Abstract
Background
There are currently limited data on the relationships between resting perfusion abnormalities, LVEF, New York Heart Association (NYHA) functional class, and exercise capacity as defined by peak VO2 and six-minute walk test in patients with heart failure and reduced left ventricular ejection fraction. Furthermore, the association between resting perfusion abnormalities and left ventricular dyssynchrony is currently unknown. This manuscript addresses The Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) gated SPECT imaging (gSPECT) substudy baseline results.
Methods
HF-ACTION was a multi-center, randomized controlled trial of aerobic exercise training vs. usual care in 2331 stable patients with LVEF ≤35% and NYHA class II–IV heart failure symptoms treated with optimal medical therapy. Subjects enrolled in the HF-ACTION sub-study underwent resting Tc 99m tetrofosmin gSPECT at baseline (n=240). Images were evaluated for extent and severity of perfusion abnormalities using a 17-segment and a 5-degree gradation severity score (SRS). LV function and dyssynchrony were assessed using validated available commercial software.
Results
The average age of patients enrolled was 59, 69% were male, 63% were white, and 33% were African-American. Of the 240 participants, 129 (54%) were ischemic and 111 (46%) were non-ischemic in etiology. The median LVEF by gated SPECT for the entire cohort was 26%. Among the nuclear variables, there was a modest correlation between LVEF and SRS (r= − 0.31, p < 0.0001) and there were stronger correlations between phase SD and SRS (r= 0.66, p< 0.0001) as well as phase SD and LVEF (r= −0.50, p< 0.0001). Patients with NYHA class III symptoms had more severe and significant degrees of dyssynchrony, median phase SD 54°, than those with NYHA class II symptoms, median phase SD 39° (p value=0.001). Patients with an ischemic etiology had a higher SRS (p< 0.0001) and significantly more dyssynchrony (p< 0.0001) than those who were nonischemic. However, there was no difference in LVEF or objective measures of exercise capacity between these groups. With respect to peak VO2, there was a weak correlation with LVEF (r = 0.18, p= 0.006) and no correlation with SRS (r = −0.04, p= 0.59) or with dyssynchrony (r= −0.13, p= 0.09). A weak, but statistically significant correlation between SRS and 6-minute walk was observed (r= −0.15, p = 0.047).
Conclusions
Gated SPECT imaging can provide important information in patients with heart failure due to severe LV dysfunction including quantitative measures of global systolic function, perfusion and dyssynchrony. These measurements are modestly but significantly related to symptom severity and objective measures of exercise capacity.
Introduction
Heart failure (HF) currently affects more than 5 million Americans with an estimated 500,000 new diagnoses each year 1. Advances in medical therapy have lead to significant improvements in morbidity and mortality in patients with HF and reduced left ventricular function. In addition to medical therapy, recognition of sudden death due to ventricular dysrhythmias and recent advances in device therapy with implantable cardioverter defibrillators (ICD) have further added to the armamentarium of treatment options for patients with HF 2–4. More recently, the emergence of ventricular dyssynchrony and subsequent treatment with cardiac resynchronization therapy (CRT) in appropriately selected HF patients has been shown to provide incremental improvements in morbidity and mortality in addition to standard background medical therapy 5–7. Despite these major improvements in treatment, the overall morbidity and mortality of patients diagnosed with HF remains high. HF also continues to be a major contributor to the economic burden of cardiovascular care in the United States with an estimated annual cost of approximately $33 billion 8. Given the increasing number of patients, persistently high morbidity and mortality, and escalating economic costs of HF, improvements in risk stratification and attention to less expensive and potentially beneficial therapies such as exercise training are warranted.
The Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION), an NIH/NHLBI funded study, is the largest randomized study of exercise therapy in HF patients to date. The overall HF-ACTION study design and rationale, including inclusion and exclusion criteria, has been previously published 9. Radionuclide imaging has been evaluated as a technique to noninvasively assess myocardial perfusion patterns to help distinguish between ischemic and nonischemic etiologies for HF and provide additional prognostic information 10–15. More recently, evaluation with technetium (Tc) 99m gSPECT imaging allowed for the simultaneous evaluation of perfusion defects, function, and left ventricular volumes that provided a more accurate and reproducible way to noninvasively distinguish between HF of ischemic versus nonischemic etiologies 16, 17. Additionally, the prognostic significance of perfusion defects as assessed by the summed rest score (SRS) on SPECT myocardial perfusion imaging has been well established 18–20.
Recent developments in gSPECT imaging have led to an automated method to extract amplitude and phase from regional LV count changes during gated SPECT imaging as a way to objectively quantify left ventricular dyssynchrony 21–24. Preliminary studies utilizing this novel software application have shown it to be a highly reproducible method with good correlation to traditional echocardiographic markers of dyssynchrony 25–28. However, the relationships of left ventricular dyssynchrony and resting perfusion defects with ischemic etiology, symptom severity, and exercise capacity have not been evaluated. The HF-ACTION gated single-photon emission tomography (gSPECT) substudy provided a valuable opportunity to examine these relationships, and included assessments of resting perfusion abnormalities, left ventricular function, mechanical dyssynchrony, NYHA class, peak VO2, and six-minute walk distance.
Methods
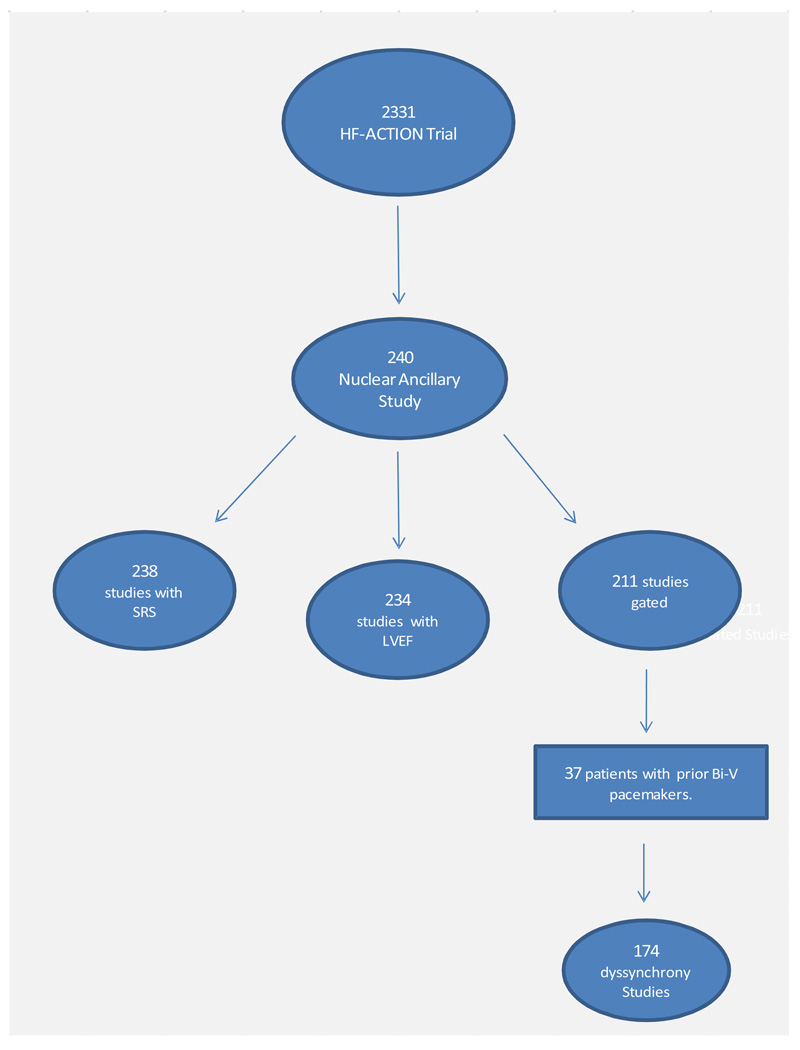
The overall design and rationale for the nuclear substudy of the HF-ACTION trial has been previously published 29. A total of 240 patients were enrolled from the overall study population, of which baseline SRS data was available on 238 (Figure 1). All subjects enrolled in the substudy underwent resting gated perfusion SPECT imaging with a minimum dose of 25 mCi Tc 99m tetrofosmin at baseline and again at 12 months. All images were obtained with a minimum of 30 seconds per step and a minimum of 60 frames at 8 frames per cycle. A standard 64 by 64 matrix low energy/high-resolution collimator was used. It was recommended that a 180° rotation be acquired from a 45° right anterior oblique to a 45° left posterior oblique position. For gated imaging, the R-R window used was within a 20% window of the average electrocardiogram cycle length. The short axis data sets were generated using Butterworth filtering followed by filtered back projection reconstruction and oblique reorientation. SPECT images were processed using standard commercially available software (Emory Toolbox, Emory University; Atlanta, GA). Each nuclear study was independently interpreted and clinically reviewed by 2 experienced nuclear medicine/nuclear cardiology physicians centralized at the nuclear core laboratory who were blinded to the patient information, including randomized treatment assignment. Perfusion defects were semi-quantified using 5 gradations and reported using a 17-segment model for resting perfusion defects to calculate a summed rest score (SRS). LV function and volumes were quantified via automated software processing. For the purposes of phase analysis to evaluate left ventricular mechanical dyssynchrony, phase standard deviation (SD) and bandwidth were evaluated. This method for phase analysis has been incorporated into the Emory Cardiac Toolbox (Emory University/Syntermed, Atlanta, GA). A representative phase histogram is shown in Figure 2.
Figure 1.
240 patients from the overall HF-ACTION trial were enrolled in the nuclear substudy. 238 studies were included for the SRS analysis (2 studies not interpretable). LVEF and volumes were unavailable in 6 studies. There were 29 non-gated studies (i.e. multigated acquisition, MUGA) where LVEF and ventricular volumes were reported, but dyssynchrony analysis could not be performed. An additional 37 patients with bi-ventricular pacemakers were excluded from the dyssynchrony analysis.
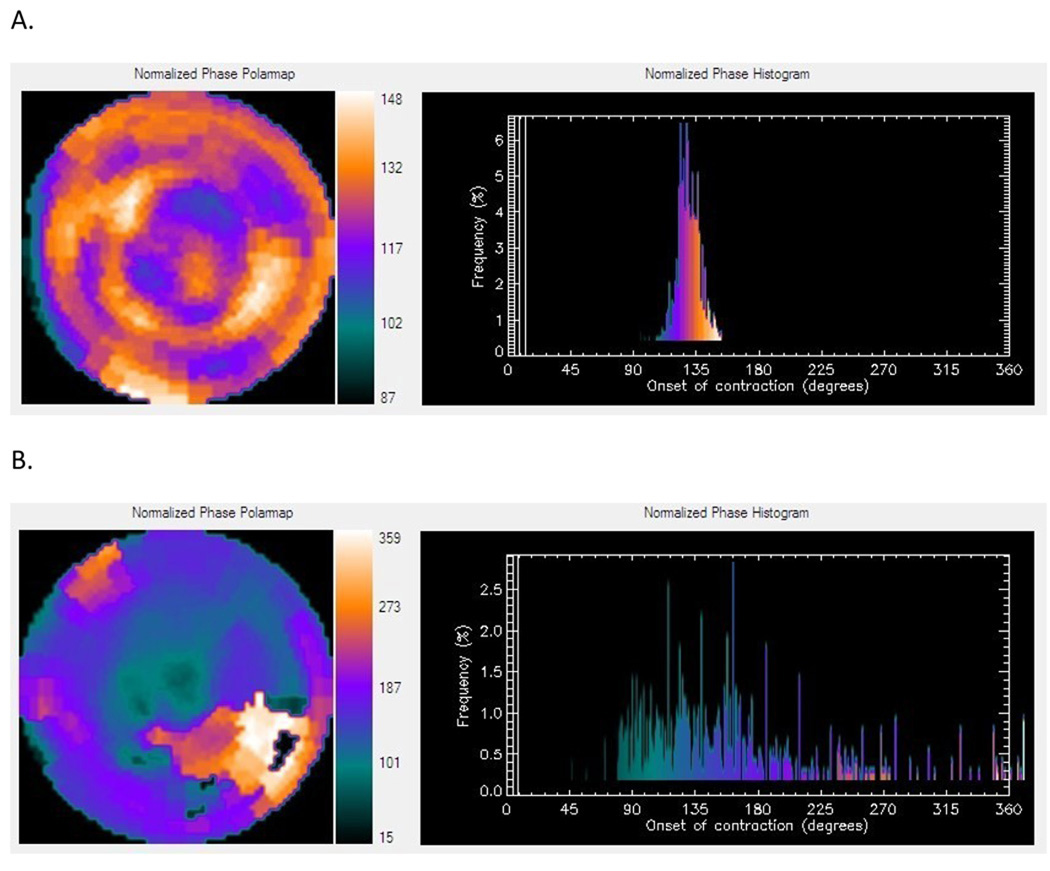
Figure 2.
Representative phase histograms. A) Normal phase histogram: The X-axis represents the timing of one cardiac cycle (R-R interval) normalized in degrees. The Y-axis represents the percent of myocardium demonstrating the onset of mechanical contraction during any particular phase of the cardiac cycle. The color maps have 256 levels with the minimum level corresponding to black and the maximum level corresponding to white. B) Abnormal phase histogram showing a wide bandwidth indicating a delayed onset of myocardial contraction representing significant left ventricular mechanical dyssynchrony.
For descriptive summaries of baseline data, counts and percentages for categorical variables were presented. For continuous measures, emphasis was given to medians and interquartile ranges. Hypothesis tests comparing two groups (ischemic versus non-ischemic etiology of HF, or NYHA class II vs. III) were performed using Wilcoxon rank sum tests. P-values for correlations were derived from an approximate t distribution, using n – 2 degrees of freedom. Scatter plots of the data were used to check for nonlinear relationships between the variables. In HF-ACTION, ischemic etiology was defined as having at least 1 of the following: angiographic stenosis of ≥ 75% in at least 1 major epicardial vessel, history of MI, history of revascularization procedure, or evidence of significant perfusion defect in the setting of ischemic symptoms 29.
Results
The baseline demographics and medical history for the patients enrolled in the nuclear substudy of the HF-ACTION trial are shown in Table 1. Data are presented by ischemic and non-ischemic strata since data have shown different characteristics and outcomes for these two groups of HF patients. The average age of patients enrolled was 59, 69% were male, 63% were white, and 33% were African-American. Of the 240 participants, 129 (54%) were ischemic and 111 (46%) were non-ischemic, which reflects a very balanced but unusual distribution given the predominance of ischemic etiology in the United States. Of the total group, 65% of the patients had a medical history significant for hypertension, 67% for hyperlipidemia, 33% for diabetes mellitus, and 16% for atrial fibrillation or atrial flutter. A total of 128 patients (53%) had a previous history of ICD placement and 44 patients (18%) had a history of bi-ventricular pacemaker placement. Background medical therapy at the time of enrollment is listed in Table 2. There was a very high utilization rate of ACE-I or ARB (94%), beta blocker (96%), and aspirin therapy (73%). Also of note, 48% of the total cohort was on an aldosterone antagonist, 40% were on digoxin, and 72% were on a loop diuretic.
Table 1.
Demographics and Medical History by Stratum
| Medical history | Ischemic (N=129) |
Non-ischemic (N=111) |
Total (N=240) |
|---|---|---|---|
| Age | |||
| Median (25th, 75th) | 63 (55, 70) | 56 (47, 64) | 59 (51, 68) |
| Mean (S.D.) | 63 (11) | 55 (13) | 59 (12) |
| Sex | |||
| Female | 22 (17%) | 52 (47%) | 74 (31%) |
| Male | 107 (83%) | 59 (53%) | 166 (69%) |
| Race | |||
| Black or African American | 27 (21%) | 51 (46%) | 78 (33%) |
| White | 93 (72%) | 57 (51%) | 150 (63%) |
| Other | 9 (7%) | 3 (3%) | 12 (5%) |
| BMI | |||
| Median (25th, 75th) | 29 (26, 33) | 31 (27, 39) | 30 (26, 35) |
| Mean (S.D.) | 30 (6) | 33 (8) | 31 (7) |
| CAD | 107 (83%) | 0 | 107 (45%) |
| Atrial fibrillation/flutter | 23 (18%) | 15 (14%) | 38 (16%) |
| Hypertension | 84 (66%) | 70 (63%) | 154 (65%) |
| Hyperlipidemia | 107 (83%) | 53 (48%) | 160 (67%) |
| Smoking status | |||
| Never | 38 (30%) | 47 (42%) | 85 (36%) |
| Current | 24 (19%) | 20 (18%) | 44 (18%) |
| Past | 66 (52%) | 44 (40%) | 110 (46%) |
| Stroke | 16 (12%) | 11 (10%) | 27 (11%) |
| Diabetes | 55 (43%) | 23 (21%) | 78 (33%) |
| COPD | 27 (21%) | 12 (11%) | 39 (17%) |
| PVD | 12 (9%) | 3 (3%) | 15 (6%) |
| Anemia | 23 (21%) | 14 (17%) | 37 (20%) |
| Mild | 19 (18%) | 12 (15%) | 31 (16%) |
| Moderate to severe | 4 (4%) | 2 (2%) | 6 (3%) |
| CABG | 61 (47%) | 0 | 61 (25%) |
| PCI | 63 (49%) | 0 | 63 (26%) |
| CABG or PCI | 98 (76%) | 0 | 98 (41%) |
| Pacemaker | 24 (19%) | 17 (15%) | 41 (17%) |
| AICD | 83 (64%) | 45 (41%) | 128 (53%) |
| Bi-ventricular pacemaker | 23 (18%) | 21 (19%) | 44 (18%) |
| AICD or Bi-ventricular pacemaker | 87 (67%) | 49 (44%) | 136 (57%) |
| AICD and Bi-ventricular pacemaker | 19 (15%) | 17 (15%) | 36 (15%) |
BMI= body mass index, CAD= history of myocardial infarction, mild anemia: Hgb not less than 10 g/dL but less than 12 g/dL, moderate-severe anemia: Hgb < 10 g/dL, COPD= chronic obstructive pulmonary disease, PVD= peripheral vascular disease, CABG= coronary artery bypass grafting, PCI= percutaneous coronary intervention, AICD= automated internal cardioverter-defibrillator
Table 2.
Medications by Stratum
| Medications | Ischemic (N=129) |
Non-ischemic (N=111) |
Total (N=240) |
|---|---|---|---|
| ACE-I | 86 (67%) | 82 (74%) | 168 (70%) |
| contraindicated/intolerance | 24 (19%) | 20 (18%) | 44 (18%) |
| ARB | 36 (28%) | 27 (24%) | 63 (26%) |
| ACE-I or ARB | 119 (92%) | 106 (95%) | 225 (94%) |
| ACE-I + ARB | 3 (2%) | 3 (3%) | 6 (3%) |
| Beta blocker | 123 (95%) | 107 (96%) | 230 (96%) |
| contraindicated/intolerance | 4 (3%) | 3 (3%) | 7 (3%) |
| Digoxin | 46 (36%) | 50 (45%) | 96 (40%) |
| Spironolactone | 59 (46%) | 49 (44%) | 108 (45%) |
| Eplerenone | 4 (3%) | 2 (2%) | 6 (3%) |
| Aspirin | 106 (82%) | 69 (63%) | 175 (73%) |
| Loop diuretic | 92 (71%) | 80 (72%) | 172 (72%) |
| Non-loop diuretic | 9 (7%) | 8 (7%) | 17 (7%) |
| Antiarrhythmic | |||
| Amiodarone | 18 (14%) | 12 (11%) | 30 (13%) |
| Other antiarrhythmic | 1 (1%) | 2 (2%) | 3 (1%) |
| Lipid-lowering agent | 108 (84%) | 49 (44%) | 157 (65%) |
| HMG-CoA reductase inhibitor | 86 (67%) | 39 (35%) | 125 (52%) |
| Other lipid-lowering agent | 11 (9%) | 10 (9%) | 21 (9%) |
| Clopidogrel | 35 (27 %) | 1 (1%) | 36 (15%) |
| Coumadin | 39 (30%) | 28 (25%) | 67 (28%) |
| Nitrate | 38 (29%) | 15 (14%) | 53 (22%) |
| Calcium channel blocker | 3 (2%) | 4 (4%) | 7 (3%) |
| Insulin | 23 (18%) | 7 (6%) | 30 (13%) |
| Glitazone | 5 (4%) | 2 (2%) | 7 (3%) |
| SSRI | 20 (16%) | 20 (18%) | 40 (17%) |
ACE-I= angiotensin converting enzyme- inhibitor, ARB= angiotensin II receptor blocker, SSRI= selective serotonin reuptake inhibitor
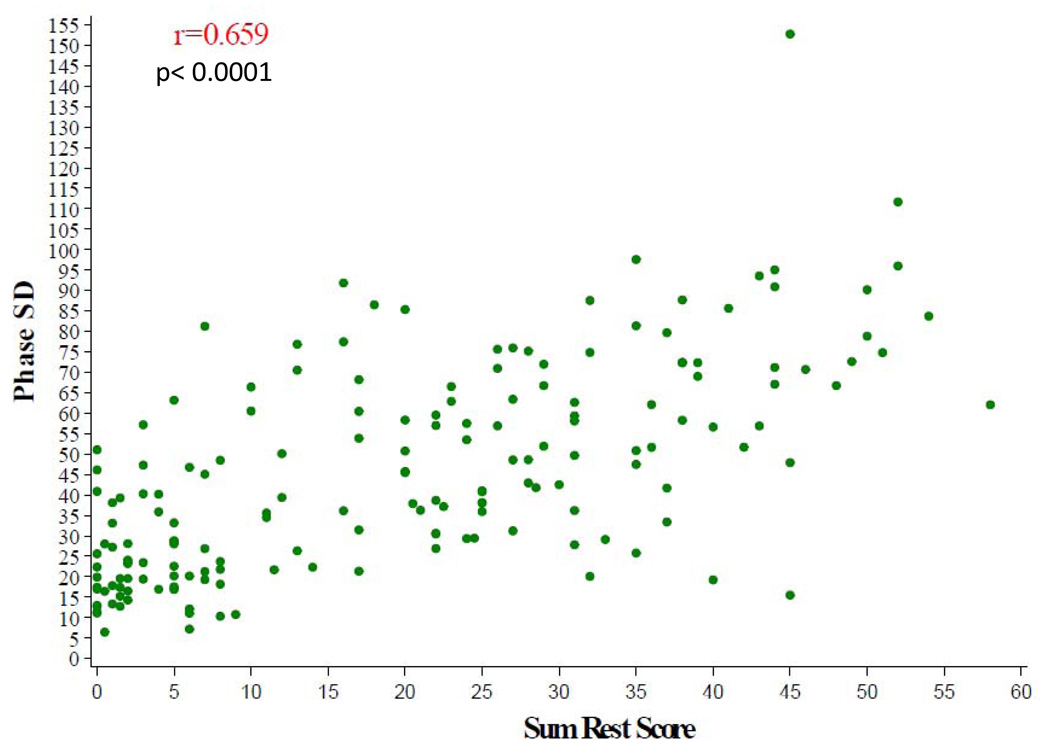
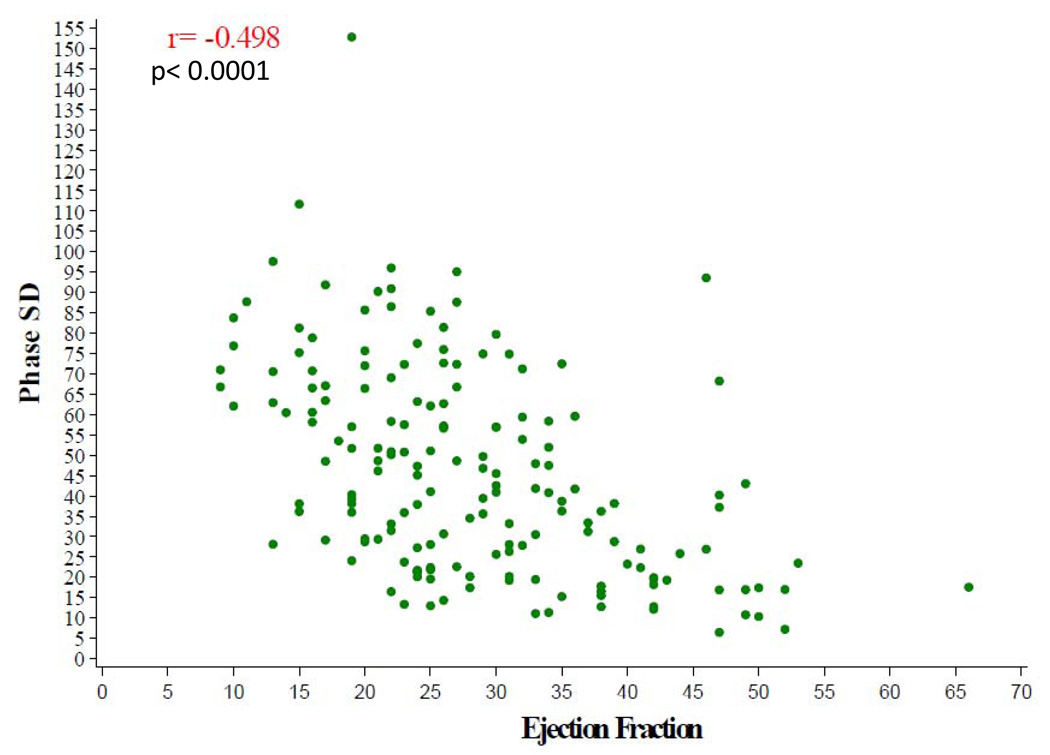
The baseline nuclear characteristics are shown in Table 3. The median LVEF by gated SPECT for the entire cohort was 26% and end systolic and end diastolic volumes were markedly enlarged with median values of 165 mL and 226 mL, respectively. Left ventricular mechanical dyssynchrony as assessed by phase analysis was clinically significant for the total cohort with a median phase SD of 41°. There were strong correlations between phase SD with baseline SRS, (r= 0.66, p< 0.0001) (Figure 3), and with gated SPECT LVEF (r= −0.50, p< 0.0001) (Figure 4). There was a statistically significant, but weaker correlation between gated SPECT LVEF and SRS (r = − 0.31, p< 0.0001).
Table 3.
Nuclear Characteristics by Stratum
| Ischemic | Non-ischemic | Total | p-value | |
|---|---|---|---|---|
| Sum Rest Score, Composite | ||||
| n, Median (25th, 75th) | 128, 29 (18, 39) | 110, 6 (2, 17) | 238, 20 (5, 31) | <0.0001 |
| Mean (S.D.) | 28 (14) | 10 (11) | 20 (15) | |
| Ejection Fraction | ||||
| n, Median (25th, 75th) | 127, 26 (21, 33) | 107, 26 (20, 38) | 234, 26 (21, 34) | 0.56 |
| Mean (S.D.) | 28 (10) | 29 (12) | 28 (11) | |
| ESV (ml) | ||||
| n, Median (25th, 75th) | 127, 167 (121, 244) | 107, 162 (102, 225) | 234, 165 (117, 238) | 0.27 |
| Mean (S.D.) | 187 (88) | 178 (101) | 183 (94) | |
| EDV (ml) | ||||
| n, Median (25th, 75th) | 127, 229 (178, 305) | 107, 224 (152, 287) | 234, 226 (168, 297) | 0.16 |
| Mean (S.D.) | 249 (93) | 236 (106) | 243 (99) | |
| Dyssynchrony Phase SD | ||||
| n, Median (25th, 75th) | 91, 57 (36, 72) | 83, 29 (19, 47) | 174, 41 (23, 63) | <0.0001 |
| Mean (S.D.) | 55 (25) | 35 (21) | 46 (25) | |
| Dyssynchrony Bandwidth | ||||
| n, Median (25th, 75th) | 91, 160 (102, 228) | 83, 82 (60, 137) | 174, 111 (71, 207) | <0.0001 |
| Mean (S.D.) | 164 (81) | 107 (67) | 137 (80) |
ESV= end systolic volume, EDV= end diastolic volume
Figure 3.
SPECT Dyssynchrony Phase SD versus Sum Rest Score
Figure 4.
SPECT Dyssynchrony Phase SD versus EF by SPECT
With respect to the ischemic versus the nonischemic HF strata, there was no difference in LVEF with a median of 26% for each group (p= 0.56). The median SRS (25th, 75th percentile) was 29 (18, 39) for the ischemic stratum and 6 (2, 17) for the nonischemic stratum (p< 0.0001). Also, there was more dyssynchrony in patients with an ischemic etiology compared with those with a nonischemic etiology, median phase SDs of 57° and 29° respectively (p< 0.0001). Interestingly, there was no significant difference in peak VO2 (p=0.35) and 6 minute walk distance (p=0.69) between these groups.
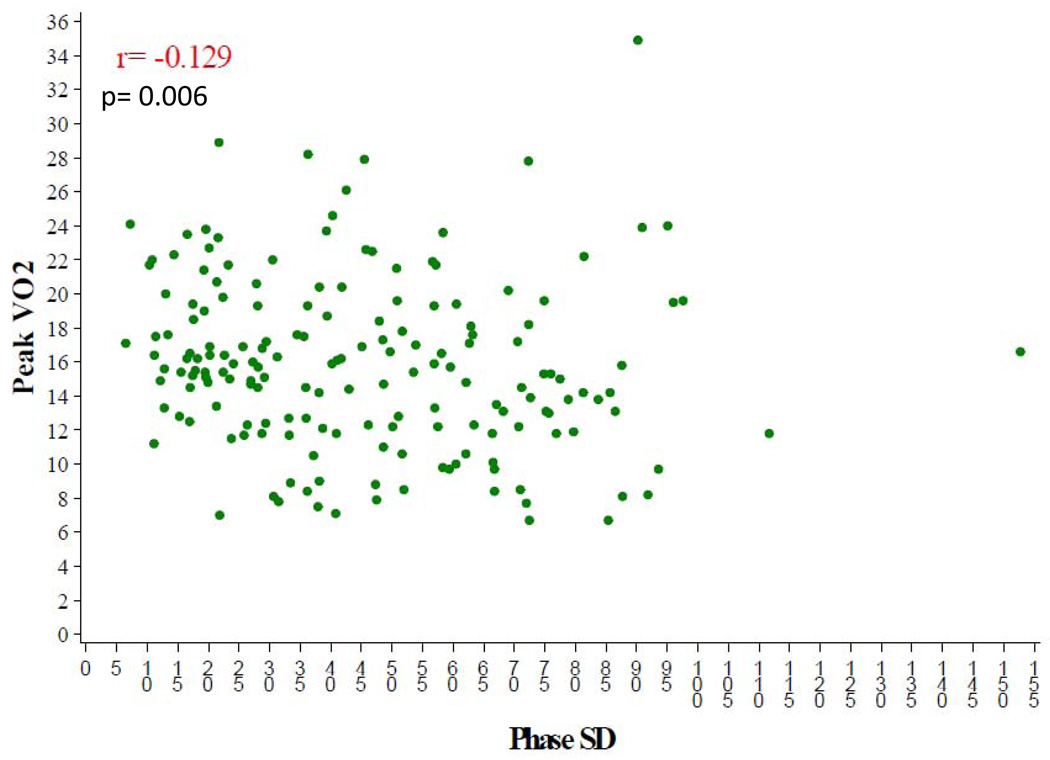
Those with NYHA class III symptoms had significantly more dyssynchrony than those with NYHA class II symptoms as measured by phase SD (median 54° versus 39°, p= 0.001) and histogram bandwidth (median 139° versus 104°, p= 0.025) (Table 4). Table 5 illustrates the baseline exercise variables for the nuclear substudy participants. The median peak VO2 was 15.3 mL/kg/min and the mean exercise duration was 10.0 minutes (IQR 7.4–12.5). The median distance in the six-minute walk test for the overall cohort was 381 meters. There was a relatively weak, but statistically significant correlation between peak VO2 and baseline LVEF, (r=0.178, p=0.006) (Figure 5). There was no correlation between peak VO2 and dyssynchrony (r= −0.13, p=0.09) or between peak VO2 and SRS (r = −0.035, p=0.59).
Table 4.
Comparison of SPECT Dyssynchrony Indices by NYHA Functional Class
| NYHA Class II | Class III | P-value | |
|---|---|---|---|
| Phase SD | |||
| n, Median (25th, 75th) | 116, 39 (20, 57) | 58, 54 (31, 75) | 0.0012 |
| Mean (S.D.) | 41 (23) | 55 (28) | |
| Bandwidth | |||
| n, Median (25th, 75th) | 116, 104 (62, 187) | 58, 139 (84, 217) | 0.0246 |
| Mean (S.D.) | 128 (78) | 155 (81) |
Table 5.
Exercise Variables by Stratum
| Exercise test variables | Ischemic | Non-ischemic | Total | p value |
|---|---|---|---|---|
| Peak VO2 (ml/kg/min) | ||||
| Median (25th, 75th) | 14.8 (12.1, 18.2) | 15.6 (12.7, 19.2) | 15.3 (12.3, 18.5) | 0.35 |
| Mean (S.D.) | 15.5 (4.9) | 15.8 (4.5) | 15.6 (4.7) | |
| Exercise duration (minutes) | ||||
| Median (25th, 75th) | 9.2 (7.4, 12.6) | 10.2 (7.5, 12.4) | 10.0 (7.4, 12.5) | 0.57 |
| Mean (S.D.) | 10.1 (4.4) | 10.1 (3.6) | 10.1 (4.0) | |
| Six-minute walk test | ||||
| Able to walk | 127 (98%) | 108 (97%) | 235 (98%) | 0.69 |
| Distance walked, meters (25th, 75th) | 378 (305, 454) | 390 (301, 454) | 381 (305, 454) | |
| HR at peak exercise (bpm) | ||||
| Median (25th, 75th) | 117 (102, 132) | 127 (110, 141) | 120 (105, 137) | 0.003 |
| Mean (S.D.) | 118 (21) | 126 (22) | 122 (22) | |
| Heart rate reserve (bpm) | ||||
| Median (25th, 75th) | 47 (35, 64) | 54 (39, 70) | 48 (36, 67) | 0.05 |
| Mean (S.D.) | 49 (20) | 54 (21) | 51 (21) |
Figure 5.
Peak VO2 versus SPECT Dyssynchrony Phase SD
Discussion
LVEF, peak VO2, and six-minute walk distance are well-described and powerful prognostic factors in patients with HF and systolic dysfunction 30–35. Several prior studies have established that there is a relative lack of correlation between the degree of left ventricular systolic dysfunction and the severity of HF symptoms and decrease in exercise capacity 36, 37. The present study examined a large group of well-defined patients with systolic LV dysfunction. We assessed radionuclide measures of LV ejection fraction, perfusion, and dyssynchrony and their relationships with subjective ratings of symptoms and objectively measured exercise capacity and 6 minute walk distance. Our findings demonstrate that more severe dyssynchrony is associated with higher SRS and lower LVEF. Furthermore, those with ischemic HF and worsened NYHA class also had more advanced degrees of dyssynchrony. Among the nuclear variables, only LVEF was significantly associated with objective measures of exercise capacity. This relationship, however, was modest at best and explained only 3% of the variability of peak exercise VO2 (r2 = 0.032).
The SRS derived from rest or stress nuclear perfusion imaging is also a validated and independent prognostic variable in the care of patients with cardiovascular disease 19, 20, 38, 39. Given the fact that LVEF, peak VO2, 6-minute walk distance, and SRS have all been previously demonstrated to be independent clinical predictors that represent very different aspects in the broad spectrum of HF patients, it is not unexpected that weaker correlations between these variables were observed.
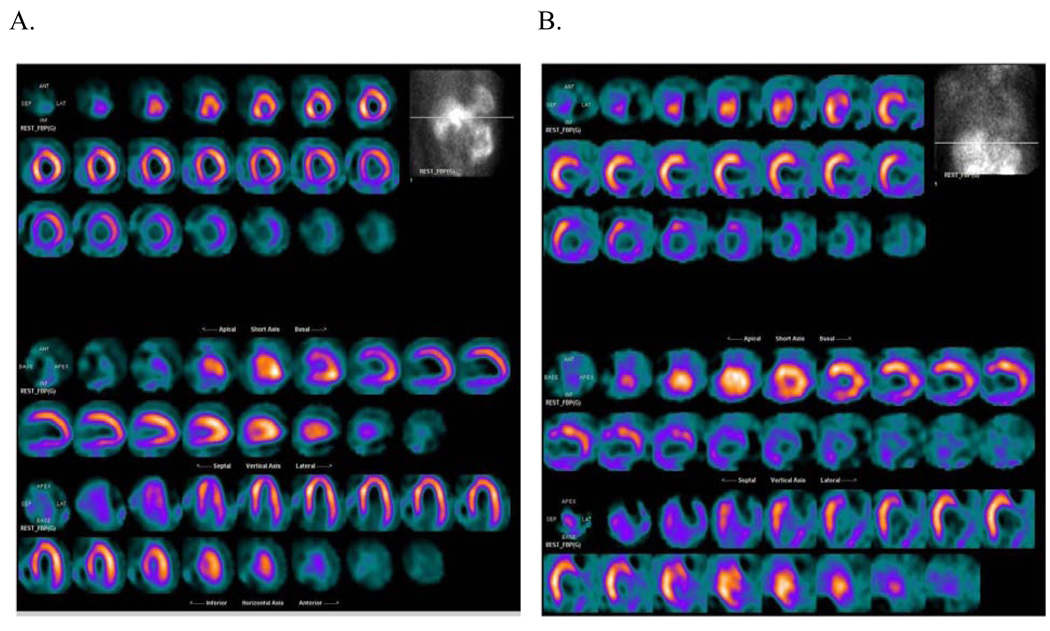
The ability to use gated SPECT perfusion imaging as a noninvasive method to distinguish ischemic from nonischemic etiologies of HF has also been described 16, 40. These data were validated in this study where the ability to distinguish ischemic from nonischemic cardiomyopathy was robust in a blinded core lab interpretation (Table 3 and Figure 6).
Figure 6.
Perfusion tomographs from the HF-ACTION nuclear substudy demonstrating A) a patient with nonischemic cardiomyopathy and a LVEF of 30%. Note that there is only mild decreased tracer activity in the basal inferior segment secondary to attenuation artifact and B) a patient with an ischemic cardiomyopathy and a LVEF of 28%. Note the severe perfusion defects and infarct in the anterolateral, inferolateral, inferior, and inferoapical segments.
CRT is approved for the treatment of HF patients with New York Heart Association class III–IV symptoms, LVEF ≤ 35%, and QRS duration ≥120 milliseconds. Quality of life, NYHA functional class, exercise capacity, ejection fraction, and mortality have all been shown to have significant improvements when CRT is applied to this patient population 5–7, 41. Despite these improvements, approximately 30% of patients fail to benefit from CRT when the current selection criteria are used 42. Noninvasive methods to assess mechanical left ventricular dyssynchrony have traditionally involved echocardiographic parameters derived from M-mode and tissue Doppler imaging. However, the recently reported Predictors of Response to Cardiac Resynchronization Therapy trial (PROSPECT) demonstrated that echocardiography did not improve patient selection for CRT and was also susceptible to high interobserver variability and a lack of reproducibility 43. A novel technique has been developed to quantify mechanical dyssynchrony using phase analysis of gated SPECT myocardial perfusion imaging 21, 25, 28, 44. Potential advantages of this technique include its automation and reproducibility 27. Phase SD and histogram bandwidth are indices derived from this application that have been shown to have a good correlation with traditional echocardiographic markers of dyssynchrony 45. An early study utilizing this technique in patients who met guideline indications for bi-ventricular pacemaker implantation demonstrated that a phase SD ≥ 43° had a reasonable sensitivity and specificity for predicting response to CRT 25.
Patients enrolled in the HF-ACTION nuclear ancillary study had significant degrees of dyssynchrony at baseline. Interestingly, those with an ischemic etiology of HF had significantly more dyssynchrony than those with non-ischemic etiology. This was also demonstrated in the strong correlation between the baseline SRS and phase SD. Not surprisingly, a declining LVEF was also associated with increasing amounts of mechanical dyssynchrony. Weaker correlations between dyssynchrony and peak VO2 as well as dyssynchrony and distance in the 6-minute walk test were observed. However, there was a significant difference in the degree of dyssynchrony between those with NYHA class III and NYHA class II symptoms. These findings are similar to those recently reported by Ypenburg et al. where an association between severity of HF symptoms and worsening mechanical dyssynchrony was also observed 46. SPECT imaging offers unique advantages over noninvasive methods to evaluate dyssynchrony that includes the simultaneous assessment of LVEF, LV volumes, and myocardial perfusion. This is an important consideration as perfusion defects and infarct location have been shown to be independent predictors of response to CRT 47–49.
The assessments of central cardiac function made in the present study had relatively modest relationships with measured peak exercise VO2. This may be partly due to the fact that exercise capacity in patients with HF involves a complex interplay between reduced cardiac output, maladaptive peripheral hemodynamics, and deranged skeletal muscle function and metabolism 50. Important peripheral effects of the HF milieu include impaired arterial formation and response to nitric oxide (NO), reduced skeletal muscle capillary density and abnormal fiber type switching, early lactic acid formation in response to exercise owing to altered intrinsic skeletal muscle metabolism and mitochondrial function, and skeletal muscle apoptosis 50.
One major limitation in the assessment of dyssynchrony in this study is that the clinical applicability of gated SPECT phase analysis has yet to be determined. Also, there are limited data with respect to dyssynchrony as defined by nuclear imaging and its relationship with patient outcomes. However, it still remains a promising new technique to assess mechanical left ventricular dyssynchrony and, with further study, may help improve patient selection for CRT. Another limitation is that of possible selection bias in this trial; which is comprised of patients willing to undergo an exercise program. Thus, relationships such as that of peak VO2 to the etiology of HF could be affected by this bias.
Conclusion
Gated SPECT imaging can provide important information in patients with HF due to severe LV dysfunction including quantitative measures of global systolic function, perfusion and dyssynchrony. These measurements are modestly but significantly related to symptom severity and objective measures of exercise capacity. Patients with the greatest degree of dyssynchrony had lower LVEF and greater amount of perfusion defect/scar. Furthermore, the novel measure of dyssynchrony appears to help discriminate ischemic from non-ischemic patients, correlates with NYHA symptom severity, and is modestly related to objective measures of exercise capacity.
Acknowledgements
A complete list of the HF-ACTION investigators is available as the last item in this supplement. This research was supported by National Institutes of Health grants: 5U01HL063747, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494,5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, 5U01HL064264, 5U01HL066461, R37AG18915, P60AG10484.
Funding: National Institutes of Health, National Heart Lung and Blood Institute and GE Healthcare
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT00047437
Conflicts
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written. Dr. Atchley is a fellow in training and is funded by a National Institutes of Health T32 research grant. Dr. Borges-Neto is part of the speakers’ bureau and advisory board and receives grants from GE Health. Dr. Ellis receives funding support from GE Health. Drs. Kraus, Iskandrian, Whellan, and Kitzman have no conflicts of interest to disclose.
References
- 1.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46(6):e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 5.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289(20):2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 8.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 9.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153(2):201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Saltissi S, Hockings B, Croft DN, Webb-Peploe MM. Thallium-201 myocardial imaging in patients with dilated and ischaemic cardiomyopathy. Br Heart J. 1981;46(3):290–295. doi: 10.1136/hrt.46.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulkley BH, Hutchins GM, Bailey I, Strauss HW, Pitt B. Thallium 201 imaging and gated cardiac blood pool scans in patients with ischemic and idiopathic congestive cardiomyopathy. A clinical and pathologic study. Circulation. 1977;55(5):753–760. doi: 10.1161/01.cir.55.5.753. [DOI] [PubMed] [Google Scholar]
- 12.Dunn RF, Uren RF, Sadick N, Bautovich G, McLaughlin A, Hiroe M, et al. Comparison of thallium-201 scanning in idiopathic dilated cardiomyopathy and severe coronary artery disease. Circulation. 1982;66(4):804–810. doi: 10.1161/01.cir.66.4.804. [DOI] [PubMed] [Google Scholar]
- 13.Tauberg SG, Orie JE, Bartlett BE, Cottington EM, Flores AR. Usefulness of thallium-201 for distinction of ischemic from idiopathic dilated cardiomyopathy. Am J Cardiol. 1993;71(8):674–680. doi: 10.1016/0002-9149(93)91009-7. [DOI] [PubMed] [Google Scholar]
- 14.Iskandrian AS, Hakki AH, Kane S. Resting thallium-201 myocardial perfusion patterns in patients with severe left ventricular dysfunction: differences between patients with primary cardiomyopathy, chronic coronary artery disease, or acute myocardial infarction. Am Heart J. 1986;111(4):760–767. doi: 10.1016/0002-8703(86)90113-4. [DOI] [PubMed] [Google Scholar]
- 15.Chikamori T, Doi YL, Yonezawa Y, Yamada M, Seo H, Ozawa T. Value of dipyridamole thallium-201 imaging in noninvasive differentiation of idiopathic dilated cardiomyopathy from coronary artery disease with left ventricular dysfunction. Am J Cardiol. 1992;69(6):650–653. doi: 10.1016/0002-9149(92)90158-u. [DOI] [PubMed] [Google Scholar]
- 16.Danias PG, Ahlberg AW, Clark BA, 3rd, Messineo F, Levine MG, McGill CC, et al. Combined assessment of myocardial perfusion and left ventricular function with exercise technetium-99m sestamibi gated single-photon emission computed tomography can differentiate between ischemic and nonischemic dilated cardiomyopathy. Am J Cardiol. 1998;82(10):1253–1258. doi: 10.1016/s0002-9149(98)00609-2. [DOI] [PubMed] [Google Scholar]
- 17.Iskandrian AE, Germano G, VanDecker W, Ogilby JD, Wolf N, Mintz R, et al. Validation of left ventricular volume measurements by gated SPECT 99mTc-labeled sestamibi imaging. J Nucl Cardiol. 1998;5(6):574–578. doi: 10.1016/s1071-3581(98)90111-8. [DOI] [PubMed] [Google Scholar]
- 18.Borges-Neto S, Shaw LK, Tuttle RH, Alexander JH, Smith WTt, Chambless M, et al. Incremental prognostic power of single-photon emission computed tomographic myocardial perfusion imaging in patients with known or suspected coronary artery disease. Am J Cardiol. 2005;95(2):182–188. doi: 10.1016/j.amjcard.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97(6):535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 20.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93(5):905–914. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12(6):687–695. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 22.Cooke CD, Ziffer J, Folks RD, Garcia EV. A count-based method for quantifying myocardial thickening from SPECT 99m Tc sestamibi studies: description of the method. J Nucl Med. 1991;32:1068. (abstract) [Google Scholar]
- 23.Cooke CD, Garcia EV, Cullom SJ, Faber TL, Pettigrew RI. Determining the accuracy of calculating systolic wall thickening using a fast Fourier transform approximation: a simulation study based on canine and patient data. J Nucl Med. 1994;35(7):1185–1192. [PubMed] [Google Scholar]
- 24.Cooke CD, Garcia EV, Cullom SJ, Faber T, Pettigrew RI. Technetium-99m sestamibi myocardial gated SPECT simulation for determining the accuracy of count-based systolic wall thickening measurements. J Nucl Med. 1993;34:96. (abstract) [Google Scholar]
- 25.Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49(16):1708–1714. doi: 10.1016/j.jacc.2007.01.063. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Garcia EV, Lerakis S, Henneman MM, Bax JJ, Trimble MA, et al. Left ventricular mechanical dyssynchrony as assessed by phase analysis of ECG-gated SPECT myocardial perfusion imaging. Echocardiography. 2008;25(10):1186–1194. doi: 10.1111/j.1540-8175.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- 27.Trimble MA, Velazquez EJ, Adams GL, Honeycutt EF, Pagnanelli RA, Barnhart HX, et al. Repeatability and reproducibility of phase analysis of gated single-photon emission computed tomography myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun. 2008;29(4):374–381. doi: 10.1097/MNM.0b013e3282f81380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trimble MA, Borges-Neto S, Smallheiser S, Chen J, Honeycutt EF, Shaw LK, et al. Evaluation of left ventricular mechanical dyssynchrony as determined by phase analysis of ECG-gated SPECT myocardial perfusion imaging in patients with left ventricular dysfunction and conduction disturbances. J Nucl Cardiol. 2007;14(3):298–307. doi: 10.1016/j.nuclcard.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 29.Bensimhon DR, Adams GL, Whellan DJ, Pagnanelli RA, Trimble M, Lee BA, et al. Effect of exercise training on ventricular function, dyssynchrony, resting myocardial perfusion, and clinical outcomes in patients with heart failure: a nuclear ancillary study of Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION); design and rationale. Am Heart J. 2007;154(1):46–53. doi: 10.1016/j.ahj.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Wong M, Staszewsky L, Latini R, Barlera S, Glazer R, Aknay N, et al. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: Valsartan heart failure trial (Val-HeFT) echocardiographic data. J Am Coll Cardiol. 2004;43(11):2022–2027. doi: 10.1016/j.jacc.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 31.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112(24):3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 32.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 33.Pardaens K, Van Cleemput J, Vanhaecke J, Fagard RH. Peak oxygen uptake better predicts outcome than submaximal respiratory data in heart transplant candidates. Circulation. 2000;101(10):1152–1157. doi: 10.1161/01.cir.101.10.1152. [DOI] [PubMed] [Google Scholar]
- 34.Roul G, Germain P, Bareiss P. Does the 6-minute walk test predict the prognosis in patients with NYHA class II or III chronic heart failure? Am Heart J. 1998;136(3):449–457. doi: 10.1016/s0002-8703(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 35.Shah MR, Hasselblad V, Gheorghiade M, Adams KF, Jr, Swedberg K, Califf RM, et al. Prognostic usefulness of the six-minute walk in patients with advanced congestive heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2001;88(9):987–993. doi: 10.1016/s0002-9149(01)01975-0. [DOI] [PubMed] [Google Scholar]
- 36.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 37.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327(10):685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 38.Marwick TH, Shaw LJ, Lauer MS, Kesler K, Hachamovitch R, Heller GV, et al. The noninvasive prediction of cardiac mortality in men and women with known or suspected coronary artery disease. Economics of Noninvasive Diagnosis (END) Study Group. Am J Med. 1999;106(2):172–178. doi: 10.1016/s0002-9343(98)00388-x. [DOI] [PubMed] [Google Scholar]
- 39.Shaw LJ, Hendel RC, Heller GV, Borges-Neto S, Cerqueira M, Berman DS. Prognostic estimation of coronary artery disease risk with resting perfusion abnormalities and stress ischemia on myocardial perfusion SPECT. J Nucl Cardiol. 2008;15(6):762–773. doi: 10.1007/BF03007357. [DOI] [PubMed] [Google Scholar]
- 40.Danias PG, Papaioannou GI, Ahlberg AW, O'Sullivan DM, Mann A, Boden WE, et al. Usefulness of electrocardiographic-gated stress technetium-99m sestamibi single-photon emission computed tomography to differentiate ischemic from nonischemic cardiomyopathy. Am J Cardiol. 2004;94(1):14–19. doi: 10.1016/j.amjcard.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 42.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, et al. Cardiac resynchronization therapy: Part 1--issues before device implantation. J Am Coll Cardiol. 2005;46(12):2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 44.Trimble MA, Borges-Neto S, Honeycutt EF, Shaw LK, Pagnanelli R, Chen J, et al. Evaluation of mechanical dyssynchrony and myocardial perfusion using phase analysis of gated SPECT imaging in patients with left ventricular dysfunction. J Nucl Cardiol. 2008;15(5):663–670. doi: 10.1016/j.nuclcard.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsan NA, Henneman MM, Chen J, Ypenburg C, Dibbets P, Ghio S, et al. Left ventricular dyssynchrony assessed by two three-dimensional imaging modalities: phase analysis of gated myocardial perfusion SPECT and tri-plane tissue Doppler imaging. Eur J Nucl Med Mol Imaging. 2008;35(1):166–173. doi: 10.1007/s00259-007-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ypenburg C, van Bommel RJ, Borleffs CJ, Bleeker GB, Boersma E, Schalij MJ, et al. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009;53(6):483–490. doi: 10.1016/j.jacc.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 47.Ypenburg C, Schalij MJ, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, et al. Extent of viability to predict response to cardiac resynchronization therapy in ischemic heart failure patients. J Nucl Med. 2006;47(10):1565–1570. [PubMed] [Google Scholar]
- 48.Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113(7):969–976. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 49.Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153(1):105–112. doi: 10.1016/j.ahj.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Duscha BD, Schulze PC, Robbins JL, Forman DE. Implications of chronic heart failure on peripheral vasculature and skeletal muscle before and after exercise training. Heart Fail Rev. 2008;13(1):21–37. doi: 10.1007/s10741-007-9056-8. [DOI] [PubMed] [Google Scholar]