Abstract
Peripheral B-cell numbers are tightly regulated by homeostatic mechanisms that influence the transitional and mature B-cell compartments and dictate the size and clonotypic diversity of the B-cell repertoire. B-lymphocyte stimulator (BLyS, a trademark of Human Genome Sciences, Inc.) plays a key role in regulating peripheral B-cell homeostasis. CD22 also promotes peripheral B-cell survival through ligand-dependent mechanisms. The B-cell subsets affected by the absence of BLyS and CD22 signals overlap, suggesting that BLyS- and CD22-mediated survival are intertwined. To examine this, the effects of BLyS insufficiency following neutralizing BLyS mAb treatment in mice also treated with CD22 ligand-blocking mAb were examined. Combined targeting of the BLyS and CD22 survival pathways led to significantly greater clearance of recirculating bone marrow, blood, marginal zone and follicular B cells than either treatment alone. Likewise, BLyS blockade further reduced bone marrow, blood and spleen B-cell numbers in CD22−/− mice. Notably, BLyS receptor expression and downstream signaling were normal in CD22−/− B cells, suggesting that CD22 does not directly alter BLyS responsiveness. CD22 survival signals were likewise intact in the absence of BLyS, as CD22 mAb treatment depleted blood B cells from mice with impaired BLyS receptor 3 (BR3) signaling. Finally, enforced BclxL expression, which rescues BR3 impairment, did not affect B-cell depletion following CD22 mAb treatment. Thus, the current studies support a model whereby CD22 and BLyS promote the survival of overlapping B-cell subsets but contribute to their maintenance through independent and complementary signaling pathways.
Keywords: B-cell depletion, B-cell homeostasis, BLyS/BAFF, CD22, mAb therapy
Introduction
In addition to the B-cell antigen receptor (BCR), cell surface CD22, CD40 and serum B-lymphocyte stimulator (BLyS, a trademark of Human Genome Sciences, Inc., Rockville, MD, USA; also known as BAFF, TALL-1, CD257 and Tnfsf13b) are the three predominant receptors/ligands necessary for mature B-cell survival in the periphery of mice (1–8). As an example, B cells develop normally in CD22-deficient (CD22−/−) mice, but recirculating bone marrow, blood and spleen marginal zone (MZ) B-cell numbers are significantly reduced, along with significantly enhanced spleen B-cell turnover (2, 9–11). CD22 is an important negative regulator of BCR signal transduction. Nonetheless, the effect of CD22 on peripheral B-cell survival is thought to be independent of the BCR and reliant on CD22–ligand interactions. B cells expressing CD22 with mutated ligand-binding domains (CD22AA and CD22Δ1-2 lines) cannot interact with broadly distributed α2,6-linked sialic acid-bearing CD22 ligands, but normal signal regulation through the CD22 cytoplasmic domain remains intact. Nonetheless, CD22AA and CD22Δ1-2 mice have reduced bone marrow, blood and MZ B-cell numbers and shortened peripheral B-cell half-lives (12). Blocking CD22–ligand interactions with mAbs also induces striking but reversible reductions in the bone marrow, blood and MZ B-cell compartments, in addition to enhancing B-cell turnover in vivo (13). Thereby, CD22 predominantly influences normal peripheral B-cell longevity in vivo through unidentified ligand-dependent mechanisms, which appear distinct from its role in regulating BCR and CD19 signaling (14).
BLyS profoundly influences peripheral B-cell homeostasis (4, 15–17). BLyS binds to three members of the tumor necrosis factor family of receptors: BLyS receptor 3 (BR3/BAFF-R), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) and B-cell maturation antigen (BCMA) (18–20). Mice deficient in BLyS or BLyS-induced signaling through BR3 have severely decreased numbers of peripheral B cells (21, 22). Sequestration of BLyS with anti-BLyS mAbs or soluble receptors (TACI-Ig and BR3-Fc fusion proteins) also leads to rapid but reversible reductions in peripheral and recirculating B cells, with no change in T cell numbers (23, 24). Notably, MZ B cells and mature recirculating B cells in the bone marrow are largely absent without intact BLyS signaling (4, 5, 22, 23). When combined with BCR ligation, BLyS acts as a potent B-cell co-stimulator (16, 17) and can also rescue self-reactive B cells from BCR-induced death (25). BR3 ligation up-regulates expression of pro-survival Bcl-2 family member proteins and promotes NF-κB activation, both of which increase B-cell survival (26). Thus, BLyS and CD22–ligand interactions are required for normal peripheral B-cell survival in vivo. In contrast, CD40 engagement regulates the survival of activated B cells, primarily within germinal centers (6, 7).
It is important to identify the signals that regulate B-cell homeostasis as alterations in B-cell survival signals may lead to the development of autoimmune disease. As such, transgenic mice that over-express BLyS develop autoimmune symptoms resembling those of systemic lupus erythematosus (27, 28). Likewise, serum BLyS levels are increased in autoimmune patients (27, 29, 30) and mice (31). The effect of directly killing B cells, as with some mAb treatments, is under investigation for the treatment of autoimmunity, with the idea that inducing autoreactive B-cell death will ameliorate pathogenic self-reactivity. In support of this, extensive depletion of mature B cells using a therapeutic CD20 mAb has shown clinical efficacy in the treatment of patients with lymphoma or autoimmune disease (32, 33) and in mouse models of autoimmunity (34–36). Enhancing our understanding of the pathways that govern peripheral B-cell survival may provide additional therapeutic targets or suggest alternative mechanisms influencing the onset of autoimmunity. In addition, the B-cell subsets affected by CD22–ligand interactions or BLyS–BR3 signaling show significant overlap, suggesting that the survival cues provided by CD22 and BR3 are intertwined. However, it has not been determined whether the effects of simultaneous CD22 and BLyS blockade have overlapping or synergistic effects on B-cell survival. Therefore, the current study identified the B-cell subsets that are dependent on CD22 and/or BLyS survival signals and whether these pathways cooperatively promote optimal B-cell survival.
Methods
Mice and mAb treatments
C57Bl/6 mice were from the mouse repository at the National Cancer Institute (NCI; Frederick, MD, USA). A/J and A/WySnJ mice were from The Jackson Laboratory (Bar Harbor, ME, USA). CD22−/− and CD22Δ1-2 mice on a B6 genetic background were as described in refs (12, 37). BclxL.Tg+ mice were generously provided by Dr Michael Farrar (University of Minnesota, Minneapolis, MN, USA) (38). All mice were housed in a specific pathogen-free barrier facility with end-point analyses carried out between 8 and 14 weeks of age. All studies and procedures were approved by the Duke University Animal Care and Use Committee.
Sterile and endotoxin-free CD22 (clone MB22-10, mouse) (13), anti-BLyS (clone 10F4, hamster; Human Genome Sciences, Inc.) (39), CD20 (MB20-11, mouse) (40) and control (hamster IgG or mouse IgG2a) mAbs were administered at 100 μg per mouse through lateral tail veins.
B-cell purification and proliferation assays
Single-cell suspensions of tissue leukocytes were generated by gentle disruption between frosted glass sides followed by RBC lysis. B cells were enriched from single-cell suspensions using a B Cell Isolation Kit (Miltenyi Biotec Inc., Auburn, CA, USA) according to the manufacturer's instructions. This method typically yielded a lymphocyte population that was >95% B220+ as determined by immunofluorescence staining and flow cytometry analysis. Purified B cells were cultured in complete RPMI 1640 medium containing 10% FBS, 1% HEPES, 1% L-glutamate, 1% Pen/Strep and 0.1% 2-mercapthoethanol. Recombinant murine BLyS (R&D Systems, Minneapolis, MN, USA), goat F(ab')2 anti-mouse IgM antibody (Cappel; ICN Biomedicals, Irvine, CA, USA) and anti-mouse CD40 mAb (clone HM40-3; hamster, no azide/endotoxin-free; BD Pharmingen, San Jose, CA, USA) were also added to the cultures as indicated. Cellular division was quantified by the incorporation of 3H-thymidine (1 μCi) added during the final 18 h of culture as measured by scintillation counting. Alternatively, B cells were labeled with 1 μM CFSE using a Vybrant CFDA-SE Cell Tracer Kit (Molecular Probes, Eugene, OR, USA) before culture, with the relative number of viable cells and the intensity of CFSE staining subsequently assessed by flow cytometry. Viability was determined by Trypan Blue exclusion or by 7-AAD staining in combination with forward-versus-side light scatter properties as determined by flow cytometry analysis.
Serum BLyS levels
Serum BLyS levels were measured by ELISA, as previously described (24). Assay plates were coated with mouse (m) BR3:human (hu) Fc fusion protein (Alexis Biochemicals, Plymouth Meeting, PA, USA) and captured BLyS was subsequently detected with a biotinylated anti-mouse BLyS mAb (clone 16D7; Human Genome Sciences, Inc.) followed by streptavidin-horse radish peroxidase detection. Results were interpolated from a mBLyS standard curve that was run on each plate.
Real-time PCR quantification of BLyS receptor transcript levels
Total RNA from 5 × 106 purified splenic+ B cells was isolated using TRIZOL Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Reverse transcribed cDNA was generated using random primers and equal amounts of RNA template. Relative amounts of transcripts for the BR3, TACI and BCMA BLyS receptors were then determined by real-time PCR using the hot-start kit, LightCycler® FastStart DNA MasterPLUS SYBR Green I, and the LightCycler Real Time PCR system (Roche, Indianapolis, IN, USA). Primer sequences used for amplification are as follows: (reference gene) CD20—5′-cctcg ccatg caacc tgctcc-3′ and 5′-ctgca gctgc cagga gtgat cc-3′; BR3—5′-gtgcc caccc agtgc aatca ga-3′ and 5′-tatgt ccagt gtccg gcgtg tg-3′; TACI—5′-gctat ggcat tctgc cccaa aga-3′ and 5′-tctgt acagg tgcgc tggct cctct-3′ and BCMA—5′-cctgc aacct gtcag cctta ctg-3′ and 5′-cctgc aacct gtcag cctta ctg-3′. Pairwise comparisons between BR3, TACI or BCMA cDNA abundance relative to CD20 cDNA abundance were made with the Relative Expression Software Tool (REST©) program (http://rest.gene-quantification.info/) for group-wise comparisons and statistical analysis. Significant differences were identified using a pairwise fixed randomization test; *P < 0.05.
Western blot analysis
Purified splenic B cells were cultured for 18 h in medium alone or in medium containing BLyS (50 ng ml−1). The cells were then lysed on ice for >2 h in TRIS buffer containing 1% NP-40, 150 mM NaCl, 0.5 M EDTA and 0.5 M NaF, supplemented with protease inhibitor cocktail, set III (Calbiochem; EMD Biosciences, San Diego, CA, USA). Cellular debris was removed by centrifugation. Whole-cell lysates were boiled for ≥5 min in reducing buffer prior to separation by PAGE on a Criterion Pre-Cast Gel (10% acrylamide). Following transfer to nitrocellulose, membranes were blotted for NF-κB2 (p100 and p52; Cell Signaling Technology, Danvers, MA, USA) or mouse β-actin (Sigma–Aldrich, St Louis, MO, USA). The membranes were then incubated with donkey anti-rabbit or goat anti-mouse antibody–HRP conjugates (Jackson ImmunoResearch, Inc., West Grove, PA, USA). Protein bands were visualized by enhanced chemiluminescence using the SuperSignal® West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL, USA).
Antibodies and flow cytometry analysis
Single-cell suspensions of mouse leukocytes were stained with predetermined optimal antibody concentrations. For intracellular staining, lymphocytes were fixed and permeabilized in BD Fix/Perm Buffer at 25°C (BD Pharmingen) and stained with predetermined concentrations of FITC-conjugated anti-mouse activated Caspase-3 mAb or anti-mouse Bcl-2 mAb (BD Pharmingen) in BD Perm/Wash Buffer at 4°C for >25 min. Data were collected on a FACSScan™, FACSCalibur™ or FACSCanto™ flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using Flowjo Software (TreeStar, Inc., Ashland, OR, USA). Antibodies used for surface staining included FITC-, PE-, PECy5- or APC-conjugated anti-mouse B220 (clone RA3-6B2), CD21/35 (7G6), CD23 (B3B4) and CD1d (1B1) mAbs from BD Pharmingen; goat anti-mouse IgM or IgD antibody (SouthernBiotech, Birmingham, AL, USA); anti-mouse IgM (11/41), CD21/35 (eBio8D9), CD93 (AA4.1), CD5 (53-7.3), CD11b (M1/70), CD19 (eBio1D3) and MHC class II (I-Ab, clone MC/114) from eBioscience, Inc. (San Diego, CA, USA).
Statistical analysis
All data are shown as mean ± SEM, unless otherwise noted. The Student's t-test was used to determine the significance of differences between sample means.
Results
B-cell depletion following anti-BLyS and CD22 mAb treatments
Whether CD22 and BLyS regulate the survival of independent B-cell subsets or affect overlapping populations was examined by disrupting both survival pathways simultaneously. Mice were treated with optimal concentrations of mAbs that sequester endogenous BLyS protein (clone 10F4) (39) or disrupt CD22–ligand interactions (clone MB22-10) (13). Anti-BLyS treatment alone reduced the absolute number of recirculating blood B cells by >50% in 4 days, with the most dramatic reduction in both frequency and number observed at day 10 (Fig. 1A). CD22 mAb treatment induced a rapid decline in blood B-cell numbers within 1 day. By day 4, the reduction in blood B-cell frequency and numbers in CD22 mAb-treated mice was maximal, with >80% reduction in total numbers compared with control mAb-treated mice. Once the majority of B cells were depleted by a single mAb, additional B-cell clearance was less obvious with combined mAb treatments. However, overall B-cell depletion was greatest in mice treated with both anti-BLyS and CD22 mAbs with B220+ B cells representing <15 and <7% of blood lymphocytes by days 4 and 10, respectively, and remained low up to 10 days. Thus, combining CD22 and BLyS mAb treatments had greater B-cell depleting effects than either treatment alone.
Fig. 1.
Complementary CD22 and BLyS–BR3 survival signals regulate B-cell homeostasis. (A) Circulating B-cell numbers following CD22 and BLyS blockade. C57Bl/6 mice were given CD22 ligand-blocking (MB22-10, open circles), anti-BLyS (10F4, closed triangles) or control (closed circles) mAbs or combined CD22 plus anti-BLyS mAbs (open triangles) on day 0. Percentages and total numbers of blood B220+ lymphocytes were determined before and after treatment by immunofluorescence staining with flow cytometry analysis. (B) Tissue B-cell numbers following CD22 and BLyS blockade as in (A). Percentages and numbers of mature recirculating bone marrow cells (BM Mature; IgM+CD93−B220+), B220+ spleen or LN B cells, splenic CD21hiCD23−B220+ MZ B cells and peritoneal cavity (PC) CD11b−CD5−CD19+ B2, CD5+CD11b+CD19+ B1a (black bars) or CD5−CD11b+CD19+ B1b (white bars) B cells were determined on day 10 after mAb treatment by immunofluorescence staining with flow cytometry analysis. Symbols and bar graphs indicate means (±SEM) for three or more mice per treatment group. Significant decreases between control and depleting mAb-treated sample means are indicated; *P ≤ 0.05; **P ≤ 0.01. Significant differences between single mAb treatment and combined anti-BLyS/CD22 mAb treatment are also indicated; †P ≤ 0.05; ††P ≤ 0.01.
Tissue B cells were also depleted in mice given both anti-BLyS and CD22 mAbs. The number of mature bone marrow B cells was reduced by 49% following anti-BLyS mAb treatment, 77% in CD22 mAb-treated mice and 88% in mice that received both anti-BLyS and CD22 mAbs for 10 days (Fig. 1B). Spleen B220+ B-cell numbers were reduced by 75, 36 and 83% in anti-BLyS mAb, CD22 mAb and combined anti-BLyS/CD22 mAb-treated mice, respectively, relative to control mAb-treated mice. Spleen CD21hiCD23− MZ B-cell numbers were reduced by >90% under all conditions when compared with control mAb-treated mice. Anti-BLyS mAb reduced lymph node (LN) B220+ B-cell numbers by 94%, CD22 mAb by 66% and combined anti-BLyS/CD22 mAb treatments by 98% compared with control mAb-treated mice. Peritoneal B2 B cells were reduced by >75% of control numbers in anti-BLyS mAb or anti-BLyS/CD22 mAb-treated mice, whereas CD22 mAb treatment alone had a mild effect. Thus, the depletion of peripheral B-cell subsets was significantly greater following combined BLyS and CD22 blockade in comparison with either treatment alone.
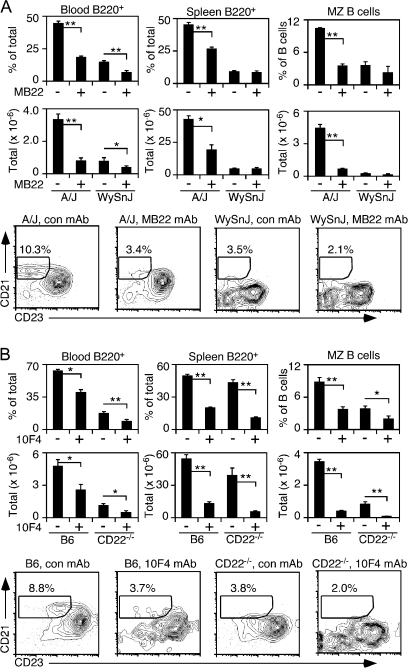
B-cell depletion following CD22 mAb treatment in A/WySnJ mice
To determine whether CD22–ligand binding affects B-cell survival through mechanisms distinct from the BLyS–BR3 pathway, the effect of CD22 mAb treatment was examined in BR3-mutant (A/WySnJ) mice that contain 90% fewer splenic B cells, including a severe reduction in MZ B cells when compared with parental A/J mice (21, 41). CD22 mAb blockade in A/J and A/WySnJ mice reduced the frequency and total number of blood B220+ B cells by ≥50% (Fig. 2A). Spleen B-cell numbers in CD22 mAb-treated A/J mice were reduced by 55% and MZ B-cell numbers were decreased by >85% in comparison with control mAb-treated A/J mice. However, spleen B220+ B cells and MZ B-cell numbers in A/WySnJ mice were not significantly altered following CD22 mAb treatment relative to control mAb treatment, likely masked by the severe survival defect of tissue-resident BR3-mutant B cells. Nonetheless, that CD22 mAb induced blood B-cell depletion in A/WySnJ mice demonstrates that CD22–ligand interactions are important for B-cell homeostasis, even in the absence of BR3 signaling.
Fig. 2.
Complementary CD22 and BLyS–BR3 survival signals regulate B-cell homeostasis in BR3 mutant (WySnJ) and CD22−/− mice. (A) Circulating and spleen B-cell frequencies and numbers in A/WySnJ and A/J mice following CD22 blockade. (B) Circulating and spleen B-cell frequencies and numbers in CD22−/− and C57BL/6 mice following anti-BLyS mAb treatment. (A and B) All mice were treated with MB22-10, anti-BLyS or control (con) mAb (100 μg) on days 0 and 5. Frequencies and total numbers of B220+ lymphocytes were determined on day 10, as in Fig. 1. Bar graphs show the means (±SEM) for three mice per treatment group and representative contour plots indicate the CD21hiCD23− gate used to identify splenic MZ B cells in each treatment group. Significant differences relative to control mAb treatment are indicated; *P ≤ 0.05; **P ≤ 0.01.
Anti-BLyS mAb depletes B cells in CD22−/− mice
To determine whether BLyS contributes to CD22−/− B-cell survival in vivo, wild-type C57Bl/6 and CD22−/− mice were given either anti-BLyS or control mAb, with blood and spleen B-cell frequencies and numbers determined 10 days later. Treatment of wild-type and CD22−/− mice with anti-BLyS mAb resulted in an ∼50% decrease in blood B220+ cell numbers when compared with control mAb-treated mice (Fig. 2B). In the spleen, anti-BLyS mAb treatment reduced B-cell numbers by 70–80% in both CD22−/− and wild-type mice. Anti-BLyS mAb treatment depleted CD21hiCD23− MZ phenotype B cells by ∼50% in both wild-type and CD22−/− mice despite the significant reduction in spleen MZ B-cell numbers already observed in CD22−/− mice (Fig. 2B). As a consequence, MZ B cells in anti-BLyS mAb-treated CD22−/− mice represented only 8% of the total MZ B-cell numbers found in control mAb-treated wild-type mice. Thereby, blood, spleen and MZ B cells from CD22−/− mice are reliant on BLyS for in vivo survival signals.
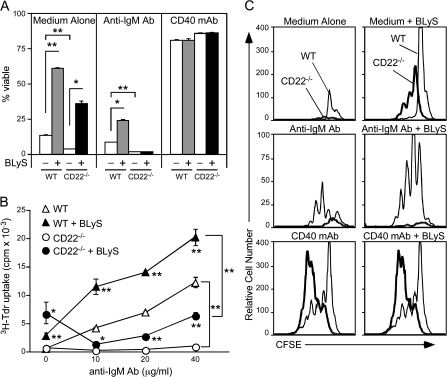
BLyS-mediated survival of CD22−/− B cells in vitro
The high B-cell turnover rate in CD22−/− mice suggests that endogenous BLyS is not sufficient to maintain normal B-cell homeostasis in the absence of CD22–ligand interactions (2, 10). To confirm this, whether BLyS enhances CD22−/− B-cell viability in vitro was examined by culturing spleen B cells from wild-type and CD22−/− mice for 3 days with medium alone, anti-IgM antibody to ligate the BCR or agonistic CD40 mAb, with or without the addition of BLyS. The percentage of viable B cells in each culture was then quantified by cell size and 7-AAD exclusion. Exogenous BLyS enhanced B-cell viability by 4.5-fold for wild-type B cells and 9.7-fold for CD22−/− B cells when compared with medium alone (Fig. 3A). In IgM-stimulated cultures, BLyS also enhanced the survival of wild-type B cells by 2.5-fold relative to cells cultured with anti-IgM antibody alone. In contrast, BCR ligation with anti-IgM antibody induced apoptosis in CD22−/− B cells after 3 days regardless of the presence or absence of BLyS (Fig. 3A). CD40 mAb-induced survival of both wild-type and CD22−/− B cells was not further enhanced by the addition of BLyS to the cultures.
Fig. 3.
CD22−/− B cells respond to BLyS survival signals but undergo BCR-induced cell death. (A) BLyS enhances unstimulated CD22−/− B-cell survival. Purified splenic B cells from wild-type and CD22−/− mice were cultured with medium alone, with anti-IgM antibody (10 μg ml−1) or with CD40 mAb (2 μg ml−1). BLyS (5 ng ml−1, filled bars) was added to the indicated cultures. Values represent the mean percentage (±SEM) of viable cells in triplicate cultures as determined by cell size and granularity (forward versus side light scatter) and 7-AAD exclusion in flow cytometry assays. (B) BLyS enhances CD22−/− B-cell survival but does not reverse BCR-induced cell death. Purified splenic B cells from wild-type (WT, triangles) and CD22−/− (circles) mice were cultured with anti-IgM antibody at the concentrations indicated (open symbols) or with anti-IgM antibody plus BLyS (5 ng ml−1, closed symbols). Cellular 3H-thymidine incorporation during the final 18 h of 3-day cultures was quantified. Values represent mean counts per minute (±SEM) from triplicate wells in a representative experiment. (C) CD22−/− B cells survive and proliferate with BLyS but die following BCR stimulation. B cells from wild-type (thin line) or CD22−/− (thick line) mice were stained with CFSE and cultured for 3 days in medium alone, with anti-IgM antibody (10 μg ml−1) or with CD40 mAb (2 μg ml−1). BLyS (5 ng ml−1) was added to the cultures as indicated (right panels). Cell viability and proliferation was determined by flow cytometry analysis based on forward versus side light scatter and CFSE dilution. (A–C) Data are representative of three independent experiments. Significant differences between sample means for each genotype are indicated; *P ≤ 0.05; **P ≤ 0.01.
Similar to what was observed for survival, culturing wild-type B cells with exogenous BLyS induced >7-fold higher levels of thymidine incorporation compared with medium alone, while CD22−/− B-cell thymidine incorporation was enhanced nearly 10-fold (Fig. 3B). When cultured with BLyS, CD22−/− B cells incorporated 2.5-fold more thymidine than wild-type B cells. Following BCR ligation, the level of thymidine uptake for wild-type B cells was enhanced 1.7- to 2.8-fold by the presence of BLyS (Fig. 3B). In contrast, thymidine uptake by CD22−/− B cells was not induced by BCR ligation. However, exogenous BLyS induced appreciable thymidine incorporation by BCR-stimulated CD22−/− B cells, but did not surpass the levels observed for CD22−/− B cells cultured with BLyS alone. The specific contribution of BLyS to CD22−/− B-cell survival and proliferation was further assessed by flow cytometric analysis. BLyS enhanced wild-type B-cell survival during 3-day cultures but did not induce cell division (Fig. 3C). In contrast, CD22−/− B cells cultured with BLyS alone underwent one or two rounds of cell division. BLyS also enhanced wild-type B-cell survival following BCR stimulation but did not increase the extent of cellular division. This contrasts with CD22−/− B cells where BCR ligation induced cell death regardless of BLyS exposure. The addition of agonistic CD40 mAb to wild-type or CD22−/− B-cell cultures enhanced B-cell survival that was not further enhanced by BLyS. Exposure to BLyS did not augment the extensive proliferation of CD22−/− B cells following agonistic CD40 ligation. Thereby, BLyS-induced proliferation of CD22−/− B cells mimics the previously described hyperproliferative B-cell response to CD40 ligation that occurs in the absence of CD22 negative regulation (37). Thus, BLyS enhances CD22−/− B-cell survival ex vivo but does not prevent BCR-induced cell death.
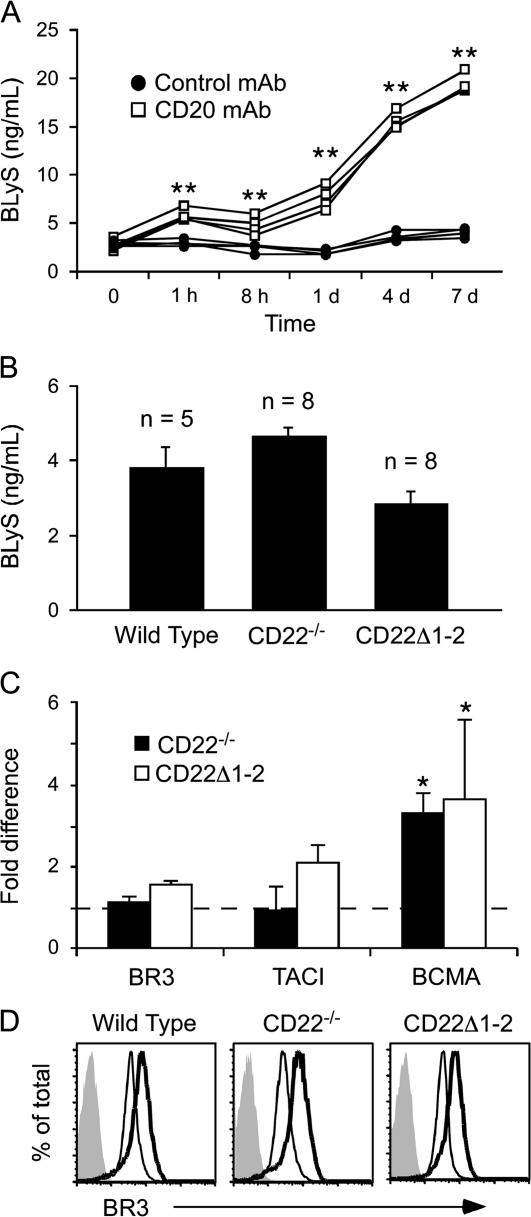
BLyS consumption in wild-type and CD22−/− mice
Serum BLyS levels were analyzed in wild-type mice and in mice lacking CD22 or CD22–ligand interactions in order to quantify BLyS binding and consumption by B cells in vivo. Serum BLyS levels in wild-type mice ranged between 3 and 5 ng ml−1 (Fig. 4A and B). However, depletion of >90% of total mature circulating and tissue B cells in wild-type mice using CD20 mAb (42) resulted in immediate (1 h) and significant increases (5- to 7-fold by day 7) in serum BLyS levels (Fig. 4A). Thereby, continuous BLyS binding and consumption by B cells maintains the normally low levels of endogenous BLyS. The level of serum BLyS in wild-type, CD22−/− and CD22–ligand binding-deficient (CD22Δ1-2) mice was similar and not significantly different (Fig. 4B). Likewise, BR3 and TACI transcript expression did not differ significantly between wild-type, CD22−/− or CD22Δ1-2 B cells when examined by reverse transcription–PCR (Fig. 4C). However, there was a roughly 3-fold increase in BCMA message levels in CD22−/− and CD22Δ1-2 B cells when compared with wild-type B cells. Despite this, BCMA protein expression was not detectable in spleen B cells from CD22−/− and CD22Δ1-2 mice by immunofluorescence staining with flow cytometry analysis (data not shown). Thus, the relative increase in BCMA transcript expression that was observed may result from the loss of CD22 negative regulation or slightly higher frequencies of activated and memory B cells since BCMA is primarily expressed by post-activated B cells (43).
Fig. 4.
CD22−/− B cells consume endogenous BLyS and express BLyS receptors. (A) In vivo B-cell depletion leads to increased serum BLyS levels. C57BL/6 mice were given B-cell-depleting CD20 mAb (n = 4, open squares) or control mAb (n = 4, closed circles) at time 0, with serum harvested at the indicated time points. Serum BLyS concentrations were determined by ELISA. (B) Serum BLyS levels are equivalent in wild-type, CD22−/− and CD22Δ1-2 mice. Bars represent mean (±SEM) BLyS concentrations in 8-week-old mice as determined by ELISA. (C) Spleen B cells from CD22−/− and CD22Δ1-2 mice express all three BLyS receptors. Relative BR3, TACI and BCMA transcript levels for wild-type, CD22−/− and CD22Δ1-2 B cells were compared by real-time PCR analysis with endogenous CD20 transcripts used as the internal standard. Transcript levels in wild-type mice were normalized to a value of 1 (dashed line) with relative mean (±SEM) fold-differences shown for CD22−/− and CD22Δ1-2 B-cell transcripts (n = 3). (A–C) Significant differences between sample means are indicated; *P ≤ 0.05; **P ≤ 0.01. (D) Activated CD22−/− and CD22Δ1-2 B cells up-regulate BR3 expression. Spleen B cells from wild-type, CD22−/− and CD22Δ1-2 mice were cultured for 17 h in medium alone (thin line) or with anti-IgM antibody (10 μg ml−1, thick line). Viable cells were assessed for cell surface BR3 expression by immunofluorescence staining with flow cytometry analysis. Histograms represent the results of three independent experiments.
Cell surface BR3 expression was also examined for wild-type, CD22−/− and CD22Δ1-2 B cells cultured with or without anti-IgM antibody for 17 h. Pronounced BCR-mediated cell death is not observed in CD22−/− B-cell cultures at this early time point (37). Surface BR3 staining revealed a single peak of near-homogeneous expression in unstimulated wild-type, CD22−/− and CD22Δ1-2 B cells (Fig. 4D). Upon activation, BR3 expression was uniformly up-regulated on B cells from mice of all three genotypes. Thus, CD22−/− and CD22Δ1-2 mice have normal serum BLyS levels, and B cells from these mice express wild-type levels of BR3 and TACI, together indicating that B cells lacking CD22 or CD22–ligand interactions bind and consume BLyS at levels similar to wild-type B cells.
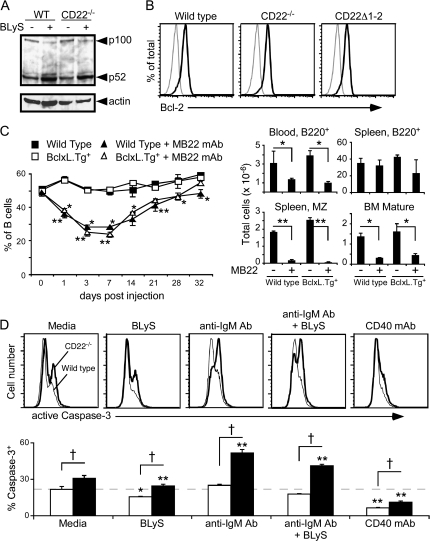
CD22 does not influence downstream mediators of BLyS–BR3 signaling
Whether CD22 expression affects BLyS-mediated processing of NF-κB2 p100 to p52 was examined by western blot analysis. Cell lysates from unstimulated wild-type and CD22−/− B-cell cultures contained similar levels of NF-κB2 p100 and its cleavage product, p52 (Fig. 5A). When the B cells were cultured with BLyS for 17 h, there was a decrease in p100 staining and an accumulation of p52 that was similar in both wild-type and CD22−/− B cells. Thus, CD22−/− B cells respond normally to BLyS–BR3 signaling with enhanced p100 processing.
Fig. 5.
CD22 and BLyS have independent effects on downstream mediators. (A) BLyS-induced NF-κB2 processing is intact in CD22−/− B cells. Whole-cell lysates of purified B cells that were cultured in medium alone or medium plus BLyS (50 ng ml−1) were subjected to western blot analysis for p100 to p52 processing. Subsequently, the membranes were stripped and reprobed for β-actin as a loading control. (B) Intracellular Bcl-2 levels are equivalent in B cells from wild-type, CD22−/− and CD22Δ1-2 mice. Splenocytes were stained for cell surface B220 expression and then fixed and permeabilized before intracellular staining with anti-Bcl-2 (think line) or isotype control (thin line) mAb. Fluorescence histograms of gated B220+ cells are representative of three or more individual mice per genotype. (C) B-cell depletion following mAb blockade of CD22 ligand binding is intact in BclxL.Tg+ mice. Wild-type and BclxL.Tg+ littermates were given CD22 or control mAb (100 μg) on day 0, with blood B220+ B-cell frequencies determined subsequently. Values represent mean (±SEM) for three or more mice per treatment group. Bar graphs indicate separate experiments where bone marrow and spleen B-cell numbers were analyzed from three or more mice per treatment group on day 10 post-treatment. (D) Caspase-3 activation is enhanced in CD22−/− B cells. Purified B cells from wild-type and CD22−/− mice were cultured in medium alone or were cultured with CD40 mAb (2 μg ml−1), anti-IgM antibody (10 μg ml−1) and/or BLyS (50 ng ml−1) for 17 h. Cells expressing active caspase 3 cleavage products were identified by intracellular staining with flow cytometry analysis. Representative histograms for wild-type (thin line) or CD22−/− (thick line) B cells are shown. Bar graphs show active caspase-3-positive B cells from wild-type (open) or CD22−/− (filled) mice. Values are mean (±SEM) frequencies of active caspase-3 positive B cells from triplicate wells in one of two independent experiments. The horizontal dashed line is shown for reference to wild-type B cells cultured in medium alone. (C and D) Significant differences between CD22−/− and wild-type B cells are indicated; †P ≤ 0.01. Within each genotype, significant differences of stimulated cultures compared with media alone are also marked; *P ≤ 0.05; **P ≤ 0.01.
BR3 signaling promotes anti-apoptotic Bcl-2 family member expression, which contributes to enhanced B-cell survival (26, 44). Enforced Bcl-2 expression also results in an accumulation of peripheral B cells in BR3-deficient or mutant mice (45, 46). However, B-cell intrinsic differences in Bcl-2 expression did not contribute to impaired CD22−/− B-cell survival since Bcl-2 levels were equivalent in wild-type, CD22−/− and CD22Δ1-2 B cells (Fig. 5B). BclxL is another downstream target of BR3 signaling, with enforced BclxL expression protecting BR3-mutant B cells from cell death in vivo (41, 44). To determine whether enforced BclxL expression could promote B-cell survival when CD22–ligand interactions were disrupted, the frequency and number of blood B cells were assessed in wild-type and BclxL.Tg+ mice given CD22 mAb. Treatment of wild-type or BclxL.Tg+ mice with control mAb did not affect bone marrow, blood or spleen B-cell frequencies. However, CD22 mAb treatment of both wild-type and BclxL.Tg+ mice significantly reduced blood B-cell numbers by day 1, with sustained depletion for >1 week (Fig. 5C). Likewise, there was a 73% decrease in mature recirculating B cells in the bone marrow and a >90% reduction in spleen MZ B-cell numbers by day 10 in both wild-type and BclxL.Tg+ mice. Therefore, enforced BclxL expression did not promote B-cell survival following disruptions in CD22 expression or CD22–ligand interactions, even though enforced BclxL expression supports BR3-deficient B-cell survival (41).
Whether CD22 deficiency affected caspase-3 activation, the terminal death effector molecule for apoptosis, was assessed following B-cell stimulation with BLyS, anti-IgM antibody or both. Roughly one-third (31 ± 1.1%) of viable CD22−/− B cells expressed active caspase-3 after culture with medium alone, which was 1.4-fold higher than for wild-type B cells (22 ± 1.3%; Fig. 5D). Culturing B cells with BLyS did not significantly change this ratio, with 15 ± 0.3% caspase-3+ wild-type and 24 ± 0.6% caspase-3+ CD22−/− B cells. However, BCR stimulation significantly increased the frequency of CD22−/− B cells expressing active caspase-3 (52 ± 1.2%), which was ∼2-fold higher than for wild-type B cells. A similar frequency of caspase-3+ CD22−/− B cells (41 ± 0.6%) was generated by anti-IgM antibody plus BLyS stimulation. In contrast, BCR ligation did not increase caspase-3 activation in wild-type B cells above that of medium alone. CD40 ligation significantly reduced caspase-3 activation in both CD22−/− and wild-type cells, consistent with CD40-enhanced survival. Thereby, CD22−/− B cells were more primed for caspase-3-induced death when compared with wild-type cells, regardless of BLyS exposure.
Discussion
Survival of the mature follicular spleen, recirculating bone marrow, blood and MZ B-cell compartments is dependent on CD22 expression and CD22–ligand interactions (9, 10, 12, 13, 47). The blood, MZ and follicular B-cell subsets also require the BLyS–BR3 pathway for survival (3, 16, 24, 48). Despite this overlap, simultaneous BLyS sequestration and mAb blockade of CD22 ligand binding led to more severe reductions in each of these compartments. For instance, BLyS blockade further reduced bone marrow, blood and spleen B-cell numbers in CD22−/− mice. Disrupting CD22–ligand interactions in BR3-mutant mice also resulted in decreased frequencies of blood B cells, although the influence of CD22-mediated survival on tissue-resident B cells was eclipsed by the severe B-cell-deficient phenotype of BR3-mutant mice. CD22−/− B-cell survival and proliferation in vitro was enhanced by the addition of BLyS, albeit not to wild-type levels, and serum BLyS consumption was similar between wild-type, CD22−/− and CD22Δ1-2 mice. Together, these data demonstrate that reduced circulating B-cell numbers and high follicular B-cell turnover in CD22−/− mice are not due to insufficient BLyS signaling to maintain B-cell homeostasis in vivo. Thereby, CD22 and BLyS promote the survival of overlapping B-cell populations through independent pathways that combine to govern peripheral B-cell longevity.
Both wild-type and CD22−/− B cells required BLyS-generated survival signals in vivo. However, increased B-cell turnover in CD22−/− and CD22Δ1-2 mice was not due to altered BR3 expression. BLyS–BR3 signaling was also intact in CD22−/− B cells in vitro, as demonstrated by enhanced BLyS-induced NF-κB activation. BLyS–BR3 signaling also influences B-cell survival in part by up-regulating Bcl-2 family proteins, including BclxL and A1 (26, 44). Pro-survival and pro-apoptotic bcl-2 family members balance mitochondrial membrane integrity, wherein disruption of this balance leads to cytochrome c release and activation of the death-promoting caspase cascade (49). However, endogenous Bcl-2 levels were not altered by CD22 deficiency. In addition, B cells with enforced BclxL expression were depleted upon CD22 mAb treatment, indicating that reduced B-cell viability in the absence of CD22–ligand interactions is independent of these pro-survival factors. Also, B-cell depletion in the current experiments was unlikely to result from altered B-cell migration since significant increases in B-cell numbers were not observed in other tissues. The absence of CD22 ligand-binding domains or mAb blockade of ligand binding was also unlikely to perturb the optimal positioning of B cells to receive BLyS signals needed for survival since serum BLyS was consumed at normal levels in CD22−/− and CD22 mAb-treated mice. Regardless, these findings collectively argue that CD22 and BLyS generate survival signals through distinct molecular pathways.
Although BLyS promoted CD22−/− B-cell survival in vivo, BLyS-induced signals did not protect CD22−/− B cells from BCR-induced cell death. These findings demonstrate that homeostatic B-cell survival and BCR-induced proliferation are uncoupled events, involving distinct molecular pathways. In support of this, CD22–ligand interactions are required for optimal B-cell survival in vivo but not for CD22-mediated regulation of BCR-induced intracellular calcium responses or intracellular SHP-1 recruitment by tyrosine-phosphorylated CD22 (12). In addition, BCR-induced cell death in CD22−/− B cells is most pronounced on the C57Bl/6 genetic background, whereas high peripheral B-cell turnover in CD22−/− mice is independent of the background strain (37). BCR-induced B-cell proliferation is dependent on induction of the c-Myc:Cul1 ubiquitin ligase pathway, which is perturbed in CD22−/− C57Bl/6 mice (37). The absence of Cul1 induction following BCR ligation of CD22−/− C57Bl/6 B cells inhibits degradation of the p27Kip1 and p21Waf1 cell cycle regulators, resulting in a block in cell cycle progression. Importantly, naive peripheral B cells are normally quiescent in vivo such that their prolonged survival does not require cellular division. Thus, CD22 regulation of B-cell survival is likely to be mediated by BCR-independent signaling pathways.
The importance of B cells for humoral immunity, autoimmune disease and lymphomagenesis has led to the development of therapies that specifically target human B cells in vivo, including the epratuzumab and belimumab mAbs that independently target CD22- and BLyS-related pathways, respectively. The studies described herein show that combined BLyS and CD22 blockade by mAbs in mice resulted in significantly greater reductions in peripheral B-cell numbers than either treatment alone. Although the in vivo mechanism of action for epratuzumab is unknown, a combination strategy targeting CD22 and BLyS may be beneficial in humans as well. In addition, since neither anti-BLyS nor CD22 mAbs deplete B cells through antibody-dependent cellular cytotoxicity in mice (13), deficiencies and allelic differences in Fc-receptor function or macrophage activation should not affect B-cell depletion following anti-BLyS and/or CD22 mAb treatments. Thereby, accelerated B-cell turnover by combined BLyS- and CD22-directed therapies may offer notable benefits in the treatment of some diseases.
Total B-cell depletion following CD20 mAb treatment resulted in significantly increased serum BLyS levels. Increased BLyS levels have also been observed in patients treated with rituximab, a chimeric CD20 mAb that is used to deplete human B cells (50, 51). Excess BLyS production is associated with autoimmune disease progression in some mouse models including NZB/NZW F1 mice and BLyS transgenic mice and in humans with systemic lupus erythematosus, rheumatoid arthritis and Sjögen's syndrome (52). Thereby, targeting B cells for depletion while not controlling the resulting increase in available BLyS may contribute to or hasten disease recurrence. Since the development and progression of autoimmune disease are multi-factorial in origin, it is prudent to have multiple treatments available, including mAbs that target unrelated B-cell survival pathways, as demonstrated here, in order to limit B-cell viability and combat disease. Thus, combination therapies that actively control serum BLyS levels and hasten B-cell turnover may complement other B-cell depletion strategies while also contributing to the clearance of disease-promoting B cells.
The lifespan of spleen and LN B cells may be influenced by additional survival pathways since a small B-cell population remained following simultaneous BLyS and CD22 blockade. In spleen, these remaining cells were predominantly IgMhi and CD23neg, likely representing recent emigrants from the bone marrow and splenic B1 B cells (data not shown). Peritoneal cavity B1a and B1b cell numbers were not significantly altered by simultaneous CD22 and BLyS mAb treatment. Correspondingly, genetically modified knock out and mutant mouse strains have previously demonstrated that peritoneal and splenic B1 B cells are not dependent on BLyS or CD22–ligand interactions for their maintenance in vivo (3, 12). Thus, continued splenic and peritoneal B1 B-cell survival following anti-BLyS or CD22 mAb treatment is consistent with their alternative survival requirements as compared with follicular B2 cells.
Collectively, these data support a model whereby B cells must integrate varied signals, including those from the BLyS–BR3 axis, CD22–ligand binding and antigen-receptor-mediated tonic signaling for their homeostatic maintenance in vivo. Peripheral B-cell tolerance relies on appropriate signals and competition for limited resources to dictate ‘fitness’ and promote survival of the naive, pre-immune B-cell repertoire. Thus far, BLyS has filled this role as the primary determinant of peripheral B-cell longevity and pool size, with CD22 providing additional necessary survival signals as demonstrated in this study. Further understanding how these signals govern peripheral B-cell survival and selection may thereby lead to important therapeutic advances in our ability to influence lymphoma growth, tolerance and autoimmune disease.
Funding
National Institutes of Health (AI56363); Southeastern Regional Center of Excellence for Emerging Infections and Biodefense (U54 AI057157). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
We thank Dr Guglielmo M. Venturi for thoughtful discussions and critical reading of this manuscript and Latoya McElreth for technical assistance.
Authorship contributions
S.H.S. carried out the bulk of the experiments and data collection; K.Y. generated and analyzed data; S.H.S., K.M.H., J.C.P. and T.F.T. assisted in study design and data analysis, discussed the results and wrote the manuscript; C.D.W. and T-S.M. provided essential reagents, generated and analyzed data, discussed the results and reviewed the manuscript.
Disclosure of Conflicts of Interest
T.F.T. is a shareholder and consultant for Angelica Therapeutics, Inc. and consultant for MedImmune, Inc. No other conflicts are reported.
References
- 1.Tedder TF, Tuscano J, Sato S, Kehrl JH. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu. Rev. Immunol. 1997;15:481. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 2.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signaling. Curr. Biol. 1997;7:133. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 3.Lentz VM, Hayes CE, Cancro MP. Bcmd decreases the life span of B-2 but not B-1 cells in A/WySnJ mice. J. Immunol. 1998;160:3743. [PubMed] [Google Scholar]
- 4.Harless SM, Lentz VM, Sah AP, et al. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr. Biol. 2001;11:1986. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 5.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 6.Gordon J. CD40 and its ligand: central players in B lymphocyte survival, growth, and differentiation. Blood Rev. 1995;9:53. doi: 10.1016/0268-960x(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 7.Kawabe T, Naka T, Yoshida K, et al. The immune responses in the CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 8.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 9.Sato S, Miller AS, Inaoki M, et al. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 10.Otipoby KL, Andersson KB, Draves KE, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 11.O'Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 12.Poe JC, Fujimoto Y, Hasegawa M, et al. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat. Immunol. 2004;5:1078. doi: 10.1038/ni1121. [DOI] [PubMed] [Google Scholar]
- 13.Haas KM, Sen S, Sanford IG, Miller AS, Poe JC, Tedder TF. CD22 ligand binding regulates normal and malignant B lymphocyte survival in vivo. J. Immunol. 2006;177:3063. doi: 10.4049/jimmunol.177.5.3063. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto M, Bradney AP, Poe JC, Steeber DA, Tedder TF. Modulation of B lymphocyte antigen receptor signal transduction by a CD19/CD22 regulatory loop. Immunity. 1999;11:191. doi: 10.1016/s1074-7613(00)80094-1. [DOI] [PubMed] [Google Scholar]
- 15.Treml JF, Hao Y, Stadanlick JE, Cancro MP. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem. Biophys. 2009;53:1. doi: 10.1007/s12013-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 17.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 1999;189:1747. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, Dixit VM. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat. Immunol. 2000;1:37. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Bressette D, Carrell JA, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J. Biol. Chem. 2000;275:35478. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 20.Marsters SA, Yan M, Pitti RM, Haas PE, Dixit VM, Ashkenazi A. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr. Biol. 2000;10:785. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 21.Lentz VM, Cancro MP, Nashold FE, Hayes CE. Bcmd governs recruitment of new B cells into the stable peripheral B cell pool in the A/WySnJ mouse. J. Immunol. 1996;157:598. [PubMed] [Google Scholar]
- 22.Yan M, Brady JR, Chan B, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr. Biol. 2001;11:1547. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 23.Gross JA, Dillon SR, Mudri S, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. Impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 24.Scholz JL, Crowley JE, Tomayko MM, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc. Natl Acad. Sci. USA. 2008;105:15517. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J. Exp. Med. 2000;192:953. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J. Clin. Invest. 2002;109:59. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 30.Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, Sato S. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. 2006;54:192. doi: 10.1002/art.21526. [DOI] [PubMed] [Google Scholar]
- 31.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 32.Anolik JH, Barnard J, Cappione A, et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 33.Looney RJ, Anolik JH, Campbell D, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa M, Hamaguchi Y, Yanaba K, et al. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am. J. Pathol. 2006;169:954. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouaziz JD, Yanaba K, Venturi GM, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc. Natl Acad. Sci. USA. 2007;104:20882. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu C, Deng S, Wong FS, Wen L. Anti-CD20 treatment prolongs syngeneic islet graft survival and delays the onset of recurrent autoimmune diabetes. Ann. N. Y. Acad. Sci. 2008;1150:217. doi: 10.1196/annals.1447.032. [DOI] [PubMed] [Google Scholar]
- 37.Poe JC, Haas KM, Uchida J, Lee Y, Fujimoto M, Tedder TF. Severely-impaired B lymphocyte proliferation, survival and induction of the c-Myc:Cullin 1 ubiquitin ligase pathway resulting from CD22 deficiency on the C57BL/6 genetic background. J. Immunol. 2004;172:2100. doi: 10.4049/jimmunol.172.4.2100. [DOI] [PubMed] [Google Scholar]
- 38.Fang W, Mueller DL, Pennell CA, et al. Frequent aberrant immunoglobulin gene rearrangements in pro-B cells revealed by a bcl-xL transgene. Immunity. 1996;4:291. doi: 10.1016/s1074-7613(00)80437-9. [DOI] [PubMed] [Google Scholar]
- 39.Zekavat G, Rostami SY, Badkerhanian A, et al. In vivo BLyS/BAFF neutralization ameliorates islet-directed autoimmunity in nonobese diabetic mice. J. Immunol. 2008;181:8133. doi: 10.4049/jimmunol.181.11.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida J, Lee Y, Hasegawa M, et al. Mouse CD20 expression and function. Int. Immunol. 2004;16:119. doi: 10.1093/intimm/dxh009. [DOI] [PubMed] [Google Scholar]
- 41.Amanna IJ, Dingwall JP, Hayes CE. Enforced bcl-xL gene expression restored splenic B lymphocyte development in BAFF-R mutant mice. J. Immunol. 2003;170:4593. doi: 10.4049/jimmunol.170.9.4593. [DOI] [PubMed] [Google Scholar]
- 42.Uchida J, Hamaguchi Y, Oliver JA, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J. Exp. Med. 2004;199:1659. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004;199:91. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J. Immunol. 2002;168:5993. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 45.Rahman ZS, Manser T. B cells expressing Bcl-2 and a signaling-impaired BAFF-specific receptor fail to mature and are deficient in the formation of lymphoid follicles and germinal centers. J. Immunol. 2004;173:6179. doi: 10.4049/jimmunol.173.10.6179. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J. Immunol. 2004;173:2245. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 47.Grewal PK, Boton M, Ramirez K, et al. ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Mol. Cell. Biol. 2006;26:4970. doi: 10.1128/MCB.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider P, Takatsuka H, Wilson A, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J. Exp. Med. 2001;194:1691. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7:1182. doi: 10.1038/sj.cdd.4400781. [DOI] [PubMed] [Google Scholar]
- 50.Cambridge G, Stohl W, Leandro MJ, Migone TS, Hilbert DM, Edwards JC. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54:723. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- 51.Lavie F, Miceli-Richard C, Ittah M, Sellam J, Gottenberg JE, Mariette X. Increase of B cell-activating factor of the TNF family (BAFF) after rituximab treatment: insights into a new regulating system of BAFF production. Ann. Rheum. Dis. 2007;66:700. doi: 10.1136/ard.2006.060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr. Opin. Immunol. 2007;19:327. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]