Abstract
Objective
To test the hypothesis that lower birth order amplifies the positive association between socioeconomic status and central adiposity in young adult males from a lower-income, developing country context.
Design
The Cebu Longitudinal Health and Nutrition Survey is an ongoing community-based, observational study of a one year birth cohort (1983).
Subjects
970 young adult males, mean age 21.5 y (2005).
Measurements
Central adiposity measured by waist circumference; birth order; perinatal maternal characteristics including height, arm fat area, age, and smoking behavior; socioeconomic status at birth and in young adulthood.
Results
Lower birth order was associated with higher waist circumference and increased odds of high waist circumference, even after adjustment for socioeconomic status in young adulthood, and maternal characteristics that could impact later offspring adiposity. Furthermore, the positive association between socioeconomic status and central adiposity was amplified in individuals characterized by lower birth order.
Conclusions
This research has failed to reject the mismatch hypothesis, which posits that maternal constraint of fetal growth acts to program developing physiology in a manner that increases susceptibility to the obesogenic effects of modern environments.
Keywords: Mismatch, Developmental Origins of Health and Disease, Fetal origins, Maternal constraint, Birth order, Central obesity
Introduction
Obesity is a serious challenge to global public health (1). Public health interventions have traditionally targeted individual-level behaviors affecting dietary intake and physical activity, but these efforts have had no success at reducing obesity prevalences. Consequently, there is growing interest in how social and physical environments influence obesity risk, and efforts to identify mutable, environmental causes of obesity are underway (2–10). However, it is equally important to explain heterogeneity in adiposity among individuals that share an environment. Some people are clearly more susceptible to obesogenic environments than others, but why? One explanation is genetic variation; that some people posses thrifty genes that increase their susceptibility to modern, obesogenic environments (11). However, the importance of thrifty genes and the degree to which they could explain the modern obesity pandemic is still debated (e.g. 12, 13).
Another explanation is Gluckman and Hanson’s mismatch hypothesis (14–22), which falls under the broader Developmental Origins of Health and Disease (DOHaD) paradigm (23). Briefly, it posits that maternal constraint of fetal growth can signal the developing fetus to prepare for a poor nutritional environment. Highlighted causes of maternal constraint include maternal body size, age, diet, and birth order (14, 24). The hypothesized fetal response to these signals is an integrated set of adjustments in the way energy is handled in the body (19). These adjustments are thought to enhance fitness via improved survival or fecundity during lean times, but could lead to obesity and related metabolic disorders in a nutritionally abundant environment. Ultimately, the mismatch hypothesis posits that prenatal influences can modify how we experience our postnatal environment, and could at least partly explain why some people are more susceptible to obesogenic environments than others.
There is substantial evidence that constraints on fetal growth are associated with offspring obesity later in life. For example, maternal exposure to famine during gestation is associated with increased risk of obesity in the adult offspring (25, 26). Other studies have found an inverse association between birth weight and central adiposity once body mass index (BMI) is accounted for, though these associations tend to be mild, and are not consistently detected (27). Maternal smoking has also been associated with both lower birth size and subsequent obesity later in life (28, 29). However, most previous studies have not explicitly tested the hypothesis that obesity results from an interaction between the constraint of fetal growth and later environment. Instead, most studies have estimated direct associations between fetal development and later obesity, irrespective of the postnatal environment. Given the mismatch hypothesis, failure to account for this interaction could lead to underestimation of the relationship between fetal growth and obesity.
Using data from a birth cohort of young adult Filipino males, we tested the hypothesis that lower birth order modified the association between central obesity and socio-economic status (SES), a useful proxy for the obesogenic environment in this context (30, 31). We also examined the influence of birth order on birth size, and explored BMI growth curves (from birth to young adulthood) in groups defined by firstborn status and SES. Although birth order is a hypothesized prenatal influence on later disease (24), and has been associated with reduced birth size (24, 29, 32–34) and increased risk of central adiposity (e.g. 35, 36, 37) and diabetes (38–40), it has not been adequately investigated in epidemiological studies.
Methods
Study design and sample
Data are from the Cebu Longitudinal Health and Nutrition Survey (CLHNS), a community-based, one-year birth cohort study in Metropolitan Cebu (pop 1.9 million), Philippines. The region includes 270 administratively defined communities called barangays (average area 2.65 km2) comprising a 720 km2 contiguous area. A single stage cluster sampling procedure was used to randomly select 33 barangays, and pregnant women residing in these barangays were recruited for the study in 1982 and 1983. Those who gave birth between May 1, 1983, and April 30, 1984, were included in the sample. More than 95% of identified women agreed to participate. A baseline interview was conducted among 3,327 women during their 6th or 7th month of pregnancy. Another survey took place immediately after birth; there were 3,080 non-twin live births which make up the CLHNS birth cohort. Subsequent surveys were conducted bimonthly to age 2, then in 1991, 1994, 1998, 2002, and 2005 (n=1885, 61% retention). For this analysis we used a sample of young adult males still enrolled in the CLHNS in 2005 (mean age 21.5 y) with complete case data (n=970; 98% of the 2005 sample, 59% of the original sample at birth). Males included in this analysis sample did not differ at from the remainder of the cohort at baseline (by t-test; p≤0.05) in mean 1983 household assets, birth length, ponderal index, maternal height, maternal AFA, maternal age, or birth order. However, the analysis sample did have slightly higher birth weights (difference 0.05 kg; 95% CI 0.01 to 0.09). Because the socio-environmental determinants of obesity are more complex and poorly understood in females (30, 31), we have excluded them from this analysis.
Measures
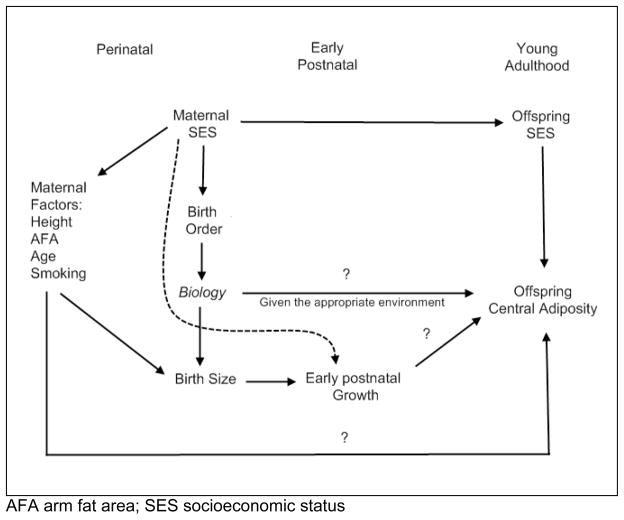
The theoretical model that describes the hypothesized relationships among variables included in this analysis is given in figure 1. The primary exposure, birth order, was assessed during the baseline interview (1983). Birth order is represented continuously or as firstborn status (versus all others) in our analyses.
Figure 1.
Theoretical model
First we estimated the impact of birth order on birth size for gestational age. Birth weights (kg) for infants born at home (62%) were measured by trained birth attendants with Salter hanging scales. The remainder, born at hospitals or clinics, were weighed on clinical scales. Lengths (cm) were measured within 6 days of birth using custom made length boards. Ponderal index is a measure of body mass independent of length (though unfortunately it can not distinguish lean mass from fat mass) and was calculated as weight kg/length m3. Gestational age was estimated from the mother’s self-reported date of her last menstrual period. For cases where this date was unknown, when pregnancy complications occurred, or when the infant was born weighing less than 2.5 kg, gestational age was clinically assessed using the Ballard method (41).
To isolate the impact of birth order on birth size, we controlled for several possible confounders. Birth order is inversely associated with maternal SES at birth due to reduced fertility in high SES mothers. SES, in turn, is positively associated with maternal age, height, and arm fat area, and inversely associated with smoking, each of which are determinants of birth size. Failure to control for these factors could obscure any relationship between birth order and birth size. We measured maternal SES at baseline using an assets-based index that reflects longer-term wealth and living standards. This SES index was calculated using a principal components analysis of data on ownership of a variety of household assets at baseline (e.g. television, land, etc) (42, 43). Maternal height was measured with a folding stadiometer. AFA was calculated from mid-upper arm circumference and triceps skinfold thickness (44) during the second or third trimester of pregnancy. Maternal smoking was represented dichotomously (yes/no), irrespective of the number of cigarettes smoked. Indicator variables were used to represent younger (<20 years) and older (>35 years) maternal ages, versus a reference age (20–35 years).
Then we estimated the impact of birth order on central adiposity in young adulthood. The primary outcome, waist circumference (WC), was measured by trained interviewers in 2005 at the midpoint between the bottom of the ribs and the top of the iliac crest. High WC was defined using a fairly low cut point of WC>85 cm that may be more appropriate in Asian populations (45). We again controlled for maternal age, height, and AFA, and smoking because each of these variables could have a developmental effect on later central adiposity. We also controlled for offspring SES in 2005, again using the same continuous index derived from a principal components analysis of 2005 household assets ownership (42, 43).
Analytical methods
First we used multivariable linear models to estimate the effect of birth order and firstborn status on birth weight, length, or ponderal index. Nonlinear effects of birth order were tested using quadratic and cubic terms. We then used multivariable linear models to estimate impact of birth order or firstborn status on young adult WC, or log odds of high WC. Again, nonlinear effects of birth order were tested using quadratic terms. We report both the crude estimates and estimates adjusted for potential confounders.
We then added an interaction between birth order (or firstborn status) and 2005 SES (our proxy variable for the post-natal environment) using the appropriate product term. Under the mismatch hypothesis, our expectation was that the positive effects of SES on central adiposity would be amplified in individuals with lower birth orders. Additional interactions were tested between SES and the other prenatal variables in the model that could also moderate the effects of SES under the mismatch hypothesis (maternal age, height, AFA, and smoking). Interactions were considered significant and reported when the corresponding p < 0.10.
All linear models included random intercepts to account for potential dependence among observations caused by the cluster-randomized design of the CLHNS that could lead to biased standard errors for estimated regression coefficients. Because we are not otherwise interested in interpreting estimated random effects for this analysis, we do not report them. All reported p-values are two-sided. All models were run using Stata, version 10.0 (Stata corp., College Station, Texas).
In an exploratory analysis, we evaluated the mean BMI growth curves from birth to young adulthood of four groups defined by firstborn status (versus not) and high versus low maternal SES measured at birth (defined by the median). This was to help evaluate whether firstborn status coupled with high SES was associated with a postnatal growth trajectory characterized by early catch up growth. Heights and weights were recorded bimonthly from birth to age two, and at ages 8.5y, 11.5y, 16y, 19y, and 21.5y. BMI was calculated as weight kg/height m2.
Results
Sample characteristics are reported in table 1. The sample in 2005 had a mean age of 21.5y. They were characterized by low mean WC (72.2 cm; sd 7.6) and body mass index (21.0; sd 3.1). Only 6% were classified as having high WC. The median birth order was 3, and ranged from 1–15. 22% were classified as firstborn. A higher proportion of firstborns compared to higher order births were preterm (21.6% versus 15.2%, chi2 p=0.027) or small for gestational age (36.6% versus 23.5%, chi2 p<0.000).
Table 1.
Sample characteristics for 970 young adult Filipino males enrolled in the Cebu Longitudinal Health and Nutrition Survey
| Variables | Values | ||
|---|---|---|---|
| Birth order | Median (Range) | 3 | (1 to 15) |
| Firstborn | % | 22.2 | |
| Waist circumference (cm) | Mean(SD) (Range) | 72.2(7.6) | (56.5 to 112) |
| High waist circumference (>85cm) | % | 6.1 | |
| Birth weight (kg) | Mean(SD) (Range) | 3.0(0.43) | (0.9 to 4.2) |
| Birth length (cm) | Mean(SD) (Range) | 49.3(2.0) | (39.74 to 55.5) |
| Ponderal index (kg/m3) | Mean(SD) (Range) | 25.2(3.0) | (13.6 to 40.6) |
| Gestational age (wks) | Mean(SD) (Range) | 38.7(2.1) | (30 to 44) |
| Maternal age at birth (yrs) | Mean(SD) (Range) | 26.7(6.0) | (14.9 to 45.6) |
| Maternal height (cm) | Mean(SD) (Range) | 150.7(5.0) | (136.1 to 166.1) |
| Maternal AFA (cm2) | Mean(SD) (Range) | 14.8(5.5) | (3.8 to 50.6) |
| Smokes | % | 12.4 | |
| SES (1983) | Mean(SD) (Range) | 0(2.1) | (−2.0 to 8.0) |
| SES (2005) | Mean(SD) (Range) | 0(2.9) | (−3.4 to 16.0) |
AFA arm fat area; SES socioeconomic status
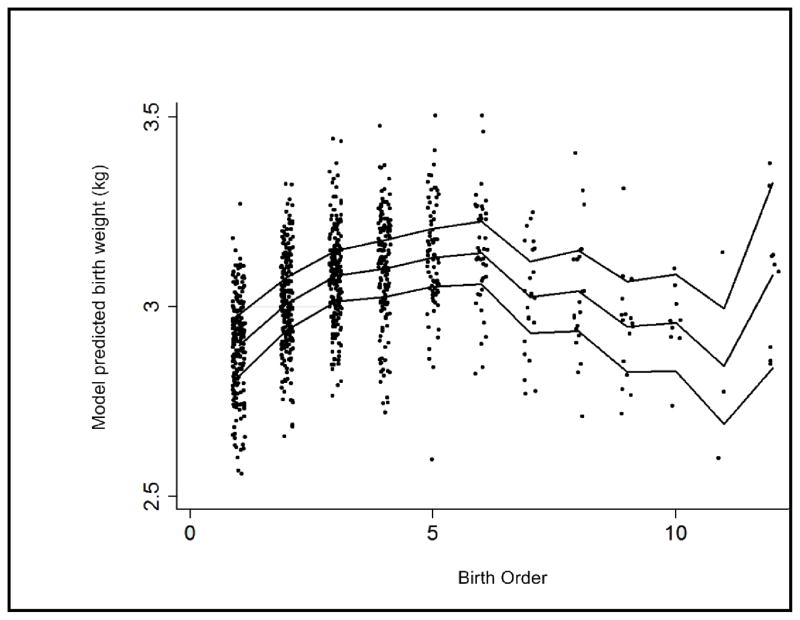
Firstborn status was associated with reduced birth weight, length, and ponderal index, adjusted for gestational age (table 2). The relationship between continuously measured birth order and birth weight, length, or ponderal index, was best described by a third order polynomial model (table 2, figure 2), though the impact of birth order on length after adjustment was not significant at p<0.05. Higher order births were increasingly larger up to the sixth born. Birth weight then decreased as birth order increased. The final upward trend was likely spurious, due to the small sample sizes at that end of the birth order distribution. The model was not altered by the exclusion of two outliers with recorded birth weights under 1 kg. Young maternal age, height and AFA were important determinants of birth length but not ponderal index; and maternal smoking, like birth order, was an important determinant of ponderal index but not length.
Table 2.
Mutilivariable linear models estimating the impact of birth order or firstborn status on birth weight, length, and ponderal index.
| Dependent Variable | ||||||
|---|---|---|---|---|---|---|
| Birth weight (g) | Birth length (cm) | Ponderal index* (kg/m3) | ||||
| Birth order models | ||||||
| Birth order | 157.27 | (76.82 to 237.68) | 0.14 | (−0.25 to 0.52) | 11.31 | (5.49 to 17.10) |
| Birth ordersquared | −23.12 | (−38.95 to −7.66) | −0.02 | (−0.09 to −0.06) | −1.72 | (−2.85 to −0.61) |
| Birth order cubed | 1.02 | (0.20 to 1.84) | 0.0003 | (−0.004 to 0.004) | 0.08 | (0.02 to 0.14) |
| Gestational age (wks) | 44.67 | (32.50 to 56.82) | 0.22 | (0.16 to 0.28) | 0.77 | (−0.11 to 1.65) |
| Maternal age <20 yrs | −47.15 | (−131.47 to 37.17) | −0.51 | (−0.91 to −0.11) | 3.48 | (−2.64 to 9.60) |
| Maternal age 20–35 yrs | REF | REF | REF | |||
| Maternal age >35 yrs | −55.41 | (−157.47 to 46.65) | −0.28 | (−0.77 to 0.20) | −1.60 | (−9.00 to 5.78) |
| Maternal height (cm) | 11.70 | (6.51 to 16.89) | 0.07 | (0.04 to 0.09) | −0.02 | (−0.39 to 0.36) |
| Maternal AFA (cm2) | 7.12 | (2.28 to 11.96) | 0.04 | (0.02 to 0.06) | −0.10 | (−0.45 to 0.26) |
| Smokes | 78.78 | (−158.61 to 1.05) | 0.13 | (−0.25 to 0.51) | −8.81 | (−14.59 to −3.02) |
| SES (1983) | 6.60 | (−6.29 to 19.50) | 0.05 | (−0.01 to 0.11) | −0.36 | (−1.31 to 0.58) |
| Firstborn models | ||||||
| Firstborn | −183.98 | (−252.92 to −115.04) | −0.25 | (−0.58 to −0.75) | −11.71 | (−16.71 to −6.71) |
| Gestational age (wks) | 44.74 | (32.65 to 56.82) | 0.22 | (0.16 to 0.28) | 0.80 | (−0.09 to 1.68) |
| Maternal age <20 yrs | −45.61 | (−128.69 to 37.46) | −0.46 | (−0.86 to −0.07) | 0.29 | (−3.13 to 8.96) |
| Maternal age 20–35 yrs | REF | - | REF | REF | ||
| Maternal age >35 yrs | −38.66 | (−124.05 to 46.74) | −0.39 | (−0.80 to 0.01) | 0.19 | (−4.26 to 8.14) |
| Maternal height (cm) | 11.69 | (6.52 to 16.86) | 0.07 | (0.04 to 0.09) | −0.03 | (−0.41 to 0.34) |
| Maternal AFA (cm2) | 7.63 | (2.81 to 12.44) | 0.04 | (0.02 to 0.06) | −0.05 | (−0.41 to 0.30) |
| Smokes | −70.77 | (−149.28 to 7.75) | 0.10 | (−0.27 to 0.48) | −7.54 | (−13.26 to −1.82) |
| SES (1983) | 7.16 | (−5.58 to 19.90) | 0.05 | (−0.01 to 0.11) | −0.36 | (−1.30 to 0.58) |
Coefficients reported as a change in independent variable associated with a tenth of a point change in ponderal index.
AFA arm fat area; REF reference category; SES socioeconomic status
Point estimates and 95% confidence intervals reported as Estimate (Lower Limit to Upper Limit)
Figure 2.
Cubic relationship between birth order and model predicted birth weight (adjusted for maternal age, height, arm fat area, smoking and socioeconomic status).
Firstborn status and lower birth order were associated with WC and log odds of high WC (table 3). The association between birth order and WC (or log odds of high WC) was linear (versus the nonlinear association we observed between birth order and birth size). After adjustment for potential confounders, this relationship was attenuated. Maternal height and AFA were consistent, positive predictors of central obesity. We failed to detect non-linearities in these variables using quadratic terms that tested the hypothesis that there was risk at both ends of their respective distributions. Maternal smoking and age were not meaningful predictors of WC. Model results were not appreciably altered when the natural log of WC was used, nor when preterm birth (yes/no), small for gestational age (yes/no), or birth size variables were included as covariates (models not shown).
Table 3.
Unadjusted and Mutilivariable linear models estimating the impact of birth order or firstborn status on waist circumference or log odds of high waist circumference.
| Dependent Variable | ||||
|---|---|---|---|---|
| WC (cm) | OR High WC (>85cm) | |||
| Unadjusted models | ||||
| Birth order | −0.36 | (−0.57 to −0.15) | 0.76 | (0.64 to 0.91) |
| Firstborn | 1.21 | (0.08 to 2.34) | 1.88 | (1.06 to 3.34) |
| Adjusted birth order models | ||||
| Birth order | −0.51 | (−0.76 to −0.27) | 0.73 | (0.59 to 0.90) |
| SES (2005) | 0.66 | (0.50 to 0.82) | 1.22 | (1.13 to 1.32) |
| Maternal age <20 yrs | −1.21 | (−2.58 to 0.16) | 1.19 | (0.52 to 2.72) |
| Maternal age 20–35 yrs | REF | - | REF | - |
| Maternal age >35 yrs | 1.52 | (0.18 to 3.21) | 1.68 | (0.55 to 5.14) |
| Maternal height (cm) | 0.23 | (0.14 to 0.32) | 1.11 | (1.04 to 1.18) |
| Maternal AFA (cm2) | 0.19 | (0.11 to 0.28) | 1.07 | (1.03 to 1.12) |
| Maternal Smoking | 0.79 | (−0.58 to 2.16) | 1.55 | (0.55 to 4.33) |
| Adjusted firstborn models | ||||
| Firstborn | 1.51 | (0.32 to 2.70) | 1.71 | (0.85 to 3.42) |
| SES (2005) | 0.67 | (0.52 to 0.83) | 1.22 | (1.13 to 1.32) |
| Maternal age <20 yrs | −1.11 | (−2.56 to 0.35) | 1.41 | (0.59 to 3.35) |
| Maternal age 20–35 yrs | REF | - | REF | - |
| Maternal age >35 yrs | −0.11 | (−1.58 to 1.36) | 0.85 | (0.31 to 2.34) |
| Maternal height (cm) | 0.23 | (0.14 to 0.32) | 1.10 | (1.04 to 1.17) |
| Maternal AFA (10 cm2) | 0.19 | (0.11 to 0.27) | 1.07 | (1.02 to 1.12) |
| Maternal smoking | 0.51 | (−0.85 to 1.88) | 1.28 | (0.47 to 3.52) |
AFA arm fat area; OR odds ratio; REF reference category; SES socioeconomic status; WC waist circumference
Point estimates and 95% confidence intervals reported as: Estimate (LowerLimit to Upper Limit)
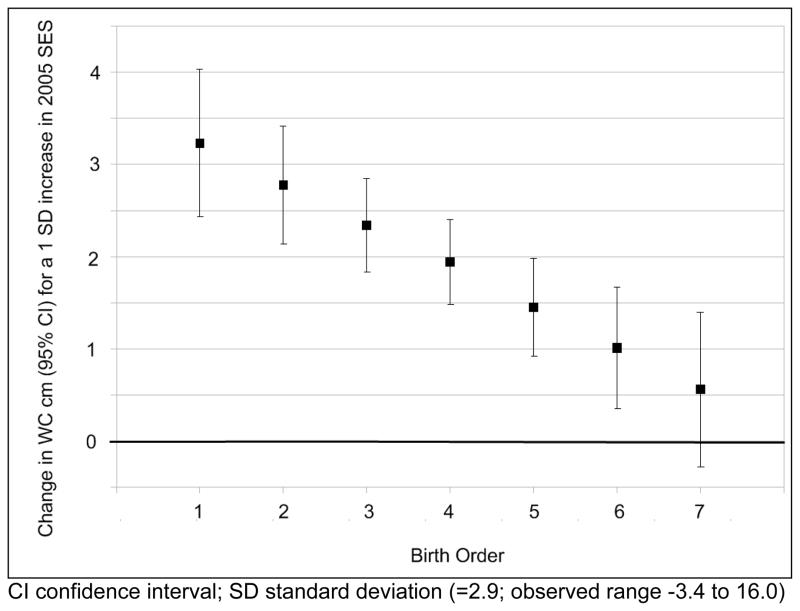
We detected a meaningful interaction between 2005 SES and birth order that was consistent with the mismatch hypothesis (table 4). For example, based on the estimated coefficients, a one SD increase in SES (2.9; observed range −3.4 to 16.0) would be associated with a 149% increase in odds of high WC (OR 2.49; 95% CI 1.65 to 3.76). However, the same increase in SES coupled with an increase in birth order would be associated with a 114% increase in odds of high WC (OR 2.14; 95% CI 1.58 to 2.89). The interaction could be viewed as a reduction in the positive impact of SES among individuals with higher birth orders (see figure 3). We found no evidence for a similar interaction between SES and the other prenatal variables (maternal age, height, AFA, smoking). We repeated our models, replacing 2005 SES with the same measure from 1983 to ascertain the relative importance of early versus later SES (model not shown). The estimated impact of early SES on central obesity was weaker than that of current SES, and there was no interaction between birth order and early SES in any model.
Table 4.
Mutilivariable linear models testing whether associations between socioeconomic status and waist circumference, or log odds of high waist circumference, are modified by birth order or firstborn status.
| Dependent Variable | ||||
|---|---|---|---|---|
| WC (cm) | OR High WC | |||
| Birth order models | ||||
| Birth order | −0.56 | (−0.80 to −0.32) | 0.77 | (0.63 to 0.95) |
| SES (2005) | 1.10 | (0.83 to 1.37) | 1.37 | (1.19 to 1.57) |
| Maternal age <20 yrs | −1.14 | (−2.50 to 0.22) | 1.23 | (0.53 to 2.86) |
| Maternal age 20–35 yrs | REF | - | REF | - |
| Maternal age >35 yrs | 1.52 | (−0.17 to 3.20) | 1.65 | (0.54 to 5.08) |
| Maternal height (cm) | 0.23 | (0.14 to 0.32) | 1.11 | (1.04 to 1.18) |
| Maternal AFA (cm2) | 0.20 | (0.12 to 0.28) | 1.08 | (1.03 to 1.13) |
| Maternal smoking | 0.73 | (−0.63 to 2.09) | 1.42 | (0.51 to 3.96) |
| Birth order X SES interaction | −0.15 | (−0.23 to −0.08) | 0.95 | (0.90 to 1.00) |
| Firstborn models | ||||
| Firstborn | 1.38 | (0.19 to 2.57) | 0.97 | (0.40 to 2.36) |
| SES (2005) | 0.55 | (0.37 to 0.74) | 1.14 | (1.03 to 1.26) |
| Maternal age <20 yrs | −0.90 | (−2.35 to 0.56) | 1.76 | (0.71 to 4.38) |
| Maternal age 20–35 yrs | REF | - | REF | - |
| Maternal age >35 yrs | −0.07 | (−1.53 to 1.40) | 0.86 | (0.31 to 2.38) |
| Maternal height (cm) | 0.23 | (0.14 to 0.32) | 1.10 | (1.04 to 1.17) |
| Maternal AFA (cm2) | 0.19 | (0.11 to 0.28) | 1.07 | (1.03 to 1.12) |
| Maternal smoking | 0.41 | (−0.95 to 1.78) | 1.16 | (0.41 to 3.14) |
| Firstborn X SES interaction | 0.44 | (0.10 to 0.79) | 1.24 | (1.04 to 1.48) |
AFA arm fat area; OR odds ratio; REF reference category; SES socioeconomic status; WC waist circumference
Point estimates and 95% confidence intervals reported as (Lower Limit to Upper Limit)
Figure 3.
The impact of young adult socioeconomic status on waist circumference is amplified for lower birth orders.
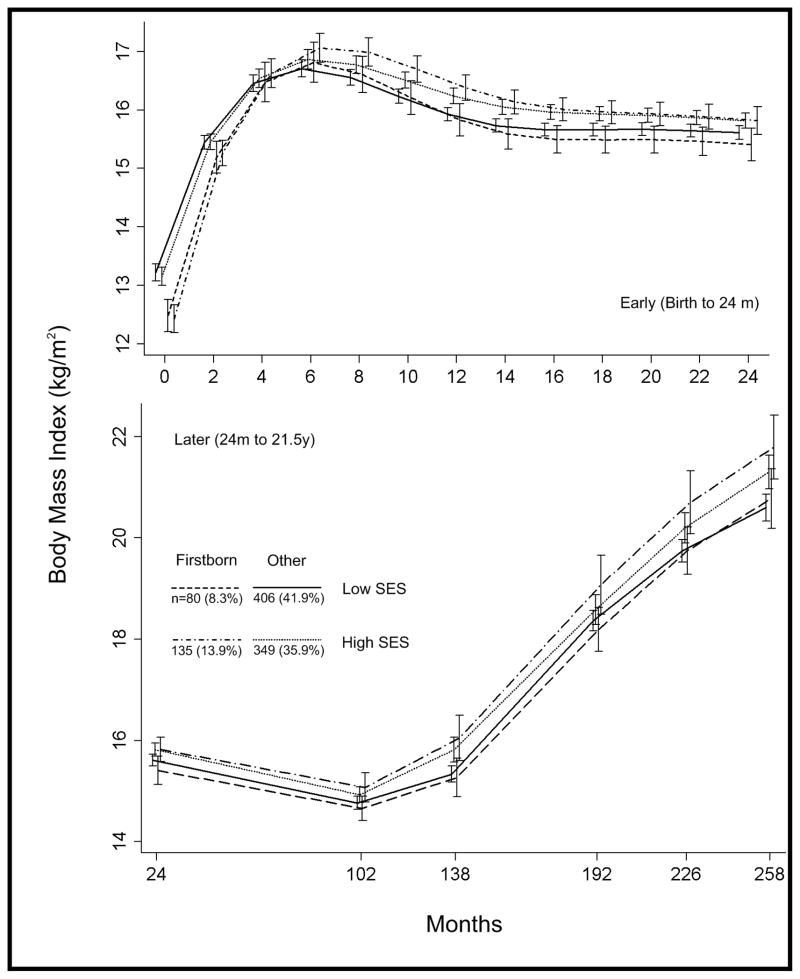
Mean BMI growth curves of four groups based on firstborn status and SES are displayed in figure 4. The high SES, firstborn group was characterized by low BMI at birth and rapid early postnatal gains in BMI. They had the largest mean BMI by six months, though at two years they were not distinguishable from the other high SES group. However, they had the largest increase in BMI across childhood and adolescence, resulting in the highest mean BMI in young adulthood. While BMI growth curves among high SES individuals were differentiated by firstborn status, there was no apparent impact of firstborn among lower SES individuals.
Figure 4.
Mean body mass index growth curves (with 95% confidence intervals) for groups of males defined by firstborn status and socioeconomic status at birth.
Discussion
The DOHaD paradigm broadly posits that environmental influences on prenatal and early postnatal development can alter physiology and/or behavior in a manner that increases risk of metabolic diseases, including obesity, in adulthood (23). Interest in the developmental origins of disease intensified after David Barker’s observation that the geographical distribution of neonatal mortality in England and Wales in 1911–15 closely corresponded to CVD mortality from the same areas in 1968–78 (46). Because most neonatal deaths at that time were attributed to low birth weight, Barker hypothesized that poor fetal nutrition was acting to program the body’s physiology in ways that adapted the offspring for a life of food insecurity while increasing “susceptibility to the effects of an affluent diet.” While the DOHaD paradigm was initially met with a great deal of skepticism (47), it now finds a great deal of support, and DOHaD research has intensified over the past 20 years. This support is largely based on a large body of research illustrating that birth size is associated with later disease in a variety of human cohorts (23). However, it is now well understood that for the DOHaD paradigm to move forward, researchers must move beyond investigating birth size (48–51) and start testing specific hypotheses focused on upstream determinants of the fetal environment.
Our goal was to test the mismatch hypothesis, which posits that maternal constraint of fetal growth increases susceptibility to the obesogenic effects of modern environments. Maternal constraint refers to the set of normal, non-pathological factors through which the mother limits fetal growth (24). Maternal constraint of fetal growth is important to ensure that the developing fetus does not outgrow the pelvic canal of its mother (22). The impact of maternal constraint was illustrated most famously by Walton and Hammond (52) who found that upon cross breeding large Shire horses and smaller Shetland ponies, the size of the foal at birth was primarily dependent on the size of the mare. Gluckman and Hanson’s focus on maternal constraint is notable because it is a normal process that operates in all pregnancies to some degree (24). This contrasts with the idea that the influences on fetal development which lead to later disease are caused by nutritional insults (53). The importance of nutritional insults has been well illustrated in studies which found that maternal exposure to the Dutch Hunger Winter (1944–45) during early pregnancy was associated with increased rates of obesity in young adult male offspring (25), and increased BMI and WC among 50 year old female offspring (26). Associations between maternal smoking and later offspring obesity have also been reported (28), further illustrating the potential impact of environmental insults during fetal development on later obesity. However, the idea that maternal constraint, a normal, non-pathological influence on the fetal environment, can impact offspring obesity has not been well investigated in humans (24). There is indirect evidence in the form of studies reporting an inverse association between birth size and later central adiposity (reflected by WC or skinfold ratios) (27); however birth size is a non-specific indicator of the fetal environment that reflects both normal and abnormal influences.
Among the forms of maternal constraint, we chose to focus on birth order and its impact on later obesity for several reasons. First, lower birth order is associated with reduced size at birth (32). We estimated associations between both firstborn status and continuously measured birth order with birth weight, length, and ponderal index. The results from our subsample of CLHNS males were consistent with earlier findings from an analysis of a larger subset of the CLHNS birth cohort: firstborns were both shorter and thinner at birth than higher order births (54). Other cohort studies have also confirmed that firstborns are both shorter and thinner at birth (e.g. 29). Our model results also indicated that the relationship between birth weight and birth order was best described by a third order polynomial relationship. While we posited that the increase in weights at the high end of the birth order distribution was likely spurious (due to small sample sizes (e.g. only 4% of males in the analysis sample were of birth order nine or above), it is interesting that a study of sheep found a similarly unexpected increase in birth weight at a parity of nine (55).
Previous studies have also reported associations between firstborn status and later obesity in adulthood. For example, firstborn status was associated with a four fold increase in odds of adiposity (skinfold thickness>85th percentile) in a cohort of young adult African Americans after adjustment for other perinatal measures including maternal BMI, education, and household size (35). Ravelli and Belmont, using data from a cohort of 19 year old Dutch males, found that being an only child was associated with obesity (56), though there was no apparent association between lower birth order and obesity in larger families. We found that firstborn status, and lower birth order in general, were associated with increased risk of central adiposity reflected by WC in young adult males.
However, under the mismatch hypothesis, the effects of birth order should be more apparent if the individual has experienced a nutritionally abundant postnatal environment. In high income countries, where nutritional energy abundance seems to be the norm, this interaction is less important (i.e. most people are experiencing the environment required to express the deleterious effect of reduced fetal growth). However, in a lower-income country such as the Philippines, where there is greater variation in nutritional environments and the prevalence of underweight is still considerable, the nature of this interaction is critical. By exploiting this environmental variation, we were able to test the mismatch hypothesis.
To test for this interaction, we used a measure of SES based on household assets in young adulthood as a crude but useful proxy measure for the obesogenic environment for males in our study. In previous analyses, we used a spatial clustering method to investigate obesogenic environments in this sample (57). We found that SES reflected in 2005 household assets was an important predictor of central adiposity in males that largely explained the spatial clustering (57) in high WC that we observed. Consequently, we concluded that the 2005 SES was a crude but useful measure to identify obesogenic environments, and took advantage of this to test the mismatch hypothesis in the CLHNS males. However, we found that the socio-environmental determinants of obesity were much more complex in the females, and at this point have not developed a useful way to estimate their exposure to obesogenic environments that would facilitate their inclusion in this analysis.
We confirmed our prediction under the mismatch hypothesis in the males: low birth order amplified the impact of SES on young adult central adiposity. While the idea that disease arises from a discordance between the pre- and postnatal nutritional environment is not a new one for DOHaD researchers, to our knowledge, only one previous study has explicitly tested for an interaction between prenatal variables and environment measured in young adulthood; Barker et al. found that the risk of coronary heart disease associated with low social class were amplified in men who were born thin at birth (58).
Rapid postnatal growth is also a hypothesized determinant of later obesity (29, 59). Because birth order is associated with higher maternal SES (due to reduced fertility among high SES women), our expectation was that firstborns would be more likely to experience an early postnatal environment that would promote rapid catch-up growth. This contrasts to other maternal constraints on fetal growth such as young age, or small body size, which tend to be associated with lower SES and thus a poorer postnatal environment that instead promotes growth faltering. We explored mean BMI growth curves in subgroups based on firstborn status and SES at birth, finding that being firstborn and having a high SES was associated with lower birth BMI followed by a rapid increase in BMI through early infancy. Furthermore, this group also had the largest mean BMI in young adulthood. The early trajectory was similar to that found among firstborns in the ALSPAC study who were thin at birth and also experienced rapid catch-up growth (29). However, due to the exploratory nature of this analysis, we were not able to determine whether the rapid postnatal growth we observed mediates the impact of birth order and SES on later young adult BMI, or whether rapid postnatal growth is a downstream indicator of aspects of fetal growth and development that impact later obesity risk independently of the postnatal growth pattern. We did try to test the hypothesis that early rather than later environment was a more important modifier of birth order by replacing SES in young adulthood with SES measured at birth in our interaction models. We found that estimated impact of early SES on later central obesity was weaker than that of current SES, and there was no interaction between birth order and early SES on young adult WC in any model. However, this is an admittedly crude attempt at elucidating the nature of this timing and more research is clearly needed.
While our analysis failed to reject the mismatch hypothesis, we must interpret our results cautiously. Lower birth order is a complex exposure variable that is likely associated with a variety of pre- and postnatal factors that we were not able to account for. Birth order is linked to household size which may influence body size in adulthood. However, household size likely reflects different exposures at various points across the life course and thus its inclusion in our linear models is somewhat problematic. We tried to crudely account for this influence by including a measure of household size averaged across the study period (results not reported). While this inclusion attenuated the estimated effects of birth order on WC, it did not affect the interaction between birth order and SES. In a lower income country like the Philippines where underweight is still prevalent, it is also possible that higher WC associated with lower birth order is due to improvement in overall nutrition found in higher SES individuals from smaller families. We tried to account for this by estimating the impact of birth order on both continuously measured WC and on the upper end of the WC distribution. We also looked at the joint impact of birth order and SES on young adult height (results not reported), finding that continuously measured birth order and SES affected height similarly to WC, though the joint impact of firstborn status and SES seemed to be confined to WC. Another competing hypothesis that we did not explicitly test was the possibility that lower order births, particularly firstborns, are allocated more food during their childhood development (60, 61). However, this mechanism predicts a strong association between lower birth order and relatively better lifecourse nutrition (usually reflected by height) in large, poorer families whose overall nutritional environment is limited. Thus it seems unlikely to account for the increased risk of central obesity that our results suggest.
More research on the underlying mechanisms explaining the relationship between birth order, fetal growth, and later disease are clearly needed. If low birth order is truly related to increased disease risk in adulthood in the manner described by the mismatch hypothesis, then a better understanding of the biological mechanisms connecting birth order and fetal growth will likely provide important insights for intervention efforts. More research is also need to evaluate whether rapid postnatal growth is a mediator of the hypothesized impact of birth order on later obesity. This is especially important as birth order is clearly not a target for public health intervention. The interaction between pre- and post-natal nutritional environments requires more explicit testing in human populations, ideally using data from prospective longitudinal birth cohort studies. The global public health impact of the mismatch hypothesis with respect to birth order could be critical, as obesogenic environments become more common, and the proportion of lower order pregnancies among humans increases.
Supplementary Material
Contributor Information
Darren L Dahly, University of Leeds, Centre for Epidemiology and Biostatistics.
Linda S Adair, University of North Carolina, Department of Nutrition, Carolina Population Center.
References
- 1.WHO. Obesity: preventing and managing the global epidemic. World Health Organization Technical Report Series. 2000 [PubMed] [Google Scholar]
- 2.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: Where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 3.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 4.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–335. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- 5.Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health. 2006;126:262–267. doi: 10.1177/1466424006070487. [DOI] [PubMed] [Google Scholar]
- 6.Popkin BM, Duffey K, Gordon-Larsen P. Environmental influences on food choice, physical activity and energy balance. Physiol Behav. 2005;86:603–613. doi: 10.1016/j.physbeh.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 7.Booth KM, Pinkston MM, Poston WSC. Obesity and the built environment. J Am Diet Assoc. 2005;105:110–117. doi: 10.1016/j.jada.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55:125–139. doi: 10.1016/s0277-9536(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 9.Entwisle B. Putting people into place. Demography. 2007;44:687–703. doi: 10.1353/dem.2007.0045. [DOI] [PubMed] [Google Scholar]
- 10.Black JL, Macinko J. Neighborhoods and obesity. Nutrition Reviews. 2008;66:2–20. doi: 10.1111/j.1753-4887.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 11.Neel JV. Diabetes mellitus: A “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 12.Prentice AM, Hennig BJ, Fulford AJ. Evolutionary origins of the obesity epidemic: natural selection of thrifty genes or genetic drift following predation release|[quest] Int J Obes. 2008;32:1607–1610. doi: 10.1038/ijo.2008.147. [DOI] [PubMed] [Google Scholar]
- 13.van der Sande MA, Ceesay SM, Milligan PJ, Nyan OA, Banya WA, Prentice A, et al. Obesity and undernutrition and cardiovascular risk factors in rural and urban Gambian communities. Am J Public Health. 2001;91:1641–1644. doi: 10.2105/ajph.91.10.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- 16.Gluckman PD, Hanson MA. Living with the Past: Evolution, Development, and Patterns of Disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman P, Hanson M. Mismatch: Why Our World No Longer Fits Our Bodies. Vol. 8. Oxford University Press; Oxford, UK: 2006. p. 219. [Google Scholar]
- 18.Pike KC, Hanson MA, Godfrey KM. Developmental mismatch: consequences for later cardiorespiratory health. BJOG. 2008;115:149–157. doi: 10.1111/j.1471-0528.2007.01603.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuzawa CW, Gluckman PD, Hanson MA, Beedle AS. Evolution in health and disease. 2. Oxford University Press; Oxford: 2007. Evolution, developmental plasticity, and metabolic disease. [Google Scholar]
- 20.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- 21.Gluckman PD, Cutfield W, Hofman P, Hanson MA. The fetal, neonatal, and infant environments—the long-term consequences for disease risk. Early Hum Dev. 2005;81:51–59. doi: 10.1016/j.earlhumdev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20:527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med. 2004;9:419–425. doi: 10.1016/j.siny.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 26.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 27.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 28.Power C, Jefferis B. Fetal environment and subsequent obesity: A study of maternal smoking. Int J Epidemiol. 2002;31:413–419. [PubMed] [Google Scholar]
- 29.Ong K, Preece MA, Emmett PM, Ahmed ML, Dunger DB. Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast-feeding: Longitudinal birth cohort study and analysis. Pediatr Res. 2002;52:863–867. doi: 10.1203/00006450-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Dahly D, Emch M, Gordon-Larsen P, Adair L. The spatial distribution of overweight and obesity among a birth cohort of young adult Filipinos (Cebu Philippines, 2005) 2009 doi: 10.1038/nutd.2013.21. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahly DL, Gordon-Larsen P, Popkin BM, Kaufman J, Adair LS. Socio-environmental determinants of obesity in young adult Filipinos. 2009 Manuscript under review. [Google Scholar]
- 32.Cogswell ME, Yip R. The influence of fetal and maternal factors on the distribution of birthweight. Semin Perinatol. 1995;19:222–240. doi: 10.1016/s0146-0005(05)80028-x. [DOI] [PubMed] [Google Scholar]
- 33.Miller JE. Birth order, interpregnancy interval and birth outcomes among Filipino infants. Journal of Biosocial Science. 1994;26:243–259. doi: 10.1017/s0021932000021271. [DOI] [PubMed] [Google Scholar]
- 34.Seidman DS, Ever-Hadani P, Stevenson DK, Slater PE, Harlap S, Gale R. Birth order and birth weight reexamined. Obstet Gynecol. 1988;72:158. [PubMed] [Google Scholar]
- 35.Stettler N, Tershakovec AM, Zemel BS, Leonard MB, Boston RC, Katz SH, et al. Early risk factors for increased adiposity: a cohort study of African American subjects followed from birth to young adulthood. Am J Clin Nutr. 2000;72:378–383. doi: 10.1093/ajcn/72.2.378. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh JR, Bandyopadhyay AR. Income, birth order, siblings, and anthropometry. Hum Biol. 2006;78:733–741. doi: 10.1353/hub.2007.0012. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Sekine M, Chen X, Kanayama H, Yamagami T, Kagamimori S. Sib-size, birth order and risk of overweight in junior high school students in Japan: Results of the Toyama Birth Cohort Study. Prev Med. 2007;44:45–51. doi: 10.1016/j.ypmed.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Cardwell CR, Carson DJ, Patterson CC. Parental age at delivery, birth order, birth weight and gestational age are associated with the risk of childhood Type 1 diabetes: a UK regional retrospective cohort study. Diabetic Medicine. 2005;22:200–206. doi: 10.1111/j.1464-5491.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 39.Stene LC, Magnus P, Lie RT, Sovik O, Joner G. Maternal and paternal age at delivery, birth order, and risk of childhood onset type 1 diabetes: population based cohort study. Br Med J. 2001;323:369. doi: 10.1136/bmj.323.7309.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bingley PJ, Douek IF, Rogers CA, Gale EAM. Influence of maternal age at delivery and birth order on risk of type 1 diabetes in childhood: Prospective population based family study. Br Med J. 2000;321:420–424. doi: 10.1136/bmj.321.7258.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. J Pediatr. 1979;95:769–774. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- 42.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vyas S, Kumaranayake L. Constructing socio-economic status indices: How to use principal components analysis. Health Policy Plan. 2006;21:459. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 44.Lohman T, Roche A, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; Champaign, Il: 1988. [Google Scholar]
- 45.Bei-Fan Z. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr. 2002;11:685–693. [PubMed] [Google Scholar]
- 46.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 47.Couzin J. Quirks of fetal environment felt decades later. Science. 2002;296:2167–2169. doi: 10.1126/science.296.5576.2167. [DOI] [PubMed] [Google Scholar]
- 48.Gillman MW. Epidemiological challenges in studying the fetal origins of adult chronic disease. Int J Epidemiol. 2002;31:294–299. [PubMed] [Google Scholar]
- 49.Langley-Evans SC. Developmental programming of health and disease. Proc Nutr Soc. 2007;65:97–105. doi: 10.1079/pns2005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law CM. Significance of birth weight for the future. Arch Dis Child Fetal Neonatal Ed. 2002;86:F7–8. doi: 10.1136/fn.86.1.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barker DJP. Fetal origins of cardiovascular and lung disease. M. Dekker; New York: 2001. [Google Scholar]
- 52.Walton A, Hammond J. The maternal effects on growth and conformation in Shire horse-Shetland pony crosses. Proc R Soc Lond B Biol Sci. 1938:311–335. [Google Scholar]
- 53.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 54.Miller JE. Birth outcomes by mother’s age at first birth in the Philippines. Int Fam Plan Perspect. 1993:98–102. [Google Scholar]
- 55.Gardner DS, Buttery PJ, Daniel Z, Symonds ME. Factors affecting birth weight in sheep: Maternal environment. Reproduction. 2007;133:297–307. doi: 10.1530/REP-06-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ravelli GP, Belmont L. Obesity in nineteen-year-old men: Family size and birth order associations. Am J Epidemiol. 1979;109:66–70. doi: 10.1093/oxfordjournals.aje.a112660. [DOI] [PubMed] [Google Scholar]
- 57.Dahly D. The spatial distribution of obesity and overweight among a birth cohort of young adult Filipinos (Cebu, 2005) 2009 doi: 10.1038/nutd.2013.21. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barker DJP, Forsen T, Uutela A, Osmond C, Eriksson JG. Size at birth and resilience to effects of poor living conditions in adult life: Longitudinal study. Br Med J. 2001;323:1273. doi: 10.1136/bmj.323.7324.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong KK, Loos RJF. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatrica. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 60.Horton S. Birth order and child nutritional status: Evidence from the Philippines. Econ Dev Cult Change. 1988;36:341–354. [Google Scholar]
- 61.Horton S. Child nutrition and family size in the Philippines. J Dev Econ. 1986;23:161–176. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.