single bands (Fig. 2A, lower panels), and co-staining of these preparations with probes for the Z-line marker alpha-actinin (not shown) revealed that each phalloidin stained band is centered on a single Z-line, as expected. However, increasing stretch resulted in the Hsp27 localization pattern showing increasingly separated doublet bands found progressively outward from the center of phalloidin stained F-actin bands. Finally, in sarcomeres stretched to the longest distances (Fig. 2A, bottom panel), the Hsp27 doublets are spatially distinct from the staining pattern for F-actin. These data are inconsistent with direct interaction between Hsp27 and F-actin.
Next, we assessed the relationship between Hsp27 and a marker for the titin A/I boundary. Fig. 2B is a representative image showing co-localization of Hsp27 and the T11 epitope marking the A/I boundary within a myofibril stretched to an average sarcomere length of 4.4 μm. As shown previously for mammalian muscle cells [60], T11 staining was characterized by doublet bands in all preparations. Myofibrils in these preparations could not be stretched as greatly as those shown in Fig. 2A without loss of defined T11 staining. However, in this and all similar preparations, T11 (red) flanked Hsp27 immunolocalization (green) bands and doublets. Therefore, these data indicate that the site of Hsp27 binding in the sarcomere lies between the Z-line and the A/I boundary recognized by the T11 antibody.
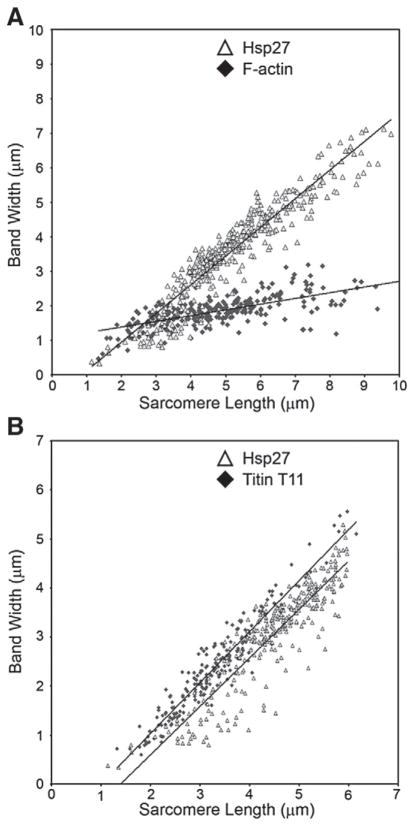
To further assess the relationship between Hsp27, F-actin and titin within cardiomyocytes, we measured doublet band separation for both Hsp27 and T11 staining as well as the width of phalloidin stained F-actin bands in the heart preparations described above. These dimensions were then plotted against the length of associated sarcomeres, and resulting scatter plots (Fig. 3A) were subjected to linear regression. Slopes of the regression lines, which indicate the increase of measured doublet separation (Hsp27) or band length (F-actin) as sarcomere length increased, were 0.8299 for Hsp27 (empty triangle) and 0.1658 for F-actin (solid diamond). Statistical analysis indicated that the slopes for F-actin and Hsp27 were significantly different (p<0.001). These results demonstrate that the measured width of phalloidin stained F-actin bands changes only slightly as sarcomeres are stretched, a finding consistent with the sliding filament model of muscle contraction. In contrast, the separation between Hsp27 doublet bands increases nearly 8 fold greater than the F-actin band lengths over the same sarcomere stretch distances.
Fig. 3.
Comparison of Hsp27 band doublet width with F-actin band length and T11 binding epitope separation in stretched myofibrils. (A) Data points are doublet band separation (Y axis) for Hsp27 (empty triangle) and F-actin band length (solid diamond) plotted against the length of associated sarcomere (X-axis). Slopes of the regression lines are significantly different (p≤0.05). (B) Separation of Hsp27 band doublets (empty triangle) and the titin T11 epitope (solid diamond) plotted against the length of associated sarcomeres. Slopes of the two regression lines are not statistically different (p>0.05). Fig. 3A shows measurements from 249 sarcomeres for Hsp27 and F-actin obtained from preparations of three hearts. Fig. 3B shows measurements from 391 sarcomeres probed for Hsp27 and 221 sarcomeres probed with T11 antibody obtained from preparations of three hearts.
We repeated this analysis for data obtained from samples probed with Hsp27 antiserum (Fig. 3B, open triangles) and the T11 antibody, which recognizes the A/I boundary region of titin (solid diamond). The resulting regression lines have slopes of 0.9879 for Hsp27 and 1.0398 for T11 and are not significantly different (p=0.11). The similarity of regression slopes calculated from these data strongly suggests a link between Hsp27 and the titin molecule within the sarcomere. It should be noted that the slope for Hsp27 doublet separation presented here is different than that calculated for Fig. 3A. This is due to our inability to detect T11 staining in myofibrils stretched greater than 6 μm. If the Hsp27 data set shown in Fig. 3A is limited to values less than 6 μm, the calculated slope is not significantly different from that shown in Fig. 3B (data not shown).
Heat shock-induced association of Hsp27 with myofibrils is not altered by stretching
It is possible that stretching can alter the association of Hsp27 with myofibrils or revealed Hsp27 binding sites not present under normal physiological conditions. To address these issues, we assessed whether the amounts of Hsp27 present in detergent-insoluble protein fractions were different in resting length and stretched preparations. Fig. 4A shows an anti-Hsp27 Western blot loaded with equal amounts of detergent-insoluble protein per lane. Consistent with results obtained in studies of mammalian muscle tissues and results shown in Fig. 1, the total amount of detergent-insoluble Hsp27 is dramatically greater in heat-shocked zebrafish heart tissue than in control preparations for both resting length (rest) and stretched (str) samples (compare samples labeled CN and HS in Fig. 4A). However, the amount of Hsp27 detected in samples prepared from resting length and stretched tissues were not different within each experimental group (compare samples labeled unstr and str in Fig. 4A). Analysis of percent IOD values for Hsp27 measured from this and 3 additional independent experiments is shown in Fig. 4B revealing a small, but not statistically significant, increase in the amount of detergent-insoluble Hsp27 following stretch with or without heat shock. In contrast, heat shock highly and significantly increased the amount of insoluble Hsp27 in both stretched and unstretched tissues. We conclude that mechanical stretch has only a minor role, if any, in causing redistribution of Hsp27 in this model.
We also considered that alternative Hsp27 binding targets might be revealed or altered by our stretching protocol. To address this issue, we lysed muscle tissues to remove unbound Hsp27 prior to stretching and compared the distribution pattern of Hsp27 and alpha-actinin in these preparations (Fig. 4C, bottom panel) with that seen in myofibrils stretched while intact (Fig. 4C, top panel). However, the localization patterns of Hsp27 (green) in relation to that of the Z-line marker alpha-actinin (red) in these two types of preparations were indistinguishable. Together, data shown in Fig. 4 indicate that the amount and location of Hsp27 recruited to myofibrils is largely independent of stretching, but is instead primarily determined by the heat shock treatment.
Extraction of F-actin does not significantly remove Hsp27 from detergent-insoluble protein fractions
To further assess the relationship between actin filaments and the Hsp27 associated with myofibrils, we extracted F-actin from detergent-insoluble pellets obtained from heat-shocked myocardial tissues by incubation with 1 M NaSCN. Fig. 5A shows duplicate Western blots of equal amounts of these samples probed with antibodies detecting Hsp27 and alpha-sarcomeric actin. Percent IOD values measured from this and three other independent experiments (Fig. 5B) show that the amount of Hsp27 in pellets (left panel) does not significantly decrease following NaSCN extraction (p=0.10). However, average percent IOD values for sarcomeric actin (right panel) decreased by nearly 60% in these preparations (p=0.02). Similarly to results published by Golenhofen et al. examining rat Hsp25 [55], our results indicate that NaSCN extracts detergent-insoluble actin from zebrafish myocardial pellets much more efficiently than Hsp27. We also examined the distribution of actin filaments and Hsp27 in detergent-insoluble pellets (Fig. 5C). Results of this analysis show that a total of 45 min of incubation in PBS alone did not noticeably alter the localization pattern of F-actin or Hsp27 associated with myofibrils (compare Fig. 5C, top left with Fig. 1B). In contrast, F-actin, as measured by the presence of rhodamine-labeled phalloidin, was largely removed from pellets by incubation with 1 M NaSCN (Fig. 5C, lower, right). However, Hsp27 remained in pellets after NaSCN extraction and maintained a striated distribution pattern indicative of association with remaining sarcomeric components (Fig. 5C, lower left). The T11 antibody is not suitable for immunoblotting, and we did not conduct Western blot analysis of titin in the present study. However, previous authors have demonstrated titin’s resistance to NaSCN extraction at the concentrations used here [52].
Hsp27 does not colocalize with myosin or desmin in heat-shocked cardiac muscle
Finally, recent studies have shown that Hsp27 can protect a variety of cytoskeletal filament proteins from degradation in stressed myocytes [22,41]. To address whether the association of Hsp27 with myofibrils in heat-shocked myocardium could be correlated with other components of myofibrils, we probed stretched myofibril preparations with Hsp27 antiserum (green, Fig. 6) and antibodies recognizing desmin (red, Fig. 6A) and myosin (red, Fig. 6B). Images shown in Fig. 6A reveal that desmin is primarily detected in stretched myofibrils in single bands and distinct from Hsp27 doublet bands. In addition, as shown in Fig. 6B, myosin localization patterns show no obvious co-localization with Hsp27 in stretched myofibril preparations.
Discussion
Hsp27 is associates with the titin filament system but not actin filaments in heat-shocked zebrafish cardiac muscle
Hsp27/Hspb1 is a small heat shock protein that participates in a variety of cytoprotective mechanisms, including reduction of reactive oxygen species levels [11], anti-apoptotic signaling [12,13,29–33] and the maintenance of cytoskeletal integrity [4]. The mechanisms underlying these functions are not fully understood, and Hsp27 does not appear to exhibit all of these functions in an individual cell type. Expression of Hsp27 has been shown to inhibit cell death and promote contractile function of mammalian cardiomyocytes after ischemia/reperfusion injury [7,8,22,61]. A common feature of the stress response in both muscle and many non-muscle cells of mammals including humans is the translocation of Hsp27 to the cytoskeleton, specifically to actin filament arrays in non-muscle cells and to the Z- and less commonly M-lines for sarcomeres in striated muscle cells. Understanding the interaction between Hsp27 and its cytoskeletal targets is essential to understanding its mechanism of cytoprotection. The present study focuses on further defining the role of Hsp27 in cardiac muscle cells and establishing the zebrafish as a model for determining mechanisms involved in Hsp27-based cardioprotection. We report that Hsp27 is diffusely distributed in the cytoplasm of adult zebrafish cardiomyocytes under control conditions and associates with a single band centered on the Z-line in resting length zebrafish myofibrils after heat shock. This translocation and distribution pattern is identical to that observed in mammalian hearts in response to ischemia/reperfusion injury [36,39,40,44]. Therefore, heat shock of the poikilotherm zebrafish appears to be an appropriate model system to analyze the interaction between Hsp27 and its cytoskeletal target in the myocardium.
Although previous studies have supported the view that Hsp27 associates with actin or actin filaments in stressed cells (see [4] for review), our results demonstrate that the association of zebrafish Hsp27 with myofibrils is independent of actin filaments. In zebrafish cardiac tissues, phalloidin stained actin filaments within myofibrils appeared as a single band centered on each sarcomere’s Z-line. Measured widths of these bands were only slightly increased in stretched myofibrils compared to resting length myofibrils, consistent with previously published data from electron micrographs and the sliding filament model of muscle contraction. In contrast, Hsp27 was localized to doublet bands in stretched myofibrils, still centered on the Z-line. The distance of separation between Hsp27 doublet bands was variable and correlated positively with the length of stretched sarcomeres, while the width of phalloidin stained F-actin regions was largely independent of sarcomere length. These data are not consistent with a model in which Hsp27 binds along the length of actin filaments. Additionally, Hsp27 bands were spatially separated from phalloidin stained regions of myofibrils in extremely stretched myofibrils. These data are also inconsistent with binding of Hsp27 to actin filament ends. In contrast, Hsp27 maintained a consistent relationship with T11 stained titin domains, and slopes of regression analysis for both markers, performed on measured Hsp27 and T11 band separation as a function of associated sarcomere length, were indistinguishable. Biochemical extraction of actin filaments from detergent-insoluble fractions of heat-shocked hearts had little effect on either the amount of Hsp27 present in these fractions or the association of Hsp27 with remaining myofibrillar structures. Finally, stretched cardiac myofibrils co-labeled with probes for myosin, alpha-actinin or desmin all failed to reveal an association with Hsp27. Together these data strongly support the hypothesis that Hsp27 does not associate with actin filaments in heat-shocked zebrafish cardiomyocytes, and instead indicate that Hsp27 associates with the titin filament system in muscle cells subjected to an experimental stress.
Does zebrafish Hsp27 model the behavior of mammalian Hsp27 orthologues?
Interaction between the related small heat shock protein alpha B-crystallin and titin filaments has been previously demonstrated [51,52]. Although several laboratories have documented the expression of a zebrafish alpha B-crystallin orthologue [62], this protein has not been detected in zebrafish cardiac or skeletal muscle. Therefore, it is possible that zebrafish Hsp27 plays the role carried out by alpha B-crystallin in mammalian systems. However, translocation of mammalian Hsp27 to myofibrils in mammalian muscle tissues, which also express endogenous alpha B-crystallin, is well documented [36,39,40,44]. The reported Hsp27 localization pattern in resting length mammalian muscles is indistinguishable from the pattern observed in our studies of resting length zebrafish myofibrils. Sequence comparison of mammalian and zebrafish Hsp27 and alpha B-crystallin proteins also show that zebrafish Hsp27 is much more closely related to mammalian orthologues than alpha B-crystallin in either system [54]. For example, three phosphorylated serines (S15, S78, and S82 in human Hsp27) and a cysteine (C137 in human Hsp27) have previously been shown to play roles in the regulation of mammalian Hsp27 function [63, 64]. All four of these amino acids are conserved in zebrafish Hsp27 but absent in alpha B-crystallin proteins from both systems. Finally, significant interactions between alpha B-crystallin and desmin in cardiomyocytes have been established [52,65]. Here, we show that Hsp27 does not colocalize with desmin after stretch, with Hsp27 being excluded from the desmin localization pattern. We conclude that the association of zebrafish Hsp27 with the titin filament system likely mimics the behavior of mammalian Hsp27 proteins.
Implications for understanding the target and function of Hsp27 in cardiomyocytes
Previously proposed mechanisms for Hsp27 cytoprotection in cardiomyocytes have largely focused on the potential of Hsp27 to act as a general protein chaperone for unfolded actin and other muscle proteins either by acting in concert with the high molecular weight heat shock protein Hsp70 (reviewed in [1]) or stabilization of actin filament arrays through lateral or filament end interactions [4,6,48–50]. In addition, Hsp27 expression has been linked to enhanced resistance of myofibrillar proteins troponin [22] and desmin [41] to degradation in injured muscle cells. However, the distribution of Hsp27 in injured muscle cells is often reported to be highly restricted to specific regions of myofibrils. The identification of the binding target for Hsp27 under these conditions may therefore provide important insight into its regulation and function. Here, we report that although the distribution of Hsp27 in resting length myofibrils of heat-shocked zebrafish cardiomyocytes is consistent with data from other systems, its localization as revealed by stretching of myofibrils, is independent of the actinomyosin cytoskeleton, as well as desmin. Instead, the distribution of Hsp27 is consistent with a protein that binds to the I-band region of the titin filament system. Within this region, Hsp27 may bind to either titin itself or a titin-associated protein. In light of these novel data, we briefly consider possible targets for Hsp27 in this model system and implications for Hsp27 function.
Titin integrity is critical to the maintenance of myofibril structure (reviewed in [66]), and a direct interaction of Hsp27 with titin could result in the stabilization of myofibrils in injured myocytes. However, our data do not strongly support this mechanism. The region of titin between the Z-line and A/I boundary that recruits Hsp27 has been well characterized. Within the I-band region of the titin molecule, there are several repeating immunoglobulin-like (Ig) domains, a PEVK domain, and the N2A and N2B alternatively spliced domains. The Ig and PEVK domains are among the first to unfold following stretch and are responsible for much of the passive tension created by the titin molecule in overstretched sarcomeres [67]. These domains could be a target for a general chaperone function of Hsp27 upon stress-induced unfolding. However, the lack of increase in detergent-insoluble Hsp27 following stretch argues against this possibility. The Hsp27 homologue, alpha B-crystallin has also been shown to bind to the N2B domain [51,55]. However, we have observed that Hsp27 binds myofibrils in zebrafish skeletal muscle [54], as well as cardiac muscle, and others have reported similar results for mammalian skeletal muscle. As zebrafish [68] and mammalian [69] skeletal muscle do not express N2B containing titin splice isoforms, the N2B domain is also an unlikely target.
Instead of binding to titin itself, Hsp27 could bind to a titin-associated protein following heat shock. The I-band region of titin is a scaffold for a number of signaling proteins, including DRAL/FHL2 [70] and cardiac ankyrin repeat protein [71]. The interaction of Hsp27 with these proteins could have profound implications for myocytes. Additionally, the N2A domain found in both skeletal and some cardiac splice isoforms of titin is a binding site for p94 [60,72], also known as calpain 3. Although there is no calpain 3 in cardiac muscle, a similar localization pattern has been reported for the ubiquitous calpain 1 [73]. Low level calpain activation is necessary for normal sarcomeric turnover [74], but myocyte injury is often correlated with a massive calcium influx leading to an overactivation of calpain activity [75]. Therefore, Hsp27 may interact with, and perhaps inactivate, calpains following stress similarly to the Hsp27-based inactivation of procaspase 3 cleavage [30]. An inhibition of calpain activity by Hsp27 could explain various data concerning Hsp27-based protection of cytoskeletal components. Also, as calpains participate in necrosis and apoptosis [76,77], an interaction with Hsp27 could explain the apparent anti-necrotic and anti-apoptotic activities of Hsp27 in various cell types. Although further study is needed to resolve these issues, based on the above discussion we feel available data most strongly suggest direct interaction of Hsp27 with regulatory proteins associated with titin filaments, rather than titin itself.
Conclusion
This study demonstrates that Hsp27 translocates to myofibrils of zebrafish cardiomyocytes following heat shock. Localization of Hsp27 in relation to other cytoskeletal proteins revealed that Hsp27 is not associated with actin, actin associated proteins, myosin or desmin. Instead, we demonstrate for the first time that Hsp27 is associated with the titin filament system in heat-shocked cardiomyocytes. Hsp27 translocation is only stimulated following thermal stress as stretch alone is insufficient to recruit Hsp27 to the titin filaments. Further characterization of this interaction may prove essential to determining the cytoprotective mechanisms of Hsp27 within the myocardium of zebrafish and mammalian systems.
Acknowledgments
We thank Ryan Middleton for expert assistance with zebrafish husbandry and Dr Henk Granzier (University of Arizona) for critical reading of a draft of this manuscript. Support of this work by the National Science Foundation (IOS- 0818993 to EAS) and the NIH Biotechnology Training Program at Washington State University T32-GM008336 (to NRT) is gratefully acknowledged.
References
- 1.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 2.Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47:7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- 3.Siddique M, Gernhard S, von Koskull-Doring P, Vierling E, Scharf KD. The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones. 2008;13:183–197. doi: 10.1007/s12192-008-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- 6.Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med. 2001;31:1624–1632. doi: 10.1016/s0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- 7.Vander Heide RS. Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2002;282:H935–H941. doi: 10.1152/ajpheart.00660.2001. [DOI] [PubMed] [Google Scholar]
- 8.Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- 9.Bonham RT, Fine MR, Pollock FM, Shelden EA. Hsp27, Hsp70, and metallothionein in MDCK and LLC-PK1 renal epithelial cells: effects of prolonged exposure to cadmium. Toxicol Appl Pharmacol. 2003;191:63–73. doi: 10.1016/s0041-008x(03)00226-6. [DOI] [PubMed] [Google Scholar]
- 10.Wu W, Welsh MJ. Expression of the 25-kDa heat-shock protein (HSP27) correlates with resistance to the toxicity of cadmium chloride, mercuric chloride, cis-platinum(II)-diammine dichloride, or sodium arsenite in mouse embryonic stem cells transfected with sense or antisense HSP27 cDNA. Toxicol Appl Pharmacol. 1996;141:330–339. doi: 10.1006/taap.1996.0290. [DOI] [PubMed] [Google Scholar]
- 11.Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- 12.Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 14.Tucker NR, Ustyugov A, Bryantsev AL, Konkel ME, Shelden EA. Hsp27 is persistently expressed in zebrafish skeletal and cardiac muscle tissues but dispensable for their morphogenesis. Cell Stress Chaperones. 2009;14:521–533. doi: 10.1007/s12192-009-0105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gernold M, Knauf U, Gaestel M, Stahl J, Kloetzel PM. Development and tissue-specific distribution of mouse small heat shock protein hsp25. Dev Genet. 1993;14:103–111. doi: 10.1002/dvg.1020140204. [DOI] [PubMed] [Google Scholar]
- 16.Pauli D, Tonka CH, Tissieres A, Arrigo AP. Tissue-specific expression of the heat shock protein HSP27 during Drosophila melanogaster development. J Cell Biol. 1990;111:817–828. doi: 10.1083/jcb.111.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clemen CS, Fischer D, Roth U, Simon S, Vicart P, Kato K, Kaminska AM, Vorgerd M, Goldfarb LG, Eymard B, Romero NB, Goudeau B, Eggermann T, Zerres K, Noegel AA, Schroder R. Hsp27-2D-gel electrophoresis is a diagnostic tool to differentiate primary desminopathies from myofibrillar myopathies. FEBS Lett. 2005;579:3777–3782. doi: 10.1016/j.febslet.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 18.Thompson HS, Scordilis SP, Clarkson PM, Lohrer WA. A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiol Scand. 2001;171:187–193. doi: 10.1046/j.1365-201x.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- 19.Feasson L, Stockholm D, Freyssenet D, Richard I, Duguez S, Beckmann JS, Denis C. Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J Physiol. 2002;543:297–306. doi: 10.1113/jphysiol.2002.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Zhang X, Qian B, Min X, Gao X, Li C, Cheng Y, Huang J. Over-expression of heat shock protein 27 attenuates doxorubicin-induced cardiac dysfunction in mice. Eur J Heart Fail. 2007;9:762–769. doi: 10.1016/j.ejheart.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation. 1997;96:4343–4348. doi: 10.1161/01.cir.96.12.4343. [DOI] [PubMed] [Google Scholar]
- 22.Lu XY, Chen L, Cai XL, Yang HT. Overexpression of heat shock protein 27 protects against ischaemia/reperfusion-induced cardiac dysfunction via stabilization of troponin I and T. Cardiovasc Res. 2008;79:500–508. doi: 10.1093/cvr/cvn091. [DOI] [PubMed] [Google Scholar]
- 23.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 24.Bryantsev AL, Kurchashova SY, Golyshev SA, Polyakov VY, Wunderink HF, Kanon B, Budagova KR, Kabakov AE, Kampinga HH. Regulation of stress-induced intracellular sorting and chaperone function of Hsp27 (HspB1) in mammalian cells. Biochem J. 2007;407:407–417. doi: 10.1042/BJ20070195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryantsev AL, Loktionova SA, Ilyinskaya OP, Tararak EM, Kampinga HH, Kabakov AE. Distribution, phosphorylation, and activities of Hsp25 in heat-stressed H9c2 myoblasts: a functional link to cytoprotection. Cell Stress Chaperones. 2002;7:146–155. doi: 10.1379/1466-1268(2002)007<0146:dpaaoh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikari AS, Sridhar Rao K, Rangaraj N, Parnaik VK, Mohan Rao C. Heat stress-induced localization of small heat shock proteins in mouse myoblasts: intranuclear lamin A/C speckles as target for alphaB-crystallin and Hsp25. Exp Cell Res. 2004;299:393–403. doi: 10.1016/j.yexcr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Bryantsev AL, Chechenova MB, Shelden EA. Recruitment of phosphorylated small heat shock protein Hsp27 to nuclear speckles without stress. Exp Cell Res. 2007;313:195–209. doi: 10.1016/j.yexcr.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marin-Vinader L, Shin C, Onnekink C, Manley JL, Lubsen NH. Hsp27 enhances recovery of splicing as well as rephosphorylation of SRp38 after heat shock. Mol Biol Cell. 2006;17:886–894. doi: 10.1091/mbc.E05-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cyto-chrome c-mediated caspase activation by sequestering both procaspase-3 and cytochrome c. Gene Expr. 2001;9:195–201. doi: 10.3727/000000001783992605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandey P, Farber R, Nakazawa A, Kumar S, Bharti A, Nalin C, Weichselbaum R, Kufe D, Kharbanda S. Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene. 2000;19:1975–1981. doi: 10.1038/sj.onc.1203531. [DOI] [PubMed] [Google Scholar]
- 31.Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo AP, Orrenius S. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6:49–58. doi: 10.1379/1466-1268(2001)006<0049:hpmotc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- 33.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- 34.Hoch B, Lutsch G, Schlegel WP, Stahl J, Wallukat G, Bartel S, Krause EG, Benndorf R, Karczewski P. HSP25 in isolated perfused rat hearts: localization and response to hyperthermia. Mol Cell Biochem. 1996;160–161:231–239. doi: 10.1007/BF00240054. [DOI] [PubMed] [Google Scholar]
- 35.Leger JP, Smith FM, Currie RW. Confocal microscopic localization of constitutive and heat shock-induced proteins HSP70 and HSP27 in the rat heart. Circulation. 2000;102:1703–1709. doi: 10.1161/01.cir.102.14.1703. [DOI] [PubMed] [Google Scholar]
- 36.Lutsch G, Vetter R, Offhauss U, Wieske M, Grone HJ, Klemenz R, Schimke I, Stahl J, Benndorf R. Abundance and location of the small heat shock proteins HSP25 and alphaB-crystallin in rat and human heart. Circulation. 1997;96:3466–3476. doi: 10.1161/01.cir.96.10.3466. [DOI] [PubMed] [Google Scholar]
- 37.Koh TJ, Escobedo J. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am J Physiol Cell Physiol. 2004;286:C713–C722. doi: 10.1152/ajpcell.00341.2003. [DOI] [PubMed] [Google Scholar]
- 38.Paulsen G, Vissing K, Kalhovde JM, Ugelstad I, Bayer ML, Kadi F, Schjerling P, Hallen J, Raastad T. Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R844–R853. doi: 10.1152/ajpregu.00677.2006. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto K, Urushidani T, Nagao T. Translocation of HSP27 to sarcomere induced by ischemic preconditioning in isolated rat hearts. Biochem Biophys Res Commun. 2000;269:137–142. doi: 10.1006/bbrc.2000.2233. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida K, Aki T, Harada K, Shama KM, Kamoda Y, Suzuki A, Ohno S. Translocation of HSP27 and MKBP in ischemic heart. Cell Struct Funct. 1999;24:181–185. doi: 10.1247/csf.24.181. [DOI] [PubMed] [Google Scholar]
- 41.Blunt BC, Creek AT, Henderson DC, Hofmann PA. H2O2 activation of HSP25/27 protects desmin from calpain proteolysis in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1518–1525. doi: 10.1152/ajpheart.00269.2006. [DOI] [PubMed] [Google Scholar]
- 42.Neuhofer W, Muller E, Burger-Kentischer A, Beck FX. Hypertonicity affects heat shock protein 27 and F-actin localization in Madin–Darby canine kidney cells. Kidney Int Suppl. 1998;67:S165–S167. doi: 10.1046/j.1523-1755.1998.06735.x. [DOI] [PubMed] [Google Scholar]
- 43.Loktionova SA, Ilyinskaya OP, Gabai VL, Kabakov AE. Distinct effects of heat shock and ATP depletion on distribution and isoform patterns of human Hsp27 in endothelial cells. FEBS Lett. 1996;392:100–104. doi: 10.1016/0014-5793(96)00792-2. [DOI] [PubMed] [Google Scholar]
- 44.Sakamoto K, Urushidani T, Nagao T. Translocation of HSP27 to cytoskeleton by repetitive hypoxia-reoxygenation in the rat myoblast cell line, H9c2. Biochem Biophys Res Commun. 1998;251:576–579. doi: 10.1006/bbrc.1998.9518. [DOI] [PubMed] [Google Scholar]
- 45.Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- 46.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mairesse N, Horman S, Mosselmans R, Galand P. Antisense inhibition of the 27 kDa heat shock protein production affects growth rate and cytoskeletal organization in MCF-7 cells. Cell Biol Int. 1996;20:205–212. doi: 10.1006/cbir.1996.0025. [DOI] [PubMed] [Google Scholar]
- 48.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 49.Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem Cell Biol. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]
- 50.Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bullard B, Ferguson C, Minajeva A, Leake MC, Gautel M, Labeit D, Ding L, Labeit S, Horwitz J, Leonard KR, Linke WA. Association of the chaperone alphaB-crystallin with titin in heart muscle. J Biol Chem. 2004;279:7917–7924. doi: 10.1074/jbc.M307473200. [DOI] [PubMed] [Google Scholar]
- 52.Golenhofen N, Arbeiter A, Koob R, Drenckhahn D. Ischemia-induced association of the stress protein alpha B-crystallin with I-band portion of cardiac titin. J Mol Cell Cardiol. 2002;34:309–319. doi: 10.1006/jmcc.2001.1513. [DOI] [PubMed] [Google Scholar]
- 53.Zhu Y, Boglomovas J, Labeit S, Granzier H. Single molecule force spectroscopy of cardiac titin’s N2B element-effects of the molecular chaperone alpha B-crystallin with disease causing mutations. J Biol Chem. 2009;284:13914–13923. doi: 10.1074/jbc.M809743200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao L, Bryantsev AL, Chechenova MB, Shelden EA. Cloning, characterization, and heat stress-induced redistribution of a protein homologous to human hsp27 in the zebrafish Danio rerio. Exp Cell Res. 2005;306:230–241. doi: 10.1016/j.yexcr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Golenhofen N, Perng MD, Quinlan RA, Drenckhahn D. Comparison of the small heat shock proteins alphaB-crystallin, MKBP, HSP25, HSP20, and cvHSP in heart and skeletal muscle. Histochem Cell Biol. 2004;122:415–425. doi: 10.1007/s00418-004-0711-z. [DOI] [PubMed] [Google Scholar]
- 56.Westerfield M. The Zebrafish Book: A Guide for Laboratory use of Zebrafish (Danio rerio) University of Oregon Press; Eugene: 2007. [Google Scholar]
- 57.Stack SM, Brown DB, Dewey WC. Visualization of interphase chromosomes. J Cell Sci. 1977;26:281–299. doi: 10.1242/jcs.26.1.281. [DOI] [PubMed] [Google Scholar]
- 58.Hojlund K, Wrzesinski K, Larsen PM, Fey SJ, Roepstorff P, Handberg A, Dela F, Vinten J, McCormack JG, Reynet C, Beck-Nielsen H. Proteome analysis reveals phosphorylation of ATP synthase beta-subunit in human skeletal muscle and proteins with potential roles in type 2 diabetes. J Biol Chem. 2003;278:10436–10442. doi: 10.1074/jbc.M212881200. [DOI] [PubMed] [Google Scholar]
- 59.Furst DO, Osborn M, Nave R, Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J Cell Biol. 1988;106:1563–1572. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keira Y, Noguchi S, Minami N, Hayashi YK, Nishino I. Localization of calpain 3 in human skeletal muscle and its alteration in limb-girdle muscular dystrophy 2A muscle. J Biochem. 2003;133:659–664. doi: 10.1093/jb/mvg084. [DOI] [PubMed] [Google Scholar]
- 61.Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM. Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol. 2004;99:392–394. doi: 10.1007/s00395-004-0483-6. [DOI] [PubMed] [Google Scholar]
- 62.Posner M, Kantorow M, Horwitz J. Cloning, sequencing and differential expression of alphaB-crystallin in the zebrafish, Danio rerio. Biochim Biophys Acta. 1999;1447:271–277. doi: 10.1016/s0167-4781(99)00155-4. [DOI] [PubMed] [Google Scholar]
- 63.Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999;274:18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- 64.Diaz-Latoud C, Buache E, Javouhey E, Arrigo AP. Substitution of the unique cysteine residue of murine Hsp25 interferes with the protective activity of this stress protein through inhibition of dimer formation. Antioxid Redox Signal. 2005;7:436–445. doi: 10.1089/ars.2005.7.436. [DOI] [PubMed] [Google Scholar]
- 65.Bennardini F, Wrzosek A, Chiesi M. Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res. 1992;71:288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- 66.Hein S, Schaper J. Weakness of a giant: mutations of the sarcomeric protein titin. Trends Mol Med. 2002;8:311–313. doi: 10.1016/s1471-4914(02)02372-9. [DOI] [PubMed] [Google Scholar]
- 67.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94:284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 68.Seeley M, Huang W, Chen Z, Wolff WO, Lin X, Xu X. Depletion of zebrafish titin reduces cardiac contractility by disrupting the assembly of Z-discs and A-bands. Circ Res. 2007;100:238–245. doi: 10.1161/01.RES.0000255758.69821.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Granzier H, Labeit S. Structure-function relations of the giant elastic protein titin in striated and smooth muscle cells. Muscle Nerve. 2007;36:740–755. doi: 10.1002/mus.20886. [DOI] [PubMed] [Google Scholar]
- 70.Lange S, Auerbach D, McLoughlin P, Perriard E, Schafer BW, Perriard JC, Ehler E. Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci. 2002;115:4925–4936. doi: 10.1242/jcs.00181. [DOI] [PubMed] [Google Scholar]
- 71.Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333:951–964. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, Sorimachi N, Shimada H, Tagawa K, Maruyama K, et al. Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J Biol Chem. 1995;270:31158–31162. doi: 10.1074/jbc.270.52.31158. [DOI] [PubMed] [Google Scholar]
- 73.Raynaud F, Fernandez E, Coulis G, Aubry L, Vignon X, Bleimling N, Gautel M, Benyamin Y, Ouali A. Calpain 1-titin interactions concentrate calpain 1 in the Z-band edges and in the N2-line region within the skeletal myofibril. FEBS J. 2005;272:2578–2590. doi: 10.1111/j.1742-4658.2005.04683.x. [DOI] [PubMed] [Google Scholar]
- 74.Kramerova I, Kudryashova E, Venkatraman G, Spencer MJ. Calpain 3 participates in sarcomere remodeling by acting upstream of the ubiquitin–proteasome pathway. Hum Mol Genet. 2005;14:2125–2134. doi: 10.1093/hmg/ddi217. [DOI] [PubMed] [Google Scholar]
- 75.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 76.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- 77.Lu T, Xu Y, Mericle MT, Mellgren RL. Participation of the conventional calpains in apoptosis. Biochim Biophys Acta. 2002;1590:16–26. doi: 10.1016/s0167-4889(02)00193-3. [DOI] [PubMed] [Google Scholar]