Abstract
Glial fibrillary acidic protein (GFAP) is the major intermediate filament protein of astrocytes in the vertebrate central nervous system. Increased levels of GFAP are the hallmark feature of gliosis, a non-specific response of astrocytes to a wide variety of injuries and disorders of the CNS, and also occur in Alexander disease where the initial insult is a mutation within the coding region of GFAP itself. In both settings, excess GFAP may cause or exacerbate astrocyte dysfunction. With the goal of finding drugs that reduce the expression of GFAP, we have devised screens to detect changes in GFAP promoter activity or protein levels in primary cultures of mouse astrocytes in a 96-well format. We have applied these screens to libraries enriched in compounds that are already approved for human use by the FDA. We report that several compounds are active at micromolar levels in suppressing the expression of GFAP. Treatment of mice for 3 weeks with one of these drugs, clomipramine, causes nearly 50% reduction in the levels of GFAP protein in brain.
INTRODUCTION
Glial fibrillary acidic protein (GFAP) is the major intermediate filament protein in astrocytes, a macroglial cell type of the vertebrate central nervous system with diverse regulatory and support functions (1). Expression is markedly up-regulated in the context of gliosis, the reactive response by astrocytes to nearly all types of injury and disease in the CNS (2). In addition, mutations within the coding region of GFAP cause Alexander disease, a fatal neurodegenerative disorder initially described in young children but also having juvenile and adult-onset forms (3,4). These mutations lead to the formation of complex protein aggregates within the cytoplasm of astrocytes known as Rosenthal fibers and in some as-yet undefined manner cause astrocyte dysfunction with catastrophic effects on neurons and oligodendroglia (5). Unlike other intermediate filament disorders, where dominant negative mutations are most common, the GFAP mutations causing Alexander disease appear to reflect gain of function. Increasing evidence points to the accumulation of GFAP protein above a toxic threshold as central to the pathogenesis of this disease (6).
Drug discovery efforts for rare disorders such as Alexander disease are hampered by the high costs associated with the development and testing of new compounds. However, existing drugs often have unexpected and useful properties when evaluated in particular applications. Recently, a consortium of laboratories cooperated in efforts to screen libraries of FDA-approved drugs to identify novel treatments for several neurodegenerative disorders (7). A screen for enhancers of glutamate transporter expression in amyotrophic lateral sclerosis led to the identification of beta-lactam antibiotics as a promising class of compounds to pursue (8). Similarly, a screen for inhibitors of caspase-3 activation caused by the expression of mutant androgen receptors identified several drugs that could be useful in the treatment of spinobulbar muscular atrophy (9).
Since a prevailing hypothesis regarding Alexander disease is that GFAP accumulation contributes to astrocyte dysfunction, we hypothesized that drugs or compounds that reduce GFAP levels would be beneficial. GFAP expression is largely but not exclusively regulated at the transcriptional level (10,11), and regulatory elements suitable for directing the expression of reporter genes in vivo have been identified in both human and mouse GFAP genes (12,13). With the goal of finding drugs or compounds that reduce GFAP, we have adapted primary cultures of mouse astrocytes to a 96-well format and devised screens to detect changes in either GFAP promoter activity or protein levels. For the promoter assay, we derived astrocytes from recently developed lines of transgenic mice expressing dual luciferase reporter genes under the control of the human GFAP and GAPDH promoters (14). We report results suggesting that several compounds may be active at micromolar levels in suppressing the expression of GFAP.
RESULTS
Assay optimization and validation
To develop 96-well cell-based screening assays for suppressors of GFAP expression, we cultured primary astrocytes from a mouse line expressing the firefly luciferase reporter under the control of the human GFAP promoter (14). The same cells were also used for a cell-based ELISA to detect the effects on GFAP protein levels. Pilot experiments optimized cell density, time in culture and assay conditions. Signals in the luciferase assay consistently yielded values that were 4000-fold above background (established from non-transgenic mice). Signals in the ELISA assay were 10-fold above background (i.e. no cells or astrocytes derived from GFAP-null mice).
Selection of chemical libraries and conditions of primary screen
To identify the compounds that decrease GFAP promoter activity and/or reduce GFAP protein levels, we chose to screen the Known Bioactive Library (KBA01) prepared by the UW-Small Molecule Screening Facility (University of Wisconsin, Madison, USA). The KBA01 library consists of 2880 compounds comprising the combined collections of two original small molecule libraries: the Prestwick Chemical Library® and the Spectrum collection. Most compounds in the Prestwick Chemical Library® are marketed drugs with known safety and bioactivity in humans. The Spectrum collection provided a wide range of biological activities and structural diversity of clinically useful drugs, natural products and non-drug chemicals. Compounds in the KBA01 library were stored as concentrated stocks in DMSO. Cells were tested for response to compounds at 10 µm after 48 h of exposure, beginning 24 h after plating into the 96-well plates (passage 2). The final concentration of DMSO used after dilution for the primary screen (1% in DMEM) had no effect on the luciferase or cell-based ELISA results.
Primary screening
A total of 36 plates were used to conduct the primary screen. Background in the luciferase assay was so low (<0.1%) that no correction was applied. Signals in the cell-based ELISA were corrected for background as determined in wells with the medium only. Z′-factors were calculated for each plate in the screen, using the following formula from Zhang et al. (15) [standard deviation (σ) and mean (μ) signals for positive (pos) and negative (neg) controls]:
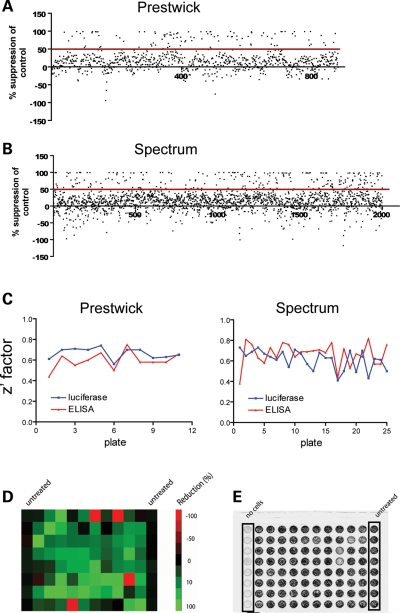
The Z′-factors averaged 0.64 for the luciferase assay and 0.63 for the cell-based ELISA, well above the recommended minimum of 0.5. Aggregate results are shown in Figure 1. Our criterion for a hit was a 50% or greater reduction in the luciferase or ELISA signals after exposure to compounds. Overall, our primary screen identified 356 (12.4%) compounds that qualified as hits in the luciferase screen and 60 (2.1%) as hits in the ELISA assay (Fig. 1, Table 1), though some were duplicates between the two libraries.
Figure 1.
Primary screening of 2880 bioactive molecules to identify suppressors of GFAP expression. (A and B) Scatter plots of response to compounds in the Prestwick and Spectrum libraries, presented as percent reduction in luciferase signal from GFAP-luciferase transgenic astrocytes after exposure to compounds at 10 µm for 48 h. (C) Z′-factor for each 96-well plate used in primary screening for both luciferase and cell-based ELISA assays. Eighty compounds were tested per plate. Plate numbers were arbitrary. The average Z′-factor for all luciferase plates in the Prestwick screen was 0.67 and in the Spectrum screen was 0.61. The average Z′-factor for all cell-based ELISA plates in the Prestwick screen was 0.6 and in the Spectrum screen was 0.67. (D) Sample plate from primary screen using luciferase assay, with activity levels illustrated by Heat Map. Cells treated with compounds (columns 2–11) were compared with untreated cells (columns 1 and 12). Green represents the maximal reduction and red represents the maximal activation, compared with control luciferase activity. (E) Sample plate from primary screen using cell-based ELISA, imaged using a Storm 860 phosphoimager. Cells treated with compounds (columns 2–11) were compared with untreated cells (column 12). Background was assessed in wells containing medium only (column 1).
Table 1.
Primary screen of 2880 compounds
| Library | % reduction | Compounds n (%) |
|
|---|---|---|---|
| Promoter screen | Protein screen | ||
| Prestwick | <50 | 772 | 856 |
| 50–75 | 37 (4.2%) | 20 (2.3%) | |
| 75–90 | 30 (3.4%) | 4 (0.5%) | |
| 90–100 | 41 (4.6%) | 0 | |
| Spectrum | <50 | 1752 | 1964 |
| 50–75 | 105 (5.3%) | 33 (1.7%) | |
| 75–90 | 52 (2.6%) | 3 (0.1%) | |
| 90–100 | 91 (4.5%) | 0 | |
Summary of results from primary screen of 2880 compounds derived from the Prestwick and Spectrum libraries, in both luciferase (GFAP promoter) and ELISA (GFAP protein) assays. Cells were exposed to each compound at 10 µm for 48 h, using one well per compound. No corrections were made for potential effects on cell survival.
To determine whether any of the hits identified in the first round of screening reflected non-specific toxicity, we subjected 400 compounds to repeat cell treatments and luciferase/ELISA assays, and in addition measured cell viability in parallel plates using the CellTiter-glo assay. Cells were again exposed to 10 µm of each compound for 48 h. Our criterion for acceptable viability was >70% survival. Under these conditions, compounds were again identified that reduced signals by >50% and without causing toxicity (nine in the luciferase assay and eight in the ELISA assay).
To further validate these results, we selected 38 compounds for dose–response curves, using independently purchased stocks. In addition to those cited above, we considered an additional group of compounds that caused at least >35% reduction in each round of screening, with an average of >40% reduction when combined (13 in the luciferase assay and 34 in the ELISA assay, six of these represented in both assays). We paid particular attention to compounds with known ability to cross the blood–brain barrier. However, some compounds were unavailable outside of the initial library collections and could not be further tested. We repeated the luciferase and cell viability assays after exposures for 48 h to 0–20 µm of each compound. In this third screen, eight of the compounds again achieved >50% reductions in GFAP promoter activity (in this particular experiment, none of the compounds caused >50% in GFAP protein). Table 2 provides the results of these assays for the top 10 compounds, ranked according to percent reduction in GFAP promoter activity (the complete listing of all 38 evaluated in this screen is given in Supplementary Material). At the highest doses, some compounds displayed cytotoxicity. The maximum tolerable dose for cell culture (‘MTDcc’) is presented as the highest concentration of drug showing acceptable cell viability (i.e. >70%).
Table 2.
Tertiary screen of 38 compounds (top 10 shown)
| Compounds | % survival at 10 µm | MTDcc (µm) | % suppression of GFAP promoter |
|---|---|---|---|
| Diaziquone | 80 | >20 | 86 |
| Clomipramine | 83 | 12.5 | 66 |
| Chrysophanol | 86 | 12.5 | 66 |
| Amitriptyline | 102 | >20 | 63 |
| Chlorprothixene | 79 | 17.5 | 60 |
| EGCDG | 84 | 17.5 | 57 |
| Tamoxifen citrate | 60 | 7.5 | 52 |
| Mundoserone | 88 | >20 | 50 |
| Amlodipine | 98 | 12.5 | 44 |
| Embelin | 63 | 7.5 | 37 |
Top 10 lead compounds based on percent reduction in GFAP promoter activity in the tertiary screen. A dose–response association was evaluated after exposure to each compound at various concentrations (0.1, 0.5, 1, 5, 10, 15, 20 µm) for 48 h. MTDcc indicates the highest concentration that reduced cell viability <30%. Each compound and concentration were analyzed in quadruplicate wells.
Response of cultured astrocytes to long-term treatment with selected compounds
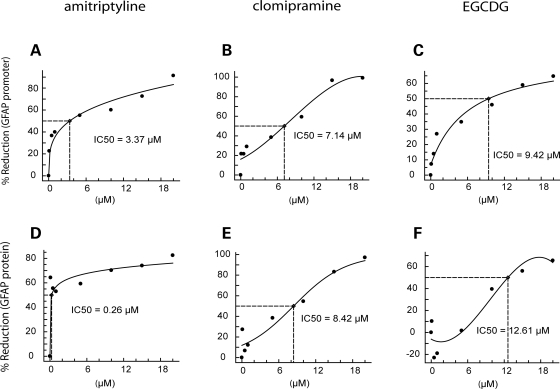
To evaluate the possibility that the observed reductions in GFAP promoter depend on length of treatment and assess whether changes in protein levels also occur, we performed dose–response analyses for seven of the compounds listed in Table 2 using 10 days of exposure. GFAP protein levels were measured using a sandwich ELISA. Cytotoxicity was often observed at 15–20 µm. However, consistent reductions in luciferase activity and GFAP protein levels with acceptable cell viability were observed in cells exposed to amitriptyline, clomipramine and epigallocatechin 3,5-digallate (EGCDG) (Fig. 2). IC50s were typically in the low micromolar range, and little or no activity was observed at nanomolar concentrations.
Figure 2.
Dose–response curves for amitriptyline, clomipramine and EGCDG evaluated for percent reduction in GFAP promoter (top row) or protein levels (bottom row). Astrocytes from GFAP-luciferase transgenic mice were exposed to each compound at 0–20 µm for 10 days. IC50s were calculated from fitted curves (see Materials and Methods). Toxicity was observed for all three compounds at the highest dose, 20 µm. Each point represents the mean of triplicate wells.
Response of mice to clomipramine: acute effects
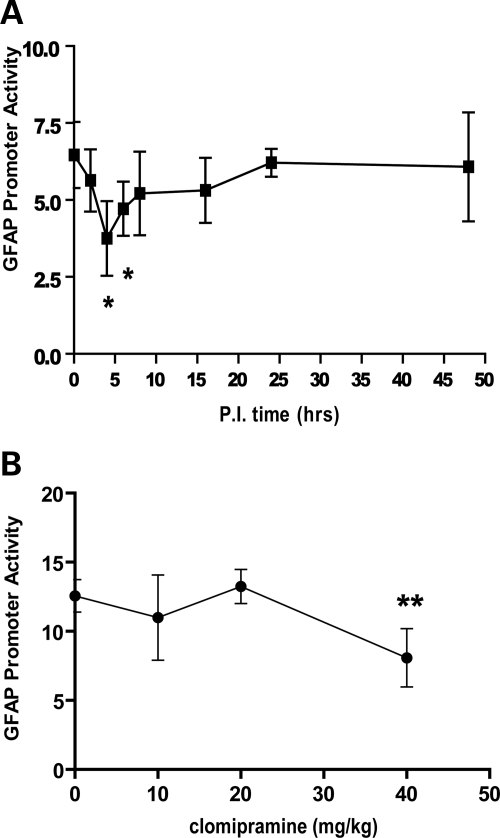
Compounds that reduce GFAP expression in vitro might not do so in vivo, due to differences in astrocyte physiology or pharmacokinetic limitations. We therefore tested whether clomipramine, administered systemically to mice, influenced GFAP promoter activity and protein levels in brain. Clomipramine, a tricyclic antidepressant, was selected for this study based on its high ranking in the cell culture assay and its known ability to cross the blood–brain barrier. Dual luciferase reporter mice (the source of primary astrocytes for the initial screening assays) were treated with single injections of 40 mg/kg, IP, and brains harvested at various time points after injection from 0 to 48 h. For these experiments, the measurement of GFAP promoter activity was performed using the Dual-Glo assay, which takes advantage of the second transgene that encodes Renilla luciferase under the control of the housekeeping GAPDH promoter. Normalizing the GFAP signal to the GAPDH signal was previously found to reduce inter-animal variability (14). As shown in Figure 3A, clomipramine treatment caused a transient reduction in promoter activity that peaked at 4–6 h after injection. Promoter activity returned to normal by 24 h. There were no changes in GFAP protein levels during this brief time period (data not shown). We next treated dual luciferase mice with varying doses of clomipramine (0–40 mg/kg, IP), and brains were collected at 4 h after injection for the measurement of GFAP promoter activity. As shown in Figure 3B, a single injection of 40 mg/kg produced a 36% reduction in promoter activity, whereas doses of 5–20 mg/kg were without effect. Clomipramine concentrations were determined in plasma and brain following single IP injections of the 40 mg/kg dose. At 1 h following injection, the concentrations of clomipramine were in a range comparable to that found effective in cultured astrocytes (6 and 3 µm for brain and plasma, respectively).
Figure 3.
Acute effects of clomipramine on GFAP promoter activity in vivo. (A) Tg172-9 mice (males, 3–4 months old) were injected with clomipramine using a dose of 40 mg/kg, IP. Brains were collected at various times post-injection and analyzed for GFAP promoter activity using the Dual-Glo assay. (B) Tg172-9 mice (females, 3–4 months old) were injected with various doses of clomipramine, IP (0, 5, 10, 20 or 40 mg/kg). Controls were injected with vehicle only. Brains were collected at 4 h post-injection and analyzed for GFAP promoter activity using the Dual-Glo assay. Graphs present the mean ± SD (n = 4 at each data point). Units on the Y-axis are expressed as the ratio of firefly to Renilla luciferase signals, multiplied by 100. Statistical significance was evaluated by the unpaired t-test (*P < 0.05).
Response of mice to clomipramine: chronic effects
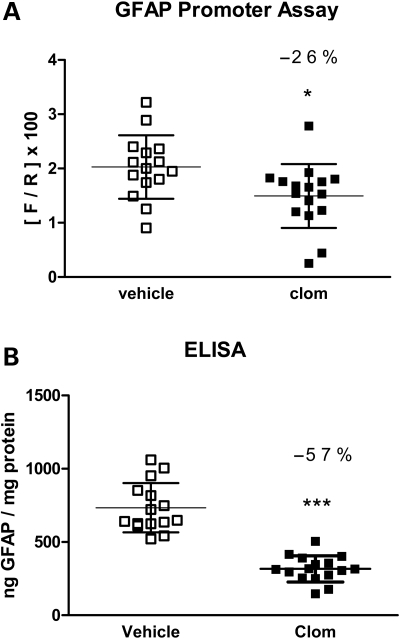
To achieve significant reductions of GFAP protein in vivo almost certainly requires chronic treatments, given the slow turnover of this protein (16). However, pilot studies using daily injections of 40 mg/kg clomipramine resulted in weight loss after 2 weeks and were discontinued. We therefore extended the interval between doses to 2 days and treated dual luciferase mice with 40 mg/kg clomipramine IP for 3 weeks without any apparent ill effects. Controls were injected with vehicle alone. One day after the last injection, brains were harvested and assayed for both GFAP promoter activity and GFAP protein. Clomipramine-treated mice displayed a 26% reduction in GFAP promoter activity (Fig. 4A, *P < 0.05, unpaired t-test). Most remarkably, these same mice displayed a 57% reduction in GFAP protein (Fig. 4B, ***P < 0.001, unpaired t-test). There was no significant change in the activity from the housekeeping GAPDH promoter in the same mice (data not shown).
Figure 4.
Long-term effects of clomipramine on GFAP promoter activity and protein levels in vivo. Tg172.9 mice were injected with 40 mg/kg clomipramine in H2O, intraperitoneally, every other day for 21 days (n = 16). Another group of mice were injected with the same volume of vehicle alone (n = 15). Brains were bisected sagittally with one half analyzed for GFAP promoter activity using the Dual-Glo assay and the other half analyzed for GFAP protein using the sandwich ELISA. (A) Clomipramine-treated mice show a 26% reduction in promoter activity compared with vehicle-treated controls. (B) Clomipramine-treated mice show a 57% reduction in total GFAP content compared with vehicle-treated controls. Graphs present the mean ± SD (*P < 0.05, ***P < 0.001, unpaired t-test).
Response of Alexander disease cell culture model to clomipramine
We considered the possibility that the response to compounds identified above might be different in astrocytes whose baseline expression of GFAP is elevated above normal, as occurs in the context of disorders such as Alexander disease (17–19). We therefore tested the response of astrocytes derived from TgGFAP-wt mice, a mouse model of Alexander disease that constitutively over-expresses wild-type human GFAP and that forms Rosenthal fibers identical to those in the human disease (20). Primary cultures were derived from whole brains of newborn TgGFAP-wt mice as previously described (20) and studied during passage 1, the period when Rosenthal fibers initially form. At 1 day in vitro (DIV), 5 µm clomipramine or vehicle as a control were added to the culture medium. After varying durations of drug treatment (re-feeding every 3 days), cells were fixed and stained with anti-GFAP antibodies for immunofluorescence microscopy. Exposure to clomipramine caused a reduction in staining intensity for GFAP, and a delay in the appearance of GFAP aggregates, although eventually the percentage of cells with aggregates reached the same levels as in vehicle-treated cells (Fig. 5).
Figure 5.
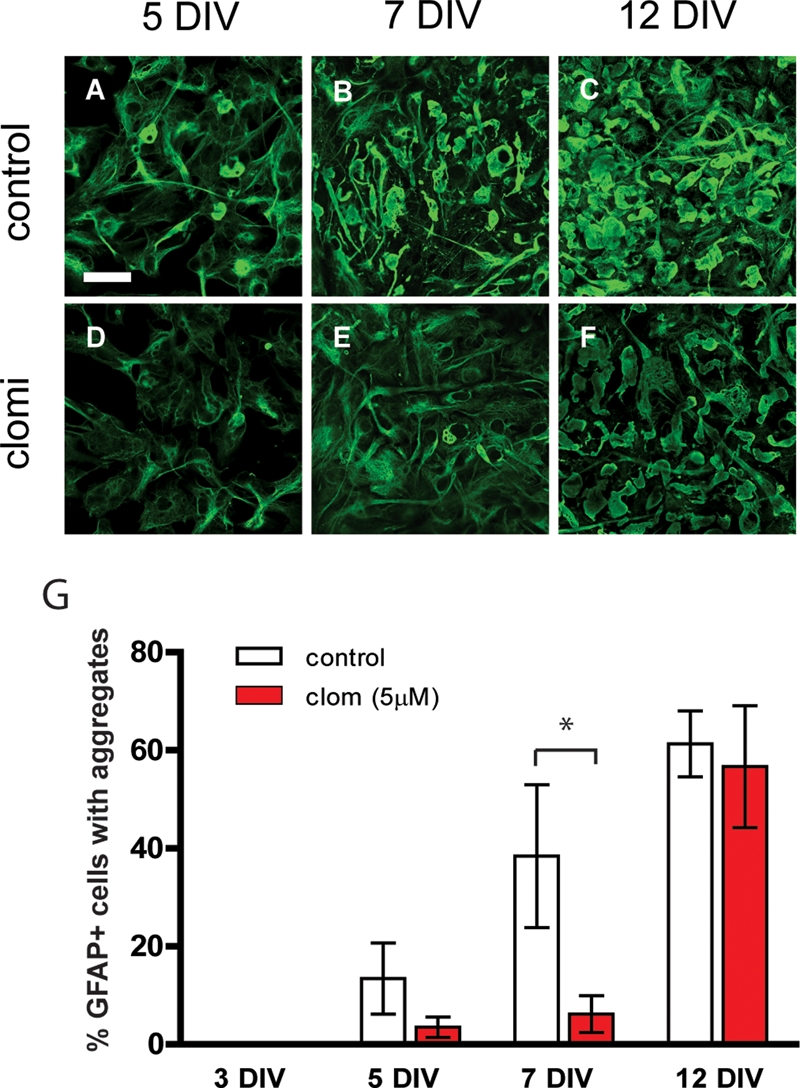
Effects of clomipramine on GFAP and cytoplasmic inclusions in astrocytes cultured from TgGFAP-wt mice. (A, D and G) Cells with GFAP aggregates, expressed as a percentage of GFAP-positive cells, begin to appear at 5 DIV in both untreated (12.5%) and drug-treated (3.3%) TgGFAP-wt cultures, but the difference is not significant. (B, E and G) At 7 DIV, drug-treated cultures contain significantly fewer cells bearing GFAP aggregates compared to untreated cultures (5.8% versus 36.0%). (C, F and G) At 12 DIV, the percent of GFAP aggregate-bearing cells is similar irrespective of drug treatment. The filamentous staining for GFAP appeared lower in drug-treated compared with vehicle-treated cells at all time points. Graphs present the mean ± SEM (n = 3 at each data point). Statistical significance was evaluated by the unpaired t-test (*P < 0.05). Scale bar = 50 µm. Data are representative of two independent experiments.
DISCUSSION
We sought to identify compounds capable of reducing GFAP levels in brain, with the long-term goal of developing a treatment for disorders such as Alexander disease where excessive accumulation of GFAP appears to be a central element of the disease. Reducing GFAP levels may also be useful in the context of chronic gliosis, the nearly universal response of astrocytes to diverse injuries or diseases of the central nervous system. Using primary cultures of mouse astrocytes, we screened 2880 compounds for their ability to reduce GFAP promoter activity or protein immunoreactivity after short-term (i.e. 48 h) exposure. After three rounds of screening, eight compounds consistently reduced expression below our arbitrary criteria for a ‘hit’, 50% reduction with no more than 30% cell death. Among this group, there were few evident structure–function relations, with the exceptions of clomipramine and amitriptyline which are both tricyclic antidepressants that share a common three-ring backbone. While primary astrocyte cultures were chosen as a practical system in which to conduct the initial screens, it is important to realize the many ways in which cultured astrocytes differ from astrocytes in vivo, which exist in a complex anatomical and physiological context. One compound, clomipramine, was selected to investigate whether it could also reduce the expression of GFAP in vivo. Single doses of clomipramine resulted in transient reductions in GFAP promoter activity. Of most importance, repeated administration of clomipramine over a 3-week period resulted in a substantial reduction in the total level of GFAP.
Clomipramine, like other tricyclic antidepressants, has diverse effects on the central nervous system that are often attributed to the inhibition of uptake of serotonin and to a lesser extent norepinephrine (21–23). Which of these activities are responsible for the antidepressant effects is not clear. In addition, the acute effects of clomipramine may be very different from chronic effects, where many changes in cell signaling pathways and gene expression may occur (24,25). It is interesting that clinical efficacy in humans typically requires treatment over a period of weeks, similar to the time frame in which changes take place in GFAP.
Clomipramine is currently approved by the FDA for the treatment of obsessive-compulsive disorder (26,27), but a number of off-label uses exist for conditions such as enuresis (28), narcolepsy/cataplexy (29), premature ejaculation (30), depression (31) and anxiety (32). As might be expected, clomipramine can have numerous potential side effects as well, including a spectrum of anti-cholinergic effects and induction of seizures (24). Some side effects, such as hyperglycaemia, could reflect direct actions on serotonin receptors (33), some of which are present on astrocytes (34). Whether the concentrations we found effective in cultured astrocytes (5–10 µm) accurately predict the responsiveness of astrocytes in vivo is not known. However, we found that the concentration of clomipramine in brain at least transiently reaches this range following IP injection. The plasma concentration of clomipramine (sometimes considered in combination with the active metabolite, desmethyl-clomipramine) that is considered therapeutic in humans is in the range of 0.3–1.75 µm (31) (reviewed in 24,25,35), close to the levels necessary for suppression of the GFAP promoter in mice.
We do not yet know how clomipramine causes the reduction in GFAP levels. Its effect on primary astrocytes suggests a direct action on these cells. The transcriptional regulation of GFAP is still poorly understood. The transcription factors Stat3 (36,37), NF1 (38), NFκB (38), AP-1 (39) and pCREB (40) have all been proposed to contribute to its regulation. However, most of these studies were performed in cell culture and await confirmation in vivo, where regulatory mechanisms can be quite different (41). In addition, the actions of clomipramine in vivo could be further augmented or even supplanted by indirect actions via neurons or microglia (42).
Any attempt to reduce the overall levels of GFAP protein must consider the rates of both synthesis and degradation. One might assume that the dual luciferase reporter mice using the GFAP promoter indirectly monitor changes in synthesis and respond on a relatively short time scale to various stimuli affecting transcription. Indeed, the effects of clomipramine on GFAP promoter activity were seen within hours of drug administration. However, if GFAP is similar to neurofilaments in being a long-lived protein (43,44), lengthy periods of time may have to elapse before significant reductions in protein take place, even in the face of substantial reductions in synthetic rate. The actual degradation rate of GFAP has been documented only three times, with inconsistent results. Using primary cultures of rat astrocytes, Chiu and Goldman (45) found biphasic kinetics of GFAP degradation, with half-times on the order of ∼12–18 h for 40% of the GFAP and 8 days for the remainder, whereas Morrison et al. (46) found only monophasic kinetics, with a half-life of 7.5 days. More relevant for eventual therapeutic applications is the GFAP degradation rate in vivo. This rate has only been measured once, in mouse spinal cord, where GFAP displayed a half-life of approximately 9 weeks (16). In this light, our finding that only 3 weeks of treatment with clomipramine leads to a ∼50% reduction in GFAP protein is surprising. Our results suggest either that GFAP half-life in vivo is considerably shorter than previously observed or that turnover varies by anatomic site or background strain of mouse. Alternatively, clomipramine may synergistically effect both synthesis and degradation so as to accelerate the drop in total protein levels.
Our findings raise a number of interesting questions. First, the initial screen employed primary cultures of astrocytes derived from wild-type mice, with normal levels of GFAP expression. We subsequently found that clomipramine was at least partially effective in astrocytes containing elevated levels of GFAP and Rosenthal fibers, as occurs in Alexander disease. However, it is not known whether clomipramine and other candidate compounds will be useful for the broad category of disorders with reactive gliosis, where the reactive phenotypes and changes in gene expression may be different from Alexander disease (2,47–49). Second, we have currently studied the effects of only one compound in vivo (clomipramine), and several other compounds shown in Table 2 appear worthy of further investigation. For instance, the top-ranked compound from the culture-based screen, diaziquone, suppressed GFAP promoter activity by over 80%. However, diaziquone is an alkylating agent that was initially developed as an anti-neoplastic agent and has a very short half-life in plasma of only 33 min (50), both features rendering it less appealing for long-term administration. Decisions about which other compounds from Table 2 should be pursued should take into account a number of factors, including their ability to penetrate the blood–brain arrier and range of potential side effects.
MATERIALS AND METHODS
Primary astrocyte culture and compound treatment
Enriched cortical primary astrocyte cultures were obtained from FVB-Tg(GFAP-luc,GAPDH-luc)172.9Mes/J mice (JR#9638) at post-natal days 1–2 and grown as previously described (14,20). After 2–3 weeks, cultures were treated with 0.25% trypsin-EDTA (Invitrogen) to yield a single cell suspension and washed five times with DMEM (Invitrogen). Cell density was measured with the Vi-CELL cell counter (Beckman Coulter) or hemocytometer by the trypan-blue exclusion method. Cells were plated in poly-d-lysine-coated white 96-well plates (Becton Dickinson) for luciferase activity assay or poly-d-lysine-coated black-wall/clear-bottom 96-well plates (Beckton Dickinson) for cell-based ELISA assay. Under these conditions, the cell cultures typically consist of >95% GFAP-positive cells. For the primary and secondary screens, cells were plated at a density of 5000 cells/well in 100 µl media with the Biomek FX liquid handler (Beckman Coulter) at the Small Molecule Screening Facility (University of Wisconsin-Madison). Cells were maintained in DMEM supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Candidate drugs and compounds for screening were obtained from two sources, the Prestwick Chemical Library® (http://www.prestwickchemical.fr/) and the Spectrum collection (http://www.msdiscovery.com/spectrum.html), both available as 10 mm stocks in DMSO. These stocks were further diluted to 1 mm in DMSO for long-term storage. For simplicity of presentation, all members of these two libraries will be referred to as ‘compounds’ in this report.
For both the primary and secondary screens, 80 compounds were screened per 96-well plate. Two columns (eight wells each) were reserved as controls. In the luciferase assay, these two columns served as positive controls (i.e. maximum GFAP signal), consisting of astrocytes grown in the absence of any compound. In pilot studies, we found that background in the luciferase assay (either from empty wells or wells containing non-transgenic astrocytes) was essentially zero, so no negative control wells were deemed necessary. In the cell-based ELISA, one column served as positive controls (again, astrocytes grown in the absence of compounds), and one column as negative controls (no astrocytes). Pilot studies using cells from GFAP-null mice (51) showed that ∼10% of the signal obtained from wells with wild-type astrocytes represented background. For simplicity in the primary screen, we used wells devoid of cells to define ELISA signal from background. Treatment with compounds commenced at 24 h after dispersion into the 96-well plates (passage 2), and continued for 48 h, at a single concentration of 10 m in medium with 1% DMSO. In subsequent dose–response and cytotoxicity assays, cells were exposed to a range of concentrations of selected compounds (0.1–20 m) for varying lengths of time. Investigators were blinded to the identity of compounds until the primary screens were completed.
Luciferase activity assay
For detection of firefly luciferase activity in cultured astrocytes, the manufacturer's protocol (E 2610, Promega) was modified and optimized to give reliable signal intensities in a 96-well format. After exposure to compounds, luciferase activity in the primary and secondary screenings was determined by adding 30 µl of Bright-Glo reagent (Promega) to the 100 µl of media and cells of each well in the white 96-well plates with the Biomek FX liquid handler (Beckman Coulter). After incubation for 1 min at RT, signals were measured with the Safire II plate reader using Magellan software (Tecan). For some assays that were aimed at confirming results of the initial screens, the same amount of Bright-Glo reagent was applied to each well in 96-well plates and signals were detected with the GloRunner Microplate Luminometer (Turner Biosystems). For the detection of both firefly and Renilla luciferase activities in tissues from dual transgenic mice, the Dual-glo luciferase assay was performed as previously described (14).
Cell based ELISA for quantitation of GFAP
For the primary and secondary screens, 5000 cells were plated per well in poly-d-lysine-coated clear-bottom/black-wall 96-well plates (Becton Dickinson) and treated with compounds for 48 h as described above. Cells were then fixed with 100% methanol for 20 min at RT. Cells were then rinsed five times with 0.1% Triton X-100/PBS for 5 min per wash. After blocking with LI-COR Odyssey blocking buffer® (LI-COR) at RT for 1.5 h, cells were incubated with rabbit anti-GFAP antibody (1:5000, DAKO) diluted in Odyssey blocking buffer® overnight at 4°C. Plates were rinsed five times with 0.1% Tween-20/PBS for 5 min per wash and incubated with IRDye™ 800CW goat anti-rabbit (1:800, LI-COR) diluted in Odyssey blocking buffer® containing 0.5% Tween-20 for 1 h at RT. Cells were washed five times for 5 min each with 0.1% Tween-20/PBS. Plates were scanned with the Odyssey® Infrared Imaging System (LI-COR) using the 800 nm channel. Liquid handling was performed using the µFill reagent dispenser (Bio-Tek Instruments, Inc.).
Cell viability
Cells were plated in poly-d-lysine-coated white bottom 96-well plates at a density of 5000 cells/well. After exposure to each compound (duplicate wells per compound), 50 µl of CellTitier-Glo Luminescent Cell Viability Assay reagent (G7571, Promega) was added to the 100 µl of media present in each well, and the luminescent signal (reflecting intracellular ATP level) was detected with either a Victor 3-V plate reader (Perkin Elmer) or GloRunner Microplate Luminometer (Turner Biosystems). Cell viability was expressed as a percent of non-treated control cells.
Sandwich ELISA for quantitation of GFAP protein
Cells grown in poly-d-lysine-coated clear-bottom 96-well plates (Becton Dickinson) were washed three times in PBS and then lysed at RT in 1% SDS/PBS, 2 mm EDTA and 50 mm Tris, pH 7.5, supplemented with Complete Proteinase Inhibitor Cocktail (Roche Applied Biosciences, cat. #11836145001) (20). The extracts were harvested from each well and the total GFAP content of each well was measured by a sandwich ELISA as described previously (20). The ELISA signals were normalized to total protein concentration of comparable wells from replicate 96-well plates, lysed in 1% SDS-proteinase K, using the BCA Protein Assay Kit (Thermo Scientific).
Immunofluorescent microscopy
Cells were grown in poly-lysine-coated four-well chamber slides (Nunc Nalge) at a density of 40 000 cells/well. After various periods of culture and drug treatment, slides were rinsed twice in PBS and fixed with 4% paraformaldehyde for 10 min at RT. Cells were then rinsed three times in PBS and permeabilized with 100% EtOH for 2 min. After more rinses in PBS, slides were blocked with 5% donkey serum in 0.1% Triton X-100/PBS for 1 h at RT and then incubated with a mouse monoclonal anti-GFAP antibody (1:1000, Chemicon) overnight at 4°C. The cells were then rinsed three times in PBS and incubated with Alexa-488 conjugated anti-mouse antibody (1:500, Molecular Probes). Coverslips were applied using Vectashield hard-set mounting medium containing DAPI (Vector Laboratories). Images were obtained from a Nikon C1 confocal microscope at the Cellular and Molecular Neuroscience Core of the Waisman Center at the University of Wisconsin-Madison. The proportion of GFAP immunoreactive cells containing GFAP inclusions was determined at each time point by counting 71–251 cells taken from three independent fields.
Clomipramine treatments of mice
In acute dosing studies, adult mice from the TgGFAP/GAPDH-Dual-Glo line (Tg172.9, FVB/N background) (males, 3–4 months old) were treated once with 0–40 mg/kg clomipramine (Sigma, C7291) freshly dissolved in water, via intra-peritoneal injections (4 mg/ml stock concentration, 10 ml/kg injection volume). At various times after injection, brains were harvested for the measurement of GFAP promoter activity using the Dual-Glo luciferase assay and GFAP protein using a sandwich ELISA as previously described (14). In some animals, clomipramine concentrations in brain and serum (collected from axillary arteries) were determined by HPLC analysis at the Pharmacokinetics Core Facility of the UW-Madison Comprehensive Cancer Center. In chronic dosing studies, adult mice of the same transgenic line (females, 2–4-month old at the onset of treatment) were given IP injections of clomipramine at 40 mg/kg, every other day for 3 weeks. Injections were given in the afternoon, between 3 and 5 PM. Controls were transgenic littermates injected with vehicle alone. At the end of the 3-week course of injections (and 1 day after the last injection), brains were harvested for the analysis of GFAP promoter activity and protein levels as described above. All animal studies were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee.
Data analysis
For the results of luciferase assays, heat maps (Java Treeview) (52) were generated to indicate the percent reduction in GFAP promoter activity. Percent reduction was displayed by a decrease in luminescent signals of compound treated wells compared with untreated controls with the Java Treeview 1.1.1 software (General Public License). Potency of drugs referred to as IC50 was calculated by generating percent reduction curves fitted for the plots with the XLfit Software (IDBS). For comparing two groups, statistical significance was considered if P < 0.05 by t-test or two-way ANOVA (Prism 3.02, GraphPad).
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the National Institutes of Health [HD046599, NS060120 and NS042803 to A.M., HD03352 to the Waisman Center and CA014520 to the University of Wisconsin Cancer Center]. Additional support was provided by the Palamaro and Juanma Funds and by the Wisconsin Alumni Research Foundation through the Lead Discovery Initiative. W.C. was supported in part by the Wayne and Jean Roper Distinguished Graduate Fellowship and by the Palamaro and Juanma Funds.
Supplementary Material
ACKNOWLEDGEMENT
We thank Denice Springman and Channi Kaur for technical assistance and Daniel M. Bolt for consultation on statistical analysis.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Eng L.F., Ghirnikar R.S., Lee Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem. Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. doi:10.1023/A:1007677003387. [DOI] [PubMed] [Google Scholar]
- 2.Eng L.F., Ghirnikar R.S. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. doi:10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 3.Brenner M., Johnson A.B., Boespflug-Tanguy O., Rodriguez D., Goldman J.E., Messing A. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 2001;27:117–120. doi: 10.1038/83679. [DOI] [PubMed] [Google Scholar]
- 4.Li R., Johnson A.B., Salomons G., Goldman J.E., Naidu S., Quinlan R., Cree B., Ruyle S.Z., Banwell B., D'Hooghe M., et al. GFAP mutations in infantile, juvenile, and adult forms of Alexander disease. Ann. Neurol. 2005;57:310–326. doi: 10.1002/ana.20406. doi:10.1002/ana.20406. [DOI] [PubMed] [Google Scholar]
- 5.Quinlan R.A., Brenner M., Goldman J., Messing A. GFAP and its role in Alexander disease. Exp. Cell Res. 2007;313:2077–2087. doi: 10.1016/j.yexcr.2007.04.004. doi:10.1016/j.yexcr.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagemann T.L., Connor J.X., Messing A. Alexander disease-associated glial fibrillary acidic protein mutations in mice induce Rosenthal fiber formation and a white matter stress response. J. Neurosci. 2006;26:11162–11173. doi: 10.1523/JNEUROSCI.3260-06.2006. doi:10.1523/JNEUROSCI.3260-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heemskerk J., Tobin A.J., Bain L.J. Teaching old drugs new tricks. Trends Neurosci. 2002;25:494–496. doi: 10.1016/s0166-2236(02)02236-1. doi:10.1016/S0166-2236(02)02236-1. [DOI] [PubMed] [Google Scholar]
- 8.Rothstein J.D., Patel S., Regan M.R., Haenggeli C., Huang Y.H., Bergles D.E., Jin L., Hoberg M.D., Vidensky S., Chung D.S., et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. doi:10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 9.Piccioni F., Roman B.R., Fischbeck K.H., Taylor J.P. A screen for drugs that protect against the cytotoxicity of polyglutamine-expanded androgen receptor. Hum. Mol. Genet. 2004;13:437–446. doi: 10.1093/hmg/ddh045. doi:10.1093/hmg/ddh045. [DOI] [PubMed] [Google Scholar]
- 10.Brenner M. Structure and transcriptional regulation of the GFAP gene. Brain Pathol. 1994;4:245–257. doi: 10.1111/j.1750-3639.1994.tb00840.x. doi:10.1111/j.1750-3639.1994.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 11.Laping N.J., Teter B., Nichols N.R., Rozovsky I., Finch C.E. Glial fibrillary acidic protein: regulation by hormones, cytokines, and growth factors. Brain Pathol. 1994;4:259–275. doi: 10.1111/j.1750-3639.1994.tb00841.x. doi:10.1111/j.1750-3639.1994.tb00841.x. [DOI] [PubMed] [Google Scholar]
- 12.Mucke L., Oldstone M.B.A., Morris J.C., Nerenberg M.I. Rapid activation of astrocyte-specific expression of GFAP-lacZ transgene by focal injury. New Biol. 1991;3:465–474. [PubMed] [Google Scholar]
- 13.Brenner M., Kisseberth W.C., Su Y., Besnard F., Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J. Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho W., Hagemann T.L., Johnson D.A., Johnson J.A., Messing A. Dual transgenic reporter mice as a tool for monitoring expression of GFAP. J. Neurochem. 2009;110:343–351. doi: 10.1111/j.1471-4159.2009.06146.x. doi:10.1111/j.1471-4159.2009.06146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J.H., Chung T.D., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high-throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. doi:10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 16.DeArmond S.J., Lee Y.-L., Kretzschmar H.A., Eng L.F. Turnover of glial filaments in mouse spinal cord. J. Neurochem. 1986;47:1749–1753. doi: 10.1111/j.1471-4159.1986.tb13084.x. doi:10.1111/j.1471-4159.1986.tb13084.x. [DOI] [PubMed] [Google Scholar]
- 17.Hagemann T.L., Gaeta S.A., Smith M.A., Johnson D.A., Johnson J.A., Messing A. Gene expression analysis in mice with elevated glial fibrillary acidic protein and Rosenthal fibers reveals a stress response followed by glial activation and neuronal dysfunction. Hum. Mol. Genet. 2005;14:2443–2458. doi: 10.1093/hmg/ddi248. doi:10.1093/hmg/ddi248. [DOI] [PubMed] [Google Scholar]
- 18.Tang G., Xu Z., Goldman J.E. Synergistic effects of the SAPK/JNK and the proteasome pathway on glial fibrillary acidic protein (GFAP) accumulation in Alexander disease. J. Biol. Chem. 2006;281:38634–38643. doi: 10.1074/jbc.M604942200. doi:10.1074/jbc.M604942200. [DOI] [PubMed] [Google Scholar]
- 19.Tian R.J., Gregor M., Wiche G., Goldman J.E. Plectin regulates the organization of glial fibrillary acidic protein in Alexander disease. Am. J. Pathol. 2006;168:888–897. doi: 10.2353/ajpath.2006.051028. doi:10.2353/ajpath.2006.051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho W., Messing A. Properties of astrocytes cultured from GFAP over-expressing and GFAP mutant mice. Exp. Cell Res. 2009;315:1260–1272. doi: 10.1016/j.yexcr.2008.12.012. doi:10.1016/j.yexcr.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlsson A., Corrodi H., Fuxe K., Hökfelt T. Effect of antidepressant drugs on the depletion of intraneuronal brain 5-hydroxytryptamine stores caused by 4-methyl-alpha-ethyl-meta-tyramine. Eur. J. Pharmacol. 1969;357:366. doi: 10.1016/0014-2999(69)90113-7. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson A. Structural specificity for inhibition of [14C]-5-hydroxytryptamine uptake by cerebral slices. J. Pharm. Pharmacol. 1970;22:729–732. doi: 10.1111/j.2042-7158.1970.tb08419.x. [DOI] [PubMed] [Google Scholar]
- 23.Ross S.B., Renyi A.L., Ogren S.O. Inhibition of the uptake of noradrenaline and 5-hydroxytryptamine by chlorphentermine and chlorimipramine. Eur. J. Pharmacol. 1972;17:107–112. doi: 10.1016/0014-2999(72)90276-2. doi:10.1016/0014-2999(72)90276-2. [DOI] [PubMed] [Google Scholar]
- 24.Baldessarini R.J. In: The Pharmacological Basis of Therapeutics. Brunton L.R., Lazo J.S., Parker K.L., editors. New York: McGraw-Hill; 2006. pp. 429–459. [Google Scholar]
- 25.Wille S.M., Cooreman S.G., Neels H.M., Lambert W.E. Relevant issues in the monitoring and the toxicology of antidepressants. Crit. Rev. Clin. Lab. Sci. 2008;45:25–89. doi: 10.1080/10408360701713112. doi:10.1080/10408360701713112. [DOI] [PubMed] [Google Scholar]
- 26.Stern R.S., Marks I.M., Mawson D., Luscombe D.K. Clomipramine and exposure for compulsive rituals: II. Plasma levels, side effects and outcome. Br. J. Psychiatry. 1980;136:161–166. doi: 10.1192/bjp.136.2.161. doi:10.1192/bjp.136.2.161. [DOI] [PubMed] [Google Scholar]
- 27.Insel T.R., Murphy D.L., Cohen R.M., Alterman I., Kilts C., Linnoila M. Obsessive-compulsive disorder. A double-blind trial of clomipramine and clorgyline. Arch. Gen. Psychiatry. 1983;40:605–612. doi: 10.1001/archpsyc.1983.04390010015002. [DOI] [PubMed] [Google Scholar]
- 28.Glazener C.M., Evans J.H., Peto R.E. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst. Rev. 2003;3:CD002117. doi: 10.1002/14651858.CD002117. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro W.R. Treatment of cataplexy with clomipramine. Arch. Neurol. 1975;32:653–656. doi: 10.1001/archneur.1975.00490520023002. [DOI] [PubMed] [Google Scholar]
- 30.Haensel S.M., Rowland D.L., Kallan K.T. Clomipramine and sexual function in men with premature ejaculation and controls. J. Urol. 1996;156:1310–1315. doi:10.1016/S0022-5347(01)65576-9. [PubMed] [Google Scholar]
- 31.Faravelli C., Ballerini A., Ambonetti A., Broadhurst A.D., Das M. Plasma levels and clinical response during treatment with clomipramine. J. Affect. Disord. 1984;6:95–107. doi: 10.1016/0165-0327(84)90011-9. doi:10.1016/0165-0327(84)90011-9. [DOI] [PubMed] [Google Scholar]
- 32.Caillard V., Rouillon F., Viel J.F., Markabi S. Comparative effects of low and high doses of clomipramine and placebo in panic disorder: a double-blind controlled study. French University Antidepressant Group. Acta Psychiatr. Scand. 1999;99:51–58. doi: 10.1111/j.1600-0447.1999.tb05384.x. doi:10.1111/j.1600-0447.1999.tb05384.x. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto Y., Inoue K., Yamada J. The tricyclic antidepressant clomipramine increases plasma glucose levels of mice. J. Pharmacol. Sci. 2003;93:74–79. doi: 10.1254/jphs.93.74. doi:10.1254/jphs.93.74. [DOI] [PubMed] [Google Scholar]
- 34.Walz W. In: Neuroglia. Kettenmann H., Ransom B.R., editors. New York: Oxford University Press; 1995. pp. 346–353. [Google Scholar]
- 35.Balant-Gorgia A.E., Gex-Fabry M., Balant L.P. Clinical pharmacokinetics of clomipramine. Clin. Pharmacokinet. 1991;20:447–462. doi: 10.2165/00003088-199120060-00002. doi:10.2165/00003088-199120060-00002. [DOI] [PubMed] [Google Scholar]
- 36.Takizawa T., Nakashima K., Namihira M., Ochiai W., Uemura A., Yanagisawa M., Fujita N., Nakao M., Taga T. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. doi:10.1016/S1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 37.Herrmann J.E., Imura T., Song B.B., Qi J.W., Ao Y., Nguyen T.K., Korsak R.A., Takeda K., Akira S., Sofroniew M.V. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. doi:10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krohn K., Rozovsky I., Wals P., Teter B., Anderson C.P., Finch C.E. Glial fibrillary acidic protein transcription responses to transforming growth factor-β1 and interleukin-1β are mediated by a nuclear factor-1-like site in the near-upstream promoter. J. Neurochem. 1999;72:1353–1361. doi: 10.1046/j.1471-4159.1999.721353.x. doi:10.1046/j.1471-4159.1999.721353.x. [DOI] [PubMed] [Google Scholar]
- 39.Masood K., Besnard F., Su Y., Brenner M. Analysis of a segment of the human glial fibrillary acidic protein gene that directs astrocyte-specific transcription. J. Neurochem. 1993;61:160–166. doi: 10.1111/j.1471-4159.1993.tb03551.x. doi:10.1111/j.1471-4159.1993.tb03551.x. [DOI] [PubMed] [Google Scholar]
- 40.Besnard F., Brenner M., Nakatani Y., Chao R., Purohit H.J., Freese E. Multiple interacting sites regulate astrocyte-specific transcription of the human gene for glial fibrillary acidic protein. J. Biol. Chem. 1991;266:18877–18883. [PubMed] [Google Scholar]
- 41.Lee Y., Su M., Messing A., Brenner M. Astrocyte heterogeneity revealed by expression of a GFAP-LacZ transgene. Glia. 2006;53:677–687. doi: 10.1002/glia.20320. doi:10.1002/glia.20320. [DOI] [PubMed] [Google Scholar]
- 42.Hwang J., Zheng L.T., Ock J., Lee M.G., Kim S.H., Lee H.W., Lee W.H., Park H.C., Suk K. Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology. 2008;55:826–834. doi: 10.1016/j.neuropharm.2008.06.045. doi:10.1016/j.neuropharm.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 43.Millecamps S., Gowing G., Corti O., Mallet J., Julien J.P. Conditional NF-L transgene expression in mice for in vivo analysis of turnover and transport rate of neurofilaments. J. Neurosci. 2007;27:4947–4956. doi: 10.1523/JNEUROSCI.5299-06.2007. doi:10.1523/JNEUROSCI.5299-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan A.D., Sasaki T., Rao M.V., Kumar A., Kanumuri V., Dunlop D.S., Liem R.K., Nixon R.A. Neurofilaments form a highly stable stationary cytoskeleton after reaching a critical level in axons. J. Neurosci. 2009;29:11316–11329. doi: 10.1523/JNEUROSCI.1942-09.2009. doi:10.1523/JNEUROSCI.1942-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiu F.C., Goldman J.E. Synthesis and turnover of cytoskeletal proteins in cultured astrocytes. J. Neurochem. 1984;42:166–174. doi: 10.1111/j.1471-4159.1984.tb09713.x. doi:10.1111/j.1471-4159.1984.tb09713.x. [DOI] [PubMed] [Google Scholar]
- 46.Morrison R.S., de Vellis J., Lee Y.L., Bradshaw R.A., Eng L.F. Hormones and growth factors induce the synthesis of glial fibrillary acidic protein in rat brain astrocytes. J. Neurosci. Res. 1985;14:167–176. doi: 10.1002/jnr.490140202. doi:10.1002/jnr.490140202. [DOI] [PubMed] [Google Scholar]
- 47.Eddleston M., Mucke L. Molecular profile of reactive astrocytes–Implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. doi:10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pekny M., Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. doi:10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 49.Sofroniew M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. doi:10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schilsky R.L., Kelley J.A., Ihde D.C., Howser D.M., Cordes R.S., Young R.C. Phase I trial and pharmacokinetics of aziridinylbenzoquinone (NSC 182986) in humans. Cancer Res. 1982;42:1582–1586. [PubMed] [Google Scholar]
- 51.McCall M.A., Gregg R.G., Behringer R.R., Brenner M., Delaney C.L., Galbreath E.J., Zhang C.L., Pearce R.A., Chiu S.Y., Messing A. Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc. Natl Acad. Sci. USA. 1996;93:6361–6366. doi: 10.1073/pnas.93.13.6361. doi:10.1073/pnas.93.13.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saldhana A.J. Java Treeview-extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.