Abstract
Several MHC class II alleles linked with autoimmune diseases form unusually low-stability complexes with class II-associated invariant chain peptides (CLIP), leading us to hypothesize that this is an important feature contributing to autoimmune pathogenesis. We recently demonstrated a novel post-endoplasmic reticulum (ER) chaperoning role of the CLIP peptides for the murine class II allele I-Ed. In the current study, we tested the generality of this CLIP chaperone function using a series of invariant chain (Ii) mutants designed to have varying CLIP affinity for I-Ag7. In cells expressing these Ii CLIP mutants, I-Ag7 abundance, turnover and antigen presentation are all subject to regulation by CLIP affinity, similar to I-Ed. However, I-Ag7 undergoes much greater quantitative changes than observed for I-Ed. In addition, we find that Ii with a CLIP region optimized for I-Ag7 binding may be preferentially assembled with I-Ag7 even in the presence of higher levels of wild-type Ii. This finding indicates that, although other regions of Ii interact with class II, CLIP binding to the groove is likely to be a dominant event in assembly of nascent class II molecules with Ii in the ER.
Keywords: antigen presentation/processing, autoimmunity, CLIP, MHC
Introduction
The MHC class II allele I-Ag7 has been the subject of extensive work at the intersection of the fields of autoimmunity and MHC class II-restricted antigen presentation. It is best known as the class II allele associated with diabetes in the non-obese diabetic (NOD) mouse (1) but is also associated with spontaneous mouse models of arthritis [K/BxN or KRN model (2, 3)] and multiple sclerosis [Biozzi AB/H mice (4)]. I-Ag7 shares sequence homology and notable biochemical features with human DQ and rat RT1.B class II alleles associated with autoimmune diabetes in these species [reviewed in (5, 6)]. Mouse strains that express I-Ag7 but do not develop spontaneous autoimmunity have been reported to mount greater than normal immune responses to antigens (4) and to have an unusually high incidence of self-reactive T cells in the periphery [reviewed in (5)].
Many unusual characteristics have been attributed to I-Ag7, including a low propensity to form SDS-stable dimers, low inherent dimer stability, a peptide-binding motif dominated by a single pocket and lack of a highly conserved salt bridge (β57D-α76R) [reviewed in (5)]. Most remarkable is the combination in I-Ag7 of all these features, each of which can be found alone in other class II alleles. Despite knowledge of these features and of various antigens that stimulate I-Ag7-restricted autoimmune responses, the mechanism of disease association with I-Ag7 (or other disease-associated alleles) remains unknown. Our laboratory has been investigating whether aberrant interaction with chaperones in the class II pathway, in particular invariant chain (Ii), is the key shared feature of these alleles.
Three accessory molecules, invariant chain (Ii), DM, and DO, influence assembly, transport and loading of class II molecules [reviewed in (7, 8)]. Nascent class II molecules are assembled onto Ii trimers in the endoplasmic reticulum (ER). Ii directs trafficking of these nonameric (αβ)3Ii3 complexes from the ER, through the Golgi apparatus, and into endosomal class II loading compartments. Class II alleles vary in their dependence on Ii for efficient assembly and egress from the ER (9). In endosomal compartments, Ii is degraded, leaving a nested set of CLIP peptides in the class II peptide-binding groove. MHC class II alleles differ widely in their affinity for CLIP (10, 11). Exchange of CLIP for other peptides is promoted by the peptide exchange catalyst DM, which is also thought to stabilize empty MHC class II and edit the repertoire of bound peptides, favoring stable peptide/MHC class II complexes. Another non-classical class II molecule, DO, is a negative regulator of DM function, expressed in a subset of antigen-presenting cells (APCs), including B cells, thymic medullary epithelial cells and specific types of dendritic cells [(12, 13) and references therein]. Reduced DM function (e.g. due to low DM expression or to co-expression of DO) generally results in the accumulation of class II/CLIP complexes. However, class II alleles with low affinity for CLIP can release CLIP peptides without DM (14, 15).
Our laboratory demonstrated that rheumatoid arthritis (RA)-associated human class II alleles (DR*0401, *0404 and *0405) form less stable complexes with CLIP than closely related non-RA-associated alleles (DR*0402 and *0403) (14). This led us to propose that low affinity for CLIP is a critical shared feature of disease-linked MHC class II alleles and contributes to disease pathogenesis. Evidence from others supports our hypothesis, as a disproportionate number of disease-associated alleles have been found to have low affinity for CLIP [reviewed in (8)]. Recently, we have extended our model by defining a novel chaperoning role of CLIP peptides for the murine class II allele, I-Ed (16). Here, we use Ii CLIP mutants to demonstrate that variation in CLIP affinity affects half-life, abundance and antigen presentation for I-Ag7, which also has low affinity for wild-type (wt) CLIP (15). Our data argue that improving CLIP region affinity for the I-Ag7 binding groove influences I-Ag7/Ii assembly.
Methods
Cell lines and antibodies
A20 is a B-lymphoma cell line from a Balb/c mouse [H-2d haplotype; American Type Culture Collection (ATCC) clone TIB-208] (17). 3A5 is a derivative of A20, which lacks H-2Mα (murine DMα) expression (18, 19). 293T cells were kindly provided by G. Azar and J. Thibodeau (University of Montreal, Quebec, Canada). Phoenix-A cells are a retroviral packaging cell line (gift of G.P. Nolan, Stanford University, Stanford, CA, USA). A BDC2.5 T hybridoma (clone BA3) was produced by R. Creusot in the laboratory of C.G. Fathman (Stanford University) using activated primary T cells from BDC2.5 transgenic mice (20) and the BW5147 thymoma cell line. 10-2.16 is a monoclonal mouse IgG2b antibody that recognizes a subset of I-A β-chains (including I-Ag7 but not I-Ad) (21, 22). B21-2 is a monoclonal rat IgG2b antibody that recognizes several I-A β-chains (including I-Ab,d,p,q,u,v) but not I-E (18, 23). B21-2 also detects I-Ag7, as it stains 2A-12 cells (described below) but not parental, untransfected 293T cells (data not shown). MK-D6 is a monoclonal mouse IgG2a antibody that recognizes I-Adβ (but not I-Aa,b,f,k,s or I-E) (24, 25) and does not cross-react with I-Ag7 [(25) and our own FACS staining using 2A-12 cells (data not shown)]. MK-D6 effectively blocks T-cell access to I-Ad (26, 27). 10-2.16, B21-2 and MK-D6 hybridomas were obtained from ATCC (clones TIB-93, TIB-229 and HB-3, respectively). Purified, FITC-conjugated OX-6 (IgG1 anti-rat RT1B antibody that cross-reacts with I-Ag7) was obtained from Serotec (Oxford, UK). FITC-conjugated M5/114 [rat IgG2b that recognizes many mouse I-A and I-E molecules, including I-Ab,d,q and I-Ed,k but not I-Ak,s,f (28)] was obtained from eBioscience (San Diego, CA, USA). We determined that M5/114 does not recognize I-Ag7, as it does not bind 2A-12 cells (data not shown). 14-4-4S is a monoclonal mouse IgG2a antibody to the common I-Eα chain (29). Purified, FITC-conjugated 14-4-4S was obtained from Southern Biotech (Birmingham, AL, USA). Purified anti-H-2M antibody (rat IgG1) was obtained from BD Pharmingen (San Jose, CA, USA). Biotinylated anti-FLAG antibody (clone M2; Sigma Aldrich, St Louis, MO, USA) was used together with streptavidin-tricolor (formerly CALTAG, now Invitrogen, Carlsbad, CA, USA). The following antibodies were used together with appropriate HRP-conjugated secondary antibodies for western blotting: anti-β-actin (mouse IgG1; Sigma Aldrich), In-1 (rat anti-mouse Ii; BD Biosciences Pharmingen, San Diego, CA, USA), anti-tetra-His (mouse IgG1; Qiagen, Hilden, Germany) and 10-2.16.
Vectors and transfections
A cDNA for I-Ag7β was obtained by reverse transcription (RT)–PCR from NOD spleen RNA and was cloned into pBMN-IB (gift of G. Nolan, Stanford University) or into pBUD (Invitrogen). A cDNA for I-Adα was obtained by RT–PCR from A20 RNA and cloned into the second site in pBUD so that a single pBUD vector could be used to express I-Ag7 (I-Adα/I-Ag7β). A cDNA for murine DMα was obtained by RT–PCR from A20 RNA and cloned into a pBMN vector. Methods for site-directed mutagenesis, cloning and retroviral transduction have been described (30). Briefly, mutant p31 murine Ii cDNAs were generated by site-directed mutagenesis, using overlap extension PCR and high-fidelity Pfu polymerase, with a pGEM-mIi-p31 construct as the original template (gift of E.K. Bikoff, University of Oxford, Oxford, UK). Ii, I-Ag7β, I-Adα and DMα cDNAs were cloned into the appropriate vectors and verified by sequencing. 293T cells were transfected with pBUD-I-Ag7 by calcium phosphate precipitation and selected with Zeocin (Invitrogen). Single-cell clones were obtained by limiting dilution. The clone 2A-12 (293T + I-Ag7, clone 12) expresses a moderate level of I-Ag7 compared with other clones obtained in the same experiment. For transient transfection screening, pBMN-Ii-IN constructs were introduced into 2A-12 by calcium phosphate precipitation. For transfection of A20 and 3A5 lines, pBMN vectors with I-Ag7β, Ii or DMα were transfected into Phoenix-A cells by calcium phosphate precipitation. Phoenix-A cell supernatant containing retroviral particles was harvested and used to infect A20 and 3A5 cells. Stable polyclonal populations expressing the appropriate constructs were obtained by blasticidin selection for I-Ag7β or G418 (neomycin) selection for Ii. As I-Ad and I-Ag7 have identical α-chains, transfected I-Ag7β assembles with endogenous I-Adα to form the I-Ag7 αβ dimer in A20 and 3A5 cells. No drug selection was performed for transient transfection with DMα.
Flow cytometry
Cells were stained on ice with antibodies described above. For cell surface FACS with 2A-12 cells, propidium iodide was used to exclude dead/dying cells. For combined cell surface and intracellular staining, surface staining was performed first, followed by fixation and permeabilization using the Cytofix/Cytoperm kit (BD Pharmingen) and intracellular staining. Data were collected using a FACScan or FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and CellQuest Pro software (BD Biosciences) and were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). The mean fluorescence intensity (MFI) of isotype controls was routinely under 10 (data not shown). MFI of staining on cells expressing mutant Ii was normalized to the appropriate (untagged, 6× His-tagged or 3× FLAG-tagged) wt control within the same experiment: 100% × MFImut/MFIwt = MFI of mutant as % of wt. For 2A-12 transient transfection experiments in Fig. 1(A), data represent staining of polyclonal populations from multiple (three to seven) independent transfections, with staining performed between 1 and 4 days after transfection. For A20.g7 and 3A5.g7 Ii transfectants in Fig. 1(B), data represent staining of stable polyclonal populations from two independent transfections/selections. For Fig. 1(C), cell surface I-Ag7 staining was assessed on DM-positive or -negative populations within a culture of stable 3A5.g7 Ii transfectants exposed to retrovirus for transient transfection with murine DMα, with staining in the days immediately following transfection, with two independent transfections.
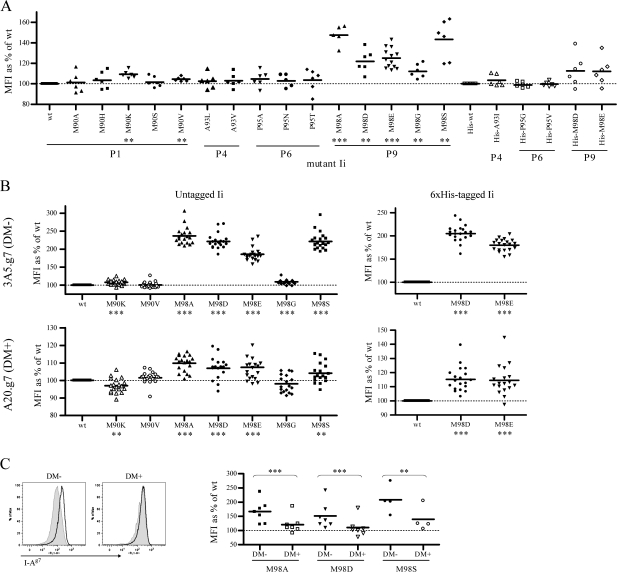
Fig. 1.
Select Ii CLIP mutants increase cell surface abundance of I-Ag7. Cell surface levels of I-Ag7 on various cells were assessed by FACS with the mAb OX-6-FITC. The MFI of isotype controls was routinely under 10 (data not shown). MFI of staining on cells expressing mutant Ii is normalized to the appropriate (untagged, 6×His-tagged or 3×FLAG-tagged) wt control within the same experiment. (A) 293T cells were stably transfected with I-Ag7 and single-cell clones were obtained by limiting dilution. A clone expressing moderate levels of I-Ag7 (2A-12) was used to screen Ii mutants for an effect on cell surface levels of I-Ag7. 2A-12 cells were transiently transfected with wt or mutant Ii constructs (closed symbols, untagged Ii; open symbols, 6×His-tagged Ii), and cell surface levels of I-Ag7 were assessed on day 1, 2, 3 and/or 4 after transfection. Data in this figure are from three to seven independent transfections for each mutant. Statistical significance was determined by paired Student’s t-test: **P < 0.01; ***P < 0.001. All seven indicated comparisons remain statistically significant after sequential Bonferroni correction for multiple comparisons. (B) 3A5 and A20 cells expressing I-Ag7 were stably transfected with wt or mutant Ii. Data represent cell surface staining of I-Ag7 on polyclonal populations from two independent transfections and selections. Statistical significance was determined by paired Student’s t-test: *P < 0.05; **P < 0.01; ***P < 0.001. All indicated comparisons remain statistically significant after sequential Bonferroni correction for multiple comparisons. (C) 3A5.g7 cells stably expressing 3xFLAG-tagged wt or mutant Ii were transiently transfected with murine DMα (H-2Mα) by retroviral transduction. In the following days, cells were stained for cell surface I-Ag7 and intracellular DM and 3xFLAG-Ii and were analyzed by FACS. Data are from two independent transfections for M98A and M98D and one transfection for M98S. Left: sample histograms showing cell surface I-Ag7 staining on DM− and DM+ populations of 3A5.g7 cells stably expressing 3xFLAG-wt Ii (shaded) or 3xFLAG-M98A Ii (open) and transiently transfected with DM. Right: summary of multiple experiments. In this panel only, MFI represents median fluorescence intensity of I-Ag7 stains (in all other panels, MFI represents mean fluorescence intensity). Note that separation of DM+ and DM- populations by gating in this assay (with this anti-DM reagent) is incomplete, even for A20 versus 3A5. Thus, even conservative DM+/− gates include a proportion of events from the overlapping populations, and the absolute value of changes between populations calculated in this manner is an underestimate (e.g. see reduced scale of mutant Ii effect in cells in DM− gate in 1C versus scale of mutant Ii effect in completely DM− populations in 1B). Nonetheless, the reduction in mutant Ii effect on cell surface I-Ag7 in the presence of DM is statistically significant as determined by paired Student’s t-test: M98A, P = 0.0005; M98D, P = 0.0005; M98S, P = 0.0025.
Pulse–chase and immunoprecipitation
The radiolabeling/co-immunoprecipitation (IP) assay used in Fig. 2, in which persistence of class II/CLIP complexes is used to determine relative class II/CLIP affinity, is a modified version of previously described methods (14, 16, 31). Cells were washed in cysteine/methionine-free DMEM (Gibco, Invitrogen Corporation) and starved for 1–2 h (at 37°C, 5% CO2) in starvation medium (Cys/Met-free DMEM + 10% dialyzed fetal bovine serum + 2 mM L-glutamine) and then labeled for 4–24 h as indicated in figure legends (at 37°C, 5% CO2) with 125 uCi ml−1 ExpreSS [35S] labeling mix (PerkinElmer, Boston, MA, USA). Labeled cells were lysed in buffer containing 6 mM CHAPS, 50 mM Tris–HCl and 150 mM NaCl (pH 8) plus 1 mM phenylmethylsulphonylfluoride and complete protease inhibitors (Roche Diagnostics, Mannheim, Germany). Lysates were clarified by centrifugation and precleared several times with combinations of normal mouse serum, rabbit-anti-mouse IgG (Zymed, South San Francisco, CA, USA), Pansorbin (heat-killed fixed Staphylococcus aureus cells: Calbiochem/EMD Biosciences, La Jolla, CA, USA) and Protein A or Protein G sepharose (formerly Amersham Pharmacia Biotech, now GE Healthcare, Piscataway, NJ, USA). Samples were normalized either for starting cell number at time 0 or for counts following IP of I-Ag7, using a 1450 microbeta beta-counter (Perkin Elmer/Wallac, Turku, Finland) as indicated in text or figure legends. I-Ag7 was immunoprecipitated by incubating lysates with Protein A or G sepharose beads coated with 10-2.16 concentrated supernatant. Proteins were eluted from the beads by boiling in reducing SDS sample buffer and separated by SDS–PAGE. Gels were treated with Amplify (Amersham Biosciences, UK), dried under vacuum and exposed to radiography film (Kodak, Rochester, NY, USA).
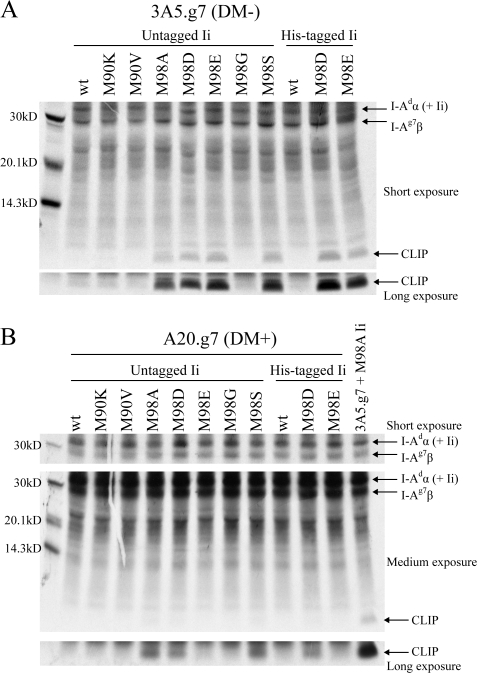
Fig. 2.
Ii CLIP mutants that increase cell surface I-Ag7 are high-CLIP-affinity mutants within a physiological range of class II/CLIP affinity. 3A5.g7 (A) or A20.g7 (B) cells stably transfected with wt or mutant Ii were labeled with [35S] cysteine and methionine (A, overnight and B, 8 h), and I-Ag7 (I-Adα/I-Ag7β) was immunoprecipitated with the anti-I-Aβ antibody 10-2.16, which does not recognize I-Adβ. Samples were normalized for counts after I-Ag7 IP and analyzed by SDS–PAGE. (Note that the I-Adα band also contains full-length Ii co-precipitated with I-Ag7.) (A) Mutations in Ii that increase cell surface I-Ag7 levels also cause increased persistence of I-Ag7/CLIP co-IP in the absence of DM. Shown: one representative experiment of four. (B) High-affinity mutant CLIP is efficiently removed by DM. Shown: one representative experiment of two.
For Fig. 4(C and D), a more traditional pulse–chase assay with 1-h starve, 1-h pulse and the designated chase times (in complete media with cold Cys/Met) was used to assess turnover of I-Ag7 (details of media, lysis, IP and gels as described above, with samples normalized for starting cell number at time 0). Densitometry was performed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
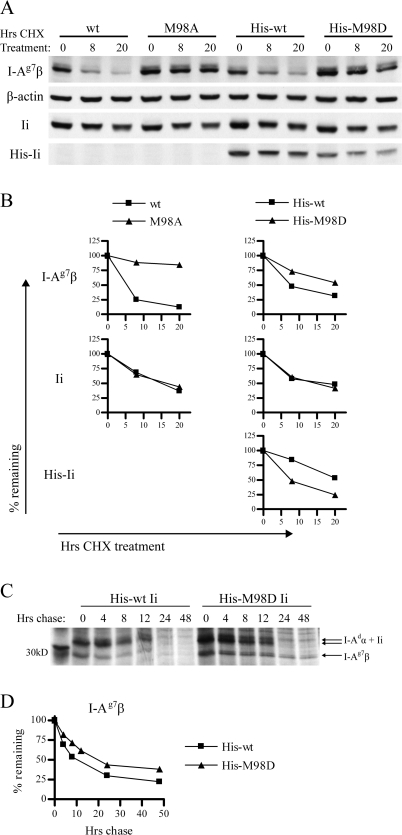
Fig. 4.
Increased affinity of CLIP for I-Ag7 increases survival time of I-Ag7 and decreases survival time of Ii. (A) 3A5.g7 cells stably expressing wt or mutant Ii were treated with CHX to block de novo protein synthesis. The level of surviving I-Ag7, actin, Ii and His-Ii was assessed by western blot at the indicated time points (using 10-2.16, anti-actin, In-1 and anti-Tetra-His primary antibodies, respectively). Samples are normalized to represent the same number of live cell equivalents (c.eq.) per lane. At the chosen dose of CHX (10 μg ml−1), 3A5 cells ceased to expand but retained >90% viability. Untreated cells expanded exponentially and maintained constant levels of I-Ag7 (per live c.eq.) over the period of observation (data not shown). One representative experiment of two is shown. Each experiment was performed with duplicate gels (one gel with high c.eq. and one gel with low c.eq.) to ensure that detection was in the quantitative range of the assay for each of the four antibodies. Shown: 2x106 c.eq. per lane for I-Ag7β and His-Ii blots and 0.4x106c.eq. per lane from the same samples for actin and total Ii blots. Not shown: the effect of His-M98E on turnover of I-Ag7β and His-Ii was slightly reduced compared with that of His-M98D. (B) Densitometry of the bands shown in A: band intensity was normalized to β-actin band intensity at the same time point and then expressed as % remaining compared with the 0-h time point to illustrate rate of turnover. (C) 3A5.g7 cells stably transfected with His-wt or His-M98D Ii were starved (1 h), labeled with [35S] cysteine and methionine (1 h) and chased for the indicated times. I-Ag7 (I-Adα/I-Ag7β) was immunoprecipitated with 10-2.16. Samples were normalized for starting cell number at time 0 and analyzed by SDS–PAGE. (D) Densitometry of the I-Ag7β bands in C: band intensity was normalized to the 0-h time point from the same sample (shown as % remaining) to illustrate turnover. (12-h time point is omitted for the His-wt Ii series due to distortion of the gel at this lane.)
Immunoblotting
Cells were harvested and lysed in buffer containing 6 mM CHAPS, as described above. For analysis of steady-state levels of I-Ag7, protein content of lysates was determined by Bradford assay, and samples were normalized for protein amount and boiled in reducing SDS sample buffer. For analysis of the half-life of I-Ag7, cells were treated with 10 μg ml−1 cycloheximide (CHX). Samples were collected at the indicated time points, lysed in buffer containing 6 mM CHAPS and normalized for live cell equivalents before boiling in reducing SDS sample buffer. For co-IP experiments demonstrating differential assembly of wt and mutant Ii with I-Ag7, lysates were normalized either for cell equivalents or for total protein amounts and were precleared and immunoprecipitated as described above, using 10-2.16 for I-Ag7, anti-tetra-His antibody for His-tagged Ii or In-1 for total Ii. Samples were separated by SDS–PAGE and transferred to Immobilon PVDF membrane (Millipore, Bedford, MA, USA). Binding of primary antibodies to the membrane was detected by HRP-conjugated secondary antibodies followed by Western Lightning enhanced chemiluminescence (ECL) substrates (PerkinElmer Life Sciences, Boston, MA, USA) and exposure to Hyperfilm ECL (Amersham Biosciences). Densitometry was performed using a Bio-Rad GS-710 densitometer and QuantityOne software (Bio-Rad, Hercules, CA, USA).
T-cell stimulation assays
APCs (105 A20.g7 or 3A5.g7 Ii transfectants) were incubated for 30–60 min with MK-D6 to pre-block I-Ad. BDC2.5 T-hybridoma cells (105) and different concentrations of 1040-79 (p79) mimetope peptide (AVRPLWVRME) (32) were added, and cultures were incubated overnight at 37°C. The p79 peptide was synthesized and purified by the Stanford PAN facility. All cultures were done in duplicate. Culture supernatants were collected at 20–24 h and assayed for IL-2 with the BD OptEIA mouse IL-2 ELISA kit and BD OptEIA TMB substrate (BD Biosciences).
Statistical methods
Normalized data (MFI for FACS experiments, or band intensity for densitometry of western blots, normalized to wt within the same experiment) from wt and mutant Ii transfectants were compared using a paired Student’s t-test (GraphPad Prism; GraphPad Software, San Diego, CA, USA) or a Wilcoxon signed ranks test, as indicated in figure legends. Within each figure, we corrected for multiple comparisons using sequential Bonferroni adjustment (33).
Results
Screening of Ii CLIP mutants for effect on cell surface abundance of I-Ag7
Ii CLIP mutations likely to increase the affinity of CLIP for I-Ag7 were chosen based on published peptide-binding motifs [studies reviewed in (5) and (34–36)] and were incorporated into a murine p31 Ii cDNA by site-directed mutagenesis. Previously, we showed that Ii CLIP mutants with increased affinity for I-Ed increased the cell surface levels of I-Ed (16). We therefore used an assay of cell surface class II levels to rapidly screen our mutant Ii panel for effects on I-Ag7. 293T cells were transfected with I-Ag7 and a clone (2A-12) with moderate cell surface I-Ag7 expression was chosen for further studies. 2A-12 cells were transiently transfected with wt or mutant Ii constructs, and cell surface I-Ag7 was measured by FACS 1–4 days after transfection (Fig. 1A). We observed increased cell surface levels of I-Ag7 in the presence of Ii with M98D or M98E mutations, which are known to be high-affinity P9 anchors for I-Ag7 [reviewed in (5) and (34)]. Among Ii constructs with single amino acid changes at the P1, P4, P6 or P9 anchor residues, five additional Ii mutants (M90K and V at P1 and M98A, G and S at P9) increased cell surface I-Ag7. Transfection with wt Ii or His-wt Ii did not alter cell surface levels of I-Ag7 as compared with untransfected 2A-12 cells (data not shown).
The seven mutations with a statistically significant effect in this assay were stably transfected into I-Ag7-expressing DM+ (A20.g7) and DM− (3A5.g7) B-cell lines, derived as described in Methods. Again, transfection of wt Ii (or His-wt Ii or 3xFLAG-wt Ii) had no effect on cell surface abundance of I-Ag7 (data not shown). The effect of P1 mutants M90K and M90V and P9 mutant M98G on cell surface I-Ag7 levels appeared diminished in 3A5.g7 compared with 2A-12, while the effects of P9 mutants M98A, D, E and S appeared greatly amplified in 3A5.g7 (Fig. 1B). Ii CLIP mutants generally had a reduced effect on cell surface I-Ag7 in A20.g7 compared with 3A5.g7 (Fig. 1B). Effects of mutant Ii on cell surface I-Ag7 were comparable for untagged, His-tagged and 3xFLAG-tagged Ii constructs (Fig. 1B and data not shown). Notably, the effects of Ii mutants on cell surface I-Ag7 are clear despite the fact that, in A20.g7 and 3A5.g7 cells, small amounts of transfected Ii must compete with excess endogenous wt Ii for assembly with I-Ag7.
We were interested in determining whether the reduced effect of Ii mutants on cell surface I-Ag7 in A20.g7 compared with 3A5.g7 cells was a result of DM co-expression in A20.g7 cells or of different levels of expression of transfected Ii. We first measured levels of His-Ii (wt) in transfected A20 and 3A5 cells, using western blot of whole cell lysates normalized for micrograms of protein and found equivalent levels of Ii expression (data not shown). However, 3A5 cells differ from their A20 parent line in several parameters (size, growth rate, etc.). Thus, we also performed experiments in which murine DMα (H-2Mα) was transiently transfected into stable 3A5.g7 Ii transfectants to reconstitute murine DM expression (Fig. 1C). As the transfection efficiency is significantly <100%, this approach allowed comparison of cell surface I-Ag7 levels in DM+ and DM− cells in the same culture, with all other cellular parameters held constant (including transfected Ii levels, confirmed by anti-FLAG staining, data not shown). These experiments explicitly confirmed that the effect of mutant Ii on I-Ag7 cell surface abundance is reduced or (as in the sample FACS plots shown, Fig. 1C left) almost completely masked in the presence of DM.
There is evidence that Ii may serve as a chaperone for DM (37) and that DM can affect abundance of class II alleles (38, 39). Intracellular FACS to detect levels of intracellular DM in wt or mutant Ii A20.g7 transfectants showed no detectable change in total cellular levels of DM (data not shown). Thus, Ii CLIP mutants apparently affect class II alleles directly in A20 transfectants (as in the absence of DM in 3A5 transfectants) rather than indirectly by modulating DM levels.
Contrary to the pattern observed for most Ii mutants, we observed a modest drop in cell surface I-Ag7 in A20.g7 transfected with M90K Ii compared with wt Ii. Similar anomalous effects are suggested for I-Ag7 with M98G Ii (Fig. 1), for I-Ed with A93F and A93I Ii (16) and for the human DR*0402 allele (which has moderate to high affinity for wt CLIP) in the presence or absence of DM (data not shown). This is surprising as class II occupancy with stable high-affinity peptides is generally associated with increased complex stability and longevity [reviewed in (40)], and there is currently no clear explanation for these exceptions.
Ii CLIP mutants that increase cell surface I-Ag7 have high affinity for CLIP
To confirm that Ii mutants that increase cell surface levels of I-Ag7 in 2A-12, 3A5.g7, and A20.g7 cells are high-CLIP-affinity mutants, we used a metabolic-labeling/co-IP assay in which increased persistence of class II/CLIP complexes in the absence of DM indicates increased class II/CLIP affinity [as in (14, 16)] (Fig. 2A). Co-IP of wt CLIP with I-Ag7 in 3A5.g7 cells was not detected, indicating spontaneous release of wt CLIP in the absence of DM. The four Ii mutants with the greatest effects on cell surface I-Ag7 (M98A, D, E and S) resulted in clearly detectable CLIP co-IP with I-Ag7. It is likely that the remaining mutants that affect cell surface I-Ag7 (M90K, M90V and M98G) increase the affinity of CLIP for I-Ag7 but to an insufficient degree for detection by co-precipitation of I-Ag7/CLIP complexes in detergent lysates.
High-affinity Ii CLIP mutants are within a physiological range of CLIP affinity
Mutant CLIP/class II complexes within a physiological range of class II/CLIP affinity should persist in the absence of DM but should remain DM susceptible. In DM+ A20.g7 cells, CLIP co-IP with I-Ag7 for the four highest affinity Ii CLIP mutants is largely (M98A, D and S) or completely (M98E) abolished (Fig. 2B), indicating efficient removal of CLIP by DM despite the increased CLIP/I-Ag7 affinity. In these experiments, CLIP/I-Ag7 complexes were analyzed immediately following radiolabeling (no chase) so that at least some of the CLIP/I-Ag7 complexes recovered from these cells are recently generated and have not yet encountered DM. However, a pulse–chase experiment (40-min pulse and 0-, 2-, 4-, 8-, 12- and 20-h chase) with A20.g7 cells transfected with M98A Ii indicates that a small cohort of stable M98A CLIP/I-Ag7 complexes accumulates over time (data not shown). For naturally occurring class II alleles with moderate to high affinity for wt CLIP, a small number of CLIP/class II complexes can be detected at the cell surface, even in the presence of DM (41). This usually does not represent absolute resistance of these complexes to DM editing, but rather reflects the fact that DM activity is limiting within physiologic kinetic windows due to substoichiometric levels of DM expression compared with class II, negative regulation of DM by DO, and preferential association of DM with empty class II [reviewed in (8) and (42–44)]. Thus, our data suggest that the high-CLIP-affinity Ii mutants for I-Ag7 are appropriately removed by DM and are within a physiological range of CLIP affinity.
In high-CLIP-affinity Ii transfectants, total cellular abundance and half-life of I-Ag7 are increased
High-CLIP-affinity Ii mutants caused increased total cellular I-Ag7, detected by western blotting of whole cell lysates from 3A5.g7 Ii transfectants (Fig. 3). This result suggests that an increased cellular pool rather than enhanced distribution to the cell surface is the basis of increased surface class II. Increased total cellular abundance of I-Ag7 is associated with increased survival time of I-Ag7 molecules in the presence of high-CLIP-affinity Ii mutants. We used CHX to block de novo synthesis in 3A5.g7 cells and determined remaining protein levels at various time points by western blot (Fig. 4A and B). Within the time frame of the assay (0–20 h), β-actin levels remained constant, while total Ii levels dropped at comparable rates in wt and mutant Ii transfectants. In contrast, I-Ag7β levels decline rapidly and dramatically in the presence of wt Ii but are significantly stabilized in the presence of high-CLIP-affinity Ii mutants, demonstrating an increased half-life and reduced turnover. The effects of three Ii mutants on I-Ag7 turnover in this assay are generally consistent with the hierarchy observed in effects on cell surface and total cellular abundance of I-Ag7 in 3A5.g7 cells: M98A > His-M98D > His-M98E. Increased half-life of I-Ag7 in the presence of high-CLIP-affinity Ii mutants is also observed in traditional pulse–chase experiments. The I-Ag7 β-chain is stabilized in 3A5.g7 transfectants stably expressing His-M98D Ii (Fig. 4C and D) or M98A Ii (data not shown) compared with (His-tagged or untagged) wt Ii. The I-Ad α-chain from precipitated I-Ag7 dimers also demonstrates this difference in turnover (Fig. 4C), although at time points through 12 h, the α-chain is poorly resolved from co-precipitated Ii in one-dimensional analysis.
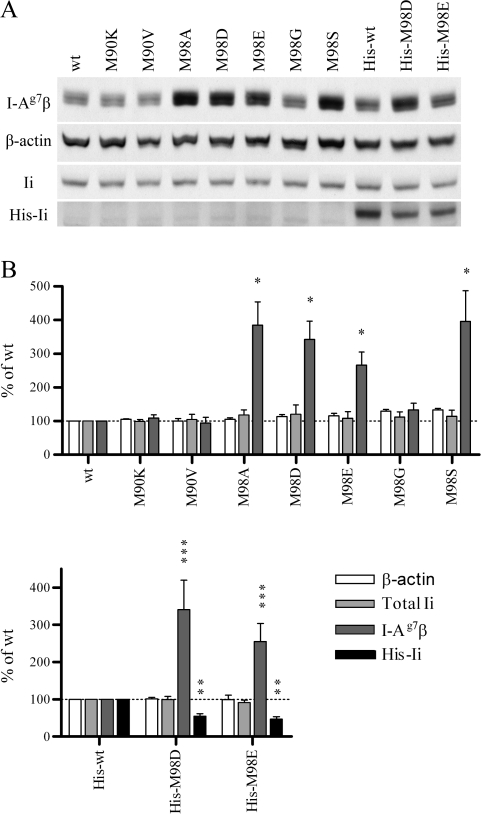
Fig. 3.
In high-CLIP-affinity Ii transfectants, total cellular abundance of I-Ag7 is increased and abundance of high-CLIP-affinity Ii is decreased. (A) Steady-state levels of I-Ag7β, β-actin, total Ii and transfected 6×His-Ii in stable 3A5.g7 Ii transfectants were assessed by western blotting (antibodies: 10-2.16, anti-actin, In-1 and anti-Tetra-His, respectively). Lysates were normalized for protein amount (shown: 25 μg per lane). One representative experiment of several is shown. (B) Densitometry from several experiments (normalized to the appropriate untagged or 6×His-tagged wt Ii sample within each experiment). Statistical significance was determined by Wilcoxon signed ranks test: *P = 0.0156; **P = 0.0078; ***P < 0.005. All indicated comparisons remain statistically significant after sequential Bonferroni correction for multiple comparisons.
High-CLIP-affinity Ii mutants have decreased half-life and are preferentially assembled with I-Ag7 compared with wt (low-CLIP-affinity) Ii
Interestingly, increased abundance of I-Ag7 is accompanied by a reduction in the steady-state levels of transfected high-CLIP-affinity Ii compared with transfected wt Ii (His-M98D and His-M98E versus His-wt; Fig. 3). An increased rate of turnover of high-CLIP-affinity Ii compared with wt (low-CLIP-affinity) Ii observed in our CHX treatment experiments (Fig. 4A and B) is likely sufficient to explain this difference in steady-state His-Ii levels. In addition, the observed effects of increased CLIP affinity on I-Ag7 are detectable despite small amounts of transfected mutant Ii compared with a large excess of endogenous wt Ii. We hypothesized that preferential assembly of high-CLIP-affinity Ii mutants with I-Ag7 and subsequent degradation of Ii molecules upon arrival in endosomes would explain both the effective competition with endogenous wt Ii and the enhanced turnover of high-CLIP-affinity Ii mutants.
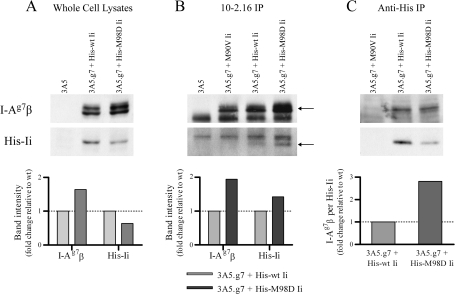
To determine whether Ii mutants with higher (CLIP) affinity preferentially assemble with I-Ag7 in the ER, we used various antibodies to IP different cohorts of class II/Ii complexes from stable 3A5.g7 Ii transfectants and western blotting to determine the proportion of class II and Ii chains recovered (Fig. 5). Ii/class II complexes under these experimental conditions are most likely recovered as nonameric Ii3(αβ)3 complexes (45), preventing complete purification of individual Ii/αβ trimers. In cells expressing multiple forms of Ii (e.g. p31/33/41/43), homotrimerization is not favored, either by specific protein–protein interactions or by physical proximity during synthesis. Thus, an apparently random mix of homotrimers and mixed trimers results [e.g. as in (46)]. Consequently, in cells where expression levels of different Ii populations are relatively comparable, we would expect random co-precipitation of all available Ii populations in most nonameric complexes. This would prevent detection of preferential assembly of specific Ii subpopulations with particular class II alleles and vice versa. However, in our transfectants, we estimate that there is at least 10-fold more endogenous Ii than transfected Ii, based on lysate amounts and film exposure times required for optimal detection of total versus His-tagged Ii in western blot experiments. Additionally, the total amount of Ii in transfected versus untransfected cells is indistinguishable by western blot even with careful lysate titrations, where differences of ∼10% are normally detectable (data not shown). The majority of Ii trimers in these cells, therefore, would include no His-tagged Ii. Thus, the preferential inclusion of I-Ag7 in nonameric complexes with His-tagged high-CLIP-affinity Ii (and vice versa) should be detectable through exclusion of the large majority of nonameric complexes lacking His-tagged Ii from the co-precipitates and consequent enrichment of Ii/class II complexes including mutant Ii and I-Ag7.
Fig. 5.
Preferential assembly of class II/Ii complexes with increased class II/CLIP affinity. Western blotting was used to detect proportions of I-Ag7β (using 10-2.16 antibody) and transfected, His-tagged Ii (using anti-Tetra His antibody) in whole cell lysates (A, 106 live cell equivalents) or in class II/Ii complexes IPed from whole cell lysates by 10-2.16 (B, anti-I-Ag7β IP from 107 cell equivalents) or by anti-Tetra-His (C, anti-His-tagged Ii IP from 107 cell equivalents) antibodies. In (B), the I-Ag7β and His-Ii bands are indicated by arrows. In the I-Ag7 blot (upper panel), the lower band is the light chain of the 10-2.16 antibody used for the IP, recognized by the goat-anti-mouse IgG2b–HRP secondary reagent in the western blot. In the anti-His blot (middle panel), the upper band is a non-specific band sometimes detected by the anti-Tetra-His antibody, detectable upon lengthy exposure of the film. One representative experiment of three is shown. Densitometry was performed as described in Methods.
In whole cell lysates, we observed an increase in steady-state abundance of total cellular I-Ag7β and decrease in total cellular His-Ii in the presence of a high-CLIP-affinity Ii mutant as compared with wt Ii (His-M98D versus His-wt Ii, Fig. 5A, as in Fig. 3). 10-2.16 IP from these lysates detects total cellular I-Ag7 and only that proportion of His-Ii that is associated (in nonamers) with I-Ag7, excluding any free His-Ii and His-Ii associated with nonamers containing only non-I-Ag7 class II molecules (I-Ed or I-Ad) (Fig. 5B). We again observed higher total cellular levels of I-Ag7 in the presence of a high-CLIP-affinity Ii mutant (His-M98D). However, despite the lower total cellular levels of His-M98D Ii, more His-M98D Ii than His-wt Ii co-IPed with I-Ag7. This increased co-IP of His-M98D Ii was not simply due to a proportional IP with increased amounts of nascent I-Ag7: a metabolic labeling experiment with a short (10-min) pulse and no chase demonstrated that the rates of I-Ag7 synthesis (and thus the amount of nascent I-Ag7β in the ER available for assembly with I-Adα and Ii) are indistinguishable in cells with His-wt versus His-M98D Ii (data not shown). Even after metabolic labeling overnight, as shown in Fig. 2(A), differences in I-Ag7 abundance are modest. These data argue against both an artifactual difference in synthesis and a major effect on I-Ag7 abundance by Ii chaperoning during synthesis. Thus, the increased I-Ag7 signal in His-M98D cells reflects higher abundance of mature (non-Ii-associated) I-Ag7 due to increased half-life of the molecules in the presence of high-CLIP-affinity Ii (Fig. 4), and the increase in co-IPed His-M98D Ii compared with His-wt Ii is observed despite equivalent levels of nascent I-Ag7 in these cells.
Conversely, anti-His IP from the same lysates detects total cellular His-Ii and only that portion of I-Ag7 that is associated (in nonamers) with His-Ii, excluding free I-Ag7β, mature I-Ag7 dimers, and I-Ag7 associated with endogenous wt Ii homotrimers (Fig. 5C). We observed lower levels of His-M98D Ii than His-wt Ii, despite similar rates of His-Ii synthesis (as determined by radiolabeling with a short pulse and no chase, data not shown). However, approximately equivalent amounts of I-Ag7β are co-IPed with His-Ii in both cell lines. Thus, high-CLIP-affinity His-M98D Ii co-precipitates more I-Ag7 per amount of His-Ii than His-wt Ii (Fig. 5C, densitometry).
Preferential assembly of high-CLIP-affinity Ii with I-Ag7 is the most likely explanation for skewed inclusion of these molecules in the same nonameric complexes. The detection of this bias despite some masking of the results by co-IP of non-targeted Ii and class II chains included in these nonamers argues that this preferential pairing is actually more dramatic than can be detected using these particular methods. Selective assembly of Ii with class II alleles based on CLIP affinity is consistent with a model in which CLIP association with the peptide-binding groove of class II is the crucial step in class II/Ii complex formation, despite the interaction of other regions of Ii with class II [reviewed in (7, 8, 40)]. Thus, assembly of class II/Ii complexes with high CLIP/class II affinity is probably favored, allowing small amounts of transfected mutant Ii to compete effectively with excess endogenous wt Ii for assembly with the targeted class II allele.
In parallel experiments in which In-1 was used to co-IP total cellular Ii (endogenous + transfected) and Ii-associated class II from 3A5.g7 cells, we were unable to detect a consistent increase in the amount of I-Ag7 precipitated with total Ii in the presence of untagged M98A or M98D Ii compared with wt Ii (data not shown). Additionally, we stained for surface class II levels on our panel of 3A5.g7 Ii transfectants [using OX-6 for I-Ag7, B21-2 for total I-A (I-Ad + I-Ag7), M5/114 for I-Ad + I-Ed, and 14-4-4S for I-Ed] and observed by FACS that cell surface abundance of I-Ad and I-Ed is unaffected by variation in I-Ag7 abundance or by the presence of the Ii mutants (data not shown). Thus, while the affinity of the Ii CLIP region influences the proportion of wt versus mutant Ii molecules assembled with any given class II allele, this selectivity does not detectably affect the total amount of I-Ag7 (or I-Ad or I-Ed) that is assembled with Ii in these cells. This implies that neither Ii nor I-Adα amounts are limiting for ER assembly of class II/Ii complexes in this system and that competition between I-Ag7β and I-Adβ for assembly with I-Adα does not detectably affect the proportion of assembled class II alleles (and therefore does not explain the amplified effect of M98A, D, E and S Ii mutants on cell surface I-Ag7 in 3A5.g7 cells compared with 2A-12 cells, where there is no additional β-chain to compete for assembly with I-Adα). This does not rule out the possibility that CLIP/class II affinity may influence competition in more physiological settings: other studies have shown that competition between multiple class II chains (and Ii) for a common partner during assembly can affect the final repertoire of class II dimers and that this competition may be influenced by efficiency of CLIP binding [reviewed in (40, 47)].
Antigen presentation via unsupervised peptide exchange is reduced in cells expressing high-CLIP-affinity Ii mutants
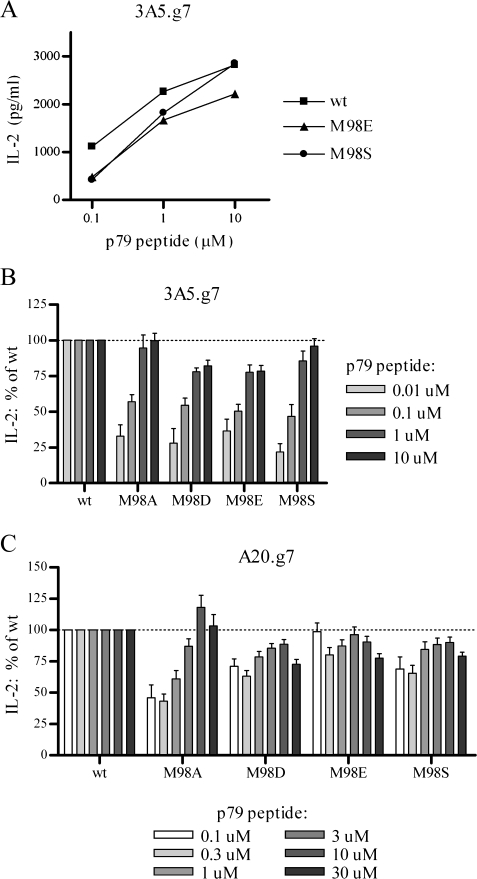
To determine the effect of Ii CLIP mutants on peptide presentation by I-Ag7, we used stable 3A5.g7 or A20.g7 Ii transfectants to present exogenously added p79 mimetope peptide to a BDC2.5 T-cell hybridoma. Despite 2- to 4-fold increases in surface I-Ag7 molecules in 3A5.g7 cells with high-CLIP-affinity Ii mutants, presentation of the p79 peptide was substantially diminished (Fig. 6A and B). The effect is most pronounced at lower, more physiologically relevant peptide concentrations and is largely (M98D and E) or completely (M98A and S) overcome by the addition of excess peptide. As expected, the effect of high-affinity CLIP mutants on antigen presentation is reduced in DM+ A20.g7 cells, where the majority of CLIP peptides are efficiently removed by DM and replaced by diverse peptides during DM-supervised peptide editing and only the small portion of class II that stochastically escapes DM editing is affected by the retained mutant CLIP (Fig. 6C). Despite high sequence homology of I-Ag7 with I-Ad, the p79 peptide is not efficiently presented to the BDC2.5 hybridoma by I-Ad: presentation of p79 by parental 3A5 cells (without transfected I-Ag7β) was below the level of detection (data not shown). Nonetheless, as a precaution, we used the I-Ad-specific mAb MK-D6 for pre-blocking and throughout the incubation with antigen and T cells.
Fig. 6.
High-CLIP-affinity Ii reduces unsupervised peptide loading. Presentation of the I-Ag7-restricted 1040-79 (p79) mimetope peptide by stable 3A5.g7 or A20.g7 Ii transfectants was detected by a BDC2.5 T-cell hybridoma. IL-2 production was measured by ELISA. (A) Presentation of exogenously added peptide by 3A5.g7 (DM-) Ii transfectants. One representative experiment of five is shown. Data shown as mean of highly reproducible duplicates. (B and C) Summary of data from multiple experiments. For each antigen concentration, IL-2 production is normalized to wt within the same experiment. (B) Presentation of exogenously added p79 peptide by 3A5.g7 transfectants; n = 5 for 0.1, 1 and 10 μM peptide and n = 2 for 0.01 μM peptide. (C) Presentation of exogenously added p79 peptide by A20.g7 transfectants; n = 5 for 0.1, 1 and 10 μM peptide and n = 4 for 0.3, 3 and 30 μM peptide.
Discussion
The affinity of I-Ag7 for wt CLIP has been somewhat controversial because of a report that NOD splenic B cells express more cell surface class II/CLIP complexes than splenic B cells from other strains (48). However, the balance of available evidence demonstrates that I-Ag7 has remarkably low affinity for wt CLIP (15, 31, 34, 49–52), and our findings confirm that I-Ag7 behaves as a low-CLIP-affinity allele in multiple assays. CLIP variants with mutations that increase their affinity for I-Ag7 perform a chaperoning role independent of full-length Ii by reducing I-Ag7 turnover, with consequent increased expression levels, and by constraining peptide exchange to the traditional DM-supervised pathway. Our current findings with I-Ag7 corroborate our previous findings with I-Ed (16) and support the conclusion that chaperoning by CLIP is a general mechanism of class II regulation. The effects on both alleles are substantially reduced in the presence of DM, indicating that CLIP chaperoning in DM+ animals is likely to have the highest impact in cells where DM function is limited.
Interestingly, the effects of high-CLIP-affinity Ii mutants on I-Ag7 are of much greater magnitude than similar effects on I-Ed (16), even though I-Ed has almost immeasurably low affinity for wt CLIP (10, 11). It is likely that this increased dependence on CLIP chaperoning is due to lower inherent dimer stability of I-Ag7 compared with I-Ed. Indeed, this would be consistent with the finding that assembly and expression of haplotype-mismatched dimers show increased dependence on Ii [reviewed in (40)]. We have previously proposed that overall class II phenotypes and expression levels may be dictated by an interplay of DM activity, CLIP affinity and inherent αβ dimer stability (8).
We find it intriguing that small uncharged P9 anchors (M98A and S) have different effects than large acidic (negatively charged) P9 anchors (M98D and E). P9 D and E anchors are generally thought to be optimal for peptide binding to I-Ag7, but it has become clear that these anchors are not required, that small uncharged P9 anchors (such as P9 A and S) are also preferred and that smaller contributions may also be made by interactions at other traditional and non-traditional anchor pockets [data presented here, studies reviewed in (5), and (34–36)]. An extensive molecular modeling study predicts a pH-dependent peptide-binding motif for I-Ag7 such that peptides with acidic P9 anchors are highly preferred during peptide loading in endosomes (low pH), but less stable at the cell surface (neutral pH), while peptides with small uncharged anchors that survive endosomal pH are more energetically favorable at the cell surface, resulting in a ‘locking into place’ effect (36). These predictions are partially substantiated by a study that used IC-50 values to assess binding to soluble I-Ag7 of CLIP variants with single amino acid substitutions at P9 (15). Data from this study argue that P9 A and S are highly favorable anchors in the context of a CLIP peptide at both neutral and acidic pH and that binding of at least one mutant (M98A) at acidic versus neutral pH conforms to the pH-dependent motif model.
CLIP, first in the context of full-length Ii and then as an independent peptide, moves from the ER (neutral pH) to endosomes (acidic) to the cell surface (neutral) and finally to recycling compartments (mildly acidic), with different possible consequences for large charged versus small uncharged P9 anchors at each phase. In our system, M98A and S Ii mutants have the greatest effect on I-Ag7 half-life and abundance, consistent with a possible advantage during assembly in the ER and extended survival at the cell surface due to the preference for small uncharged P9 anchors in neutral environments. In contrast, M98D and E CLIP may provide the greatest advantage for I-Ag7/CLIP survival during the initial stay in (acidic) endosomes. Unlike most Ii CLIP mutants for I-Ag7 (and I-Ed), variants with large acidic (negatively charged) P9 CLIP anchors (M98D and E) inhibited peptide presentation even in the presence of excess exogenous p79 peptide (Fig. 6B). Peptide presentation in this context is a complicated balance of varying I-Ag7 abundance and a pH-dependent binding motif, but this result likely reflects the especially strong preference for P9 D or E anchors at low pH (making bound M98D or E CLIP particularly difficult to displace), as most ‘cell surface’ peptide exchange probably occurs in recycling compartments with somewhat reduced pH. Large charged and small uncharged P9 anchors may also have distinct advantages at different stages of the class II pathway based on their ability to affect overall I-Ag7 conformation (53).
Various experimental systems have indicated that a subset of class II alleles can be successfully assembled into dimers in the absence of Ii (e.g. I-Ag7 in our 2A-12 cells). Thus, it is often assumed that α- and β-chains form dimers first and subsequently bind to Ii trimers. However, several studies provide evidence that individual α- or β-chains are capable of associating with Ii and of binding peptides or unfolded segments of larger proteins, including the CLIP region of Ii, in a manner dependent on standard binding groove/anchor interactions [reviewed in (40, 47, 54)]. Our findings that small amounts of transfected mutant Ii compete effectively with an excess of endogenous wt (low-affinity) Ii for I-Ag7 and I-Ed binding and that high-CLIP-affinity Ii is disproportionately represented in nonameric complexes with I-Ag7 (and vice versa), likely reflecting preferential assembly of high-CLIP-affinity Ii/class II complexes, do not distinguish between these models but argue that CLIP contact is a critical step in nonamer assembly that is ultimately dominant over other regions of Ii/class II interaction. This would predict effects on allelic expression, particularly in cells where Ii levels are not in molar excess over class II dimers or where multiple chains compete for a common partner. This pivotal role for CLIP in class II/Ii assembly is also consistent with the importance of groove occupancy for completion of class II folding, release from general ER chaperones, and overcoming kinetic or energetic limitations to stable subunit associations (e.g. mitigating the extensive electrostatic repulsion within the positively charged P9 pocket of I-Ag7) (8, 36, 40).
We previously described several models for how varying class II/CLIP affinity may influence susceptibility to autoimmunity, including effects related to stability, abundance and half-life of class II as well as modulation of access to non-traditional pathways of antigen presentation that circumvent proper supervision by DM (8, 14, 16). Studies in animal models of autoimmunity will be necessary to demonstrate a direct effect of varying CLIP affinity on disease pathogenesis, and the Ii CLIP mutants characterized in this study will be valuable tools in this endeavor. As class II MHC alleles define the single greatest genetic risk factor for the majority of autoimmune disorders, a potential opportunity for clinical intervention remains untapped until the mechanism of this association is clearly defined. We currently believe that a general deficiency in interaction of class II alleles with chaperones in the class II pathway, including Ii [this study and (8, 14, 16)] and possibly DM (55), establishes a context of vulnerability to autoimmunity. Within this context, more specific features of the class II alleles along with the influence of background genes may determine the specificity of an autoimmune reaction and the target organs.
Funding
National Institutes of Health (NIH) (E.D.M); NIH Immunology Training Grant (T32 AI07290 to C.H.R. and O.C.); the National Science Foundation (C.H.R.); the Arthritis Foundation (E.D.M.); the Juvenile Diabetes Research Foundation (E.D.M.); the American College of Rheumatology’s Research and Education Foundation (E.D.M.).
Acknowledgments
We thank our collaborators for generously sharing important reagents as indicated in Methods. We also thank Dr Kathryn Haskins and Gene Barbour for their assistance with related experiments.
References
- 1.Acha-Orbea H, McDevitt HO. The first external domain of the nonobese diabetic mouse class II I-A beta chain is unique. Proc. Natl Acad. Sci. USA. 1987;84:2435. doi: 10.1073/pnas.84.8.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 3.Kyburz D, Corr M. The KRN mouse model of inflammatory arthritis. Springer Semin. Immunopathol. 2003;25:79. doi: 10.1007/s00281-003-0131-5. [DOI] [PubMed] [Google Scholar]
- 4.Liu GY, Baker D, Fairchild S, et al. Complete characterization of the expressed immune response genes in Biozzi AB/H mice: structural and functional identity between AB/H and NOD A region molecules. Immunogenetics. 1993;37:296. doi: 10.1007/BF00187458. [DOI] [PubMed] [Google Scholar]
- 5.Suri A, Levisetti MG, Unanue ER. Do the peptide-binding properties of diabetogenic class II molecules explain autoreactivity? Curr. Opin. Immunol. 2008;20:105. doi: 10.1016/j.coi.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nepom GT, Erlich H. MHC class-II molecules and autoimmunity. Annu. Rev. Immunol. 1991;9:493. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- 7.Alfonso C, Karlsson L. Nonclassical MHC class II molecules. Annu. Rev. Immunol. 2000;18:113. doi: 10.1146/annurev.immunol.18.1.113. [DOI] [PubMed] [Google Scholar]
- 8.Busch R, Rinderknecht CH, Roh S, et al. Achieving stability through editing and chaperoning: regulation of MHC class II peptide binding and expression. Immunol. Rev. 2005;207:242. doi: 10.1111/j.0105-2896.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- 9.Bikoff EK, Germain RN, Robertson EJ. Allelic differences affecting invariant chain dependency of MHC class II subunit assembly. Immunity. 1995;2:301. doi: 10.1016/1074-7613(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 10.Liang MN, Beeson C, Mason K, McConnell HM. Kinetics of the reactions between the invariant chain (85-99) peptide and proteins of the murine class II MHC. Int. Immunol. 1995;7:1397. doi: 10.1093/intimm/7.9.1397. [DOI] [PubMed] [Google Scholar]
- 11.Sette A, Southwood S, Miller J, Appella E. Binding of major histocompatibility complex class II to the invariant chain-derived peptide, CLIP, is regulated by allelic polymorphism in class II. J. Exp. Med. 1995;181:677. doi: 10.1084/jem.181.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornell TM, Burster T, Jahnsen FL, et al. Human dendritic cell expression of HLA-DO is subset specific and regulated by maturation. J. Immunol. 2006;176:3536. doi: 10.4049/jimmunol.176.6.3536. [DOI] [PubMed] [Google Scholar]
- 13.Denzin LK, Fallas JL, Prendes M, Yi W. Right place, right time, right peptide: DO keeps DM focused. Immunol. Rev. 2005;207:279. doi: 10.1111/j.0105-2896.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 14.Patil NS, Pashine A, Belmares MP, et al. Rheumatoid arthritis (RA)-associated HLA-DR alleles form less stable complexes with class II-associated invariant chain peptide than non-RA-associated HLA-DR alleles. J. Immunol. 2001;167:7157. doi: 10.4049/jimmunol.167.12.7157. [DOI] [PubMed] [Google Scholar]
- 15.Hausmann DH, Yu B, Hausmann S, Wucherpfennig KW. pH-dependent peptide binding properties of the type I diabetes-associated I-Ag7 molecule: rapid release of CLIP at an endosomal pH. J. Exp. Med. 1999;189:1723. doi: 10.1084/jem.189.11.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinderknecht CH, Belmares MP, Catanzarite TL, et al. Posttranslational regulation of I-Ed by affinity for CLIP. J. Immunol. 2007;179:5907. doi: 10.4049/jimmunol.179.9.5907. [DOI] [PubMed] [Google Scholar]
- 17.Kim KJ, Kanellopoulos-Langevin C, Merwin RM, Sachs DH, Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 1979;122:549. [PubMed] [Google Scholar]
- 18.Dang LH, Lien LL, Benacerraf B, Rock KL. A mutant antigen-presenting cell defective in antigen presentation expresses class II MHC molecules with an altered conformation. J. Immunol. 1993;150:4206. [PubMed] [Google Scholar]
- 19.Russell HI, York IA, Rock KL, Monaco JJ. Class II antigen processing defects in two H2d mouse cell lines are caused by point mutations in the H2-DMa gene. Eur. J. Immunol. 1999;29:905. doi: 10.1002/(SICI)1521-4141(199903)29:03<905::AID-IMMU905>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 21.Oi VT, Jones PP, Goding JW, Herzenberg LA, Herzenberg LA. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr. Top. Microbiol. Immunol. 1978;81:115. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- 22.Braunstein NS, Germain RN. Allele-specific control of Ia molecule surface expression and conformation: implications for a general model of Ia structure-function relationships. Proc. Natl Acad. Sci. USA. 1987;84:2921. doi: 10.1073/pnas.84.9.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinman RM, Nogueira N, Witmer MD, Tydings JD, Mellman IS. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J. Exp. Med. 1980;152:1248. doi: 10.1084/jem.152.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J. Exp. Med. 1981;153:1198. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boitard C, Bendelac A, Richard MF, Carnaud C, Bach JF. Prevention of diabetes in nonobese diabetic mice by anti-I-A monoclonal antibodies: transfer of protection by splenic T cells. Proc. Natl Acad. Sci. USA. 1988;85:9719. doi: 10.1073/pnas.85.24.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes KA, Mitchell RN. Detection of functional class II-associated antigen: role of a low density endosomal compartment in antigen processing. J. Exp. Med. 1995;181:1715. doi: 10.1084/jem.181.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loss GE, Jr., Elias CG, Fields PE, Ribaudo RK, McKisic M, Sant AJ. Major histocompatibility complex class II-restricted presentation of an internally synthesized antigen displays cell-type variability and segregates from the exogenous class II and endogenous class I presentation pathways. J. Exp. Med. 1993;178:73. doi: 10.1084/jem.178.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharya A, Dorf ME, Springer TA. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J. Immunol. 1981;127:2488. [PubMed] [Google Scholar]
- 29.Ozato K, Mayer NM, Sachs DH. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982;34:113. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Doebele RC, Busch R, Scott HM, Pashine A, Mellins ED. Determination of the HLA-DM interaction site on HLA-DR molecules. Immunity. 2000;13:517. doi: 10.1016/s1074-7613(00)00051-0. [DOI] [PubMed] [Google Scholar]
- 31.Peterson M, Sant AJ. The inability of the nonobese diabetic class II molecule to form stable peptide complexes does not reflect a failure to interact productively with DM. J. Immunol. 1998;161:2961. [PubMed] [Google Scholar]
- 32.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J. Immunol. 2001;166:908. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 33.Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Statist. 1979;6:65. [Google Scholar]
- 34.Reich EP, von Grafenstein H, Barlow A, Swenson KE, Williams K, Janeway CA., Jr. Self peptides isolated from MHC glycoproteins of non-obese diabetic mice. J. Immunol. 1994;152:2279. [PubMed] [Google Scholar]
- 35.Reizis B, Eisenstein M, Mor F, Cohen IR. The peptide-binding strategy of the MHC class II I-A molecules. Immunol. Today. 1998;19:212. doi: 10.1016/s0167-5699(97)01238-3. [DOI] [PubMed] [Google Scholar]
- 36.Moustakas AK, Routsias J, Papadopoulos GK. Modelling of the MHC II allele I-A(g7) of NOD mouse: pH-dependent changes in specificity at pockets 9 and 6 explain several of the unique properties of this molecule. Diabetologia. 2000;43:609. doi: 10.1007/s001250051350. [DOI] [PubMed] [Google Scholar]
- 37.Koonce CH, Bikoff EK. Dissecting MHC class II export, B cell maturation, and DM stability defects in invariant chain mutant mice. J. Immunol. 2004;173:3271. doi: 10.4049/jimmunol.173.5.3271. [DOI] [PubMed] [Google Scholar]
- 38.Koonce CH, Wutz G, Robertson EJ, Vogt AB, Kropshofer H, Bikoff EK. DM loss in k haplotype mice reveals isotype-specific chaperone requirements. J. Immunol. 2003;170:3751. doi: 10.4049/jimmunol.170.7.3751. [DOI] [PubMed] [Google Scholar]
- 39.Rinderknecht CH, Roh S, Pashine A, et al. DM influences the abundance of major histocompatibility complex class II alleles with low affinity for class II-associated invariant chain peptides via multiple mechanisms. Immunology. 2010 doi: 10.1111/j.1365-2567.2010.03282.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Germain RN. Binding domain regulation of MHC class II molecule assembly, trafficking, fate, and function. Semin. Immunol. 1995;7:361. doi: 10.1006/smim.1995.0041. [DOI] [PubMed] [Google Scholar]
- 41.Doebele RC, Pashine A, Liu W, et al. Point mutations in or near the antigen-binding groove of HLA-DR3 implicate class II-associated invariant chain peptide affinity as a constraint on MHC class II polymorphism. J. Immunol. 2003;170:4683. doi: 10.4049/jimmunol.170.9.4683. [DOI] [PubMed] [Google Scholar]
- 42.Kropshofer H, Arndt SO, Moldenhauer G, Hammerling GJ, Vogt AB. HLA-DM acts as a molecular chaperone and rescues empty HLA-DR molecules at lysosomal pH. Immunity. 1997;6:293. doi: 10.1016/s1074-7613(00)80332-5. [DOI] [PubMed] [Google Scholar]
- 43.Glazier KS, Hake SB, Tobin HM, Chadburn A, Schattner EJ, Denzin LK. Germinal center B cells regulate their capability to present antigen by modulation of HLA-DO. J. Exp. Med. 2002;195:1063. doi: 10.1084/jem.20012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramachandra L, Kovats S, Eastman S, Rudensky AY. Variation in HLA-DM expression influences conversion of MHC class II alpha beta:class II-associated invariant chain peptide complexes to mature peptide-bound class II alpha beta dimers in a normal B cell line. J. Immunol. 1996;156:2196. [PubMed] [Google Scholar]
- 45.Newcomb JR, Cresswell P. Structural analysis of proteolytic products of MHC class II-invariant chain complexes generated in vivo. J. Immunol. 1993;151:4153. [PubMed] [Google Scholar]
- 46.Lamb CA, Cresswell P. Assembly and transport properties of invariant chain trimers and HLA-DR-invariant chain complexes. J. Immunol. 1992;148:3478. [PubMed] [Google Scholar]
- 47.Koch N, McLellan AD, Neumann J. A revised model for invariant chain-mediated assembly of MHC class II peptide receptors. Trends Biochem. Sci. 2007;32:532. doi: 10.1016/j.tibs.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Bhatnagar A, Milburn PJ, Lobigs M, Blanden RV, Gautam AM. Nonobese diabetic mice display elevated levels of class II-associated invariant chain peptide associated with I-Ag7 on the cell surface. J. Immunol. 2001;166:4490. doi: 10.4049/jimmunol.166.7.4490. [DOI] [PubMed] [Google Scholar]
- 49.Stratmann T, Apostolopoulos V, Mallet-Designe V, et al. The I-Ag7 MHC class II molecule linked to murine diabetes is a promiscuous peptide binder. J. Immunol. 2000;165:3214. doi: 10.4049/jimmunol.165.6.3214. [DOI] [PubMed] [Google Scholar]
- 50.Munz C, Hofmann M, Yoshida K, et al. Peptide analysis, stability studies, and structural modeling explain contradictory peptide motifs and unique properties of the NOD mouse MHC class II molecule H2-A(g7) Eur. J. Immunol. 2002;32:2105. doi: 10.1002/1521-4141(200208)32:8<2105::AID-IMMU2105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 51.Suri A, Vidavsky I, van der Drift K, Kanagawa O, Gross ML, Unanue ER. In APCs, the autologous peptides selected by the diabetogenic I-Ag7 molecule are unique and determined by the amino acid changes in the P9 pocket. J. Immunol. 2002;168:1235. doi: 10.4049/jimmunol.168.3.1235. [DOI] [PubMed] [Google Scholar]
- 52.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J. Clin. Invest. 2005;115:2268. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardiner A, Richards KA, Sant AJ, Arneson LS. Conformation of MHC class II I-A(g7) is sensitive to the P9 anchor amino acid in bound peptide. Int. Immunol. 2007;19:1103. doi: 10.1093/intimm/dxm081. [DOI] [PubMed] [Google Scholar]
- 54.Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu. Rev. Immunol. 1994;12:259. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 55.Fallang LE, Roh S, Holm A, et al. Complexes of two cohorts of CLIP peptides and HLA-DQ2 of the autoimmune DR3-DQ2 haplotype are poor substrates for HLA-DM. J. Immunol. 2008;181:5451. doi: 10.4049/jimmunol.181.8.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]