Abstract
Computational investigations of ligand-directed selectivities in Ullmann-type coupling reactions of methanol and methylamine with iodobenzene by β-diketone- and 1,10-phenanthroline-ligated Cu(I) complexes are reported. Density functional theory (DFT) calculations with several functionals were performed on both the nucleophile formation and aryl halide activation steps of these reactions. The origin of ligand-directed selectivities in N- vs. O-arylation reactions as described in a previous publication (J. Am. Chem. Soc. 2007, 129, 3490–3491) were studied and explained. The selectivities observed experimentally are not derived from initial Cu(I)-nucleophile formation, but from the subsequent steps involving aryl halide activation. The arylation may occur via single-electron transfer (SET) or iodine atom transfer (IAT), depending on the electron-donating ability of the ligand and nucleophile. Mechanisms involving either oxidative addition/reductive elimination or sigma-bond metathesis are disfavored. SET mechanisms are favored in reactions promoted by the β-diketone ligand; N-arylation is predicted to be favored in these cases, in agreement with experimental results. The phenanthroline ligand promotes O-arylation reactions via IAT mechanisms in preference to N-arylation reactions, which occur via SET mechanisms; this result is also in agreement with experimental results.
Introduction
Ullmann and Goldberg first reported the coupling of C-C and C–N bonds by copper complexes more than a century ago.1 Until recently, these protocols remained underutilized due to limitations such as low yields, limited scope and lack of selectivity. However, recent reports have generated renewed interest in Ullmann-type reactions in both academic and industrial settings by demonstrating the use of chelating ligands such as β-diketones,2,3 1,2-diamines,4 phenanthrolines,5,6 bipyridines,7 α-amino acids,8 and others.9–14 Increased activity and broadened substrate scope are achieved when these ligands are used in combination with bases such as K3PO4, Cs2CO3 and K2CO3 in copper-catalyzed arylations.
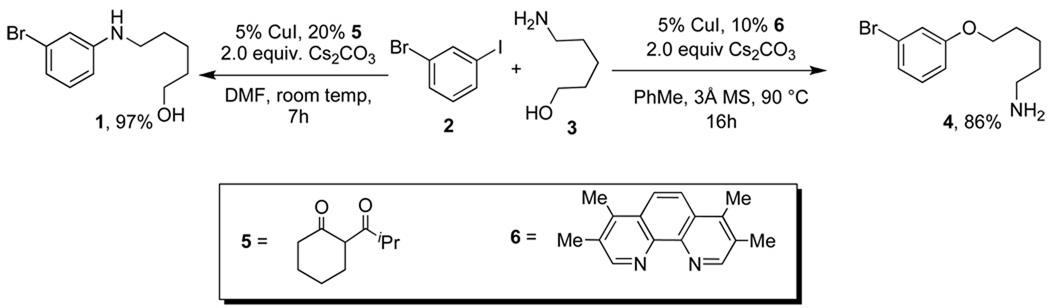
Building on preliminary studies demonstrating copper-catalyzed N- and O-arylation reactions of β-amino alcohols,15 the Buchwald group recently investigated ligand effects on the selectivities of related reactions. β-Diketone 5 promoted the formation of N-arylated products in CuI-catalyzed reactions of 5-amino-1-pentanols with iodoarenes in DMF, but O-arylated products were formed in toluene by switching to the 1,10-phenanthroline ligand, 6 (Scheme 1).16 The β-diketone ligand 5 typically promoted N-arylation to O-arylation in >20:1 ratio; the phenanthroline ligand, 6, typically promoted the formation of a 16:1 ratio of O-arylated product to N-arylated product. A recent examination demonstrated that O-selective reactions are also formed with picolinic acid and N1,N2-dimethylcyclohexane-1,2-diamine (CyDMEDA).17
Scheme 1.
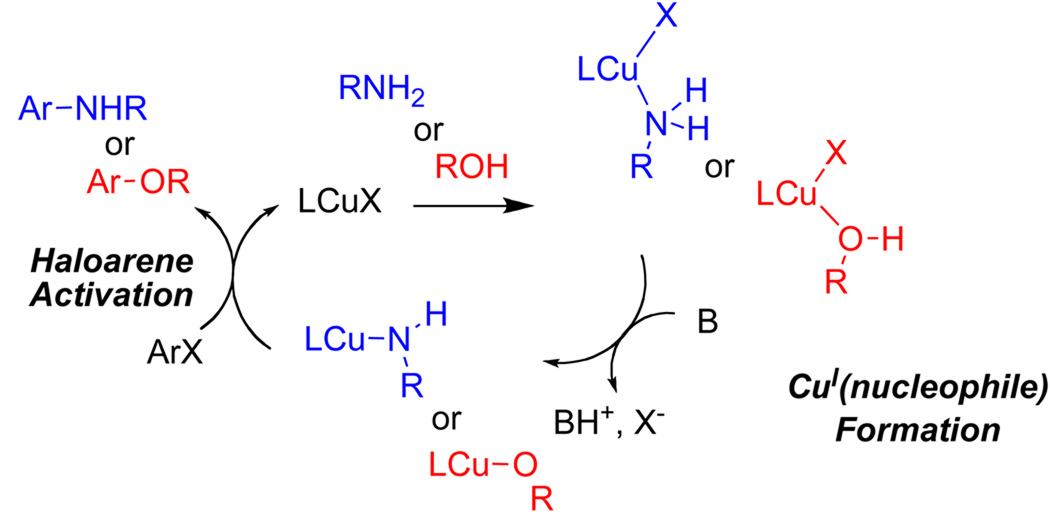
The proposed catalytic cycle is shown in Scheme 2. The CuI(nucleophile) complex is formed via coordination of the alcohol or the amine with the CuI(halide) and subsequent elimination of hydrogen halide. The CuI(nucleophile) complex then reacts with aryl halide to give the arylated products and regenerate the CuI(halide) catalyst. It was proposed16 that ligands influence whether the alcohol or the amine substituent becomes coordinated to the ligated CuI(halide), and influence whether the O-bound or N-bound nucleophiles are more acidic. The pKa of the alcohol bound to (phenanthroline)CuI is presumably much lower than that of the bound amine, thereby leading to the favored formation of the (phenanthroline)CuI(methoxide) complex and, eventually, to O-arylation. In contrast, the electrophilicity of CuI is lowered by the anionic β-diketone ligand; this presumably disfavors binding of the alcohol, and increases the affinity of the amine for CuI. Deprotonation of the bound amine leads to the formation of the N-arylated product.
Scheme 2.
Alternatively, the selectivity differences might arise in the step involving arene activation and coupling with the coordinated nucleophile. This study differentiates between these proposals.
The formation of ligated CuI(nucleophile) complexes in Ullmann-type reactions has been studied experimentally recently; 18,19 the formation of the ligated CuI(nucleophile) complex and subsequent product are highly dependent on the concentration of the chelating ligand. CuI is multiply ligated by the nucleophile at low ligand concentrations; aryl halide activation only occurs after formation of the LCuI(nucleophile) species at intermediate ligand concentrations.18,19 Catalytic activity is diminished at higher ligand concentrations.
A computational study by Guo and coworkers showed the intermediacy of the LCuI(nucleophile) complexes versus other potential copper species in the coupling of aryl halides with amides.20 This investigation confirmed that the concentration of the LCuI(nucleophile) complex far exceeds concentrations of other potential copper species. Additionally, the barrier for oxidative addition of the aryl halide to the LCuI(nucleophile) complex is lower than barriers for reactions involving other complexes. Computational investigations by Tye et al. showed that the reaction of PhI with LCuI(nucleophile) has a lower barrier for oxidative addition in comparison with other potential CuI complexes.19
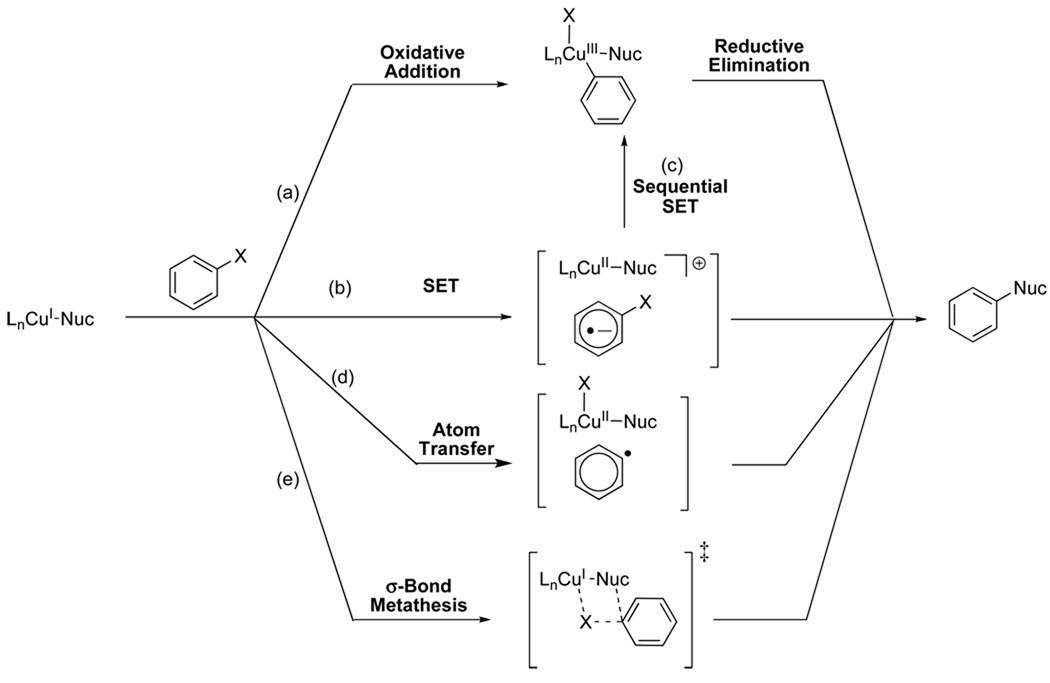
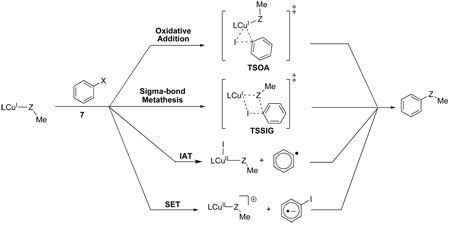
These reports demonstrate that reactions catalyzed by CuI proceed via the initial formation of a CuI(nucleophile) species, but there is no consensus on the mechanisms of subsequent steps involving aryl halide activation. Possible mechanisms based on early work by Kochi,21 Whitesides,22 Johnson23 and Cohen24 are summarized in Scheme 3. The most widely accepted mechanism involves oxidative addition of the CuI(nucleophile) complex to the aryl halide leading to the formation of a CuIII intermediate (Scheme 3a). An alternative proposal involves single-electron transfer (SET) from the CuI(nucleophile) complex to the aryl halide resulting in the formation of a radical pair comprising the radical anion of the aryl halide and a CuII species, i.e. an SRN1 mechanism (Scheme 3b). This radical pair could be directly converted into products, or could form the CuIII intermediate, after a subsequent single electron-transfer (Scheme 3c). An atom transfer mechanism has also been postulated, involving transfer of the halide atom from the aryl halide (Scheme 3d). A recent study has suggested that a four-centered sigma-bond metathesis mechanism (Scheme 3e) could occur.25 A mechanism involving nucleophilic aromatic substitution via a π-complexed organocuprate intermediate has also been proposed,26 but there is scant experimental evidence to support this hypothesis.
Scheme 3.
There is less experimental evidence for mechanisms of aryl halide activation in Ullmann-type reactions involving ligated CuI-complexes. Hida and coworkers have demonstrated that bromoanthraquinone radical anions could be detected by EPR spectroscopy in Ullmann-type reactions promoted by 2-aminoethanol, suggesting that the mechanism involves the oxidation of the CuI species to CuII.27 Whether a CuIII complex was ever formed, or the product was formed directly, could not be ascertained from these investigations. Bethell and coworkers observed that products consistent with the formation of a CuIII intermediate are formed in related reactions of bromoanthraquinone with primary amines.28 Huffman and Stahl have demonstrated that N-arylation occurs via the coupling of CuIII(aryl) species with nitrogen-based nucleophiles.29 This result could be accounted for by the initial formation of CuIII(aryl)(nucleophile) intermediates which reductively eliminate to form C–N coupling products. These results suggest that reductive elimination from CuIII intermediates can occur but do not rule out mechanisms involving formation of CuI or CuII intermediates prior to aryl activation.
Finally, Tye et al. performed experimental investigations which appear to rule out the intermediacy of aryl free radicals and CuII intermediates in related reactions.19 Based on these experiments, the authors concluded that these reactions occur via mechanisms involving either concerted oxidative addition to form a CuIII intermediate or inner-sphere electron transfer. In a notable series of experiments, ligated CuI(nucleophile) complexes were reacted with an aryl halide ortho-substituted with an allyloxy substituent that serves as a radical clock. Only products consistent with C–N coupling and reduction of the arylhalide were formed; cyclized products corresponding to the initial formation of an aryl radical and subsequent cyclization were not detected, suggesting that the reaction of the putative aryl radical with the CuII(nucleophile) complex must be faster than intramolecular cyclization. In fact, results provided in this manuscript suggest that CuII intermediates are too short-lived to be detectable, consistent with these experiments. In another set of experiments, two aryl bromides and an aryl chloride with different reduction potentials but similar rates for halide dissociation from the aryl halide radical anion were reacted with CuI(nucleophile) complexes. While both aryl bromides reacted to form the C–N coupled product, no reaction was observed with the aryl chloride even though it had a greater potential for reduction than the aryl bromides. Based on the differing results obtained with these aryl halides, this experiment could indicate that outer-sphere electron-transfer leading to the formation of the radical anion does not occur. However, this experiment could also imply that aryl chlorides, even with greater potentials for reduction, are less likely to coordinate to copper than aryl bromides to facilitate electron transfer. In fact, few examples are known of the use of aryl chlorides in Cu-catalyzed Ullmann-type reactions. Such reactions typically require forcing conditions, the use of very electron-rich ligands or the use of electron-poor aryl chlorides.30–33 It has not been determined that the mechanisms for reactions involving aryl chlorides are identical to those involving aryl bromides and aryl iodides. Therefore, the fact that no reaction occurs in some examples involving aryl chlorides is not conclusive evidence that aryl halide radical anions are not formed during these types of reactions.
Previous computational investigations by Tye et al.19 and by Guo and coworkers20 on the mechanisms of Ullmann-type reactions have only focused on mechanisms involving oxidative addition; alternative mechanisms involving SET, atom transfer and sigma-bond metathesis were not explored.
The primary goal of this study was the elucidation of the mechanism and source of ligand-directed N- vs. O-selectivities in Ullmann-type arylation reactions. Computational studies with density functional methods suggest that the observed selectivities arise from single electron transfer or iodine atom transfer processes in which short-lived radical pairs are formed before rapidly being converted to products.
Computational Methodology
All calculations were carried out with the Gaussian0334 suite of computational programs. The B3LYP35,36 and MPWB1K37 density functional theory (DFT) methods were used for geometry optimizations and single point energy calculations, respectively.38 The 6–31+G(d,p) basis set was employed for the C, H, N and O atoms for calculations involving B3LYP, and the MG3S39 basis set was employed for calculations involving MPWB1K. Both methods employed the LANL2DZ effective core potentials of Hay and Wadt with double-ζ basis sets for Cu, I and Cs. These calculations were augmented by geometry optimizations with the CPCM40 solvation method with UAKS cavities. Solvent parameters for acetonitrile (ε=36.64) were employed, although DMF (ε=36.71) was used in experiments involving the β-diketone ligand.41 No parameters are available in Gaussian03 for the DMF solvent. Calculations for reactions involving the 1,10-phenanthroline ligand involved the CPCM model for toluene. The Gibbs free energies presented in this article are derived from MPWB1K electronic energies, plus zero-point energy, thermal and entropy corrections from B3LYP calculations, plus solvation energy corrections from the CPCM method.
Results and Discussion
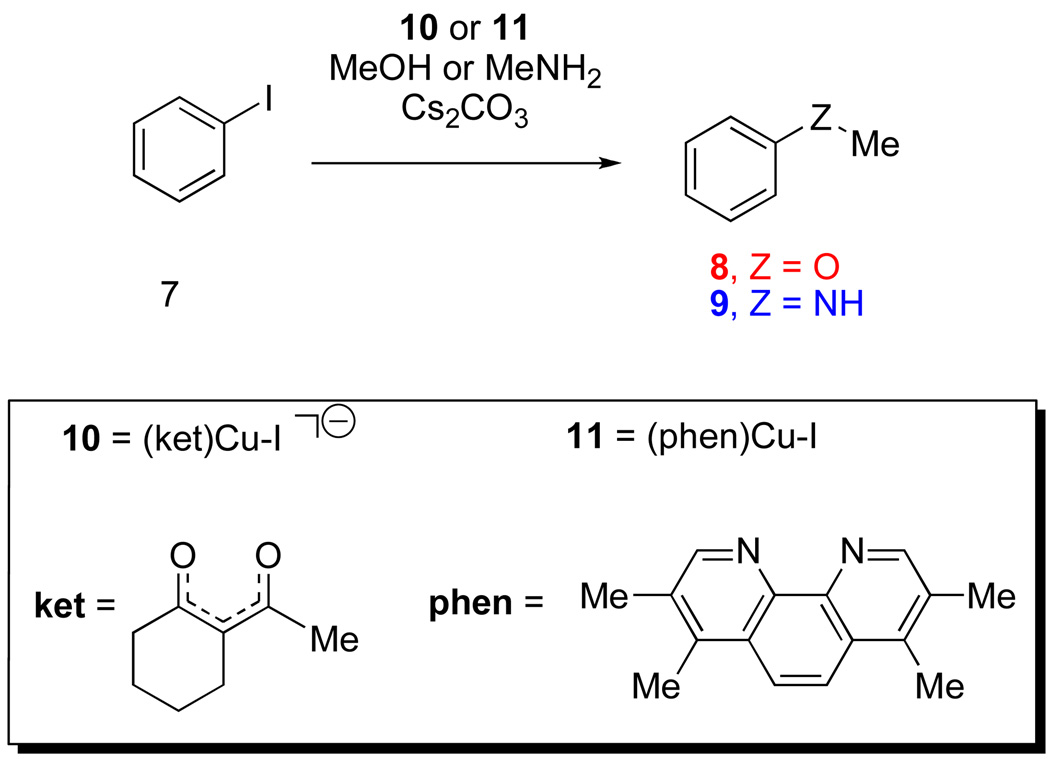
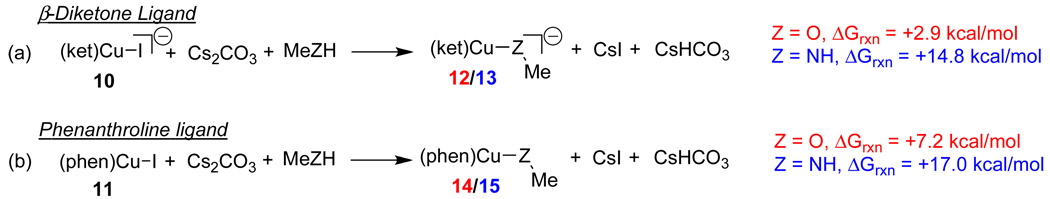
Computational models of reagents used in experiments were employed in an effort to reduce the computational cost associated with these calculations. Computational investigations were performed on separate reactions of iodobenzene with methanol or with methylamine, as models of the aminoalcohols used experimentally. The reactions involving CuI complexes, 10 and 11 (Figure 1) were studied. All energies shown hereafter are referenced to energies for the reactions of methanol and methylamine with iodobenzene catalyzed by the ligated CuI(iodide) complexes.
Figure 1.
Computational models of reagents studied in N- and O-arylation reactions.
The formation of the ligated CuI(nucleophile) complexes was first considered (Figure 2). The formation of the CuI(methoxide) complex is preferred over the CuI(methylamido) complex with both ligands. With the β-diketone ligand, the reaction to form the CuI(methoxide) complex 12 is 12 kcal/mol more exergonic than the reaction to give the CuI(methylamido) complex, 13. With the phenanthroline ligand, the CuI(methoxide) complex is formed in a 10 kcal/mol more exergonic reaction than that of the CuI(methylamido) complex. These results suggest that the observed experimental selectivities most likely do not arise due to the nature of the ligated CuI(nucleophile) complexes, but occur in the aryl halide activation step of the reaction.
Figure 2.
Reaction free energies in kcal/mol in solution for CuI(nucleophile) formation from the reactions between CuI(iodide) complexes 10 and 11 and the methanol or methylamine nucleophiles. Energies are given for reactions with methanol and methylamine, respectively.
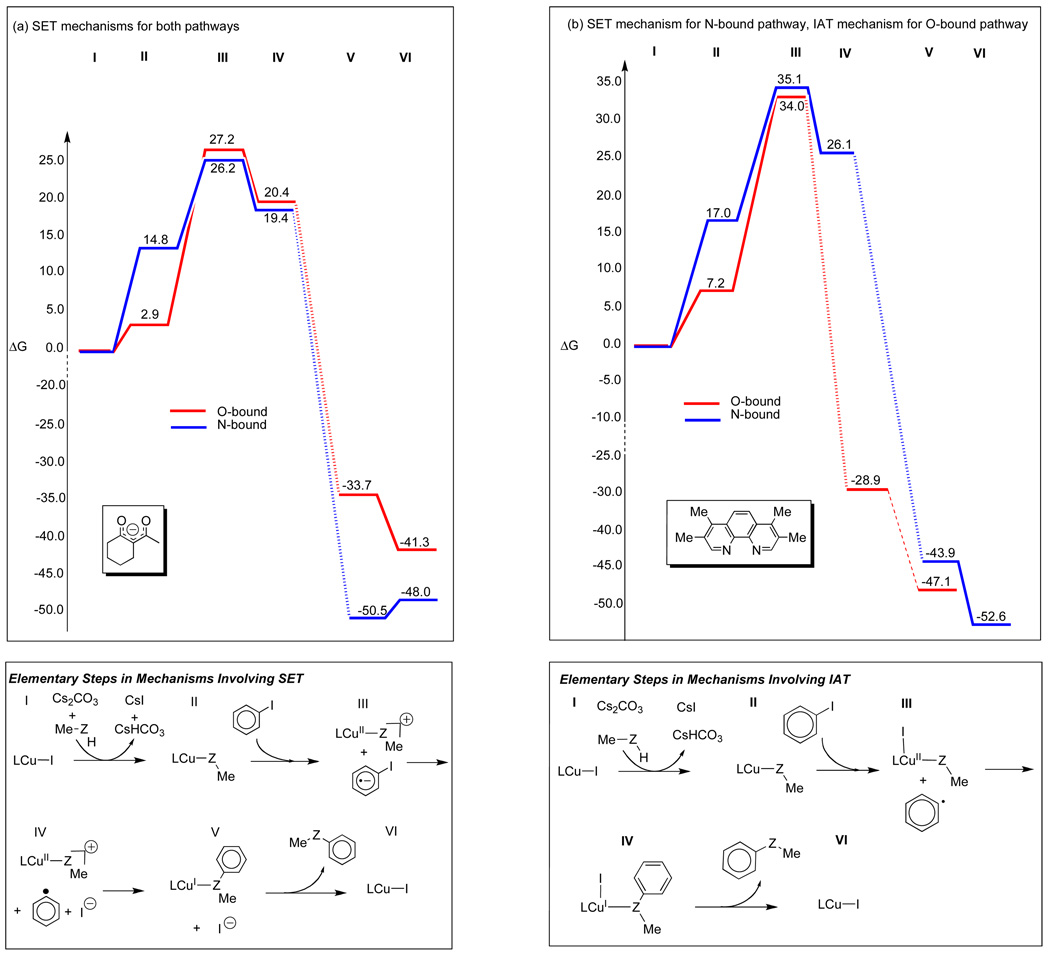
Computed free energies for key species in possible mechanisms for aryl halide activation of β-diketone- and phenanthroline-bound CuI complexes are shown in Table 1. The activation energies for oxidative addition of iodobenzene to the CuI(nucleophile) complexes are much larger than energies computed for key complexes in the SET or IAT mechanisms. The transition structure for oxidative addition to 12 has an energy of 65 kcal/mol. A similar transition state could not be found for oxidative addition of iodobenzene to 13; but the barrier should be higher than the energy of the complex formed after oxidative addition, which has an energy of 55 kcal/mol.
Table 1.
Free energies in kcal/mol for key stationary points in mechanisms of ligand-promoted Ullmann-type N- and O-arylation reactions. (Z = O, NH)
| Cu(ZMe) formation |
TSOA | TSSig | IAT | SET | Product formation |
|
|---|---|---|---|---|---|---|
| (ket)Cu complexes | ||||||
| (MeO)-bound (12) | 2.9 | 64.6 | 57.1 | 32.9 | 27.2 | −41.3 |
| (MeNH)-bound (13) | 14.8 | 55.0a | 65.6 | 41.1 | 26.2 | −48.0 |
| (phen)Cu complexes | ||||||
| (MeO)-bound (14) | 7.2 | 43.2 | 43.4 | 34.0 | 43.6 | −47.1 |
| (MeNH)-bound (15) | 17.0 | 53.7 | 50.9 | 39.6 | 35.1 | −52.6 |
Energy of the oxidative addition complex, see text for details.
Similarly, activation barriers for the oxidative addition of iodobenzene to (phen)CuI(nucleophile) complexes are prohibitively large; 43 and 54 kcal/mol are required for oxidative addition of iodobenzene to the O-bound and N-bound complexes 14 and 15, respectively. The oxidative addition steps involve the transformation of CuI complexes with closed shell d10 electron configurations into CuIII complexes with d8 electron configurations with two unpaired electrons. The barriers for sigma-bond metathesis are also unreasonably large, almost isoenergetic with barriers for oxidative addition.
Barriers for mechanisms involving the transfer of the iodine atom (IAT) from iodobenzene to the CuI complexes and for single-electron transfer (SET) from the CuI complexes to iodobenzene were estimated from the energies of the completely separated CuII(nucleophile) complexes and the iodobenzene ionic radical or benzene radical formed by these processes. Transition states corresponding to iodine atom transfer in these reactions could not be located after many attempts.
Activation free energies for SET mechanisms can also be estimated from the standard free energies of these reactions through Marcus-Hush theory and related formulations. The outer-sphere SET Marcus-Hush theory model is applicable when initial SET proceeds via the formation of an intermediate.42,43 Activation energies due to SET involving electron transfer and accompanying cleavage of the aryl halide bond (i.e. concerted SET) can be derived from Savéant’s model.42,44 “Sticky” SET mechanisms are involved when concerted electron transfer results in the formation of a radical/ion pair in the solvent cage; Savéant’s model can be extended to these cases. The supporting information that accompanies this article demonstrates that activation free energies for SET estimated by Marcus theory are only slightly larger than energies for the formation of the intermediates as presented in Table 1.
The IAT and SET mechanisms require much lower activation energies than oxidative addition or sigma-bond metathesis. The energies required for IAT to form O-bound and N-bound (ket)CuII complexes and the phenyl radical, are 33 and 41 kcal/mol, respectively. SET from 12 and 13 to form the O-bound and N-bound (ket)CuII complexes and the iodobenzene radical anion requires only 27 and 26 kcal/mol, respectively (Table 1). These results suggest that the electron-rich β-diketone ligand promotes the SET mechanism, in which the electron is transferred from the CuI(nucleophile) complex. Although the N-bound CuI(nucleophile) complex is less stable than the O-bound CuI(nucleophile) complex, the N-bound pathway is favored in the SET mechanism since the amido substituent is a better electron-donor than the methoxide substituent, and facilitates electron transfer. This is in agreement with experimentally observed N-selective arylation in reactions promoted by the β-diketone ligand.
The β-diketone and phenanthroline ligands exhibit marked differences in selectivities for mechanisms involving single-electron transfer and iodine atom transfer. In contrast to the β-diketone ligand, the neutral, less electron-rich phenanthroline ligand increases the barriers for SET to 44 and 35 kcal/mol, respectively, for O-bound and N-bound (phen)CuI complexes. In contrast, barriers for IAT are much less sensitive to the effects of ligands. O- and N-bound (phen)CuI complexes require 34 and 40 kcal/mol for the IAT pathway, respectively, very similar to reactions involving the β-diketone complexes. Thus, when phenanthroline is used as a ligand, IAT and SET mechanisms have similar barriers, and may both occur depending on the nucleophile. The Cu-catalyzed O-arylation reaction proceeds via IAT while N-arylation proceeds via SET. This result is a notable departure from reactions involving CuI complexes ligated with the β-diketone ligand in which SET processes are favored for reactions involving both types of nucleophiles. Overall, IAT from iodobenzene to the O-bound CuI complex 14 is more favorable (ΔGIAT = 34 kcal/mol) than SET from the N-bound CuI complex 15 to iodobenzene (ΔGSET = 35 kcal/mol).45 This selectivity is in agreement with the experimentally favored O-selective reactions involving the phenanthroline ligand.46 Finally, we note that the SET reactions involving the β-diketone ligand have comparatively lower energies (26–27 kcal/mol) than reactions involving the phenanthroline ligand (34–35 kcal/mol). This is consistent with the fact that lower temperatures were required to promote reactions with the β-diketone ligand than with the phenanthroline ligand (room temperature vs. 90 °C).
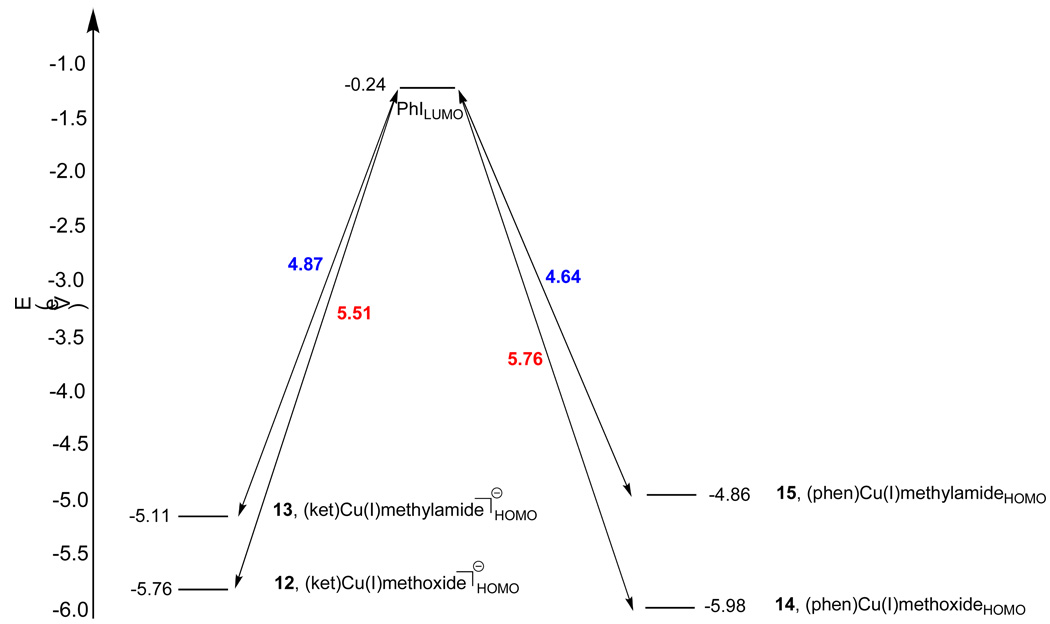
Frontier molecular orbital (FMO) analysis47 of the interactions of the CuI(nucleophile) complexes with iodobenzene reveals that iodobenzene always interacts more favorably with the HOMOs (highest occupied molecular orbitals) of the N-bound CuI(nucleophile) complexes than those of the O-bound complexes. As shown in Figure 3, the CuI(methylamido) complexes 13 and 15 possess higher-lying HOMOs than their analogous CuI(methoxide) complexes, 12 and 14. Consequently, these CuI(methylamido) complexes interact more favorably with the LUMO (lowest unoccupied molecular orbital) of iodobenzene. This is consistent with the fact that amido compounds are generally more electron-rich than alkoxides and therefore possess higher-lying HOMOs that will interact more favorably with electrophiles.48
Figure 3.
Interactions of frontier molecular orbitals of 12, 13, 14 and 15 with the LUMO of iodobenzene.
Stronger FMO interactions between the N-bound complexes and electrophiles results in the selective formation of N-arylated products with the β-diketone ligand. However, O-arylation is promoted by the phenanthroline ligand. This preference is controlled by stronger Cu-O binding in the ligated CuI(nucleophile) complexes. The fact that both N-arylation and O-arylation products are formed despite the inherent >10 kcal/mol preference for the formation of O-bound intermediates suggests that the selectivities are caused by subtle differences in the electronic properties of the phenanthroline and β-diketone ligands in those intermediates which are manifested in the SET or IAT steps of these reactions.
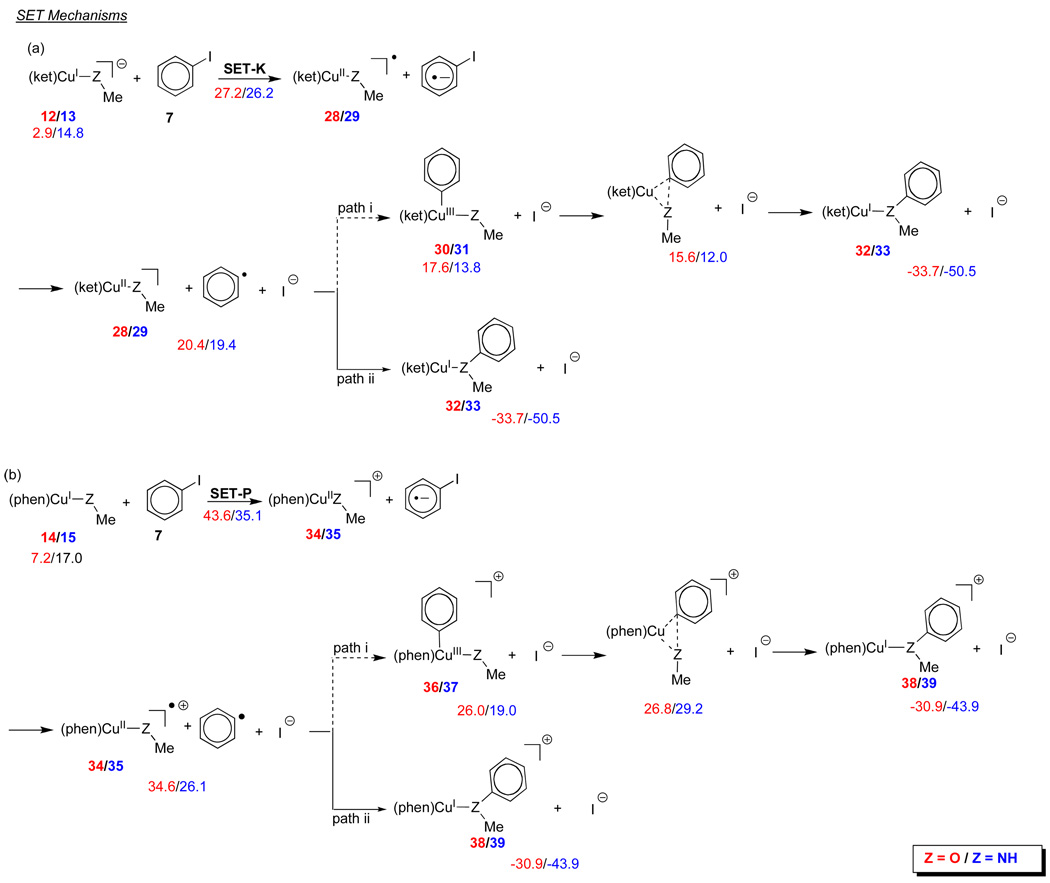
Further analysis of the IAT and SET mechanisms reveals important insights into the nature of the intermediates on the potential energy surfaces of these reactions. Addition of the aryl radical to the metal center of the CuII complex formed by IAT can lead to the formation of ligated CuIII(iodide) complexes, which can then reductively eliminate and form product-ligated CuI complexes (Figure 4 a-b, paths i). These pathways can be ruled out because of the high energies calculated for these CuIII complexes in comparison with the radical intermediates. The alternative pathways involving addition of the phenyl radical to the heteroatom of the nucleophile moiety (Figure 4 a–b, paths ii) are more likely. These are highly exergonic reactions due to the formation of the more stable CuI complexes in which the anisole and N-methylaniline complexes are bound to the metal center.
Figure 4.
Free energies of intermediates in IAT mechanisms involving intermediates 12, 13, 14 and 15. Energies are given for reactions with methanol and methylamine, respectively.
Two separate pathways are also possible in SET mechanisms as shown in Figure 5. The initially formed iodobenzene radical anion fragments to form the iodide anion and the phenyl radical. The phenyl radical could add to the metal center of the ligated CuII(nucleophile) in complexes 28/29 and 34/35 to form CuIII complexes 30/31 and 36/37 from the β-diketone and phenanthroline ligands, respectively. Reductive elimination from these complexes results in the formation of complexes 32/33 and 38/39 in which anisole and N-methylaniline are bound to the metal center. The more likely pathways are highly exothermic process, and involve direct formation of the more stable CuI complexes, 32/33 and 38/39, by attachment of the phenyl radical to the oxygen or nitrogen atoms that are directly attached to the metal center. Mechanisms involving sequential electron transfers can usually be ruled out because of the high energies of the CuIII intermediates compared to the CuI species. Then again, CuIII intermediates such as 30/31 and 36/37 are similar to the CuIII complexes proposed as intermediates in work done by Huffman and Stahl.29
Figure 5.
Free energies of intermediates in SET mechanisms involving intermediates (a) 12, 13 and (b) 14 and 15. Energies are given for reactions with methanol and methylamine, respectively.
In closing, we have provided the detailed energetic profiles for the β-diketone and phenanthroline-promoted reactions of methylamine and methanol with iodobenzene (Figure 7). These mechanisms, whether they involve SET or IAT, involve a CuI/CuII couple. CuIII intermediates are predicted to be inaccessible owing to their high energies. The large exothermicities of reactions involving the formation of CuI(product) complexes from the CuII intermediates suggest that CuII intermediates formed during SET or IAT are likely to be too short-lived to be detectable, in agreement with the observations of Hida and coworkers.27 We postulate that these intermediates are generated, not as free radicals, but as caged radical pairs, that are rapidly converted to products before adventitious reactions can occur. This proposal could rationalize the lack of experimental evidence for the presence of CuII intermediates as well as aryl radicals or aryl halide radical anions in related CuI-catalyzed reactions,19,27 although we do not rule out the possibility that those reactions proceed via alternative mechanisms.
Figure 7.
Free energy profiles for (a) β-diketone and (b) phenanthroline-promoted CuI-catalyzed reactions of methanol and methylamine with iodobenzene. Energies are given for reactions with methanol and methylamine, respectively.
Overall, our results suggest that for phenanthroline- and β-diketone-promoted CuI-catalyzed Ullmann-type reactions of methanol and methylamine with iodobenzene, experimental selectivities arise in the aryl halide activation step of the reaction and not in the nucleophile formation step. Both ligands promote the formation of CuI(methoxide) intermediates in preference to CuI(methylamido) intermediates. The mechanism in the aryl halide activation step is determined by the electron-donating ability of the ligand and the nucleophile. The electron-rich β-diketone ligand promotes SET reactions involving both types of nucleophiles. The rate of SET is faster in reactions involving CuI(methylamido) complexes than in reactions involving the CuI(methoxide) complexes thereby leading to selective N-arylation; this selectivity is enough to overcome the inherent preference for the formation of the (ket)CuI(methoxide) complex. By contrast, the less electron-rich phenanthroline ligand promotes SET in the reaction involving the CuI(methylamido) complex, but promotes IAT in the reaction involving the CuI(methoxide) complex. The combined rate due to the formation of the (phen)CuI(methoxide) and concomitant IAT is faster than the combined rate due to formation of (phen)CuI(methylamido) and SET leading to the formation of O-arylated products.
Supplementary Material
Acknowledgement
We are grateful to the National Institute of General Medical Sciences (NIGMS), National Institutes of Health (NIH) for financial support (Grants GM-58160 to SLB and GM-36700 to KNH), and to the National Science Foundation via the National Center for Supercomputing Applications (NCSA) for supercomputing resources.
Footnotes
Supporting Information Available. Energies and Cartesian coordinates of stationary points from MPWB1K and B3LYP calculations. Complete citation of reference 34. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Ullmann F, Bielecki J. Chem. Ber. 1901;34:2174–2185. [Google Scholar]; (b) Ullmann F. Chem. Ber. 1903;36:2382–2384. [Google Scholar]; (c) Ullmann F, Sponagel P. Chem. Ber. 1905;38:2211–2212. [Google Scholar]; (d) Goldberg I. Chem. Ber. 1906;39:1691–1692. [Google Scholar]
- 2.Shafir A, Buchwald SL. J. Am. Chem. Soc. 2006;128:8742–8743. doi: 10.1021/ja063063b. [DOI] [PubMed] [Google Scholar]
- 3.de Lange B, Lambers-Verstappen MH, Schmieder-van de Vondervoort L, Sereinig N, de Rijk R, de Vries AHM, de Vries JG. Synlett. 2006:3105–3109. [Google Scholar]
- 4.(a) Klapars A, Huang X, Buchwald SL. J. Am. Chem. Soc. 2002;124:7421–7428. doi: 10.1021/ja0260465. [DOI] [PubMed] [Google Scholar]; (b) Antilla JC, Klapars A, Buchwald SL. J. Am. Chem. Soc. 2002;124:11684–11688. doi: 10.1021/ja027433h. [DOI] [PubMed] [Google Scholar]
- 5.Goodbrand HB, Hu NX. J. Org. Chem. 1999;64:670–674. [Google Scholar]
- 6.Kiyomori A, Marcoux J-F, Buchwald SL. Tetrahedron Lett. 1999;40:2657–2660. [Google Scholar]
- 7.(a) Gujadhur R, Venkataraman D, Kintigh JT. Tetrahedron Lett. 2001;42:4791–4793. [Google Scholar]; (b) Gujadhur RK, Bates CG, Venkataraman D. Org. Lett. 2001;3:4315–4317. doi: 10.1021/ol0170105. [DOI] [PubMed] [Google Scholar]
- 8.(a) Ma D, Cai Q, Zhang H. Org. Lett. 2003;5:2453–2455. doi: 10.1021/ol0346584. [DOI] [PubMed] [Google Scholar]; (b) Ma D, Zhang Y, Yao J, Wu S, Tao F. J. Am. Chem. Soc. 1998;120:12459–12467. [Google Scholar]
- 9.Chen YJ, Chen HH. Org. Lett. 2006;8:5609–5612. doi: 10.1021/ol062339h. [DOI] [PubMed] [Google Scholar]
- 10.Cristau H-J, Cellier PP, Spindler J-F, Taillefer M. Chem.-Eur. J. 2004;10:5607–5622. doi: 10.1002/chem.200400582. [DOI] [PubMed] [Google Scholar]
- 11.Shen R, Porco JA. Org. Lett. 2000;2:1333–1336. doi: 10.1021/ol005800t. [DOI] [PubMed] [Google Scholar]
- 12.Fagan PJ, Hauptman E, Shapiro R, Casalnuovo A. J. Am. Chem. Soc. 2000;122:5043–5051. [Google Scholar]
- 13.Kwong FY, Klapars A, Buchwald SL. Org. Lett. 2002;4:581–584. doi: 10.1021/ol0171867. [DOI] [PubMed] [Google Scholar]
- 14.Kwong FY, Buchwald SL. Org. Lett. 2003;5:793–796. doi: 10.1021/ol0273396. [DOI] [PubMed] [Google Scholar]
- 15.Job GE, Buchwald SL. Org. Lett. 2002;4:3703–3706. doi: 10.1021/ol026655h. [DOI] [PubMed] [Google Scholar]
- 16.Shafir A, Lichtor PA, Buchwald SL. J. Am. Chem. Soc. 2007;129:3490–3491. doi: 10.1021/ja068926f. [DOI] [PubMed] [Google Scholar]
- 17.Maiti D, Buchwald SL. J. Am. Chem. Soc. 2009;131:17423–17429. doi: 10.1021/ja9081815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Strieter ER, Bhayana B, Buchwald SL. J. Am. Chem. Soc. 2009;131:78–88. doi: 10.1021/ja0781893. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Strieter ER, Blackmond DG, Buchwald SL. J. Am. Chem. Soc. 2005;127:4120–4121. doi: 10.1021/ja050120c. [DOI] [PubMed] [Google Scholar]
- 19.(a) Tye JW, Weng Z, Giri R, Hartwig JF. Angew. Chem. 2010;49:2185–2189. doi: 10.1002/anie.200902245. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tye JW, Weng Z, Johns AM, Incarvito CD, Hartwig JF. J. Am. Chem. Soc. 2008;130:9971–9983. doi: 10.1021/ja076668w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S-L, Liu L, Fu Y, Guo Q-X. Organometallics. 2007;26:4546–4554. [Google Scholar]
- 21.(a) Jenkins CL, Kochi JK. J. Am. Chem. Soc. 1972;94:856–865. [Google Scholar]; (b) Kochi JK. J. Am. Chem. Soc. 1957;79:2942–2948. [Google Scholar]
- 22.(a) Whitesides GM, Kendall PE. J. Org. Chem. 1972;37:3718–3725. [Google Scholar]; (b) Whitesides GM, Fischer WF, Jr., San Filippo J, Jr., Bashe RW, House HO. J. Am. Chem. Soc. 1969;91:4871–4882. [Google Scholar]
- 23.Johnson CR, Dutra GA. J. Am. Chem. Soc. 1973;95:7783–7785. [Google Scholar]
- 24.(a) Cohen T, Cristea I. J. Am. Chem. Soc. 1976;98:748–753. [Google Scholar]; (b) Cohen T, Lewarchik RJ, Tarino JZ. J. Am. Chem. Soc. 1974;96:7753–7760. [Google Scholar]; (c) Cohen T, Wood J, Dietz AG., Jr. Tetrahedron Lett. 1974:3555–3558. [Google Scholar]
- 25.Van Allen D. Amherst: University of Massachusetts; 2004. [Google Scholar]
- 26.(a) Paine AJ. J. Am. Chem. Soc. 1987;109:1496–1502. [Google Scholar]; (b) Couture C, Paine AJ. Can. J. Chem. 1985;63:111–120. [Google Scholar]; (c) Weingarten H. J. Org. Chem. 1964;29:977–978. [Google Scholar]; (d) Weingarten H. J. Org. Chem. 1964;29:3624–3626. [Google Scholar]
- 27.Arai S, Hida M, Yamagishi T. Bull. Chem. Soc. Jpn. 1978;51:277–282. [Google Scholar]
- 28.Bethell D, Jenkins IL, Quan PM. J. Chem. Soc., Perkin Trans. II. 1985:1789–1795. [Google Scholar]
- 29.Huffman LM, Stahl SS. J. Am. Chem. Soc. 2008;130:9196–9197. doi: 10.1021/ja802123p. [DOI] [PubMed] [Google Scholar]
- 30.Klapars A, Antilla JC, Huang XH, Buchwald SL. J. Am. Chem. Soc. 2001;123:7727–7729. doi: 10.1021/ja016226z. [DOI] [PubMed] [Google Scholar]
- 31.Gajare AS, Toyota K, Yoshifuji M, Ozawa F. Chem. Commun. 2004:1994–1995. doi: 10.1039/b408232j. [DOI] [PubMed] [Google Scholar]
- 32.Xia N, Taillefer M. Chem. Eur. J. 2008;14:6037–6039. doi: 10.1002/chem.200800436. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Zhang YY, Zhu LB, Lan JB, Xie RG, You JS. J. Am. Chem. Soc. 2007;129:13879–13886. doi: 10.1021/ja073633n. [DOI] [PubMed] [Google Scholar]
- 34.Frisch MJ, et al. Gaussian 03. Wallingford CT: Gaussian, Inc; 2004. Revision E.01. [Google Scholar]
- 35.(a) Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]; (b) Becke AD. J. Chem. Phys. 1993;98:1372–1377. [Google Scholar]; (c) Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 36.(a) Stephens PJ, Devlin FJ, Ashvar CS, Bak KL, Taylor PR, Frisch MJ. ACS Symp. Ser. 1996;629:105. [Google Scholar]; (b) Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ. J. Phys. Chem. 1994;98:11623–11627. [Google Scholar]; (c) Hehre WJ, Radom L, Schleyer PV, Pople JA. Ab Initio Molecular Orbital Theory. New York: Wiley; 1986. [Google Scholar]
- 37.(a) Becke AD. J. Chem. Phys. 1996;104:1040–1046. [Google Scholar]; (b) Adamo C, Barone V. J. Chem. Phys. 1998;108:664–675. [Google Scholar]; (c) Zhao Y, Truhlar DG. J. Phys. Chem. A. 2004;108:6908–6918. [Google Scholar]
- 38.A number of computational methods were evaluated for their abilities to correctly predict the observed selectivities. These studies are summarized in the Supporting Information that accompanies this article.
- 39.Lynch BJ, Truhlar DG. J. Phys. Chem. A. 2003;107:3898–3906. [Google Scholar]
- 40.(a) Cossi M, Rega N, Scalmani G, Barone V. J. Comput. Chem. 2003;24:669–681. doi: 10.1002/jcc.10189. [DOI] [PubMed] [Google Scholar]; (b) Barone V, Cossi M. J. Phys. Chem. A. 1998;102:1995–2001. [Google Scholar]; (c) Andzelm J, Kölmel C, Klamt A. J. Chem. Phys. 1995;103:9312–9320. [Google Scholar]; (d) Klamt A, Schüürmann G. J. Chem. Soc. Perkin Trans. 1993;2:799–805. [Google Scholar]
- 41.The selectivities of reactions involving the β-diketone-promoted Cu-catalyzed coupling of aminopentanol with aryl iodides in butyronitrile are similar to those in DMF.
- 42.For a recent review of the estimation of activation free energies using Marcus theory, see Houmam A. Chem. Rev. 2008;108:2180–2237. doi: 10.1021/cr068070x. For a practical demonstration of the estimation of activation free energies using Marcus theory, see Lin CY, Coote ML, Gennaro A, Matyjaszewski K. J. Am. Chem. Soc. 2008;130:12762–12774. doi: 10.1021/ja8038823.
- 43.(a) Marcus RA, Sutin N. Biochem. Biophys. Acta. 1982;811:265–322. [Google Scholar]; (j) Marcus RA. Faraday Discuss. Chem. Soc. 1982;74:7–15. [Google Scholar]; (b) Marcus RA. Theory and Applications of Electron Transfers at Electrodes and in Solution. In: Rock PA, editor. Special Topics in Electrochemistry. New York: Elsevier; 1977. pp. 61–179. [Google Scholar]; (c) Marcus RA. J. Chem. Phys. 1965;43:679–701. [Google Scholar]; (d) Marcus RA. Annu. Rev. Phys. Chem. 1964;15:155–196. [Google Scholar]; (e) Hush NS. Trans. Faraday Soc. 1961;57:557–580. [Google Scholar]; (f) Marcus RA. Can. J. Chem. 1959;37:155–163. [Google Scholar]; (g) Hush NS. J. Chem. Phys. 1958;28:962–972. [Google Scholar]; (h) Marcus RA. J. Chem. Phys. 1956;24:979–989. [Google Scholar]; (i) Marcus RA. J. Chem. Phys. 1957;26:872–877. [Google Scholar]; (j) Marcus RA. J. Chem. Phys. 1956;24:966–978. [Google Scholar]
- 44.(a) Savéant J-M. Acc. Chem. Res. 1992;26:455–461. [Google Scholar]; (b) Savéant J-M. J. Am. Chem. Soc. 1987;114:10595–10602. [Google Scholar]; (c) Savéant J-M. J. Am. Chem. Soc. 1987;109:6788–6795. [Google Scholar]; (d) Savéant J-M. Dissociative Electron Transfer. In: Mariano PS, editor. Advances in Electron Transfer Chemistry. Vol. 4. New York: JAI Press; 1994. pp. 53–116. [Google Scholar]
- 45.Marcus-Hush and Savéant theories were also used to estimate activation free energies for SET from reaction free energies for formation of the ligated Cu(II) intermediates and iodobenzene radical ions shown in Table 1 (see supporting information for details). The estimated activation free energies for SET from (ket)Cu(methoxide) and (ket)Cu(methylamido) to iodobenzene are 28.1 and 26.6 kcal/mol, respectively; SET from (phen)CuI(methoxide) and (phen)Cu(methylamido) to iodobenzene require energies of 38.0 and 35.6 kcal/mol, respectively. These barriers are similar to the reaction free energies presented in Table 1.
-
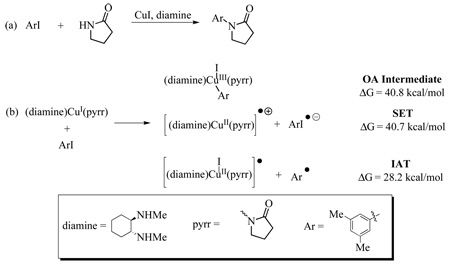
46.For the purpose of comparison with the results presented in the main article, we have computed the energies for SET, IAT and OA-complex formation in the reaction of 2-pyrrolidinone (pyrr) with 3,5-dimethyliodobenzene (ArI) catalyzed by CuI and N1,N2-dimethylcyclohexane-1,2-diamine (diamine) (see figure (a) below; for further details see references 16 and 18). As shown below in figure (b), SET is isoenergetic with the formation of the OA intermediate (ΔG = 41 kcal/mol). Significantly, IAT is more favorable than either SET or OA (ΔG = 28 kcal/mol). This suggests that as a general trend, electron-rich β-diketones promote reactions via SET, while phenanthrolines, diamines and other less electron-rich ligands promote reactions via IAT.
- 47. Fukui K, Fujimoto H. Bull. Chem. Soc. Jpn. 1967;40:2018–2025. Fukui K, Fujimoto H. Bull. Chem. Soc. Jpn. 1969;42:3399–3409. Fukui K, Fortschr Chem. Forsch. 1970;15:1. Fukui K. Acc. Chem. Res. 1971;4:57–64. Houk KN. Acc. Chem. Res. 1975;8:361–369. For reviews, see Houk KN. In: Pericyclic Reactions. Marchand AP, Lehr RE, editors. Vol. 2. New York: Academic Press; 1977. Fukui K. Angew. Chem. Int. Ed. Engl. 1982;21:801–809.
- 48.Kozmin SA, Green MT, Rawal VH. J. Org. Chem. 1999;64:8045–8047. doi: 10.1021/jo981563k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.